Abstract
The drug intestinal permeability (Peff) measure has been widely used as one of the main factors governing both the rate and/or extent of drug absorption (Fabs) in humans following oral administration. In this communication we emphasize the complexity behind and the care that must be taken with this in-vivo Peff measurement. Intestinal permeability, considering the whole of the human intestine, is more complex than generally recognized, and this can lead to misjudgment regarding Fabs and Peff in various settings, e.g. drug discovery, formulation design, drug development and regulation. Setting the adequate standard for the low/high permeability class boundary, the different experimental methods for the permeability measurement, and segmental-dependent permeability throughout the human intestine due to different mechanisms, are some of the main points that are discussed. Overall, the use of jejunal Peff as a surrogate for extent of absorption is sound and scientifically justified; a compound with high jejunal Peff will have high Fabs, eliminating the risk for misclassification as a BCS Class I drug. Much more care should be taken, however, when jejunal Peff does not support a high-permeability classification; a thorough examination may reveal high-permeability after all, attributable to e.g. segmental-dependent permeability due to degree of ionization or transporter expression. In this situation, the use of multiple permeability experimental methods, including the use of metabolism, which except of luminal degradation requires absorption, is prudent and encouraged.
Keywords: intestinal permeability, fraction dose absorbed, biopharmaceutics classification system, oral absorption
The drug intestinal permeability measure has been widely used as one of the main factors governing both the rate and/or extent of drug absorption (Fabs) in humans following oral administration1-3. Extensive scientific research has established that a good correlation exists between the human jejunal permeability (Peff) measured using single-pass perfusion techniques and the fraction of dose absorbed from an immediate-release, rapidly dissolving, dosage form4, 5. The best practice to determine Fabs is from pharmacokinetic/mass-balance studies in humans3, 4, 6-8. The complexity and cost of carrying out these studies are very high, as they require radiolabelled API for the validation of high drug and metabolites recovery 9, 10. Since the FDA uses Fabs as the basis for high-permeability BCS classification2, an alternative channel of using Peff as a surrogate for Fabs was suggested by the FDA BCS guidance2. In this communication we emphasize the complexity behind and the care that must be taken with this in-vivo permeability measurement. Human intestinal permeability (HIP), considering the whole of the human intestine, is more complex than generally recognized, and this can lead to misjudgment regarding Fabs and Peff in various settings, e.g. drug discovery, formulation design, drug development and regulation.
The fundamental basis of the biopharmaceutics classification system (BCS) is the application of Fick's first law to a membrane transport (regardless of mechanism), revealing that the drug flux (mass/area/time) through the intestinal wall is equal to the permeability times the concentration of the drug at the intestinal membrane surface. This is a boundary condition on the fundamental mass balance equation for drug absorption7. This is a local mass balance requirement, pertaining to each point along the intestinal membrane; the permeability, in general, must be considered as position and time dependent. This dependence may be due to drug concentration changes (e.g. in carrier-mediated transport), changes in luminal fluid content, physiological/biochemical variations, water absorption, cellular differentiation, up/down regulation of drug transporters, membrane structure/composition variations along the intestine, etc. However, theoretically reliable prediction of extent of absorption can be made based on this mass-balance, resulting in the equation:
| Eq 1 |
as was developed in the first presentation of the BCS1. Absorption, as defined in equation 1, is taken to be the transport of drug, via diffusion and/or carrier mediated transport 11, 12, across the intestinal membrane and/or through the tight-junctions, and thus, ‘in the body’.
