Abstract
Rationale
A delicate balance between protein synthesis and degradation maintains cardiac size and function. TRIM63 encoding Muscle RING Finger 1 (MuRF1) maintains muscle protein homeostasis by tagging the sarcomere proteins with ubiquitin for subsequent degradation by the Ubiquitin-Proteasome System (UPS).
Objectives
To determine the pathogenic role of TRIM63 in human hypertrophic cardiomyopathy (HCM).
Methods and Results
Sequencing of TRIM63 gene in 302 HCM probands (250 Caucasians) and 339 controls (262 Caucasians) led to identification of two missense (p.A48V and p.I130M) and a deletion (p.Q247*) variants exclusively in the HCM probands. These three variants were absent in 751 additional controls screened by TaqMan assays. Likewise, rare variants were enriched in the Caucasian HCM population (11/250, 4.4% vs. 3/262, 1.1%, respectively, p=0.024). Expression of the mutant TRIM63 was associated with mislocalization of TRIM63 to sarcomere Z disks, impaired auto-ubiquitination, reduced ubiquitination and UPS-mediated degradation of myosin heavy chain 6, cardiac myosin binding protein C, calcineurin (PPP3CB), and p-MTOR in adult cardiac myocytes. Induced expression of the mutant TRIM63 in the mouse heart was associated with cardiac hypertrophy, activation of the MTOR-S6K and calcineurin pathways and expression of the hypertrophic markers, which were normalized upon turning off expression of the mutant protein.
Conclusions
TRIM63 mutations, identified in patients with HCM, impart loss-of-function effects on E3 ligase activity and are likely causal mutations in HCM. The findings implicate impaired protein degradation in the pathogenesis of HCM.
Keywords: Genetics, mutation, hypertrophic cardiomyopathy, ubiquitin, hypertrophy
INTRODUCTION
A delicate balance between protein synthesis and degradation maintains muscle trophic homeostasis, including cardiac size and function. A shift in protein homeostasis in favor of synthesis, resulting in increased cell protein content, is an established mechanism for cardiac hypertrophy. 1 The molecular pathways responsible for enhanced protein synthesis and ensuing cardiac hypertrophy have been extensively characterized. 2 In contrast, the potential contributions of the protein degradation pathways, including the ubiquitin-proteasome system (UPS), in maintaining cardiac protein homeostasis and the pathogenesis of cardiac hypertrophy are less well understood. 3-7
Hypertrophic cardiomyopathy (HCM) is a relatively common genetic disorder and a prototypic form of cardiac hypertrophy. 8, 9 Over a dozen causal genes, coding for thick, thin and Z disk proteins of sarcomeres, have been identified in probands and families with HCM. 8, 9 Cardiac hypertrophy, notwithstanding the genetic-based diagnosis, is the clinical diagnostic hallmark of HCM and a major determinant of mortality and morbidity. 10-12 HCM is the most common cause of sudden cardiac death (SCD) in the young athletes and an important cause of morbidity in the older adults. 9, 13
Cardiac hypertrophy in HCM is considered secondary to activation of a diverse array of intracellular signaling pathways that collectively promote protein synthesis. 14, 15 The role of protein degradation, the opposite end of the spectrum from protein synthesis, in the pathogenesis of HCM is less well recognized. 16, 17 Identification and functional characterization of TRIM63 encoding MuRF1 and FBXO32 encoding F-box protein 32 (a.k.a. Atrogin 1 or MAFbx) have raised considerable interest in the role of these molecular regulators of cardiac protein degradation in maintaining cardiac structure and function in cardiomyopathies. 4, 5, 18-20
TRIM63 (protein ID:Q969Q1), also known as muscle-specific RING finger protein 1 (MuRF1), is an E3 ubiquitin ligase that is expressed selectively in cardiac and skeletal muscles. 18 TRIM63 is capable of polyubiquitination and UPS-mediated degradation of thick filament proteins MYH6 and MYBPC3. 21-23 Over-expression of MuRF1 (TRIM63) antagonizes cardiac myocyte hypertrophic response to agonists and increases susceptibility to heart failure in response to pressure overload. 4,24 In contrast, deficiency of MuRF1 (MuRF1-/-) exaggerates cardiac hypertrophic response to aortic banding. 6 In view of the increasing recognition of protein degradation pathways in regulating muscle trophic state, we set to delineate the pathogenic role of TRIM63 in cardiomyopathies. Accordingly, we screened HCM probands for mutations in TRIM63, identified and functionally characterized three mutations that invoke impaired protein degradation as a likely pathogenic mechanism for cardiac hypertrophy in human HCM.
METHODS
An expanded METHODS section is provided as Online data.
Ethical approval
The Institutional Review Board approved the studies in humans. The participants signed informed consents. The use of mice conformed to the Guide for the Care and Use of Laboratory Animals published by the US National Institutes of Health and was approved by the Institutional Animal Care and Use Committee.
Study population
The discovery study population was comprised of 302 (250 Caucasians) probands with HCM and 339 (262 Caucasians) controls individuals, all evaluated by history, physical examination, ECG and echocardiography. Additionally 751 control individuals were screened by the TaqMan assays for the specific rare variants identified in the HCM probands.
TRIM63 variants
The coding and exon-intron boundary regions of TRIM63 were sequenced by Sanger sequencing (Online Table I). The sequence output was analyzed using Variant Reporter software and manually by two individuals and compared with the TRIM63 reference sequence (hg19, Chromosome 1, NC_000001.10, 26377798..26394121, complement). Variant genotyping was performed using the Allelic Discrimination Assays on a 7900HT SDS (Online Table I).
Multiple sequence alignment
Regions encompassing the mutant amino acids from multiple species were aligned using ClustalW (http://www.ebi.ac.uk/Tools/msa/clustalw2/).
Exclusion of known common causal genes for HCM
TRIM63 mutation carriers were screened for mutations in MYH7, MYBPC3, TNNT2, TNNI3, TPM1 and ACTC1, known relatively common genes for HCM, by Sanger sequencing. 9
Genotyping
Family members were genotyped for five short tandem repeat (STR) DNA markers located near the TRIM63 locus on 1p34-p33 by PCR and capillary electrophoresis on an ABI 3730xl system 25 (Online Figure I).
Cloning and site directed mutagenesis
Trim63 cDNA was synthesized from mouse total cardiac RNA by reverse transcription and was tagged with a Flag at the 3′ end. The mutations were introduced by site directed mutagenesis (Online Table I).
Recombinant lenti- and adenoviruses
Flag-tagged WT, p.A48V, p.I130M and p.Q247* Trim63 cDNAs were cloned into the lentiviral expression vector. The lentiviral plasmid containing the Trim63 cDNA and the packaging plasmids were transfected into 293T cells to generate the viruses.
Flag-tagged Trim63 cDNAs were cloned into adenovirus expression vector by homologous recombination. The plasmid DNA was mixed with Lipofectamine™ 2000 and transfected to 293A cells. Cells were harvested 7-10 days post-transfection, when reached ~80% cytopathicity. The crude adenovirus lysates were prepared by freeze/thaw cycles. Viruses were amplified to generate higher-titer viral stocks.
HeLa-His/Biotin-Ubiquitin cells
A MSCV retroviral plasmid expressing a His6-Biotin-Ubiquitin fusion protein and retrovirus packaging plasmids were co-transfected into 293T cells. Retroviruses were then used to infect the HeLa cells. Cells were then selected under blasticidin selection to generate stable HeLa-His/Bio-Ub cells.
Adult ventricular myocytes
Adult cardiac myocytes were isolated from four to six months old mice (FVB background) upon retrograde perfusion and enzymatic digestion with collagenase Type II and were reintroduced to calcium at a final concentration of 200μM CaCl2, in the presence of 2mM ATP. Myocytes were counted in a hemocytometer and placed on laminin-coated plates in a CO2 incubator at 37°C.
Adenoviral Infection
Adult cardiac myocytes were transduced with the recombinant adenoviruses at a multiplicity of infection of 100 for 4 hours. 26-28
Doxycycline-inducible wild type and mutant TRIM63 transgenic mice
Cardiac-restricted inducible tet-off transgenic mice expressing TRIM63WT, TRIM63A48V, TRIM63I130M and TRIM63Q247* were generated by the conventional methods 29, 30. The regulator mice (Myh6-tTA) and the tet-O target vector were kind and generous gifts from Dr. Robbins. 29, 30
Immunoblotting
Immunoblotting was performed using aliquots of 30ug of protein extracts as published. 31
Co-IP
To detect auto-ubiquitination, aliquots of 2μg/ml of Biotin were added to culture media of Hela-His/Bio-Ubiquitin cells transduced with the recombinant lentiviruses. Cells were cultured in the presence of MG132, a proteasome inhibitor, and a protease inhibitor cocktail. Cell lysates were sonicated to shear DNA and cell membranes. Strepavidin-Agarose Slurry was added to precipitate biotinylated proteins followed by electrophoresis and immunoblotting.
Likewise, 500ug aliquots of adult cardiac myocytes proteins, extracted in the presence of MG132, were pre-cleared with IgG and Protein A/G agarose beads, incubated with an anti Ubiquitin antibody and treated with Protein A/G agarose beads. The immunoprecipitates were used for immunoblotting.
Immunofluorescence
Immunofluorescence was performed per published methods. 31 Briefly, co-localization of ubiquitin and TRIM63 in HeLa-His/Bio-Ub cells was detected upon treating the cells with biotin to label ubiquitin. The cells were incubated with a rabbit polyclonal anti Flag antibody followed by incubation with a mixture of a donkey anti rabbit antibody conjugated with Alexa Fluor® 350 and Streptavidin conjugated with Texas Red. Co-localization in myocytes was performed using a monoclonal anti Flag antibody conjugated to Cy3 and the nuclei were counter-stained with DAPI. To detect co-localization of TRIM63 and α-actinin, adenovirally transduced adult cardiac myocytes were fixed, permeabilized and incubated with mouse monoclonal anti α-actinin. The secondary antibody was donkey anti mouse conjugated with Alexa Fluor 488 dye.
Creatine kinase (CK) enzymatic assay
Aliquots of protein (10μg) were mixed with the reconstituted reagent and incubated at room temperature. The optical density of each sample was read using a microplate reader at absorbance wavelength of 340nm at 10 min, 25 min and 40min. CK activities were calculated based on colorimetric readouts.
