Abstract
Dendritic cells (DCs) are the most potent antigen-presenting cells and are the mediators of T cell immunity. Many investigators have explored the potential of using DCs as a vaccine for tumour-derived antigens in immunotherapy of B cell malignancies, and the results have been disappointing. To search for better tumour antigens to improve the efficacy of DC-based immunotherapy in myeloma, we evaluated and compared the efficacy of the vaccination of DCs pulsed with idiotype (Id) or tumour lysate in the 5TGM1 myeloma mouse model. Our results showed that Id- or tumour lysate-pulsed DC vaccines protected mice efficiently against developing myeloma, retarded tumour growth, induced tumour regression against established tumour and protected surviving mice from tumour rechallenge. The therapeutic responses were associated with an induction of strong humoral immune responses, including anti-Id or anti-lysate antibodies, and cellular immune responses including myeloma-specific CD8+ cytotoxic T lymphocytes, CD4+ type 1 T helper cells and memory T cells in mice receiving Id- or tumour lysate-pulsed DC vaccines. In addition, our studies showed that tumour lysate-pulsed DCs were more potent vaccines than the Id-pulsed DC vaccines to promote anti-tumour immunity in the model. This information will be important for improving the strategies of DC-based immunotherapy for patients with myeloma and other B cell tumours.
Keywords: dendritic cells, idiotype, immunotherapy, multiple myeloma, tumour lysate
Introduction
Despite progress in the treatment of multiple myeloma (MM) by using conventional chemotherapies and supportive therapeutics in combination with stem cell transplantation, this disease still remains fatal in the majority of patients. MM is characterized by the clonally expansion of malignant plasma cells within the bone marrow [1,2]. Thus, new treatments are required to eradicate minimal residual myeloma cells. Tumour-specific, antigen-based immunotherapy holds great promise for this purpose in patients with MM as well as other B cell tumours [3–5].
Dendritic cells (DCs) are the most potent antigen-presenting cells (APCs) and the most potent mediator of immunity to prime naive T cells into effector cells by capture and processing of antigens, expression of co-stimulatory molecules, migration to lymphoid organs and secretion of cytokines to initiate immune responses [6,7]. Thus far, many investigators have developed and optimized the methods to use DCs as APCs for tumour-derived antigens in vaccination and immunotherapy for various cancers [8–10].
In MM, recent studies have shown that DCs pulsed with idiotype (Id) protein, which is a tumour-specific antigen because of the unique antigenic structure in its variable regions, generated Id-specific cytotoxic T lymphocytes (CTLs). The CTLs recognized and lysed the myeloma cells [11,12]. In addition, recent clinical studies have shown that Id-pulsed DC vaccination enhanced Id-specific humoral and cellular immune responses to treat tumour in patients with MM [13–16]. On the other hand, myeloma cells by themselves may contain a multitude of tumour antigens that can stimulate an increase of T cell responses against tumour and can lead to an induction of stronger anti-myeloma responses [5]. A recent study has shown that DCs pulsed with myeloma cell lysate generated myeloma-specific CTLs, and these T cells were efficient in killing primary myeloma cells [17]. Moreover, DCs fused with myeloma cells not only induced anti-tumour humoral and cellular immune responses but also extended the survival of tumour-established mice [18–20]. Thus far, many investigators have examined various DC-based immunotherapies, such as Id- or myeloma cell-based DC vaccines, for the treatment of patients with MM as well as other B cell malignancies. However, whether the different forms of DC-based vaccines have the same efficacy in MM has not been determined.
Therefore, in this study we compared and evaluated the effects of Id-pulsed DC vaccine and myeloma tumour cell lysate-pulsed DC vaccine at preventing or treating myeloma and priming myeloma-specific immune responses in the 5TGM1 myeloma mouse model, derived originally from 5T33 myeloma cells [21,22]. Such a study is important for the selection and use of a more potent DC-based vaccination for further clinical immunotherapeutic strategies in patients.
Materials and methods
Mice and cell lines
C57BL/KaLwRij mice were purchased from Harlan CPB (Zeist, the Netherlands) and maintained in an American Association of Laboratory Animal Care-accredited animal facility. This study was approved by the Institutional Animal Care and Use Committee of the University of Texas MD Anderson Cancer Center. All mice were 6–8 weeks old at the beginning of each experiment. The 5TGM1 murine myeloma cell line was cultured in Iscove's modified Dulbecco's media (IMDM; Invitrogen, Grand Island, NY, USA) supplemented with 10% heat-inactivated fetal bovine serum (FBS; Thermo Scientific, Rockford, IL, USA), 100 U/ml penicillin–streptomycin and 2 mM L-glutamine (both from Invitrogen). The B16 melanoma cell line, originated from C57BL/6 mice, was purchased from American Type Culture Collection (ATCC; Rockville, MD, USA) and cultured in IMDM.
