Abstract
Objectives
To determine if serum markers for collagen I and III synthesis, the C-terminal peptide from Pro-collagen-I (PICP) and the N-terminal peptide from Pro-Collagen III (PIIINP), correlate with LA fibrosis and post-operative AF.
Background
Atrial fibrillation (AF) after cardiac surgery is associated with adverse outcomes. We recently demonstrated that left atrial (LA) fibrosis is associated with post-operative AF in patients with no previous history of AF.
Methods
Fifty-four patients having cardiac surgery without a history of AF consented to left and right atrial biopsies, and a pre-operative peripheral blood draw. Picrosirius red staining quantified the percentage of fibrosis, and RT-PCR assessed atrial tissue mRNA transcripts involved in the fibrosis pathway. PICP and PIIINP levels were measured using an enzyme immunosorbant assay.
Results
Eighteen patients developed AF, while 36 remained in normal sinus rhythm (NSR). Left atrial fibrosis was higher in patients who developed AF vs. NSR (6.13 ±2.9% vs. 2.03± 1.9%, p =0.03). LA mRNA transcripts for collagen I, III, transforming growth factor, and angiotensin were 1.5-2.0 fold higher in AF patients. Serum PICP and PIIINP levels were highest in AF vs. NSR (PICP: 451.7±200 vs. 293.3±114 ng/ml, p=0.006; PIIINP: 379±286 vs. 191.6±162 pg/ml, p=0.01). Further there was a linear correlation between LA fibrosis and serum PICP levels (R2= 0.2; p = 0.01), and of the markers only PICP was independently associated with AF.
Conclusions
This demonstrates that serum PICP and PIIINP levels correlate with the presence of left atrial fibrosis and may act as a predictor for post-operative AF even in the absence of previous history of AF.
Keywords: Atrial Fibrillation, Fibrosis, Cardiac Surgery
Introduction
Atrial fibrillation (AF) occurs in ~30% of patients following cardiac surgery and contributes significantly to the post-operative morbidity, mortality, and costs (1). We recently demonstrated that post-surgical AF was maintained by left atrial (LA) sources and associated with pre-operative fibrosis (2). We hypothesized that left ventricular hypertrophy and diastolic dysfunction contribute to LA dilatation and stretch promoting extracellular matrix remodeling, manifested in elevated serum and tissue markers for fibrosis aiding to the prediction of post-operative AF, in patients who have no previous history of AF.
Fibrosis in the atria primarily consists of type I and III collagen, which is synthesized by fibroblasts and myofibroblasts, and is regulated by a cascade of fibro-proliferative signals including Transforming Growth factor Beta (TGF-β) and Angiotensin II(ANG) working in concert to increase collagen secretion (3). Collagen I and III are secreted as pro-collagen precursors containing amino- and carboxyl-terminal pro-peptides (4) that are released into the serum by proteases after collagen deposition. While these pro-peptides correlate with the rate of collagen synthesis (4), type I collagen degradation results in the release of a carboxyl terminal telopeptide of collagen I (CITP) (4-6). Previous authors have analyzed levels of the carboxyl terminal peptide from pro-collagen I (PICP), CITP, and the amino terminal peptide from Pro-collagen type III (PIIINP) to monitor ventricular fibrosis during hypertension and congestive heart failure (4-6). However, the utility of these peptides as markers to predict a collagen substrate necessary for the development of AF is controversial (7,8) and has not been determined in patients undergoing cardiothoracic surgery.
We hypothesized that patients without a history of arrhythmias, who developed AF following cardiac surgery have 1: left ventricular hypertrophy and diastolic dysfunction resulting in LA stretch reflective of an increased left atrial diameter; 2: increased left atrial mRNA expression of collagen I and III, TGFβ, and Angiotensin II; 3: an increased percentage of left atrial fibrosis; 4: higher serum levels of PICP and PIIINP; and that serum markers for collagen synthesis would correlate with the percentage of left atrial fibrosis.
Methods
Patient Recruitment
After approval from the Institutional Review Board, 54 patients scheduled for cardiac surgery consented to atrial biopsies and evaluation of PICP, PIIINP and CITP levels. Medical records were reviewed to identify any of the exclusion criteria: <18 years of age, AF history, previous sternotomy, history of hyperthyroidism, pulmonary fibrosis, cirrhosis, or scleroderma. In addition, all patients were interviewed prior to the operation to verify that they had no known history of AF. All patients were monitored by continuous telemetry throughout their hospitalization for the presence of AF, and were treated following the Society of Thoracic Surgery guidelines after an open heart procedure (1 Atrial fibrillation was defined as an irregularly irregular rhythm without P-waves for a period of at least 15 minutes as observed by a physician(2). All patients who developed AF were given full disclosure and treated following the Society of Thoracic Surgery guidelines (1,2).
Pre-operative electrocardiograms were obtained on the day of surgery, magnified to 6400X and the duration from the onset of the p-wave to the return of the isoelectric line was determined by one blinded investigator. Pre-operative echocardiograms were obtained in all patients. Detailed methods regarding the echocardiogram measurements can be found in the data supplement.
