Summary
Perilipin family proteins (Plins) coat the surface of intracellular neutral lipid storage droplets in various cell types. Studies across diverse species demonstrate that Plins regulate lipid storage metabolism through recruitment of lipases and other regulatory proteins to lipid droplet surfaces. Mammalian genomes have distinct Plin gene members and additional protein forms derived from specific mRNA splice variants. However, it is not known if the different Plins have distinct functional properties. Using biochemical, cellular imaging and flow cytometric analyses, we now show that within individual cells of various types, the different Plin proteins preferentially sequester to separate pools of lipid storage droplets. By examining ectopically expressed GFP fusions and all endogenous Plin protein forms, we demonstrate that different Plins sequester to different types of lipid droplets that are composed of either triacylcerides or cholesterol esters. Furthermore, Plins with strong association preferences to triacylceride (or cholesterol ester) droplets can re-direct the relative intracellular triacylceride–cholesterol ester balance toward the targeted lipid. Our data suggest diversity of Plin function, alter previous assumptions about shared collective actions of the Plins, and indicate that each Plin can have separate and unique functions.
Key words: PLIN, ADRP, TIP47, LSDP5, S3-12, Triacylglyceride, Cholesterol, Fatty acids, Lipolysis
Introduction
Intracellular neutral lipid storage droplets (LSDs) are unique organelles that store metabolic precursors of cellular energy, membrane biosynthesis, steroid hormone synthesis, and signaling (Farese and Walther, 2009; Kimmel et al., 2010; Londos et al., 2005). LSDs contain different lipids [e.g. triacylglycerides (TAGs) or cholesteryl esters (CEs)] at their core, surrounded by a phospholipid monolayer. LSD surfaces in organisms as diverged as mammals, Drosophila and Dictyostelium are targeted by an evolutionarily related family of proteins (Kimmel et al., 2010; Lu et al., 2001; Miura et al., 2002), the Perilipins (Plins). Mammalian genomes have five distinct Plin gene members and additional protein forms derived from specific mRNA splice variants (Kimmel et al., 2010).
Plin1 (Perilipin 1) is the major LSD coat protein in adipocytes and steroidogenic cells (Greenberg et al., 1993; Servetnick et al., 1995). Other Plins exhibit different expression patterns. Plin2 is the predominant, but not exclusive, form in liver (Dalen et al., 2006), whereas Plin5 is primarily expressed in oxidative tissues, including heart, soleus muscle, and brown fat (Dalen et al., 2007; Wolins et al., 2006; Yamaguchi et al., 2006). Based on Plin1 function (Martinez-Botas et al., 2000; Sztalryd et al., 2003; Tansey et al., 2001; Wang et al., 2009), the Plins are viewed as fundamental regulators of lipolytic activity. Loss of Plin1 (Martinez-Botas et al., 2000; Tansey et al., 2001) or Plin2 (also known as ADRP) (Chang et al., 2006) in mice significantly reduces intracellular lipid levels in adipocytes and hepatocytes, respectively. Furthermore, heterozygous loss-of-function mutations in human PLIN1 leads to a familial partial lipodystrophy, supporting a required role for Perilipin in TAG storage within human adipocyte LSDs (Gandotra et al., 2011). Regardless, little is known of lipid interaction specificity of the various Plins. Here, we show that distinct Plins differentially sequester to either TAG- or CE-specific LSDs and can alter relative intracellular TAG or CE levels toward the preferentially targeted lipid. These data demonstrate and emphasize diverse functions for the different Plins.
Results
Exogenous fatty acids and cholesterol differentially stabilize accumulation of Plin protein family members
Intracellular LSDs accumulate substantially when cells are cultured overnight in the presence of high concentrations of various exogenous lipids (Xu et al., 2005). Since Plins primarily sequester to LSD surfaces (Miura et al., 2002), we determined if different Plins exhibited differential regulation in response to either fatty acids or cholesterol, lipids that mobilize separate pathways.
Y1 mouse adrenocortical cells have robust capacity for steroid hormone synthesis and accumulate TAG and CE LSDs as energy and metabolic precursor stores. Further, steroidogenic cells are able to synthesize all 4 Plin1 mRNA splice variants (Servetnick et al., 1995; Xu et al., 2005) and express all other Plin genes.
Y1 cells were cultured under standard conditions or in medium supplemented with oleic acid and/or cholesterol. Endogenous Plin proteins were quantified in whole cell lysates by specific immunoblotting (Fig. 1A). In general, none of the Plins exhibited significant accumulation in unsupplemented medium. However, significant Plin accumulation differences were observed in the presence of oleic acid or cholesterol. The two major Plin1 variants of steroidogenic cells, Plin1a and Plin1c, exhibited reciprocal patterns. Plin1a was enhanced by oleic acid, but not by cholesterol, whereas the Plin1c response was exactly opposite (Fig. 1A). The effects were largely activating, since the expressions of Plin1a and Plin1c were not diminished in cells cultured simultaneously with oleic acid and cholesterol. Plin1b and Plin1d proteins are not easily detected in Y1 cells (Servetnick et al., 1995), although Plin1b appears to be regulated similarly to Plin1a (Fig. 1A).
Fig. 1.
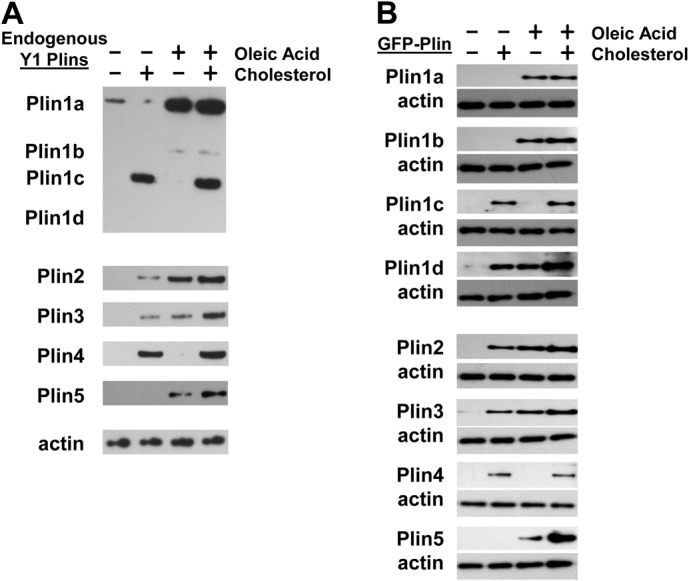
Differential accumulation of Plins in cells cultured in the absence or presence of fatty acid and/or cholesterol. (A) Y1 adrenal cells were cultured overnight in the absence or presence of oleic acid and/or cholesterol. Whole cell lysates were prepared and endogenous Plins assayed by immunoblotting. Data are representative of three experiments. (B) McARH7777 rat liver cells were transiently transfected with the indicated GFP–Plin-expressing constructs and cultured overnight in the absence or presence of oleic acid and/or cholesterol. Whole cell lysates were prepared and GFP–Plin assayed by immunoblotting. Data are representative of three experiments.
Plin2 and Plin3 (also known as TIP47) accumulate similarly regardless of the exogenous lipid moiety, although Plin2 may be slightly more responsive to oleic acid. Conversely, Plin4 (also known as S312) and Plin5 (also known as LSDP5) show extreme lipid specificity, largely mimicking that of Plin1c and Plin1a, respectively (Fig. 1A).
Since exogenous lipids may have differential regulatory effects on the transcription or translation of endogenous Plin mRNAs and, thus, indirectly impact Plin protein accumulation, we also examined the effects of oleic acid and cholesterol using GFP–Plin protein fusions expressed from identical constitutively active promoter vectors. McARH7777 rat hepatoma cells were transiently transfected with vectors to separately express each GFP–Plin protein fusion and cultured under standard conditions or in medium supplemented with oleic acid and/or cholesterol.
The GFP–Plin proteins in McARH7777 cells showed identical responses to oleic acid and cholesterol as their endogenous counterparts in Y1 cells (Fig. 1A,B). GFP–Plin1a and GFP–Plin5 were specifically responsive to the positive effects of oleic acid, whereas GFP–Plin1c and GFP–Plin4 were only detected in the presence of cholesterol (Fig. 1B). GFP–Plin2 and GFP–Plin3 did not show a significant preference to either lipid. The responses of Plin1b and Plin1d were more clear in this heterologous expression system. Plin1b is structurally most similar to Plin1a (Lu et al., 2001) and GFP–Plin1b behaves identically to both Plin1a and GFP–Plin1a. Plin1d is the smallest variant (Lu et al., 2001) and GFP–Plin1d shows limited lipid preference, acting more like Plin2 and Plin3.