Echocardiography
Transthoracic (M mode and 2D) and Doppler echocardiography was performed to assess cardiac function in the age- and sex-matched non-transgenic and transgenic animals using an HP 5500 Sonos echocardiography unit equipped with a 15-MHz linear transducer. 31-33
Cardiac morphology and histology
The heart was explanted, fixed by perfusion and photographed. Ventricular weight was determined after excising the heart at the atrioventricular groove to isolate atria and the great blood vessels. Thin myocardial sections were stained with H&E, and Masson trichrome. Collagen volume fraction was determined by morphometric analysis using ImageTool 3.0 analysis software (http://ddsdx.uthscsa.edu/dig/itdesc.html). 33, 34
Myocyte cross sectional area (CSA) was determined after staining of the cryosections with 2μg/ml wheat germ agglutinin conjugated with Texas-Red. The sections were mounted in Hard SetTM mounting medium and examined under fluorescence microscopy. 33
Enrichment of ubiquitinated cardiac proteins
Heart protein lysates (250 μg) were incubated with Tandem Ubiquitin Binding Entities (TUBEs) agarose beads. Beads were collected by centrifugation, washed with TBS-T and the ubiquitin-enriched proteins were eluted for immunoblotting.
Turning off and on expression of the TRIM63Q247* transgene
To shut down the expression of the transgene, three-month old non-transgenic and double transgenic mice were fed with doxycycline at a 500ug/ml concentration in water for 30 days. Doxycycline was then withdrawn in half of the treated animals in order to re-introduce expression of the transgene. After 72 hours of re-expression of the transgene, mice were euthanized for molecular analysis.
qPCR
The mRNA levels of selected markers were quantified by qPCR using specific TaqMan Gene expression assays, as published 31. The list of the probes is shown in Online Table I.
Statistics
Differences among the groups (mean ±SD) were compared by one-way analysis of variance for normally distributed continuous variables. Bonferroni correction for multiple-comparisons was applied. Variables that did not follow a normal distribution pattern were compared by Kruskal-Wallis test. Differences in the categorical variables were compared by Chi Square test. All statistical analyses were performed using STATA v. 10.1.
The authors had full access to the data and take responsibility for its integrity. All authors have read and agree to the manuscript as written.
RESULTS
Characteristics of the study population
The discovery study population included 302 probands including 250 Caucasians with HCM and 339 (262 Caucasians) control individuals. Characteristics of the study population were largely similar to those published. 35 In brief, males comprised 54% of the HCM study population. The mean age of the HCM population was 51.4 ± 16.5 years and the mean interventricular septal thickness was 1.9 ± 0.47 cm (Online Table II).
TRIM63 variants
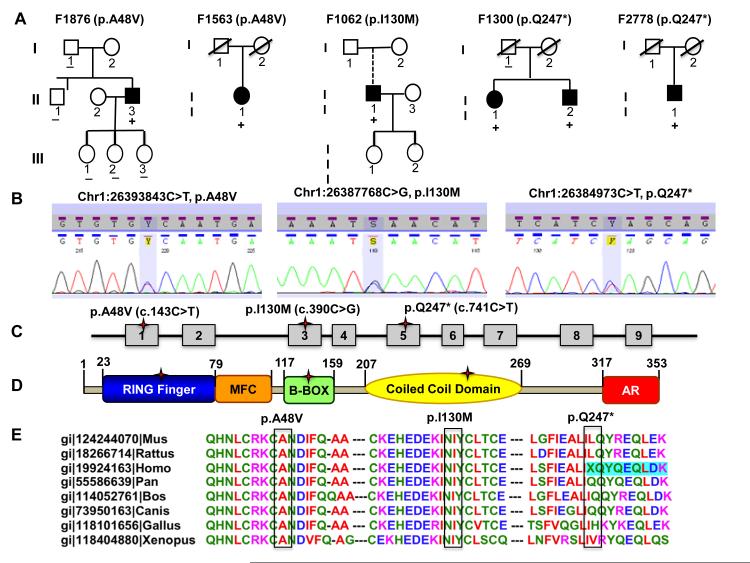
Five rare variants (MAF<0.01) and one common variant (MAF=0.17) were detected in the study population (Online Table III). All rare variants were detected in the Caucasians. The prevalence of the rare variants was enriched in the Caucasian probands with HCM, as the rare variants were present in 11/250 (4.4%) probands with HCM and 3/262 (1.1%) of the control individuals (Χ2=5.1, p=0.024). The rare TRIM63 (RefSeq:NP_115977) variants p.A48V (Chr1:26393843C>T, p.Ala48Val), p.I130M (Chr1:26387768C>G, p.Ile130Met) and p.Q247* (Chr1:26384973C>T, p.Glu247Ter) were exclusive to five probands with HCM and absent in 339 control individuals that were sequenced and 751 control individuals that were screened by TaqMan assays. The p.A48V and p.Q247* occurred in two unrelated families and were present in the affected but not in unaffected members of the families (Figure 1, Panel A). The sizes of the families, nevertheless, were inadequate for co-segregation or a formal genetic linkage. Genotyping of the family members for five STR markers and 21 single nucleotide polymorphisms (SNPs) at the TRIM63 locus (1p34-1p33) showed unique haplotypes for individuals with the p.A48V mutation. The finding suggests an independent origin of the mutation, assuming no recombination event at the region between the TRIM63 and the marker. However, individuals with the p.Q247* shared the same haplotype for the STR markers and SNPs. Hence, an independent origin of the p.Q247* in these two families could not be established (Online Figure I). The p.E269K SNP (rs61749355, mRNA position 941, c.805G>A, NM_032588.2, GAG>AAΓ) was identified in 2 control individuals and 6 probands with HCM. A rare p.R195C variant was identified in a single control individual, who had no history of medical problems and had a normal ECG and echocardiogram. A known common SNP p.E237K (rs2275950, mRNA position 845, G>A) was detected at equal frequency in HCM probands and controls (MAF: 0.23 and 0.18, respectively, X2=5.0, p=0.082). The complete list of non-synonymous and other variants is shown in Online Table III.
Figure 1. TRIM63 mutations.
A. Pedigrees of families with TRIM63 mutations. The full circles (female) and squares (male) indicate the affected individuals. Those with the mutations are identified with the + sign. A broken connecting line indicates an adopted person. The slash through sign indicates a deceased person. B. Electrophoregram of three TRIM63 mutations identified in HCM probands. C and D. Topographic location of the TRIM63 mutations on exons and protein, respectively. E. Multiple sequence alignment showing evolutionary conservation of the affected amino acids.
The p.A48V and p.I130M affected highly conserved amino acids and the p.Q247* variant led to deletion of 106 aa from the 353 aa long full-length protein (Figure 1 B-E). Residues 48 and 130 are located in RING-type (aa 23-79) and B-box-type (117-159) domains of Zinc finger motif in TRIM63 protein, respectively. Residue 130 is also located at interacting domain with titin (TTN), which includes residues 74 to 218. The p.Q247* mutation is located in the coil-coiled domain (aa 207-269), which typically provides mechanical stability to the proteins. PolyPhen2 prediction positioned the missense variants at the probably damaging category (highest). 36 Considering the human molecular genetic data, the p.A48V, p.I130M and p.Q247* were referred to as “mutations”.
Exclusion of known causal genes for HCM
None of the five HCM probands who carried the TRIM63 mutations had a putative causal variant in MYH7, MYBPC3, TNNT2, TNNI3, TPM1, and ACTC1, known relatively common genes for HCM. 9
Phenotypic expression of TRIM63 mutations
The phenotype in mutation carrier was notable for moderate to severe left ventricular hypertrophy associated with left ventricular outflow tract obstruction in four out of six individuals requiring either surgical septal myectomy or transcatheter septal ablation (Online Table IV).
Mutations impair auto-ubiquitination of TRIM63
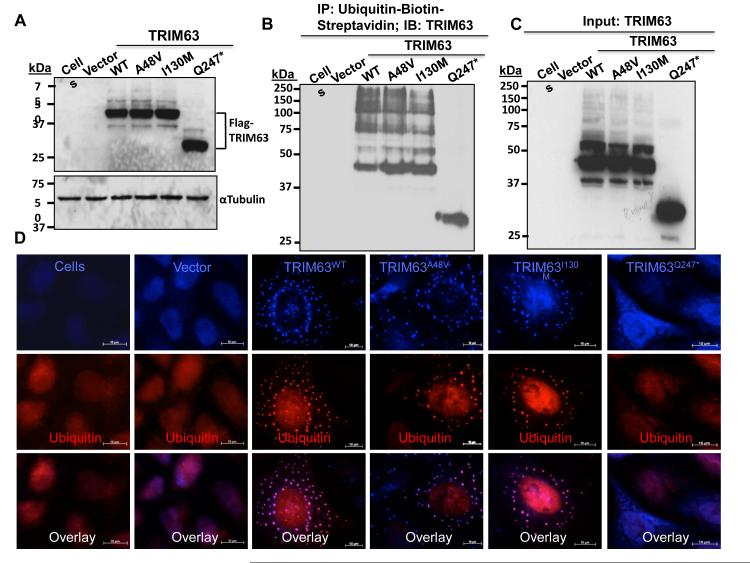
E3 ligases are known to undergo auto-ubiquitination, particularly in the absence of their primary substrates. This intrinsic feature of E3 ligases afforded the opportunity to test the effects of the p.A48V, p.I130M and p.Q247* mutations on TRIM63 ligase activity. 37 Transduction of the HeLa-His/Biotin-Ubiquitin cells with the recombinant lentiviruses expressing either Flag-tagged TRIM63WT, TRIM63A48V, TRIM63I130M or TRIM63Q247* followed by co-immunoprecipitation (Co-IP) showed near complete loss of auto-ubiquitination in cells transduced with the TRIM63Q247* lentiviral construct (Figure 2A-C). Likewise, immunofluorescence staining of the transduced HeLa-His/Biotin-Ubiquitin cells for ubiquitin and TRIM63 (Flag) showed no discernible auto-ubiquitinated TRIM63 in the cells transduced with the TRIM63Q247* viruses (Figure 2D). Quantitative analysis also showed 60 to 70% reductions in the levels of auto-ubiquitinated TRIM63A48V and TRIM63I130M (Online Figure II).
Figure 2. Effects of the mutations on auto-ubiquitination of TRIM63.
A. Immunoblot showing expression of the wild type (WT) and mutant TRIM63 proteins in HeLa cells transduced with the recombinant lentiviruses. B. Auto-ubiquitination of the WT and mutant TRIM63 in MG132 treated HeLa-His/Bio-Ub cells detected by Co-immunoprecipitation (Co-IP). Biotinylated ubiquitin was precipitated with streptavidin-conjugate agarose beads and probed with an anti Flag antibody. C. Immunoblot of the input protein probed with an anti Flag (TRIM63) antibody. D. Immunofluorescence staining of auto-ubiquitinated TRIM63 in HeLa-His/Bio-Ub cells treated with MG132. TRIM63 is detected using an anti Flag antibody and biotinylated ubiquitin with a streptavidin-conjugated anti ubiquitin antibody. Percent auto-ubiquitinated TRIM63 aggregates, as determined by the percentage of co-localized ubiquitin and TRIM63 proteins, were 76.7 ± 13%, 31.5 ± 8%, 21.0 ± 5% and 0% in the TRIM63WT, TRIM63A48V, TRIM63I130M and TRIM63Q247*, respectively (N= 20 cells per group, ANOVA p<0.001 and p<0.05 any of the mutant TRIM63 vs TRIM63WT).
