Abstract
Sensorineural hearing loss results from damage to the hair cells of the organ of Corti and is irreversible in mammals. While hair cell regeneration may prove to be the ideal therapy after hearing loss, prevention of initial hair cell loss could provide even more benefit at a lower cost. Previous studies have shown that the deletion of Atoh1 results in embryonic loss of hair cells while the absence of Barhl1, Gfi1, and Pou4f3 leads to the progressive loss of hair cells in newborn mice. We recently reported that in the early embryonic absence of N-Myc (using Pax2-Cre), hair cells in the organ of Corti develop and remain until at least seven days after birth, with subsequent progressive loss. Thus, N-Myc plays a role in hair cell viability; however, it is unclear if this is due to its early expression in hair cell precursors and throughout the growing otocyst as it functions through proliferation or its late expression exclusively in differentiated hair cells. Furthermore, the related family member L-Myc is mostly co-expressed in the ear, including in differentiated hair cells, but its function has not been studied and could be partially redundant to N-Myc. To test for a long-term function of the Mycs in differentiated hair cells, we generated nine unique genotypes knocking out N-Myc and/or L-Myc after initial formation of hair cells using the well-characterized Atoh1-Cre. We tested functionality of the auditory and vestibular systems at both P21 and four months of age and under the administration of the ototoxic drug cisplatin. We conclude that neither N-Myc nor L-Myc is likely to play important roles in long-term hair cell maintenance. Therefore, it is likely that the late-onset loss of hair cells resulting from early deletion of the Mycs leads to an unsustainable developmental defect.
Keywords: L-Myc, N-Myc, Hair Cell, Hearing, Prevention, Regeneration
1. Introduction
Sensorineural hearing loss and vestibular ataxia are common symptoms of a variety of human disorders resulting from damage to inner ear organ of Corti or vestibular hair cells. Hair cell damage can result from developmental malformations, post-developmental insults (Dror and Avraham, 2010), or late-onset effects of mutations (Fasquelle et al., 2011) of either cochlear (hearing) or vestibular (balance) hair cells. Regardless of etiology, loss of hair cells is irreversible in mammals. Current treatment for individuals with hearing loss is cochlear implants, and while these prostheses provide substantial improvement to those with profound hearing loss, they cannot fully restore hearing and may perform poorly in many user environments (Bent et al., 2009; Chang and Fu, 2006; Colletti et al., 2011; Gifford and Revit, 2010; Peterson et al., 2010). Translational research aims to find a route to restore hearing through regeneration of new hair cells but this avenue not only requires the knowledge of how to make a long-term viable hair cell, but also the technical ability to replace the damaged cells while retaining the highly complex and delicate organization of the organ of Corti with a precise mix of cell types in a specific topology (Kopecky and Fritzsch, 2011; Pan et al., 2012b). While initial results to generate hair cells in vitro are promising (Oshima et al., 2010), the ability to treat patients through replacement of damaged hair cells must be considered a secondary option behind hair cell loss prevention. In short, individuals who are at risk for hair cell loss may be given transient therapeutic intervention through enhancing the inner ear hair cells’ natural ability to guard against insult and age to reduce hearing loss. This requires an understanding of the molecular basis of hair cell development and of late-onset hair cell loss.
During normal hair cell development, neurosensory cell precursors undergo proliferation to increase the total number of cells, during which time these precursor cells are resistant to differentiation. As levels of proto-oncogenes decrease and differentiation transcription factors (TFs) increase, quiescent neurosensory cell precursors are capable of differentiating into either sensory neurons, sensory hair cells, or supporting cells. This balance of initial proliferation and subsequent differentiation consists of multiple feedback loops; including the interactions between the basic Helix-Loop-Helix (bHLH) Myc proto-oncogene family and the bHLH differentiation TFs NeuroD1 and Atoh1, necessary for the formation of neurons and hair cells, respectively (Jahan et al., 2010; Jones et al., 2006; Pan et al., 2012a). After the initiation of hair cell differentiation by Atoh1 (Bermingham et al., 1999), a cascade of transcription factors promote long-term survival of the organ of Corti, including Barhl1, Gfi1, and Pou4f3, with the loss of each of these resulting in progressive hair cell death (Chellappa et al., 2008; Li et al., 2002; Pauley et al., 2008; Wallis et al., 2003). Manipulation of these, or a subset of these, genes coupled with other yet-to-be defined genes may play a future therapeutic role in protecting hair cells. To this end, our recent data on Pax2-Cre N-Myc single conditional knockout (CKO) mice suggests a previously unexplored importance of the proto-oncogene N-Myc on long-term hair cell maintenance (Dominguez-Frutos et al., 2011; Kopecky et al., 2011; Kopecky et al., 2012a). Pax2-Cre N-Myc CKO mice have an initial formation of both cochlear and vestibular hair cells with subsequent loss of cochlear hair cells (Kopecky, et al., 2011) beginning around postnatal day 21 (P21) and complete loss of cochlear hair cells by nine months of age (Kopecky et al., 2012a). However, vestibular hair cells remained until at least nine months of age.
Myc plays many roles in the body, but its main role historically is proliferation control as it functions as a proto-oncogene (Eisenman, 2001; Hatton et al., 2006; Knoepfler et al., 2002; Knoepfler et al., 2006; Young et al., 2011; Zindy et al., 2006). In the ear, only N-Myc and L-Myc, not C-Myc, are present (Dominguez-Frutos et al., 2011; Kopecky et al., 2011; Romand et al., 1994) such that the balance of proliferation and differentiation in the inner ear is, in part, controlled by the N-Myc and L-Myc nodes. We hypothesized that the loss of hair cells in the Pax2-Cre N-Myc CKO cochlea was due to either formation of inherently abnormal, and therefore unstable, hair cells (delayed-effect) or that N-Myc was responsible for the continued maintenance of hair cells (late-effect). To distinguish between these alternative hypotheses, we needed to first consider the potential late-effect of the Mycs in hair cells prior to exploration of the possibility of long-term instability of hair cell development (delayed-effect). In this paper, we explore the potential role of the Myc family in long-term hair cell maintenance.
In support of the ‘late-effect’ argument, we previously reported co-expression of both N-Myc and L-Myc in hair cells at P0 in the wild-type (WT) C57BL/6J mice (Kopecky et al., 2011), well after proliferation in the inner ear ends (Matei et al., 2005), indicating the potential for a secondary, non-proliferative role of N-Myc and L-Myc in differentiated hair cells, consistent with Myc’s other non-proliferative functions in the body, including roles in cell metabolism and cell death (Conacci-Sorrell and Eisenman, 2011; Dang, 2010; Sloan and Ayer, 2010). It is therefore possible that the secondary upregulation of N-Myc and L-Myc, specifically in the hair cells, is needed to avoid the belated death of cochlear hair cells that occurs when N-Myc is deleted earlier in development as in our Pax2-Cre N-Myc mice which deleted N-Myc around embryonic day 8.5, shortly after formation of the otic placode.
Our working hypothesis is that N-Myc and L-Myc act in partial redundancy to provide long-term maintenance to cochlear hair cells separate from their early roles in proliferation and in the absence of the two ear expressed Mycs, specifically in differentiated hair cells, hair cells will undergo cell death similar to that seen in the Pax2-Cre N-Myc CKO mice. To test this hypothesis, we generated a series of single CKOs and double CKOs that deleted N-Myc, L-Myc, or both after initial hair cell formation using Atoh1-Cre (Matei et al., 2005; Pan et al., 2012a). We restricted our investigation to organ of Corti hair cell development, organ of Corti organization, and cochlear hair cell functionality with auditory brainstem response (ABR) at P21 and four months of age and tested the P21 dCKO mice using ABR three days after the injection of cisplatin. We also assessed gravito-inertial vestibular functionality using the Noldus Catwalk System. While we tested all genetic variants, we report the analysis of one line, Atoh1-Cre N-Myc f/f L-Myc f/f (referred to as dCKO), since it should have the most severe phenotype.
2. Results
2.1 Normal Appearing Hair Cells are Retained Despite Delayed Absence of N-Myc and L-Myc
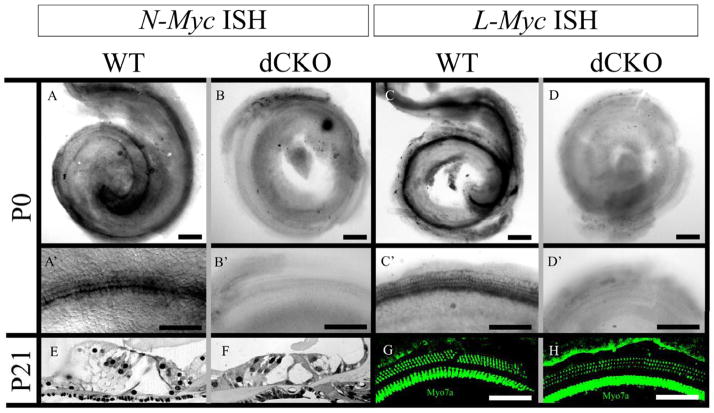
Both N-Myc and L-Myc were present in control littermates at P0 in the cochlea as revealed with whole mount in situ hybridizations (Figure 1A and 1C). Magnification of whole mounted cochleae showed the presence of N-Myc and L-Myc specifically in the hair cells (Figures 1A, A′ and 1C, C′). In the dCKO, neither N-Myc nor L-Myc was seen in the cochlea (Figure 1B and 1D) or in the cochlear hair cells (Figure 1B′ and 1D′). Despite the absence of both Mycs at P0, a normal organ of Corti was seen at three weeks of age in both WT and dCKO mice with one row of inner hair cells, three rows of outer hair cells, surrounded by supporting cells, shown by epoxy resin sections of the middle turn of the cochlea (Figure 1E and 1F). There also was no apparent difference of myosin VIIa immunohistochemistry between WT and dCKO mice at P21, also shown at the middle turn of the cochlea (Figure 1G and 1H).