Differential sub-cellular localizations of tagged fatty acid and cholesteryl markers
Data (Fig. 1) suggest that the sequestration and stabilization of individual Plins to different classes of LSDs may influence the specific accumulation of particular Plin proteins. To examine this further, we established conditions to preferentially tag and purify TAG- and CE-specific LSDs.
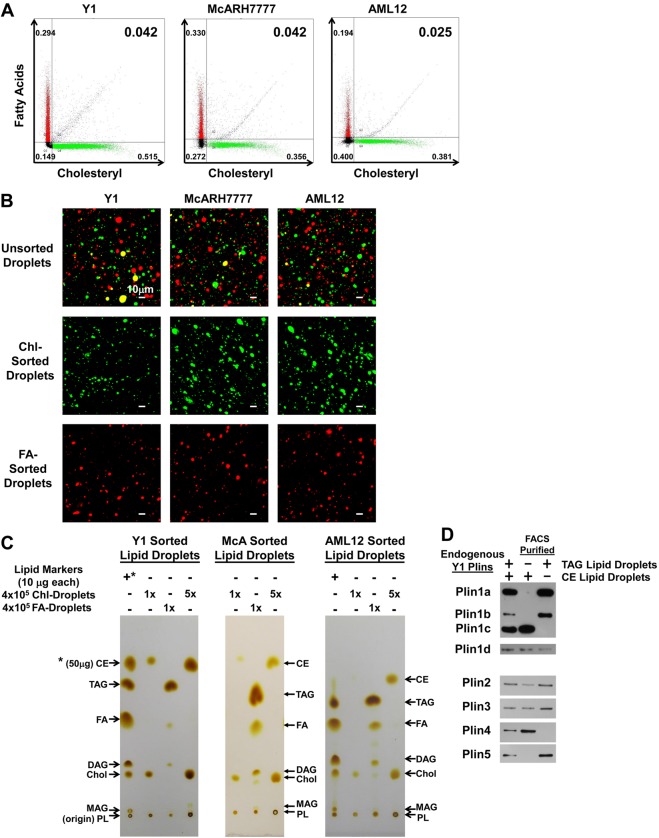
Y1, McARH7777 and AML12 cells were cultured overnight in the presence of both oleic acid and cholesterol, plus BODIPY 558/568 C12 [as a fluorescent fatty acid (FA) dye marker] and cholesteryl BODIPY 500/510 FL C12 [as a fluorescent cholesteryl (Chl) dye marker] and imaged (Fig. 2A). All three types of cells showed definitive separation of FA (red) and Chl (green) markers into distinct LSDs clusters. Few sectors show any colocalization, a pattern similarly observed in 4T1 mouse mammary tumor cells, primary mouse liver cells, C2C12 mouse myoblasts, 3T3-L1 mouse fibroblasts, J774A.1 mouse monocyte-macrophages, and CHOK1 Chinese hamster ovary cells (supplementary material Fig. S1A). In general, the different markers, though distinctly separate, were largely intermingled. However, the McARH7777 cells (Fig. 2A) were most distinctive. Droplets with tagged FAs (hereafter referred to as FA-tagged droplets) segregated to entirely separate sub-cellular regions to those with tagged Chl (hereafter referred to as Chl-tagged droplets). FA-tagged droplets were polarized to the cell periphery, while Chl-tagged droplets were centrally localized. Less distinctive labeling and polarized separation of the droplets is seen in the McARH7777 cells cultured for shorter incubation periods (supplementary material Fig. S1B).
Fig. 2.
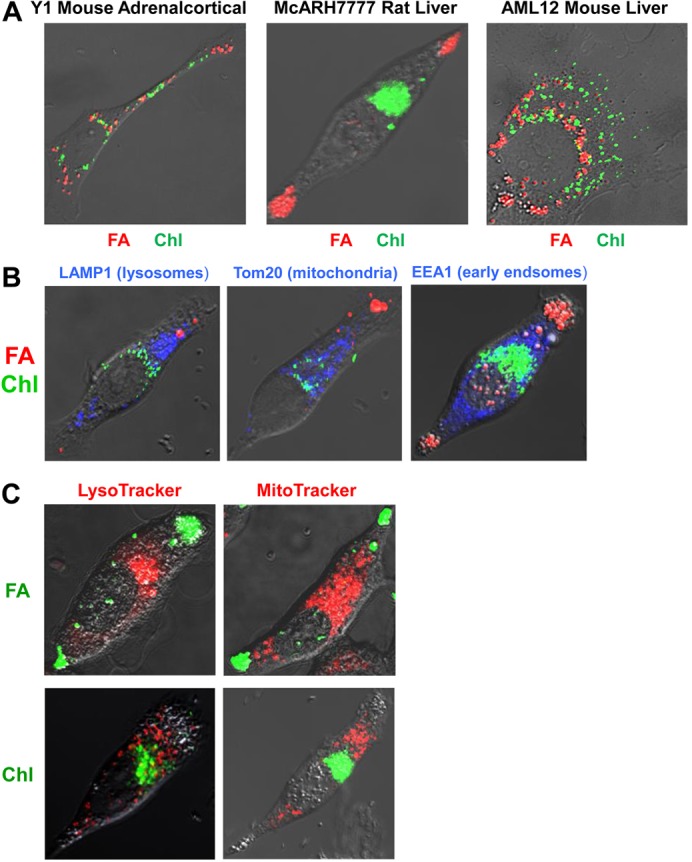
Spatially distinct intracellular accumulations of fatty acid and cholesteryl dye markers. (A) Y1 adrenal, McARH7777 rat liver and AML12 mouse liver cells were cultured overnight in the presence of oleic acid and cholesterol, plus fatty acid BODIPY 558/568 C12 (FA) and cholesteryl BODIPY 500/510 FL C12 (Chl). Representative confocal images are shown with red and green indicating the localization of FA and Chl, respectively. (B) McARH7777 cells were cultured overnight in the presence of oleic acid and cholesterol, plus fatty acid BODIPY 558/568 C12 and cholesteryl BODIPY 500/510 FL C12 and probed for organelle localization using immunofluorescence detection of LAMP1 (Lysosomal-associated membrane protein 1), TOM2 (Translocase of outer mitochondrial membranes 20 kDa) and EEA1 (Early endosome antigen 1). (C) McARH7777 cells were cultured overnight in the presence of oleic acid and cholesterol, plus fatty acid BODIPY 500/510 C12 or cholesteryl BODIPY 500/510 FL C12 and probed for organelle localization by staining with organelle-specific markers.
The polarized localizations of FA- and Chl-tagged droplets suggested that it might be possible to distinguish separate associations of these distinct droplets. McARH7777 cells were cultured with oleic acid, cholesterol, and FA- or Chl-tagged fluorescent markers, and co-stained using antibodies against several organelle-specific proteins (Fig. 2B) or organelle-specific dye trackers (Fig. 2C). In McARH7777 cells, the FA-tagged droplets localized entirely separate from lysosomes, mitochondria, and early endosomes (Fig. 2B,C). The Chl-tagged droplets and other organelles are more centrally localized and perinuclear. Nonetheless, there was nominal overlap of Chl-tagged droplets with these other structures (Fig. 2B,C). Lysosomes and mitochondria remained largely separate, and there was minimal intermingling with early endosomes. The data indicate that the FA or Chl fluorescent tags do not broadly label these other organelles.
FACS purification of LSDs enriched in either TAG or CE
To determine if FA- and Chl-tagged LSDs had distinct lipid compositions, we first developed conditions for their separate purifications. Labeled Y1, McARH7777 and AML12 cells were lysed, LSDs floated by gradient centrifugation, and the FA- and Chl-fluorescently tagged LSDs separated by FACS (Fig. 3A; supplementary material Fig. S2A–C). The FA- and Chl-specific labelings were relatively similar and reproducible. Generally, >70% of the particles were dye tagged in dual label experiments. FACS separations were distinct with <5% of unsorted particles carrying both FA and Chl dye markers (Fig. 3A,B). The particles also showed reasonable size homogeneity; ∼80% of all particles had diameters of 2–6 µm. Experiments with a mixture of labeled and unlabeled lysates and particles showed <2% dye marker transfer and/or non-specificity (supplementary material Fig. S2A–C).
Fig. 3.