Preparation of idiotype protein and tumour lysate
The 5TGM1 myeloma cells were cultured in hybridoma serum-free medium (Invitrogen) and mouse immunoglobulin (Ig)G2b Id protein, secreted by the 5TGM1 myeloma cells, was purified from cell culture supernatant using Hi-Trap Protein-A affinity chromatography (GE Healthcare, Piscataway, NJ, USA), as described previously [23]. Id protein and keyhole limpet haemocyanin (KLH; EMD Biosciences, La Jolla, CA, USA) conjugate was made using glutaraldehyde (Sigma, St Louis, MO, USA) to enhance the immunogenicity of the Id protein, as described previously [24]. Briefly, conjugation was performed at room temperature using a 1:1 mixture (w/w) of Id protein and KLH in 0·1% glutaraldehyde, followed by dialysis against phosphate-buffered saline (PBS; Mediatech, Manassas, VA, USA) for 24 h.
Tumour lysate was prepared by five rounds of freezing and thawing to disrupt the 5TGM1 myeloma cells. Cell debris was removed by centrifugation, and cell lysate was filtered by using a 0·2-µm syringe filter. The total proteins were quantified and conjugated with KLH using 0·1% glutaraldehyde.
Generation of dendritic cells
DCs were generated from bone marrow stem cells of syngeneic mice, as described previously [25,26]. Briefly, bone marrow mononuclear cells were cultured at a density of 1 × 106 cells/ml in RPMI-1640 complete medium with 20 ng/ml granulocyte–monocyte colony-stimulating factor (GM-CSF; R&D Systems, Minneapolis, MN, USA) and 10% heat-inactivated FBS at 37°C in 5% CO2. At day 4, medium was replaced with fresh medium containing GM-CSF, and at day 8, immature DCs were pulsed for 8 h with Id-KLH or 5TGM1 myeloma cell lysate-KLH proteins at a concentration of 100 µg/ml, followed by the addition of tumour necrosis factor (TNF)-α (10 ng/ml) and interleukin (IL)-1β (10 ng/ml; both from R&D Systems) for 48 h to induce DC maturation. Mature DCs were collected and used to vaccinate the mice.
Vaccination of mice
Each experiment included four groups of mice and was repeated twice. In prophylaxis studies, mice were vaccinated subcutaneously into the flank by a weekly injection of 1 × 106/100 µl/mouse of Id-KLH-pulsed DCs or tumour lysate-KLH-pulsed DCs for a total of three injections. Following each vaccination, GM-CSF (200 ng/day per mouse) was injected subcutaneously adjacent to the vaccination sites for 3 consecutive days. Control mice received injections of PBS or 1 × 106/mouse of unpulsed DCs. One week after the final vaccination, mice were challenged intravenously with 1 × 106 5TGM1 myeloma cells and tumour burden was monitored by measuring serum IgG2b Id protein. In therapeutic studies, mice were injected intravenously with 1 × 106 5TGM1 myeloma cells. Ten days later, when myeloma growth was established, mice were treated with Id-KLH-pulsed DC vaccine or tumour lysate-KLH-pulsed DC vaccine, as described previously. Mice were killed humanely when moribund or hind-leg paralysis developed.
Detection of IgG2b idiotype protein, anti-idiotype, anti-lysate or anti-KLH antibodies
Enzyme-linked immunoabsorbent assay (ELISA) was used to measure titres of anti-Id, anti-lysate or anti-KLH antibodies, as described previously [24]. When detecting anti-Id antibodies, horseradish peroxidase (HRP)-conjugated goat anti-mouse IgG (Southern Biotech, Birmingham, AL, USA) was pre-absorbed against Id protein to reduce unspecific binding. The same assay was also used to measure the serum IgG2b Id protein for the monitoring of tumour burden, as described previously [27].
Immunophenotyping
Fluorescent isothiocyanate (FITC)-, phycoerythrin (PE)- or allophycocyanin (APC)-conjugated monoclonal antibodies (mAbs) against CD11c, CD40, CD80, CD86 and major histocompatibility complex (MHC) class II for DCs or CD4, CD8, and CD44 for T cells (all from eBioscience, San Diego, CA, USA) were added to splenocytes, incubated for 30 min at 4°C, washed twice and analysed using a FACSCalibur flow cytometer (BD Biosciences, San Jose, CA, USA).
Detection of cytokine production
Intracellular staining of IFN-γ was performed using a Cytofix/Cytoperm kit (BD Biosciences), according to the manufacturer's instructions. Mice were vaccinated subcutaneously three times every week with Id-KLH-pulsed DCs or tumour lysate-KLH-pulsed DCs, and PBS or unpulsed DCs were injected as controls. One week later, splenocytes were isolated from the mice, cultured with Id protein, tumour lysate or irradiated (80 Gy) 5TGM1 myeloma cells for 24 h, and GolgiPlug (BD Biosciences) was added for the final 6 h before staining to inhibit cytokine secretion. T cells were stained with FITC-conjugated anti-CD8 or -CD4 mAbs, followed by fixation and permeabilization, staining with APC-conjugated anti-IFN-γ mAbs (eBiosciences), and after washing were analysed using a FACSCalibur flow cytometer.