Atrial Biopsy
Three to five millimeter biopsies were obtained from the left and right atrial appendage. The biopsy was divided, and placed in 10% buffered formalin and RNAlater™ (Ambion). RNA was extracted, transcribed, and messenger RNA (mRNA) levels for the gene of interest were normalized to 18s- ribosomal RNA (9,10). Further details regarding the mRNA methods can be found in the data supplement.
Histology
Specimens were embedded in paraffin and sectioned at 6μm. Slides were stained with picrosirius red as previously described (2,11), and examined with a Bioquant imaging system to quantify the area of fibrosis and atrial muscle. Epicardial, endocardial, and perivascular fibrosis, were excluded from the analysis. The percentage of fibrosis within the atrial muscle from each sample was calculated. All samples were measured in triplicate by a single investigator.
PICP, CITP, and PIIINP
Serum from all 54 patients was obtained pre-operatively as well as from 10 healthy subjects with no known cardiac disease. All samples were obtained from a peripheral vein, the serum extracted, and stored at -80° C. PICP (Takara Biomedical), CITP (Orion Diagnostica), and PIIINP (Usyn) levels were measured by enzyme linked immunosorbant assay according the manufacturers’ specifications and based on previous data (12,13). The sensitivity (lower detection limit) for each assay was PICP: 10 ng/ml, CITP: 1.0 μg/L, and PIIINP: 20 pg/ml, and the intra-assay variations were PICP: 6.0 %, CITP: 3.3 %, and PIIINP: 1.8 %. All samples were run in duplicate and measured at 450λ.
Statistical Analyses
For complete statistical methods see the online supplement
Results
Patient Variables
Of the 54 patients enrolled, 36-remained in NSR and 18-developed new onset post-operative AF. Patient variables (Table 1) demonstrated increased age as the only factor associated with AF development. From the pre-operative echocardiography data shown in Table 2, increased LA diameter, greater than mild valve disease, the number of patients with class II or III diastolic dysfunction, and increased left ventricular mass index were all associated with post-operative AF.
Table 1.
Patient Variables
| NSR =36 | AF after Surgery=18 | P value | |
|---|---|---|---|
| Age (years) | 64.5±8.5 | 73.3±7.8 | 0.001* |
| Male Gender | 29 (81%) | 14 (78 %) | 1.0 |
| BSA m2 | 2.08±0.23 | 2.01±0.23 | 0.4 |
| Medical History | |||
| Hypertension | 28 (78%) | 15 (89%) | 0.5 |
| COPD | 3 (8%) | 0 (0%) | 0.5 |
| Hyperthyroidism | 0 | 0 | 1.0 |
| CAD | 32 (100%) | 16 (94%) | 0.6 |
| Tobacco Abuse | 19 (53%) | 13 (72%) | 0.2 |
| Alcohol Abuse | 1 (3%) | 0 | 1.0 |
| Pre-operative Medications | |||
| ACE Inhibitor | 13(36%) | 6 (33%) | 1.0 |
| ARB | 5 (14%) | 1 (6%) | 0.7 |
| Beta Blocker | 16 (44%) | 12 (65%) | 0.2 |
| Statin | 21 (58%) | 11 (61%) | 1.0 |
| P-Wave Duration (sec) | 0.12±0.01 | 0.12±0.01 | 0.9 |
| Operation | |||
| CABG | 31 (94%) | 14(77%) | 0.5 |
| Valve | 1 (3%) | 1 (6%) | 1.0 |
| CABG/Valve | 1 (3%) | 3 (17%) | 0.1 |
Abbreviations: AF-Atrial Fibrillation; ACE, Angiontensin Converting Enzyme inhibitors; BSA, Body surface area; ARB, Angiotensin Receptor Blockers; CABG-Coronary Artery Bypass Graft; COPD, Chronic Obstructive Pulmonary Disease; HTN-Hypertension; NSR-Normal Sinus Rhythm; NYS CHF- New York State Congestive Heart Failure; Valve, Valvular Heart Disease.
Denotes Statistical Significance
Table 2.
Trans-Esophageal Echocardiogram Parameters Examined
| Echo Parameter | NSR n=36 | AF after Surgery n=18 | P value |
|---|---|---|---|
| Ejection Fraction % | 51.1±8 | 49.3±12 | 0.6 |
| > Mild Valve Disease | 2 (6%) | 6 (35%) | 0.004* |
| LA Diameter (cm) | 4.8 ±0.7 | 5.2±0.5 | 0.02* |
| RA Diameter (cm) | 4.9±0.8 | 4.8±0.6 | 0.5 |
| Diastolic Dysfunction | 21(58%) | 12 (75%) | 0.3 |
| > Class I DD | 1(3%) | 4 (25%) | 0.02* |
| LV Mass Index (g/m2) | 245.7 ±78 | 305.7±98 | 0.03* |
| E Wave (cm/sec) | 67.4±13 | 71.5±27 | 0.6 |
| A Wave (cm/sec) | 65.1±16 | 67.3 ±18 | 0.7 |
| A Wave Duration(sec) | 0.15±0.02 | 0.14±0.02 | 0.3 |
| E’ Wave(cm/sec) | 10.2±2.8 | 8.8±2.9 | 0.1 |
| A’ Wave(cm/sec) | 10.1±3.4 | 9.0±3.1 | 0.3 |
| E/A | 1.1±0.4 | 1.1±0.6 | 0.8 |
| E’/A’ | 1.1±0.3 | 1.1±0.5 | 0.8 |
Abbreviations: AF- Atrial Fibrillation, DD-Diastolic Dysfunction, LV-Left Ventricular, LA-Left Atrial NSR- Normal Sinus Rhythm, RA-Right Atrial.