Differential localization of Plins to TAG or CE droplets. Y1 adrenal, McARH7777 rat liver and AML12 mouse liver cells were cultured overnight in the presence of oleic acid and cholesterol, plus fatty acid BODIPY 558/568 C12 (FA) and cholesteryl BODIPY 500/510 FL C12 (Chl). (A) Representative FACS profiles of purified FA- and Chl-tagged lipid droplets are shown, with relative distributions of total lipid droplet numbers indicated in the different quadrants (FA−/Chl−, FA+/Chl−, FA−/Chl+ and FA+/Chl+); blue represents dual signals of the FA and Chl probes. Y1 cells: 25,000 total droplets; McARH7777 cells: 20,000 total droplets; AML12 cells: 25,000 total droplets. (B) Confocal images of unsorted and FACS sorted FA- and Chl-tagged lipid droplets from Y1, McARH7777 and AML12 cells. (C) Lipids isolated from sorted FA- and Chl-droplets were separated by TLC in parallel with lipid markers and detected by staining with iodine vapor. 1× indicates 2×105 LSD particles; 5× indicates 1×106 LSD particles. Marker lanes have 50 µg CE (*) and 10 µg each of TAG, FA, diacylglycerides (DAG), cholesterol (Chol), MAG and phospholipid (PL). PLs do not migrate from the origin in this system. (D) Proteins were prepared from unsorted and FACS-sorted TAG- or CE-LSD isolated from Y1 cells. Endogenous Plin proteins were assayed by immunoblotting. Identical numbers of lipid droplet particles were loaded in each lane. Data are representative of three experiments.
Lipids were extracted from sorted FA- and Chl-tagged LSDs and analyzed and quantified by thin layer chromatography (Fig. 3C; Table 1; supplementary material Fig. S3A). Total LSD content was similar within and between cell types. The Chl-tagged droplets were primarily comprised of cholesteryl ester and a smaller amount of cholesterol; we were unable to detect triacylglycerols, fatty acids and related metabolites in the sorted Chl-tagged droplets. Conversely, the isolated FA-tagged droplets were comprised primarily of triacylglycerol and minor quantities of metabolites (di- and mono-acylglycerides and fatty acids); small amounts of cholesterol were present, but cholesteryl ester was not detected. Thus, we have established conditions to fluorescently tag, image and purify LSDs specific to either CE or TAG.
Table 1.
Lipid specificities in FACS-purified lipid droplets
| Lipid | Chl-tagged droplets | FA-tagged droplets | ||||
| Y1 | McA | AML12 | Y1 | McA | AML12 | |
| CE | 90 µg | 65 µg | 70 µg | ND (<3 µg) | ND (<3 µg) | ND (<3 µg) |
| TAG | ND (<0.5 µg) | ND (<0.5 µg) | ND (<0.5 µg) | 70 µg | 100 µg | 100 µg |
| FA | ND (<0.5 µg) | ND (<0.5 µg) | ND (<0.5 µg) | 10 µg | 15 µg | 20 µg |
| DAG | ND (<0.5 µg) | ND (<0.5 µg) | ND (<0.5 µg) | 20 µg | 10 µg | 10 µg |
| Chol | 50 µg | 15 µg | 15 µg | 3–5 µg | 3–5 µg | 3–5 µg |
| MAG | ND (<2 µg) | ND (<2 µg) | ND (<2 µg) | 15 µg | 10 µg | 10 µg |
Chl- and FA-tagged lipid droplets from Y1, McARH7777 and AML12 cells were separated by FACS, and lipid profiles analyzed by TLC (see Fig. 3C). Values (from 2×106 droplets) were extrapolated (±30%) from relative staining intensity data in comparison to standards (see supplementary material Fig. S3B) for CE, cholesteryl ester; TAG, triacylglycerol; FA, fatty acid; DAG, diacylglycerol; Chol, cholesterol; and MAG, monoacylglycerol. Phospholipid spot staining was similar for each, but did not resolve from the origin. ND, not detected.
We also traced the cell fate of the BODIPY precursor markers (supplementary material Fig. S3B). The native BODIPY–Chl migrates during TLC at a unique position that differs from both cholesterol and cholesterol ester and retains its identical motility even after integration within the CE droplets. Thus, BODIPY–Chl appears to be incorporated directly into CE droplets and is not significantly metabolized into other droplet-specific lipids during the time frame studied. BODIPY–FA migrates during TLC less far than does untagged FA, but changes mobility when integrated into TAG droplets. We suggest that this represents the incorporation of the BODIPY–FA into TAG, which migrates less far than untagged TAG and distinctly from phospholipids. Regardless, the FA and Chl markers represent specific tracer tags that allow us to separately purify the distinct populations of LSDs that are biochemically enriched in either TAG or CE, hereafter referred to as TAG-LSDs and CE-LSDs, respectively.
Plin associations with TAG- or CE-LSDs
We next examined Plin protein associations with isolated TAG- or CE-LSDs. Y1 cells were cultured with oleic acid and cholesterol, plus FA- and Chl-fluorescent markers. LSDs were floated, TAG- or CE-LSDs purified by FACS, and associations of Y1 endogenous Plins within unsorted LSDs or to TAG- or CE-specific populations then assayed by specific immunoblotting.
All of the Plin proteins were detected in the unsorted LSD population (Fig. 3D), consistent with previous data (Fig. 1A,B). The relative increase in endogenous Plin1b and Plin1d signals probably reflects the enrichment of these forms in floating droplets compared to whole cell lysates (see Fig. 1A, Fig. 3D). In this assay, the individual Plins show distinct sequestration to TAG- or CE-specific LSDs. Plin1a, Plin1b and Plin5 are restricted to TAG populations, whereas Plin1c and Plin4 localize with the CE-LSDs (Fig. 3D). Plin2, Plin3 and Plin1d show limited association preference (Fig. 3D).
Sub-cellular localization of GFP-Plins to TAG- or CE-LSDs
The whole cell accumulations of specific Plin proteins in response to either oleic acid or cholesterol correlate with the associations of the same Plins, to either TAG- or CE-LSDs (Fig. 1, Fig. 3D). Still, we wished to investigate specific Plin/droplet associations by alternative methods. Since McARH7777 cells accumulate TAG- and CE-LSDs that are completely separated (Fig. 2A), we could visually observe if GFP–Plins exhibited parallel localization differences. In addition we could quantify the relative distributions of GFP–Plins among CE- or TAG-LSDs by FACS.
McARH7777 cells were transiently transfected individually with vectors that specifically encode different eGFP–Plin fusions and cultured with oleic acid and cholesterol plus either BODIPY 558/568 C12 (TAG marker) or cholesteryl BODIPY 576/589 C11 (CE marker). Both dye markers have excitation/emission spectra that differ from eGFP (488/509). GFP/TAG and GFP/CE experiments were always performed in pairs and data analyzed in parallel to assess reciprocal responses (Figs 4, 5, 6; supplementary material Fig. S4).
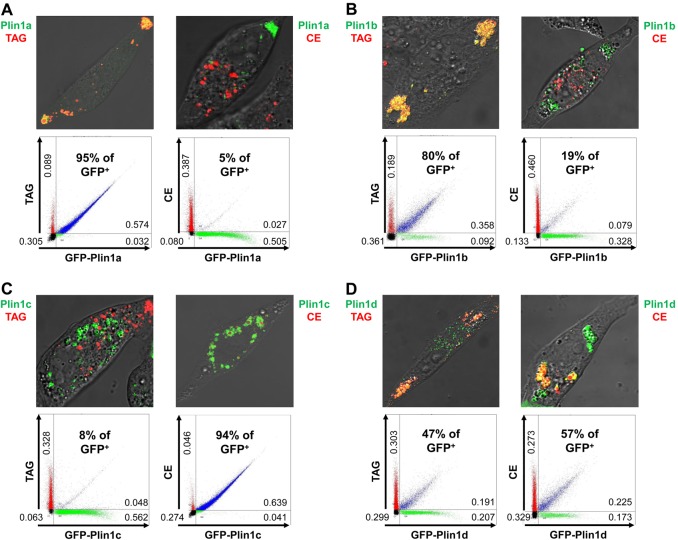
Fig. 4.
Differential localization of Plin1 proteins to TAG or CE lipid droplets. McARH7777 cells were transiently transfected with the indicated GFP–Plin1-expressing constructs and cultured overnight in the presence of oleic acid and cholesterol, plus either BODIPY 558/568 C12 (TAG) or cholesteryl BODIPY 576/589 C11 (CE). Representative confocal images are shown for each with red indicating the localization of either TAG or CE droplets, green indicating localization of GFP–Plin1 proteins, and yellow (or rings) indicating colocalization. Representative FACS profiles of TAG- and GFP-labeled lipid droplets or of CE- and GFP-labeled lipid droplets are shown, with relative distributions of total particle numbers indicated in the different quadrants, and the relative Plin1 variant localizations indicated as a percentage of the total GFP+ signal that co-sorts (blue) with either TAG+ or CE+ tags. Each experiment was performed at least three times (see Fig. 6). (A) Plin1a (25,000 total droplets for each population); (B) Plin1b (20,000 total droplets for each population); (C) Plin1c (25,000 total droplets for each population); (D) Plin1d (10,000 total droplets for each population).
