Summary
In the last five years, the zebrafish (Danio rerio) has rapidly gained popularity as a model system for studying leukocyte migration and trafficking in vivo. The optical clarity of zebrafish embryos, as well as the potential for genetic manipulation and the development of tools for live imaging, have provided new insight into how leukocytes migrate in response to directional cues in live animals. This Commentary discusses recent progress in our understanding of how leukocytes migrate in vivo, including the role of intracellular signaling through phosphatidylinositol 3-kinase (PI3K) in both random and directed migration. The importance of leukocyte reverse migration in the resolution of inflammation will also be discussed. Finally, we will highlight how zebrafish models have helped to provide new insight into leukocyte migration and the way in which migration is altered in disease.
Key words: Chemotaxis, Migration, Neutrophil, Zebrafish
Introduction
Chemotaxis is the directed movement of cells in response to environmental cues. It is observed in diverse cell systems, ranging from bacteria to eukaryotes. The steps involved in chemotaxis include the sensing of attractants and the coupling of these cues to the intracellular motility machinery that drives directed cell movement. A key component of the chemotactic motility of eukaryotic cells is establishing cell polarity with leading edge actin polymerization and trailing edge actomyosin-based contraction and cell detachment. In multi-cellular organisms, chemotaxis is fundamental to normal development and the recruitment of immune cells to sites of tissue injury or infection.
Most of the progress in understanding chemotaxis has been achieved through the study of the single-celled organism, Dictyostelium discoideum, and from isolated mammalian neutrophils in two-dimensional (2D) environments in vitro (Van Haastert and Devreotes, 2004). It is only recently that zebrafish have gained popularity as a model system to study leukocyte migration and trafficking in vivo. The immune system of zebrafish and mammals is highly conserved on both the cellular and molecular level (Lieschke and Trede, 2009). Zebrafish develop immune cells, including lymphocytes, mast cells, dendritic cells, eosinophils, macrophages and neutrophils, produce chemokines and cytokines, including tumor necrosis factor alpha (TNF-α), interferon gamma (IFN-γ), granulocyte colony-stimulating factor (GCSF, also known as CSF3), interleukin 8 (IL8) and C-X-C chemokine receptor type 4 (CXCR4) (Bobe and Goetz, 2001; van der Sar et al., 2006; Grayfer and Belosevic, 2009; Liongue et al., 2009; Oehlers et al., 2010; Walters et al., 2010; Lopez-Munoz et al., 2011), and utilize conserved molecules that are involved in pattern recognition, such as Toll-like receptors (TLRs), nucleotide-binding oligomerization domain molecules (NODs), Asc-type amino acid transporter 1 (ASC1) and myeloid differentiation primary response protein 88 (Myd88) (Masumoto et al., 2003; van der Sar et al., 2006; Laing et al., 2008; Oehlers et al., 2011).
The most abundant circulating leukocytes in both human and zebrafish are neutrophils (Lieschke et al., 2001). In response to inflammatory stimuli, such as tissue injury or infection, neutrophils rapidly infiltrate the damaged tissue compartment, where they phagocytose, degranulate, produce reactive oxygen species (ROS) or extrude their DNA to form extracellular nuclear traps (NETs) (Nathan, 2006; Borregaard, 2010). As the first line of defense, neutrophils function to contain the invading agents and produce inflammatory mediators that regulate adaptive immunity (Nathan, 2006). Macrophages are comprised of a heterogeneous group of cells and are generally recognized as specialized phagocytic cells that provide diverse roles in host defense (Gordon and Taylor, 2005; Mosser and Edwards, 2008). Macrophages ingest foreign substances, infectious microbes, cancer cells and apoptotic debris, including neutrophils. They can also affect adaptive immunity by secreting chemokines, cytokines or by functioning as antigen presenting cells.
The ability to rapidly sense and infiltrate damaged tissues is crucial for leukocyte function and host defense. Absent or reduced neutrophil function in response to infection is associated with primary immunodeficiency (Zuelzer et al., 1964), whereas over-activation of the neutrophil response underlies the pathogenesis of various diseases, including ischemia, reperfusion-induced tissue damage and autoinflammatory diseases, amongst others (Summers et al., 2010). This Commentary discusses recent progress in our understanding of leukocyte forward and reverse chemotaxis in vivo in zebrafish. We also discuss zebrafish models of human immunodeficiency that have provided new insight into leukocyte migration during development and disease. Details of the ontogeny of zebrafish early immune cells and established tools for live-cell imaging in zebrafish have been reviewed elsewhere (Crosier et al., 2008; Mathias et al., 2009a) and will therefore not be covered here.
Chemotaxis – from ‘in vitro’ to ‘in vivo’
Studies of 2D migration in vitro provide limited information about cell motility within interstitial tissues and lack physiological relevance. Research using neutrophils that adhere to 2D artificial substrates in the presence of different chemoattractants has yielded inconsistent and even contradictory results. For example, the requirement for phosphatidylinositol-3-kinase (PI3K) during neutrophil chemotaxis is context-dependent in vitro. PI3K-γ is essential for murine neutrophil migration on fibrinogen-coated surfaces, but is dispensable for migration on glass (Ferguson et al., 2007). Treatment of human neutrophils in vitro with PI3K inhibitors abolishes migration towards selected chemoattractants, such as IL8 and leukotriene B4, but not towards others, including formyl–Met–Le–Phe (fMLP) or complement 5a (Heit et al., 2002).
In addition, the modes and mechanisms of 2D and three-dimensional (3D) migration can be different. An example that highlights this difference is the recently discovered discrepancy between the role of integrins in leukocyte migration in two and three dimensions by using mouse models. Integrin-mediated adhesion is required for leukocyte migration on 2D surfaces in vitro. However, murine dendritic cells that have been depleted of all integrin receptors are able to migrate in interstitial tissues in three dimensions (Lämmermann et al., 2008). In contrast to adhesive migration on 2D surfaces (Lammermann and Sixt, 2009), integrin-independent 3D migration is driven by actin-network expansion, which promotes protrusive actin-based flow at the leading edge. Although integrin-mediated migration is relevant to certain steps during leukocyte migration in vivo, including leukocyte transmigration, cell–cell interactions and/or retention in tissues, interstitial 3D amoeboid migration can be independent of integrin-mediated adhesion in vivo. Collectively, these studies discussed above highlight the importance of developing relevant in vivo systems to characterize cell migration in live animals.
Mice and zebrafish have distinct but complementary advantages as models for understanding leukocyte motility (Trede et al., 2004) (Table 1). A broad collection of knockout mice, including tissue-specific knockouts, is available. However, intravital imaging in mice has limited temporal and spatial resolution and often requires invasive surgical procedures (Goodarzi et al., 2003). The recent improvements using intravital imaging of inflammation in the ear of mice in vivo (Li et al., 2012) has minimized the preparation time and increased the resolution, but still requires invasive procedures. By contrast, zebrafish are transparent during embryonic development and provide a non-invasive platform to observe leukocyte behavior and subcellular molecular events at a high resolution in vivo. An exciting example of the types of studies that can be carried out in zebrafish is the visualization of the direct derivation of multipotential hematopoietic stem cells from the aortic endothelium (Bertrand et al., 2010; Herbomel and Kissa, 2010). There are, however, some caveats to the zebrafish system. Leukocytes in mice are primarily recruited to inflammatory sites from the bloodstream (Nourshargh et al., 2010). By contrast, during embryonic development in zebrafish, leukocytes can be recruited directly from hematopoietic tissue to sites of tissue injury, thereby bypassing the extravasation process (Yoo and Huttenlocher, 2011). Therefore, leukocyte recruitment in zebrafish embryos primarily reflects leukocyte interstitial chemotaxis, whereas in mice, both extravasation and interstitial chemotaxis are crucial for leukocyte infiltration. In addition, the adaptive immune system, including T and B cells, does not mature until after the early larval period (Trede et al., 2004), making it an ideal system in which to study the innate immune system alone. Moreover, morpholino oligonucleotides (MOs) provide a unique tool for globally suppressing gene expression in zebrafish embryos. These stable nucleic acid analogs bind pre-mRNA to prevent splicing or the initiation of translation (Morcos, 2007). MOs usually reliably suppress gene expression for 2 or more days post fertilization. Further differences between zebrafish and mouse models are summarized in Table 1.