Denotes Statistical Significance
Left and Right Atrial Biopsies
Of the 54 patients enrolled, there were 10 patients in whom biopsies were not analyzed. In three patients there was no observable LA appendage, and therefore biopsies were not obtained. In two patients inspection of the left or right atrial biopsy demonstrated only fat and an absence of muscle, and these biopsies were not analyzed. Last, in 5 patients the atrial appendage was thought to be excessively friable, thin, and at risk for post-operative bleeding and therefore, a biopsy was not taken.
RT-PCR
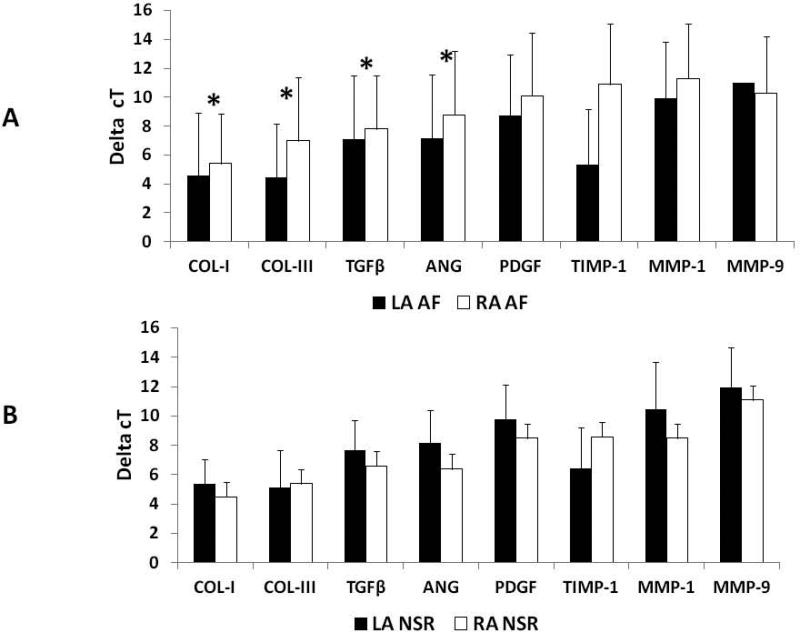
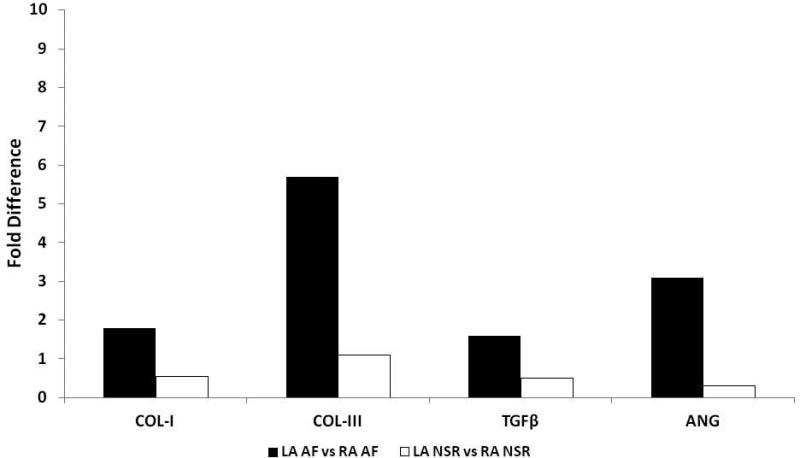
Patient characteristics from the 44 patients who had biopsies are presented in table 3 of the online supplement. The expression of 18s rRNA was similar in the left and right atria of all patients (Table 4, online supplement). Comparisons in the expression patterns of the mRNA transcripts from the entire patient population between left and right atrial samples are shown in table 5 of the online supplement. The only significant difference was a 9.1 fold increase in TIMP-1 expression from LA samples (p=0.002). The mRNA expression of relevant transcripts by delta cT values between those patients who remained in NSR and those who developed AF are provided in figures 1A and 1B. All of the transcripts analyzed involved in structural remodeling had a mean increase in expression within the left atrium of patients who developed AF compared to the left atrium of patients who remained in NSR. To gain a greater appreciation for the relevance in differences between delta cT values that were statistically significant, the delta cT values were converted to a percent fold change (Figure 2). Collagen I and III as well as TGFβ and ANG were all significantly increased by nearly2.0 fold in LA samples from those patients who developed AF.
Figure 1. Delta cT values for mRNA transcripts.