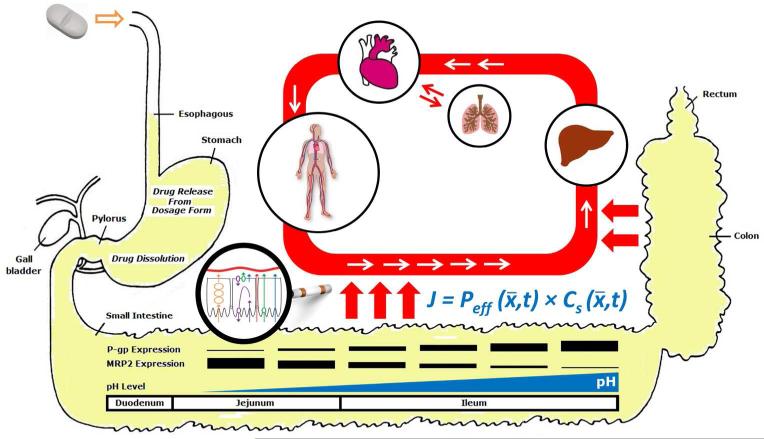
However, jejunal permeability (alone) may not always adequately predict Fabs. As noted above, the effective permeability (Peff) in Equation 1 is, in general, position and time dependent (Figure 1), the (surface) area integration is over the entire intestine, and the time integration is from zero (time of dosing) until tres, the residence time of the (whole) intestine. Equation 1, based on Gauss’ Theorem (Divergence theorem), relates drug lost from the intestinal lumen (luminal volume) to the drug transported through the intestinal wall. This, of course, excludes luminal and brush border degradation in the ‘lumen’ prior to absorption. The experimental studies in humans determine the jejunal Peff, a 10 cm segment of the intestine approximately 40 cm distal to the gastro-duodenal junction, and represent the average Peff of that 10 cm of proximal jejunum. The Peff of this segment, which is the best human measurement available today, may not represent the Peff of the whole of the human intestine and thus may not always adequately predict Fabs as required by Equation 1. This view was explicitly discussed in the BCS presentation paper1, and was later efficiently implemented in physiologically-based pharmacokinetic (PBPK) modeling software e.g. GastroPlus®, that divide the intestine into segments to account for regional-dependent permeability and metabolism13, 14. While for nonionizable and transcellular passively absorbed compounds the regional differences in the permeability along the intestine may be small, for drugs that are ionizable and/or their absorption is carrier-mediated, complex position-dependent permeability can be expected, as will be discussed below15. The FDA definition of Fabs>90% for high-permeability classification, i.e. the low/high permeability class boundary for classification of drugs, was initially conservative based on the need to consider these more complex permeability concerns. Notably, the European Medicines Agency, EMA, that initially followed the Fabs>90% definition for high-permeability classification, has recently decreased the permeability class boundary to 85% fraction of dose absorbed16.
Figure 1.
Schematic illustration of (some of) the complexity behind absorption processes and the permeability measure, considering the whole of the human intestine, as discussed in this communication.
The β-blocker metoprolol has been widely used as a marker for the low/high permeability class boundary, that is, if a compound shows higher Peff than metoprolol then it is considered to be high-permeability, and vice versa. The corresponding FDA's Fabs based high-permeability classification states that a drug substance is classified as high-permeability when the fraction of dose absorbed in humans is 90% or higher2. Examination of the human intestinal absorption of metoprolol reveals that it is in fact completely (100%) absorbed17, as evidenced by radiolabel mass-balance studies in five human subjects, thus making metoprolol a conservative reference drug for the low/high permeability class boundary. Indeed, the angiotensin II blocker losartan has been shown to have a lower human Peff than that of metoprolol yet a high fraction dose absorbed based on mass-balance. Similarly, isotretinoin was reported to have 90% fraction dose absorbed yet a human Peff lower than that of metoprolol (Table 1). The original choice of metoprolol as a reference drug was intentionally conservative in the initial guidance, to minimize the chances for exceptions to the dissolution standard that was going to be recommended for ‘Biowaivers’.
Table 1.
Literature human jejunal permeability (Peff) and fraction dose absorbed (Fabs) values for metoprolol, losartan and isotretinoin. Metoprolol is a conservative reference drug for the low/high permeability class boundary, as losartan and isotretinoin show lower Peff yet high Fabs.
The results of more recent studies show that not only does metoprolol represent an overly conservative boundary reference drug, it carries an additional complexity. We have shown that metoprolol's intestinal permeability in the rat is segmental-dependent; as a basic drug (pKa=9.5) with Log P value ~2, the pH changes along the small intestine were significant enough to lead to 5-fold higher permeability in the ileum (pH 7.5) than in the jejunum (pH 6.5). This raises the question of choice of Peff reference value even for metoprolol. An IR oral dose of metoprolol has been shown to be completely absorbed in the upper small intestine in both humans and animal models; Jobin et al have used the intubation technique in humans to show that 60% of a metoprolol oral dose is absorbed from the duodenum, and an additional 20% is absorbed from the following 30 cm of the jejunum, leading to absorption of 80% of the dose from the upper 50 cm of the small intestine18. Masaoka et al have shown that in rats, 90% of an oral metoprolol dose is absorbed prior to the jejunum, and the remaining 10% is absorbed in the upper jejunum19. It follows, hence, that metoprolol's Peff value at pH 7.5 (ileum) is not likely to be physiologically relevant, in terms of mass of drug absorbed, for an IR dosage form; rather, the permeability at pH 6.5, the average pH of the human jejunum (and the pH used in human jejunal permeability studies), would govern metoprolol's in-vivo intestinal absorption from an IR dosage form, and this permeability value allows the complete absorption of metoprolol. This analysis points out a possible extension to the regulatory high-permeability criterion: taking metoprolol's jejunal Peff at pH 6.5 as the benchmark for high-permeability, it is suggested that if a compound matches/exceeds this threshold anywhere in the intestine, and not necessarily in the jejunum, it is a high-permeability compound with complete absorption (Fabs>90%). This illustrates the issue of segmental-dependent permeability, in this case due to luminal degree of ionization and its effect on the (complex) intestinal membrane permeability, a very relevant consideration as will be discussed below.