Mutations impair ubiquitination of TRIM63 substrates in adult cardiac myocytes
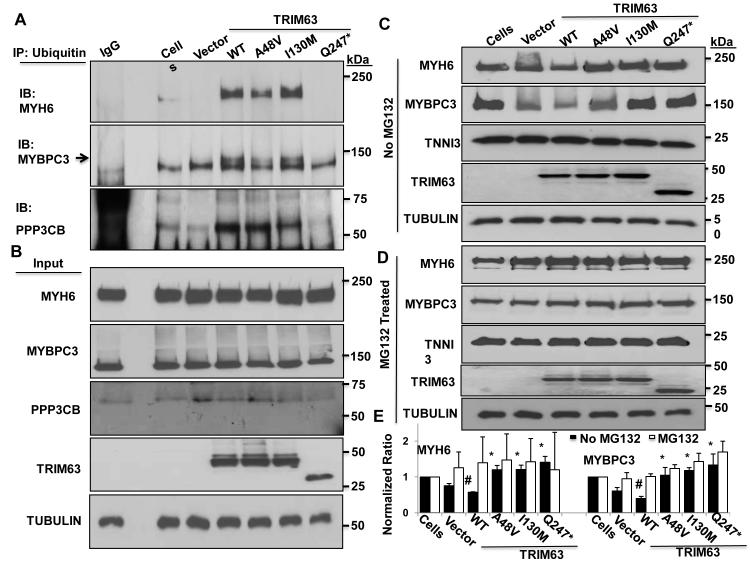
To detect whether mutations affected binding to and ubiquitination of TRIM63 known substrates, cardiac myocytes were transduced with the recombinant adenoviruses and levels of ubiquitinated and total MYH6 and MYBPC3 were determined by Co-IP and immunoblotting. Co-IP of ubiquitinated MYH6 and MYBPC3 with an anti ubiquitin antibody, performed in the presence of MG132 to prevent degradation of ubiquitinated proteins, showed a near complete absence of ubiquitinated MYH6 and MYBPC3 in cardiac myocytes transduced with Ad5/CMV/TRIM63Q247* viruses (Figure 3, A and B). As compared to TRIM63WT, level of co-precipitated MYH6 and MYBPC3 were modestly reduced in cardiac myocytes transduced with recombinant viruses expressing TRIM63A48V protein but was largely unchanged in myocytes transduced with the TRIM63I130M viruses (Figure 3, A and B). Because FBXO32 (Atrogin 1 or MAFbx), a muscle E3 ligase, is known to target calcineurin, we determined ubiquitination of PPP3CB (protein phosphatase 3 catalytic subunit β) in cardiac myocytes transduced with the WT or mutant TRIM63 constructs. 38 As shown in Figure 3, A and B, level of ubiquitinated PPP3CB was increased in myocytes transduced with the TRIM63WT construct as compared to non-transduced myocytes. In contrast, level of ubiquitinated PPP3CB was drastically reduced in cardiac myocytes transduced with TRIM63Q247* viruses and moderately in the TRIM63I130M group.
Figure 3. Effects of the TRIM63 mutations on ubiquitination of MYH6, MYBPC3 and PPP3CB in adult cardiac myocytes.
A. IP was performed using an anti ubiquitin antibody and immunoblotting with anti MYH6, anti MYBPC3 and anti PPP3CB antibodies. B. Blots representing input proteins and α-TUBULIN (loading control). C and D. Immunoblots of MYH6 and MYBPC3 in the absence or presence of MG132, respectively. E. Quantitative data for MYH6 and MYBPC3 levels in the absence (black columns) or presence (open columns) of MG132 (N=3 per group, # p<0.05 compared to cells alone and *<0.05 compared to TRIM63WT).
Mutations impair UPS-mediated degradation of MYH6 and MYBPC3 in adult cardiac myocytes
To determine effects of impaired ubiquitination of mutant TRIM63 on protein levels of known substrates, we performed immunoblotting to detect and quantify MYH6 and MYBPC3 protein levels in cardiac myocytes transduced with the recombinant viruses in the presence or absence of MG132. As shown in Figure 3C, levels of MYH6 and MYBPC3 were reduced significantly in cardiac myocytes transduced with the adenoviruses expressing TRIM63WT in the absence of MG132, as compared to non-transduced cells. The findings confirm the previous data identifying thick filament proteins as targets of TRIM63. 39 In contrast, levels of MYH6 and MYBPC3 protein were increased in cells treated with the adenoviruses expressing TRIM63A48V, TRIM63I130M and TRIM63Q247* as compared to myocytes expressing TRIM63WT (Figure 3, C and E). Treatment with MG132 equalized levels of MYH6 and MYBPC3 in the experimental groups, supporting UPS mediated degradation of MYH6 and MYBPC3 in TRIM63WT group (Figure 3, D and E). Protein level of cardiac troponin I was unchanged in cells expressing the TRIM63WT protein and was similar in all experimental groups.
Mutations reduce localization of TRIM63 to sarcomere Z disk
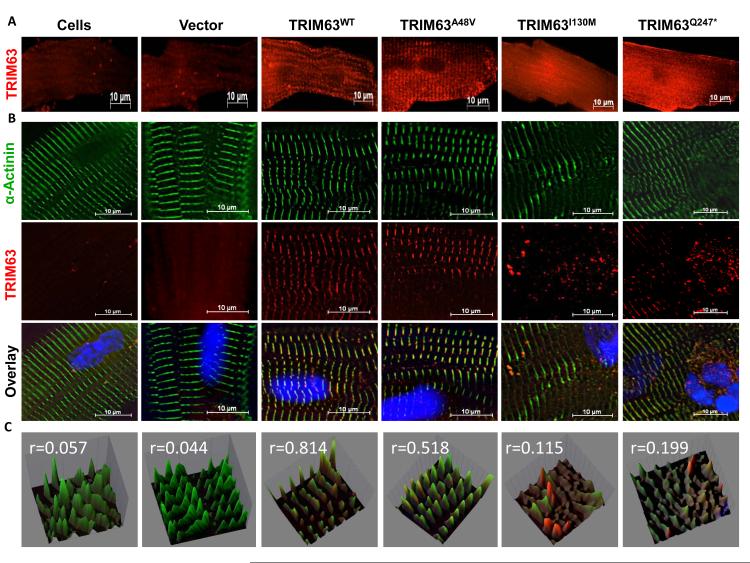
To determine whether TRIM63 mutations affected its localization in the sarcomere, cardiac myocytes were transduced with the recombinant viruses and co-stained with anti Flag (TRIM63) and anti α-actinin antibodies. As shown in Figure 4, TRIM63WT was co-localized with α-actinin at the Z disk (r=0.814). In contrast, TRIM63I130M and TRIM63Q247* had a diffuse expression pattern with minimal co-localization with α-actinin (r=0.115 and r=0.199, respectively). TRIM63A48V showed a moderate reduction in co-localization with α-actinin (r=0.518).
Figure 4. Reduced localization of mutant TRIM63 proteins to Z disks in cardiac myocytes.
A. Low-magnification immunofluorescence images of transduced adult cardiac myocytes expressing either a WT or a mutant TRIM63 protein. B. Deconvolution images of myocytes at Z disk regions after co-staining with anti α-ACTININ (green) and anti Flag (red) antibodies and the corresponding overlay images. C. Quantitative spectral display of Z disks stained for α-ACTININ and TRIM63 in the overlay images. Correlation coefficient (r value) between the two colors in each image is shown (A value of 0 indicates no correlation and 1 a perfect co-localization).
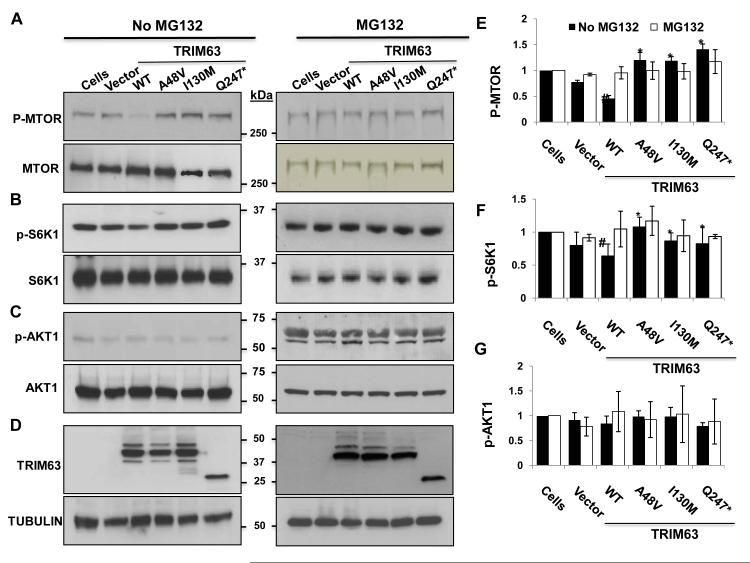
Mutations impair UPS-mediated degradation of MTOR-S6K hypertrophic signaling pathway in adult cardiac myocytes
To identify the mechanism(s) by which TRIM63 mutations cause HCM, we focused on the mammalian target of rapamycin (MTOR) pathway, not only because it is a major cardiac hypertrophic signaling pathway but also because F-box proteins, such as FBXW7 are known to target phosphorylated MTOR (p-MTOR) for ubiquitination and degradation. 40 Immunoblot analysis of protein extracts of adult cardiac myocytes transduced with the recombinant adenoviruses in the absence of the UPS inhibitor MG132 showed reduced level of p-MTOR in myocytes expressing the TRIM63WT protein (Figure 5A). In contrast, p-MTOR protein level in cardiac myocytes transduced with the mutant TRIM63 constructs was significantly higher as compared to the TRIM63WT group. Inhibition of the UPS by MG132 led to equalization of the p-MTOR level in in all experimental groups including in the TRIM63WT group. Total MTOR protein level was unchanged in all experimental groups in both MG132 treated and untreated cells.
Figure 5. Levels of selected signal regulators of cardiac hypertrophy in adult cardiac myocytes.