In some experiments, ELISA was used to measure secreted IFN-γ from T cells. Splenocytes were restimulated for 3 days with irradiated 5TGM1 myeloma cells. Supernatants were collected and the cytokine amounts were quantified, using commercially available ELISA kits (eBioscience), according to the manufacturer's instructions. All assays were performed in duplicate.
Antigen-specific T cell proliferation assay
Splenocytes were prelabelled with 5 µM of 5(6)-carboxyfluorescein diacetate succinimidyl ester (CFSE; Invitrogen) for 10 min at 37°C. After washing, labelled cells were seeded and restimulated with Id protein, tumour lysate or irradiated 5TGM1 myeloma cells for 5 days. After that, the cells were stained with APC-conjugated anti-CD8 or -CD4 mAbs for 30 min, washed and analysed by flow cytometer to detect dilution of CFSE.
Cytotoxicity assay
The standard 51Cr-release assay was performed to measure the cytotoxicity of the T cells against 5TGM1 myeloma cells [28]. As no myeloma cell lines from C57BL mice are available, B16 melanoma cells were used as control target cells. Target cells were labelled with 50 µCi of 51Cr-sodium chromate (PerkinElmer, Waltham, MA, USA) for 1 h and incubated with various numbers of T cells in 96-well U-bottomed tissue culture plates in RPMI-1640 complete medium. After 4 h, 50% of the supernatants were collected, and radioactivity was measured by a gamma-counter. All assays were performed in triplicate. Results are shown as mean percentage 51Cr-release calculated as follows: [(sample counts – spontaneous counts)/(maximum counts – spontaneous counts)] × 100.
Statistical analysis
Student's t-test was used to compare various experimental groups. P < 0·05 was considered statistically significant. Survival was evaluated from the day of tumour injection until death, and the Kaplan–Meier test was used to compare mouse survival between the groups. All data are shown as mean ± standard deviation.
Results
Tumour lysate-pulsed DC vaccine or idiotype-pulsed DC vaccine protected mice from developing myeloma
In the prophylactic study, mice received three weekly subcutaneous vaccinations with 1 × 106/mouse of Id-KLH-pulsed DCs or tumour lysate-KLH-pulsed DCs. Control mice received injections of PBS or unpulsed DCs. One week after the final vaccination, 1 × 106 5TGM1 myeloma cells were challenged intravenously, and tumour burden was monitored by measuring circulating IgG2b Id protein. As shown in Fig. 1a, two of 10 mice receiving Id-KLH-pulsed DC vaccine (P < 0·05, compared with mice receiving PBS or unpulsed DCs) and three of 10 mice receiving tumour lysate-KLH-pulsed DC vaccine (P < 0·01, compared with mice receiving PBS or unpulsed DCs) displayed no increase in serum IgG2b Id protein and showed no sign of myeloma. In contrast, all mice receiving injections of PBS or unpulsed DCs developed myeloma. Mouse survival data, summarizing all 10 mice per group, are shown in Fig. 1b. All mice receiving PBS or unpulsed DCs died within 60 days after tumour injection, whereas 20 and 30% of mice receiving Id-KLH-pulsed DCs and tumour lysate-KLH-pulsed DCs, respectively, survived without detectable tumours. The Kaplan–Meier test showed that mice receiving tumour lysate-KLH-pulsed DCs had better survival than those treated with Id-KLH-pulsed DCs (P < 0·05). These results show that tumour lysate-pulsed DC vaccine provides better protection than Id-pulsed DC vaccine in mice against developing myeloma.
Fig. 1.
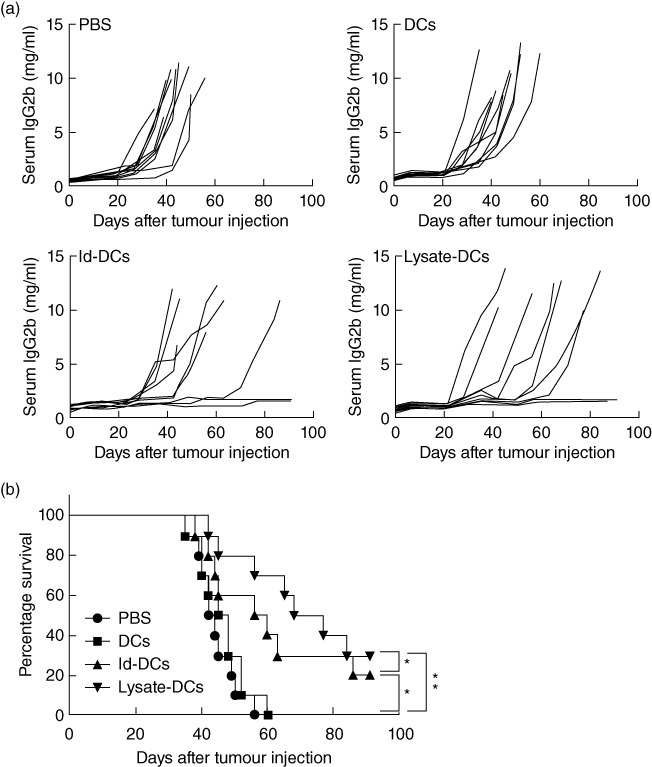
In-vivo protective effect of dendritic cell (DC) vaccines. (a) Tumour burden measured as levels of serum immunoglobulin (Ig)G2b idiotype (Id) protein in mice (10 per group) receiving phosphate-buffered saline (PBS), unpulsed DCs (DCs), idiotype-keyhole limpet haemocyanin (Id-KLH)-pulsed DC vaccine (Id-DCs) or tumour lysate-KLH-pulsed DC vaccine (lysate-DCs). Pooled results of two experiments performed are shown. (b) Survival data of mice (10 per group, summarized from two experiments) receiving PBS, unpulsed DCs, Id-KLH-pulsed DC vaccine or tumour lysate-KLH-pulsed DC vaccine. C57BL/KaLwRij mice received three weekly subcutaneous injections of Id-KLH-pulsed DC vaccine or tumour lysate-KLH-pulsed DC vaccine. PBS and unpulsed DCs served as controls. One week after the third vaccination, all mice were challenged intravenously with 1 × 106 5TGM1 myeloma cells. Serum samples were collected weekly, and tumour burden was monitored by measuring circulating IgG2b Id protein. *P < 0·05; **P < 0·01.