We have recently addressed the distinctive absorption characteristics of the β-blocker sotalol, for which low apparent permeability across Caco-2 cell monolayers has been reported, but also a high fraction dose absorbed (Fabs>90%) in humans has been reported 20, 21. We have shown that, similarly to metoprolol, sotalol's intestinal permeability in the rat was segmental-dependent, with higher Peff at distal small intestinal regions (with higher average pH) than in proximal segments. At any given small intestinal segment and pH, sotalol's permeability was lower than that of metoprolol; however, most significanly, sotalol's permeability in the ileum at pH 7.5 exceeded that of metoprolol in the jejunum at pH 6.5 and matched metoprolol's Peff in the middle small intestine, pH 7.0 (Table 2). Since the ileum accounts for more than half of the human small intestinal length, an oral dose of sotalol would have an apparent high-permeability (i.e. greater than that of metoprolol in the jejunum) throughout a significant portion of the small intestinal residence time (~2 hours), resulting in its high fraction dose absorbed22. Significantly, the human tmax values of sotalol and metoprolol from an IR product support this analysis; metoprolol reaches Cmax within ~1 hr, indicating rapid absorption from the proximal small intestine (following gastric emptying) consistent with the independent studies noted above, while sotalol's Cmax is reached only after ~4 hr, strongly suggesting higher rate and extent of absorption from the distal parts of the small intestine. This analysis illustrates the limitations, as well as the care that must be used, in making a case for high/low permeability classification based merely on jejunal Peff values and comparison to metoprolol; sotalol would be falsely classified as low-permeability based on jejunal Peff value determined at pH 6.5, but when more thoroughly evaluated, the permeability basis for sotalol's nearly complete oral absorption is apparent, and the correct high-permeability classification was made. This case also stresses the care that must be taken when relying on (solely) Caco-2 studies for permeability characterization, and the limitations of this experimental method in representing the intestinal membrane permeability process in all of its complexity. Yet, very notably, published results thus far indicate that there are no known false-positives in Caco-2 permeability studies, indicating the significance/strength of this experimental method 20, 23, 24. That is, if a compound shows high-permeability across Caco-2 cell monolayer in a well validated and conducted study, it will have high Fabs.
Table 2.
Effective permeability values (×105 cm/sec) obtained for sotalol and metoprolol after in-situ single-pass perfusion to the rat proximal jejunum at pH 6.5, mid-small intestine at pH 7.0, and to the distal ileum at pH 7.5 22. Mean (S.D.); n=6. Sotalol's ileal permeability exceeds/matches metoprolol's jejunal/mid small intestinal Peff respectively, resulting in its high Fabs.
| Proximal Jejunum, pH 6.5 | Mid-Small Intestine, pH 7.0 | Distal Ileum, pH 7.5 | |
|---|---|---|---|
| Metoprolol | 3.7 (0.4) | 5.6 (0.6) | 20 (4.5) |
| Sotalol | 0.4 (0.05) | 1.7 (0.6) | 5.1 (0.6) |
Equation 1 requires one to consider the permeability, in all of its complexity, and in order to be valid requires one to consider the whole of the intestine, under the luminal conditions of dosing and is valid based on mass balance if one uses or determines the correct (complex) permeability. Further, metoprolol is absorbed, based on mass-balance studies, to 100%, while a high-permeability drug is defined at Fabs>90%. Thus the permeability value for high-permeability classification should be adjusted down to reflect this difference. It was suggested that the β-blocker labetalol, which exhibits lower Peff than metoprolol yet 95% Fabs, could serve as a more reflective marker for the low/high permeability class boundary.
