Summary
Scribble was originally identified as a Drosophila protein that regulates epithelial polarity and formation of the basolateral surface. The mammalian orthologue, Scrib, is evolutionarily conserved, but does not appear to be necessary for apical-basolateral epithelial polarity. Instead, it is implicated in the regulation of cell survival, protein trafficking, adhesion and migration. A key issue is to understand the molecular pathway by which Scrib participates in these processes. We have investigated Scrib using a three-dimensional epithelial cell culture system. We show a novel association between the leucine-rich repeat domain of Scrib and the co-chaperone Sgt1 and demonstrate that these proteins are necessary for epithelial morphogenesis and tubulogenesis following hepatocyte growth factor (HGF) stimulation. The molecular chaperone HSP90 is also required for Sgt1 association with Scrib, and both Sgt1 and HSP90 are needed to ensure proper Scrib protein levels. Furthermore, reduced Scrib stability, following inhibition of Sgt1–HSP90, lowers the cellular abundance of the Scrib–βPix–PAK complex. Inhibition of any member of this complex, Scrib, βPix or PAK, is sufficient to block HGF-mediated epithelial morphogenesis. The identification of Scrib as an Sgt1–HSP90 client protein required for three-dimensional cell migration suggests that chaperone-mediated regulation of polarity protein stability and homeostasis is an unappreciated mechanism underlying dynamic rearrangements during morphogenesis.
Key words: Chaperone, Migration, Morphogenesis, Polarity
Introduction
The proper formation and maintenance of epithelial tissues is of central importance to human development and disease. Epithelial cells are characterized by a polarized cellular architecture that is critical to their function. This polarity is defined by distinct apical and basolateral membrane domains separated by intercellular tight junctions. A great deal of research has focused on identifying the molecular components and pathways essential to the establishment and proper maintenance of epithelial polarity. One such component, the Drosophila protein Scribble (Scrib in mammals), was originally identified as having a tumor-suppressor-like function in preventing overgrowth of imaginal tissues (Bilder et al., 2000; Bilder and Perrimon, 2000). Drosophila Scribble is localized to the basolateral membrane in epithelial cells and is thought to function as a molecular scaffold to help assign basolateral membrane identity; mutation of Scribble causes a loss of epithelial polarity. Puzzlingly, in mammals, loss of Scrib does not cause obvious defects in epithelial polarity establishment, though Scrib has been shown to have other critical roles in epithelia (reviewed by Bilder, 2004; Humbert et al., 2008; Nelson, 2009). A central issue is to understand both the molecular pathways regulating Scrib as well as the downstream mechanisms of Scrib function.
Scrib is a member of the LAP (leucine-rich repeat and PDZ) family of proteins. The leucine-rich repeat (LRR) domain of Scrib is critical to Scrib function and membrane targeting (Albertson et al., 2004; Zeitler et al., 2004). Despite the fundamental importance of the LRR domain to Scrib function, the molecular mechanisms of this domain remain relatively unknown (Kallay et al., 2006; Legouis et al., 2003). In contrast, the PDZ domain region of Scrib is required for the physical association of at least nine other proteins including the Rho-family guanine nucleotide exchange factor (GEF) βPix/ARHGEF7 (Audebert et al., 2004; Humbert et al., 2008; Nelson, 2009). A Scrib–βPix complex was shown to be essential for Scrib-dependent recruitment of Rac1 and Cdc42 to the leading edge of migrating cells, oriented directional migration, vesicle trafficking in neurons, receptor recycling in thyroid cells and inhibition of mammary tumorigenesis (Audebert et al., 2004; Dow et al., 2007; Lahuna et al., 2005; Nola et al., 2008; Osmani et al., 2006; Zhan et al., 2008). Additionally, βPix can interact with p21-activated kinase (PAK) and facilitate the formation of a tripartite complex with Scrib that is necessary for migration of two-dimensional (2D)-cultured cells (Nola et al., 2008). A study in Drosophila also demonstrated a reciprocal requirement between Scrib and PAK for their proper localization (Bahri et al., 2010). It remains unclear how widespread a requirement PAK signaling is for proper Scrib function, but it has been reported that reduction of Scrib can affect a number of signaling pathways other than PAK, depending on the cellular context (reviewed in Humbert et al., 2008).
A number of studies have highlighted critically important mechanisms regulating cellular Scrib protein levels including: stabilization by the intermediate filament cytoskeleton, ubiquitin-mediated proteolysis, cleavage by caspases and transcriptional repression through microRNAs (Nakagawa and Huibregtse, 2000; Phua et al., 2009; Sone et al., 2008; Vaira et al., 2012). One important additional mechanism by which cells can regulate the stability and maturation of certain proteins is through the use of molecular chaperones. A chaperone-mediated role in Scrib stabilization and function is currently unknown.
Sgt1 (suppressor of G2 allele of Skp1) is a chaperone with conserved functions in the regulation of kinetochore complex assembly and innate immunity (Davies and Kaplan, 2010; Kadota et al., 2010; Kitagawa et al., 1999; Steensgaard et al., 2004). Sgt1 (also called Sugt1) interacts with the chaperone protein HSP90 and this has led to a model of Sgt1 acting as a co-chaperone by linking HSP90 to a subset of client proteins (Catlett and Kaplan, 2006). Sgt1 seems to be preferentially associated with a number of proteins containing LRR domains and may regulate their stability and/or the conformational activation of LRR protein complexes (Austin et al., 2002; Azevedo et al., 2002; da Silva Correia et al., 2007; Dubacq et al., 2002; Mayor et al., 2007; Stuttmann et al., 2008). Intriguingly, mutation of Drosophila Sgt1 was recently demonstrated to result in reduced cortical levels of Scribble in neuroblasts (Andersen et al., 2012). The molecular mechanism for this function of Sgt1 is unknown.
In this study, we have identified a novel association between the poorly understood LRR domain of Scrib and Sgt1. We propose that this interaction is required to recruit HSP90 to Scrib and that together Sgt1and HSP90 serve a crucial function in regulating Scrib protein stability. Reduction of Scrib, Sgt1 or HSP90 causes defects in a three-dimensional (3D) model of HGF-mediated epithelial morphogenesis. Furthermore, we provide evidence that βPix and PAK are downstream effectors of Scrib in this process.
Results
Sgt1 is associated with the leucine-rich repeat region of Scrib
Using full-length human Scrib, we performed a BLAST protein homology search to identify Scrib homologs in S. cerevisiae. This approach revealed that the LRR domain of Scrib, but not other regions of the protein, has significant homology to several proteins. Specifically, the LRR domain of Scrib shares the greatest sequence similarity with the LRR region of yeast adenylyl cyclase, Cyr1p/Cdc35p. We reasoned that the LRR domains of these two proteins might have similar functions mediated through conserved protein–protein interactions. In an attempt to understand these functions and identify new mammalian proteins that bind to the LRR domain of Scrib, we employed a candidate approach based on proteins known to interact specifically with the LRR domain of Cyr1p. One such protein is S. cerevisiae Sgt1, a co-chaperone-like protein that, in addition to regulating Cyr1p function, has a role in kinetochore assembly. Mammalian Sgt1 has been shown to participate in innate immunity and kinetochore assembly (Kadota et al., 2010; Steensgaard et al., 2004). Very little is known about mammalian Sgt1 function outside of these processes.
Using two separate Scrib antibodies, we immunoprecipitated endogenous Scrib from equal amounts of MDCK cell lysate. The immunoprecipitates (IPs) were then subjected to western blot analysis to detect Sgt1. Notably, endogenous Sgt1 was detected in both Scrib IP samples, but not in the IPs from two distinct control antibodies (Fig. 1A). A protein closely related to Sgt1, CacyBP/SIP, was not detected in Scrib IPs further supporting the specificity of the interaction between Scrib and Sgt1 (not shown). Additionally, we expressed a GFP tagged, human Sgt1 fusion protein in MDCK cells and subjected cell lysates to immunoprecipitation with an anti-GFP antibody. Scrib was observed specifically in the immunoprecipitates from GFP–Sgt1-expressing cells, but not in immunoprecipitates from mock-transfected cells (Fig. 1B). We conclude that Scrib and Sgt1 form a complex in MDCK cells.
Fig. 1.
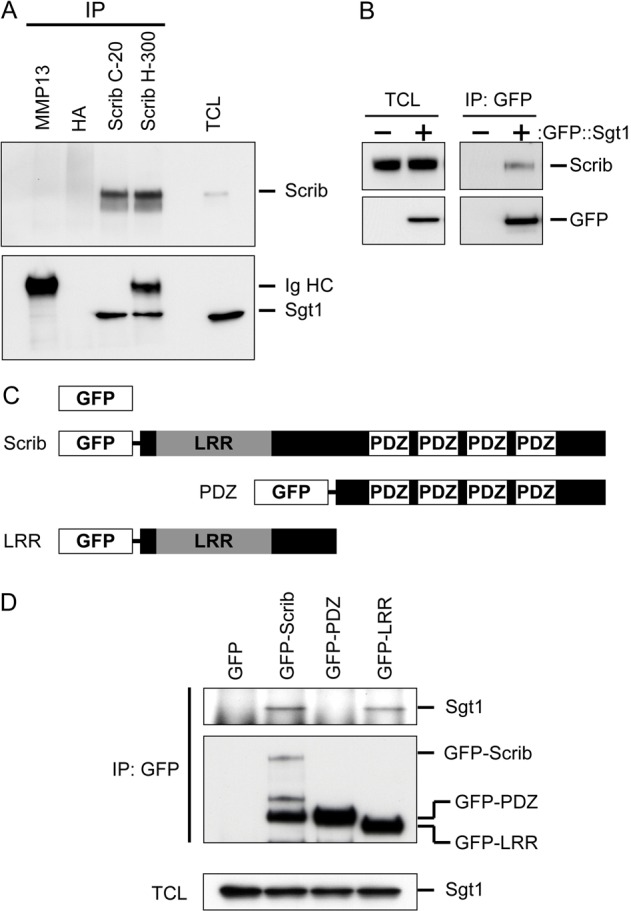
Sgt1 forms a complex with the LRR domain of Scrib. (A) Equal amounts of the same MDCK total cell lysate (TCL) were subjected to immunoprecipitation with two control antibodies (MMP13 and HA) and two anti-Scrib antibodies. IPs were subjected to SDS-PAGE and western blot analysis and examined for the presence of Scrib and Sgt1. Rabbit immunoglobulin heavy chain is also visible when using an anti-rabbit-HRP secondary antibody. The amount of Sgt1 protein immunoprecipitated by the Scrib C-20 antibody was determined to be 0.86% (±0.03%) of total Sgt1 protein levels. (B) GFP immunoprecipitation of mock and GFP-Sgt1-transfected MDCK cells. IPs were blotted to verify equal amounts of Scrib were present in the starting TCL. Scrib was only detectable in the IPs from GFP–Sgt1-expressing cells. (C) Illustration of the GFP fusion proteins that were expressed in MDCK cells to map the domain requirements for Sgt1 association. (D) Anti-GFP immunoprecipitation of the fusion proteins shown in B. IPs were blotted to detect the presence of precipitated GFP fusion proteins and Sgt1. Equal volumes of TCL were also blotted to show equal amounts of Sgt1 were available in the starting material. Using an antibody directed against GFP, we observed similar amounts of GFP–PDZ and GFP–LRR in IPs. Full-length GFP–Scrib appeared to be expressed at a lower level and a number of smaller GFP proteins were detected. These bands are probably degradation products from GFP–Scrib.