Tumour lysate-pulsed DC vaccine or idiotype-pulsed DC vaccine was therapeutic against established myeloma
To examine and compare the efficacy of tumour lysate-pulsed DC vaccine or Id-pulsed DC vaccine in treating established myeloma, mice were first challenged intravenously with 5TGM1 myeloma cells. Ten days later, vaccinations were given to tumour-bearing mice. As shown in Fig. 2a, myeloma-bearing mice receiving injections of PBS or unpulsed DCs all died of myeloma with large tumour burdens, whereas one of 10 mice receiving Id-KLH-pulsed DC vaccine (P < 0·05, compared with mice receiving PBS or unpulsed DCs) and one of 10 mice receiving tumour lysate-KLH-pulsed DC vaccine (P < 0·01, compared with mice receiving PBS or unpulsed DCs) displayed no increase in serum IgG2b Id protein and showed no sign of myeloma. Based on the survival curve (Fig. 2b) from all 10 mice per each group, mice receiving PBS and unpulsed DCs all died within 53 days, respectively, after tumour injection, while 10% of mice receiving Id-KLH-pulsed DC vaccine and tumour lysate-KLH-pulsed DC vaccine, respectively, survived without detectable tumours. These results demonstrate that tumour lysate-pulsed DC vaccine or Id-pulsed DC vaccine retarded tumour growth efficiently and induced tumour regression in some treated mice.
Fig. 2.
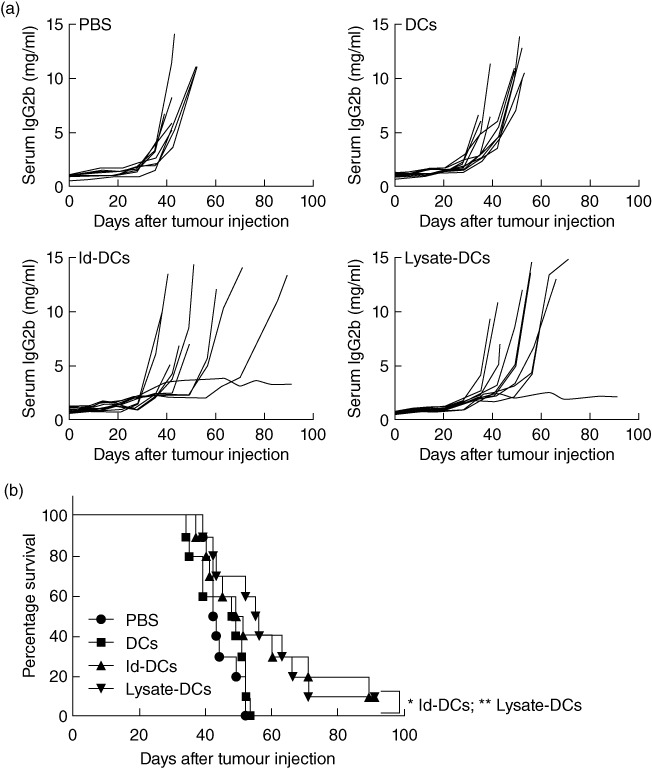
In-vivo therapeutic effect of dendritic cell (DC) vaccines in myeloma-bearing mice. (a) Tumour burden measured as levels of serum immunoglobulin (Ig)G2b idiotype (Id) protein in mice (10 per group) receiving phosphate-buffered saline (PBS), unpulsed DCs (DCs), idiotype-keyhole limpet haemocyanin (Id-KLH)-pulsed DC vaccine (Id-DCs) or tumour lysate-KLH-pulsed DC vaccine (lysate-DCs). Pooled results of two experiments performed are shown. (b) Survival curve of mice (10 per group, summarized from two experiments) receiving PBS, unpulsed DCs, Id-KLH-pulsed DC vaccine or tumour lysate-KLH-pulsed DC vaccine. C57BL/KaLwRij mice were challenged with 1 × 106 5TGM1 myeloma cells, and 10 days following tumour injection, three weekly vaccinations with Id-KLH-pulsed DC vaccine or tumour lysate-KLH-pulsed DC vaccine were given. PBS and unpulsed DCs served as controls. *P < 0·05; **P < 0·01.
