Abstract
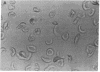
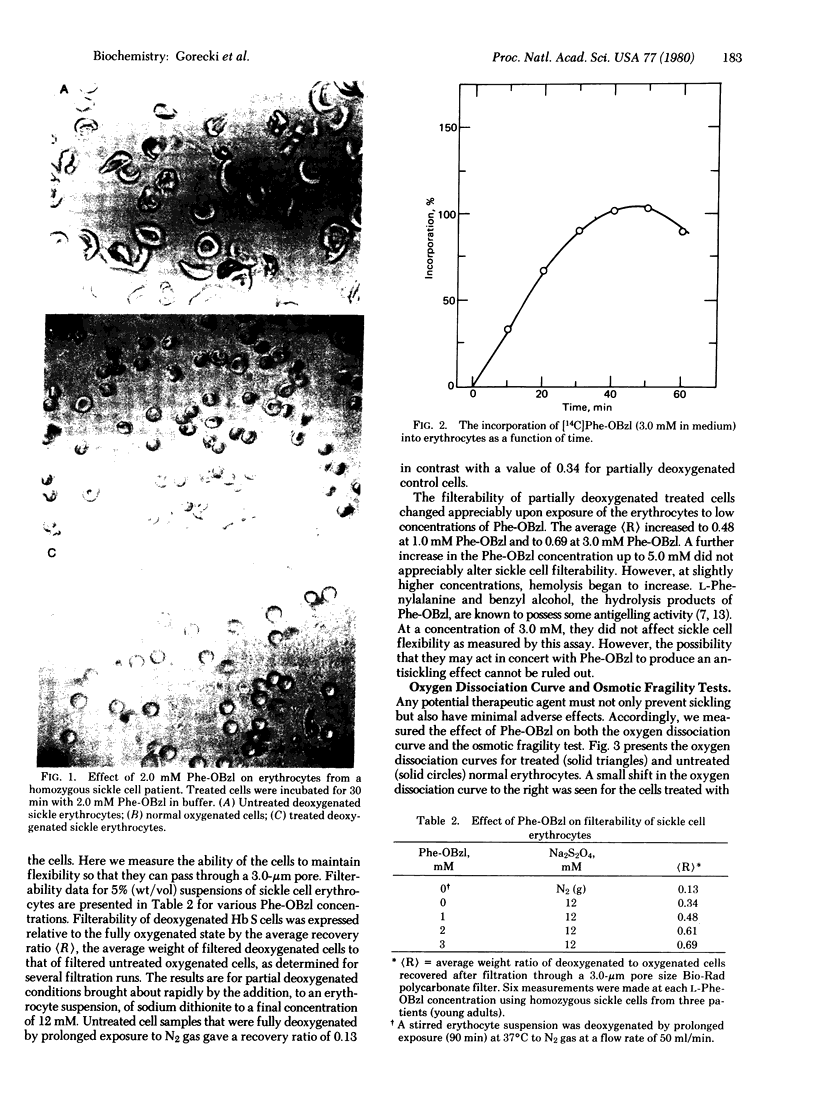
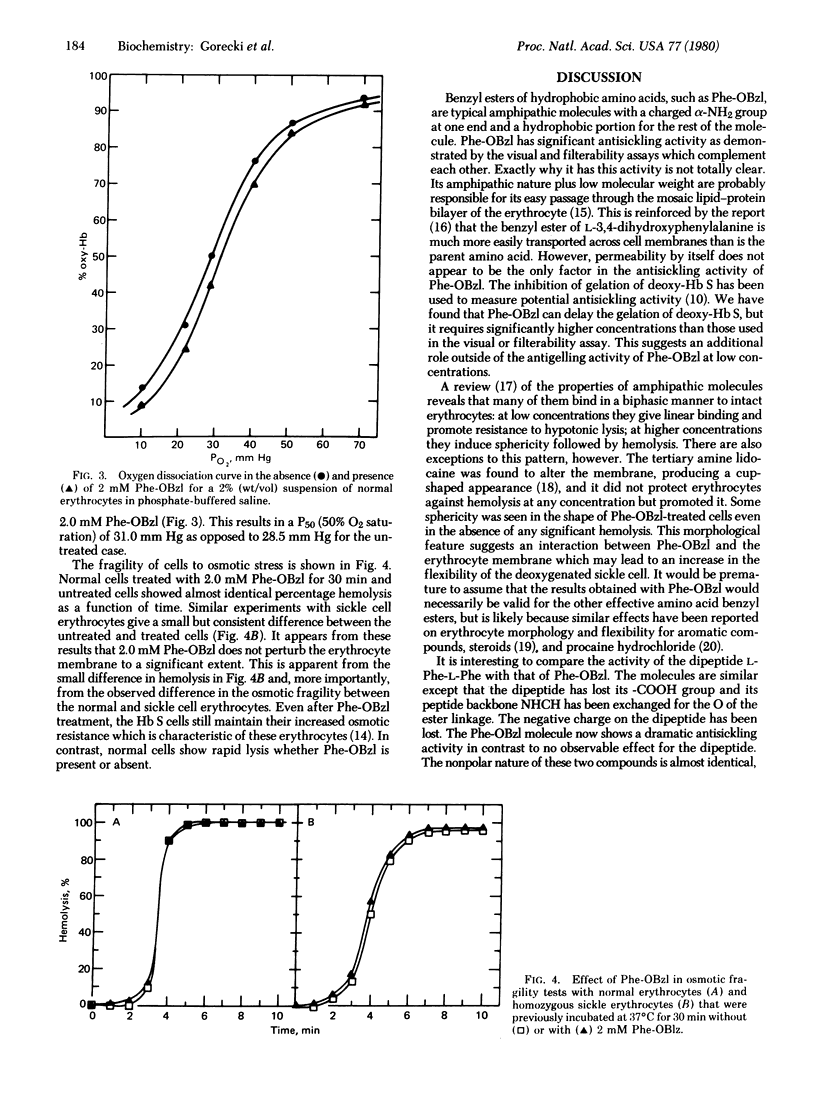
The sickling of homozygous sickel cells upon deoxygenation is inhibited in the presence of 3 mM L-phenylalanine benzyl ester (Phe-OBzl) or benzyl esters of other aromatic or hydrophobic amino acids. Phe-OBzl was found to permeate into erythrocytes rapidly, and the deoxygenated cells maintained considerable flexibility as measured by their ability to pass through 3-micron pores. The osmotic fragility of the cells was unchanged and the oxygen dissociation curve was shifted slightly from a 50% saturation values of 28.5 mm Hg to 31.0 mm Hg. At lower concentrations of Phe-OBzl some antisickling activity was seen. The Phe-OBzl antisickling activity may involve both binding to deoxyhemoglobin S and modification of the erythrocyte membrane. This class of compounds has considerable potential as therapeutic agents for the treatment of sickle cell disease.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Baker R., Powars D., Haywood L. J. Restoration of the deformability of "irreversibly" sickled cells by procaine hydrochloride. Biochem Biophys Res Commun. 1974 Jul 24;59(2):548–556. doi: 10.1016/s0006-291x(74)80015-x. [DOI] [PubMed] [Google Scholar]
- Bodor N., Sloan K. B., Higuchi T., Sasahara K. Improved delivery through biological membranes. 4. Prodrugs of L-dopa. J Med Chem. 1977 Nov;20(11):1435–1445. doi: 10.1021/jm00221a014. [DOI] [PubMed] [Google Scholar]
- Brewer G. J., Oelshlegel F. J., Jr Antisickling effects of zinc. Biochem Biophys Res Commun. 1974 Jun 4;58(3):854–861. doi: 10.1016/s0006-291x(74)80495-x. [DOI] [PubMed] [Google Scholar]