Based on our candidate approach strategy, we predicted that the Scrib–Sgt1 protein interaction should be mediated through the LRR domain of mammalian Scrib. To test this, we expressed GFP only, full-length GFP–Scrib, a fusion protein containing only the LRR region of Scrib and a fusion protein containing the PDZ region of Scrib but lacking the LRR domain (Fig. 1C). Using an anti-GFP antibody, we immunoprecipitated the fusion proteins from expressing MDCK cell lines. When these IPs were probed for the presence of Sgt1, only full-length Scrib and the LRR domain containing fusion protein were able to co-immunoprecipitate Sgt1 (Fig. 1D). These results validate our candidate approach and suggest that we have identified a novel protein interaction between the LRR region of Scrib and Sgt1.
A pool of Scrib and Sgt1 is localized to intermediate filaments
After determining that Scrib and Sgt1 co-immunoprecipitate, we investigated whether the two proteins shared a similar subcellular distribution. Immunofluorescent staining of Sgt1 in subconfluent MDCK cells grown on a 2D surface revealed a broad cytoplasmic distribution as well as staining at the midbody similar to what has been previously described (not shown) (Martins et al., 2009; Steensgaard et al., 2004). Despite this broad distribution, Sgt1 could clearly be seen enriched along a filamentous network (Fig. 2A). It has recently been reported that a pool of Scrib localizes to intermediate filaments (Phua et al., 2009). We examined whether Sgt1 shared this colocalization with the intermediate filament cytoskeleton by co-staining subconfluent MDCK cells with Sgt1 and vimentin antibodies. Sgt1 showed colocalization with vimentin filaments and this colocalization could be observed more clearly by pre-permeabilizing cells before fixation (Fig. 2A). Scrib showed a similar, albeit more punctate, enrichment on vimentin filaments in agreement with the earlier study (Fig. 2A) (Phua et al., 2009). When we expressed human Scrib fused to the fluorescent protein Venus, this filamentous staining was enhanced and the fusion protein was clearly colocalized with Sgt1 in subconfluent MDCK cells (Fig. 2A).
Fig. 2.
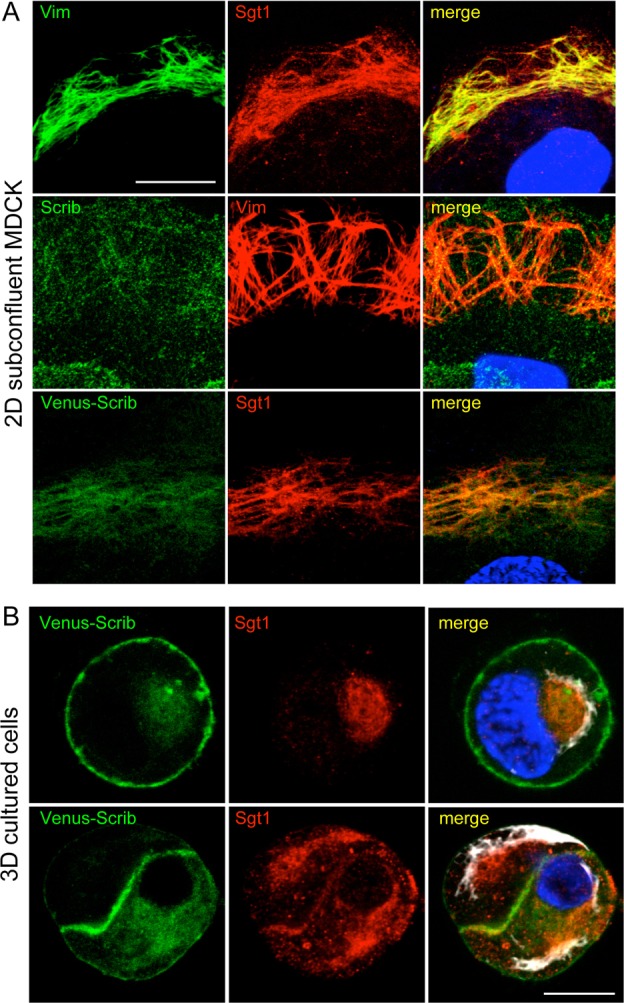
Scrib and Sgt1 colocalize with the intermediate filament cytoskeleton. Immunofluorescence images of fixed and antibody-stained MDCK cells. Nuclei were stained with Hoechst (blue in the merged images). Colocalization appears as yellow in the merged image. (A) Subconfluent 2D-cultured MDCK cells were fixed and stained with the indicated antibodies. Both Sgt1 and Scrib exhibited a filamentous staining pattern that overlapped extensively with vimentin. Expression of a full-length Venus–Scrib fusion protein enhances the filamentous localization of Scrib and colocalizes with Sgt1 staining. (B) Cross sections of one- and two-cell Matrigel-grown MDCK cells. Scrib is mainly localized to the plasma membrane and cell junctions, but there is also a smaller intracellular pool. Sgt1 shows colocalization with the intracellular pool of Scrib in 3D culture. The vimentin cytoskeleton, shown in white, is closely associated with intracellular Scrib and Sgt1. Nuclei are shown in blue in the merged images. Scale bars: 10 µm.
We, and others, have shown that when MDCK and other epithelial cells are grown as 3D cysts in a thick gel of extracellular matrix material, such as Matrigel, the cells more closely resemble epithelial cells in vivo (Bryant and Mostov, 2008). When we localized Scrib in 3D Matrigel cultures, Scrib was strongly localized along the plasma membrane and at cell–cell junctions with a smaller pool of intracellular Scrib also detectable (Fig. 2B). Sgt1 did not share the plasma membrane distribution of Scrib, but could be seen partially colocalized with the intracellular pool of Scrib (Fig. 2B). Although we lacked the fine resolution imaging in our 3D cultures to visualize individual vimentin intermediate filaments, we observed vimentin-rich clusters closely associated with the pool of colocalized Scrib and Sgt1 (Fig. 2B). Similar to what has been shown for Scrib localization with vimentin (Phua et al., 2009), we saw less colocalization between Scrib, Sgt1 and vimentin in well-polarized cells (not shown). This could indicate that the Scrib–Sgt1 interaction is preferentially occurring during epithelial rearrangements such as migration. RNAi reduction of Scrib or Sgt1 did not noticeably affect the ability of the other to localize with vimentin in MDCK cells (not shown). This data fits well with previous work demonstrating that the PDZ domain region of Scrib was necessary and sufficient for Scrib localization to intermediate filaments (Phua et al., 2009), and our observation that the Sgt1–Scrib association occurs independently of the PDZ domain. Together, our data show that Sgt1 is localized with a pool of Scrib, not at the plasma membrane, but closely associated with the vimentin intermediate filament cytoskeleton.
RNAi of Scrib or Sgt1 decreased cyst extension formation in response to HGF
To examine the functional role of Scrib and Sgt1 in MDCK cells, we generated lentiviral shRNAs targeting two unique regions of both canine Scrib and Sgt1 (Fig. 3A) (Qin et al., 2005). Scrib and Sgt1 lentiviral shRNA-infected cells, grown in Matrigel, formed 3D cysts and properly localized the apical membrane protein gp135/podocalyxin to the apical/luminal surface of cysts (Fig. 3B). The adherens junction associated protein β-catenin was also localized correctly to the basolateral surface of cysts, indicating that some degree of adherens-junction-mediated cell–cell adhesion remained intact in Scrib- and Sgt1-RNAi-treated cells. The apical actin network of Scrib- and Sgt1-RNAi-treated cells also appeared indistinguishable from control-treated cells (Fig. 3C). The normal apico-basolateral distribution of these markers, as well as the apically oriented Golgi marker GM130 (not shown), lead us to conclude that the ability of cells, having reduced levels of Scrib or Sgt1, to properly polarize into cysts and form adherens junctions remains largely intact. This data agrees with previous studies showing that RNAi knockdown of Scrib in mammalian cells failed to cause dramatic apico-basal polarity defects (Dow et al., 2007; Qin et al., 2005). Despite the relatively normal polarity of Scrib- and Sgt1-RNAi-treated cysts, we observed an accumulation of cells in cyst lumens (Fig. 3B; supplementary material Fig. S1). These luminal cells frequently had fragmented nuclei and increased cleaved caspase3 antibody staining suggesting they were undergoing apoptosis (supplementary material Fig. S1). These observations are similar to those recently published showing that loss of Scrib in MDCK cells results in increased apoptosis that can be enhanced by competition with neighboring wild-type cells (Norman et al., 2012).
Fig. 3.