A: Delta cT values in left atrial mRNA expression for patients who developed Atrial Fibrillation (AF) and those who remained in Normal Sinus Rhythm (NSR). B: Delta cT values in right atrial mRNA expression for patients who developed Atrial Fibrillation (AF) and those who remained in Normal Sinus Rhythm (NSR). Abbreviations: Collagen-I(COL-I), Collagen-III(COL-III), Transforming Growth Factor Beta(TGFβ), Platelet Derived Growth Factor (PDGF), a Angiotensin (ANG), Tissue Inhibitor of Metalloprotienase-1(TIMP-1) and Matrix Metalloprotienase 1 and 9 (MMP-1 and-9). *Denotes statistical significance
Figure 2. Fold difference in mRNA transcripts.
mRNA expression by converting the delta cT values from figures 1A and 1B into fold differences by using the following equation: Fold difference (mean ΔcTLA_NSR - mean ΔcTLA_AF) = 2(mean ΔcTLA_NSR - mean ΔcTLA_AF). The data produced provide a visual representation of the fold change after statistical analysis demonstrated significance for the delta cT values, and therefore there are no standard deviation bars. Abbreviations: Collagen-I(COL-I), Collagen-III(COL-III), Transforming Growth Factor Beta(TGFβ), and Angiotensin (ANG) Atrial Fibrillation(AF) Normal Sinus Rhythm (NSR). *Denotes statistical significance
Fibrosis and AF
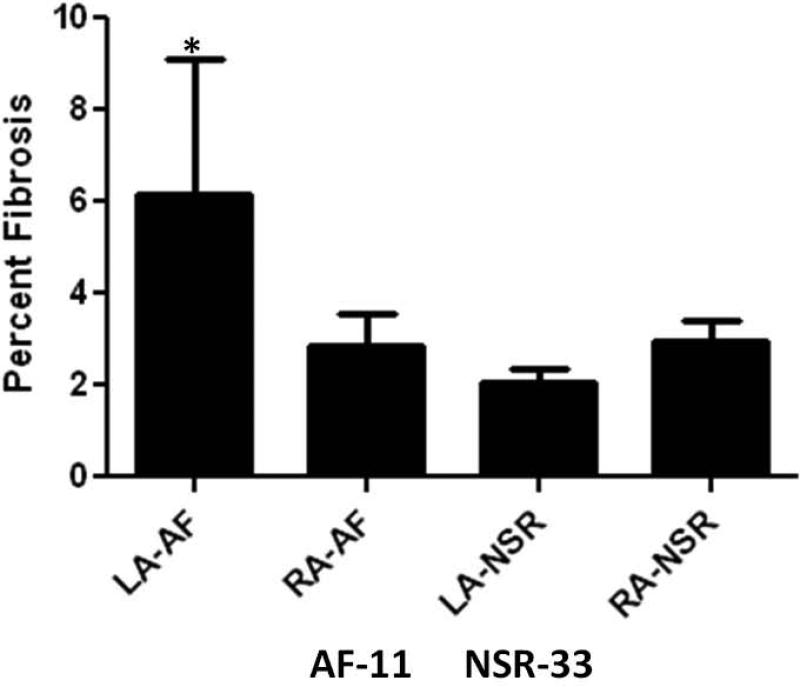
Interstitial fibrosis manifested as winding strands within the atrial muscle was observed in all left and right atrial biopsies. There were no significant differences between the LA and RA biopsies in the area of tissue available for analysis (LA:3.7 ± 2.8 mm2 vs. RA:3.0 ± 2.3 mm2, p =0.12), or the overall percentage of fibrosis between LA and RA samples (3.1±2.8% vs. 2.9±2.4%, p=0.63). There was no difference in RA fibrosis between patients who developed post-operative AF and those who remained in NSR (2.83 ±2.3% vs. 2.94±2.4%, p=0.4). However, as shown in figure 3, the percent area occupied by fibrosis within each sample was significantly higher in the LA samples of those patients who developed post-operative AF (6.13 ±2.4% vs. 2.03± 1.9%; p = 0.03). These results are consistent with data published previously (2).
Figure 3. Percent Fibrosis.