When carrier-mediated intestinal permeability, both influx and efflux, contribute to the drug absorption process, the expression levels of the relevant transporters along the intestine may lead to a segmental dependent permeability phenomenon. For instance, we have shown that the expression level of the efflux transporter P-glycoprotein (P-gp) along the rat small intestine is regional-dependent and follows a gradient, increasing from the proximal to the distal segments25, 26. The same trend, although with higher variability, has been shown in humans as well27, 28. We have also shown that the permeability of the H2 blockers and P-gp substrates cimetidine and famotidine varies along the intestine, and is inversely correlated with the P-gp expression levels in the different segments25, 29. The same segmental-dependent permeability, at the same magnitude (approximately 2-fold) was reported for cimetidine intestinal absorption in humans30, showing the correlation between human and rat segmental-dependent permeability, also when carrier-mediated transport is involved. In the case of (solely) P-gp substrates, the jejunal Peff may still adequately correlate with Fabs in spite of the potential segmental-dependent permeability and the decreased Peff downstream, because high-permeability in the jejunum will likely allow the compound a high extent of absorption from an IR drug product regardless of the subsequent permeability in the more distal intestinal segments, similarly to the analysis of metoprolol's absorption. On the other hand, we have shown that the expression of the efflux transporter MRP2 throughout the rat small intestine follows an opposite gradient, decreasing from the proximal to the distal segments26. In this case, a (solely) MRP2 substrate may show low jejunal Peff, but sufficiently high ileal permeability to enable high Fabs. This may lead to misclassification when judging merely by the jejunal permeability. Additional complexity may rise when multiple transporters are involved in the intestinal absorption of the drug26, 31, 32. It should be noted that this analysis is relevant only for low/borderline permeability compounds; intestinal transporters will have no or little apparent effect on the intestinal absorption of drugs with significantly high permeability32-34.
The segmental-dependent intestinal permeability of drugs along the human intestine, and its relevance to the overall absorption, is (at present) largely unknown for most drugs. Regional infusions of the human intestine have been performed and support the segmental dependence of permeability, but due to the unknown- and time-dependent surface area, a permeability value cannot be calculated. Given the lack of direct experimental data in humans, especially human ileal permeability, the best we can do today is to evaluate segmental dependence in animal models that can provide a strong indication of the expected situation in humans35-39. Indeed, it was recognized in the FDA guidance on BCS that animal data, particularly rat small intestinal perfusion, can be correlated very well with human jejunal permeability2. Further it was recognized that tissue culture methods, e.g. the commonly used Caco-2 cell monolayers, could reduce the need for human and animal experiments but carried much more uncertainty since the biochemical and cellular expression of proteins and other cellular components and developmental differences (compared to human intestine) are difficult to quantify or unknown. Thus, a stronger scientific case, in the absence of human data, would need to be made for a convincing high-permeability classification. The requirement for 20 reference or validation compounds was made for these (among other) reasons, so these could be used to validate a case for high-permeability determination 24. Overall, human data, particularly human fraction absorbed would be the ‘gold’ standard in the determination of high-permeability; however permeability can be estimated, with varying degrees of confidence, much more easily than mass balance studies can be performed.
Notwithstanding the complexity behind the permeability value, human jejunal Peff values of 42 compounds with various absorption mechanisms determined principally at Uppsala University and at the University of Michigan over a period of 18 years have been shown to have an excellent correlation with the fraction dose absorbed in humans4, 6, 8. Overall, therefore, the use of jejunal Peff as a surrogate for extent of absorption is sound and scientifically justified; a compound with high jejunal permeability (exceeding that of metoporolol's jejunal permeability) will certainly have a high fraction dose absorbed, eliminating the risk for misclassification as a BCS Class I drug. Much more care should be taken, however, when jejunal Peff does not support a high-permeability classification; a thorough examination may reveal high-permeability after all, attributable to e.g. segmental-dependent permeability due to degree of ionization or transporter expression (whatever the underlying biochemical mechanism for this dependence). In this situation, the use of multiple permeability experimental methods, including the use of metabolism, which except of luminal degradation requires absorption, as suggested by the BDDCS40, 41, is prudent and encouraged 3, 20, 23.