Figure 1. The complete loss of N-Myc and L-Myc in inner ear hair cells does not affect overall organ of Corti organization or hair cell cytoarchitecture.
N-Myc (A) and L-Myc (C) are expressed in the cochlea after birth. Their expression is prominent in cochlear hair cells (A′ and C′). Using Atoh1-Cre, N-Myc (B) and L-Myc (D) were completely removed from inner ear hair cells, specifically noted is their absence in the organ of Corti (B′ and D′). However, this absence had no noticeable effect on overall architecture of the organ of Corti as three rows of outer hair cells and one row of inner hair cells were present throughout the cochlea along with the proper arrangement of supporting cells seen in epoxy resin sections of WT (E) and dCKO (F) mice as well as with myosin VIIa immunohistochemistry of WT (G) and dCKOs (H), shown near the middle turn. Scale bar = 100μm.
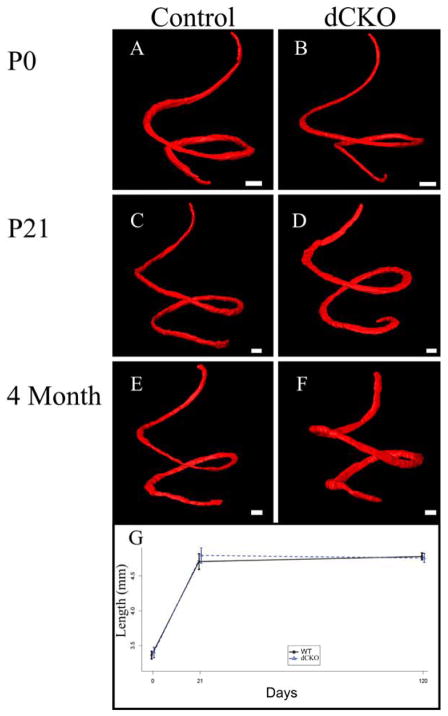
To fully analyze whether there was any notable hair cell loss or any abnormalities with cochlear development, we performed myosin VIIa immunohistochemistry on four P0 WT and dCKO ears (Figure 2A and 2B), six P21 WT and dCKO ears (Figure 2C and 2D), and six four month old WT and dCKO ears (Figure 2E and 2F), imaged the cochleae with confocal microscopy, and subsequently performed manual segmentation followed by three dimensional reconstructions, as previously described (Kopecky et al., 2012b). Segmentation of the organ of Corti allowed for three dimensional comparison and length quantification between WT and dCKO mice at P0, P21, and four months of age. While there was substantial lengthening of the organ of Corti from P0 to P21 for both WTs (3.36+/−0.094mm vs. 4.71+/−0.27mm; p<0.001) and dCKOs (3.40+/−0.13mm vs. 4.79+/−0.27mm; p<0.001), there was no significant change between P21 and four months of age for WTs (4.71+/−0.27mm vs. 4.78+/−0.062mm; p=0.558) and dCKOs (4.79+/−0.27mm vs. 4.76+/−0.17mm; p=0.779) (Figure 2G). These WT length measurements were consistent with previously published data (Kopecky et al., 2011; Kopecky et al., 2012a; Pan et al., 2011). Furthermore, equivalence testing confirmed that P0 WT and P0 dCKO mice (df=26) were identical and P21 WT and P21 dCKO mice (df=26) as well as four month WT and four month dCKO mice (df=26) were the same (Figure 3A). Our data suggest that the loss of both N-Myc and L-Myc in differentiated hair cells had no effect on the length of the organ of Corti (Figure 2) or its overall development (Figure 1); this contrasted with the disorganization and loss of hair cells as well as the reduction in length of the organ of Corti in the Pax2-Cre N-Myc CKO mice at P21 as previously reported (Dominguez-Frutos et al., 2011; Kopecky et al., 2011; Kopecky et al., 2012a). Figs 2 and 3
Figure 2. The loss of N-Myc and L-Myc does not change the normal morphologic development of the organ of Corti.
Myosin VIIa positive hair cells and rhodamine stained organ of Corti nuclei were segmented at P0 in WT (A) and dCKO mice (B), P21 (C and D) mice, and four months of age (E and F) mice. Segmented hair cells were three dimensionally reconstructed to provide an accurate measurement of the length of the organ of Corti at these three time points. From P0 to four months of age, the organ of Corti lengthened (G); however, this increase in length was similar between WT and dCKO mice. Scale bar = 100μm.
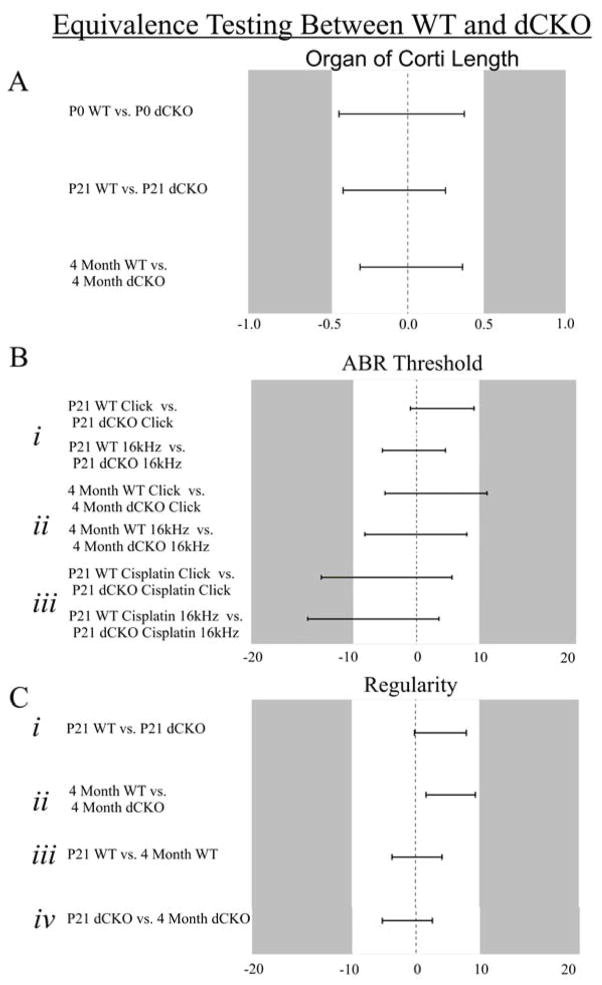
Figure 3. Equivalence testing confirms equality (or near equality) between groups for all parameters tested between WT and dCKO mice.
Equivalence testing allowed for the identification of similar groups and was based on the estimated means and the confidence interval (CI) for the difference in means. An absolute difference of Δ was determined such that a difference less than Δ essentially coincided with equality of means. Any pair of means with a CI that was completely contained in −Δ to +Δ was considered equivalent. The Δ values for organ of Corti length, ABR, and regularity, were determined to be 0.5mm, 10dB, and 10%, respectively. P0 WT and P0 dCKO, P21 WT and P21 dCKO, and four month WT and four month dCKO organ of Corti lengths were equivalent (A). At P21, click and 16 kHz ABR responses were equivalent between WT and dCKO (Bi). At four months of age, click ABR responses were nearly equivalent while 16 kHz ABR responses were equivalent (Bii). After cisplatin treatment, neither click nor 16 kHz ABR responses were equivalent at the 10dB threshold; however, were equivalent at the 15dB threshold (Biii). All regularity measurements: P21 WT vs. P21 dCKO (Ci), four month WT vs. four month dCKO (Cii), P21 WT vs. four month WT (Ciii), and P21 dCKO vs. four month dCKO (Civ), were equivalent.
1.12.2 Presence of Hair Cells at P21 and Four Months of Age Corresponds to Normal Auditory Functioning in dCKO Mice
While the cochlear hair cells had no noticeable histological (Figure 1) nor morphological (Figure 2) defects in the absence of N-Myc and L-Myc, it could be possible that their normal functionality was nevertheless compromised. We tested cochlear hair cell functionality using auditory brainstem response (ABR) and vestibular hair cell functionality using the Noldus Catwalk System, using established protocols (Kopecky et al., 2012a). We tested over 220 mice and all nine allelic variations of our Atoh1-Cre mutants for ABR at both P21 and four months of age. Our initial objective in generating the nine genotypes was to assess the relative contribution and possible redundancy of the two ear expressed Mycs. However, throughout our data analysis, there were no differences noted between the WT, single CKOs, and dCKO mice; thus, our results and discussion present data only for the WT and dCKO mice. All of the data can be directly viewed in the tables to assess the various CKOs.