- Cerami A., Manning J. M. Potassium cyanate as an inhibitor of the sickling of erythrocytes in vitro. Proc Natl Acad Sci U S A. 1971 Jun;68(6):1180–1183. doi: 10.1073/pnas.68.6.1180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DANON D. A RAPID MICRO METHOD FOR RECORDING RED CELL OSMOTIC FRAGILITY BY CONTINUOUS DECREASE OF SALT CONCENTRATION. J Clin Pathol. 1963 Jul;16:377–382. doi: 10.1136/jcp.16.4.377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Isaacs W. A., Hayhoe F. G. Steroid hormones in sickle-cell disease. Nature. 1967 Sep 9;215(5106):1139–1142. doi: 10.1038/2151139a0. [DOI] [PubMed] [Google Scholar]
- Kubota S., Yang J. T. Oligopeptides as potential antiaggregation agents for deoxyhemoglobin S. Proc Natl Acad Sci U S A. 1977 Dec;74(12):5431–5434. doi: 10.1073/pnas.74.12.5431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lubin B. H., Pena V., Mentzer W. C., Bymun E., Bradley T. B., Packer L. Dimethyl adipimidate: a new antisickling agent. Proc Natl Acad Sci U S A. 1975 Jan;72(1):43–46. doi: 10.1073/pnas.72.1.43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Naccache P., Sha'afi R. I. Patterns of nonelectrolyte permeability in human red blood cell membrane. J Gen Physiol. 1973 Dec;62(6):714–736. doi: 10.1085/jgp.62.6.714. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nigen A. M., Manning J. M. Inhibition of erythrocyte sickling in vitro by DL-glyceraldehyde. Proc Natl Acad Sci U S A. 1977 Jan;74(1):367–371. doi: 10.1073/pnas.74.1.367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Noguchi C. T., Schechter A. N. Effects of amino acids on gelation kinetics and solubility of sickle hemoglobin. Biochem Biophys Res Commun. 1977 Jan 24;74(2):637–642. doi: 10.1016/0006-291x(77)90350-3. [DOI] [PubMed] [Google Scholar]