The average percentage of fibrosis organized by post-operative rhythm (Abbreviations: AF-Atrial Fibrillation, LA-Left atrial, NSR-Normal Sinus Rhythm, RA-Right Atrial). *Denotes statistical significance
Serum markers of collagen synthesis/degradation and AF
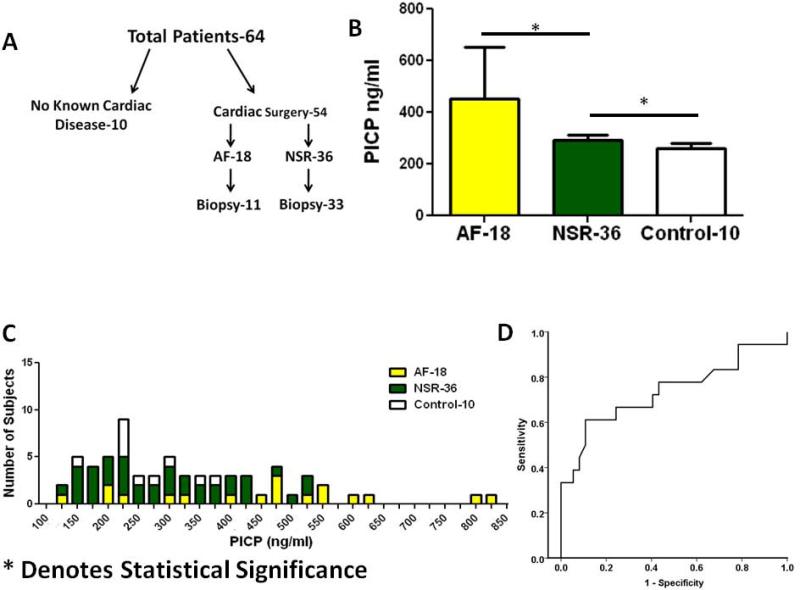
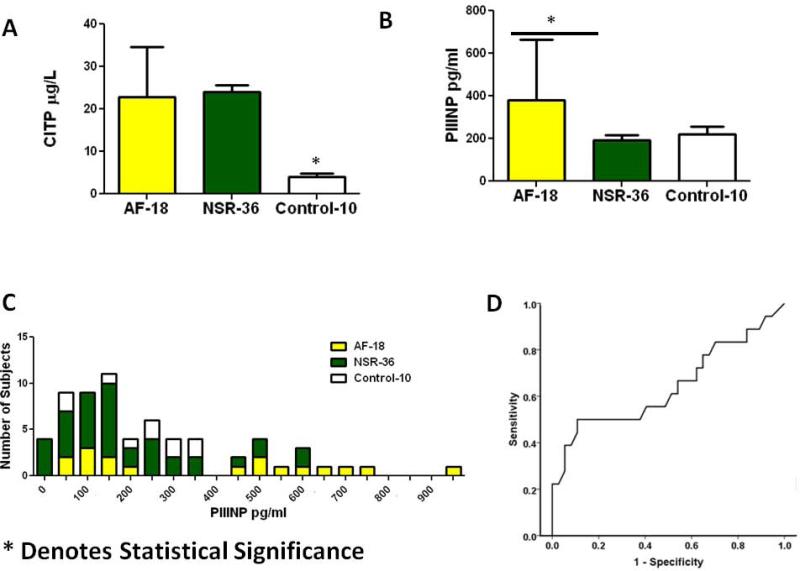
Serum PICP, PIIINP, and CITP levels were obtained from 54 patients and 10 control subjects (figure 4A). The 10 control subjects had no history of cardiac disease, were annually followed by a health care provider, and were currently not taking any medications (Table 6 online supplement). PICP levels in patients who developed post-operative AF (451.7 ± 200ng/ml) were significantly higher than those who remained in NSR (293.3 ±114, p= 0.006) and those without a known history of cardiac disease (260.4 ±66, p=0.001) (Figure 4B). Figure 4C provides a histogram in which patient values have been placed in 50 ng/ml bins of PICP and demonstrates that the majority of patients who developed AF had higher PICP values than those who remained in NSR. Further figure 4D presents a ROC curve comparing the development of AF and PICP and demonstrates an overall area under the curve of 0.75.
Figure 4. Increased PICP levels.
A: Schematic of blood samples obtained from patients tested for PICP, CITP, and PIIINP; B Average values for serum PICP levels in control patients, patients developing AF, and patients remaining in NSR after surgery; C: Histogram of serum PICP values in control and surgical patients; D: PICP ROC curve Abbreviations: AF-Atrial Fibrillation, PICP- Carboxyl terminal peptide from Pro-Collagen I, NSR-Normal Sinus Rhythm. *Denotes statistical significance
Similar to PICP levels, CITP levels were lowest in the healthy control group (4.1±2.2μg/L) compared patients who underwent cardiac surgery (23.7±9.7 μg/L, p=0.002). However, there was no significant difference between patients who developed AF (22.8 11.7μg/L) and those who remained in NSR (24.1± 8.7μg/L) (figure 5A).
Figure 5. PIIINP and CITP levels.
A: CITP levels from patients who developed atrial fibrillation, normal sinus rhythm, and control patients; B: PIIINP levels from patients who developed atrial fibrillation, normal sinus rhythm, and control patients; C: Histogram of serum PIIINP values in control and surgical patients demonstrating; D PIIINP ROC curve.
Abbreviations: AF-Atrial Fibrillation, CITP-C Terminal telopeptide from Pro-Collagen I, PIIINP- N Terminal peptide from Pro-Collagen III, NSR-Normal Sinus Rhythm. *Denotes statistical significance
Higher PIIINP levels also correlated with the development of post-operative AF. As shown in figure 5B, PIIINP levels in patients who developed AF (AF:379±286 vs. NSR:191.6±162 pg/ml, p = 0.01. Unlike PICP and CITP levels, there was no significant difference between patients having surgery and the healthy controls (238.4±107; p = 0.2). Similar to PICP the histogram in figure 5C represents 50 pg/ml bins of PIIINP, and demonstrates a lower utility when compared to PICP in differentiating between patients who developed post-operative AF and those who remained in NSR. In figure 5D the ROC curve further validates this point demonstrating an area under the curve of 0.64.