At P21, WT littermates had a click ABR threshold response of 37.82+/−0.63dB and dCKOs had a similar threshold response of 41.91+/−1.09dB. We also tested tone pip threshold responses at 2 kHz, 4 kHz, 8 kHz, 12 kHz, 16 kHz, 18 kHz, 20 kHz, 24 kHz, and 28 kHz. All mice had a standard tone response curve with 16 kHz representing the most sensitive response (Figure 4A, Table 1). We tested P21 WT and dCKO mice at both the click (df=1330) response as well as the 16kHz (df=1330) response for equivalence and confirmed that both groups were identical (Figure 3Bi).
Figure 4. ABR threshold between WT and dCKO mice is the same at both P21 and four months of age.
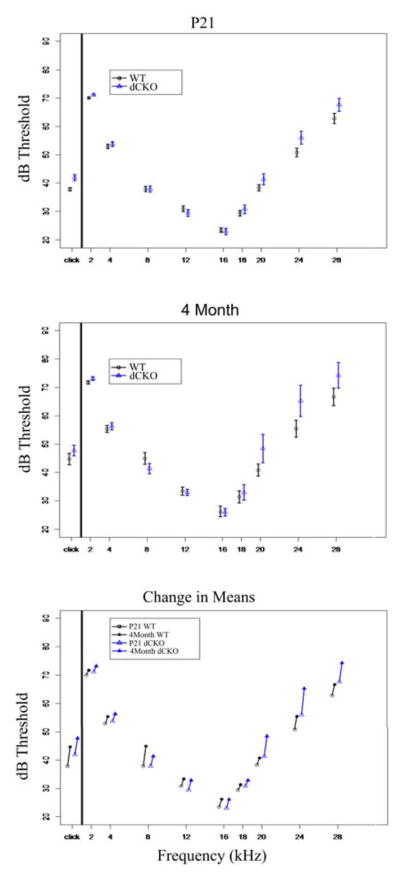
Mice were tested using both click (broadband noise) and tone pip (frequency specific) ABR. At P21 (A), WT mice (circles) and dCKO mice (triangles), there were no noticeable differences between the two groups and both groups had their most sensitive hearing at 16 kHz. At four months of age (B), both groups performed worse than P21 mice, and both groups were equivalent or near equivalent at both the click response and 16 kHz. When assessing the difference between the means of P21 and four month WT and dCKO (C), there was a significant increase in threshold for both groups at click; however, both groups had nearly identical threshold shifts.
Table 1.
P21 ABR
| Genotype | #Animals | Click | 2kHz | 4kHz | 8kHz | 12kHz | 16kHz | 18kHz | 20kHz | 24kHz | 28kHz |
|---|---|---|---|---|---|---|---|---|---|---|---|
| WT | 50 | 37.82+/−0.63 | 70.14+/−0.32 | 52.92+/−0.78 | 37.88+/−0.96 | 30.9+/−0.97 | 23.48+/−0.82 | 29.45+/−0.94 | 38.31+/−1.09 | 50.82+/−1.57 | 62.86+/−1.80 |
| Atoh1-Cre N-Myc f/f L-Myc f/f (dCKO) | 34 | 41.91+/−1.09 | 71.24+/−0.36 | 53.76+/−0.82 | 37.88+/−1.08 | 29.5+/−1.11 | 23.05+/−1.13 | 30.82+/−1.44 | 41.32+/−1.93 | 56.00+/−2.30 | 67.71+/−2.35 |
| Atoh1-Cre N-Myc +/+ L-Myc f/+ | 3 | 45.00+/−6.24 | 72.00+/−2.00 | 49.00+/−3.00 | 34.00+/−3.00 | 30.00+/−3.61 | 24.00+/−5.29 | 33.00+/−8.19 | 41.00+/−8.89 | 54.00+/−7.81 | 62.00+/−8.72 |
| Atoh1-Cre N-Myc +/+ L-Myc f/f | 5 | 42.60+/−4.39 | 70.60+/−1.75 | 56.20+/−3.50 | 41.80+/−3.09 | 33.40+/−3.60 | 28.00+/−3.15 | 35.20+/−4.41 | 44.20+/−6.26 | 58.60+/−4.49 | 68.20+/−5.58 |
| Atoh1-Cre N-Myc f/+ L-Myc f/f | 19 | 37.26+/−1.32 | 68.89+/−0.47 | 52.32+/−1.45 | 38.42+/−1.28 | 30.05+/−0.86 | 22.63+/−0.91 | 26.74+/−1.06 | 36.68+/−1.70 | 47.74+/−1.95 | 58.47+/−2.35 |
| Atoh1-Cre N-Myc f/f L-Myc f/+ | 23 | 43.78+/−1.51 | 71.61+/−0.63 | 54.91+/−1.05 | 40.13+/−1.12 | 32.09+/−1.23 | 25.43+/−1.42 | 33.00+/−1.69 | 43.43+/−2.00 | 57.91+/−2.27 | 68.57+/−2.36 |
| Atoh1-Cre N-Myc f/f L-Myc +/+ | 4 | 41.25+/−2.25 | 70.75+/−0.75 | 54.25+/−3.33 | 40.75+/−2.56 | 32.5+/−2.60 | 25.75+/−4.80 | 35.50+/−5.12 | 46.00+/−5.05 | 55.75+/−9.20 | 64.75+/−9.28 |
| Atoh1-Cre N-Myc f/+ L-Myc f/+ | 2 | 37.50+/−1.50 | 70.00+/−0.00 | 44.50+/−1.50 | 31.00+/−3.00 | 23.50+/−1.50 | 20.50+/−4.50 | 29.50+/−7.50 | 38.50+/−10.50 | 58.00+/−15.00 | 65.50+/−16.50 |
At four months of age, WT littermates had a click ABR threshold response of 44.66+/−1.99dB and dCKOs had a similar threshold response of 47.73+/−1.87dB. We also tested tone pip threshold responses at 2 kHz, 4 kHz, 8 kHz, 12 kHz, 16 kHz, 18 kHz, 20 kHz, 24 kHz, and 28 kHz. All mice had a standard tone response curve with 16 kHz representing the most sensitive response (Figure 4B, Table 2). Equivalence testing confirmed that at 16 kHz four month WT and four month dCKO mice were identical (df=1330) and at the click response the two groups were nearly identical (df=1330) (Figure 3Bii).
Table 2.
Four Month ABR
| Genotype | #Animals | Click | 2kHz | 4kHz | 8kHz | 12kHz | 16kHz | 18kHz | 20kHz | 24kHz | 28kHz |
|---|---|---|---|---|---|---|---|---|---|---|---|
| WT | 27 | 44.66+/−1.99 | 71.84+/−0.53 | 55.44+/−1.26 | 44.93+/−2.04 | 33.37+/−1.45 | 26.22+/−1.80 | 31.33+/−2.09 | 40.78+/−2.14 | 55.44+/−2.99 | 66.75+/−3.08 |
| Atoh1-Cre N-Myc f/f L-Myc f/f (dCKO) | 11 | 47.73+/−1.87 | 73.27+/−0.63 | 56.36+/−1.30 | 41.36+/−1.83 | 32.91+/−1.09 | 26.09+/−1.30 | 32.09+/−2.69 | 48.45+/−4.98 | 65.36+/−5.52 | 74.36+/−4.57 |
| Atoh1-Cre N-Myc +/+ L-Myc f/+ | 3 | 45.00+/−3.46 | 72.00+/−1.00 | 55.00+/−1.73 | 41.00+/−2.00 | 33.00+/−4.00 | 26.00+/−2.00 | 30.00+/−1.00 | 38.00+/−2.65 | 52.00+/−4.58 | 74.00+/−5.00 |
| Atoh1-Cre N-Myc +/+ L-Myc f/f | 6 | 41.00+/−3.44 | 70.50+/−0.92 | 55.00+/−1.55 | 38.50+/−1.69 | 30.00+/−2.28 | 22.50+/−2.84 | 32.50+/−3.17 | 52.00+/−3.71 | 66.00+/−4.36 | 74.50+/−3.85 |
| Atoh1-Cre N-Myc f/+ L-Myc f/f | 8 | 46.13+/−1.69 | 72.25+/−0.49 | 55.75+/−1.36 | 38.88+/−1.88 | 28.75+/−1.36 | 22.75+/−1.58 | 31.75+/−2.52 | 47.88+/−4.81 | 63.63+/−4.99 | 72.25+/−4.97 |
| Atoh1-Cre N-Myc f/f L-Myc f/+ | 14 | 50.57+/−1.66 | 73.86+/−0.58 | 58.64+/−1.30 | 46.86+/−1.76 | 35.29+/−1.43 | 30.36+/−1.38 | 38.29+/−2.19 | 52.64+/−3.62 | 65.50+/−4.05 | 76.43+/−3.08 |
| Atoh1-Cre N-Myc f/f L-Myc +/+ | 5 | 45.60+/−3.47 | 73.00+/−1.90 | 59.20+/−3.87 | 43.60+/−2.40 | 34.00+/−4.14 | 27.40+/−4.18 | 31.00+/−4.45 | 40.00+/−5.45 | 52.00+/−8.27 | 61.00+/−8.49 |
| Atoh1-Cre N-Myc f/+ L-Myc f/+ | 5 | 42.00+/−2.32 | 71.20+/−0.73 | 50.80+/−1.53 | 37.00+/−1.90 | 28.00+/−1.90 | 22.60+/−2.20 | 28.00+/−2.32 | 43.00+/−4.35 | 60.40+/−4.49 | 73.60+/−4.39 |
There was an observed increase in threshold between P21 and four months of age for both the WT littermates and dCKO mice across all frequencies (Figure 4C). We tested for a time effect at click and 16kHz and found significance for WT (t(75)=4.0590, p=0.0005) and dCKO (t(43)=2.6594, p=0.0438) mice at the click frequency, but as stated above, WT and dCKO mice were found to be nearly identical at the 16kHz frequency for each respective time point (i.e. both groups hearing statistically worsened from P21 to four months, but the hearing worsened equally between the two groups).