Table 1.
Comparison of zebrafish embryos and mice as models for leukocyte migration
| Mice | Zebrafish embryos | |
| General | ||
| Degree of conservation compared with humans | Mammalian | Vertebrate |
| Optical clarity | − | + |
| In vivo image resolution | Cellular | Molecular |
| Cost to maintain | High | Low |
| Generation time | 3 months | 3–6 months |
| Number of animals used for each experiment | Small | Large |
| Fully sequenced genome | + | + |
| Developmental stage | Various | Embryonic |
| Inbred strains | + | − |
| Genome duplication | − | + |
| Leukocytes | ||
| Accurate model of human hematopoiesis | + | + |
| Well-characterized immune system | + | +/− |
| Conserved innate immune system | + | + |
| Presence of adaptive immunity | + | − |
| Suitable model for leukocyte random motility | − | + |
| Suitable model for leukocyte transmigration | + | + |
| Suitable model for leukocyte interstitial migration | +/− | + |
| Tools | ||
| Use of MO for transient gene depletion | − | + |
| Reverse genetics | Knock-out, knock-in, tissue-specific knockout, inducible system | TALENs, ZFNs, knock-in, tissue-specific knockdown, inducible system |
| Forward genetics | + | +/gynogenetic diploid screen |
| Large-scale WISH screens | +/− | + |
| In vivo chemical screens | − | + |
| Administration of blocking antibodies | i.v. | Limited antibodies available |
TALENs, TAL effector nucleases; WISH, whole-mount in situ hybridization; ZFN, zinc-finger nucleases; i.v., intravenous; +, yes; −, no.
Neutrophil and macrophage migration in zebrafish embryos
The development of transgenic zebrafish lines with fluorescently labeled neutrophils and/or macrophages has revolutionized our ability to dissect the molecular mechanisms that regulate leukocyte motility and trafficking during normal homeostasis and following acute or chronic inflammation (summarized in Table 2) (Lawson and Weinstein, 2002; Ward et al., 2003; Hsu et al., 2004; Mathias et al., 2006; Renshaw et al., 2006; Hall et al., 2007; Mathias et al., 2009b; Feng et al., 2010; Ellett et al., 2011; Gray et al., 2011; Wittamer et al., 2011). During zebrafish development, neutrophils comprise two distinct populations: a relatively stationary population in the caudal hematopoietic tissue, and a spontaneously highly motile population in the rostral mesenchyme (Yoo and Huttenlocher, 2011). Therefore, zebrafish provide a unique tool to separate the effects of chemical or genetic perturbations on spontaneous neutrophil motility (within the rostral mesenchyme) and induced chemotaxis (to sites of infection or wounds) (Yoo and Huttenlocher, 2011). By using zebrafish lines that express transgenes that encode fluorescently tagged proteins and are driven by the myeloperoxidase (mpx) promoter to primarily label neutrophils, a robust and rapid recruitment of neutrophils to the sites of tissue injury is observed following tail transection (Renshaw et al., 2006) or needle injury of the tail or ventral fin (Mathias et al., 2006). Transgenic lines using the lysozyme C promoter (Hall et al., 2007) also contain labeled neutrophils (Feng et al., 2010). More recently, by using photoconversion and cell tracking, it has been shown that neutrophils can be recruited to a ventral fin wound directly from the caudal hematopoietic tissue (Yoo and Huttenlocher, 2011). Subsequently, neutrophils repeatedly migrate between the wound and the vasculature and finally resolve the inflammatory response through reverse migration back to the vasculature (Mathias et al., 2006; Yoo and Huttenlocher, 2011).
Table 2.
Existing reporter lines used to visualize myeloid cells in zebrafish
| Reporter line (ZFIN genotype) | Promoter origin | Cell type labeled | Reference | Notes |
| Tg(spi1:EGFP)pA301 | Spi1/Pu.1 | Myeloid cells | (Ward et al., 2003) | Embryonic stage examined |
| Tg(zpu.1:EGFP)df5 | Spi1/Pu.1 | Myeloid, early lymphatic cells | (Hsu et al., 2004) | Both adult and embryos examined |
| Tg(fli1a:EGFP)y1 | Fli1a | Macrophages, Endothelial cells | (Lawson and Weinstein, 2002; Feng et al., 2010) | Primitive macrophages labeled |
| Tg(mpx:GFP)i113/i114 | Mpx | Neutrophils | (Renshaw et al., 2006) | Lower expression in primitive macrophages |
| Tg(mpx:GFP)uwm1 | Mpx | Neutrophils | (Mathias et al., 2006; Mathias et al., 2009b) | Lower expression in primitive macrophages |
| Tg(lyz:EGFP)nz117 | Lyz/lysC | Neutrophils | (Hall et al., 2007; Feng et al., 2010) | Expression in primitive macrophages |
| Tg(lyz:dsRed)nz50 | Lyz/lysC | Neutrophils | (Hall et al., 2007; Feng et al., 2010) | Expression in primitive macrophages |
| CLGY463(YFP) | Myc enhancer | Neutrophils | (Meijer et al., 2008) | A subset of neutrophils labeled |
| Tg(mpx:mCherry) | Mpx | Neutrophils | (Yoo et al., 2010) | Lower expression in macrophages |
| Tg(mpx:Dendra2) | Mpx | Neutrophils | (Walters et al., 2010; Yoo et al., 2010; Yoo and Huttenlocher, 2011) | Lower expression in macrophages |
| Tg(fms:GAL4.VP16)i186 | Fms (CSF1R) | Macrophages | (Gray et al., 2011) | |
| Tg(mpeg1:mCherry) | Mpeg1 | Macrophages | (Ellett et al., 2011) | Active only in the embryo and larvae |
| Tg(mpeg1:EGFP) | Mpeg1 | Macrophages | (Ellett et al., 2011) | Active only in the embryo and larvae |
| Tg(mpeg1:Gal4-VP16) | Mpeg1 | Macrophages | (Ellett et al., 2011) | Active only in the embryo and larvae |
| Tg(mhc2dab:GFP) | Mhc2dab | B lymphocytes, eosinophils, macrophages and DCs | (Wittamer et al., 2011) | Adult animal |
| Tg (cd45:DsRed) | Cd45 | Myeloid cells and T lymphocytes | (Wittamer et al., 2011) | |
| Tg(mhc2dab:GFP and cd45:DsRed) | Mhc2dab, cd45 | Macrophages and DCs | (Wittamer et al., 2011) |
DCs, dendritic cells.