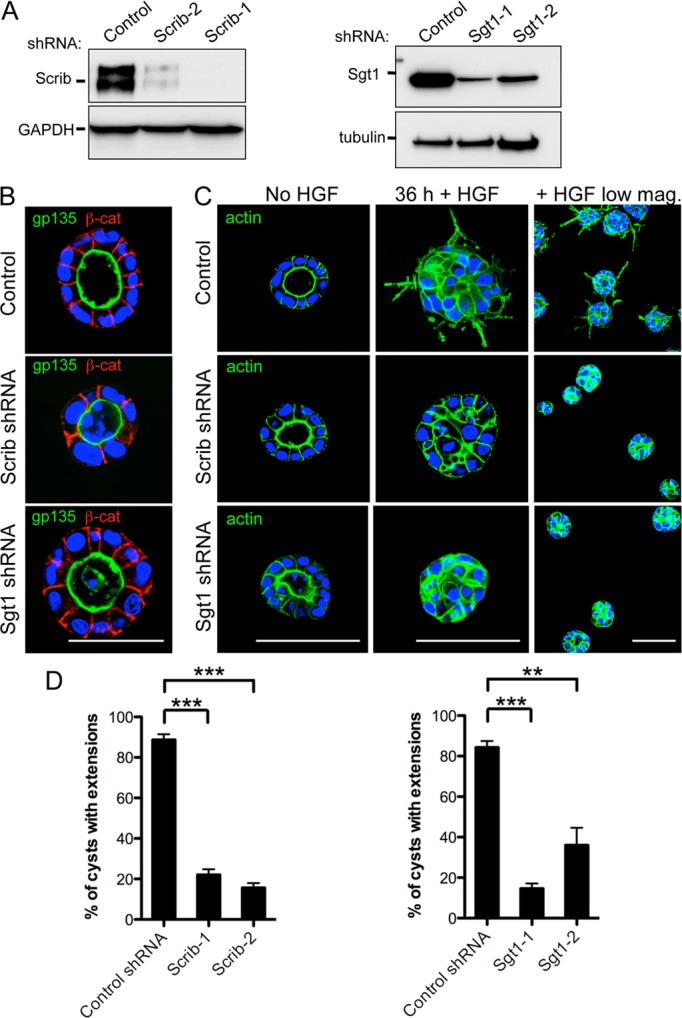
Reduction of Scrib or Sgt1 protein levels blocks HGF-mediated extension formation. (A) Western blot of MDCK total cell lysates treated with either a control shRNA or shRNAs directed against Scrib or Sgt1. GAPDH was included as a loading control for the lysates. (B) Fixed MDCK cysts were stained with the apical domain marker gp135 and the adherens junction marker β-catenin (β-cat) as indicated. Nuclei were stained with Hoechst (blue). Localization of gp135 and β-catenin is indistinguishable between control cysts and Scrib or Sgt1 RNAi cysts. (C) 4-day-old control-shRNA- or Scrib- and Sgt1-shRNA-infected cysts were induced with 50 ng/ml HGF for 36 hours followed by fixation. Actin was visualized by fluorescently labeled phalloidin staining (green) and nuclei were stained with Hoechst dye (blue). A lower magnification image of the cysts (right) provides a wider field image of the results. (D) Quantification of the HGF-induced extension formation assay results. The Y-axis represents the percentage of cysts with one or more extensions. **P<0.01, ***P<0.001, Dunnett's multiple comparison test. Scale bars: 50 µm (B) and 100 µm (C).
Previous studies have implicated Scrib in the regulation of adhesion and migration. Depending on the cellular context, Scrib can act as a positive or negative regulator of migratory behaviors (reviewed by Humbert et al., 2008). In 2D-cultured MDCK cells, RNAi of Scrib resulted in decreased cell–cell adhesion and increased non-directional migration (Qin et al., 2005). When 3D-cultured MDCK cysts are treated with HGF, cells initially undergo a partial epithelial to mesenchymal transition and send out actin-rich extensions from their basal surface (Montesano et al., 1991; O'Brien et al., 2002; Pollack et al., 1998). These extensions are the first step in HGF-induced MDCK tubulogenesis. We wanted to analyze the role Scrib and Sgt1 played in regulating this HGF-mediated MDCK cell morphogenesis. We first grew control, Sgt1 and Scrib RNAi cysts for 4 days in Matrigel. When control-shRNA-treated cysts are induced with HGF, a majority of cysts produce one or more actin-rich extensions within 36 hours (Fig. 3C,D). In contrast, Scrib- and Sgt1-RNAi-treated cysts show a statistically significant decrease in their ability to produce actin-rich extensions protruding from their basal surface following HGF induction (Fig. 3C,D). The extensions that did form on these cysts were often shorter in length than the extensions on control cysts (not shown). Collectively, this data demonstrates that Scrib and Sgt1 are necessary for HGF signaling and play a positive role in the morphogenetic rearrangements of polarized cells in 3D culture.
Scrib is a client of the Sgt1–HSP90 chaperone complex
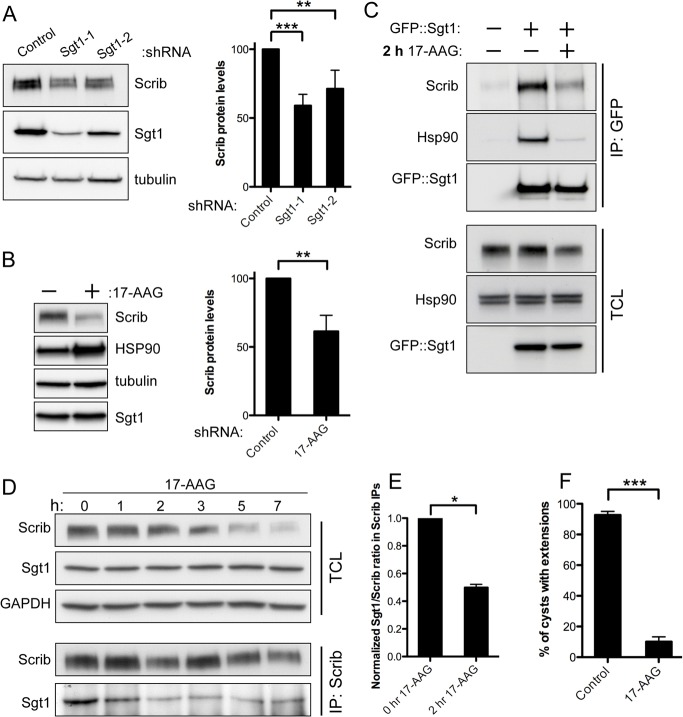
Sgt1 displays properties of a chaperone in both its kinetochore assembly role and innate immune receptor function. Moreover, Sgt1 functions as an HSP90 co-chaperone in these processes. No mammalian Sgt1-dependent HSP90 client protein has yet been identified outside of kinetochore assembly and innate immunity. To investigate whether Scrib could be an Sgt1–HSP90 client, we first examined total Scrib protein levels by western blot following Sgt1 RNAi. Normalized Scrib protein levels were significantly reduced by up to 41% following Sgt1 shRNA treatment relative to control-shRNA-treated cells (Fig. 4A). In addition to being statistically significant, this level of Scrib protein reduction is similar to what has been observed for other Sgt1 client proteins following Sgt1RNAi (Davies and Kaplan, 2010).
Fig. 4.
Scrib is an Sgt1–HSP90 client protein. (A) Total cell lysates for Sgt1-shRNA-treated cells were probed for the indicated proteins and compared to control cell lysates. Scrib protein levels were normalized using an anti-tubulin loading control and quantified for a minimum of three separate experiments. Dunnett's multiple comparison test was used. (B) Total cell lysates for 0.5 µM 17-AAG-treated cells were probed for the indicated proteins and compared to control cell lysates. Data was quantified as in A. (C) Immunoprecipitation of GFP from GFP-Sgt1- or mock-transfected cells after 2 hours of 0.5 µM 17-AAG treatment. Although the total levels of Scrib protein remain similar at this early timepoint, a dramatic reduction in the association between Sgt1 and both HSP90 and Scrib can be seen following western blotting of the IPs for these proteins. (D) Timecourse, in hours (h), of 0.5 µM 17-AAG-treated MDCK cells. Treated cell lysates were also used to IP Scrib and blot for the presence of Sgt1. Following the first hour of 17-AAG-mediated HSP90 inhibition, the amount of Sgt1 associated with Scrib was reduced. This reduction in Scrib–Sgt1 association occurred prior to the loss of Scrib protein stability, apparent at the 3 hour timepoint. (E) Quantification of the Sgt1/Scrib ratio in Scrib IPs after 2 hours of 17-AAG treatment. Ratios were normalized to untreated control sample IPs. (F) 4-day-old cysts were treated for 48 hours with HGF in the presence or absence of 50 nM 17-AAG. After 48 hours cells were fixed and stained for actin. Percentage of cysts forming extensions was quantified for three separate experiments. 17-AAG-treated cysts with cleaved caspase-3 staining were excluded from the extension formation analysis. *P<0.05, **P<0.01, ***P<0.001.
To determine if Scrib is also an HSP90 client, we used the well-characterized, high-specificity HSP90 inhibitor 17-(allylamino)-17demethoxygeldanamycin (17-AAG) to disrupt HSP90 function and examined the effect on Scrib levels (Trepel et al., 2010). Following 16 hours of 17-AAG treatment, we observed a significant reduction in Scrib protein levels relative to that of control cell levels (Fig. 4B). Consequently, Scrib stability appears to require both the chaperone HSP90 and co-chaperone Sgt1 in MDCK cells.
Previous work has shown that HSP90 activity is required for the formation of Sgt1–HSP90 client protein complexes and client stabilization; loss of complex formation precedes destabilization of client proteins (Mayor et al., 2007). To determine what effect HSP90 function has on the association of Scrib and Sgt1, we treated Sgt1-transfected MDCK cells with 17-AAG for 2 hours to block HSP90 activity. This short duration of 17-AAG treatment had little effect on the overall levels of Scrib protein. However, when we examined GFP–Sgt1 IPs from 17-AAG-treated cells, we observed a marked decrease in the amount of both Scrib and HSP90 that was able to associate with GFP–Sgt1 (Fig. 4C). This result indicates that, similar to what has been observed for other Sgt1–HSP90 client proteins, HSP90 activity is required for the formation of an Sgt1–HSP90–Scrib complex.