Immunoblots showing levels of p-MTOR and total MTOR (A), p-S6K1 and total S6K1 (B), and p-AKT1 and total AKT1 (C) in the presence and absence of MG132. D. Represents expression of WT and mutant TRIM63 in the experimental groups. E, F and G. show quantitative levels of p-MTOR, p-S6K1 and p-AKT1, respectively, in the absence (black columns) and presence (open columns) of MG132 (N=3 per group, # p<0.05 TRIM63WT compared to control cells; * p<0.05 Mutant TRIM63 groups compared to TRIM63WT).
To further investigate reduced p-MTOR levels in the TRIM63WT group, we detected and quantified levels of total and p-S6K1 and p-AKT1, downstream targets of MTORC1 and MTORC2, respectively, by immunoblotting. In accord with the reduced level of p-MTOR, level of p-S6K1 but not total S6K1 was also significantly reduced in cardiac myocytes expressing TRIM63WT but not in any of the mutant TRIM63 groups (Figure 5B). In contrast, protein level of p-AKT1 and total AKT1 were unchanged in cardiac myocytes transduced with the WT or mutant Trim63 constructs. Treatment with MG132 normalized reduced level of p-S6K1 in the TRIM63WT group and equalized in all groups.
To determine whether p-MTOR was the direct target of TRIM63, we performed Co-IP using an anti Flag antibody in immunoprecipitation and an anti p-MTOR antibody for immunoblotting and repeated the Co-IP in the reverse order. Despite six independent Co-IP reactions and using different antibodies, no significant Co-IP of p-MTOR with TRIM63 in any of the experimental groups was detected. Likewise, no significant Co-IP of p-S6K1 with TRIM63 was detected in any of the experimental groups. The null results of Co-IP studies might simply reflect the experimental conditions and yet might also indicate an indirect targeting of p-MTOR by TRIM63 through targeting other components of the MTORC1 complex. Therefore, we determined levels of p-Raptor, total Raptor, and GβL by immunoblotting. Levels of p-Raptor and GβL were largely unchanged and there were not significant differences in their levels among the experimental groups (Online Figure III).
Mutations had no discernible effects on CK activity
TRIM63 is implicated in regulating CK enzymatic activity. 41 To determine whether mutations affected CK enzymatic activity, CK enzymatic activity was quantified serially in adult mouse cardiac myocytes transduced with the recombinant adenoviruses. TRIM63 mutations had no significant effects on CK protein levels or CK enzymatic activity (Online Figure IV).
Inducible expression of mutant TRIM63 leads to cardiac hypertrophy
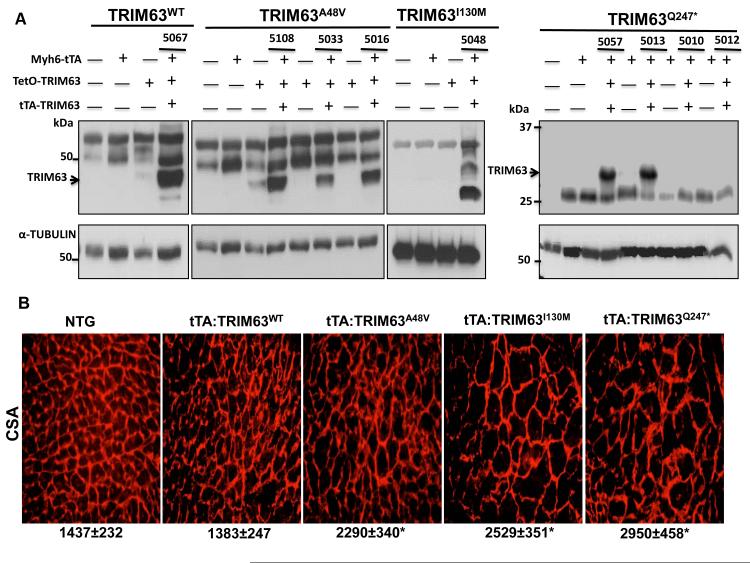
Because each human genome contains about 100 loss-of-function variants 42, to further substantiate the causal role of the TRIM63 mutations identified in human patients with HCM, we generated Doxycycline-inducible double transgenic mice, transcriptionally regulated by the Myh6 promoter 29, 30. Expression of the transgene was detected by immunoblotting of cardiac protein extracts in three tTA:TRIM63A48V, one tTA:TRIM63I130M, two tTA:TRIM63Q247* and one tTA:TRIM63WT independent lines (Figure 6A). To determine relative levels of the Flag-tagged transgene TRIM63 and endogenous TRIM63 proteins, cardiac protein extracts were probed by immunoblotting using a pan-TRIM63 antibody. Level of the transgene protein was lower than that of the endogenous TRIM63 protein in tTA:TRIM63WT, tTA”TRIM63A48A and tTA:TRIM63Q247* but was higher in the tTA:TRIM63I130M double transgenic mice (Online Figure V). Morphological and histological analysis showed increased ventricular weight/body weight by approximately 15 to 20% (Online Figure VI) and myocyte CSA by about 1.6 to 2-fold in the three mutant TRIM63 groups, particularly in tTA:TRIM63Q247* group, as compared to tTA:TRIM63WT or non-transgenic mice (Figure 6B). There was also an approximately 3-fold increase in interstitial fibrosis in the tTA:TRIM63I130M group as compared to non-transgenic mice (Online Figure VII). Interstitial fibrosis was not increased significantly in other mutant TRIM63 groups.
Figure 6. Cardiac and myocyte hypertrophy in Doxycycline-inducible WT and mutant TRIM63 transgenic mice.
A. Immunoblots showing expression of the transgene protein detected using an anti Flag antibody in double transgenic lines (without Doxycycline). Bands other than those corresponding to TRIM63 are thought to represent nonspecific antibody reactivity. There were no discernible differences in the expression levels of the transgene among different lines of the same gene, with the exception of the line 5033 in the TRIM63A48V group, which had a lower level of transgene protein. Therefore, TRIM63WT line 5067, TRIMA48V line 5016, TRIM63I130M line 5048 and for TRIM63Q247* line 5057 were used in the analysis. B. Gross cardiac morphology. C. Left ventricular weight/body weight ratio (mean ±SD, N=6 to 12 mice per group). D. Representative WGA stained thin myocardial sections reflective of myocyte cross-sectional area. E. Myocyte cross sectional area (mean ± SD), was measured in over 2,000 myocytes per mouse and three to four mice per group. # p<0.05 compared to non-transgenic mice, * p<0.05 compared to TRIM63WT. Molecular size markers on the right side of the panels relate only to the TRIM63Q247* group.
Echocardiographic evaluation of cardiac size and function showed increased left ventricular wall thickness, mass and mass index in the mutant TRIM63 groups, except for the TRIM63I130M, as compared to non-transgenic or tTA:TRIM63WT mice (Online Table V). Left ventricular fractional shortening was similar to that in the non-transgenic mice, except in the TRIM63I130M, which was decreased modestly. Expression of the TRIM63WT was associated with increased left ventricular end diastolic diameter and modestly decreased wall thickness, as compared to non-transgenic mice. However, left ventricular fractional shortening was preserved.
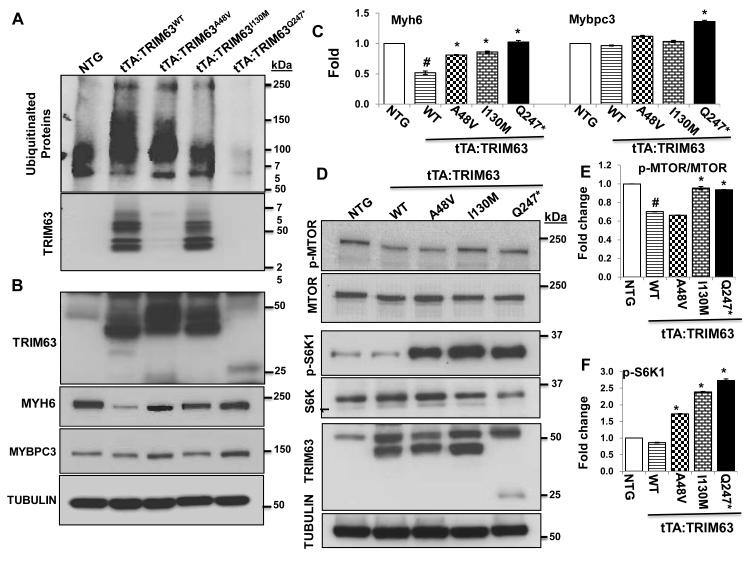
Mutations reduce ubiquitination of total protein, MYH6 and MYBPC3 and activate the MTOR-S6K pathway in the heart
To assess the effects of the mutations on cardiac protein ubiquitination, we enriched myocardial protein extracts for ubiquitinated proteins by passing the extracts through Tandem Ubiquitin Binding entities (TUBEs) agarose beads and probing the enriched proteins by immunoblotting using an anti Ubiquitin antibody. The findings were notable for a greater than two-fold increase in the level of ubiquitinated cardiac proteins in the tTA:TRIM63WT mice (Figure 7A). In contrast, levels of ubiquitinated total protein were reduced in the mutant tTA:TRIM63 groups, as compared to TRIM63WT mice and was near absent in the tTA:TRIM63Q247* mice. These findings are in accord with the in vitro data in the HeLa-His/Biotin-Ubiquitin cells and Co-IP studies in virally transduced adult cardiac myocytes. Likewise, level of ubiquitinated TRIM63A48V and TRIM63Q247* were reduced significantly but less so in the TRIM63I130M as compared to TRIM63WT (Figure 7A, lower panel).
Figure 7. Reduced ubiquitination of cardiac proteins in mutant TRIM63 transgenic mice.
A. Immunoblot showing levels of ubiquitinated cardiac proteins detected probed with an anti ubiquitin antibody. The lower panel represents ubiquitinated TRIM63 enriched for ubiquitination and probed with an anti Flag antibody. B. Immunoblots showing levels of TRIM63, MYH6, MYBPC3 and α-TUBULIN proteins, the latter as a control for loading conditions. C. Quantitative data on MYH6 and MYBPC3 levels. D. Levels of p-MTOR, total MTOR, p-S6K1 and total S6K1 along with panels showing expression of the transgene proteins and α-TUBULIN. E and D. Quantitative data on protein levels of p-MTOR and p-S6K1. N=3-4 mice per group, # p<0.05 compared to non-transgenic mice, * p<0.05 compared to TRIM63WT.
To analyze levels of the specific sarcomere proteins, we determined protein levels of TRIM63, MYH6 and MYBPC3 in the cardiac protein extracts by immunoblotting. As shown in Figure 7, panels B and C, MYH6 level were reduced in the tTA:TRIM63WT mice, as compared to non-transgenic mice but were higher in the mutant tTA:TRIM63 groups as compared to the tTA:TRIM63WT mice but equal to that in the non-transgenic mice, in accord with the in vitro data in cultured cardiac myocytes. MYBPC3 level was only increased in the tTA:TRIM63Q247* mouse heart.