Our laboratory has also reported that a population of cells that are referred to as inflammatory macrophages also express low levels of the neutrophil protein Mpx, and are labeled with the mpx promoter (Mathias et al., 2009b). These macrophages are positive for the macrophage marker macrophage colony-stimulating factor 1 receptor (Csf1r), and they migrate towards wound sites and phagocytose melanocyte debris. Early macrophage responses to tissue injury have also been reported by using a transgenic zebrafish line that expresses GFP under the friend leukemia integration 1a (fli1a) promoter, which labels both the endothelium and early macrophages (Redd et al., 2006). Detailed functional and behavioral comparisons between neutrophils and macrophages during wound inflammation were not feasible until the more recent identification of the macrophage specific genes, macrophage expressed gene 1 (mpeg1) (Ellett et al., 2011) and cfms (which encodes Csf1r) (Gray et al., 2011). Macrophages display a slower, more directional migration to tissue wounds compared with neutrophils, and dwell more persistently at the wound margin before wound resolution (Ellett et al., 2011).
Macrophage- or neutrophil-deficient transgenic lines have been used to determine whether neutrophils and macrophages affect one another during the recruitment phase. Targeted ablation of macrophages with bacterial nitroreductase (Gray et al., 2011) and a zebrafish congenital neutropenia model (Walters et al., 2010) have indicated that macrophages and neutrophils respond to tissue injury normally in the absence of the respective other cell lineage. Real-time imaging of neutrophil–macrophage interactions has also revealed that macrophages phagocytose neutrophils, either whole or in part, during the wound resolution phase (Ellett et al., 2011). In accordance with mammalian studies (Savill et al., 1989), macrophage phagocytosis of neutrophils might clear apoptotic neutrophils at the wound site, or alternatively, contribute to antigen sampling.
In addition to acute neutrophilic inflammation, zebrafish provide a powerful model system to study chronic neutrophilic inflammation. In an insertional mutagenesis screen for zebrafish that exhibit abnormal tissue distribution of neutrophils, Mathias and colleagues have identified a mutant line with an insertion in the gene encoding the hepatocyte growth factor activator (hail). This line is characterized by chronic neutrophil infiltration in the tail fin and an epidermal proliferative phenotype that is reminiscent of psoriasis (Mathias et al., 2007). Similar neutrophil behavior has been observed in two additional ‘chronic inflammation’ zebrafish lines that were identified in the same screen (Dodd et al., 2009; Walters et al., 2009). Neutrophils and macrophages are also recruited to oncogene-transformed melanoblasts and goblet cells within the larval skin, which supports the idea that transformed cells represent a ‘chronic wound’ that is characterized by persistent leukocyte infiltration (Feng et al., 2010). Using live imaging, Feng and colleagues have reported that both neutrophils and macrophages engulf transformed cells. Interestingly, preventing leukocyte infiltration impairs the growth of transformed cell clones, which indicates that leukocytes probably provide trophic support for the growth of transformed cells (Feng et al., 2010).
Cell polarity during neutrophil chemotaxis in vivo
Neutrophil amoeboid migration is characterized by the dynamic coordination of protrusion at the leading edge and actomyosin-mediated retraction at the trailing edge. During random migration, neutrophils extend pseudopods that bifurcate, and subsequently, one pseudopod can become stabilized to determine the direction of migration (reviewed by Insall, 2010). Cell polarity is crucial to neutrophil migration both in vitro and in vivo. In vivo imaging with different actin probes [LifeAct, which labels total F-actin and UtrCH (the actin-binding domain of utrophin), which labels more stable populations of F-actin] has revealed that dynamic F-actin localizes to the leading edge and stable F-actin predominantly localizes to the tail of rapidly migrating zebrafish neutrophils in vivo (Yoo et al., 2010).
Chemoattractive signals produced at the site of tissue injury must translate into precise directional cues that guide directed migration (Van Haastert and Devreotes, 2004; Iglesias and Devreotes, 2008). Therefore, chemotaxis can be broken down into two separate steps: gradient sensing and cell polarization (Devreotes and Janetopoulos, 2003). Directional sensing does not require actin polymerization and is mediated by recognition of chemoattractants by their cell surface receptors (usually G-protein-coupled receptors). The initiation of intracellular signaling cascades results in asymmetric localization and activation of key signaling molecules, including PI3K, phospholipase C (PLC) and members of the Rho GTPase family, such as Cdc42 and Rac, which promote the localized nucleation and polymerization of F-actin at the leading edge of neutrophils (Weiner, 2002). The asymmetric distribution of the PI3K product phosphatidylinositol (3,4,5)-triphosphate [PtdIns(3,4,5)P3], at the front of the cell is a hallmark of cell polarization in neutrophils and other amoeboid cells (Fig. 1) (Weiner, 2002; Kolsch et al., 2008; Afonso and Parent, 2011).
Fig. 1.
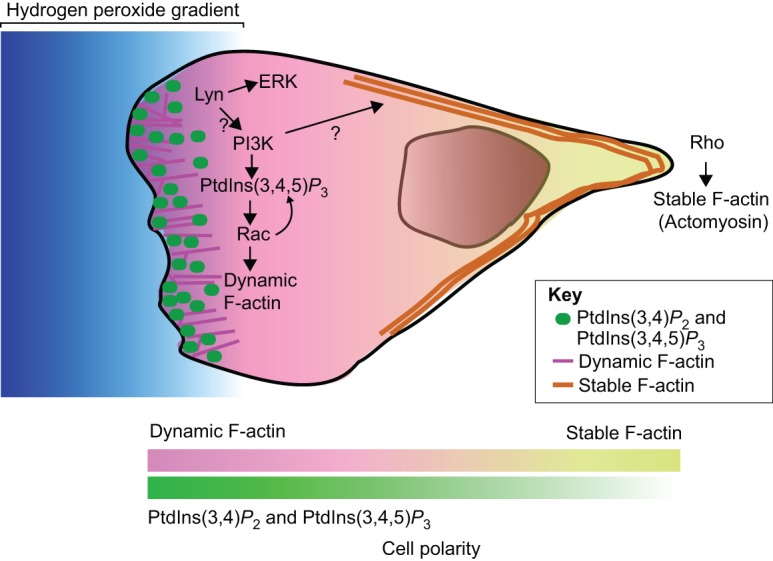
Cell polarity during neutrophil migration in zebrafish. Polarized activation of PI3K is required for cell motility (Weiner, 2002; Kolsch et al., 2008). The products of PI3K, PtdIns(3,4)P2 and PtdIns(3,4,5)P3, accumulate at the cell front and activate downstream signaling components, including Rac GTPases, which lead to polymerization of dynamic F-actin and protrusion of the leading edge. Rac further activates PI3K, thereby forming a positive feedback loop that amplifies cell polarity and promotes neutrophil migration (Kolsch et al., 2008; Yoo et al., 2010). PI3K also regulates neutrophil tail retraction through unknown mechanisms (Yoo et al., 2010). With regards to neutrophils, a hydrogen peroxide gradient is generated by injured tissue (Niethammer et al., 2009) and the Src family kinase, Lyn, is oxidized and activated by hydrogen peroxide (Yoo et al., 2011), which leads to activation of ERK and/or PI3K and directed migration. The lines at the bottom of the diagram (‘cell polarity’) indicate the gradient of changes in actin dynamics from more dynamic F-actin at the front of the cell to a stable population in the rear, and asymmetric enrichment of PtdIns(3,4)P2 and PtdIns(3,4,5)P3 at the front of migrating neutrophils.