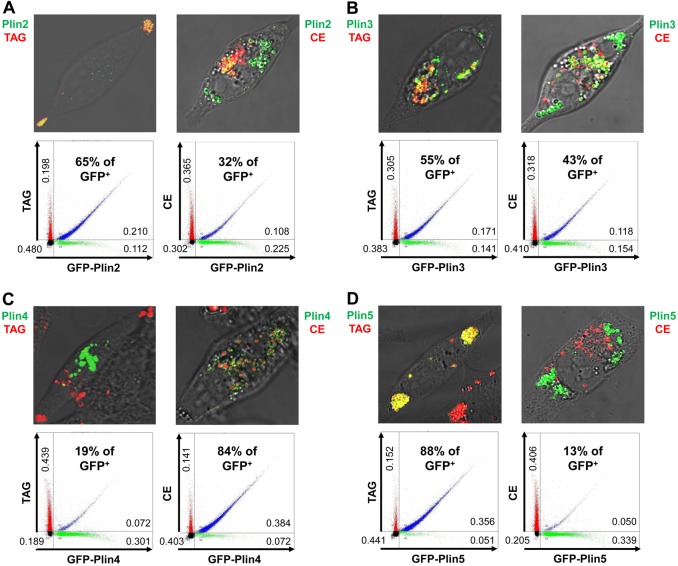
Fig. 5.
Differential localization of Plin2, -3, -4 and -5 proteins to TAG or CE lipid droplets. McARH7777 cells were transiently transfected with the indicated GFP–Plin-expressing constructs and cultured overnight in the presence of oleic acid and cholesterol, plus either BODIPY 558/568 C12 (TAG) or cholesteryl BODIPY 576/589 C11 (CE). Representative confocal images are shown for each, with red indicating the localization of either TAG or CE droplets, green indicating localization of GFP–Plin proteins, and yellow (or rings) indicating colocalization. Representative FACS profiles of TAG- and GFP-labeled lipid droplets or of CE- and GFP-labeled lipid droplets are shown, with relative distributions of total particle numbers indicated in the different quadrants, and the relative Plin2–5 variant localizations indicated as a percentage of total GFP+ signal that co-sorts (blue) with either TAG+ or CE+ tags. Each experiment was performed at least three times (see Fig. 6). (A) Plin2 (15,000 total droplets for each population); (B) Plin3 (20,000 total droplets for each population); (C) Plin4 (25,000 total droplets for each population); (D) Plin5 (10,000 total droplets for each population).
Fig. 6.
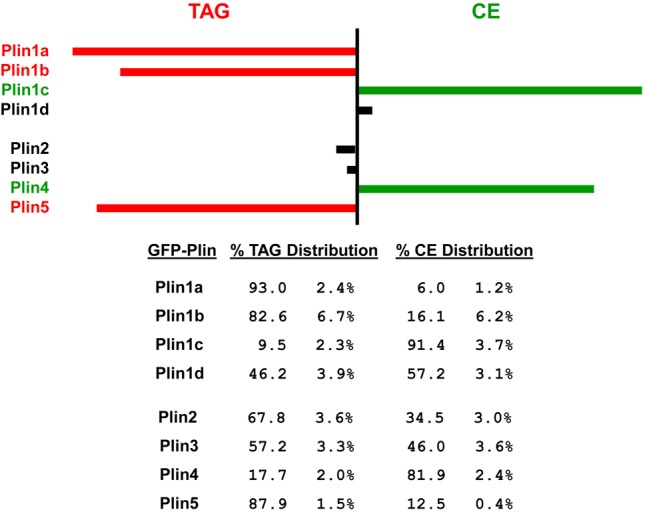
Relative distributions of Plin family proteins to TAG- or CE-specific intracellular lipid storage droplets. McARH7777 cells were transiently transfected with the indicated GFP–Plin-expressing constructs and cultured overnight in the presence of oleic acid and cholesterol, plus either BODIPY 558/568 C12 (FA) or cholesteryl BODIPY 576/589 C11 (Chl) dye markers. TAG or CE distributions are the percentage of the total GFP+ signal that co-sorts, respectively, with either FA+ or Chl+ tags (see Figs 4, 5). Data are from at least three independent, paired experiments, where the TAG+CE sum for each pair was 98–102%. Values are the means ± standard deviation. Plin1a, Plin1b and Plin5 show strong preference for localization to TAG-LSDs, with Plin1a consistently exhibiting a stronger TAG signal than Plin1b or Plin5. Plin1c and Plin4 show strong preference for localization to CE-LSDs, with Plin1c consistently exhibiting a stronger CE signal than did Plin4. Plin1d, Plin2 and Plin3 exhibit less specific localization. Values are ± standard deviation.
GFP–Plin1a localizes to the polarized periphery of McARH7777 cells and primarily with TAG-LSDs (Fig. 4A, Fig. 6). In contrast, while CE-LSDs are sequestered to the cell interior, none of the GFP–Plin1a signal colocalizes with the CE marker (Fig. 4A). FACS profiles of cell populations support these conclusions; ∼95% of expressed GFP–Plin1a co-sorts with the TAG marker, while only ∼5% GFP–Plin1a co-sorts with CE-LSDs (Fig. 4A, Fig. 6).
GFP–Plin1b also exhibits a localization preference for TAG-LSDs compared to CE-LSDs, although the FACS profiles show slightly less specificity (Fig. 4B, Fig. 6).
GFP–Plin1c shows the expected reciprocal pattern to GFP–Plin1a (see Fig. 1, Fig. 3D). GFP–Plin1c localizes to the cell interior, surrounding CE-LSDs, and distinctly separate from the polarized TAG-LSDs (Fig. 4C). The FACS data are consistent, where >90% of GFP–Plin1c sorts with CE and separate from TAG (Fig. 4C, Fig. 6).
The GFP fusions of Plin1d, Plin2, and Plin3 show minimal preference for TAG- or CE-LSDs and segregate with both (Fig. 4D, Fig. 5A,B). All 3 protein fusions are found in both interior and peripheral cell regions, colocalize with both TAG- and CE-LSDs within the cell, and co-segregate with both by FACS, but to varying degrees (Fig. 4D, Fig. 5A,B, Fig. 6).
GFP–Plin4 and GFP–Plin5 exhibit reciprocal LSD preferences and are, thus, respectively, similar to Plin1c and Plin1a (see Figs 1, 3). While GFP–Plin4 sequesters with CE-LSDs in the cell interior (Fig. 5C), GFP–Plin5 polarizes with TAG-LSDs to the cell periphery (Fig. 5D). FACS data substantiate these preferences; ∼85% of GFP–Plin4 co-sorts with a CE marker, while ∼85% of GFP–Plin5 segregates with TAG (Fig. 5C,D, Fig. 6).
Plins with TAG- or CE-binding preferences can alter cellular TAG/CE distributions
Loss of Plin1 and Plin2 in mice alters the targeted accumulation of lipid levels in defined cell types (Martinez-Botas et al., 2000; Tansey et al., 2001; Chang et al., 2006). We were, thus, interested to determine if there were a preferential relationship among individual Plin proteins, their lipid targeting preference, and the cellular accumulation of either TAG or CE. We first attempted siRNA approaches to deplete Plins targeted to either TAG- or CE-LSDs. Data from other systems clearly show that depletion of one Plin protein type results in compensation by other Plins (Martinez-Botas et al., 2000; Tansey et al., 2001; Chang et al., 2006; Sztalryd et al., 2006). Further, Plin2 and Plin3 are expressed in most cells and exhibit no TAG or CE targeting preference. Thus, experiments directed toward defining Plin effects on TAG or CE levels required us to simultaneously target multiple Plins in any individual cell. Several cultured cells lines were selected but we were unsuccessful in depleting any of the TAG- or CE-LSD specific Plins in combination with Plin2 and Plin3. As we were unable to deplete all Plins associated with either TAG- or CE-LSDs, we sought an alternative approach.
When we compared FACS analyses of GFP–Plin1a- and GFP–Plin1c-expressing cells (Fig. 4), we noticed a conspicuous difference in the relative distributions of TAG- and CE-LSDs. More than 60% of total LSD particles in Plin1a-expressing cells were tagged by the TAG dye marker (Fig. 4A), whereas the Plin1c-expressing cells were predominantly (>60%) populated with CE-containing LSDs (Fig. 4C). These data suggested that the ectopic expression of Plins with specific lipid targeting preferences might quantitatively alter the balance of TAG/CE levels in individual cells. We thus analyzed the relative TAG/CE distributions in cells expressing various Plin proteins.