Atrial fibrosis correlates with serum peptide levels
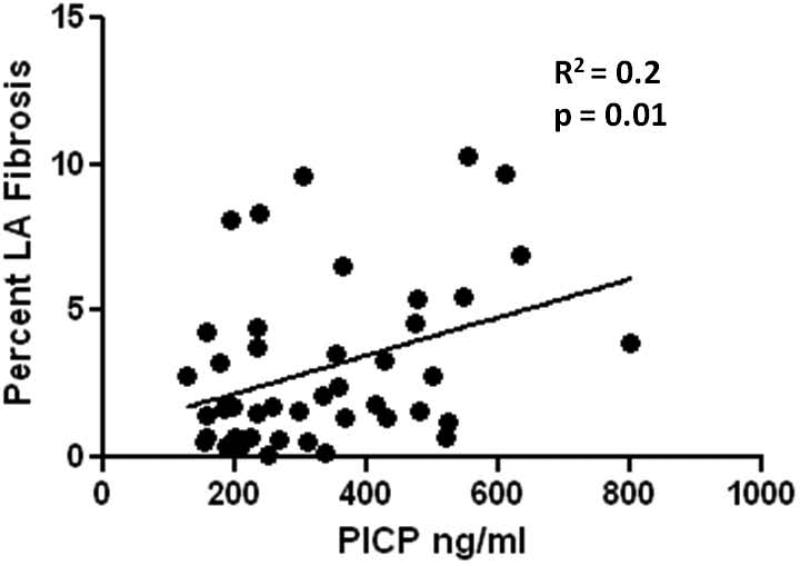
To determine whether the systemic synthesis and degradation of collagen I and III correlates with the level of atrial fibrosis we attempted to match the percentage of left and right atrial fibrosis with the serum PICP, CITP, and PIIINP levels. As demonstrated in Figure 6, LA fibrosis did correlate significantly with PICP levels (R2=0.2, p = 0.01). However, we found no correlation between the percentage of RA fibrosis and PICP values (R2 = 0.04, p = 0.3). In addition, no significant correlations were demonstrable between the percentage of LA or RA fibrosis and CITP or PIIINP levels.
Figure 6. Correlation between PICP and Left Atrial Fibrosis.
Linear regression of the percentage of left atrial fibrosis and serum PICP level.
Serum peptide levels and patient variables
We performed a multivariate analysis to determine whether the serum markers contribute significantly to the development of post-operative AF over and above patient variables such as age, which is an important predictor for the development of AF. Only age and PICP levels provided a significant contribution to the development of post-operative AF over other patient variables. (Age: Odds Ratio 1.13, p value 0.01; PICP: Odds Ratio 1.01, p value 0.02)
As age was the most important patient variable in the development of AF, we constructed ROC curves comparing individual PICP and PIIINP levels to age, as well as adjusting PICP and PIIINP levels to age. Figures 1 and 2 within the online supplement demonstrate that the area's under the curve for PICP, PIIINP, and age were 0.74, 0.67, and 0.77 respectively. Construction of ROC curves incorporating PICP along with age was 0.84 while the area under the curve for PIIINP along with age was 0.81.
Discussion
The main findings of this study are as follows: Patients without a history of AF who develop post-operative AF have 1) increased left ventricular mass index and left atrial diameter measured from pre-operative echocardiograms; 2) left atria with increased levels of Collagen I and III, TGFβ and ANG expression demonstrated by mRNA transcripts; 3: increased percentage of left atrial fibrosis from within the left atrial appendage; 4: increased serum PICP and PIIINP levels which correlate positively with the development of AF in the post-operative phase of cardiac surgery. Further, in patients who developed post-operative AF serum PICP levels correlate with the percentage of left but not right atrial fibrosis.
Among all patient variables, age was the most important factor in the development of post-operative AF. The accumulation of fibrosis and collagen in the atria resulting from hypertension, valvular disease, and diastolic dysfunction provide a likely mechanism for this correlation (14). Diastolic dysfunction has been shown to correlate with left atrial stretch and collagen deposition (5,6). Left ventricular mass index quantifies left ventricular hypertrophy and diastolic dysfunction, and is increased in our group of patients who developed post-operative AF, along with LA diameter, an indicator of stretch.
Analysis of gene expression patterns in patients who developed AF demonstrated increased pro-fibrotic markers in left atrial tissue. Within the entire population there was no difference in collagen I and III expression between left and right atrial samples. However, in patients who developed post-operative AF, collagen I and III were increased in the left atrium, and may further validate the hypothesis that increased LA pressures associated with hypertrophy and diastolic dysfunction result in atrial fibrosis and post operative AF risk (1,2). Of the pro-fibrotic marker analyzed only TGFβ and ANG were significantly increased in the LA tissue of patients who developed post-surgical AF. These two markers are integral to collagen synthesis (3), and provide a molecular mechanism for the increased percentage of LA fibrosis in AF patients. Interestingly, these transcripts were increased only in the left atrium; however, left and right atrial transcript differences have been previously reported (2, 15,16). Microarray analysis demonstrated 624 genes, 41% of which were expressed higher within the left vs right atrial myocardium (15). Further, an increased expression of the angiotensin receptors has been found within the LA myocardium of patients who develop AF (16). Our data further supports previous work which demonstrates left and right atrial differences (2).