PI3K was initially identified as a key signaling component involved in gradient sensing during amoeboid chemotaxis, including neutrophil-directed migration. However, PI3K is only required for leukocyte migration in some 2D environments (Afonso and Parent, 2011). Recently, the role of PI3K in neutrophil migration in vivo has been clarified further. Using both genetic and pharmacological approaches in zebrafish, we have shown that PI3K activity is required for both random and directed neutrophil motility (Yoo et al., 2010), thereby, suggesting that PI3K and the generation of PtdIns(3,4,5)P3 act as a key motor at the leading edge that drives cell protrusion and is necessary for motility. Live imaging of the dynamic localization of PtdIns(3,4,5)P3 and phosphatidylinositol (3,4)-biphosphate [PtdIns(3,4)P2] in zebrafish neutrophils has revealed that PI3K is activated at the cell front regardless of the direction of cell movement, either towards or away from a laser wound. Therefore, although the accumulation of PtdIns(3,4,5)P3 at the leading edge is an early marker of cell polarity and gradient sensing, it is probably a key player in mediating localized protrusion and motility rather than gradient sensing.
PI3K has been implicated in the localized activation of Rac GTPases at the leading edge to mediate dynamic actin-based cell protrusion (Weiner et al., 2002). Recent in vivo analyses in zebrafish have provided support for a positive feedback loop between PI3K and Rac in mediating localized protrusion (Yoo et al., 2010). In fact, localized activation of Rac in zebrafish neutrophils by using a photoactivatable version of Rac results in protrusions that are enriched in PtdIns(3,4,5)P3 and PtdIns(3,4)P2, and is sufficient to guide cell migration, which indicates that Rac can directly activate PI3K in vivo. However, when PI3K is inhibited, localized activation of Rac only rescues membrane protrusion, but not the polarization of F-actin dynamics or cell migration of zebrafish neutrophils. These findings indicate that PI3K is required for cell polarization and motility in a manner that is separable from Rac-mediated leading edge protrusion. Although the detailed mechanisms by which PI3K coordinates polarity during cell migration are still not well understood, in vivo analysis in zebrafish has demonstrated that PI3K is required for both Rac-based F-actin polymerization at the leading edge during pseudopod formation and the polarity of F-actin dynamics that is necessary for efficient tail retraction.
The role of another cytoskeletal system, microtubules, in leukocyte migration has also been investigated in zebrafish macrophages (Redd et al., 2006). De-stabilization of microtubules with nocodazole impairs macrophage recruitment to wounds, which can be restored with the Rho kinase inhibitor Y-27632. These observations suggest that microtubules are involved in establishing or maintaining the polarity of Rho activation, which is required for macrophage recruitment in zebrafish.
Sensing environmental cues
The signals generated at sites of tissue injury or infection that induce leukocyte attraction are probably complex and dynamic, and are not yet fully understood. Until recently, the prevailing view was that leukocytes were attracted to wounds by the release of ‘danger signals’, including ATP, uric acid, lipids, DNA and nuclear proteins (Škoberne et al., 2004; Martin and Leibovich, 2005). However, a recent study in zebrafish larvae has reported the surprising finding that tissue wounding induces the rapid formation of a hydrogen peroxide gradient, which forms an essential first step in leukocyte recruitment (Niethammer et al., 2009). This study positioned hydrogen peroxide as a crucial regulator of initial rapid leukocyte recruitment to acute injury. Hydrogen peroxide has also recently been identified as a key factor that mediates leukocyte attraction to transformed cells in zebrafish (Feng et al., 2010). Interestingly, subsequent studies have reported that hydrogen peroxide is not required for neutrophil recruitment to sites of bacterial infection, which indicates that different cues sensitize neutrophils to tissue injury or infection (Deng et al., 2012). Although future studies are still necessary to confirm the physiological relevance of hydrogen-peroxide-mediated leukocyte attraction to wounds in mammals, the studies discussed above provide a possible therapeutic intervention niche for the treatment of inflammation at tumor or wound sites, and represent a major advance in wound biology.
Until recently, the way in which hydrogen peroxide signals that are generated by the wound are sensed by neutrophils remained a mystery. However, we have identified a member of the Src family kinases, Lyn, as a leukocyte redox sensor that detects reactive oxygen species (ROS) at sites of tissue injury and mediates leukocyte wound attraction (Yoo et al., 2011). Exposure to ROS leads to oxidation of a cysteine residue on Lyn, which activates its kinase activity. The activation of Lyn leads to the activation of extracellular signal regulated kinase (ERK) pathways, which are also required for neutrophil wound attraction. In addition, it is well known that Lyn can activate PI3K in vitro (Pleiman et al., 1994). Therefore, it is highly attractive to speculate that activation of Lyn by ROS can lead to asymmetric PI3K activation in leukocytes, which then drives cell polarization and migration along a ROS gradient.
Neutrophil reverse migration
The term ‘reverse neutrophil migration’ is used to refer to migration of neutrophils away from a site of tissue injury back into the vasculature or lymphatic system to resolve inflammation. The previous dogma was that neutrophilic inflammation was resolved by apoptosis within tissues and by macrophage-mediated phagocytosis (El Kebir and Filep, 2010). However, non-invasive imaging in zebrafish has shown that neutrophils migrate away from an acute injury during the resolution phase (Fig. 2) (Mathias et al., 2006). A subsequent study reported that only a small percentage of neutrophils at wounds undergo apoptosis (Loynes et al., 2010), supporting the idea that inflammation is primarily resolved through neutrophil reverse migration in zebrafish larvae. The speed and directionality of forward and reverse migration are similar, which indicates that the signals that mediate forward and reverse migration are equally strong (Mathias et al., 2006). The recent application of advanced imaging techniques using photoconversion has demonstrated that neutrophils display repeated backwards and forwards movements between the wound site and the vasculature (Yoo and Huttenlocher, 2011). Moreover, neutrophils that become photolabeled at the wound disperse randomly throughout the body, and persist in the vasculature and other diverse tissues for at least two days after the acute injury. However, the physiological relevance of the dissemination of wound-sensitized neutrophils is not clear. The limitation of these studies is that the observations made at the zebrafish larval stage might not represent neutrophil behavior in mature higher vertebrates or humans. Nevertheless, several studies have supported the idea that human neutrophils can undergo reverse migration (Buckley et al., 2006; Mulero et al., 2011). Furthermore, neutrophil reverse migration has recently been confirmed with confocal intravital microscopy in mice after ischemia–reperfusion injury (Woodfin et al., 2011). The molecular mechanism underlying neutrophil reverse migration is largely unknown. A recent study in zebrafish has reported that activation of the oxygen-sensing transcription factor hypoxia-inducible factor1a (HIF1A), either using pharmacological approaches or genetic manipulation, reduces the levels of both neutrophil apoptosis and reverse migration (Elks et al., 2011). In addition, a secreted E. coli mucinase also seems to affect neutrophil reverse migration (Szabady et al., 2009).
Fig. 2.
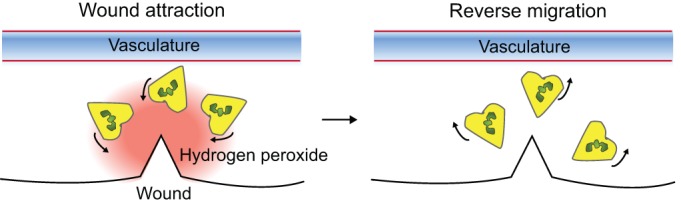
Neutrophil reverse migration. Following tissue injury, neutrophils are rapidly attracted to the injury site by a tissue gradient of hydrogen peroxide (Niethammer et al., 2009). The majority of neutrophils migrate back to the vasculature (reverse migration) during resolution of inflammation (Mathias et al., 2006; Yoo and Huttenlocher, 2011). The underlying mechanisms that regulate neutrophil reverse migration are not known.