Immunobloting was performed to detect and quantify levels of the main components of TORC1 complex. There was an approximately 30% reduction in p-MTOR level in the tTA:TRIM63WT group as compared to non-transgenic mice. Notably, p-S6K1 level in the heart was dramatically increased in the mutant tTA-TRIM63 group, as compared to non-transgenic or TRIM63WT mice (Figure 7, D-F).
Because over-expression of TRIM63 has been shown to affect Mybpc3 mRNA level, we also assessed mRNA levels of Myh6, Mybpc3, Mtor, Ppp2cb and Rps6kb1 (S6k) in the heart of WT and mutant TRIM63 transgenic animals. 20 There were no significant differences in the mRNA levels of the selected molecules (Online Figure VIII).
Switching on expression of mutant TRIM63 proteins in the heart activates the MTOR-S6K and calcineurin-NFAT1 pathways
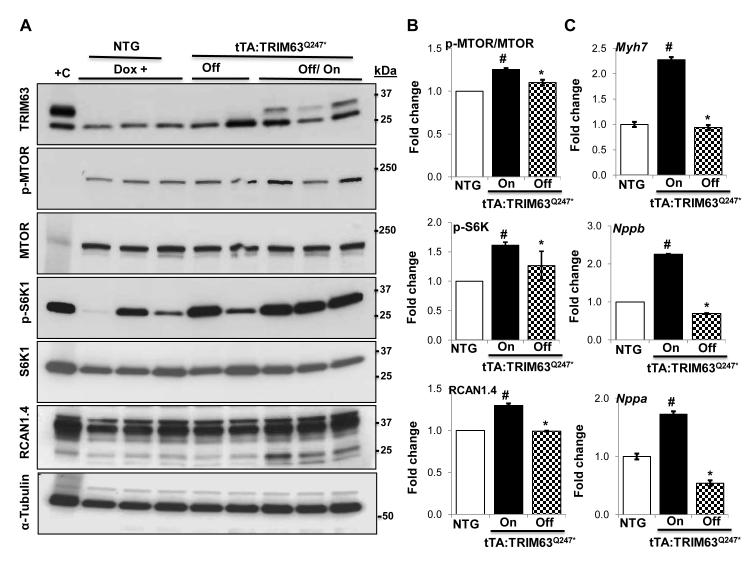
The switch on and off bigenic mouse approach afforded the opportunity to turn off expression of the mutant TRIM63 protein and then analyze induction of expression of the molecular regulators and markers of cardiac hypertrophy. In this set of experiments tTA:TRIM63Q247* was used as this mutation was associated with the most dramatic phenotype in isolated adult cardiac myocytes and in transgenic mice. Accordingly, expression of the TRIM63Q247* was turned off in the tTA:TRIM63Q247* mice upon daily administration of Doxycycline for 30 days and then it was turned on in half of the animals – by withdrawing Doxycycline (Online Figure IX). Immunoblotting performed three days after turning on expression of the mutant transgene showed concordant increase in the expression levels of p-MTOR, pS6K1 and RCAN1.4 and normalization of these proteins upon turning off in the mutant TRIM63 bigenic mice (Figure 8, A and B). In conjunction with activation of the hypertrophic signaling pathways, mRNA levels of Myh7, Nppa and Nppb, quantified by qPCR, were increased three days after turning on and were normal upon turning off expression of the mutant TRIM63Q247* protein (Figure 8, C). Echocardiographic assessment of cardiac size and function showed normal wall thickness and systolic function during the period when the transgene expression was shut down as well as three days after turning on expression of TRIM63Q247* protein.
Figure 8. Induction of cardiac hypertrophic markers upon turning off and on expression of the mutant TRIM63 protein.
A. Immunoblots representing levels of the transgene TRIM63, p-MTOR, total MTOR, p-S6K1, total S6K1, RCAN1.4 and α-TUBULIN upon withdrawal (turning on) and administration of Doxycycline (turning on) expression of the TRIM63Q247*. B. Quantitative data for the p-MTOR, p-S6K1 and RCAN1.4. The comparisons were made between off and on states of the transgene and between transgenic on mode and non-transgenic groups. TRIM63WT mice were not included in these experiments. C. qPCR data on relative mRNA levels of selected hypertrophic markers Myh7, Nppa and Nppb. (N= 3 per group, # p<0.05 compared to non-transgenic mice, * p<0.05 compared to TRIM63WT).
DISCUSSION
We provide human molecular genetic and in vitro and in vivo functional evidence to implicate TRIM63, encoding MuRF1, an E3 ubiquitin ligase, as a likely causal gene for human HCM. The p.A48V, p.I130M and p.Q247* variants were exclusive to the HCM study population (Caucasians) and were not detected in over 500 control Caucasian individuals. The small size of the families was not permissive to genotype-phenotype co-segregation analysis, which is a limitation of the study. The nonsense (p.Q247*) and the p.A48V variants recurred in two families. The nonsense variant led to premature truncation and loss of approximately 1/3rd of the protein. The p.A48V and p.I130M variants affected highly conserved amino acids and were predicted – in silico - to be probably damaging to protein structure and function. Functionally, the variants had loss-of-function effects on E3 ubiquitin ligase activity, as detected in specialized Ubiquitin-tagged HeLa cells, virally transduced adult cardiac myocytes and transgenic hearts. Furthermore, expression of the mutant TRIM63 in the heart through inducible transgenesis led to cardiac and myocyte hypertrophy in mice, activation of the MTOR-S6K and calcineurin-RCAN1.4 pathways and expression of the hypertrophic markers. The truncating TRIM63Q247* had the most prominent in vitro and in vivo phenotypic effects. Collectively, the human molecular genetic data along with the functional studies in adult cardiac myocytes and transgenic mice support the causal role of TRIM63 in the pathogenesis of human HCM. The findings implicate impaired degradation of cardiac proteins as a pathogenic mechanism for cardiac hypertrophy and activation of the MTOR-S6K and calcineurin-RCAN1.4 pathways as the responsible pathways involved in the pathogenesis of HCM caused by TRIM63 mutations.
The main biological function of UPS in the heart is clearance of the misfolded and damaged proteins. Likewise, TRIM63 (MuRF1) and FBX032 (commonly known as atrogin-1 or MAFbx), the major components of the UPS in the heart, are implicated in the removal of mutant sarcomere proteins in HCM. 20 In addition, a mismatch between UPS capacity and accumulation of substrates is commonly observed in various forms of cardiac hypertrophy and dysfunction, which might actively participate in the pathogenesis of cardiomyopathies. 3, 5, 16, 17, 43 The findings of the present study extend the biological functions of UPS in the heart and provide direct genetic and functional evidence to implicate defective protein degradation as the initial impetus for induction of cardiac hypertrophy in HCM. These discoveries advocate the notion that cardiac hypertrophy might also be a disease of impaired protein degradation.
TRIM63 mutations led to loss of E3 ligase activity, as reflected by reduced auto-ubiquitination and ubiquitination of the known substrates. The mutations, however, exhibited variable functional effects and cardiac phenotypes, which are not unanticipated but rather inherent to phenotypic expression of mutations in various genes, as best illustrated for the phenotypic plasticity of LMNA mutations. 44 The phenotype was more prominent for the truncating TRIM63Q247* mutation, which apparently, at least in part, had escaped the Nonsense Mediated Decay (NMD) mechanism, an established mechanism for haploinsufficiency resulting from the nonsense mutations in HCM. 17, 45 Stable expression of the truncated TRIM63, observed in HeLa cells transduced with a lentiviral construct, in adult cardiac myocytes infected with an adenoviral construct and in the heart of transgenic mice, might reflect the use of cDNA in these experiments as opposed to the genomic fragment. Alternatively, the relatively distal location of the p.Q247* mutation from the translation initiation site might afford the opportunity for the stable expression of a 246 aa protein. The truncated TRIM63 protein exhibited a near total loss of the E3 ligase activity, as demonstrated by near total loss of auto-ubiquitination and ubiquitinylation of TRIM63 substrates MYH6 and MYBPC3. In addition, the level of ubiquitinated calcineurin (PPP3CB) was drastically lower in cardiac myocytes transduced with the TRIM63Q247* viruses. Thus, the biological and functional data are the strongest for the TRIM63Q247*.
Expression of TRIM63WT was associated with reduced levels of MYH6 and MYBPC3, known substrates for TRIM63. 39 However, protein level of cardiac troponin I was unchanged, which has been observed in some but not other studies. 39,23 Reduced MYH6 and MYBPC3 levels and increased level of ubiquitinated PPP3CB, were associated with mild thinning of the interventricular septum and left ventricular enlargement as compared to non-transgenic mice but no significant change in left ventricular mass, mass index or fractional shortening at the baseline. Over-expression of TRIM63WT in the heart is known to render the heart susceptible to failure in response to stress. 4
The findings also implicate activation of MTOR-S6K (MTORC1 pathway) and calcineurin in the pathogenesis of HCM caused by the TRIM63 mutations and hence, links the protein degradation and synthesis pathways. These findings suggest MTORC1 and PPP3CB but not MTORC2 pathways, as the potential direct or indirect targets of TRIM63 in the heart. Despite reduced levels of p-MTOR and PPP3CB upon over-expression of TRIM63, we could not demonstrate co-precipitation of TRIM63 and p-MTOR or PPP3CB. Thus, the changes in the p-MTOR, p-S6K1, and RCAN1.4 levels might be secondary to activation of the hypertrophic program instigated by the TRIM63 mutations. Nonetheless, activation of p-MTOR/p-S6K and calcineurin/RCAN1.4 pathways in isolated myocytes and the mouse heart support the causal role of TRIM63 variants in the pathogenesis of HCM. The precise mechanism(s) by which a homeostatic shift in favor of reduced protein degradation instigates a cardiac hypertrophic response remain unknown.