Tumour lysate-pulsed DC vaccine or idiotype-pulsed DC vaccine was potent at inducing tumour antigen-specific antibody responses in vivo
To elucidate the immunological mechanisms underlying these phenomena, we first examined vaccine-induced humoral immune responses. Serum samples were collected from mice after the third vaccination, and titres of anti-Id, anti-lysate and anti-KLH antibodies were measured by ELISA. Id-KLH-pulsed DC vaccine induced significantly higher titres of anti-Id (Fig. 3a) and anti-KLH (Fig. 3c) antibodies than PBS or unpulsed DCs controls in treated tumour-free mice (P < 0·01, compared with mice receiving PBS or unpulsed DCs). However, compared with tumour-free mice, the titres of anti-Id antibodies (Fig. 3d) in tumour-bearing mice were not significantly different between PBS or unpulsed DCs controls, while the titres of anti-KLH antibodies in tumour-bearing mice (Fig. 3f) were comparable to those found in tumour-free mice. Mice receiving tumour lysate-KLH-pulsed DC vaccine had significantly higher titres of anti-lysate (Fig. 3b) and anti-KLH (Fig. 3c) antibodies than PBS or unpulsed DCs controls in treated tumour-free mice (P < 0·01, compared with mice receiving PBS or unpulsed DCs) as well as in tumour-bearing mice (Fig. 3e,f; P < 0·01, compared with mice receiving PBS or unpulsed DCs). These results suggest a role for the humoral immune responses in protecting mice from developing myeloma induced by the tumour lysate-pulsed DC vaccine or Id-pulsed DC vaccine. Moreover, the lower titres of anti-Id antibodies in tumour-bearing mice than tumour-free mice may be the result of binding and neutralizing of the antibodies by the large amounts of circulating IgG2b Id protein, rather than the inability of the mice to mount humoral immune responses against the antigens.
Fig. 3.
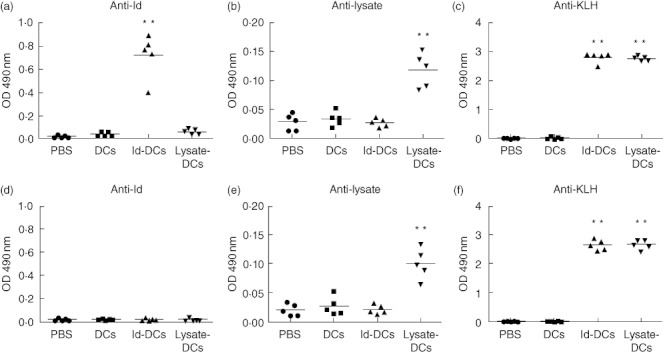
Humoral immune responses in mice receiving dendritic cell (DC) vaccines. Titres of serum anti-idiotype (Id) (a), anti-lysate (b) and anti-keyhole limpet haemocyanin (KLH) (c) antibodies in tumour-free mice; and titres of anti-Id (d), anti-lysate (e) and anti-KLH (f) antibodies in tumour-bearing mice after three weekly subcutaneous injections of phosphate-buffered saline (PBS), unpulsed DCs, Id-KLH-pulsed DC vaccine or tumour lysate-KLH-pulsed DC vaccine. Shown are the absorbance values of enzyme-linked immunosorbent assay (ELISA) results at 1 : 200 dilution of serum from mice (five per group) 1 week after the third vaccination, and the bars represent the mean absorbance in each group. Representative results from one of two independent experiments are shown. **P < 0·01, compared with PBS or unpulsed DC controls.