Zebrafish as a disease model
As described above, zebrafish have emerged as a useful model to study neutrophil migration. Zebrafish are also rapidly gaining popularity as a model system to study human disease by providing new insight into disease pathogenesis and a platform for drug screening (Fig. 3). Here, we specifically focus on zebrafish disease models where defects in leukocyte migration contribute to disease pathogenesis.
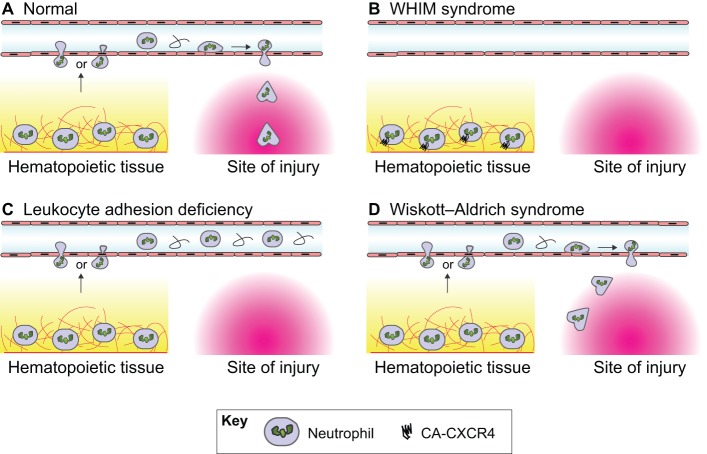
Fig. 3.
Schematic diagram showing different disease models in zebrafish. (A) In mammals, under normal conditions, tissue injury leads to neutrophils becoming mobilized from the hematopoietic tissue, migrating into the vasculature and exiting the vasculature at sites that are close to the site of injury. Neutrophil migration across the endothelium occurs either through transcellular (i.e. through the cell body) or paracellular (i.e. through cell–cell junctions) routes. Neutrophils then undergo interstitial migration to reach the site of injury. In zebrafish, neutrophils can be recruited directly from the hematopoietic tissue to wounds in certain contexts (Yoo et al., 2011). (B) In patients with WHIM syndrome (Walters et al., 2010), neutrophils are retained in hematopoietic tissue by constitutively active CXCR4 signaling and are, therefore, absent from the vasculature or site of injury. (C) In patients with leukocyte adhesion deficiency as a result of a dominant inhibitory mutation in Rac2 (Deng et al., 2011), neutrophils are released into the vasculature, but are not able to migrate out of the vasculature and are, therefore, absent at sites of injury. (D) In patients with Wiskott-Aldrich syndrome, which is caused by a WASP deficiency (Cvejic et al., 2008), neutrophils are not able to efficiently migrate to the site of tissue injury.
The Wiskott-Aldrich syndrome protein (WASP) is an actin-binding protein that is specific to hematopoietic cells. WASP deficiency leads to the primary immunodeficiency Wiskott-Aldrich syndrome, which is characterized by recurrent infections, autoimmunity and bleeding disorders. Morpholino-mediated knockdown of Wasp1 in zebrafish embryos does not affect the development of leukocytes, but results in the substantially reduced recruitment of neutrophils and macrophages to sites of acute tissue injury (Cvejic et al., 2008). Detailed live imaging of migrating cells has revealed that Wasp1-deficient neutrophils make more frequent stops en route to the wound and are also less directional owing to, at least in part, defects in pseudopod selection. The study by Cvejic and colleagues not only confirmed the physiological role of WASP in immune function, but also provided a basic understanding of leukocyte chemotaxis by highlighting a role for WASP in pseudopod selection during leukocyte motility.
Another example of a zebrafish model for human primary immunodeficiency is the zebrafish WHIM transgenic model. Warts, Hypogammaglobulinemia, Infections and Myelokethaxis (WHIM) syndrome is driven by constitutive C-X-C chemokine receptor 4 (Cxcr4) signaling (Walters et al., 2010). Expression of a truncated form of Cxcr4, which has impaired internalization, in zebrafish neutrophils results in the retention of neutrophils within hematopoietic tissues and prevents their recruitment to sites of tissue injury. This ‘stickiness’ of neutrophils within the caudal hematopoietic tissue (CHT) is dependent on the Cxcr4 ligand stromal cell-derived factor 1 alpha (Sdf1a), because depleting endogenous Sdf1a rescues the WHIM phenotype in zebrafish. Detailed live imaging in the CHT of zebrafish has revealed that neutrophils in the CHT increase local motility following tail clipping, indicating that neutrophils in the CHT can sense and respond to wound-generated signals, but are unable to traffic out of the hematopoietic tissue in the WHIM model. This zebrafish WHIM model also provides a tool to screen for agents that regulate neutrophil mobilization and might provide therapeutic benefit in WHIM syndrome, for example, through treatment with G-CSF (Bohinjec and Andoljsek, 1992).
Leukocyte adhesion deficiency is a primary immunodeficiency that is characterized by abnormal neutrophil distribution with increased circulating neutrophils (neutrophilia) and absent recruitment to peripheral tissues. The dominant negative D57N point mutation in the hematopoietic-specific Rac isoform Rac2 has been identified in patients with leukocyte adhesion deficiency-like syndromes (Ambruso et al., 2000; Williams et al., 2000). However, whether this mutation is sufficient to cause the disease had not been determined until recently. Expression of Rac2(D57N) in zebrafish neutrophils fully recapitulates the human disease (Deng et al., 2011). Live time-lapse imaging has revealed that Rac2-deficient neutrophils have impaired 3D motility in mesenchymal tissue, but are released from the CHT at increased frequency. These observations indicate that Rac2 is required for active retention of neutrophils in the CHT, but is dispensable for the mobilization process. In addition, excessive neutrophil retention in the CHT that is induced by constitutive CXCR4 signaling is attenuated by the expression of Rac2(D57N), which positions CXCR4–Rac2 signaling as a key mechanism that mediates neutrophil retention. Collectively, this study by Deng and colleagues has demonstrated that expression of Rac2(D57N) in neutrophils is sufficient to cause immunodeficiency and provides new insight into the role of Rac2 in neutrophil homeostasis during development and disease conditions.
Concluding remarks and future perspectives
During the last decade, zebrafish have provided a powerful tool to analyze leukocyte biology in live animals. The optical clarity of zebrafish embryos allows an unprecedented resolution of signaling events on both the cellular and molecular level in an intact organism. These reports have uncovered previously unknown mechanisms that are crucial to basic processes underlying neutrophil motility and inflammation, particularly the identification of hydrogen peroxide as a signal that mediates neutrophil recruitment and the observation of reverse neutrophil migration during resolution of inflammation. Substantial evidence also supports the importance of zebrafish to model human disease and to aid in drug discovery.
The identification of new mechanisms that resolve neutrophil-mediated inflammation by reverse migration in zebrafish have recently been confirmed in mouse models, highlighting how these two models systems can complement the investigation of neutrophil motility and inflammation. We anticipate that zebrafish will continue to provide a powerful tool to uncover fundamental mechanisms of neutrophil motility and trafficking in vivo that are relevant to human immunity.
Challenges remain to identify the signaling dynamics at wounded tissue, the vasculature and within leukocytes during attraction and resolution phases of the inflammatory response. Because the inhibition of hydrogen peroxide production only inhibits the first wave of neutrophil wound infiltration, the factors that mediate the later sustained phase of neutrophil recruitment are still unknown. Furthermore, the physiological signals or the loss of specific signals that drive neutrophil reverse migration are also unknown. It is probable that neutrophils are exposed to competing gradients of chemoattractants and that an intracellular signaling hierarchy determines the direction of migration. Detailed tissue-specific gene-expression profiles during the initiation and resolution phase of inflammation will provide clues to these fundamental processes. The real-time observation of the dynamics of environmental cues, such as the use of hydrogen peroxide sensor HyPer, will provide detailed information about the onset and resolution of tissue-generated signals. Additional tools to tweak the subcellular activation of signaling molecules, such as photo-activatable Rac, will help to dissect the signaling events that regulate neutrophil forward and reverse migration. We anticipate that zebrafish will be instrumental in elucidating mechanisms that regulate leukocyte migration in vivo and will continue to provide new insight into the onset and resolution of inflammation.