Several alternative mechanisms might also account or influence the phenotypic consequences of TRIM63 mutations. The truncating mutation, which eliminates a major part of the coiled coil domain, might interfere with the mechanical stability of the protein and affect its interactions with proteins other than MYH6 and MYBPC3. The p.A48V mutation is located in RING type domain, which mediates ubiquitin-ligase activity by binding to ubiquitination enzymes as well as the substrates. 46 The p.I130M and p.Q247* also affected subcellular distribution of TRIM63 and its co-localization with the α-actinin. The p.I130M mutation is located in the B-box type domains of Zinc finger motif, which is also the interacting domain with titin (residues 74 to 218 on TRIM63 and repeats A168/A169 in titin), adjacent to the titin kinase domain. 47, 48 TRIM63 is involved in regulating muscle trophic homeostasis through interactions with a number of proteins including glycogen phosphorylase and FOXO (Forkhead box, subgroup O). 47 Whether TRIM63 mutations influence titin kinase activity or ubiquitination of other substrates is unknown. The crystal structure of the B-Box domain of TRIM63 has been resolved. 49 The isoleucine at position 130 resides in a groove near the interacting domain with the muscle type CK. 50 Substitution of methionine, which has a side branch, for isoleucine might affect its interactions with CK. However, under the conditions of our experiments, we detected no significant effects of over expression of wild type or mutant TRIM63 on CK enzymatic activity in virally transduced cardiac myocytes. It is also possible that reduced E3 ligase activity of the mutant TRIM63 proteins impairs clearance of misfolded and damaged sarcomere proteins, which might partially incorporate into the sarcomere, and cause sarcomere dysfunction leading to cardiac hypertrophy and dysfunction. Likewise, reduced clearance of damaged and misfolded TRIM63 substrate proteins could lead to their accumulation in the endoplasmic reticulum, inducing an unfolded protein response, which might contribute to the pathogenesis of cardiac phenotype. 51 Therefore, various alternative or additive mechanisms – beyond those delineated in the present studies - might be responsible for the hypertrophic effects of TRIM63 mutations in humans. Although we did not detect significant differences in the levels of WT and mutant TRIM63 proteins in transduced cardiac myocytes and in the transgenic hearts, differences, beyond the sensitivity of the immunoblotting, could exist in the expression levels of WT and mutant TRIM63 that might confound data interpretation.
In conclusion, we provide molecular genetic evidence in humans and functional data in isolated cardiac myocytes and in inducible transgenic mouse models to implicate TRIM63 as a likely causal gene for human HCM. The discoveries provide evidence for impaired degradation of sarcomere proteins as a mechanism for the pathogenesis of HCM in humans. Consequently, sarcomeres are not only the contractile units of striated muscles but are also signaling hubs for regulation of muscle trophic homeostasis and hence, cardiac size and function.
Supplementary Material
Online Figure I. Genotypes of the family members for short tandem repeat (STR) markers near the TRIM63 locus. A. Selected STR markers at the 1p34-p33 locus and their chromosomal position.
B. Affected members of the F1876 and F1563 families, who carry the p.A48V mutation do not share a common allele for the D1S2644 marker, suggesting an independent origin, provisional to no recombination event in the region between the TRIM63 and the marker.
C. Affected members of F1330 and F2778 who carry the p.Q247* variant shared a common allele for the markers tested. Hence, they might not be independent in origin.
Online Figure II. Percent auto-ubiquitinated TRIM63 aggregates, as determined by the percentage of co-localized ubiquitin and TRIM63 proteins (N= 20 cells per group, ANOVA p<0.001; # p<0.05, TRIM63WT vs. Cell alone; * any of the mutant TRIM63 groups vs. TRIM63WT.
Online Figure III. Effects of TRIM63 mutations on selected components of TORC1 complex: A. Immunoblots of additional components of mTORC1, namely p-Raptor, total raptor and GβL, in transgenic mice expressing either a wild type (TRIM63WT) or one of the three mutant TRIM63 (TRIM63A48V, TRIM63I130M and TRIM63Q247*). The differences in the protein levels of the components of TORC1 complex were not significant. Quantitative data are shown in panel B.
Online Figure IV. Effects of TRIM63 mutations on CK protein level and enzymatic activity: A. Immunoblots showing expression of WT and mutant TRIM63 (TRIM63A48V, TRIM63130M and TRIM63Q247*) in adult cardiac myocytes transduced with the recombinant adenoviruses. B. Immunoblot showing expression level of muscle CK in cardiac myocytes in the experimental groups. C. Enzymatic activity of muscle CK at three different time points after addition of the substrates among the experimental groups. D. Quantitative level of CK enzymatic activity at 30 minutes after initiation of the reaction among the experimental groups
Online Figure V. Expression level of endogenous and transgene TRIM63, FBXO32 and TRIM55 in the hearts of tTA:TRIM63 transgenic mice. Immunoblots showing expression of the endogenous and transgene TRIM63 proteins, detected using a pan-TRIM63 antibody, FBXO32 and TRIM55. Protein level of the Flag-tagged transgene wild type (TRIM63WT) and mutant TRIM63A48V and TRIM63Q247* proteins were lower than that of the endogenous TRIM63. However, transgene TRIM63I130M protein level was higher than the endogenous TRIM63 protein level.
Online Figure VI. Ventricular weight /Body Weight. * p<0.05 Bonferroni corrected pairwise p value between the mutant and wild type (WT) TRIM63 transgenic mice
Online Figure VII. Cardiac Histology in inducible transgenic mice: H&E (upper panels) and Masson trichrome (MT) stained thin myocardial section (low and high magnifications) from non-transgenic, wild type bigenic (tTA:TRIM63WT and mutant bigenic TRIM63 mice. N= 3-6 per group.
* p<0.05 compared to non-transgenic (NTG)
Online Figure VIII. mRNA levels of selected TRIM63 targets in transgenic mice: Panel A. mRNA levels of Myh6, Mybpc3, Mtor, Rps6kb1 (S6K), Ppp2cb and Gapdh in the experimental groups (N=3 per group). No significant differences were detected in the mRNA levels of the selected genes between non-transgenic (NTG) and wild type TRIM63 (tTA:TRIM63WT) mice.
Panel B. shows the actual amplification plots of selected mRNA in the experimental groups. The plots are practically super-imposed. Plots for Myh6 are not included as the experiments were done on a separate run.
Online Figure IX. Experimental approach to turning Off and On expression of TRIM63Q247* in the heart. Doxycycline was administered to 3-month old mice for 30 days to turn off expression of the transgene. A control group of non-transgenic mice was also treated with Doxycycline for 30 days. At the end of 30 days of treatment, Doxycycline was withdrawn in half of the mice in each group to turn on expression of the transgene. Levels of selected molecules was detected three days after turning on expression of the transgene.
Online Table I A. Sequence of oligonucleotide primers used in Sanger Sequencing of TRIM63 exons
B. Sequence of Oligonucleotide primers used in 5′ Nuclease (TaqMan) Assay (Assay by Design)
C. Oligonucleotide primers used in site directed mutagenesis
D. TaqMan probes used in the qPCR reactions
Online Table II Demographic and Phenotypic Characteristics of the Study Population
Online Table III A. Rare Non-synonymous Variants Detected in The Study Population
C. Synonymous, Non-coding and Deletion Variants Detected in the Study Population
Online Table IV Phenotypic Characteristics of HCM Probands with Rare TRIM63 Variants
Online Table V Echocardiographic phenotype in Trim63 Inducible transgenic mice
Novelty and Significance.
What Is Known?
Hypertrophic cardiomyopathy (HCM), a single gene disorders with Mendelian patterns of inheritance, is a major cause of sudden cardiac death in the young and morbidity in the adult.
Mutations in over a dozen genes, encoding for sarcomere proteins are known to cause HCM.
The molecular mechanisms responsible for the phenotypic expression of HCM are diverse but largely involve impaired sarcomere structure and function.
Impaired ubiquitin-proteasome system (UPS) is implicated in the pathogenesis of HCM phenotype.
What New Information Does This Study Contribute?
We identified three rare variants in TRIM63 as likely causal mutations in small families with HCM and showed that rare variants in TRIM63 were enriched in HCM cases.
TRIM63 rare variants were loss-of-function variants and impaired E3 ubiquitin ligase activity of TRIM63 against its known substrates MYH6 and MYBPC3.
The findings also implicated MTOR and PPPC3B as potential new targets of TRIM63.
With identification of the common causal genes for HCM, the focus has been shifted to identification of the uncommon causal genes, typically in sporadic cases and small families. However, molecular genetic data in sporadic cases and small families are often inconclusive because of the abundance of the common and rare including functional variants in each genome. The genetic approaches need to be complemented with molecular mechanistic studies to strengthen the causal role of the novel or rare variants identified in sporadic cases or small families. In the present study, we provide human molecular genetic data in conjunction with the mechanistic studies in adult cardiac myocytes and inducible transgenic mice to implicate TRIM63 as a likely causal gene for human HCM. The findings illustrate the diversity of the molecular genetic causes of HCM and emphasize the complexity of the mechanisms involved in the pathogenesis of HCM. Successful prevention and treatment of HCM is likely to necessitate delineation of the specific genes and the molecular pathways involved in its pathogenesis. Such will provide the opportunity for specific interventions targeted to specific genes and pathways in order to prevent the evolving phenotype and reverse the established HCM.
ACKOWLEDGMENTS
The authors wish to acknowledge scientific guidance of Tony Eissa, M.D., Mark Entman. M.D., and Heinrich Taegtmeyer, M.D., Ph.D. who served on the thesis advisory committee for SNC.
SOURCES OF FUNDING This work is supported in part by grants from NHLBI (R01-HL088498 and R34HL105563); NIA (R21 AG038597-01), Burroughs Wellcome Award in Translational Research (#1005907), TexGen Fund from Greater Houston Community Foundation and George and Mary Josephine Hamman Foundation.