To more carefully assess whether loss of the Sgt1–Scrib interaction following HSP90 inhibition might be a critical step preceding the destabilization of Scrib protein levels, we performed a timecourse where we simultaneously examined Scrib protein levels and the Scrib–Sgt1 association following addition of 0.5 µM 17-AAG. We detected a reduction in the amount of Sgt1 in Scrib IPs at the first timepoint of 1 hour and saw this reduction continue until it reached a maximum by hours 2 and 3 (Fig. 4D). Quantification of the Sgt1/Scrib ratio in Scrib IPs after 2 hour 17-AAG treatment confirmed that HSP90 activity is critical for the Scrib–Sgt1 interaction (Fig. 4E). Scrib total protein levels remained fairly constant until the 3rd hour of treatment when they began to decrease (Fig. 4D). These results confirmed that loss of the Sgt1–Scrib association preceded Scrib protein destabilization; additionally, the kinetics of these two events looked strikingly similar to those seen for another Sgt1–HSP90 client protein, NALP3, following HSP90 activity inhibition (Mayor et al., 2007). This data, combined with the observation that RNAi reduction of Sgt1 leads to Scrib destabilization, suggest that the reduced Scrib stability observed following 17-AAG treatment is not likely an indirect or non-specific effect of inhibitor treatment on Scrib stability, but instead is a direct consequence of the failure to form the Sgt1–HSP90 chaperoned Scrib complex. We conclude that Scrib is an Sgt1–HSP90 client protein.
If HSP90 functions together with Sgt1 to stabilize Scrib protein levels, we would predict that inhibition of HSP90 following 17-AAG treatment should block HGF-mediated extension formation. We therefore simultaneously treated MDCK cysts with 17-AAG and HGF and assayed extension formation. Although many cysts had a disorganized structure and were positive for the apoptotic marker cleaved caspase-3 after this extended 48 hour treatment, we scored extension formation on apparently healthy cysts that were negative for cleaved caspase-3 staining. In these cysts, we saw a significant reduction in extension formation relative to untreated control cysts (Fig. 4F). The ability of 17-AAG to block HGF-mediated extension formations in our assay agrees with the literature demonstrating a requirement for HSP90 in HGF-mediated 2D cell migration (Xie et al., 2005). Although HSP90 is known to target a broad spectrum of clients that could be required for extension formation, the identification of Scrib as an Sgt1–HSP90 client provides a potential contributing mechanism for the effects of 17-AAG on HGF-mediated epithelial morphogenesis.
Scrib forms a complex with PAK and Sgt1–HSP90 regulates the availability of that complex
To understand how Scrib might be mechanistically linked to the regulation of cyst extension formation, we decided to investigate potential Scrib effectors in this process. The PDZ domain of Scrib has previously been shown to form a complex with the Rho-family GEF βPix/ARHGEF7 and this association enables Scrib to form a tripartite complex with p21-activated kinase (PAK) (Nola et al., 2008). Both βPix and PAK also have well-established roles in regulating aspects of cell migration (Frank and Hansen, 2008). We reasoned that Scrib, βPix and PAK could be forming a complex involved in the regulation of extension formation. We first asked whether Scrib and activated PAK were localized in extensions in a manner analogous to their colocalization at the leading edge of 2D migrating cells. Indeed, extensive immunofluorescent colocalization of Scrib and a phosphorylated form of PAK (pPAK), thought to indicate the activated state, was observed at actin-rich areas in extensions (Fig. 5A). This close association of Scrib and pPAK in extensions argues that a Scrib–βPix–PAK complex is likely to play a role in extensions.
Fig. 5.
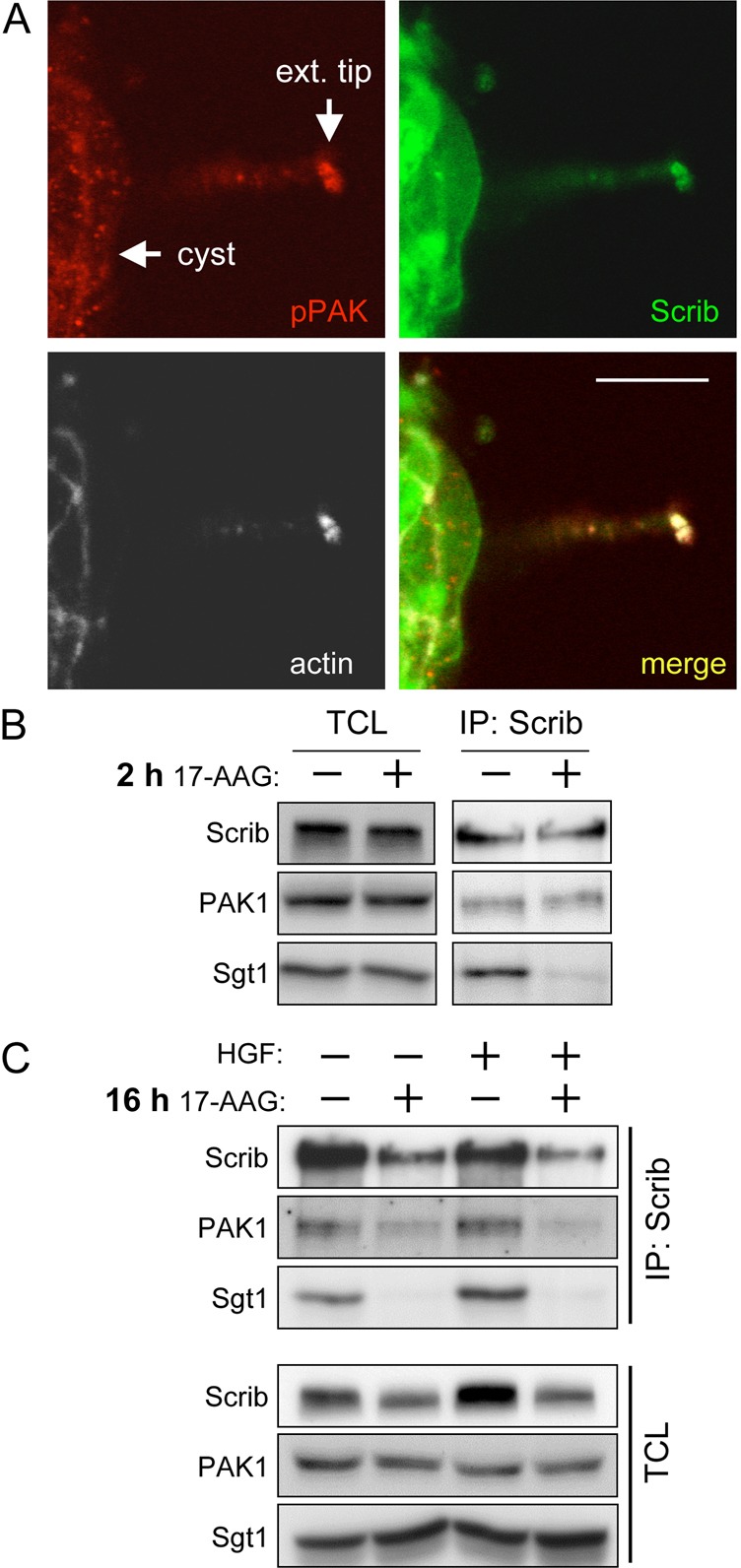
Pak is a potential effector of Scrib. Inhibition of Sgt–HSP90 reduces total Scrib–PAK complex abundance indirectly through decreased Scrib protein levels. (A) MDCK Venus–Scrib-expressing cysts were treated with HGF for 48 hours. Cysts were then fixed and stained with a pPAK antibody (red) and for actin (white). Arrows denote either the edge of the cyst from which the extension has grown or the tip/end of the extension (ext. tip) where Scrib, pPAK and actin show maximum colocalization. Scale bar: 10 µm. (B) Scrib IPs from cells treated for 2 hours with 0.5 µM 17-AAG and control cells were subjected to SDS-PAGE alongside total cell lysate input samples (TCL) and western blotted for the indicated proteins. Despite similar levels of total protein loading, 0.5 µM 17-AAG treatment reduced the association of Sgt1 with Scrib. Conversely, equal amounts of PAK were found in both 17-AAG-treated and untreated Scrib IPs, suggesting Sgt1–HSP90 is not directly required for the Scrib–PAK association. (C) Scrib IPs of cells treated for 16 hours with 0.5 µM 17-AAG and control cells that were either treated with (+) HGF or left untreated (–) were subjected to SDS-PAGE alongside total cell lysate input samples (TCL) and western blotted for the indicated proteins. 17-AAG treatment reduced Scrib protein levels and the amount of Scrib and PAK that was immunoprecipitated.
To more directly determine whether Scrib formed a biochemical complex with βPix or PAK in MDCK cells we performed IPs with Scrib. We were able to detect the presence of βPix and PAK in Scrib IPs from MDCK cell lysates but not in IPs of an HA control antibody (supplementary material Fig. S2A). Although we did not detect a reproducible increase in the amount of Sgt1, βPix and PAK in Scrib IPs following HGF treatment (Fig. 5C; supplementary material Fig. S2A), the number of cells responding to HGF by producing extensions and the fraction of total cellular Scrib in these extensions is small; consequently, it may be difficult to detect any increased association by IP. Together, these data demonstrate that a Scrib–βPix–PAK complex is a likely effector of HGF signaling during morphogenetic rearrangements and tubulogenesis.