Footnotes
Funding
This work was supported by National Institutes of Health Grants [grant number GM074827 to A.H.]. Deposited in PMC for release after 12 months.
References
- Afonso P. V., Parent C. A. (2011). PI3K and chemotaxis: a priming issue? Sci. Signal. 4, pe22 10.1126/scisignal.2002019 [DOI] [PubMed] [Google Scholar]
- Ambruso D. R., Knall C., Abell A. N., Panepinto J., Kurkchubasche A., Thurman G., Gonzalez–Aller C., Hiester A., deBoer M., Harbeck R. J.et al. (2000). Human neutrophil immunodeficiency syndrome is associated with an inhibitory Rac2 mutation. Proc. Natl. Acad. Sci. USA 97, 4654–4659 10.1073/pnas.080074897 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bertrand J. Y., Chi N. C., Santoso B., Teng S., Stainier D. Y., Traver D. (2010). Haematopoietic stem cells derive directly from aortic endothelium during development. Nature 464, 108–111 10.1038/nature08738 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bobe J., Goetz F. W. (2001). Molecular cloning and expression of a TNF receptor and two TNF ligands in the fish ovary. Comp. Biochem. Physiol. B Biochem. Mol. Biol. 129, 475–481 10.1016/S1096-4959(01)00353-0 [DOI] [PubMed] [Google Scholar]
- Bohinjec J., Andoljsek D. (1992). Neutrophil-releasing activity of recombinant human granulocyte-macrophage colony stimulating factor in myelokathexis. Br. J. Haematol. 82, 169–170 10.1111/j.1365-2141.1992.tb04609.x [DOI] [PubMed] [Google Scholar]
- Borregaard N. (2010). Neutrophils, from marrow to microbes. Immunity 33, 657–670 10.1016/j.immuni.2010.11.011 [DOI] [PubMed] [Google Scholar]
- Buckley C. D., Ross E. A., McGettrick H. M., Osborne C. E., Haworth O., Schmutz C., Stone P. C., Salmon M., Matharu N. M., Vohra R. K.et al. (2006). Identification of a phenotypically and functionally distinct population of long-lived neutrophils in a model of reverse endothelial migration. J. Leukoc. Biol. 79, 303–311 10.1189/jlb.0905496 [DOI] [PubMed] [Google Scholar]
- Cvejic A., Hall C., Bak–Maier M., Flores M. V., Crosier P., Redd M. J., Martin P. (2008). Analysis of WASp function during the wound inflammatory response–live-imaging studies in zebrafish larvae. J. Cell Sci. 121, 3196–3206 10.1242/jcs.032235 [DOI] [PubMed] [Google Scholar]
- Deng Q., Yoo S. K., Cavnar P. J., Green J. M., Huttenlocher A. (2011). Dual roles for Rac2 in neutrophil motility and active retention in zebrafish hematopoietic tissue. Dev. Cell 21, 735–745 10.1016/j.devcel.2011.07.013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deng Q., Harvie E. A., Huttenlocher A. (2012). Distinct signalling mechanisms mediate neutrophil attraction to bacterial infection and tissue injury. Cell. Microbiol. 14, 517–528 10.1111/j.1462-5822.2011.01738.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Devreotes P., Janetopoulos C. (2003). Eukaryotic chemotaxis: distinctions between directional sensing and polarization. J. Biol. Chem. 278, 20445–20448 10.1074/jbc.R300010200 [DOI] [PubMed] [Google Scholar]
- Dodd M. E., Hatzold J., Mathias J. R., Walters K. B., Bennin D. A., Rhodes J., Kanki J. P., Look A. T., Hammerschmidt M., Huttenlocher A. (2009). The ENTH domain protein Clint1 is required for epidermal homeostasis in zebrafish. Development 136, 2591–2600 10.1242/dev.038448 [DOI] [PMC free article] [PubMed] [Google Scholar]
- El Kebir D., Filep J. G. (2010). Role of neutrophil apoptosis in the resolution of inflammation. ScientificWorldJournal 10, 1731–1748 10.1100/tsw.2010.169 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Elks P. M., van Eeden F. J., Dixon G., Wang X., Reyes–Aldasoro C. C., Ingham P. W., Whyte M. K., Walmsley S. R., Renshaw S. A. (2011). Activation of hypoxia-inducible factor-1α (Hif-1α) delays inflammation resolution by reducing neutrophil apoptosis and reverse migration in a zebrafish inflammation model. Blood 118, 712–722 10.1182/blood-2010-12-324186 [DOI] [PubMed] [Google Scholar]
- Ellett F., Pase L., Hayman J. W., Andrianopoulos A., Lieschke G. J. (2011). mpeg1 promoter transgenes direct macrophage-lineage expression in zebrafish. Blood 117, e49–e56 10.1182/blood-2010-10-314120 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feng Y., Santoriello C., Mione M., Hurlstone A., Martin P. (2010). Live imaging of innate immune cell sensing of transformed cells in zebrafish larvae: parallels between tumor initiation and wound inflammation. PLoS Biol. 8, e1000562 10.1371/journal.pbio.1000562 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ferguson G. J., Milne L., Kulkarni S., Sasaki T., Walker S., Andrews S., Crabbe T., Finan P., Jones G., Jackson S.et al. (2007). PI(3)Kgamma has an important context-dependent role in neutrophil chemokinesis. Nat. Cell Biol. 9, 86–91 10.1038/ncb1517 [DOI] [PubMed] [Google Scholar]
- Goodarzi K., Goodarzi M., Tager A. M., Luster A. D., von Andrian U. H. (2003). Leukotriene B4 and BLT1 control cytotoxic effector T cell recruitment to inflamed tissues. Nat. Immunol. 4, 965–973 10.1038/ni972 [DOI] [PubMed] [Google Scholar]
- Gordon S., Taylor P. R. (2005). Monocyte and macrophage heterogeneity. Nat. Rev. Immunol. 5, 953–964 10.1038/nri1733 [DOI] [PubMed] [Google Scholar]
- Gray C., Loynes C. A., Whyte M. K., Crossman D. C., Renshaw S. A., Chico T. J. (2011). Simultaneous intravital imaging of macrophage and neutrophil behaviour during inflammation using a novel transgenic zebrafish. Thromb. Haemost. 105, 811–819 10.1160/TH10-08-0525 [DOI] [PubMed] [Google Scholar]
- Grayfer L., Belosevic M. (2009). Molecular characterization of novel interferon gamma receptor 1 isoforms in zebrafish (Danio rerio) and goldfish (Carassius auratus L.). Mol. Immunol. 46, 3050–3059 10.1016/j.molimm.2009.06.004 [DOI] [PubMed] [Google Scholar]
- Hall C., Flores M. V., Storm T., Crosier K., Crosier P. (2007). The zebrafish lysozyme C promoter drives myeloid-specific expression in transgenic fish. BMC Dev. Biol. 7, 42 10.1186/1471-213X-7-42 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hall C. J., Flores M. V., Crosier K. E., Crosier P. S. (2008). Live imaging early immune cell ontogeny and function in zebrafish Danio rerio. J. Fish Biol. 73, 1833–1871 10.1111/j.1095-8649.2008.01980.x [DOI] [Google Scholar]