1.22.3 Presence of Hair Cells at P21 and Four Months of Age Corresponds to Normal Vestibular Functioning in dCKO Mice
We next wanted to determine if there was any functional defect in vestibular hair cells, which are also known to be positive for both N-Myc and L-Myc (Kopecky et al., 2011) and whose function relies on proper N-Myc and was compromised by its absence previously described in the Pax2-Cre N-Myc CKO mice (Kopecky et al., 2012a). No defects were noted at any age in the vestibular epithelia.
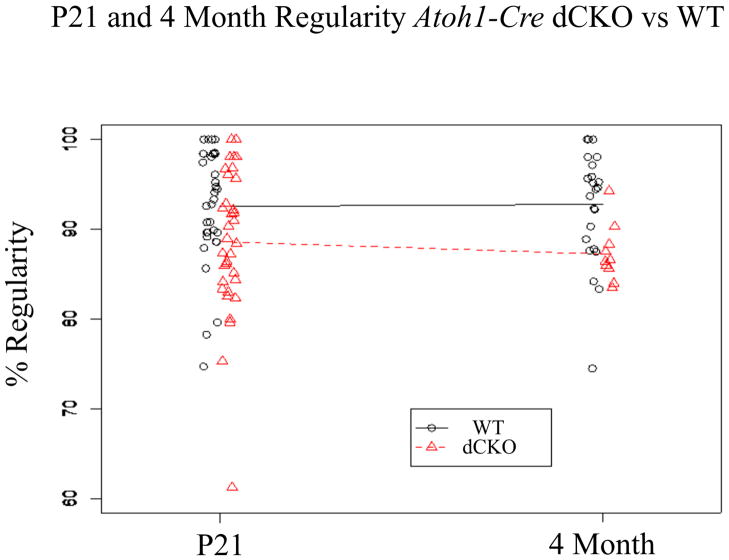
At P21, WT littermates had a gait regularity of 92.54+/−1.12% and dCKOs had a gait regularity of 88.61+/−1.38% (Figure 5, Table 3). At four months of age, WT littermates had a gait regularity of 92.77+/−1.29% and dCKOs had a gait regularity of 87.24+/−1.00% (Figure 5, Table 3). Again, equivalence testing confirmed that at both P21 (df= 62.75, Figure 3Ci) and at four months of age (df=30.66, Figure 3Cii), the WT and dCKO groups showed no difference. Furthermore, there was no time effect for either the WT (df= 50.18, Figure 3Ciii) or dCKO (df= 38.09, Figure 3Civ) mice (i.e. there was no change in performance over time), consistent with previously published data (Kopecky et al., 2012a).
Figure 5. Regularity measurements suggest that dCKO mice have normal vestibular functioning and do not suffer from ataxic gait.
The Noldus Catwalk System measured a number of gait parameters, one of which was regularity. Regularity was used to assess overall coordination of the mouse such that as coordination declined, so did percent regularity. We have previously shown that Pax2-Cre N-Myc CKO mice suffer from ataxic gait, suggested by low regularity that did not improve over time. Here, we saw that both WT and dCKO mice performed with high regularity at both P21 and four months of age and that the regularities were equivalent. All test points were plotted for both WT and dCKO with a line connecting the two means showing the change in means from P21 to four months of age. Equivalence testing confirmed the similarity of the groups at both time points and across time.
Table 3.
P21 vs. Four Month Regularity
| P21 | 4 month | ||||
|---|---|---|---|---|---|
| Genotype | #Animals | % Regularity | #Animals | % Regularity | Change |
| WT | 33 | 92.54+/−1.12 | 24 | 92.77+/−1.29 | 0.23 |
| Atoh1-Cre N-Myc f/f L-Myc f/f (dCKO) | 34 | 88.61+/−1.38 | 10 | 87.24+/−1.00 | −1.37 |
| Atoh1-Cre N-Myc +/+ L-Myc f/+ | 3 | 96.08+/−1.66 | 3 | 90.75+/−0.89 | −5.33 |
| Atoh1-Cre N-Myc +/+ L-Myc f/f | 5 | 92.53+/−3.58 | 3 | 91.74+/−3.01 | −0.79 |
| Atoh1-Cre N-Myc f/+ L-Myc f/f | 15 | 90.06+/−1.27 | 8 | 90.75+/−1.81 | 0.69 |
| Atoh1-Cre N-Myc f/f L-Myc f/+ | 16 | 91.70+/−1.72 | 9 | 87.42+/−1.46 | −4.28 |
| Atoh1-Cre N-Myc f/f L-Myc +/+ | 2 | 89.51+/−8.53 | 1 | 100.00+/−0.00 | 10.49 |
2.4 Treatment with the Ototoxic Drug Cisplatin Affects WT and dCKO Mice Equally
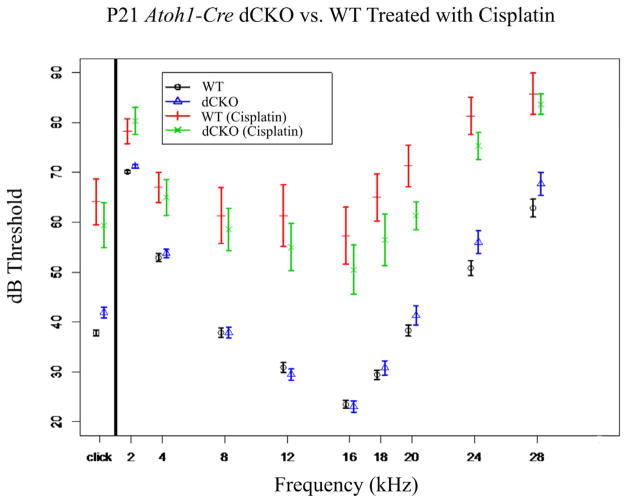
While the absence of N-Myc and L-Myc in hair cells did not lead to any noticeable histological (Figure 1), morphological (Figure 2), or behavioral abnormalities (Figures 4 and 5) when deleted after hair cell formation at either P21 or four months of age, confirmed by equivalence tests (Figure 3), it is possible that either the time lapse was not long enough or that N-Myc and L-Myc simply act to protect against certain metabolic insults, rather than play a role in age related cochlear hair cell defects. If N-Myc and L-Myc play a role in hair cell maintenance, it might be possible that they are important in cell metabolism, and given the Mycs’ nearly ubiquitous roles in cancer, alter the hair cell’s susceptibility to the anti-neoplastic drug cisplatin. If this were the case, we would expect a difference in the threshold shift between WT mice and dCKO mice treated with cisplatin. WT mice treated with 20 mg/g mouse cisplatin had a click ABR threshold of 64.13+/−4.60dB (untreated 37.82+/−0.63dB) which represented a hearing loss threshold shift of approximately 25 dBs (Figure 6). The dCKOs treated with the same dose of cisplatin had a click ABR threshold of 59.40+/−4.49dB (untreated 41.9+/−1.09dB), which represented a similar hearing loss threshold shift of nearly 20dB (Table 4). The same relationships held true at the various frequencies tested (Figure 6). Both groups of mice experienced significant losses of hearing due to cisplatin treatment, indicating that the deafening approach we took was effective; however, there was only minimal difference between the two groups and the groups were shown to be nearly equivalent (df=1330, Figure 3Biii).
Figure 6. Cisplatin severely decreases hearing but does not disproportionally affect dCKO mice.
Ototoxic drugs, such as cisplatin are well-known to cause hearing loss, shown by large ABR threshold shifts. Upon administration of cisplatin to WT mice, a large threshold shift was seen compared to untreated mice (compare vertical red line to black circle). A nearly equal threshold shift was observed when comparing treated and untreated dCKO mice (compare green “x” with blue triangle) suggesting that while both groups of mice were negatively affected by the cisplatin treatment, the hearing loss was comparable between the two groups.