Tumour lysate-pulsed DC vaccine or idiotype-pulsed DC vaccine induced strong IFN-γ T cell immune responses against myeloma cells
To examine the vaccine-induced cellular immune responses, we analysed the type of T cell responses induced by the vaccines in tumour-free mice after vaccination with tumour lysate-KLH-pulsed DCs or Id-KLH-pulsed DCs. Splenocytes were collected and restimulated in vitro with Id protein, tumour lysate or irradiated 5TGM1 myeloma cells for 24 h. Intracellular cytokine staining showed that Id-KLH-pulsed DC vaccine or tumour lysate-KLH-pulsed DC vaccine, but not PBS or unpulsed DCs, induced significantly increased percentages of IFN-γ-expressing CD4+ (Fig. 4a) and CD8+ T cells (Fig. 4b) in response to Id protein (P < 0·01, compared with mice receiving PBS or unpulsed DCs) or tumour lysate (P < 0·01, compared with mice receiving PBS or unpulsed DCs). IFN-γ-expressing CD4+ and CD8+ T cells were induced in mice receiving Id-KLH-pulsed DC vaccine (P < 0·01, compared with mice receiving PBS or unpulsed DCs) or tumour lysate-KLH-pulsed DC vaccine (P < 0·01, compared with mice receiving PBS or unpulsed DCs) in response to 5TGM1 myeloma cells compared with PBS or unpulsed DCs controls. In addition, the numbers of 5TGM1 myeloma cell-stimulated IFN-γ-secreting CD8+, but not CD4+ T cells in mice receiving tumour lysate-KLH pulsed DC vaccine, was significantly higher than in mice treated with Id-KLH-pulsed DC vaccine (P < 0·05). The percentages of IFN-γ-expressing CD4+ (Fig. 4c) and CD8+ T cells (Fig. 4d) were summarized from three independent experiments. Similar results were obtained using ELISA assay, measuring the secretion of IFN-γ of splenocytes in response to 5TGM1 myeloma cells from the four different groups of mice (Fig. 4e). These findings demonstrate that tumour lysate-KLH-pulsed DC vaccine or Id-KLH-pulsed DC vaccine induced predominantly tumour-specific IFN-γ CD8+ and CD4+ T cell responses. In addition, tumour lysate-pulsed DC vaccine induced stronger IFN-γ T cell immune responses than Id-pulsed DC vaccine.
Fig. 4.
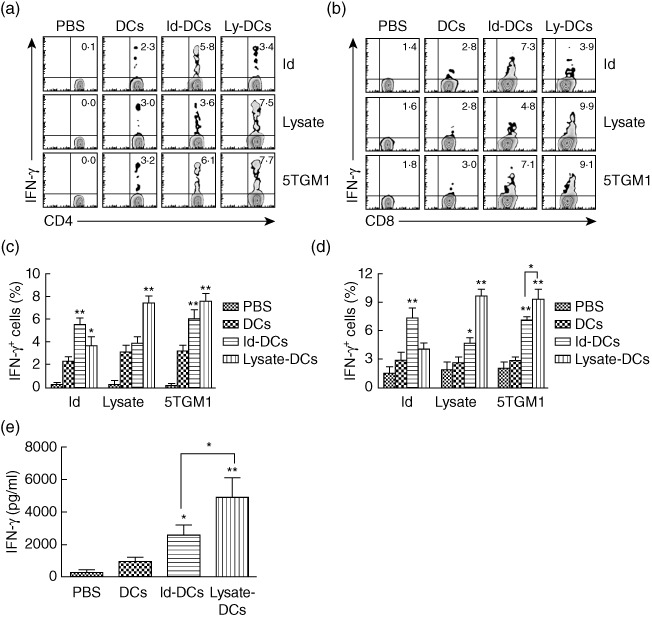
Cellular immune responses in mice receiving dendritic cell (DC) vaccines. Flow cytometry analyses showing the expression of interferon (IFN)-γ in gated CD4+ (a) or CD8+ (b) T cells in response to idiotype (Id) protein, tumour lysate (lysate) or irradiated 5TGM1 myeloma cells (5TGM1). Values in each dot-plot represent the percentages of CD4+ or CD8+ T cells expressing IFN-γ. Summarized data for percentages of IFN-γ-expressing CD4+ (c) or CD8+ (d) T cells from three different experiments are shown. In these studies, tumour-free mice (three per group) were vaccinated with three weekly subcutaneous injections of Id-keyhole limpet haemocyanin (KLH)-pulsed DC vaccine (Id-DCs) or tumour lysate-KLH-pulsed DC vaccine (lysate-DCs). Phosphate-buffered saline (PBS) and unpulsed DCs (DCs) served as controls. One week later, splenocytes were isolated, pooled and restimulated with Id protein, tumour lysate or irradiated 5TGM1 myeloma cells for 24 h. (e) Amount of secreted IFN-γ by tumour-specific T cells in splenocytes of mice that were restimulated with irradiated 5TGM1 myeloma cells for 3 days. Cytokine in cell culture media was quantified by enzyme-linked immunosorbent assay (ELISA). Representative results from one of three independent experiments are shown. The error bars represent stand deviations of three independent experiments. **P < 0·01, compared with PBS or unpulsed DC controls.