- Heit B., Tavener S., Raharjo E., Kubes P. (2002). An intracellular signaling hierarchy determines direction of migration in opposing chemotactic gradients. J. Cell Biol. 159, 91–102 10.1083/jcb.200202114 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hsu K., Traver D., Kutok J. L., Hagen A., Liu T. X., Paw B. H., Rhodes J., Berman J. N., Zon L. I., Kanki J. P.et al. (2004). The pu.1 promoter drives myeloid gene expression in zebrafish. Blood 104, 1291–1297 10.1182/blood-2003-09-3105 [DOI] [PubMed] [Google Scholar]
- Iglesias P. A., Devreotes P. N. (2008). Navigating through models of chemotaxis. Curr. Opin. Cell Biol. 20, 35–40 10.1016/j.ceb.2007.11.011 [DOI] [PubMed] [Google Scholar]
- Insall R. H. (2010). Understanding eukaryotic chemotaxis: a pseudopod-centred view. Nat. Rev. Mol. Cell Biol. 11, 453–458 10.1038/nrm2905 [DOI] [PubMed] [Google Scholar]
- Kissa K., Herbomel P. (2010). Blood stem cells emerge from aortic endothelium by a novel type of cell transition. Nature 464, 112–115 10.1038/nature08761 [DOI] [PubMed] [Google Scholar]
- Kölsch V., Charest P. G., Firtel R. A. (2008). The regulation of cell motility and chemotaxis by phospholipid signaling. J. Cell Sci. 121, 551–559 10.1242/jcs.023333 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laing K. J., Purcell M. K., Winton J. R., Hansen J. D. (2008). A genomic view of the NOD-like receptor family in teleost fish: identification of a novel NLR subfamily in zebrafish. BMC Evol. Biol. 8, 42 10.1186/1471-2148-8-42 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lämmermann T., Sixt M. (2009). Mechanical modes of ‘amoeboid’ cell migration. Curr. Opin. Cell Biol. 21, 636–644 10.1016/j.ceb.2009.05.003 [DOI] [PubMed] [Google Scholar]
- Lämmermann T., Bader B. L., Monkley S. J., Worbs T., Wedlich–Söldner R., Hirsch K., Keller M., Förster R., Critchley D. R., Fässler R.et al. 2008). Rapid leukocyte migration by integrin-independent flowing and squeezing. Nature 45351–55 10.1038/nature06887 [DOI] [PubMed] [Google Scholar]
- Lawson N. D., Weinstein B. M. (2002). In vivo imaging of embryonic vascular development using transgenic zebrafish. Dev. Biol. 248, 307–318 10.1006/dbio.2002.0711 [DOI] [PubMed] [Google Scholar]
- Li J. L., Goh C. C., Keeble J. L., Qin J. S., Roediger B., Jain R., Wang Y., Chew W. K., Weninger W., Ng L. G. (2012). Intravital multiphoton imaging of immune responses in the mouse ear skin. Nat. Protoc. 7, 221–234 10.1038/nprot.2011.438 [DOI] [PubMed] [Google Scholar]
- Lieschke G. J., Trede N. S. (2009). Fish immunology. Curr. Biol. 19, R678–R682 10.1016/j.cub.2009.06.068 [DOI] [PubMed] [Google Scholar]
- Lieschke G. J., Oates A. C., Crowhurst M. O., Ward A. C., Layton J. E. (2001). Morphologic and functional characterization of granulocytes and macrophages in embryonic and adult zebrafish. Blood 98, 3087–3096 10.1182/blood.V98.10.3087 [DOI] [PubMed] [Google Scholar]
- Liongue C., Hall C. J., O’Connell B. A., Crosier P., Ward A. C. (2009). Zebrafish granulocyte colony-stimulating factor receptor signaling promotes myelopoiesis and myeloid cell migration. Blood 113, 2535–2546 10.1182/blood-2008-07-171967 [DOI] [PubMed] [Google Scholar]
- López–Muñoz A., Sepulcre M. P., Roca F. J., Figueras A., Meseguer J., Mulero V. (2011). Evolutionary conserved pro-inflammatory and antigen presentation functions of zebrafish IFNγ revealed by transcriptomic and functional analysis. Mol. Immunol. 48, 1073–1083 10.1016/j.molimm.2011.01.015 [DOI] [PubMed] [Google Scholar]
- Loynes C. A., Martin J. S., Robertson A., Trushell D. M., Ingham P. W., Whyte M. K., Renshaw S. A. (2010). Pivotal Advance: Pharmacological manipulation of inflammation resolution during spontaneously resolving tissue neutrophilia in the zebrafish. J. Leukoc. Biol. 87, 203–212 10.1189/jlb.0409255 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martin P., Leibovich S. J. (2005). Inflammatory cells during wound repair: the good, the bad and the ugly. Trends Cell Biol. 15, 599–607 10.1016/j.tcb.2005.09.002 [DOI] [PubMed] [Google Scholar]
- Masumoto J., Zhou W., Chen F. F., Su F., Kuwada J. Y., Hidaka E., Katsuyama T., Sagara J., Taniguchi S., Ngo–Hazelett P.et al. (2003). Caspy, a zebrafish caspase, activated by ASC oligomerization is required for pharyngeal arch development. J. Biol. Chem. 278, 4268–4276 10.1074/jbc.M203944200 [DOI] [PubMed] [Google Scholar]
- Mathias J. R., Perrin B. J., Liu T. X., Kanki J., Look A. T., Huttenlocher A. (2006). Resolution of inflammation by retrograde chemotaxis of neutrophils in transgenic zebrafish. J. Leukoc. Biol. 80, 1281–1288 10.1189/jlb.0506346 [DOI] [PubMed] [Google Scholar]
- Mathias J. R., Dodd M. E., Walters K. B., Rhodes J., Kanki J. P., Look A. T., Huttenlocher A. (2007). Live imaging of chronic inflammation caused by mutation of zebrafish Hai1. J. Cell Sci. 120, 3372–3383 10.1242/jcs.009159 [DOI] [PubMed] [Google Scholar]
- Mathias J. R., Walters K. B., Huttenlocher A. (2009a). Neutrophil motility in vivo using zebrafish. Methods Mol. Biol. 571, 151–166 10.1007/978-1-60761-198-1_10 [DOI] [PubMed] [Google Scholar]
- Mathias J. R., Dodd M. E., Walters K. B., Yoo S. K., Ranheim E. A., Huttenlocher A. (2009b). Characterization of zebrafish larval inflammatory macrophages. Dev. Comp. Immunol. 33, 1212–1217 10.1016/j.dci.2009.07.003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morcos P. A. (2007). Achieving targeted and quantifiable alteration of mRNA splicing with Morpholino oligos. Biochem. Biophys. Res. Commun. 358, 521–527 10.1016/j.bbrc.2007.04.172 [DOI] [PubMed] [Google Scholar]
- Mosser D. M., Edwards J. P. (2008). Exploring the full spectrum of macrophage activation. Nat. Rev. Immunol. 8, 958–969 10.1038/nri2448 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mulero V., Sepulcre M. P., Rainger G. E., Buckley C. D. (2011). Editorial: Neutrophils live on a two-way street. J. Leukoc. Biol. 89, 645–647 10.1189/jlb.0111013 [DOI] [PubMed] [Google Scholar]
- Nathan C. (2006). Neutrophils and immunity: challenges and opportunities. Nat. Rev. Immunol. 6, 173–182 10.1038/nri1785 [DOI] [PubMed] [Google Scholar]
- Niethammer P., Grabher C., Look A. T., Mitchison T. J. (2009). A tissue-scale gradient of hydrogen peroxide mediates rapid wound detection in zebrafish. Nature 459, 996–999 10.1038/nature08119 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nourshargh S., Hordijk P. L., Sixt M. (2010). Breaching multiple barriers: leukocyte motility through venular walls and the interstitium. Nat. Rev. Mol. Cell Biol. 11, 366–378 10.1038/nrm2889 [DOI] [PubMed] [Google Scholar]