We next investigated the role of Sgt1–HSP90 on the Scrib–βPix–PAK complex. Sgt1 may affect HGF-mediated extension formations by directly facilitating the formation of this tripartite complex. Alternatively, Sgt1 could indirectly regulate the complex by stabilizing Scrib protein levels. We reasoned that a 2 hour 17-AAG treatment that disrupts the Scrib–Sgt1 association, but does not decrease Scrib protein levels, might also lead to decreased association between Scrib and PAK. Interestingly, despite the loss of the Scrib–Sgt1 interaction after 2 hour 17-AAG treatment, we observed unchanged PAK levels in Scrib IPs relative to untreated cells (Fig. 5B). This result indicates that Sgt1–HSP90 does not directly facilitate the Scrib–PAK interaction. We then examined what would happen to Scrib–PAK complex formation following a 16 hour 17-AAG treatment where we observed reduced Scrib protein levels. As expected, 16 hour treatment with 17-AAG reduced Scrib protein levels and consequently, we immunoprecipitated less Scrib from cell lysates (Fig. 5C). Importantly, we observed reduced PAK in these Scrib immunoprecipitates. This demonstrates that, although Sgt1–HSP90 does not directly facilitate the association of Scrib with PAK, loss of Scrib stability following HSP90 inhibition affects the overall abundance of Scrib and Scrib–PAK complex available to the cell.
Scrib and Sgt1 regulate PAK localization
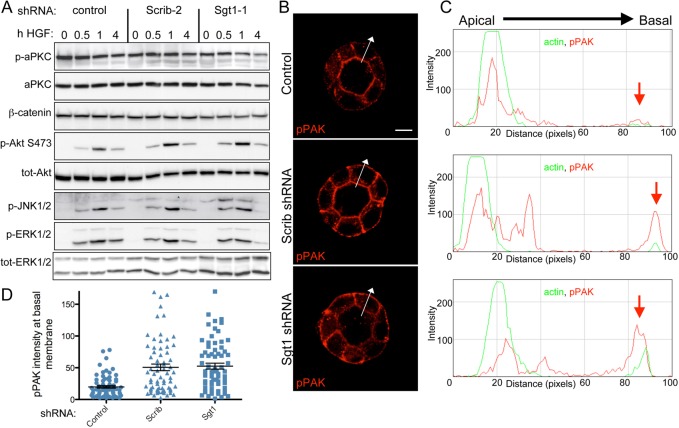
Scrib has been implicated in the regulation of a number of signaling proteins including: aPKC, JNK, ERK, and PAK (Humbert et al., 2008; Leong et al., 2009; Nola et al., 2008). Previous work has also demonstrated that ERK is required for HGF-mediated extension formation and tubulogenesis of MDCK cells cultured in 3D (O'Brien et al., 2004). To determine if the extension formation defects we saw in Scrib and Sgt1 RNAi cells could be due to defects in any of these signaling pathways downstream of HGF, we examined total cell lysates from 3D-cultured MDCK cells. Significantly, we saw no change in the HGF regulated activation of ERK or JNK in the Scrib and Sgt1 RNAi cells relative to cells expressing a control shRNA (Fig. 6A). Similarly, we saw no difference in the activation/phosphorylation profiles for aPKC and Akt from Scrib and Sgt1 RNAi 3D cyst total cell lysates relative to control lysates (Fig. 6A). Together, these results suggest that failure to activate ERK, JNK, aPKC or PI3K signaling is unlikely to explain the extension defects we see and point to an alternative pathway for Scrib and Sgt1 in the regulation of HGF signaling.
Fig. 6.
RNAi of Scrib or Sgt1 leads to mislocalization of pPAK (T423). (A) Total cell lysates were harvested from MDCK cysts following treatment with HGF for the indicated times (in hours). Lysates were then run on SDS-PAGE gels and subjected to western blot analysis for the indicated proteins. (B) Immunofluorescence staining of MDCK control shRNA or Scrib and Sgt1 shRNA cysts with an antibody that recognizes phosphorylation of Pak1/2 at position Thr423/402 (red). Arrows are drawn through cyst walls at the location where images were subjected to linescan analysis. (C) Linescan analysis using ImageJ software to plot the intensity of actin staining (green line) and pPAK staining (red line) along the apico-basal axis of a cell in the cyst wall shown by arrows in B. Red arrow indicates the location of the basal membrane of cells. The signal intensity peak for actin identifies the apical surface of individual cells in the cyst. (D) Intensity of pPAK staining at the basal surface was quantified using linescans through numerous cysts and plotting as a scatter plot. Scale bar: 10 µm.
Due to the observed association between Scrib and PAK in MDCK cells, we next examined pPAK in Scrib and Sgt1 RNAi cysts. PAK can be regulated by a number of signaling pathways and the spatiotemporal regulation of its activity is critical to its functions (Bokoch, 2003; Kumar et al., 2009). Consequently, we stained cysts with an antibody that specifically recognized phosphorylation of threonine 423/402 on Pak1/2 to gain spatially important information on PAK activity. Staining of control shRNA cysts revealed both junctional and apical pPAK (Fig. 6B). When we stained Scrib and Sgt1 RNAi cysts, this apical and junctional staining remained; however, unlike control cysts, we observed increased basal membrane accumulation of pPAK (Fig. 6B,C). We further quantified distribution of pPAK along a line drawn through the middle of the cell and running from the apical to the basal surface, as shown in Fig. 6B,C. The signal from basal-localized pPAK (or for comparison actin) is indicated by the red arrow in each panel. Quantification of multiple cysts following shRNA treatment is shown in Fig. 6D and confirms that loss of Scrib or Sgt1 causes an accumulation of pPAK at the basal membrane. Due to the lack of extensions in Scrib and Sgt1 RNAi cysts, it was not possible to determine if pPAK was correctly localized in these actin-rich structures following HGF treatment. Taken together with our previous observation that Scrib forms a complex with βPix and PAK, the data suggest that Scrib and Sgt1 are required for the proper localization of a pool of pPAK in MDCK cysts.
PAK and βPix are required for cyst extension formation
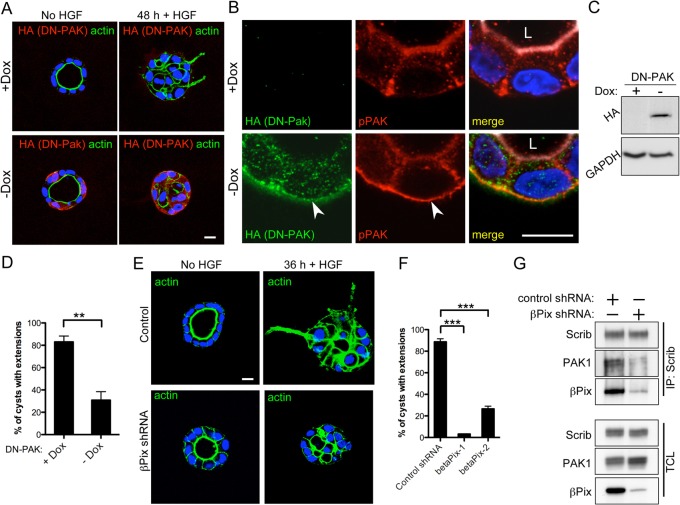
If the Scrib–βPix–PAK complex is indeed required for HGF-mediated extension formation, inhibition of βPix and PAK should cause the same defects in this process that were observed following Scrib RNAi. We first investigated what would happen when we inhibited PAK kinase activity. To test this, we employed the use of a previously established MDCK cell line expressing a full-length, kinase-dead PAK1(K299R) protein (Zegers et al., 2003). This protein functions as a dominant negative (DN) and has inducible expression under the control of a tetracycline-responsive promoter using the Tet-off system. Using this system, we grew control MDCK cysts in the presence of doxycycline (+Dox) or DN-PAK-expressing cysts in the absence of doxycycline (−Dox). Immunofluorescence staining and western blot analysis was performed to confirm the expression of the HA-tagged DN-PAK mutant protein (Fig. 7A,C). Following HGF treatment of cyst cultures, +Dox control cysts had numerous extensions after 48 hours (Fig. 7A,C). In contrast, DN-PAK-expressing cells had a statistically significant reduction in the number of extensions following HGF treatment (Fig. 7A,D). To confirm that this phenotype was not due to a clonal variation in this cell line, we tested a second DN-PAK clone and obtained similar results (not shown). These results reveal a requirement for PAK kinase activity during HGF signaling in 3D-cultured MDCK cells. Interestingly, in addition to a punctate intracellular distribution, we observed strong localization of DN-PAK along the basal surface of cysts (Fig. 7B). This localization was very similar to the mislocalization of pPAK we saw following Scrib and Sgt1 RNAi. Indeed, pPAK also strongly accumulated along the basal surface and colocalized with DN-PAK in these cysts (Fig. 7B). Together, these data indicate that disruption of the Scrib–βPix–PAK complex, either through loss of Scrib or inhibition of PAK kinase activity, can trap a pool of pPAK at the basal surface where it is presumably unable to carry out a critical function such as actin or focal adhesion remodeling.
Fig. 7.
PAK and βPix are required for HGF-mediated cyst extension formation. (A) Tet-off-regulated expression of an HA-tagged DN-PAK (K299R) kinase-dead construct. Cysts were cultured for 5 days in Matrigel and in the presence or absence of doxycycline (+/– Dox) to regulate expression. Cysts were treated with 50 ng/ml HGF for 48 hours to induce extensions. Anti-HA immunofluorescence staining (red) of fixed cysts was performed to monitor DN-PAK expression, and fluorescently labeled phalloidin (green) was used to visualize actin. Nuclei were stained with Hoechst (blue). (B) Magnification of DN-PAK-expressing cysts showing basal localization of DN-PAK. pPAK colocalizes at the basal surface with DN-PAK, but not in control cysts (arrows). Merged images also show actin, in white, marking the apical surface. L, lumen. (C) Dox-regulated expression of DN-PAK was also monitored by western blot analysis and anti-GAPDH was used as a cell lysate loading control. (D) The percentage of cysts with one or more extensions in the presence or absence of Dox was quantified. (E,F) 4-day-old cysts of control cells or cells infected with βPix lentiviral shRNA were induced with HGF (E) and the ability of cysts to form extensions was quantified (F). Dunnett's multiple comparison test was used. (G) IPs of Scrib in control- or βPix-shRNA-treated cells. Less PAK is associated with Scrib following βPix RNAi. **P<0.01, ***P<0.001. Scale bars: 10 µm.