AML12 cells were transiently transfected individually with vectors that specifically encode different Plin proteins and cultured with oleic acid and cholesterol. Cells cultured without exogenous lipids accumulated only limited levels of TAG or CE regardless of Plin expression (data not shown), whereas untransfected control cells accumulate large quantities of both TAG and CE. LSDs were isolated from the various Plin-expressing and control cells and their TAG/CE levels quantified by TLC. Cells expressing Plin1a or Plin1c were analyzed in pairs, and normalized in parallel experiments of lipid loaded and unloaded control cells (Fig. 7). Similar paired and normalized experiments were used to analyze cells expressing Plin4 or Plin5.
Fig. 7.
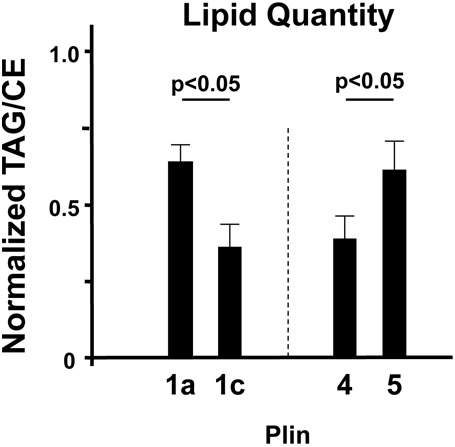
Relative change in TAG- or CE-specific intracellular lipid storage upon expression of various Plin proteins. AML12 cells were transiently transfected with the indicated GFP–Plin-expressing constructs and cultured overnight in the presence of oleic acid and cholesterol. Transfection efficiencies were confirmed by visualizing GFP fluorescence. Untransfected cells, cultured with/without exogenous oleic acid and cholesterol, were grown in parallel. LSDs were isolated by centrifugation and lipids were extracted, separated by TLC in parallel with lipid markers, and TAG and CE detected after staining with iodine vapor. Relative TAG/CE ratios were quantified in cells transfected with each specific Plin-expressing construct and analyzed in parallel with identically grown untransfected cells for normalization and TLC background correction. The TAG/CE ratio for untransfected lipid-loaded cells (controls) was set to 0.5. The relative TAG/CE-ratio of Plin1a- and Plin1c-expressing cells were always analyzed in parallel and normalized to those of control cells, and then secondarily compared to results determined for its Plin-expressing counterpart. Values >0.5 indicate a proportional increase in TAG lipid bias, whereas values <0.5 indicate a proportional increase in CE lipid bias. Relative distributions of TAG/CE levels are shown as the means ± standard deviation for each paired comparison. Data for each pair are based on three independent experiments. Plin4- and Plin5-expressing cells were similarly analyzed as pairs and internally compared as described for the Plin1a/Plin1c pair. Plin1a and Plin5 show a relative bias for cellular TAG accumulation and a strong preference for binding specificity to TAG-LSDs (see Fig. 6). Conversely, Plin1c and Plin4 show a relative increase in cellular CE levels and a strong preference for binding to CE-LSDs.
Plin1a and Plin1c exhibited largely reciprocal effects on TAG/CE levels, with an ∼60% TAG lipid bias observed in Plin1a-expressing cells and an ∼60% CE bias observed for Plin1c-expressing cells (Fig. 7). Plin5/Plin4 differences in relative TAG/CE accumulations were similarly polarized toward either TAG or CE, respectively. Thus, ectopic expression of Plins that exhibit lipid targeting preferences can polarize relative cellular lipid-type distributions. These effects are seen for both the TAG-specific (i.e. Plin1a and Plin5) and the CE-specific (i.e. Plin1c and Plin4) Plin proteins, but not for the non-preferential Plins 2, 3 and 1d (data not shown).
Discussion
We have demonstrated that distinct Plins differentially sequester to either TAG- or CE-specific LSDs, emphasizing diversity of function for the different Plins. These significant functional differences towards TAG- or CE-LSDs impact previous assumptions about commonality of Plin action. The large unilocular TAG-LSDs that are targeted by Plin1a in adipocytes are proposed to derive from nascent droplets marked by Plin2, Plin3 and Plin4 (Wolins et al., 2005). While Plin2 and Plin3 interact with TAG-LSDs, this association, unlike that of Plin1a, is not exclusive, as Plin2 and Plin3 also co-segregate with CE-LSDs. Since Plin4 primarily targets CE-LSDs, conclusions regarding linear development of Plin-specific LSD populations, based upon static global imaging of cells cultured with exogenous fatty acids (Wolins et al., 2005), must be tempered.
Plin structural domains that direct LSD targeting are still poorly defined. All Plins share an N-terminal, ∼100 amino acid PAT domain and a distal 11-mer amphipathic helical repeat (Bussell and Eliezer, 2003; Lu et al., 2001; Miura et al., 2002). C-terminal to both, Plins are increasingly diverged. Several groups have probed for LSD-interacting motifs through domain-specific expressions (Garcia et al., 2003; Hickenbottom et al., 2004; McManaman et al., 2003; Nakamura and Fujimoto, 2003; Ohsaki et al., 2006; Subramanian et al., 2004a; Subramanian et al., 2004b; Yamaguchi et al., 2006), but these studies have focused on TAG-enriched cells and ignored effects of CE-LSDs. In addition, some constructs have exposed amphipathic helices that are usually masked in endogenous Plins and, thus, may target inappropriately (Hickenbottom et al., 2004).
No simple and consistent structural model for preferential Plin associations with TG- or CE-LSDs can be easily deduced from sequence or structural motif scanning and interrogation. While the PAT and associated 11-mer domains may be involved in LSD interaction, these regions cannot be sufficient determinants for specific targeting. Although all Plin1 variants have identical N-terminal 198 residues, which include the PAT and 11-mer regions (Kimmel et al., 2010; Lu et al., 2001; Miura et al., 2002), they have dissimilar LSD targeting. Further, the PAT and 11-mer domains differ greatly among the other Plin proteins in both length and sequence. Lipid binding discrimination can also not be simply deduced by analyses of C-termini, which differ highly among Plin4, Plin5, and each of the unique Plin1 forms. Signaling or targeting motifs may instead reside in non-common segments, as hydrophobic segments are suggested to facilitate Plin1 targeting to LSDs (Garcia et al., 2003). While Plin-specific interacting proteins possibly help direct TAG- or CE-LSD recognition, one must consider that the extremely diverged single Plin species in Dictyostelium specifically targets LSDs when expressed in mammalian CHO cells (Lu et al., 2001; Miura et al., 2002). Furthermore, although Plin interactions that are unique to TAG- or CE-specific LSDs may involve the surrounding phospholipid monolayer, these would also require commonality in both diverse tissues (e.g. adrenal and liver cells) and species.
Pathogenesis associated with abnormal lipid storage has serious health consequences. Thus, understanding the mechanisms that direct lipid storage and lipolytic breakdown is paramount. The Plins regulate access of lipases to lipids stored within the LSD core (Granneman et al., 2009; Granneman et al., 2011; Martinez-Botas et al., 2000; Miyoshi et al., 2007; Sztalryd et al., 2003; Tansey et al., 2001; Wang et al., 2011; Wang et al., 2009; Yamaguchi et al., 2004), and the cellular content of Plins and accumulated LSDs seem intimately coordinated. In addition, various Plins may have significantly different regulatory effects on cellular lipolytic activity depending upon tissue context (Dalen et al., 2007; Tansey et al., 2003). Accordingly, recent attention has been directed towards possible causal linkages of aberrant Plin function with human disease. In particular, heterozygous loss-of-function mutations of PLIN1 causes a familial partial lipodystrophy in humans (Gandotra et al., 2011) and polymorphisms in human genes for Plin1 (Qi et al., 2004) and Plin4 (Richardson et al., 2011) have been associated with obesity.
Our data add novel conceptual parameters for LSD and Perilipin function and analyses. The various Plins target different classes of LSDs even within a single cell population and Plins with LSD lipid specificities can preferentially affect the accumulation of the targeted LSD class. Intriguingly, tissues such as adipose, heart and oxidative muscle, that predominantly accumulate TAG, have highest expression of Plin 1a, 1b or 5, which specifically target TAG-LSDs. Conversely, steroidogenic cells, which accumulate CE, express these Plins more poorly in comparison to Plin1c. We suggest that Plin expression may not impact all cellular lipids and LSDs equivalently, but that the actions of individual Plin types may be differentially targeted to distinct LSD classes within a given cell. It will, thus, be of interest to evaluate the full spectrum of Plin variants in broad tissue panels that exhibit differential TAG/CE biases. Our new findings underscore the view that each Plin is likely to have very separate and perhaps unique functions associated with their LSD-specific targeting.