Non-standard Abbreviations
- HCM
Hypertrophic cardiomyopathy
- TRIM63
Tripartite motif containing 63
- UPS
Ubiquitin proteasome system
- MuRF1
Muscle-specific RING Finger 1
- SCD
Sudden cardiac death
- MYH6
Myosin heavy chain 6 (or alpha)
- MYBPC3
Myosin binding protein C 3
- PPP3CB
Protein phosphatase 3 catalytic subunit, Beta isoform
- MTOR
Mammalian target of rapamycin
- WT
Wild type
Footnotes
DISCLOSURES None
This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
REFERENCES
- 1.Simpson P. Stimulation of hypertrophy of cultured neonatal rat heart cells through an alpha 1-adrenergic receptor and induction of beating through an alpha 1- and beta 1-adrenergic receptor interaction. Evidence for independent regulation of growth and beating. Circulation Research. 1985;56:884–894. doi: 10.1161/01.res.56.6.884. [DOI] [PubMed] [Google Scholar]
- 2.Heineke J, Molkentin JD. Regulation of cardiac hypertrophy by intracellular signalling pathways. Nat Rev Mol Cell Biol. 2006;7:589–600. doi: 10.1038/nrm1983. [DOI] [PubMed] [Google Scholar]
- 3.Schlossarek S, Carrier L. The ubiquitin-proteasome system in cardiomyopathies. Current Opinion in Cardiology. 2011;26:190–195. doi: 10.1097/HCO.0b013e32834598fe. [DOI] [PubMed] [Google Scholar]
- 4.Willis MS, Schisler JC, Li L, Rodriguez JE, Hilliard EG, Charles PC, Patterson C. Cardiac muscle ring finger-1 increases susceptibility to heart failure in vivo. Circ Res. 2009;105:80–88. doi: 10.1161/CIRCRESAHA.109.194928. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Mearini G, Schlossarek S, Willis MS, Carrier L. The ubiquitin-proteasome system in cardiac dysfunction. Biochim Biophys Acta. 2008;1782:749–763. doi: 10.1016/j.bbadis.2008.06.009. [DOI] [PubMed] [Google Scholar]
- 6.Willis MS, Ike C, Li L, Wang DZ, Glass DJ, Patterson C. Muscle ring finger 1, but not muscle ring finger 2, regulates cardiac hypertrophy in vivo. Circ Res. 2007;100:456–459. doi: 10.1161/01.RES.0000259559.48597.32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Willis MS, Patterson C. Into the heart: The emerging role of the ubiquitin-proteasome system. J Mol Cell Cardiol. 2006;41:567–579. doi: 10.1016/j.yjmcc.2006.07.015. [DOI] [PubMed] [Google Scholar]
- 8.Watkins H, Ashrafian H, Redwood C. Inherited cardiomyopathies. The New England Journal Of Medicine. 2011;364:1643–1656. doi: 10.1056/NEJMra0902923. [DOI] [PubMed] [Google Scholar]
- 9.Marian AJ. Hypertrophic cardiomyopathy: From genetics to treatment. Eur J Clin Invest. 2010;40:360–369. doi: 10.1111/j.1365-2362.2010.02268.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Spirito P, Bellone P, Harris KM, Bernabo P, Bruzzi P, Maron BJ. Magnitude of left ventricular hypertrophy and risk of sudden death in hypertrophic cardiomyopathy. N.Engl.J.Med. 2000;342:1778–1785. doi: 10.1056/NEJM200006153422403. [DOI] [PubMed] [Google Scholar]
- 11.Elliott PM, Gimeno B, Jr., Mahon NG, Poloniecki JD, McKenna WJ. Relation between severity of left-ventricular hypertrophy and prognosis in patients with hypertrophic cardiomyopathy. Lancet. 2001;357:420–424. doi: 10.1016/S0140-6736(00)04005-8. [DOI] [PubMed] [Google Scholar]
- 12.Olivotto I, Gistri R, Petrone P, Pedemonte E, Vargiu D, Cecchi F. Maximum left ventricular thickness and risk of sudden death in patients with hypertrophic cardiomyopathy. J Am Coll Cardiol. 2003;41:315–321. doi: 10.1016/s0735-1097(02)02713-4. [DOI] [PubMed] [Google Scholar]
- 13.Maron BJ, Doerer JJ, Haas TS, Tierney DM, Mueller FO. Sudden deaths in young competitive athletes: Analysis of 1866 deaths in the united states, 1980-2006. Circulation. 2009;119:1085–1092. doi: 10.1161/CIRCULATIONAHA.108.804617. [DOI] [PubMed] [Google Scholar]
- 14.Ashrafian H, McKenna WJ, Watkins H. Disease pathways and novel therapeutic targets in hypertrophic cardiomyopathy. Circulation Research. 2011;109:86–96. doi: 10.1161/CIRCRESAHA.111.242974. [DOI] [PubMed] [Google Scholar]
- 15.Marian AJ. Genetic determinants of cardiac hypertrophy. Curr Opin Cardiol. 2008;23:199–205. doi: 10.1097/HCO.0b013e3282fc27d9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Bahrudin U, Morisaki H, Morisaki T, Ninomiya H, Higaki K, Nanba E, Igawa O, Takashima S, Mizuta E, Miake J, Yamamoto Y, Shirayoshi Y, Kitakaze M, Carrier L, Hisatome I. Ubiquitin-proteasome system impairment caused by a missense cardiac myosin-binding protein c mutation and associated with cardiac dysfunction in hypertrophic cardiomyopathy. Journal of molecular biology. 2008;384:896–907. doi: 10.1016/j.jmb.2008.09.070. [DOI] [PubMed] [Google Scholar]
- 17.Carrier L, Schlossarek S, Willis MS, Eschenhagen T. The ubiquitin-proteasome system and nonsense-mediated mrna decay in hypertrophic cardiomyopathy. Cardiovascular Research. 2010;85:330–338. doi: 10.1093/cvr/cvp247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Bodine SC, Latres E, Baumhueter S, Lai VK, Nunez L, Clarke BA, Poueymirou WT, Panaro FJ, Na E, Dharmarajan K, Pan ZQ, Valenzuela DM, DeChiara TM, Stitt TN, Yancopoulos GD, Glass DJ. Identification of ubiquitin ligases required for skeletal muscle atrophy. Science. 2001;294:1704–1708. doi: 10.1126/science.1065874. [DOI] [PubMed] [Google Scholar]
- 19.Gomes MD, Lecker SH, Jagoe RT, Navon A, Goldberg AL. Atrogin-1, a muscle-specific f-box protein highly expressed during muscle atrophy. Proc Natl Acad Sci U S A. 2001;98:14440–14445. doi: 10.1073/pnas.251541198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Mearini G, Gedicke C, Schlossarek S, Witt CC, Kramer E, Cao P, Gomes MD, Lecker SH, Labeit S, Willis MS, Eschenhagen T, Carrier L. Atrogin-1 and murf1 regulate cardiac mybp-c levels via different mechanisms. Cardiovasc Res. 2010;85:357–366. doi: 10.1093/cvr/cvp348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Fielitz J, Kim MS, Shelton JM, Latif S, Spencer JA, Glass DJ, Richardson JA, Bassel-Duby R, Olson EN. Myosin accumulation and striated muscle myopathy result from the loss of muscle ring finger 1 and 3. J Clin Invest. 2007;117:2486–2495. doi: 10.1172/JCI32827. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Clarke BA, Drujan D, Willis MS, Murphy LO, Corpina RA, Burova E, Rakhilin SV, Stitt TN, Patterson C, Latres E, Glass DJ. The e3 ligase murf1 degrades myosin heavy chain protein in dexamethasone-treated skeletal muscle. Cell Metab. 2007;6:376–385. doi: 10.1016/j.cmet.2007.09.009. [DOI] [PubMed] [Google Scholar]
- 23.Kedar V, McDonough H, Arya R, Li HH, Rockman HA, Patterson C. Muscle-specific ring finger 1 is a bona fide ubiquitin ligase that degrades cardiac troponin i. Proc Natl Acad Sci U S A. 2004;101:18135–18140. doi: 10.1073/pnas.0404341102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Arya R, Kedar V, Hwang JR, McDonough H, Li HH, Taylor J, Patterson C. Muscle ring finger protein-1 inhibits pkc{epsilon} activation and prevents cardiomyocyte hypertrophy. J Cell Biol. 2004;167:1147–1159. doi: 10.1083/jcb.200402033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Daw EW, Chen SN, Czernuszewicz G, Lombardi R, Lu Y, Ma J, Roberts R, Shete S, Marian AJ. Genome-wide mapping of modifier chromosomal loci for human hypertrophic cardiomyopathy. Hum Mol Genet. 2007;16:2463–2471. doi: 10.1093/hmg/ddm202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Marian AJ, Yu QT, Mann DL, Graham FL, Roberts R. Expression of a mutation causing hypertrophic cardiomyopathy disrupts sarcomere assembly in adult feline cardiac myocytes. Circ Res. 1995;77:98–106. doi: 10.1161/01.res.77.1.98. [DOI] [PubMed] [Google Scholar]
- 27.Marian AJ, Zhao G, Seta Y, Roberts R, Yu QT. Expression of a mutant (arg92gln) human cardiac troponin t, known to cause hypertrophic cardiomyopathy, impairs adult cardiac myocyte contractility. Circ Res. 1997;81:76–85. doi: 10.1161/01.res.81.1.76. [DOI] [PubMed] [Google Scholar]
- 28.Zhou YY, Wang SQ, Zhu WZ, Chruscinski A, Kobilka BK, Ziman B, Wang S, Lakatta EG, Cheng H, Xiao RP. Culture and adenoviral infection of adult mouse cardiac myocytes: Methods for cellular genetic physiology. American journal of physiology. Heart and circulatory physiology. 2000;279:H429–436. doi: 10.1152/ajpheart.2000.279.1.H429. [DOI] [PubMed] [Google Scholar]
- 29.Sanbe A, Gulick J, Hanks MC, Liang Q, Osinska H, Robbins J. Reengineering inducible cardiac-specific transgenesis with an attenuated myosin heavy chain promoter. Circ Res. 2003;92:609–616. doi: 10.1161/01.RES.0000065442.64694.9F. [DOI] [PubMed] [Google Scholar]
- 30.Gulick J, Robbins J. Regulation of transgene expression using tetracycline. Curr Protoc Mol Biol. 2005 doi: 10.1002/0471142727.mb2312s71. Chapter 23:Unit 23 12. [DOI] [PubMed] [Google Scholar]
- 31.Lombardi R, da Graca Cabreira-Hansen M, Bell A, Fromm RR, Willerson JT, Marian AJ. Nuclear plakoglobin is essential for differentiation of cardiac progenitor cells to adipocytes in arrhythmogenic right ventricular cardiomyopathy. Circulation Research. 2011;109:1342–1353. doi: 10.1161/CIRCRESAHA.111.255075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Lombardi R, Dong J, Rodriguez G, Bell A, Leung TK, Schwartz RJ, Willerson JT, Brugada R, Marian AJ. Genetic fate mapping identifies second heart field progenitor cells as a source of adipocytes in arrhythmogenic right ventricular cardiomyopathy. Circ Res. 2009;104:1076–1084. doi: 10.1161/CIRCRESAHA.109.196899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Lombardi R, Bell A, Senthil V, Sidhu J, Noseda M, Roberts R, Marian AJ. Differential interactions of thin filament proteins in two cardiac troponin t mouse models of hypertrophic and dilated cardiomyopathies. Cardiovasc Res. 2008;79:109–117. doi: 10.1093/cvr/cvn078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Marian AJ, Senthil V, Chen SN, Lombardi R. Antifibrotic effects of antioxidant n-acetylcysteine in a mouse model of human hypertrophic cardiomyopathy mutation. J Am Coll Cardiol. 2006;47:827–834. doi: 10.1016/j.jacc.2005.10.041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Osio A, Tan L, Chen SN, Lombardi R, Nagueh SF, Shete S, Roberts R, Willerson JT, Marian AJ. Myozenin 2 is a novel gene for human hypertrophic cardiomyopathy. Circ Res. 2007;100:766–768. doi: 10.1161/01.RES.0000263008.66799.aa. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Adzhubei IA, Schmidt S, Peshkin L, Ramensky VE, Gerasimova A, Bork P, Kondrashov AS, Sunyaev SR. A method and server for predicting damaging missense mutations. Nat Methods. 2010;7:248–249. doi: 10.1038/nmeth0410-248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Laney JD, Hochstrasser M. Analysis of protein ubiquitination. Curr Protoc Protein Sci. 2011 doi: 10.1002/0471140864.ps1405s66. Chapter 14:Unit14 15. [DOI] [PubMed] [Google Scholar]
