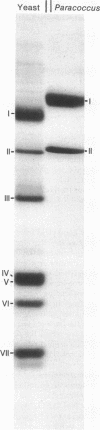
Abstract
Cytochrome c oxidase (ferrocytochrome c: oxygen oxidoreductase, EC 1.9.3.1) was purified from the cytoplasmic membrane of the bacterium Paracoccus denitrificans. The enzyme contains two heme groups (a and a3) and two copper atoms per minimal unit, oxidizes mammalian cytochrome c at a high rate, and, when incorporated into liposomes, generates an electrochemical proton gradient during cytochrome c oxidation. Sodium dodecyl sulfate/polyacrylamide gel electrophoresis reveals only two subunits of apparent molecular weights 45,000 and 28,000; they appear to correspond to the two largest mitochondrially made subunits of the seven-subunit cytochrome c oxidase isolated from yeast mitochondria. Because of its structural simplicity. Paracoccus cytochrome c oxidase offers new possibilities for exploring the mechanism of cytochrome c oxidase function.
Full text
PDF


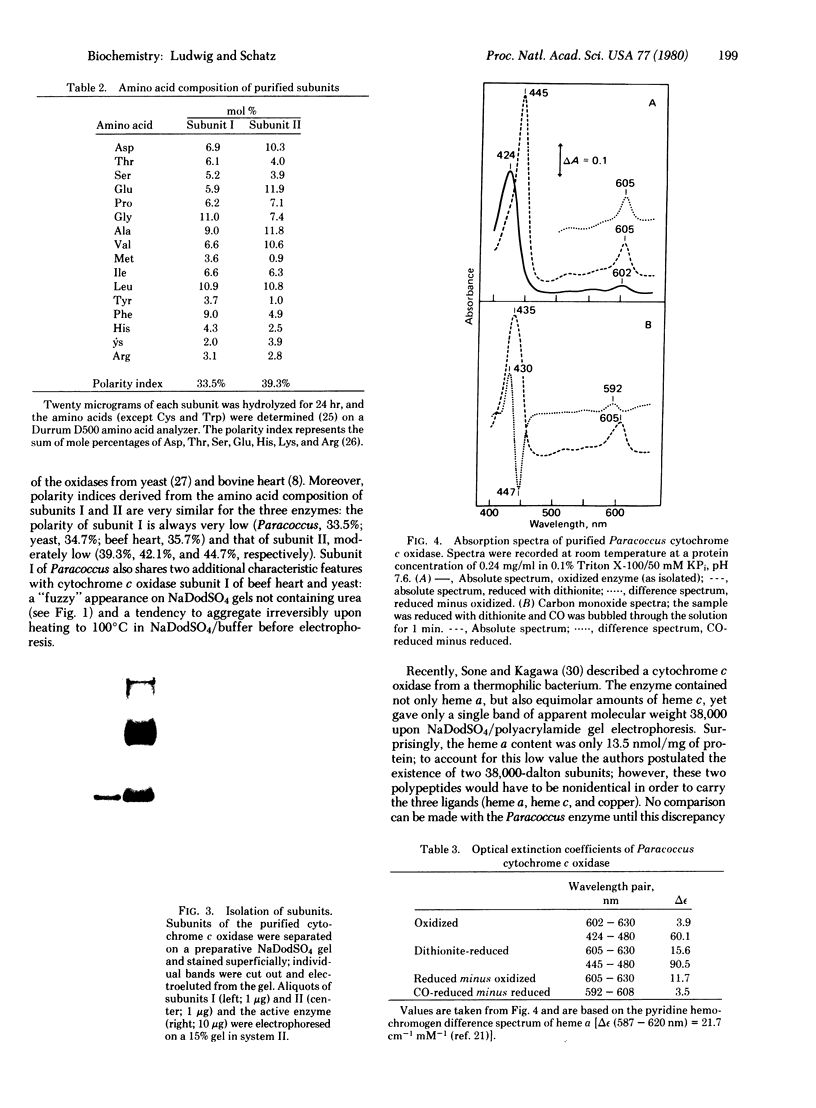

Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Buse G., Steffens G. J., Steffens G. C. Studies on cytochrome c oxidase, III. Relationship of cytochrome oxidase subunits to electron carriers of photophosphorylation. Hoppe Seylers Z Physiol Chem. 1978 Aug;359(8):1011–1013. [PubMed] [Google Scholar]
- Capaldi R. A., Vanderkooi G. The low polarity of many membrane proteins. Proc Natl Acad Sci U S A. 1972 Apr;69(4):930–932. doi: 10.1073/pnas.69.4.930. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cox J. C., Ingledew W. J., Haddock B. A., Lawford H. G. The variable cytochrome content of Paracoccus denitrificans grown aerobically under different conditions. FEBS Lett. 1978 Sep 15;93(2):261–265. doi: 10.1016/0014-5793(78)81117-x. [DOI] [PubMed] [Google Scholar]
- Deters D., Müller U., Homberger H. Breakage of yeast cells: large scale isolation of yeast mitochondria with a continuous-flow disintegrator. Anal Biochem. 1976 Jan;70(1):263–267. doi: 10.1016/s0003-2697(76)80067-x. [DOI] [PubMed] [Google Scholar]
- Douglas M. G., Butow R. A. Variant forms of mitochondrial translation products in yeast: evidence for location of determinants on mitochondrial DNA. Proc Natl Acad Sci U S A. 1976 Apr;73(4):1083–1086. doi: 10.1073/pnas.73.4.1083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Downer N. W., Robinson N. C. Characterization of a seventh different subunit of beef heart cytochrome c oxidase. Similarities between the beef heart enzyme and that from other species. Biochemistry. 1976 Jun 29;15(13):2930–2936. doi: 10.1021/bi00658a036. [DOI] [PubMed] [Google Scholar]