- Noguchi C. T., Schechter A. N. Inhibition of sickle hemoglobin gelation by amino acids and related compounds. Biochemistry. 1978 Dec 12;17(25):5455–5459. doi: 10.1021/bi00618a020. [DOI] [PubMed] [Google Scholar]
- Nozaki Y., Tanford C. The solubility of amino acids and two glycine peptides in aqueous ethanol and dioxane solutions. Establishment of a hydrophobicity scale. J Biol Chem. 1971 Apr 10;246(7):2211–2217. [PubMed] [Google Scholar]
- Palek J., Liu A., Liu D., Snyder L. M., Fortier N. L., Njoku G., Kiernan F., Funk D., Crusberg T. Effect of procaine HCLl on ATP: calcium-dependent alterations in red cell shape and deformability. Blood. 1977 Jul;50(1):155–164. [PubMed] [Google Scholar]
- Ross P. D., Subramanian S. Inhibition of sickle cell hemoglobin gelation by some aromatic compounds. Biochem Biophys Res Commun. 1977 Aug 22;77(4):1217–1223. doi: 10.1016/s0006-291x(77)80109-5. [DOI] [PubMed] [Google Scholar]
- SHEN S. C., FLEMING E. M., CASTLE W. B. Studies on the destruction of red blood cells; irreversibly sickled erythrocytes; their experimental production in vitro. Blood. 1949 May;4(5):498–504. [PubMed] [Google Scholar]
- Seeman P. The membrane actions of anesthetics and tranquilizers. Pharmacol Rev. 1972 Dec;24(4):583–655. [PubMed] [Google Scholar]
- Sheetz M. P., Singer S. J. Biological membranes as bilayer couples. A molecular mechanism of drug-erythrocyte interactions. Proc Natl Acad Sci U S A. 1974 Nov;71(11):4457–4461. doi: 10.1073/pnas.71.11.4457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Votano J. R., Gorecki M., Rich A. Sickle hemoglobin aggregation: a new class of inhibitors. Science. 1977 Jun 10;196(4295):1216–1219. doi: 10.1126/science.870976. [DOI] [PubMed] [Google Scholar]