- 38.Li HH, Kedar V, Zhang C, McDonough H, Arya R, Wang DZ, Patterson C. Atrogin-1/muscle atrophy f-box inhibits calcineurin-dependent cardiac hypertrophy by participating in an scf ubiquitin ligase complex. The Journal Of Clinical Investigation. 2004;114:1058–1071. doi: 10.1172/JCI22220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Cohen S, Brault JJ, Gygi SP, Glass DJ, Valenzuela DM, Gartner C, Latres E, Goldberg AL. During muscle atrophy, thick, but not thin, filament components are degraded by murf1-dependent ubiquitylation. J Cell Biol. 2009;185:1083–1095. doi: 10.1083/jcb.200901052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Mao JH, Kim IJ, Wu D, Climent J, Kang HC, DelRosario R, Balmain A. Fbxw7 targets mtor for degradation and cooperates with pten in tumor suppression. Science. 2008;321:1499–1502. doi: 10.1126/science.1162981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Willis MS, Schisler JC, Li L, Rodriguez JE, Hilliard EG, Charles PC, Patterson C. Cardiac muscle ring finger-1 increases susceptibility to heart failure in vivo. Circulation Research. 2009;105:80–88. doi: 10.1161/CIRCRESAHA.109.194928. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.MacArthur DG, Balasubramanian S, Frankish A, Huang N, Morris J, Walter K, Jostins L, Habegger L, Pickrell JK, Montgomery SB, Albers CA, Zhang ZD, Conrad DF, Lunter G, Zheng H, Ayub Q, DePristo MA, Banks E, Hu M, Handsaker RE, Rosenfeld JA, Fromer M, Jin M, Mu XJ, Khurana E, Ye K, Kay M, Saunders GI, Suner MM, Hunt T, Barnes IH, Amid C, Carvalho-Silva DR, Bignell AH, Snow C, Yngvadottir B, Bumpstead S, Cooper DN, Xue Y, Romero IG, Wang J, Li Y, Gibbs RA, McCarroll SA, Dermitzakis ET, Pritchard JK, Barrett JC, Harrow J, Hurles ME, Gerstein MB, Tyler-Smith C. A systematic survey of loss-of-function variants in human protein-coding genes. Science. 2012;335:823–828. doi: 10.1126/science.1215040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Carrier L, Schlossarek S, Willis MS, Eschenhagen T. The ubiquitin-proteasome system and nonsense-mediated mrna decay in hypertrophic cardiomyopathy. Cardiovasc Res. 2010;85:330–338. doi: 10.1093/cvr/cvp247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Worman HJ, Bonne G. “Laminopathies”: A wide spectrum of human diseases. Experimental Cell Research. 2007;313:2121–2133. doi: 10.1016/j.yexcr.2007.03.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Vignier N, Schlossarek S, Fraysse B, Mearini G, Kramer E, Pointu H, Mougenot N, Guiard J, Reimer R, Hohenberg H, Schwartz K, Vernet M, Eschenhagen T, Carrier L. Nonsense-mediated mrna decay and ubiquitin-proteasome system regulate cardiac myosin-binding protein c mutant levels in cardiomyopathic mice. Circ Res. 2009;105:239–248. doi: 10.1161/CIRCRESAHA.109.201251. [DOI] [PubMed] [Google Scholar]
- 46.Joazeiro CA, Weissman AM. Ring finger proteins: Mediators of ubiquitin ligase activity. Cell. 2000;102:549–552. doi: 10.1016/s0092-8674(00)00077-5. [DOI] [PubMed] [Google Scholar]
- 47.McElhinny AS, Kakinuma K, Sorimachi H, Labeit S, Gregorio CC. Muscle-specific ring finger-1 interacts with titin to regulate sarcomeric m-line and thick filament structure and may have nuclear functions via its interaction with glucocorticoid modulatory element binding protein-1. J Cell Biol. 2002;157:125–136. doi: 10.1083/jcb.200108089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Centner T, Yano J, Kimura E, McElhinny AS, Pelin K, Witt CC, Bang ML, Trombitas K, Granzier H, Gregorio CC, Sorimachi H, Labeit S. Identification of muscle specific ring finger proteins as potential regulators of the titin kinase domain. J Mol Biol. 2001;306:717–726. doi: 10.1006/jmbi.2001.4448. [DOI] [PubMed] [Google Scholar]
- 49.Mrosek M, Meier S, Ucurum-Fotiadis Z, von Castelmur E, Hedbom E, Lustig A, Grzesiek S, Labeit D, Labeit S, Mayans O. Structural analysis of b-box 2 from murf1: Identification of a novel self-association pattern in a ring-like fold. Biochemistry. 2008;47:10722–10730. doi: 10.1021/bi800733z. [DOI] [PubMed] [Google Scholar]
- 50.Koyama S, Hata S, Witt CC, Ono Y, Lerche S, Ojima K, Chiba T, Doi N, Kitamura F, Tanaka K, Abe K, Witt SH, Rybin V, Gasch A, Franz T, Labeit S, Sorimachi H. Muscle ring-finger protein-1 (murf1) as a connector of muscle energy metabolism and protein synthesis. J Mol Biol. 2008;376:1224–1236. doi: 10.1016/j.jmb.2007.11.049. [DOI] [PubMed] [Google Scholar]
- 51.Dickhout JG, Carlisle RE, Austin RC. Interrelationship between cardiac hypertrophy, heart failure, and chronic kidney disease: Endoplasmic reticulum stress as a mediator of pathogenesis. Circulation Research. 2011;108:629–642. doi: 10.1161/CIRCRESAHA.110.226803. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Online Figure I. Genotypes of the family members for short tandem repeat (STR) markers near the TRIM63 locus. A. Selected STR markers at the 1p34-p33 locus and their chromosomal position.
B. Affected members of the F1876 and F1563 families, who carry the p.A48V mutation do not share a common allele for the D1S2644 marker, suggesting an independent origin, provisional to no recombination event in the region between the TRIM63 and the marker.
C. Affected members of F1330 and F2778 who carry the p.Q247* variant shared a common allele for the markers tested. Hence, they might not be independent in origin.
Online Figure II. Percent auto-ubiquitinated TRIM63 aggregates, as determined by the percentage of co-localized ubiquitin and TRIM63 proteins (N= 20 cells per group, ANOVA p<0.001; # p<0.05, TRIM63WT vs. Cell alone; * any of the mutant TRIM63 groups vs. TRIM63WT.
Online Figure III. Effects of TRIM63 mutations on selected components of TORC1 complex: A. Immunoblots of additional components of mTORC1, namely p-Raptor, total raptor and GβL, in transgenic mice expressing either a wild type (TRIM63WT) or one of the three mutant TRIM63 (TRIM63A48V, TRIM63I130M and TRIM63Q247*). The differences in the protein levels of the components of TORC1 complex were not significant. Quantitative data are shown in panel B.
Online Figure IV. Effects of TRIM63 mutations on CK protein level and enzymatic activity: A. Immunoblots showing expression of WT and mutant TRIM63 (TRIM63A48V, TRIM63130M and TRIM63Q247*) in adult cardiac myocytes transduced with the recombinant adenoviruses. B. Immunoblot showing expression level of muscle CK in cardiac myocytes in the experimental groups. C. Enzymatic activity of muscle CK at three different time points after addition of the substrates among the experimental groups. D. Quantitative level of CK enzymatic activity at 30 minutes after initiation of the reaction among the experimental groups
Online Figure V. Expression level of endogenous and transgene TRIM63, FBXO32 and TRIM55 in the hearts of tTA:TRIM63 transgenic mice. Immunoblots showing expression of the endogenous and transgene TRIM63 proteins, detected using a pan-TRIM63 antibody, FBXO32 and TRIM55. Protein level of the Flag-tagged transgene wild type (TRIM63WT) and mutant TRIM63A48V and TRIM63Q247* proteins were lower than that of the endogenous TRIM63. However, transgene TRIM63I130M protein level was higher than the endogenous TRIM63 protein level.
Online Figure VI. Ventricular weight /Body Weight. * p<0.05 Bonferroni corrected pairwise p value between the mutant and wild type (WT) TRIM63 transgenic mice
Online Figure VII. Cardiac Histology in inducible transgenic mice: H&E (upper panels) and Masson trichrome (MT) stained thin myocardial section (low and high magnifications) from non-transgenic, wild type bigenic (tTA:TRIM63WT and mutant bigenic TRIM63 mice. N= 3-6 per group.
* p<0.05 compared to non-transgenic (NTG)
Online Figure VIII. mRNA levels of selected TRIM63 targets in transgenic mice: Panel A. mRNA levels of Myh6, Mybpc3, Mtor, Rps6kb1 (S6K), Ppp2cb and Gapdh in the experimental groups (N=3 per group). No significant differences were detected in the mRNA levels of the selected genes between non-transgenic (NTG) and wild type TRIM63 (tTA:TRIM63WT) mice.
Panel B. shows the actual amplification plots of selected mRNA in the experimental groups. The plots are practically super-imposed. Plots for Myh6 are not included as the experiments were done on a separate run.
Online Figure IX. Experimental approach to turning Off and On expression of TRIM63Q247* in the heart. Doxycycline was administered to 3-month old mice for 30 days to turn off expression of the transgene. A control group of non-transgenic mice was also treated with Doxycycline for 30 days. At the end of 30 days of treatment, Doxycycline was withdrawn in half of the mice in each group to turn on expression of the transgene. Levels of selected molecules was detected three days after turning on expression of the transgene.
Online Table I A. Sequence of oligonucleotide primers used in Sanger Sequencing of TRIM63 exons
B. Sequence of Oligonucleotide primers used in 5′ Nuclease (TaqMan) Assay (Assay by Design)
C. Oligonucleotide primers used in site directed mutagenesis
D. TaqMan probes used in the qPCR reactions
Online Table II Demographic and Phenotypic Characteristics of the Study Population
Online Table III A. Rare Non-synonymous Variants Detected in The Study Population
C. Synonymous, Non-coding and Deletion Variants Detected in the Study Population
Online Table IV Phenotypic Characteristics of HCM Probands with Rare TRIM63 Variants
Online Table V Echocardiographic phenotype in Trim63 Inducible transgenic mice