Table 4.
P21 cisplatin treated mice
| Genotype | #Animals | Click | 2kHz | 4kHz | 8kHz | 12kHz | 16kHz | 18kHz | 20kHz | 24kHz | 28kHz |
|---|---|---|---|---|---|---|---|---|---|---|---|
| WT | 9 | 64.13+/−4.60 | 78.25+/−2.45 | 67.00+/−3.00 | 61.33+/−5.60 | 61.33+/−6.21 | 57.33+/−5.70 | 65.00+/−4.74 | 71.33+/−4.16 | 81.33+/−3.77 | 85.75+/−4.16 |
| Difference Untreated vs. Treated | 26.31 | 8.11 | 14.08 | 23.45 | 30.43 | 33.85 | 35.55 | 33.02 | 30.51 | 22.89 | |
| Atoh1-Cre N-Myc f/f L-Myc f/f (dCKO) | 10 | 59.40+/−4.49 | 80.33+/−2.74 | 65.00+/−3.61 | 58.6+/−4.21 | 55.00+/−4.75 | 50.50+/−4.90 | 56.50+/−5.12 | 61.33+/−2.80 | 75.33+/−2.73 | 83.67+/−2.07 |
| Difference Untreated vs. Treated | 17.49 | 9.09 | 11.24 | 20.72 | 25.5 | 27.45 | 25.68 | 20.01 | 19.33 | 15.96 |
2.5 Summary
The delayed loss of N-Myc and L-Myc only in hair cells using a delayed-onset hair cell specific Cre line did not affect the development of the organ of Corti. In addition, the long-term retention of hair cells in dCKO mice was essentially equivalent to WTs. The functionality of the cochlear and vestibular hair cells in all Atoh1-Cre mutants seemed to be uncompromised as assessed with both ABR and the Noldus Catwalk System, respectively, at P21 and four months of age as statistical tests confirmed equivalence. Additionally, damaging the cochlear hair cells with ototoxic doses of cisplatin resulted in nearly equally large threshold shifts for both WT and dCKO mice. Overall, these data indicate that the function of expression of the Myc genes in differentiated hair cells, if any, remains unclear and is certainly not the reason why hair cells disappear in the cochlea of postnatal Pax2-Cre N-Myc CKO mice (Kopecky et al., 2011; Kopecky et al., 2012a), thus indicating that the early effect of N-Myc and L-Myc is much more profound than the late-onset effects of the Mycs.
3. Discussion
N-Myc is necessary for the normal development of the inner ear (Dominguez-Frutos et al., 2011; Kopecky et al., 2011; Kopecky et al., 2012a), and along with its family member L-Myc, both were candidates for hair cell loss prevention as mature cochlear hair cells are lost after embryonic deletion of N-Myc and both genes are co-expressed selectively in these hair cells (Kopecky et al., 2011). We further hypothesized that the Mycs regulate hair cell maintenance through their roles in metabolic pathways (Eilers and Eisenman, 2008; Nilsson et al., 2012), especially through LDH (Cairo et al., 2005; Omata et al., 1978). Given the high metabolic needs of the cochlea (Dallos, 1992; Spector and Carr, 1979) and its sensitivity to metabolic changes (Wangemann et al., 2007), the loss of metabolic homeostasis through Myc regulation may predispose hair cells to damage upon increase in metabolic stresses, which occurs upon the onset of hearing (correlating with the loss of hair cells in the Pax2-Cre N-Myc CKO mice (Kopecky et al., 2011)) or after the administration of an ototoxic drug. The Mycs regulate both LDH (which is responsible for the Warburg effect seen in cancer) and p53, which are deregulated in cancer (Chen et al., 2010; Slack et al., 2007; Torres et al., 2010) and are two proteins that may regulate pathways involved in cisplatin ototoxicity of the ear (Rybak, 2007; Rybak and Ramkumar, 2007; Rybak et al., 2007). If N-Myc and L-Myc do indeed play a metabolic role in differentiated hair cells, then the loss of N-Myc and L-Myc would result in hair cells being more susceptible to cisplatin (D’Aguanno et al., 2011; Michaelis et al., 2008; Paffhausen et al., 2007), providing an important link to not only explaining the function of the late expression of the Myc’s, but also one pathway to ameliorate cisplatin damage. Mycs also play a direct role in DNA replication, a function that the alkylating drug cisplatin inhibits. Other ototoxic drugs such as kanamycin would not directly test whether altered levels of the proto-oncogene Myc would alter susceptibility to anti-neoplastic regimens.
By knocking out these genes, separately and together, specifically in hair cells, we directly tested whether N-Myc and L-Myc play a role in hair cell survival. Our data on all the single CKOs, single CKO with the other Myc heterozygous (i.e. Atoh1-Cre N-Myc f/f L-Myc f/+), and dCKO mice confirmed similarity between all groups, thus suggesting that the absence of N-Myc and/or L-Myc in the inner ear hair cells has no profound effect on long-term survival of hair cells or hair cell resistance to ototoxic mechanisms. Despite these findings, N-Myc and L-Myc still could play an indirect role in maintenance mechanisms as both these genes play important roles in a number of pathways in the cell. Furthermore, assessment of other cell maintenance genes should be performed to rule out compensatory mechanisms in the absence of the two ear expressed Mycs. Additionally, testing hair cell loss through chemical or noise induced deafening protocols, in Myc overexpressing systems may yield additional insight into Myc maintenance mechanisms and should be performed prior to completely ruling out N-Myc and L-Myc as potential preventative options.
While our data on loss-of-function of these genes in mature hair cells does not indicate a measurable effect, these data provide clarity to the cause of the progressive hair cell loss seen in previously published Pax2-Cre N-Myc CKO mice (Dominguez-Frutos et al., 2011; Kopecky et al., 2011; Kopecky et al., 2012a). Assuming that we can rule out a major function of the Myc genes in differentiated hair cells, the delayed loss of cochlear hair cells in the Pax2-Cre N-Myc CKO must result from the early signaling of the Myc genes, likely during the tightly regulated timing of the cell cycle exit and transition to hair cell differentiation resulting in “unhealthy” hair cells that apparently die when the auditory system matures around postnatal day 16 in mice. Further assessment using tamoxifen-inducible Cre lines must be performed to better define the stage in a hair cell’s development during which the early effects on N-Myc and L-Myc absence elicits this late hair cell loss (i.e. this effect must occur after Pax2 (~E8.5) expression but prior to Atoh1 (~E14.5) expression). Defining more precisely this stage will help clarify how the Myc genes affect cell cycle exit and the expression of cell differentiation genes, such as Atoh1 and Neurod1.
Our data demonstrates that it is the yet-to-be molecularly defined early effect of the Myc genes that set the stage for the demise of fully differentiated hair cells three weeks later. To our knowledge, this is the first study to show such a delayed loss of cochlear hair cells following an early embryonic insult. Nevertheless, it is only one extreme of a continuum of function of various genes in the differentiating hair cells that results in delayed loss after near normal differentiation such as delayed losses of Atoh1 (Pan et al., 2012a), Barhl1 (Chellappa et al., 2008; Li et al., 2002), and Pou4f3 (Pauley et al., 2008). Combined, these data suggest that hearing loss in the elderly could possibly be correlated with insults to the ear dramatically earlier than their onset of demise, and much earlier than previously thought. Essentially, our data opens up the possibility that identification of such early-onset effects followed by much later demise of hair cells could provide a novel avenue for targeted intervention if understood at the molecular level. How much deregulation of Myc genes during the critical transition from proliferation to differentiation plays remains to be determined as one possible cause for such a late-onset.
4. Experimental Procedures
4.1 Mice and genotyping
We crossed Atoh1-Cre (tgAtoh1creFri) mice (Matei et al., 2005) with N-Myc f/f mice (Jackson Labs B6.129-N-Myctm1Psk/J) and L-Myc f/f mice (donated by Dr. Eisenman) to generate the following mouse lines:
WT (absence of Cre)
Atoh1-Cre N-Myc f/+ (N-Myc conditional heterozygote (het))
Atoh1-Cre N-Myc f/f (N-Myc single conditional knockout (CKO))
Atoh1-Cre L-Myc f/+ (L-Myc het)
Atoh1-Cre L-Myc f/f (L-Myc CKO)
Atoh1-Cre N-Myc f/f L-Myc f/+ (N-Myc CKO with L-Myc het)
Atoh1-Cre N-Myc f/+ L-Myc f/f (L-Myc CKO with N-Myc het)
Atoh1-Cre N-Myc f/+ L-Myc f/+ (N-Myc het and L-Myc het)
Atoh1-Cre N-Myc f/f L-Myc f/f (N-Myc and L-Myc double CKO (dCKO))
Tail biopsies were used for genomic DNA and polymerase chain reaction for genotyping was performed using the following primers (N-Myc: IMR6727 5′gtcgcgctagtaagagctgagatc 3′ IMR6729 5′ cacagctctggaaggtgggagaaagttgagcgtctcc 3′ Cre: 1 5′ cctgttttgcacgttcaccg 3′ 2 5′ atgcttctgtccgtttgccg 3′ IMR42 5′ ctaggccacagaattgaaagatct 3′ IMR43 5′ gtaggtggaaattctagcatcatcc 3′). Animal care and usage was in accordance with the University of Iowa Institutional Animal Care and Use Committee (IACUC) guidelines for the use of laboratory animals in biological research and approved (ACURF #0804066 and 1103057).