References
- 1.Amidon GL, Lennernäs H, Shah VP, Crison JR. A theoretical basis for a biopharmaceutic drug classification: the correlation of in vitro drug product dissolution and in vivo bioavailability. Pharmaceutical Research. 1995;12(3):413–420. doi: 10.1023/a:1016212804288. [DOI] [PubMed] [Google Scholar]
- 2.CDER/FDA . Guidance for industry: Waiver of in vivo bioavailability and bioequivalence studies for immediate release dosage forms based on a biopharmaceutical slassification system. Center for Drug Evaluation and Research; 2000. [Google Scholar]
- 3.Dahan A, Miller JM, Amidon GL. Prediction of solubility and permeability class membership: provisional BCS classification of the world's top oral drugs. AAPS Journal. 2009;11(4):740–746. doi: 10.1208/s12248-009-9144-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Lennernäs H. Intestinal permeability and its relevance for absorption and elimination. Xenobiotica. 2007;37(10-11):1015–51. doi: 10.1080/00498250701704819. [DOI] [PubMed] [Google Scholar]
- 5.Lennernäs H. Human jejunal effective permeability and its correlation with preclinical drug absorption models. Journal of Pharmacy and Pharmacology. 1997;49(7):627–638. doi: 10.1111/j.2042-7158.1997.tb06084.x. [DOI] [PubMed] [Google Scholar]
- 6.Lennernäs H. Human intestinal permeability. Journal of Pharmaceutical Scinces. 1998;87(4):403–410. doi: 10.1021/js970332a. [DOI] [PubMed] [Google Scholar]
- 7.Lennernäs H. Modeling gastrointestinal drug absorption requires more in vivo biopharmaceutical data: experience from in vivo dissolution and permeability studies in humans. Current Drug Metabolism. 2007;8(7):645–57. doi: 10.2174/138920007782109823. [DOI] [PubMed] [Google Scholar]
- 8.Sun D, Lennernäs H, Welage LS, Barnett JL, Landowski CP, Foster D, Fleisher D, Lee K-D, Amidon GL. Comparison of human duodenum and caco-2 gene expression profiles for 12,000 gene sequences tags and correlation with permeability of 26 drugs. Pharmaceutical Research. 2002;19(10):1400–1416. doi: 10.1023/a:1020483911355. [DOI] [PubMed] [Google Scholar]
- 9.Cook JA, Davit BM, Polli JE. Impact of biopharmaceutics classification system-based biowaivers. Molecular Pharmaceutics. 2010;7(5):1539–1544. doi: 10.1021/mp1001747. [DOI] [PubMed] [Google Scholar]
- 10.Roffey S, Obach R, Gedge J, Smith D. What is the objective of the mass balance study? A retrospective analysis of data in animal and human excretion studies employing radiolabeled drugs. Drug Metabolism Reviews. 2007;39(1):17–43. doi: 10.1080/03602530600952172. [DOI] [PubMed] [Google Scholar]
- 11.Dobson PD, Kell DB. Carrier-mediated cellular uptake of pharmaceutical drugs: an exception or the rule? Nature Reviews Drug Discovery. 2008;7(3):205–220. doi: 10.1038/nrd2438. [DOI] [PubMed] [Google Scholar]
- 12.Sugano K, Kansy M, Artursson P, Avdeef A, Bendels S, Di L, Ecker GF, Faller B, Fischer H, Gerebtzoff G, Lennernäs H, Senner F. Coexistence of passive and carrier-mediated processes in drug transport. Nature Reviews Drug Discovery. 2010;9(8):597–614. doi: 10.1038/nrd3187. [DOI] [PubMed] [Google Scholar]
- 13.Yu LX, Amidon GL. A compartmental absorption and transit model for estimating oral drug absorption. International Journal of Pharmaceutics. 1999;186(2):119. doi: 10.1016/s0378-5173(99)00147-7. [DOI] [PubMed] [Google Scholar]
- 14.Yu LX, Lipka E, Crison JR, Amidon GL. Transport approaches to the biopharmaceutical design of oral drug delivery systems: prediction of intestinal absorption. Advanced Drug Delivery Reviews. 1996;19(3):359–376. doi: 10.1016/0169-409x(96)00009-9. [DOI] [PubMed] [Google Scholar]
- 15.Tannergren C, Bergendal A, Lennernäs H, Abrahamsson B. Toward an increased understanding of the barriers to colonic drug absorption in humans: implications for early controlled release candidate assessment. Molecular Pharmaceutics. 2009;6(1):60–73. doi: 10.1021/mp800261a. [DOI] [PubMed] [Google Scholar]