Materials and Methods
Materials
Fatty-acid-free bovine serum albumin (BSA) was purchased from Fisher Scientific (Pittsburgh, PA). Phosphate-buffered saline (PBS), glutamine, fetal bovine serum (FBS), horse serum, cholesteryl BODIPY® 500/510 FL C12 [cholesteryl 4,4-difluoro-5,7-dimethyl-4-bora-3a,4a-diaza-s-indacene-3-dodecanoate; cholesteryl dye], BODIPY® 558/568 C12 [4,4-difluoro-5-(2-thienyl)-4-bora-3a,4a-diaza-s-indacene-3-dodecanoic acid; fatty acid dye], cholesteryl BODIPY® 576/589 C11 [cholesteryl 4,4-difluoro-5-(2-pyrrolyl)-4-bora-3a,4a-diaza-s-indacene-3-undecanoate; cholesteryl dye], and BODIPY® 500/510 FL C12 [4,4-difluoro-5-methyl-4-bora-3a,4a-diaza-s-indacene-3-dodecanoate; fatty acid dye] were from Invitrogen (Carlsbad, CA). MitoTracker (579/599) and LysoTracker (577/590) were also from Invitrogen (Carlsbad, CA). Antibodies to LAMP1 and EEa1 were from Abcam (Cambridge, MA) and to TOM2 was from Santa Cruz Biotechnology, Inc. (Santa Cruz, CA). The 250 µm silica gel H TLC plates were from Analtech (Newark, DE). Complete protease inhibitor cocktail tablets were from Roche Diagnostics (Indianapolis, IN). Paraformaldehyde was from Electron Microscopy Sciences (Hatfield, PA). Cell media and all other chemicals were from Sigma-Aldrich (St Louis, MO).
Cell culture
Y1 mouse adrenal-cortical, AML12 mouse liver, and McARH7777 rat hepatoma cells were obtained from the American Tissue Culture Collection (Manassas, VA). Cells were grown to subconfluence in medium supplemented with 100 µg/ml penicillin and 100 µg/ml streptomycin and incubated in humidified air containing 5% CO2 at 37°C. Y1 adrenal cells were grown in Dulbecco's modified Eagle's medium (DMEM)/Ham's nutrient mixture F-12 supplemented with 15% horse serum, 2.5% FBS and 2 mM glutamine. McARH7777 cells were grown in DMEM supplemented with 10% FBS. AML12 cells were grown in DMEM/Ham's nutrient mixture F-12 supplemented with 5 mg/ml insulin, 5 mg/ml transferrin, 5 ng/ml selenium, 10% FBS and 2 mM glutamine.
Plin–GFP fusion constructs and transfection
The perilipin expression vectors were generated using Multi-Site Gate-Way (Invitrogen, Carlsbad, California). Mouse Plins 1a, 2, 3, and 5 cDNAs were amplified from previously described pSG5 vectors (Dalen et al., 2007). Plins 1b, 1c, 1d and 4 cDNAs were amplified from Y-1 cells or adipose tissue mRNA using PfuTurbo® DNA Polymerase (Stratagene). Primers used contained overhangs for insertion into the pDONR-221 P4r-P3r vector, Kozak sequence (ACCTAG) and stop codon (CTA), and were designed using Vector NTI 10.0 (Invitrogen) with Tm set to 65°C.
PCR products were recombined into the pDONR-221 P4r-P3r vector using BP Clonase II (Invitrogen) to produce pENTR vectors. GFP was amplified from pEGFP-C1 (Clontech) and cloned into the pDONR-221 P1-P4. The V5-6x-His-Gly epitope was cloned into the pDONR-221 P3-P2 vector.
The destination vector was generated by replacing the multi-cloning site of pcDNA3 (Invitrogen) with the attR1-ccdB-chloramphenicol-attR2 cassette (R1-R2) from pLenti6/v5-DEST (Invitrogen). The R1-R2 cassette was amplified with PfuTurbo, and digested with HindIII/ApaI prior to ligation into HindIII/ApaI-digested pcDNA3 vector. The ligation mixture was transformed into ccdB Survival TR cells (Invitrogen) and clones selected on Ampicilin (100 µg/ml) and Chloramphenicol (25 µg/ml) plates. The novel pcDNA3-R1-R2 vector (pcDNA3-KTD2-DEST) was subsequently recombined with the above pENTR vectors using LR clonase II (Invitrogen) to generate the pKTD2-G-Perilipin-VH expression vectors. Due to the stop codon inserted into the pENTR-perilipin vectors, the 3-end V5-6xHis-G tag will not be translated. Correctly amplified sequences were confirmed by sequencing (Macrogen, Korea).
Transient transfection was carried out following the manufacturer's instructions (Invitrogen) using Lipofectamine LTX reagent. McARH7777 cells (cell density 6.25×104/cm2) were incubated in standard growth conditions in medium lacking antibiotic for one day and then incubated with fresh media containing plasmid DNA (250 ng/cm2), Lipofectamine LTX reagent (1 µl/cm2) and Opti-MEM (50 µl/cm2) for 1 day.
Immunoblot analyses
Proteins were separated by electrophoresis in 10% NuPAGE gels (Invitrogen, Carlsbad, CA) using MOPS running buffer and then subjected to immunoblot analyses as described (Kim et al., 2002). For immunoblot analyses, we used rabbit polyclonal antisera (1:3000) to mouse Plin1 (Servetnick et al., 1995), Plin2 (Xu et al., 2005), or Plin3 (Sztalryd et al., 2006), and guinea pig polyclonal antisera (1:3000) to human Plin4 (American Research Product, 03-GP31) and human Plin5 (Progen Biotechnik, GmbH GP34). Rabbit antibody (1:5000) to β-actin and GFP were, respectively, from Abcam (Cambridge, MA) and Invitrogen (Carlsbad, CA). Secondary antibodies to rabbit or guinea pig IgG were from Jackson ImmunoResearch (West Grove, PA) and used at 1:5000 dilution.
Lipid loading
Cells were grown overnight to <50% confluence in media supplemented with 100–200 µM oleic acid bound to fatty acid free BSA (2.5:1 mol oleic acid: mol BSA) (Dalen et al., 2006) and/or 50 µM cholesterol complexed with β-methyl cyclodextrin (8:1 mol β-methyl cyclodextrin: mol cholesterol) (Christian et al., 1997). For fatty acid and cholesteryl dye labeling, cells were grown overnight to <50% confluence in media supplemented with 100 µM oleic acid and 50 µM cholesterol, plus 1.0 µM BODIPY® 558/568 C12 (fatty acid dye) and 0.5 µM cholesteryl BODIPY® 500/510 FL C12 (cholesteryl dye). GFP–Plin expressing cells were grown overnight to <50% confluence in media supplemented with 100 µM oleic acid and 50 µM cholesterol, plus either 1.0 µM BODIPY® 558/568 C12 (fatty acid dye) or 0.5 µM cholesteryl BODIPY® 576/589 C11 (cholesteryl dye).
Lipid droplet preparation
Cells were grown overnight, washed with PBS, scraped into PBS, and pelleted by centrifugation (300 g for 5 min). The cell pellet was resuspended in 4 ml hypotonic lysis solution (50 mM HEPES pH 7.3, 0.1 M KCl, 2 mM MgCl2) at 4°C, containing protease inhibitors [20 µg/ml leupeptin, 1 mM benzamidine and 100 µM 4-(2-aminoethyl)-benzenesulfonylfluoride] and lysed by incubation on ice for 30 min. 1 ml of 50% (w/v) sucrose in lysis solution was added to the cell lysate for a total volume of 5 ml. This 10% w/v sucrose solution was layered at the bottom of an ultracentrifuge tube and a step gradient of 5%, 2.5% and 0% sucrose was carefully layered above; centrifugation (Beckman Coulter Optima XL100K Ultracentrifuge) was at 154,000 g for 1 h at 4°C. The floating lipid layer was collected using a Beckman tube slicer (Brea, CA) for immunoblotting or flow cytometry (Brasaemle et al., 2004; Hsieh and Huang, 2005).
FACS separation of fluorescently labeled lipid droplets
Lipid droplets isolated from cells labeled with fluorescent fatty acid dye, fluorescent cholesteryl dye, and/or expressing GFP were sorted using a FACSAria II cytometer (BD Biosciences). All fluorochromes were excited using a 488 nm laser. Fluorescent emissions from lipid droplets labeled with cholesteryl BODIPY® 500/510 FL C12 or with GFP-tagged perilipin proteins were collected through a 502 nm longpass filter followed by a 530/30 nm bandpass filter. Emissions from lipid droplets labeled with BODIPY® 558/568 C12 or with cholesteryl BODIPY® 576/589 C11 were collected through a 556 nm longpass filter followed by a 575/26 bandpass filter. Compensation settings for experiments with multiple fluorescent dyes were established using lipid droplets isolated from cells labeled with a single fluorescent dye (see supplementary material Figs. S1, S3). The forward scatter threshold was set at the lowest possible linear signal height of 200 (within an allowed range of 200 to 262,143), permitting detection of lipid droplets with diameters >1 µm. For sorting, ∼5000 events/second were processed and sort precision was set on the default purity mode.