Finally, it has been shown that βPix is required for the formation of the Scrib–βPix–PAK complex during 2D cell migration (Nola et al., 2008). Since reduction in the abundance of this complex through Scrib or Sgt1 RNAi blocks HGF-mediated extension formation, we predicted that reduction of βPix would also cause extension formation defects. To test this, we generated two lentiviral shRNAs that effectively targeted βPix for RNAi (supplementary material Fig. S3A). Similar to Scrib RNAi cysts, βPix RNAi cysts grown in Matrigel had normal polarity (supplementary material Fig. S3B). When we treated βPix RNAi cysts with HGF, we saw a significant decrease in the number of cysts able to form extensions (Fig. 7E,F). To confirm that Scrib had reduced association with PAK in these cells, we performed Scrib IPs in control or βPix-shRNA-treated cells. As expected, we saw reduced PAK levels in Scrib IPs from βPix shRNA cells (Fig. 7G). These experiments lead us to conclude that a Scrib–βPix–PAK complex is required for epithelial morphogenesis and that inhibition of this complex via a number of different ways, including RNAi of Scrib, Sgt1 and βPix or inhibition of PAK kinase activity, can lead to defects in this process.
Discussion
The evolutionarily conserved polarity protein Scrib plays a role in a number of fundamental cellular processes. Essential to Scrib function are the LRR domain and PDZ domains that are thought to mediate protein–protein interactions and presumably help Scrib scaffold larger molecular complexes. Despite the essential requirement of the LRR domain to Scrib function and localization, little is known about the molecular mechanism by which it functions or the protein interactions associated with it. Here we have uncovered a role for Scrib in the regulation of HGF-mediated epithelial morphogenesis. Central to the function of Scrib in this process is the co-chaperone protein Sgt1. Our data highlight a crucial and unrecognized function of the LRR domain in Scrib stability and homeostasis mediated through Sgt1 and HSP90. Loss of Sgt1–HSP90 limits the abundance of Scrib to form a complex with βPix and PAK. This Scrib–βPix–PAK complex is subsequently required to produce extensions in response to HGF (model shown in supplementary material Fig. S4).
Scrib has been reported to regulate ERK, JNK and aPKC signaling in other contexts and defects in the regulation of these pathways could explain the lack of HGF responsiveness in Scrib and Sgt1 RNAi cells (Humbert et al., 2008; Leong et al., 2009). Surprisingly, we saw no significant defects in the regulation of these pathways or in PI3 kinase/Akt signaling from both unstimulated and HGF stimulated Scrib or Sgt1 RNAi cells. Instead, we observed defects in the spatiotemporal regulation of PAK. The mislocalization of PAK following Sgt1 or Scrib RNAi as well as the localization of DN-PAK to the basal surface of cysts may reflect an accumulation of PAK at focal adhesions. It has been proposed that PAKs play a role in the dynamic regulation of focal adhesions by mechanisms that depend on its interaction with βPix. Inhibition of PAK function leads to its accumulation at focal adhesions and inhibits focal adhesion turnover (Bokoch, 2003; Frank and Hansen, 2008; Nayal et al., 2006; Zegers et al., 2003). Focal adhesion turnover is crucial for migration and defects in this process could explain the inability of Scrib deficient cysts to form extensions following addition of HGF (Gardel et al., 2010). Alternatively, the mislocalization of PAK to the basal surface might simply reflect an inability to localize PAK to another subcellular location. Additional analysis will be required to more fully understand the downstream consequences of Scrib–βPix–Pak signaling in extension formation.
A growing body of literature has demonstrated that HSP90 regulates diverse cellular functions. It accomplishes this by acting as a molecular chaperone for a wide variety of client proteins not only during times of cellular stress but also as to maintain protein homeostasis (Taipale et al., 2010). One way that HSP90 is thought to chaperone a broad spectrum of client proteins is by acting together with co-chaperones, which guide its recognition of clients and can help modulate HSP90 activity. Sgt1 is such a protein. Prior to this study, no functions or client proteins for mammalian Sgt1 were reported outside of its role in kinetochore complex assembly and innate immune receptor signaling. Although Sgt1 has been reported to chaperone a number of seemingly unrelated cellular clients, proteins containing LRR domains are overrepresented in Sgt1-dependent phenomena and the SGS domain of Sgt1 can directly associate with the LRR domain of a number of proteins (Dubacq et al., 2002; Mayor et al., 2007; Stuttmann et al., 2008). Our demonstration that the LRR domain of Scrib is necessary for Sgt1 complex formation provides additional evidence that Sgt1 may have evolved to preferentially link LRR domain containing proteins to the chaperone activity provided by HSP90.
We show that destabilization of Scrib following inhibition of Sgt1–HSP90 reduces the abundance of Scrib–PAK complexes. It is important to note that our data demonstrate that Sgt1 does not appear to be directly required for association of Scrib and PAK, but rather indirectly affects the ability of this complex to form by reducing the amount of Scrib available for the association. In fact, Sgt1–HSP90 is not likely part of the Scrib–βPix–PAK complex based on our inability to detect βPix or PAK in Sgt1 IPs and the failure of Sgt1 to colocalize with PAK in extensions (not shown). Consequently, Sgt1–HSP90 can be thought of as playing a permissive role in this signaling pathway through the regulation of Scrib levels. The identification of a function for Sgt1 in mammalian cell migration and Scrib stability points to the possibility of additional potential functions and clients for Sgt1 in mammals that remain to be discovered. Although Sgt1–HSP90 could be stabilizing a number of proteins required for migration, the identification of Scrib as an Sgt1–HSP90 client required for migration could also be relevant to the clinically important properties of HSP90 inhibition (Taipale et al., 2010).
Sgt1 and Scrib display subcellular colocalization with the intermediate filament protein vimentin. A recent study concluded that vimentin is responsible for protecting Scrib from ubiquitylation and degradation as well as regulating directional migration of 2D-cultured MDCK cells (Phua et al., 2009). Although we were unable to detect any ubiquitylation of Scrib in MDCK cells, Sgt1–HSP90 may function in concert with intermediate filaments to protect Scrib from ubiquitin-mediated degradation. Intriguingly, Sgt1 has been shown to associate with proteins of both ubiquitylating and de-ubiquitylating complexes (Kitagawa et al., 1999; Sowa et al., 2009; Zhang et al., 2008).
The proper regulation of protein stability and homeostasis has emerged as an essential regulatory mechanism for a number of proteins and the pathways they function in (Taipale et al., 2010). Despite the fundamental role for chaperones in a wide variety of cellular processes, little evidence has been presented for their role in the regulation of polarity proteins. Interestingly, another HSP90 co-chaperone, CDC-37, was shown to regulate PAR polarity complex proteins in C. elegans (Beers and Kemphues, 2006). More recently, a genetic requirement for Sgt1 in mediating cortical Scribble localization and LKB1/AMPK signaling in Drosophila neuroblasts was reported (Andersen et al., 2012). The authors of this study did not directly look at Scrib stability; however, it is possible that Sgt1 also stabilizes Scribble in Drosophila as well. These findings and our current study highlight an unappreciated role for chaperone regulated protein stability on polarity proteins. Undoubtedly, additional examples of polarity protein regulated processes that require chaperoning await discovery; indeed this may be a general mechanism for the regulation of polarity and morphogenesis.
Materials and Methods
Cell culture and reagents
MDCK II cells were grown in MEM supplemented with 5% fetal bovine serum, penicillin and streptomycin. For cyst experiments, MDCK cells were trypsinized into a single cell suspension (2×104 cells/ml) containing 2% growth-factor-reduced Matrigel. This suspension was plated into individual chambers of eight-well coverglass chamber slides coated with 6 µl 100% Matrigel and grown for 4–5 days. Το induce cyst extension formation, Matrigel grown cysts were washed once with normal growth medium and then stimulated for 24–48 hours with 50 ng/ml HGF in 2% growth factor reduced Matrigel.
Primary antibodies used in this study were: anti-β-catenin anti-Scrib (H-300 and C-20), anti-HA, anti-MMP13 (Santa Cruz Biotechnologies); mouse anti-gp135 (gift from George Ojakian); anti-cleaved caspase 3, anti-ERK1/2, anti-pERK1/2, anti-pJNK1/2, anti-Akt, anti-pAkt; anti-Pak1 (Cell Signaling); rat anti-α tubulin and rabbit anti-Sgt1 (Abcam); anti-vimentin (Sigma); anti-GAPDH and anti-βPix (Chemicon); anti-HSP90, anti-aPKC, anti-paPKC (BD Biosciences); anti-pPAK1/2/3 T423 (for IF, Biosource), anti-GFP (Invitrogen). Secondary antibodies used in this study were: Alexa-Fluor-488, -555 or -647-conjugated anti-mouse, anti-rat, anti-goat or anti-rabbit antibodies (Molecular Probes). Actin filaments were stained with Alexa-Fluor-488-, 555- or 633-conjugated phalloidin (Molecular Probes). DNA was stained with Hoechst (Molecular Probes). Immunoblotting was achieved using HRP-conjugated anti-goat, anti-rabbit or anti-mouse antibodies (Jackson Immunochemicals).
GFP::Scrib and GFP::Sgt1 expression constructs were generated by PCR amplification using either full-length mouse Scrib cDNA or full-length human Sgt1 cDNA as templates. PCR products were digested with restriction enzymes and cloned into pEGFP-C1. Constructs were then sequence verified.
RNA interference
shRNA sense oligos were designed against canine βPIX using the iRNAi freeware program. βPIX shRNA1, 5′-CCGGTGTGGTGCTACAGAATATTTTCAAGAGAAATATTCTGTAGCACCACATTTTTG-3′; βPIX shRNA2, 5′-CCGGGGAGGATCTTAGTAAGAGTTTCAAGAGAACTCTTACTAAGATCCTCCTTTTTG-3′. Scrib sense shRNA oligo sequences were based on previously published shRNA sequences targeting canine Scrib (Qin et al., 2005). Scrib shRNA1, 5′-CCGGCAGATGGTCCTCAGCAAGTTTCAAGAGAACTTGCTGAGGACCATCTGTTTTTG-3′; Scrib shRNA2, 5′-CCGGGAGGTGACACTGTGCAGCATTCAAGAGATGCTGCACAGTGTCACCTCTTTTTG-3′. Sgt shRNA 1 sequence was, 5′-CCGGGGACAGAAATTAGATAGTTTCAAGAGACACTATCTAATTTCTGTCCTTTTTG-3′, Sgt1 shRNA 2, 5′-CCGGCCCTCCTGATGATATGGAATTCAAGAGATTCCATATCATCAGGAGGGTTTTTG-3′. Sense and antisense oligos were annealed and ligated into the AgeI and EcoRI sites of the lentiviral pLKO.1 TRC cloning vector and transformed into bacteria. Positive clones were sequenced to ensure the shRNA sequences were free of errors.