- 16.EMA . Guideline on the investigation of bioequivalence. European Medicines Agency; 2010. [DOI] [PubMed] [Google Scholar]
- 17.Regardh C, Borg K, Johansson R, Johnsson G, Palmer L. Pharmacokinetic studies on the selective beta1-receptor antagonist metoprolol in man. Journal of Pharmacokinetics and Biopharmaceutics. 1974;2(4):347–64. doi: 10.1007/BF01061407. [DOI] [PubMed] [Google Scholar]
- 18.Jobin G, Cortot A, Godbillon J, Duval M, Schoeller J, Hirtz J, Bernier J. Investigation of drug absorption from the gastrointestinal tract of man. I. Metoprolol in the stomach, duodenum and jejunum. British Journal of Clinical Pharmacology. 1985;19(Suppl 2):97S–105S. doi: 10.1111/j.1365-2125.1985.tb02749.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Masaoka Y, Tanaka Y, Kataoka M, Sakuma S, Yamashita S. Site of drug absorption after oral administration: Assessment of membrane permeability and luminal concentration of drugs in each segment of gastrointestinal tract. European Journal of Pharmaceutical Scinces. 2006;29(3-4):240–250. doi: 10.1016/j.ejps.2006.06.004. [DOI] [PubMed] [Google Scholar]
- 20.Chen M-L, Yu L. The use of drug metabolism for prediction of intestinal permeability. Molecular Pharmaceutics. 2009;6(1):74–81. doi: 10.1021/mp8001864. [DOI] [PubMed] [Google Scholar]
- 21.Yang Y, Faustino PJ, Volpe DA, Ellison CD, Lyon RC, Yu LX. Biopharmaceutics classification of selected beta-blockers: solubility and permeability class membership. Molecular Pharmaceutics. 2007;4(4):608–614. doi: 10.1021/mp070028i. [DOI] [PubMed] [Google Scholar]
- 22.Dahan A, Miller JM, Hilfinger JM, Yamashita S, Yu LX, Lennernäs H, Amidon GL. High-permeability criterion for BCS classification: Segmental/pH dependent permeability considerations. Molecular Pharmaceutics. 20107(5):1827–1834. doi: 10.1021/mp100175a. [DOI] [PubMed] [Google Scholar]
- 23.Amidon KS, Langguth P, Lennernäs H, Yu L, Amidon GL. Bioequivalence of oral products and the biopharmaceutics classification system: science, regulation, and public policy. Clinical Pharmacology and Therapeutics. 2011;90(3):467–470. doi: 10.1038/clpt.2011.109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Thiel-Demby VE, Humphreys JE, St. John Williams LA, Ellens HM, Shah N, Ayrton AD, Polli JW. Biopharmaceutics classification system: Validation and learnings of an in vitro permeability assay. Molecular Pharmaceutics. 2009;6(1):11–18. doi: 10.1021/mp800122b. [DOI] [PubMed] [Google Scholar]
- 25.Dahan A, Amidon GL. Segmental dependent transport of low permeability compounds along the small intestine due to P-glycoprotein: The role of efflux transport in the oral absorption of BCS class III drugs. Molecular Pharmaceutics. 2009;6(1):19–28. doi: 10.1021/mp800088f. [DOI] [PubMed] [Google Scholar]
- 26.Dahan A, Sabit H, Amidon GL. Multiple efflux pumps are involved in the transepithelial transport of colchicine: Combined effect of P-gp and MRP2 leads to decreased intestinal absorption throughout the entire small intestine. Drug Metabolism and Disposition. 2009;37(10):2028–2036. doi: 10.1124/dmd.109.028282. [DOI] [PubMed] [Google Scholar]
- 27.Mouly S, Paine M. P-glycoprotein increases from proximal to distal regions of human small intestine. Pharmaceutical Research. 2003;20(10):1595–9. doi: 10.1023/a:1026183200740. [DOI] [PubMed] [Google Scholar]
- 28.Thorn M, Finnstrom N, Lundgren S, Rane A, Loof L. Cytochromes P450 and MDR1 mRNA expression along the human gastrointestinal tract. British Journal of Clinical Pharmacology. 2005;60(1):54–60. doi: 10.1111/j.1365-2125.2005.02389.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Dahan A, West BT, Amidon GL. Segmental-dependent membrane permeability along the intestine following oral drug administration: Evaluation of a triple single-pass intestinal perfusion (TSPIP) approach in the rat. European Journal of Pharmaceutical Scinces. 2009;36(2–3):320–329. doi: 10.1016/j.ejps.2008.10.013. [DOI] [PubMed] [Google Scholar]