Confocal laser scanning microscopy
Confocal laser scanning microscopy (CLSM) was carried out using a Zeiss LSM 510 (Jena, Germany) inverted confocal microscope with a 100× (Plan-Apochromat, NA1.40) oil objective lens. GFP and cholesteryl BODIPY® FL 500/510 FL C12 were imaged using argon 488-nm laser and a 505–530-nm BP emission filter. BODIPY® 558/568 C12 and cholesteryl BODIPY® 576/589 C11 were imaged using a He/Ne 543-nm laser excitation and a 580-nm LP emission filter. The software for confocal microscopic image generation was LSM510 software 3.2. The cells were seeded and manipulated in 35 mm glass bottom culture dishes (MatTek, Ashland, MA), fixed with 4% paraformaldehyde in PBS for 30 minutes, and washed twice for 5 minutes before observation. To visualize isolated lipid droplets, the unsorted or sorted lipid droplet populations were first mixed with an equal volume of glycerol before observation.
Lipid extraction and analyses
Lipid droplets were extracted twice with two volumes of chloroform:heptane:methanol (4:3:2; v/v/v) (Hsieh and Huang, 2007). The lipids were applied to TLC plates and separated in hexane:diethyl ether:acetic acid (70:30:1; v/v/v); the plates were stained overnight in an iodine chamber to visualize the lipids. Extracted lipids were separated in parallel to a dilution series of lipid standards applied to the same plate. The standards were cholesteryl oleate (cholesteryl ester; CE), glyceryl trioleate (triacylglycerol; TAG), oleic acid (fatty acid; FA), cholesterol (Chol), dioleoylglycerol (diacyclglycerol; DAG), DL-α-monoolein (monoacyclglycerol; MAG), and L-α-phosphatidylcholine (phospholipid; PL).
TIFF images of the iodine stained TLC plates were analyzed by ImageQuant TL with module 1D gel analyses (GE Health Life Sciences, Piscataway, NJ). Stored 8-bit grayscale images were created manually, since single lane images had various shapes and spot distances. Spot intensities were corrected for background and data exported to generate curve standards for the various lipid marker controls. Masses of the unknown lipids were extrapolated in comparison to standard curves (see supplementary material Fig. S3A).
Statistical analyses
Confocal data (Figs 4, 5, 6) are from at least three independent, paired experiments (see Fig. 3), where the TAG/CE sum for each pair was 98–102% (see Fig. 3). McARH7777 cells were transiently transfected with the indicated GFP-Plin-expressing constructs and cultured overnight in the presence of 100 µM oleic acid and 50 µM cholesterol, plus either 1 µM BODIPY 558/568 C12 (FA) or 0.5 µM cholesteryl BODIPY 576/589 C11 (Chl) dye markers. TAG or CE distributions represent the percentage of the total GFP+ signal that co-sorts, respectively, with either FA+ or Chl+ tags.
Supplementary Material
Footnotes
Funding
This work was supported by the Intramural Research Program of the National Institutes of Health; the National Institute of Diabetes and Digestive and Kidney Diseases; and a travel grant from the Henning and Johan Throne-Holst's Foundation to K.T.D. Deposited in PMC for release after 12 months.
Supplementary material available online at http://jcs.biologists.org/lookup/suppl/doi:10.1242/jcs.104943/-/DC1
References
- Brasaemle D. L., Dolios G., Shapiro L., Wang R. (2004). Proteomic analysis of proteins associated with lipid droplets of basal and lipolytically stimulated 3T3-L1 adipocytes. J. Biol. Chem. 279, 46835–46842 10.1074/jbc.M409340200 [DOI] [PubMed] [Google Scholar]
- Bussell R., Jr and Eliezer D. (2003). A structural and functional role for 11-mer repeats in alpha-synuclein and other exchangeable lipid binding proteins. J. Mol. Biol. 329, 763–778 10.1016/S0022-2836(03)00520-5 [DOI] [PubMed] [Google Scholar]
- Chang B. H., Li L., Paul A., Taniguchi S., Nannegari V., Heird W. C., Chan L. (2006). Protection against fatty liver but normal adipogenesis in mice lacking adipose differentiation-related protein. Mol. Cell. Biol. 26, 1063–1076 10.1128/MCB.26.3.1063-1076.2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Christian A. E., Haynes M. P., Phillips M. C., Rothblat G. H. (1997). Use of cyclodextrins for manipulating cellular cholesterol content. J. Lipid Res. 38, 2264–2272 [PubMed] [Google Scholar]
- Dalen K. T., Ulven S. M., Arntsen B. M., Solaas K., Nebb H. I. (2006). PPARalpha activators and fasting induce the expression of adipose differentiation-related protein in liver. J. Lipid Res. 47, 931–943 10.1194/jlr.M500459-JLR200 [DOI] [PubMed] [Google Scholar]
- Dalen K. T., Dahl T., Holter E., Arntsen B., Londos C., Sztalryd C., Nebb H. I. (2007). LSDP5 is a PAT protein specifically expressed in fatty acid oxidizing tissues. Biochim. Biophys. Acta 1771, 210–227 10.1016/j.bbalip.2006.11.011 [DOI] [PubMed] [Google Scholar]
- Farese R. V., Jr and Walther T. C. (2009). Lipid droplets finally get a little R-E-S-P-E-C-T. Cell 139, 855–860 10.1016/j.cell.2009.11.005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gandotra S., Le Dour C., Bottomley W., Cervera P., Giral P., Reznik Y., Charpentier G., Auclair M., Delépine M., Barroso I.et al. (2011). Perilipin deficiency and autosomal dominant partial lipodystrophy. N. Engl. J. Med. 364, 740–748 10.1056/NEJMoa1007487 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garcia A., Sekowski A., Subramanian V., Brasaemle D. L. (2003). The central domain is required to target and anchor perilipin A to lipid droplets. J. Biol. Chem. 278, 625–635 10.1074/jbc.M206602200 [DOI] [PubMed] [Google Scholar]
- Granneman J. G., Moore H. P., Krishnamoorthy R., Rathod M. (2009). Perilipin controls lipolysis by regulating the interactions of AB-hydrolase containing 5 (Abhd5) and adipose triglyceride lipase (Atgl). J. Biol. Chem. 284, 34538–34544 10.1074/jbc.M109.068478 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Granneman J. G., Moore H. P., Mottillo E. P., Zhu Z., Zhou L. (2011). Interactions of perilipin-5 (Plin5) with adipose triglyceride lipase. J. Biol. Chem. 286, 5126–5135 10.1074/jbc.M110.180711 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Greenberg A. S., Egan J. J., Wek S. A., Moos M. C., Jr, Londos C., Kimmel A. R. (1993). Isolation of cDNAs for perilipins A and B: sequence and expression of lipid droplet-associated proteins of adipocytes. Proc. Natl. Acad. Sci. USA 90, 12035–12039 10.1073/pnas.90.24.12035 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hickenbottom S. J., Kimmel A. R., Londos C., Hurley J. H. (2004). Structure of a lipid droplet protein; the PAT family member TIP47. Structure 12, 1199–1207 10.1016/j.str.2004.04.021 [DOI] [PubMed] [Google Scholar]
- Hsieh K., Huang A. H. (2005). Lipid-rich tapetosomes in Brassica tapetum are composed of oleosin-coated oil droplets and vesicles, both assembled in and then detached from the endoplasmic reticulum. Plant J. 43, 889–899 10.1111/j.1365-313X.2005.02502.x [DOI] [PubMed] [Google Scholar]
- Hsieh K., Huang A. H. (2007). Tapetosomes in Brassica tapetum accumulate endoplasmic reticulum-derived flavonoids and alkanes for delivery to the pollen surface. Plant Cell 19, 582–596 10.1105/tpc.106.049049 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim H. U., Hsieh K., Ratnayake C., Huang A. H. (2002). A novel group of oleosins is present inside the pollen of Arabidopsis. J. Biol. Chem. 277, 22677–22684 10.1074/jbc.M109298200 [DOI] [PubMed] [Google Scholar]