- Oehlers S. H., Flores M. V., Hall C. J., O’Toole R., Swift S., Crosier K. E., Crosier P. S. (2010). Expression of zebrafish cxcl8 (interleukin-8) and its receptors during development and in response to immune stimulation. Dev. Comp. Immunol. 34, 352–359 10.1016/j.dci.2009.11.007 [DOI] [PubMed] [Google Scholar]
- Oehlers S. H., Flores M. V., Hall C. J., Swift S., Crosier K. E., Crosier P. S. (2011). The inflammatory bowel disease (IBD) susceptibility genes NOD1 and NOD2 have conserved anti-bacterial roles in zebrafish. Dis. Model. Mech. 4, 832–841 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pleiman C. M., Hertz W. M., Cambier J. C. (1994). Activation of phosphatidylinositol-3′ kinase by Src-family kinase SH3 binding to the p85 subunit. Science 263, 1609–1612 10.1126/science.8128248 [DOI] [PubMed] [Google Scholar]
- Redd M. J., Kelly G., Dunn G., Way M., Martin P. (2006). Imaging macrophage chemotaxis in vivo: studies of microtubule function in zebrafish wound inflammation. Cell Motil. Cytoskeleton 63, 415–422 10.1002/cm.20133 [DOI] [PubMed] [Google Scholar]
- Renshaw S. A., Loynes C. A., Trushell D. M., Elworthy S., Ingham P. W., Whyte M. K. (2006). A transgenic zebrafish model of neutrophilic inflammation. Blood 108, 3976–3978 10.1182/blood-2006-05-024075 [DOI] [PubMed] [Google Scholar]
- Savill J. S., Wyllie A. H., Henson J. E., Walport M. J., Henson P. M., Haslett C. (1989). Macrophage phagocytosis of aging neutrophils in inflammation. Programmed cell death in the neutrophil leads to its recognition by macrophages. J. Clin. Invest. 83, 865–875 10.1172/JCI113970 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Škoberne M., Beignon A. S., Bhardwaj N. (2004). Danger signals: a time and space continuum. Trends Mol. Med. 10, 251–257 10.1016/j.molmed.2004.04.001 [DOI] [PubMed] [Google Scholar]
- Summers C., Rankin S. M., Condliffe A. M., Singh N., Peters A. M., Chilvers E. R. (2010). Neutrophil kinetics in health and disease. Trends Immunol. 31, 318–324 10.1016/j.it.2010.05.006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Szabady R. L., Lokuta M. A., Walters K. B., Huttenlocher A., Welch R. A. (2009). Modulation of neutrophil function by a secreted mucinase of Escherichia coli O157:H7. PLoS Pathog. 5, e1000320 10.1371/journal.ppat.1000320 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Trede N. S., Langenau D. M., Traver D., Look A. T., Zon L. I. (2004). The use of zebrafish to understand immunity. Immunity 20, 367–379 10.1016/S1074-7613(04)00084-6 [DOI] [PubMed] [Google Scholar]
- van der Sar A. M., Stockhammer O. W., van der Laan C., Spaink H. P., Bitter W., Meijer A. H. (2006). MyD88 innate immune function in a zebrafish embryo infection model. Infect. Immun. 74, 2436–2441 10.1128/IAI.74.4.2436-2441.2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van Haastert P. J., Devreotes P. N. (2004). Chemotaxis: signalling the way forward. Nat. Rev. Mol. Cell Biol. 5, 626–634 10.1038/nrm1435 [DOI] [PubMed] [Google Scholar]
- Walters K. B., Dodd M. E., Mathias J. R., Gallagher A. J., Bennin D. A., Rhodes J., Kanki J. P., Look A. T., Grinblat Y., Huttenlocher A. (2009). Muscle degeneration and leukocyte infiltration caused by mutation of zebrafish Fad24. Dev. Dyn. 238, 86–99 10.1002/dvdy.21821 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walters K. B., Green J. M., Surfus J. C., Yoo S. K., Huttenlocher A. (2010). Live imaging of neutrophil motility in a zebrafish model of WHIM syndrome. Blood 116, 2803–2811 10.1182/blood-2010-03-276972 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ward A. C., McPhee D. O., Condron M. M., Varma S., Cody S. H., Onnebo S. M., Paw B. H., Zon L. I., Lieschke G. J. (2003). The zebrafish spi1 promoter drives myeloid-specific expression in stable transgenic fish. Blood 102, 3238–3240 10.1182/blood-2003-03-0966 [DOI] [PubMed] [Google Scholar]
- Weiner O. D. (2002). Regulation of cell polarity during eukaryotic chemotaxis: the chemotactic compass. Curr. Opin. Cell Biol. 14, 196–202 10.1016/S0955-0674(02)00310-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weiner O. D., Neilsen P. O., Prestwich G. D., Kirschner M. W., Cantley L. C., Bourne H. R. (2002). A PtdInsP(3)- and Rho GTPase-mediated positive feedback loop regulates neutrophil polarity. Nat. Cell Biol. 4, 509–513 10.1038/ncb811 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Williams D. A., Tao W., Yang F., Kim C., Gu Y., Mansfield P., Levine J. E., Petryniak B., Derrow C. W., Harris C.et al. (2000). Dominant negative mutation of the hematopoietic-specific Rho GTPase, Rac2, is associated with a human phagocyte immunodeficiency. Blood 96, 1646–1654 [PubMed] [Google Scholar]
- Wittamer V., Bertrand J. Y., Gutschow P. W., Traver D. (2011). Characterization of the mononuclear phagocyte system in zebrafish. Blood 117, 7126–7135 10.1182/blood-2010-11-321448 [DOI] [PubMed] [Google Scholar]
- Woodfin A., Voisin M. B., Beyrau M., Colom B., Caille D., Diapouli F. M., Nash G. B., Chavakis T., Albelda S. M., Rainger G. E.et al. (2011). The junctional adhesion molecule JAM-C regulates polarized transendothelial migration of neutrophils in vivo. Nat. Immunol. 12, 761–769 10.1038/ni.2062 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yoo S. K., Huttenlocher A. (2011). Spatiotemporal photolabeling of neutrophil trafficking during inflammation in live zebrafish. J. Leukoc. Biol. 89, 661–667 10.1189/jlb.1010567 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yoo S. K., Deng Q., Cavnar P. J., Wu Y. I., Hahn K. M., Huttenlocher A. (2010). Differential regulation of protrusion and polarity by PI3K during neutrophil motility in live zebrafish. Dev. Cell 18, 226–236 10.1016/j.devcel.2009.11.015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yoo S. K., Starnes T. W., Deng Q., Huttenlocher A. (2011). Lyn is a redox sensor that mediates leukocyte wound attraction in vivo. Nature 480, 109–112 10.1038/nature10632 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zuelzer W. W., Evans R. K., Goodman J.1964). Myelokathexis– a new form of chronic granulocytopenia–report of a case. N. Engl. J. Med. 270699–704 10.1056/NEJM196404022701402 [DOI] [PubMed] [Google Scholar]