- 30.Sutcliffe F, Riley S, Kaser-Liard B, Turnberg L, Rowland M. Absorption of drugs from human jejunum and ileum. British Journal of Clinical Pharmacology. 1988;26:206P–207P. [Google Scholar]
- 31.Dahan A, Amidon GL. Small intestinal efflux mediated by MRP2 and BCRP shifts sulfasalazine intestinal permeability from high to low, enabling its colonic targeting. American Journal of Physiology - Gastrointestinal & Liver Physiology. 2009;297(2):G371–G377. doi: 10.1152/ajpgi.00102.2009. [DOI] [PubMed] [Google Scholar]
- 32.Giacomini K, Huang S, Tweedie D, Benet L, Brouwer K, Chu X, Dahlin A, Evers R, Fischer V, Hillgren K, Hoffmaster K, Ishikawa T, Keppler D, Kim R, Lee C, Niemi M, Polli J, Sugiyama Y, Swaan P, Ware J, Wright S, Yee S, Zamek-Gliszczynski M, Zhang L. Membrane transporters in drug development. Nature Reviews Drug Discovery. 2010;9(3):215–236. doi: 10.1038/nrd3028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Cao X, Yu LX, Barbaciru C, Landowski CP, Shin H-C, Gibbs S, Miller HA, Amidon GL, Sun D. Permeability dominates in vivo intestinal absorption of P-gp substrate with high solubility and high permeability. Molecular Pharmaceutics. 2005;2(4):329–340. doi: 10.1021/mp0499104. [DOI] [PubMed] [Google Scholar]
- 34.Custodio JM, Wu CY, Benet LZ. Predicting drug disposition, absorption/elimination/transporter interplay and the role of food on drug absorption. Advanced Drug Delivery Reviews. 2008;60(6):717. doi: 10.1016/j.addr.2007.08.043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Cao X, Gibbs S, Fang L, Miller H, Landowski C, Shin H, Lennernäs H, Zhong Y, Amidon G, Yu L, Sun D. Why is it challenging to predict intestinal drug absorption and oral bioavailability in human using rat model. Pharmaceutical Research. 2006;23(8):1675–86. doi: 10.1007/s11095-006-9041-2. [DOI] [PubMed] [Google Scholar]
- 36.Chiou W, Barve A. Linear correlation of the fraction of oral dose absorbed of 64 drugs between humans and rats. Pharmaceutical Research. 1998;15(11):1792–5. doi: 10.1023/a:1011981317451. [DOI] [PubMed] [Google Scholar]
- 37.Dahan A, Amidon GL. Grapefruit juice and its constituents augment colchicine intestinal absorption: Potential hazardous interaction and the role of P-glycoprotein. Pharmaceutical Research. 2009;26(4):883–892. doi: 10.1007/s11095-008-9789-7. [DOI] [PubMed] [Google Scholar]
- 38.Kim JS, Mitchell S, Kijek P, Tsume Y, Hilfinger J, Amidon GL. The suitability of an in situ perfusion model for permeability determinations: Utility for BCS class I biowaiver requests. Molecular Pharmaceutics. 2006;3(6):686–694. doi: 10.1021/mp060042f. [DOI] [PubMed] [Google Scholar]
- 39.Lennernäs H. Animal data: the contributions of the Ussing chamber and perfusion systems to predicting human oral drug delivery in vivo. Advanced Drug Delivery Reviews. 2007;59(11):1103–1120. doi: 10.1016/j.addr.2007.06.016. [DOI] [PubMed] [Google Scholar]
- 40.Benet L, Amidon GL, Barends D, Lennernäs H, Polli J, Shah V, Stavchansky S, Yu L. The use of BDDCS in classifying the permeability of marketed drugs. Pharmaceutical Research. 2008;25(3):483–488. doi: 10.1007/s11095-007-9523-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Wu C-Y, Benet LZ. Predicting drug disposition via application of BCS: Transport/absorption/ elimination interplay and development of a biopharmaceutics drug disposition classification system. Pharmaceutical Research. 2005;22(1):11–23. doi: 10.1007/s11095-004-9004-4. [DOI] [PubMed] [Google Scholar]