- Erecińska M., Wilson D. F. Cytochrome c oxidase: a synopsis. Arch Biochem Biophys. 1978 May;188(1):1–14. doi: 10.1016/0003-9861(78)90348-x. [DOI] [PubMed] [Google Scholar]
- Fox T. D. Five TGA "stop" codons occur within the translated sequence of the yeast mitochondrial gene for cytochrome c oxidase subunit II. Proc Natl Acad Sci U S A. 1979 Dec;76(12):6534–6538. doi: 10.1073/pnas.76.12.6534. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Henderson R., Capaldi R. A., Leigh J. S. Arrangement of cytochrome oxidase molecules in two-dimensional vesicle crystals. J Mol Biol. 1977 Jun 5;112(4):631–648. doi: 10.1016/s0022-2836(77)80167-8. [DOI] [PubMed] [Google Scholar]
- Hinkle P. C., Kim J. J., Racker E. Ion transport and respiratory control in vesicles formed from cytochrome oxidase and phospholipids. J Biol Chem. 1972 Feb 25;247(4):1338–1339. [PubMed] [Google Scholar]
- John P., Whatley F. R. The bioenergetics of Paracoccus denitrificans. Biochim Biophys Acta. 1977 Oct 5;463(2):129–153. doi: 10.1016/0304-4173(77)90006-4. [DOI] [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- Lawford H. G. Energy transduction in the mitochondrionlike bacterium Paracoccus denitrificans during carbon- or sulphate-limited aerobic growth in continuous culture. Can J Biochem. 1978 Jan;56(1):13–22. doi: 10.1139/o78-003. [DOI] [PubMed] [Google Scholar]
- Lawford H. G. Proton translocation coupled to ubiquinol oxidation in Paracoccus denitrificans. Can J Biochem. 1979 Feb;57(2):172–177. doi: 10.1139/o79-021. [DOI] [PubMed] [Google Scholar]
- Ludwig B., Downer N. W., Capaldi R. A. Labeling of cytochrome c oxidase with [35S]diazobenzenesulfonate. Orientation of this electron transfer complex in the inner mitochondrial membrane. Biochemistry. 1979 Apr 17;18(8):1401–1407. doi: 10.1021/bi00575a002. [DOI] [PubMed] [Google Scholar]
- Mason T. L., Poyton R. O., Wharton D. C., Schatz G. Cytochrome c oxidase from bakers' yeast. I. Isolation and properties. J Biol Chem. 1973 Feb 25;248(4):1346–1354. [PubMed] [Google Scholar]
- Nelson N., Deters D. W., Nelson H., Racker E. Partial resolution of the enzymes catalyzing photophosphorylation. 8. Properties of isolated subunits of coupling factor 1 from spinach chloroplasts. J Biol Chem. 1973 Mar 25;248(6):2049–2055. [PubMed] [Google Scholar]