Virus was produced by cotransfecting shRNA vectors and Virapower lentiviral vector packaging mix (Invitrogen, Carlsbad, CA) in 293FT cells. After 48 hours of virus production the virus-containing medium was collected and frozen at −80°C until it was used for infection. For viral transduction of MDCK cells, viral supernatant was added directly to non-adhered MDCK cells for 12–24 hours. Following 24 hours of recovery in fresh medium, lentiviral infected cells were selected for by the addition of 5 µg/ml puromycin.
DN-PAK expression
Expression constructs and cell lines for experiments using HA-tagged kinase-dead PAK1-(K299R) and Myc-tagged kinase-dead PAK1-(R193A, P194A, K299R) have been described previously (Sells et al., 1999; Zegers et al., 2003). For cyst extension formation assays, DN PAK1 cell lines were maintained in 20 ng/ml Doxycycline (Dox) until the time of cyst plating. Single cell suspensions were plated in Matrigel with or without Dox and allowed to develop into 4-day cysts. Cysts were then induced with 50 ng/ml HGF, with and without Dox and scored for extensions after 48 hours. PAK-KD expression was monitored by western blot analysis of total cell lysates and immunofluorescence staining.
Immunofluorescent staining
Cells were fixed using 2% paraformaldehyde. Some samples were first pre-permeabilized with 0.5% saponin for 10 min. at 4°C before fixation. All samples were blocked and permeabilized with 0.7% fish skin gelatin and permeabilized with 0.025% saponin in PBS+. Primary and secondary antibodies were applied sequentially with three washes between the primary and secondary additions. After four post-secondary washes, samples were analyzed on a Zeiss LSM510 confocal microscope. Linescan analysis of pPAK localization in cysts was performed using ImageJ software.
Immunoblotting and immunoprecipitation
Lysates for immunoblotting were prepared by directly adding SDS-PAGE sample buffer to live cells. For 2D HGF-stimulated cell lysates, cells were plated 24 hours prior to HGF treatment. Cells were then serum starved for 4 hours prior to the addition of 50 ng/ml HGF in serum-free medium for various times. 3D HGF-stimulated cyst lysates were prepared by growing MDCK cysts in 2% Matrigel for 4 days. Cysts were then serum starved in the absence of Matrigel for 2 hours. 50 ng/ml HGF was then added in serum-free medium for various times prior to harvesting lysates.
For co-immunoprecipitation experiments, subconfluent MDCK cells were lysed in a 50 mM Tris-HCl (pH 7.5), 150 mM NaCl, 0.5% NP-40 buffer augmented with proteinase and phosphatase inhibitors for 20 mins at 4°C. Lysates were spun at 15,000 g for 15 min and the supernatants were then incubated with 1 g of primary antibody for 4 hours at 4°C. Protein A/G Sepharose, preincubated with 3% BSA to block non-specific binding, was then added to the lysates for an additional 1.5 hours. Beads were washed 4× in lysis buffer followed by a final wash in PBS. Protein was eluted by direct addition of sample loading buffer to the beads and run on a 4-20% gradient SDS-PAGE gel.
Supplementary Material
Acknowledgments
We would like to thank Annette Shewan, Dave Bryant, Anirban Datta and other members of the Mostov laboratory for helpful discussions. Ian Macara kindly provided the Venus-Scrib expression plasmid.
Footnotes
Funding
This work was supported by the National Institutes of Health [grant numbers P01 AI53194, R01 DK074398, R01 DK091530 to K.E.M., R01 GM076363 to M.M.Z.]; and a Ruth Kirschstein NRSA fellowship [number F32 DK077660 to D.J.E.]. Deposited in PMC for release after 12 months.
Supplementary material available online at http://jcs.biologists.org/lookup/suppl/doi:10.1242/jcs.108670/-/DC1
References
- Albertson R., Chabu C., Sheehan A., Doe C. Q. (2004). Scribble protein domain mapping reveals a multistep localization mechanism and domains necessary for establishing cortical polarity. J. Cell Sci. 117, 6061–6070 10.1242/jcs.01525 [DOI] [PubMed] [Google Scholar]
- Andersen R. O., Turnbull D. W., Johnson E. A., Doe C. Q. (2012). Sgt1 acts via an LKB1/AMPK pathway to establish cortical polarity in larval neuroblasts. Dev. Biol. 363, 258–265 10.1016/j.ydbio.2011.12.047 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Audebert S., Navarro C., Nourry C., Chasserot–Golaz S., Lécine P., Bellaiche Y., Dupont J. L., Premont R. T., Sempéré C., Strub J. M.et al. (2004). Mammalian Scribble forms a tight complex with the betaPIX exchange factor. Curr. Biol. 14, 987–995 10.1016/j.cub.2004.05.051 [DOI] [PubMed] [Google Scholar]
- Austin M. J., Muskett P., Kahn K., Feys B. J., Jones J. D., Parker J. E. (2002). Regulatory role of SGT1 in early R gene-mediated plant defenses. Science 295, 2077–2080 10.1126/science.1067747 [DOI] [PubMed] [Google Scholar]
- Azevedo C., Sadanandom A., Kitagawa K., Freialdenhoven A., Shirasu K., Schulze–Lefert P. (2002). The RAR1 interactor SGT1, an essential component of R gene-triggered disease resistance. Science 295, 2073–2076 10.1126/science.1067554 [DOI] [PubMed] [Google Scholar]
- Bahri S., Wang S., Conder R., Choy J., Vlachos S., Dong K., Merino C., Sigrist S., Molnar C., Yang X.et al. (2010). The leading edge during dorsal closure as a model for epithelial plasticity: Pak is required for recruitment of the Scribble complex and septate junction formation. Development 137, 2023–2032 10.1242/dev.045088 [DOI] [PubMed] [Google Scholar]
- Beers M., Kemphues K. (2006). Depletion of the co-chaperone CDC-37 reveals two modes of PAR-6 cortical association in C. elegans embryos. Development 133, 3745–3754 10.1242/dev.02544 [DOI] [PubMed] [Google Scholar]
- Bilder D. (2004). Epithelial polarity and proliferation control: links from the Drosophila neoplastic tumor suppressors. Genes Dev. 18, 1909–1925 10.1101/gad.1211604 [DOI] [PubMed] [Google Scholar]
- Bilder D., Perrimon N. (2000). Localization of apical epithelial determinants by the basolateral PDZ protein Scribble. Nature 403, 676–680 10.1038/35001108 [DOI] [PubMed] [Google Scholar]
- Bilder D., Li M., Perrimon N. (2000). Cooperative regulation of cell polarity and growth by Drosophila tumor suppressors. Science 289, 113–116 10.1126/science.289.5476.113 [DOI] [PubMed] [Google Scholar]
- Bokoch G. M. (2003). Biology of the p21-activated kinases. Annu. Rev. Biochem. 72, 743–781 10.1146/annurev.biochem.72.121801.161742 [DOI] [PubMed] [Google Scholar]
- Bryant D. M., Mostov K. E. (2008). From cells to organs: building polarized tissue. Nat. Rev. Mol. Cell Biol. 9, 887–901 10.1038/nrm2523 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Catlett M. G., Kaplan K. B. (2006). Sgt1p is a unique co-chaperone that acts as a client adaptor to link Hsp90 to Skp1p. J. Biol. Chem. 281, 33739–33748 10.1074/jbc.M603847200 [DOI] [PubMed] [Google Scholar]
- da Silva Correia J., Miranda Y., Leonard N., Ulevitch R. (2007). SGT1 is essential for Nod1 activation. Proc. Natl. Acad. Sci. USA 104, 6764–6769 10.1073/pnas.0610926104 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davies A. E., Kaplan K. B. (2010). Hsp90-Sgt1 and Skp1 target human Mis12 complexes to ensure efficient formation of kinetochore-microtubule binding sites. J. Cell Biol. 189, 261–274 10.1083/jcb.200910036 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dow L. E., Kauffman J. S., Caddy J., Zarbalis K., Peterson A. S., Jane S. M., Russell S. M., Humbert P. O. (2007). The tumour-suppressor Scribble dictates cell polarity during directed epithelial migration: regulation of Rho GTPase recruitment to the leading edge. Oncogene 26, 2272–2282 10.1038/sj.onc.1210016 [DOI] [PubMed] [Google Scholar]
- Dubacq C., Guerois R., Courbeyrette R., Kitagawa K., Mann C. (2002). Sgt1p contributes to cyclic AMP pathway activity and physically interacts with the adenylyl cyclase Cyr1p/Cdc35p in budding yeast. Eukaryot. Cell 1, 568–582 10.1128/EC.1.4.568-582.2002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frank S. R., Hansen S. H. (2008). The PIX-GIT complex: a G protein signaling cassette in control of cell shape. Semin. Cell Dev. Biol. 19, 234–244 10.1016/j.semcdb.2008.01.002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gardel M. L., Schneider I. C., Aratyn–Schaus Y., Waterman C. M. (2010). Mechanical integration of actin and adhesion dynamics in cell migration. Annu. Rev. Cell Dev. Biol. 26, 315–333 10.1146/annurev.cellbio.011209.122036 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Humbert P. O., Grzeschik N. A., Brumby A. M., Galea R., Elsum I., Richardson H. E. (2008). Control of tumourigenesis by the Scribble/Dlg/Lgl polarity module. Oncogene 27, 6888–6907 10.1038/onc.2008.341 [DOI] [PubMed] [Google Scholar]
- Kadota Y., Shirasu K., Guerois R. (2010). NLR sensors meet at the SGT1-HSP90 crossroad. Trends Biochem. Sci. 35, 199–207 10.1016/j.tibs.2009.12.005 [DOI] [PubMed] [Google Scholar]