The importance of left atrial fibrosis as a substrate in the development of lone AF is well documented (2,9,11). The accumulation of fibrosis within the extracellular space prolongs conduction time between myocytes creating conditions favorable for the initiation and maintenance of reentry (11). Yet despite the acceptance of fibrosis as a substrate for the development of lone AF, there is controversy regarding its importance in the development of post-operative AF (2,17). Previous data regarding the percentage of RA fibrosis in the development of post-surgical AF has produced mixed results (17). To date there is a preponderance of evidence (18) to suggest that in the majority of AF cases, the right atrium is merely a bystander, whereas electrical sources in the left atrium are integral to the mechanism of AF maintenance (2). Our data corroborate previous work (2), demonstrating that only differences in fibrosis within the left and not the right atrium correlate with the development of post-operative AF. Therefore, it is reasonable to conclude that LA fibrosis may have a greater impact on AF initiation and maintenance than RA fibrosis (2).
Markers of collagen I synthesis (PICP) and degradation (CITP) have been compared in AF patients, typically in those who undergo catheter ablation (19, 20). However, differences in serum peptide levels were compared between AF patients and healthy controls (20). We demonstrates that there were differences in all surgical patients in PICP and CITP levels compared to healthy controls, but also a significant difference between PICP levels in patients who developed and AF and those who remained in NSR, all of which had cardiovascular disease but no previous history of AF.
PICP levels demonstrated the most significant difference among patients who developed AF, those who remained in NSR, and control patients without a history of cardiac disease. This is in contrast with a previous report which demonstrated no difference in pro-collagen-I levels between patients with AF and matched control patients with a similar cardiac history but in NSR (8). Matching patients with a similar cardiac history may negate the utility in separating patients with fibrosis. Importantly, the stoichiometric ratio between the number of collagen molecules produced, and the number of PICP molecules released is 1:1 (4). As a result PICP levels have been shown to correlate in a linear fashion with the percentage of ventricular fibrosis (R=0.4) in patients with hypertensive heart disease (5). In patients having heart surgery we demonstrate a similar correlation (R2=0.2) between the percentage of LA fibrosis, and serum PICP levels. Such a correlation serves to further validate the importance of LA fibrosis in the development of post-operative AF.
The use of PIIINP as a marker of collagen III synthesis remains controversial. Unlike PICP levels which has a stoichiomertric ratio of 1:1 with collagen deposition, PIIINP levels are less reliable (4). This may account in part for why when compared to PICP differences, PIIINP levels in patients who remained in NSR and who developed AF did not correlate with the percentage of left or right atrial fibrosis.
Limitations
Although all patients had no known history of AF, some could have had prior episodes of asymptomatic AF, as pre-operative Holter monitoring was not performed. The areas of LA and RA tissues analyzed in our study were relatively small in comparison to the overall size of each chamber, and we have made inferences based on differences only from a small sample. However, each biopsy was taken from the same location on the LA or RA, and therefore is a useful point of comparison. Ethical considerations prevented us from taking larger samples or samples from multiple locations, which would have given additional information but placed the patient at increased risk for post-operative bleeding. Therefore, tissue available for western blots for protein analysis was regrettably not available. .
Conclusion
We have demonstrated the importance of fibrosis as a substrate in the development of post-operative AF. Further testing to identify whether PICP levels could be used to predict patients who would develop AF following cardiac surgery remains unclear. It is well known that AF is one of the most frequent complications of cardiac surgery. AF affects more than one third of patients and is associated with increased morbidity and mortality and longer, more expensive hospital stays. As such, the ability to identify which patients have a pre-existing substrate, and are therefore at high risk for developing AF, could be critical for improved post-operative outcomes.
Supplementary Material
Acknowledgments
We would like to thank the Clinical Translational Science Institute at the University of Rochester for their statistical assistance.
Abbreviation List
- AF
Atrial Fibrillation
- NSR
Normal Sinus Rhythm
- LA
Left Atrial
- TGF-β
Transforming Growth factor Beta
- ANG
Angiotensin II
- CITP
C terminal telopeptide from Collagen I
- PICP
C terminal peptide from collagen I
- PIIINP
N terminal peptide from collagen II
- mRNA
messenger RNA
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Disclosures: Dr. José Jalife is a consultant for Topera, Inc. Sources of Funding: NHLBI P01-HL39707 and The Leducq Foundation (JJ).