To examine the functional properties of T cells in mice receiving tumour lysate-KLH-pulsed DC vaccine or Id-KLH-pulsed DC vaccine, we examined T cell proliferative responses induced in response to Id protein, tumour lysate or irradiated 5TGM1 myeloma cells by using CFSE dilution assay. Splenocytes from mice receiving Id-KLH-pulsed DC vaccine or tumour lysate-KLH-pulsed DC vaccine were collected, labelled with 5 µM CFSE and restimulated in vitro with Id protein, tumour lysate or irradiated 5TGM1 myeloma cells for 5 days. CD4+ (Fig. 5a) or CD8+ (Fig. 5b) T cell proliferative response was induced in mice receiving Id-KLH-pulsed DC vaccine or tumour lysate-KLH-pulsed DC vaccine in response to Id protein (P < 0·01, compared with mice receiving PBS or unpulsed DCs) or tumour lysate (P < 0·01, compared with mice receiving PBS or unpulsed DCs) compared with PBS or unpulsed DCs controls. In addition, culture of CD4+ and CD8+ T cells from mice receiving Id-KLH-pulsed DC vaccine or tumour lysate-KLH-pulsed DC vaccine resulted in high percentages of proliferating T cells cultured with irradiated 5TGM1 myeloma cells (P < 0·01, compared with mice receiving PBS or unpulsed DCs). Moreover, the 5TGM1 myeloma cell-specific CD8+ T cell proliferative responses but not CD4+ T cells in mice receiving tumour lysate-KLH-pulsed DC vaccine was significantly stronger than that induced by Id-KLH-pulsed DC vaccine (P < 0·05). The percentages of proliferating CD4+ (Fig. 5c) and CD8+ (Fig. 5d) T cells were summarized from three independent experiments. These results confirm that Id- and tumour-specific CD4+ or CD8+ T cells were generated in mice receiving Id-KLH-pulsed DC vaccine or tumour lysate-KLH-pulsed DC vaccine.
Fig. 5.
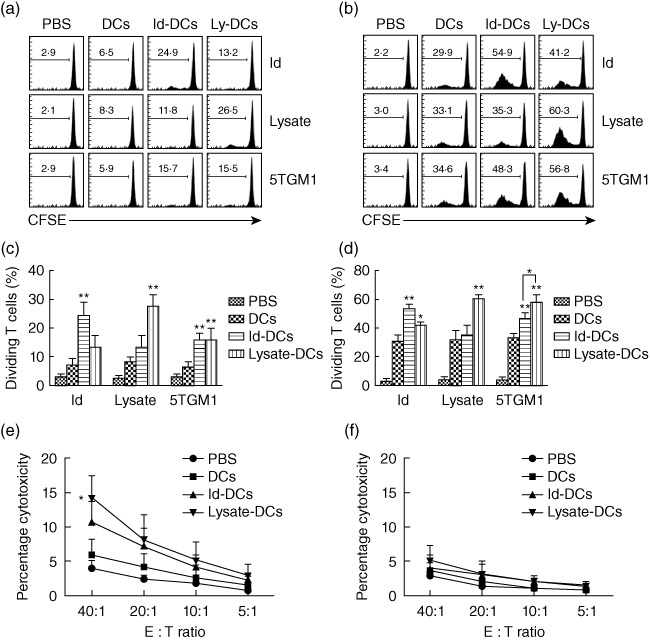
Proliferative and cytotoxic activity of T cells in mice receiving dendritic cell (DC) vaccines. 5,6-Carboxyfluorescein diacetate succinimidyl ester (CFSE) dilution assay showing the percentages of proliferating CD4+ (a) and CD8+ (b) T cells in splenocytes of mice after restimulation in vitro with idiotype (Id) protein (Id), tumour lysate (lysate) or irradiated 5TGM1 myeloma cells (5TGM1) for 5 days. Values in each histogram represent the percentages of dividing CD4+ or CD8+ T cells. Summarized data for percentages of proliferating CD4+ (c) or CD8+ (d) T cells from three different experiments are shown. Also shown is cytotoxicity activity against 5TGM1 myeloma cells (e) or B16 melanoma cells (f) of splenocytes of mice after restimulation with irradiated 5TGM1 myeloma cells for 7 days. In these studies, tumour-free mice (three per group) were vaccinated with three weekly subcutaneous injections of phosphate-buffered saline (PBS), unpulsed DCs (DCs), Id-KLH-pulsed DC vaccine (Id-DCs) or tumour lysate-KLH-pulsed DC vaccine (lysate-DCs). Splenocytes were isolated, pooled, prelabelled with 5 µM CFSE, restimulated with Id protein, tumour lysate or irradiated 5TGM1 cells for 5 days, and measured for CSFE dilution. Splenocytes were isolated, pooled and restimulated with irradiated 5TGM1 myeloma cells for 7 days and the percentage of cytotoxicity was measured by 51Cr-release assay. Representative results from one of three experiments are shown. The error bars represent standard deviations of three independent experiments. *P < 0·05; **P < 0·01, compared with PBS or unpulsed DC controls.
Interestingly, compared with PBS control mice, splenocytes from mice vaccinated with DCs alone also showed T cell activation, such as increased IFN-γ secretion (Fig. 4) and proliferation (Fig. 5), although they were significantly lower compared with mice vaccinated with Id-KLH- or tumour lysates-KLH-pulsed DCs. We believe that this was caused by T cell responses to contaminated FBS in which murine DCs were generated. As all mice except PBS controls received injections of DCs cultured in FBS, these mice were also vaccinated against FBS. Therefore, FBS-specific T cell responses were observed in all mice vaccinated with DCs.