- Kallay L. M., McNickle A., Brennwald P. J., Hubbard A. L., Braiterman L. T. (2006). Scribble associates with two polarity proteins, Lgl2 and Vangl2, via distinct molecular domains. J. Cell. Biochem. 99, 647–664 10.1002/jcb.20992 [DOI] [PubMed] [Google Scholar]
- Kitagawa K., Skowyra D., Elledge S. J., Harper J. W., Hieter P. (1999). SGT1 encodes an essential component of the yeast kinetochore assembly pathway and a novel subunit of the SCF ubiquitin ligase complex. Mol. Cell 4, 21–33 10.1016/S1097-2765(00)80184-7 [DOI] [PubMed] [Google Scholar]
- Kumar A., Molli P. R., Pakala S. B., Bui Nguyen T. M., Rayala S. K., Kumar R. (2009). PAK thread from amoeba to mammals. J. Cell. Biochem. 107, 579–585 10.1002/jcb.22159 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lahuna O., Quellari M., Achard C., Nola S., Méduri G., Navarro C., Vitale N., Borg J. P., Misrahi M. (2005). Thyrotropin receptor trafficking relies on the hScrib-betaPIX-GIT1-ARF6 pathway. EMBO J. 24, 1364–1374 10.1038/sj.emboj.7600616 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Legouis R., Jaulin–Bastard F., Schott S., Navarro C., Borg J. P., Labouesse M. (2003). Basolateral targeting by leucine-rich repeat domains in epithelial cells. EMBO Rep. 4, 1096–1102 10.1038/sj.embor.7400006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leong G. R., Goulding K. R., Amin N., Richardson H. E., Brumby A. M. (2009). Scribble mutants promote aPKC and JNK-dependent epithelial neoplasia independently of Crumbs. BMC Biol. 7, 62 10.1186/1741-7007-7-62 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martins T., Maia A. F., Steffensen S., Sunkel C. E. (2009). Sgt1, a co-chaperone of Hsp90 stabilizes Polo and is required for centrosome organization. EMBO J. 28, 234–247 10.1038/emboj.2008.283 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mayor A., Martinon F., De Smedt T., Pétrilli V., Tschopp J. (2007). A crucial function of SGT1 and HSP90 in inflammasome activity links mammalian and plant innate immune responses. Nat. Immunol. 8, 497–503 10.1038/ni1459 [DOI] [PubMed] [Google Scholar]
- Montesano R., Matsumoto K., Nakamura T., Orci L. (1991). Identification of a fibroblast-derived epithelial morphogen as hepatocyte growth factor. Cell 67, 901–908 10.1016/0092-8674(91)90363-4 [DOI] [PubMed] [Google Scholar]
- Nakagawa S., Huibregtse J. M. (2000). Human scribble (Vartul) is targeted for ubiquitin-mediated degradation by the high-risk papillomavirus E6 proteins and the E6AP ubiquitin-protein ligase. Mol. Cell. Biol. 20, 8244–8253 10.1128/MCB.20.21.8244-8253.2000 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nayal A., Webb D. J., Brown C. M., Schaefer E. M., Vicente–Manzanares M., Horwitz A. R. (2006). Paxillin phosphorylation at Ser273 localizes a GIT1-PIX-PAK complex and regulates adhesion and protrusion dynamics. J. Cell Biol. 173, 587–589 10.1083/jcb.200509075 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nelson W. J. (2009). Remodeling epithelial cell organization: transitions between front-rear and apical-basal polarity. Cold Spring Harb. Perspect. Biol. 1, a000513 10.1101/cshperspect.a000513 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nola S., Sebbagh M., Marchetto S., Osmani N., Nourry C., Audebert S., Navarro C., Rachel R., Montcouquiol M., Sans N.et al. (2008). Scrib regulates PAK activity during the cell migration process. Hum. Mol. Genet. 17, 3552–3565 10.1093/hmg/ddn248 [DOI] [PubMed] [Google Scholar]
- Norman M., Wisniewska K. A., Lawrenson K., Garcia–Miranda P., Tada M., Kajita M., Mano H., Ishikawa S., Ikegawa M., Shimada T.et al. (2012). Loss of Scribble causes cell competition in mammalian cells. J. Cell Sci. 125, 59–66 10.1242/jcs.085803 [DOI] [PMC free article] [PubMed] [Google Scholar]
- O'Brien L. E., Zegers M. M., Mostov K. E. (2002). Opinion: Building epithelial architecture: insights from three-dimensional culture models. Nat. Rev. Mol. Cell Biol. 3, 531–537 10.1038/nrm859 [DOI] [PubMed] [Google Scholar]
- O'Brien L. E., Tang K., Kats E. S., Schutz–Geschwender A., Lipschutz J. H., Mostov K. E. (2004). ERK and MMPs sequentially regulate distinct stages of epithelial tubule development. Dev. Cell 7, 21–32 10.1016/j.devcel.2004.06.001 [DOI] [PubMed] [Google Scholar]
- Osmani N., Vitale N., Borg J. P., Etienne–Manneville S. (2006). Scrib controls Cdc42 localization and activity to promote cell polarization during astrocyte migration. Curr. Biol. 16, 2395–2405 10.1016/j.cub.2006.10.026 [DOI] [PubMed] [Google Scholar]
- Phua D. C., Humbert P. O., Hunziker W. (2009). Vimentin regulates scribble activity by protecting it from proteasomal degradation. Mol. Biol. Cell 20, 2841–2855 10.1091/mbc.E08-02-0199 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pollack A. L., Runyan R. B., Mostov K. E. (1998). Morphogenetic mechanisms of epithelial tubulogenesis: MDCK cell polarity is transiently rearranged without loss of cell-cell contact during scatter factor/hepatocyte growth factor-induced tubulogenesis. Dev. Biol. 204, 64–79 10.1006/dbio.1998.9091 [DOI] [PubMed] [Google Scholar]
- Qin Y., Capaldo C., Gumbiner B. M., Macara I. G. (2005). The mammalian Scribble polarity protein regulates epithelial cell adhesion and migration through E-cadherin. J. Cell Biol. 171, 1061–1071 10.1083/jcb.200506094 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sells M. A., Boyd J. T., Chernoff J. (1999). p21-activated kinase 1 (Pak1) regulates cell motility in mammalian fibroblasts. J. Cell Biol. 145, 837–849 10.1083/jcb.145.4.837 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sone K., Nakagawa S., Nakagawa K., Takizawa S., Matsumoto Y., Nagasaka K., Tsuruga T., Hiraike H., Hiraike–Wada O., Miyamoto Y.et al. (2008). hScrib, a human homologue of Drosophila neoplastic tumor suppressor, is a novel death substrate targeted by caspase during the process of apoptosis. Genes Cells 13, 771–785 10.1111/j.1365-2443.2008.01204.x [DOI] [PubMed] [Google Scholar]
- Sowa M. E., Bennett E. J., Gygi S. P., Harper J. W. (2009). Defining the human deubiquitinating enzyme interaction landscape. Cell 138, 389–403 10.1016/j.cell.2009.04.042 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steensgaard P., Garrè M., Muradore I., Transidico P., Nigg E. A., Kitagawa K., Earnshaw W. C., Faretta M., Musacchio A. (2004). Sgt1 is required for human kinetochore assembly. EMBO Rep. 5, 626–631 10.1038/sj.embor.7400154 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stuttmann J., Parker J. E., Noël L. D. (2008). Staying in the fold: The SGT1/chaperone machinery in maintenance and evolution of leucine-rich repeat proteins. Plant Signal. Behav. 3, 283–285 10.4161/psb.3.5.5576 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taipale M., Jarosz D. F., Lindquist S. (2010). HSP90 at the hub of protein homeostasis: emerging mechanistic insights. Nat. Rev. Mol. Cell Biol. 11, 515–528 10.1038/nrm2918 [DOI] [PubMed] [Google Scholar]
- Trepel J., Mollapour M., Giaccone G., Neckers L. (2010). Targeting the dynamic HSP90 complex in cancer. Nat. Rev. Cancer 10, 537–549 10.1038/nrc2887 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vaira V., Faversani A., Dohi T., Montorsi M., Augello C., Gatti S., Coggi G., Altieri D. C., Bosari S. (2012). miR-296 regulation of a cell polarity-cell plasticity module controls tumor progression. Oncogene 31, 27–38 10.1038/onc.2011.209 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xie Q., Gao C. F., Shinomiya N., Sausville E., Hay R., Gustafson M., Shen Y., Wenkert D., Vande Woude G. F. (2005). Geldanamycins exquisitely inhibit HGF/SF-mediated tumor cell invasion. Oncogene 24, 3697–3707 10.1038/sj.onc.1208499 [DOI] [PubMed] [Google Scholar]
- Zegers M. M., Forget M. A., Chernoff J., Mostov K. E., ter Beest M. B., Hansen S. H. (2003). Pak1 and PIX regulate contact inhibition during epithelial wound healing. EMBO J. 22, 4155–4165 10.1093/emboj/cdg398 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zeitler J., Hsu C. P., Dionne H., Bilder D. (2004). Domains controlling cell polarity and proliferation in the Drosophila tumor suppressor Scribble. J. Cell Biol. 167, 1137–1146 10.1083/jcb.200407158 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhan L., Rosenberg A., Bergami K. C., Yu M., Xuan Z., Jaffe A. B., Allred C., Muthuswamy S. K. (2008). Deregulation of scribble promotes mammary tumorigenesis and reveals a role for cell polarity in carcinoma. Cell 135, 865–878 10.1016/j.cell.2008.09.045 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang M., Botër M., Li K., Kadota Y., Panaretou B., Prodromou C., Shirasu K., Pearl L. H. (2008). Structural and functional coupling of Hsp90- and Sgt1-centred multi-protein complexes. EMBO J. 27, 2789–2798 10.1038/emboj.2008.190 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.