4.2 Noldus Catwalk
The Noldus Catwalk System consists of a walkway floor containing a sheet of glass encased with a light. P21 and four month old mice were placed in a corridor 60cm long by 10cm wide and allowed to freely move. A mounted camera captured a 40cm × 10cm field from below the walkway. As the mouse entered the field of view, a run was initiated and stored on a computer. If the mouse exited the field of view within 20 seconds and there was less than a 60% variation in speed, the run was compliant. If the mouse could not exit the field of view or the run was otherwise terminated, the run was considered non-compliant. Numerous compliant runs were acquired for each mouse and were termed a trial. Between each trial, the walkway barrier was removed and the glass was cleaned to remove any excrement or smell from the preceding trial using standard glass cleaner. All mice were tested under similar conditions. After each trial, the acquired data was classified. Classification is the semi-automated process of entering which limb (RH=Right Hind, LH= Left Hind, RF= Right Front, LF= Left Front) induced the recorded print.
4.3 Auditory Brainstem Response (ABR)
Mice were anesthetized with 0.025mL/g of the anesthetic Tribromoethanol (Avertin®) and after a surgical level of anesthesia was induced, needle electrodes were inserted subcutaneously in the vertex, slightly posterior to the pinna, and in the contralateral hind-limb. A loud speaker was placed 10cm from the pinna of the test ear and computer-generated clicks were given in an open field environment in a soundproofed chamber. Click stimuli, given in decreasing 3-dB increments, were presented and electrode responses were averaged across 512 presentations using Tucker-Davis Technologies System hardware running BioSig® Software. Recorded signals were bandpass filtered (300Hz–5kHz) and 60Hz notch filtered. Identical setup but a tone pip program was given at 2 kHz through 28 kHz incrementally with a stepwise decrease in amplitude at each frequency.
4.4 Perfusion
After ABR, mice were injected intraperitoneally (IP) with greater than a 0.025mL/g of anesthetic Avertin® and after ocular and pedal reflexes ceased, 4% paraformaldehyde (PFA) was pumped continuously with a 30-gauge needle into the left ventricle. The right ventricle was opened to facilitate clearing. After fixation, heads were hemi-dissected, placed in 4% PFA, and covered with parafilm for long-term storage in 4°C.
4.5 Plastic Sections
Dissected ears were incubated for two hours with 2.5% glutaraldehyde and washed with 0.1 M phosphate buffer three times over a period of two hours. Ears were reacted in 1% osmium tetroxide for approximately one hour and rinsed with 0.1 M phosphate buffer. Samples were dehydrated with an increasing graded ethanol (EtOH) series and then incubated in a 1:1 EtOH and propylene oxide mixture. Samples were mixed with Epon 812 and propylene oxide overnight and then oriented and embedded with Epon 812 and placed in an incubator for two days at 60°C. Ears were sectioned at 2μm with a Leica Ultratome and sections were retrieved from water bath and placed on heated glass slides in sequential order. They were allowed to bake onto the glass slide and stained for one minute with Stevenel’s Blue and imaged (Nichols et al., 2008). Stained slides were rinsed with dH20 and allowed to dry before imaging. Sections were coverslipped using DMX mounting solution and allowed to dry overnight. Samples were imaged with a Nikon Eclipse 800 microscope and captured with Image-Pro.
4.6 In Situ Hybridization
Fixed embryos were prepared to avoid RNAse activity. RNA riboprobes labeled with digoxigenin were N-Myc and L-Myc (made by Jason Pecka, Creighton University). The protocol was previously described (Pan et al., 2009; Pauley et al., 2006). Briefly, ears were dissected in 0.4% PFA, washed and digested with 10μg/mL Proteinase K (Ambion, Austin, Tex. USA). The samples were incubated with riboprobe in hybridization solution (50% formamide, 2X saline sodium citrate, and 6% dextran sulfate) overnight at 60°C. Samples were washed and incubated overnight with an anti-digoxigenin antibody (Roche Diagnostics, Mannheim, Germany). Unbound antibody was washed off with phosphate buffered saline (PBS) and samples were reacted with BM Purple substrate to cause a purple reaction product in places of bound mRNA. Samples were dissected, mounted, and imaged.
4.7 Immunohistochemistry
Ears were dissected in 0.4% PFA and defatted overnight in 70% EtOH. They were removed from EtOH and blocked for two hours in blocking solution (5% normal goat serum (NGS), 0.1% Triton X-100, in PBS). Myosin VIIa (Proteus Biosciences, 25-6790) was diluted in the blocking solution at a 1: 200 ratio (primary antibody: blocking solution) and incubated for three days at 4°C. Ears were washed with PBS and Alexa Fluor 633 anti-mouse secondary antibodies were diluted in blocking buffer (1:500) and incubated for two days, blocked from light, in 4°C. Ears were mounted on a slide in glycerol and images were captured with the Leica TCS SP5 multiphoton confocal microscope, as previously described (Pan et al., 2009; Pauley et al., 2006).
4.8 Three Dimensional Reconstruction
Tissue Preparation
Ears were decalcified in 10% ethylenediaminetetracetic acid (EDTA) for four days with fresh EDTA changed daily. Ears were rinsed with PBS at least three times with each wash lasting two hours. Ears were put into 70% EtOH overnight. Ears were reacted with myosin VIIa according to the immunohistochemistry protocol above. After removal of secondary antibody, ears were washed three times, two hours each with PBS. Ears were placed in 70% EtOH overnight. 70% EtOH was replaced with 90% EtOH for one hour and then two changes of 100% EtOH for two hours each. Ears that were not processed for immunohistochemistry but rather rhodamine isothiocyanate were put in a rhodamine EtOH mixture until tissue was very lightly stained. 100% EtOH or rhodamine EtOH mixtures were removed and clearing solution was added to 100% EtOH to obtain a 1:1 ratio. Clearing solution was made with five parts methyl salicylate to three parts benzyl benzoate (MSBB). The 1:1 100% EtOH to MSBB was replaced with 100% MSBB overnight at room temperature, in dark. Ears were then put in two changes of MSBB and imaged. Protocol adapted from previously described work (Kopecky et al., 2012b)
Confocal Imaging
Ears were placed on a glass slide and Dow Corning Grease was applied to the coverslip to make a retainer for MSBB. Ears were placed in the MSBB bath and spacers were added to ensure absence of compression stresses. Using a TCS SP5 confocal microscope, Z-stacks of 5μm increments were obtained.
Segmentation and Three Dimensional Renderings
Z-stacks were loaded into Amira Version 5.4. Either the organ of Corti (rhodamine stained) or myosin VIIa positive cells were segmented. Segmented images were resampled (2×2×1), surface generated (unconstrained), smoothed (20 iterations), and the surface was viewed. Length measurements were obtained through the three dimensional measurement functions within the Amira software.
4.9 Cisplatin Treatment
Cisplatin solution was made from Sigma cis-Diammineplatinum (II) dichloride (P4394) and dissolved in 5% dimethyl sulfoxide (DMSO) at room temperature on a shaker until fully dissolved. Cisplatin was made fresh each test date. We injected both WT and dCKO P18 pups with 20mg cisplatin/kg mouse IP. We injected non-treated littermates with same volume saline. Three days later, all mice were tested for ABR. Our protocol was adapted from that used in mice previously (Hill et al., 2008; Lee et al., 2004; Parham, 2011). Saline treated mice had identical ABR values to untreated animals and were pooled.
4.10 Statistical Analyses
Each test of equivalence (H0: μ1 ≠ μ2 vs. HA:μ1 = μ2) was performed at the α=0.05 level and then adjusted for multiple comparisons using the (1–2α) *100% confidence interval (CI) for the difference between means. Any pair of means with an adjusted CI being completely contained in the interval −Δ to +Δ was considered equivalent, where Δ is the largest allowable difference for two means to be considered equivalent. The Δ values used in the study for organ of Corti length, ABR, and regularity, were 0.5mm, 10dB, and 10%, respectively. The CIs for differences in means for organ of Corti length and ABR were constructed from a two-way ANOVA (genotype x time) and three-way ANOVA (genotype x time x frequency) model fit, respectively. The CIs for regularity were constructed using independent means with the Welch t distribution approximation to allow for non-constant variance across groups. The degrees of freedom (df) associated with each equivalence test is provided. A Bonferroni adjustment for multiple comparisons was applied within each of the three respective analyses.