- Poyton R. O., Schatz G. Cytochrome c oxidase from bakers' yeast. III. Physical characterization of isolated subunits and chemical evidence for two different classes of polypeptides. J Biol Chem. 1975 Jan 25;250(2):752–761. [PubMed] [Google Scholar]
- Rubin M. S., Tzagoloff A. Assembly of the mitochondrial membrane system. IX. Purification, characterization, and subunit structure of yeast and beef cytochrome oxidase. J Biol Chem. 1973 Jun 25;248(12):4269–4274. [PubMed] [Google Scholar]
- Scholes P. B., Smith L. The isolation and properties of the cytoplasmic membrane of Micrococcus denitrificans. Biochim Biophys Acta. 1968 Feb 12;153(2):350–362. doi: 10.1016/0005-2728(68)90080-7. [DOI] [PubMed] [Google Scholar]
- Schwab A. J., Sebald W., Weiss H. Different pool sizes of the precursor polypeptides of cytochrome oxidase from Neurospora crassa. Eur J Biochem. 1972 Nov 7;30(3):511–516. doi: 10.1111/j.1432-1033.1972.tb02122.x. [DOI] [PubMed] [Google Scholar]
- Sigel E., Carafoli E. The charge stoichiometry of cytochrome c oxidase in the reconstituted system. J Biol Chem. 1979 Nov 10;254(21):10572–10574. [PubMed] [Google Scholar]
- Smith L. Bacterial cytochromes and their spectral characterization. Methods Enzymol. 1978;53:202–212. doi: 10.1016/s0076-6879(78)53025-5. [DOI] [PubMed] [Google Scholar]
- Swank R. T., Munkres K. D. Molecular weight analysis of oligopeptides by electrophoresis in polyacrylamide gel with sodium dodecyl sulfate. Anal Biochem. 1971 Feb;39(2):462–477. doi: 10.1016/0003-2697(71)90436-2. [DOI] [PubMed] [Google Scholar]
- Vanneste W. H., Ysebaert-Vanneste M., Mason H. S. The decline of molecular activity of cytochrome oxidase during purification. J Biol Chem. 1974 Dec 10;249(23):7390–7401. [PubMed] [Google Scholar]
- Veillon C., Vallee B. L. Atomic spectroscopy in metal analysis of enzymes and other biological material. Methods Enzymol. 1978;54:446–484. doi: 10.1016/s0076-6879(78)54028-7. [DOI] [PubMed] [Google Scholar]
- WILLIAMS J. N., Jr A METHOD FOR THE SIMULTANEOUS QUANTITATIVE ESTIMATION OF CYTOCHROMES A, B, C1, AND C IN MITOCHONDRIA. Arch Biochem Biophys. 1964 Sep;107:537–543. doi: 10.1016/0003-9861(64)90313-3. [DOI] [PubMed] [Google Scholar]
- Wikström M., Krab K. Proton-pumping cytochrome c oxidase. Biochim Biophys Acta. 1979 Aug 17;549(2):177–122. doi: 10.1016/0304-4173(79)90014-4. [DOI] [PubMed] [Google Scholar]