References
- 1.Mckeown PP. Executive Summary: American college of chest physicians guidelines for the prevention and management of postoperative atrial fibrillation after cardiac surgery. Chest. 2005;128:6–8. doi: 10.1378/chest.128.2_suppl.1s. [DOI] [PubMed] [Google Scholar]
- 2.Swartz MF, Fink GW, Lutz CJ, et al. Left-versus-Right Atrial Differences in Frequency Gradients, K+ Channel Transcripts, and Fibrosis in Patients Developing Atrial Fibrillation Following Cardiac Surgery. Heart Rhythm. 2009;6:1415–1422. doi: 10.1016/j.hrthm.2009.06.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Burstein B, Nattel S. Atrial fibrosis: Mechanisms and clinical relevance in atrial fibrillation. J Am Coll Cardiol. 2008;51:802–809. doi: 10.1016/j.jacc.2007.09.064. [DOI] [PubMed] [Google Scholar]
- 4.Lopez B, Gonzalez A, Querejeta R, Diez J. The use of collage-derived serum peptides for the clinical assessment of hypertensive heart disease. J of Hypertension. 2005;23(8):1445–1451. doi: 10.1097/01.hjh.0000173780.67308.f1. [DOI] [PubMed] [Google Scholar]
- 5.Querejeta R, Varo N, Lopez B, et al. Serum carboxy-terminal propeptide of procollagen type I is a marker of myocardial fibrosis in hypertensive heart disease. Circulation. 2000;101:1729–1735. doi: 10.1161/01.cir.101.14.1729. [DOI] [PubMed] [Google Scholar]
- 6.Lopez B, Gonzalez A, Beaumont J, Querejeta R, Larman M, Diez J. Identification of a potential cardiac antifibrotic mechanism of torasemide in patients with chronic renal failure. J Am Coll Cardiol. 2007;50(9):859–867. doi: 10.1016/j.jacc.2007.04.080. [DOI] [PubMed] [Google Scholar]
- 7.Tziakas DN, Chalikais GK, Stakos DA. Effect of angiotensin converting enzyme insertion/deletion genotype on collagen type I synthesis and degradation in patients with atrial fibrillation and arterial hypertension. Expert Opin Pharmacother. 2007;8(14):2225–2234. doi: 10.1517/14656566.8.14.2225. [DOI] [PubMed] [Google Scholar]
- 8.Tziakas DN, Chalikias GK, Papanas N, Stakos DA, Chatzikyriakou SV, Maltezos E. Circulating levels of collagen type I degradation marker depend on the type of atrial fibrillation. 2007;9(8):589–596. doi: 10.1093/europace/eum072. [DOI] [PubMed] [Google Scholar]
- 9.Cardin S, Libby E, Pelletier P, et al. Contrasting Gene expression profiles in two canine models of atrial fibrillation. Circ Res. 2007;100:425–433. doi: 10.1161/01.RES.0000258428.09589.1a. [DOI] [PubMed] [Google Scholar]
- 10.Epperson LE, Martin SL. Quantitative assessment of ground squirrel mRNA levels in multiple stages of hibernation. Physiol Genomics. 2002;10:93–102. doi: 10.1152/physiolgenomics.00004.2002. [DOI] [PubMed] [Google Scholar]
- 11.Tanaka K, Zlochivier S, Vikstrom K, et al. Spatial distribution of fibrosis governs fibrillation wave dynamics in the posterior left atrium during heart failure. Circ Res. 2007;101:839–847. doi: 10.1161/CIRCRESAHA.107.153858. [DOI] [PubMed] [Google Scholar]
- 12.Martos R, Baugh J, Ledwidge M, et al. Evidence of increased myocardial collagen turnover linked to diastolic dysfunction. Circulation. 2007;115:888–895. doi: 10.1161/CIRCULATIONAHA.106.638569. [DOI] [PubMed] [Google Scholar]
- 13.Berruti A, Dogliotti L, Gorzeonon G, et al. Differential patterns of bone turnover in relation to bone pain and disease extent in bone in cancer patients with skeletal metastases. Clinc Chem. 1999;45:1240–1247. [PubMed] [Google Scholar]
- 14.Anyukhovsky EP, Susonov EA, Plotnikov A, et al. Cellular electrophysiologic properties of old canine atria provide a substrate for arrhythmogenesis. Cardiovasc Res. 2002;54:462–469. doi: 10.1016/s0008-6363(02)00271-7. [DOI] [PubMed] [Google Scholar]
- 15.Kahr PC, Piccini I, Fabritz L, et al. Systematic analysis of gene expression differences between left and right atria in different mouse strains and in human atrial tissue. PLoS. 2011;6(10):e26389. doi: 10.1371/journal.pone.0026389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Boldt A, Werzel U, Weigl J, et al. Expression of angiotensin II receptors in human left and right atrial tissue in atrial fibrillation with and without underlying mitral valve disease. J Am Coll Cardiol. 2003;42:1785–1792. doi: 10.1016/j.jacc.2003.07.014. [DOI] [PubMed] [Google Scholar]
- 17.Cosgrave J, Foley JB, Flavin R, et al. Preoperative atrial histological changes are not associated with postoperative atrial fibrillation. Cardiovasc Path. 2006;15:213–217. doi: 10.1016/j.carpath.2006.04.002. [DOI] [PubMed] [Google Scholar]
- 18.Haissaguerre M, Jais P, Shah DC, et al. Spontaneous initiation of atrial fibrillation by ectopic beats originating in the pulmonary veins. N Engl J Med. 1998;339:659–666. doi: 10.1056/NEJM199809033391003. [DOI] [PubMed] [Google Scholar]
- 19.Okumura Y, Watanabe I, Nakai T, et al. Impact of biomarkers of inflammation and extracellular matrix turnover on the outcome of atrial fibrillation ablation: Importance of matrix metalloproteinase-2 as a predictor of atrial fibrillation recurrence. J Cardiovasc Electrophysiol. 2011;22(9):987–993. doi: 10.1111/j.1540-8167.2011.02059.x. [DOI] [PubMed] [Google Scholar]
- 20.Kallergis EM, Manios EG, Kanoupakis EM, et al. Extracellular matrix alterations in patients with paroxysmal and persistant atrial fibrillation: biochemical assessment of collagen type-1 turnover. J Am Coll Cardiol. 2008;52(3):211–215. doi: 10.1016/j.jacc.2008.03.045. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.