- Kimmel A. R., Brasaemle D. L., McAndrews–Hill M., Sztalryd C., Londos C. (2010). Adoption of PERILIPIN as a unifying nomenclature for the mammalian PAT-family of intracellular lipid storage droplet proteins. J. Lipid Res. 51, 468–471 10.1194/jlr.R000034 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Londos C., Sztalryd C., Tansey J. T., Kimmel A. R. (2005). Role of PAT proteins in lipid metabolism. Biochimie 87, 45–49 10.1016/j.biochi.2004.12.010 [DOI] [PubMed] [Google Scholar]
- Lu X., Gruia–Gray J., Copeland N. G., Gilbert D. J., Jenkins N. A., Londos C., Kimmel A. R. (2001). The murine perilipin gene: the lipid droplet-associated perilipins derive from tissue-specific, mRNA splice variants and define a gene family of ancient origin. Mamm. Genome 12, 741–749 10.1007/s00335-01-2055-5 [DOI] [PubMed] [Google Scholar]
- Martinez–Botas J., Anderson J. B., Tessier D., Lapillonne A., Chang B. H., Quast M. J., Gorenstein D., Chen K. H., Chan L. (2000). Absence of perilipin results in leanness and reverses obesity in Lepr(db/db) mice. Nat. Genet. 26, 474–479 10.1038/82630 [DOI] [PubMed] [Google Scholar]
- McManaman J. L., Zabaronick W., Schaack J., Orlicky D. J. (2003). Lipid droplet targeting domains of adipophilin. J. Lipid Res. 44, 668–673 10.1194/jlr.C200021-JLR200 [DOI] [PubMed] [Google Scholar]
- Miura S., Gan J. W., Brzostowski J., Parisi M. J., Schultz C. J., Londos C., Oliver B., Kimmel A. R. (2002). Functional conservation for lipid storage droplet association among Perilipin, ADRP, and TIP47 (PAT)-related proteins in mammals, Drosophila, and Dictyostelium. J. Biol. Chem. 277, 32253–32257 10.1074/jbc.M204410200 [DOI] [PubMed] [Google Scholar]
- Miyoshi H., Perfield J. W., 2nd, Souza S. C., Shen W. J., Zhang H. H., Stancheva Z. S., Kraemer F. B., Obin M. S., Greenberg A. S. (2007). Control of adipose triglyceride lipase action by serine 517 of perilipin A globally regulates protein kinase A-stimulated lipolysis in adipocytes. J. Biol. Chem. 282, 996–1002 10.1074/jbc.M605770200 [DOI] [PubMed] [Google Scholar]
- Nakamura N., Fujimoto T. (2003). Adipose differentiation-related protein has two independent domains for targeting to lipid droplets. Biochem. Biophys. Res. Commun. 306, 333–338 10.1016/S0006-291X(03)00979-3 [DOI] [PubMed] [Google Scholar]
- Ohsaki Y., Maeda T., Maeda M., Tauchi–Sato K., Fujimoto T. (2006). Recruitment of TIP47 to lipid droplets is controlled by the putative hydrophobic cleft. Biochem. Biophys. Res. Commun. 347, 279–287 10.1016/j.bbrc.2006.06.074 [DOI] [PubMed] [Google Scholar]
- Qi L., Shen H., Larson I., Schaefer E. J., Greenberg A. S., Tregouet D. A., Corella D., Ordovas J. M. (2004). Gender-specific association of a perilipin gene haplotype with obesity risk in a white population. Obes. Res. 12, 1758–1765 10.1038/oby.2004.218 [DOI] [PubMed] [Google Scholar]
- Richardson K., Louie–Gao Q., Arnett D. K., Parnell L. D., Lai C. Q., Davalos A., Fox C. S., Demissie S., Cupples L. A., Fernandez–Hernando C.et al. (2011). The PLIN4 variant rs8887 modulates obesity related phenotypes in humans through creation of a novel miR-522 seed site. PLoS ONE 6, e17944 10.1371/journal.pone.0017944 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Servetnick D. A., Brasaemle D. L., Gruia–Gray J., Kimmel A. R., Wolff J., Londos C. (1995). Perilipins are associated with cholesteryl ester droplets in steroidogenic adrenal cortical and Leydig cells. J. Biol. Chem. 270, 16970–16973 10.1074/jbc.270.28.16970 [DOI] [PubMed] [Google Scholar]
- Subramanian V., Garcia A., Sekowski A., Brasaemle D. L. (2004a). Hydrophobic sequences target and anchor perilipin A to lipid droplets. J. Lipid Res. 45, 1983–1991 10.1194/jlr.M400291-JLR200 [DOI] [PubMed] [Google Scholar]
- Subramanian V., Rothenberg A., Gomez C., Cohen A. W., Garcia A., Bhattacharyya S., Shapiro L., Dolios G., Wang R., Lisanti M. P.et al. (2004b). Perilipin A mediates the reversible binding of CGI-58 to lipid droplets in 3T3-L1 adipocytes. J. Biol. Chem. 279, 42062–42071 10.1074/jbc.M407462200 [DOI] [PubMed] [Google Scholar]
- Sztalryd C., Xu G., Dorward H., Tansey J. T., Contreras J. A., Kimmel A. R., Londos C. (2003). Perilipin A is essential for the translocation of hormone-sensitive lipase during lipolytic activation. J. Cell Biol. 161, 1093–1103 10.1083/jcb.200210169 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sztalryd C., Bell M., Lu X., Mertz P., Hickenbottom S., Chang B. H., Chan L., Kimmel A. R., Londos C. (2006). Functional compensation for adipose differentiation-related protein (ADFP) by Tip47 in an ADFP null embryonic cell line. J. Biol. Chem. 281, 34341–34348 10.1074/jbc.M602497200 [DOI] [PubMed] [Google Scholar]
- Tansey J. T., Sztalryd C., Gruia–Gray J., Roush D. L., Zee J. V., Gavrilova O., Reitman M. L., Deng C. X., Li C., Kimmel A. R.et al. (2001). Perilipin ablation results in a lean mouse with aberrant adipocyte lipolysis, enhanced leptin production, and resistance to diet-induced obesity. Proc. Natl. Acad. Sci. USA 98, 6494–6499 10.1073/pnas.101042998 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tansey J. T., Huml A. M., Vogt R., Davis K. E., Jones J. M., Fraser K. A., Brasaemle D. L., Kimmel A. R., Londos C. (2003). Functional studies on native and mutated forms of perilipins. A role in protein kinase A-mediated lipolysis of triacylglycerols. J. Biol. Chem. 278, 8401–8406 10.1074/jbc.M211005200 [DOI] [PubMed] [Google Scholar]
- Wang H., Hu L., Dalen K., Dorward H., Marcinkiewicz A., Russell D., Gong D., Londos C., Yamaguchi T., Holm C.et al. (2009). Activation of hormone-sensitive lipase requires two steps, protein phosphorylation and binding to the PAT-1 domain of lipid droplet coat proteins. J. Biol. Chem. 284, 32116–32125 10.1074/jbc.M109.006726 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang H., Bell M., Sreenevasan U., Hu H., Liu J., Dalen K., Londos C., Yamaguchi T., Rizzo M. A., Coleman R.et al. (2011). Unique regulation of adipose triglyceride lipase (ATGL) by perilipin 5, a lipid droplet-associated protein. J. Biol. Chem. 286, 15707–15715 10.1074/jbc.M110.207779 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wolins N. E., Quaynor B. K., Skinner J. R., Schoenfish M. J., Tzekov A., Bickel P. E. (2005). S3-12, Adipophilin, and TIP47 package lipid in adipocytes. J. Biol. Chem. 280, 19146–19155 10.1074/jbc.M500978200 [DOI] [PubMed] [Google Scholar]
- Wolins N. E., Quaynor B. K., Skinner J. R., Tzekov A., Croce M. A., Gropler M. C., Varma V., Yao–Borengasser A., Rasouli N., Kern P. A.et al. (2006). OXPAT/PAT-1 is a PPAR-induced lipid droplet protein that promotes fatty acid utilization. Diabetes 55, 3418–3428 10.2337/db06-0399 [DOI] [PubMed] [Google Scholar]
- Xu G., Sztalryd C., Lu X., Tansey J. T., Gan J., Dorward H., Kimmel A. R., Londos C. (2005). Post-translational regulation of adipose differentiation-related protein by the ubiquitin/proteasome pathway. J. Biol. Chem. 280, 42841–42847 10.1074/jbc.M506569200 [DOI] [PubMed] [Google Scholar]
- Yamaguchi T., Omatsu N., Matsushita S., Osumi T. (2004). CGI-58 interacts with perilipin and is localized to lipid droplets. Possible involvement of CGI-58 mislocalization in Chanarin-Dorfman syndrome. J. Biol. Chem. 279, 30490–30497 10.1074/jbc.M403920200 [DOI] [PubMed] [Google Scholar]
- Yamaguchi T., Matsushita S., Motojima K., Hirose F., Osumi T. (2006). MLDP, a novel PAT family protein localized to lipid droplets and enriched in the heart, is regulated by peroxisome proliferator-activated receptor alpha. J. Biol. Chem. 281, 14232–14240 10.1074/jbc.M601682200 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.