Differences in organ of Corti length between P0 and P21 and ABR between P21 and four months for WT and dCKO were assessed within genotype using independent t tests and adjusted for multiple comparisons using the Bonferroni adjustment. All tests were performed at the p ≤ 0.05 level.
Highlights.
Proto-oncogenes N-Myc and L-Myc are strongly expressed in organ of Corti hair cells
Late deletion of the Mycs after hair cell differentiation shows no loss of hair cells
Cisplatin application does not disproportionately affect mutant hair cells
Loss of hair cells in Pax2-Cre N-Myc mice is likely due to defect in proliferation
N-Myc and L-Myc are unlikely to play a role in therapeutic prevention of hearing loss
Acknowledgments
We thank Jackson Labs for providing the N-Myc f/f mice and Dr. Knoepfler for creating them. We thank Dr. Eisenman for providing the L-Myc f/f mice. We would also like to thank Dr. Matei for the Atoh1-Cre mice. We thank Jason Pecka for producing the N-Myc and L-Myc riboprobes. We thank Dr. Thomas Schimmang for helpful discussion with the Pax2-Cre N-Myc mice. The Leica TCS SP5 confocal microscope was purchased in part with a grant from the Roy J. Carver foundation. Grant funding for Bernd Fritzsch was provided through the NIH and NIDCD RO1-DC055095590. Funding for Benjamin Kopecky was provided by the ICTS TL1 CTSA UL1RR024979 grant. We thank the NIH P30 grant (DC010362) for making the ABR equipment and the Noldus Catwalk System available as part of the supported core facilities. We thank the University of Iowa Carver College of Medicine, Medical Scientist Training Program, and Office of the Vice President for Research for support.
Footnotes
Ethics and conflict of interest disclosure
The authors declare no conflict of interest and state that all experiments involving the use and care of animals was in accordance with EC Directive 86/609/EEC for animal experiments and Uniform Requirements for manuscripts submitted to Biomedical journals
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Bent T, Buchwald A, Pisoni DB. Perceptual adaptation and intelligibility of multiple talkers for two types of degraded speech. J Acoust Soc Am. 2009;126:2660–9. doi: 10.1121/1.3212930. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bermingham NA, Hassan BA, Price SD, Vollrath MA, Ben-Arie N, Eatock RA, Bellen HJ, Lysakowski A, Zoghbi HY. Math1: an essential gene for the generation of inner ear hair cells. Science. 1999;284:1837–41. doi: 10.1126/science.284.5421.1837. [DOI] [PubMed] [Google Scholar]
- Cairo S, De Falco F, Pizzo M, Salomoni P, Pandolfi PP, Meroni G. PML interacts with Myc, and Myc target gene expression is altered in PML-null fibroblasts. Oncogene. 2005;24:2195–203. doi: 10.1038/sj.onc.1208338. [DOI] [PubMed] [Google Scholar]
- Chang YP, Fu QJ. Effects of talker variability on vowel recognition in cochlear implants. J Speech Lang Hear Res. 2006;49:1331–41. doi: 10.1044/1092-4388(2006/095). [DOI] [PubMed] [Google Scholar]
- Chellappa R, Li S, Pauley S, Jahan I, Jin K, Xiang M. Barhl1 regulatory sequences required for cell-specific gene expression and autoregulation in the inner ear and central nervous system. Mol Cell Biol. 2008;28:1905–14. doi: 10.1128/MCB.01454-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen L, Iraci N, Gherardi S, Gamble LD, Wood KM, Perini G, Lunec J, Tweddle DA. p53 is a direct transcriptional target of MYCN in neuroblastoma. Cancer Res. 2010;70:1377–88. doi: 10.1158/0008-5472.CAN-09-2598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Colletti L, Mandala M, Zoccante L, Shannon RV, Colletti V. Infants versus older children fitted with cochlear implants: Performance over 10 years. Int J Pediatr Otorhinolaryngol. 2011 doi: 10.1016/j.ijporl.2011.01.005. [DOI] [PubMed] [Google Scholar]
- Conacci-Sorrell M, Eisenman RN. Post-translational control of Myc function during differentiation. Cell Cycle. 2011;10:604–10. doi: 10.4161/cc.10.4.14794. [DOI] [PMC free article] [PubMed] [Google Scholar]
- D’Aguanno S, D’Alessandro A, Pieroni L, Roveri A, Zaccarin M, Marzano V, De Canio M, Bernardini S, Federici G, Urbani A. New insights into neuroblastoma cisplatin resistance: a comparative proteomic and meta-mining investigation. J Proteome Res. 2011;10:416–28. doi: 10.1021/pr100457n. [DOI] [PubMed] [Google Scholar]
- Dallos P. The active cochlea. J Neurosci. 1992;12:4575–85. doi: 10.1523/JNEUROSCI.12-12-04575.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dang CV. Enigmatic MYC Conducts an Unfolding Systems Biology Symphony. Genes Cancer. 2010;1:526–531. doi: 10.1177/1947601910378742. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dominguez Frutos E, Lopez Hernandez I, Vendrell V, Neves J, Gallozzi M, Gutsche K, Quintana L, Sharpe J, Knoepfler PS, Eisenman RN, Trumpp A, Giraldez F, Schimmang T. N-myc controls proliferation, morphogenesis, and patterning of the inner ear. J Neurosci. 2011;31:7178–89. doi: 10.1523/JNEUROSCI.0785-11.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dror AA, Avraham KB. Hearing impairment: a panoply of genes and functions. Neuron. 2010;68:293–308. doi: 10.1016/j.neuron.2010.10.011. [DOI] [PubMed] [Google Scholar]
- Eilers M, Eisenman RN. Myc’s broad reach. Genes Dev. 2008;22:2755–66. doi: 10.1101/gad.1712408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eisenman RN. Deconstructing myc. Genes Dev. 2001;15:2023–30. doi: 10.1101/gad928101. [DOI] [PubMed] [Google Scholar]
- Fasquelle L, Scott HS, Lenoir M, Wang J, Rebillard G, Gaboyard S, Venteo S, Francois F, Mausset-Bonnefont AL, Antonarakis SE, Neidhart E, Chabbert C, Puel JL, Guipponi M, Delprat B. Tmprss3, a transmembrane serine protease deficient in human DFNB8/10 Mycs’ Role in Hair Cell Survival 24 deafness, is critical for cochlear hair cell survival at the onset of hearing. J Biol Chem. 2011;286:17383–97. doi: 10.1074/jbc.M110.190652. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gifford RH, Revit LJ. Speech perception for adult cochlear implant recipients in a realistic background noise: effectiveness of preprocessing strategies and external options for improving speech recognition in noise. J Am Acad Audiol. 2010;21:441–51. doi: 10.3766/jaaa.21.7.3. quiz 487–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hatton BA, Knoepfler PS, Kenney AM, Rowitch DH, de Alboran IM, Olson JM, Eisenman RN. N-myc is an essential downstream effector of Shh signaling during both normal and neoplastic cerebellar growth. Cancer Res. 2006;66:8655–61. doi: 10.1158/0008-5472.CAN-06-1621. [DOI] [PubMed] [Google Scholar]
- Hill GW, Morest DK, Parham K. Cisplatin induced ototoxicity: effect of intratympanic dexamethasone injections. Otol Neurotol. 2008;29:1005–11. doi: 10.1097/MAO.0b013e31818599d5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jahan I, Pan N, Kersigo J, Fritzsch B. Neurod1 suppresses hair cell differentiation in ear ganglia and regulates hair cell subtype development in the cochlea. PLoS One. 2010;5:e11661. doi: 10.1371/journal.pone.0011661. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jones JM, Montcouquiol M, Dabdoub A, Woods C, Kelley MW. Inhibitors of differentiation and DNA binding (Ids) regulate Math1 and hair cell formation during the development of the organ of Corti. J Neurosci. 2006;26:550–8. doi: 10.1523/JNEUROSCI.3859-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Knoepfler PS, Cheng PF, Eisenman RN. N-myc is essential during neurogenesis for the rapid expansion of progenitor cell populations and the inhibition of neuronal differentiation. Genes Dev. 2002;16:2699–712. doi: 10.1101/gad.1021202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Knoepfler PS, Zhang XY, Cheng PF, Gafken PR, McMahon SB, Eisenman RN. Myc influences global chromatin structure. EMBO J. 2006;25:2723–34. doi: 10.1038/sj.emboj.7601152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kopecky B, Fritzsch B. Regeneration of Hair Cells: Making Sense of All the Noise. Pharmaceuticals (Basel) 2011;4:848–879. doi: 10.3390/ph4060848. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kopecky B, Santi P, Johnson S, Schmitz H, Fritzsch B. Conditional deletion of N-Myc disrupts neurosensory and non-sensory development of the ear. Dev Dyn. 2011;240:1373–90. doi: 10.1002/dvdy.22620. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kopecky B, Decook R, Fritzsch B. Mutational ataxia resulting from abnormal vestibular acquisition and processing is partially compensated for. Behav Neurosci. 2012a doi: 10.1037/a0026896. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kopecky B, Johnson S, Schmitz H, Santi P, Fritzsch B. Scanning thin-sheet laser imaging microscopy elucidates details on mouse ear development. Dev Dyn. 2012b;241:465–80. doi: 10.1002/dvdy.23736. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee JE, Nakagawa T, Kita T, Kim TS, Iguchi F, Endo T, Shiga A, Lee SH, Ito J. Mechanisms of apoptosis induced by cisplatin in marginal cells in mouse stria vascularis. ORL J Otorhinolaryngol Relat Spec. 2004;66:111–8. doi: 10.1159/000079329. [DOI] [PubMed] [Google Scholar]
- Li S, Price SM, Cahill H, Ryugo DK, Shen MM, Xiang M. Hearing loss caused by progressive degeneration of cochlear hair cells in mice deficient for the Barhl1 homeobox gene. Development. 2002;129:3523–32. doi: 10.1242/dev.129.14.3523. [DOI] [PubMed] [Google Scholar]
- Matei V, Pauley S, Kaing S, Rowitch D, Beisel KW, Morris K, Feng F, Jones K, Lee J, Fritzsch B. Smaller inner ear sensory epithelia in Neurog 1 null mice are related to earlier hair cell cycle exit. Dev Dyn. 2005;234:633–50. doi: 10.1002/dvdy.20551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Michaelis M, Bliss J, Arnold SC, Hinsch N, Rothweiler F, Deubzer HE, Witt O, Langer K, Doerr HW, Wels WS, Cinatl J., Jr Cisplatin-resistant neuroblastoma cells express enhanced levels of epidermal growth factor receptor (EGFR) and are sensitive to treatment with EGFR specific toxins. Clin Cancer Res. 2008;14:6531–7. doi: 10.1158/1078-0432.CCR-08-0821. [DOI] [PubMed] [Google Scholar]
- Nichols DH, Pauley S, Jahan I, Beisel KW, Millen KJ, Fritzsch B. Lmx1a is required for segregation of sensory epithelia and normal ear histogenesis and morphogenesis. Cell Tissue Res. 2008;334:339–58. doi: 10.1007/s00441-008-0709-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nilsson LM, Forshell TZ, Rimpi S, Kreutzer C, Pretsch W, Bornkamm GW, Nilsson JA. Mouse genetics suggests cell-context dependency for Myc-regulated metabolic enzymes during tumorigenesis. PLoS Genet. 2012;8:e1002573. doi: 10.1371/journal.pgen.1002573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Omata T, Ohtani I, Ohtsuki K, Ouchi J. A detection method for lactic dehydrogenase activity in the inner ear. J Histochem Cytochem. 1978;26:313–7. doi: 10.1177/26.4.659836. [DOI] [PubMed] [Google Scholar]
- Oshima K, Shin K, Diensthuber M, Peng AW, Ricci AJ, Heller S. Mechanosensitive hair cell-like cells from embryonic and induced pluripotent stem cells. Cell. 2010;141:704–16. doi: 10.1016/j.cell.2010.03.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paffhausen T, Schwab M, Westermann F. Targeted MYCN expression affects cytotoxic potential of chemotherapeutic drugs in neuroblastoma cells. Cancer Lett. 2007;250:17–24. doi: 10.1016/j.canlet.2006.09.010. [DOI] [PubMed] [Google Scholar]
- Pan N, Jahan I, Lee JE, Fritzsch B. Defects in the cerebella of conditional Neurod1 null mice correlate with effective Tg(Atoh1-cre) recombination and granule cell requirements for Neurod1 for differentiation. Cell Tissue Res. 2009 doi: 10.1007/s00441-009-0826-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pan N, Jahan I, Kersigo J, Kopecky B, Santi P, Johnson S, Schmitz H, Fritzsch B. Conditional deletion of Atoh1 using Pax2-Cre results in viable mice without differentiated cochlear hair cells that have lost most of the organ of Corti. Hear Res. 2011;275:66–80. doi: 10.1016/j.heares.2010.12.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pan N, Jahan I, Kersigo J, Duncan JS, Kopecky B, Fritzsch B. A novel Atoh1 “self-terminating” mouse model reveals the necessity of proper Atoh1 level and duration for hair cell differentiation and viability. PLoS One. 2012a;7:e30358. doi: 10.1371/journal.pone.0030358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pan N, Kopecky B, Jahan I, Fritzsch B. Understanding the Evolution and Development of Neurosensory Transcription Factors of the Ear to Enhance Therapeutic Translation. Cell and Tissue Research. 2012b doi: 10.1007/s00441-012-1454-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parham K. Can intratympanic dexamethasone protect against cisplatin ototoxicity in mice with age related hearing loss? Otolaryngol Head Neck Surg. 2011;145:635–40. doi: 10.1177/0194599811409304. [DOI] [PubMed] [Google Scholar]
- Pauley S, Lai E, Fritzsch B. Foxg1 is required for morphogenesis and histogenesis of the mammalian inner ear. Dev Dyn. 2006;235:2470–82. doi: 10.1002/dvdy.20839. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pauley S, Kopecky B, Beisel K, Soukup G, Fritzsch B. Stem cells and molecular strategies to restore hearing. Panminerva Med. 2008;50:41–53. [PMC free article] [PubMed] [Google Scholar]
- Peterson NR, Pisoni DB, Miyamoto RT. Cochlear implants and spoken language processing abilities: review and assessment of the literature. Restor Neurol Neurosci. 2010;28:237–50. doi: 10.3233/RNN-2010-0535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Romand R, Hirning-Folz U, Ehret G. N-myc expression in the embryonic cochlea of the mouse. Hear Res. 1994;72:53–8. doi: 10.1016/0378-5955(94)90205-4. [DOI] [PubMed] [Google Scholar]
- Rybak LP. Mechanisms of cisplatin ototoxicity and progress in otoprotection. Curr Opin Otolaryngol Head Neck Surg. 2007;15:364–9. doi: 10.1097/MOO.0b013e3282eee452. [DOI] [PubMed] [Google Scholar]
- Rybak LP, Ramkumar V. Ototoxicity. Kidney Int. 2007;72:931–5. doi: 10.1038/sj.ki.5002434. [DOI] [PubMed] [Google Scholar]
- Rybak LP, Whitworth CA, Mukherjea D, Ramkumar V. Mechanisms of cisplatin-induced ototoxicity and prevention. Hear Res. 2007;226:157–67. doi: 10.1016/j.heares.2006.09.015. [DOI] [PubMed] [Google Scholar]
- Slack AD, Chen Z, Ludwig AD, Hicks J, Shohet JM. MYCN-directed centrosome amplification requires MDM2-mediated suppression of p53 activity in neuroblastoma cells. Cancer Res. 2007;67:2448–55. doi: 10.1158/0008-5472.CAN-06-1661. [DOI] [PubMed] [Google Scholar]
- Sloan EJ, Ayer DE. Myc, mondo, and metabolism. Genes Cancer. 2010;1:587–96. doi: 10.1177/1947601910377489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spector GJ, Carr C. The ultrastructural cytochemistry of peroxisomes in the guinea pig cochlea: a metabolic hypothesis for the stria vascularis. Laryngoscope. 1979;89:1–38. doi: 10.1288/00005537-197906001-00001. [DOI] [PubMed] [Google Scholar]
- Torres J, Regan PL, Edo R, Leonhardt P, Jeng EI, Rappaport EF, Ikegaki N, Tang XX. Biological effects of induced MYCN hyper-expression in MYCN-amplified neuroblastomas. Int J Oncol. 2010;37:983–91. doi: 10.3892/ijo_00000749. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wallis D, Hamblen M, Zhou Y, Venken KJ, Schumacher A, Grimes HL, Zoghbi HY, Orkin SH, Bellen HJ. The zinc finger transcription factor Gfi1, implicated in lymphomagenesis, is required for inner ear hair cell differentiation and survival. Development. 2003;130:221–32. doi: 10.1242/dev.00190. [DOI] [PubMed] [Google Scholar]
- Wangemann P, Nakaya K, Wu T, Maganti RJ, Itza EM, Sanneman JD, Harbidge DG, Billings S, Marcus DC. Loss of cochlear HCO3- secretion causes deafness via endolymphatic Mycs’ Role in Hair Cell Survival 26 acidification and inhibition of Ca2+ reabsorption in a Pendred syndrome mouse model. Am J Physiol Renal Physiol. 2007;292:F1345–53. doi: 10.1152/ajprenal.00487.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Young SL, Diolaiti D, Conacci-Sorrell M, Ruiz-Trillo I, Eisenman RN, King N. Premetazoan ancestry of the Myc-Max network. Mol Biol Evol. 2011;28:2961–71. doi: 10.1093/molbev/msr132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zindy F, Knoepfler PS, Xie S, Sherr CJ, Eisenman RN, Roussel MF. N-Myc and the cyclin-dependent kinase inhibitors p18Ink4c and p27Kip1 coordinately regulate cerebellar development. Proc Natl Acad Sci U S A. 2006;103:11579–83. doi: 10.1073/pnas.0604727103. [DOI] [PMC free article] [PubMed] [Google Scholar]