To evaluate the induction of tumour-specific CTL responses in vaccinated mice, the standard 51Cr-release assay was used. Splenocytes from mice receiving the vaccines were restimulated with irradiated 5TGM1 myeloma cells for 7 days and subjected to analysis. As shown in Fig. 5e, T cells from mice receiving tumour lysate-KLH-pulsed DC vaccine (P < 0·05, compared with mice receiving unpulsed DCs), but not Id-KLH-pulsed DC vaccine, lysed 5TGM1 myeloma cells efficiently and specifically compared with PBS or unpulsed DCs controls. No killing was observed against B16 melanoma cells (Fig. 5f). These results indicate that the tumour lysate-KLH-pulsed DC vaccine was able to induce therapeutic myeloma-specific CTL responses rather than Id-pulsed DC vaccine in vivo.
Tumour lysate-pulsed DC vaccine or idiotype-pulsed DC vaccine protected surviving mice from tumour rechallenge
Flow cytometric analysis showed that both CD4+ and CD8+ (CD44high) memory T cells were increased in mice receiving either Id-KLH-pulsed DC vaccine or tumour lysate-KLH-pulsed DC vaccine (P < 0·01, compared with mice receiving PBS or unpulsed DCs; Fig. 6a). Moreover, tumour lysate-KLH-pulsed DC vaccine induced significantly higher percentages of CD4+ CD44high memory T cells than Id-KLH-pulsed DC vaccine (P < 0·05). Quantitative expression of these molecules was summarized from three independent experiments in Fig. 6b.
Fig. 6.
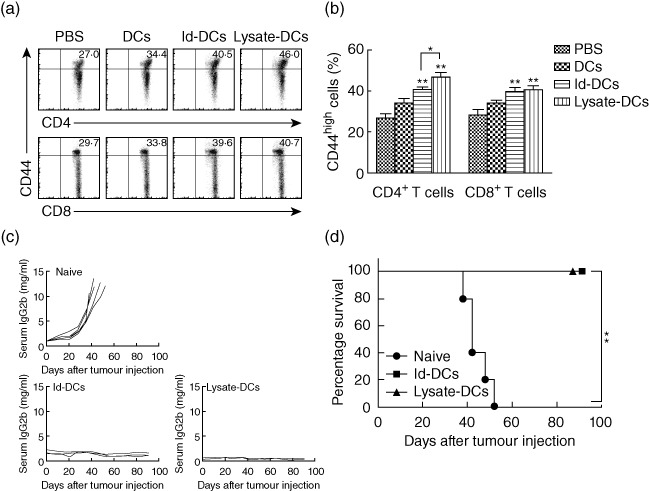
Dendritic cell (DC) vaccines protect surviving mice against tumour rechallenge. Flow cytometry analyses shows the expression of CD4+ or CD8+ T cell surface marker and CD44 memory T cell marker (a). Values in each plot represent the percentages of CD44high T cells on gated CD4+ or CD8+ T cells. (b) Summarized data for percentages of CD44high T cells on gated CD4+ or CD8+ from three different experiments are shown. In these studies, tumour-free mice (three per group) were vaccinated with three weekly subcutaneous injections of phosphate-buffered saline (PBS), unpulsed DCs (DCs), idiotype-keyhole limpet haemocyanin (Id-KLH)-pulsed DC vaccine (Id-DCs) or tumour lysate-KLH-pulsed DC vaccine (lysate-DCs). Representative results from one of three independent experiments are shown. Tumour burden measured as levels of serum immunoglobulin (Ig)G2b Id protein (c) and survival curve (d) of naive mice (n = 5) or surviving mice from prior prophylactic and therapeutic studies of Id-KLH-pulsed DC vaccine (n = 3) or tumour lysate-KLH-pulsed DC vaccine (n = 4) after tumour rechallenge. In the studies, surviving mice were rechallenged 4 months later with intravenous injection of 1 × 106 5TGM1 myeloma cells and followed for tumour burden and survival. Naive mice served as the control and were injected with the same number of 5TGM1 myeloma cells. The error bars represent standard deviations of three independent experiments. **P < 0·01, compared with PBS or unpulsed DC controls.
To examine whether the vaccines promote an induction of tumour-specific memory immunity, mice that survived without tumour burden in the prophylactic and therapeutic experiments were rechallenged with the 5TGM1 myeloma cells 4 months after the first tumour inoculation. As controls, naive mice were also injected with the tumour cells. As shown in Fig. 6c, all naive mice developed myeloma, whereas all mice receiving Id-KLH-pulsed DC vaccine and tumour lysate-KLH-pulsed DC vaccine (P < 0·01, compared with naive mice) displayed no increase in serum IgG2b Id protein and showed no sign of myeloma growth. Mouse survival data are shown in Fig. 6d; all naive mice died within 52 days from tumour injection, whereas 100% of mice receiving either Id-KLH-pulsed DC vaccine or tumour lysate-KLH-pulsed DC vaccine, respectively, survived without detectable tumour burden after tumour rechallenge (P < 0·01, compared with naive mice). These results demonstrate that tumour lysate-KLH-pulsed DC vaccine or Id-KLH-pulsed DC vaccine induced myeloma-specific memory immune responses, which protected mice efficiently from tumour rechallenge.
Discussion
DC-based vaccines require that the cells present one or more tumour antigens to the T cells. A number of antigens associated with various tumours have been explored for immunotherapy. Id protein, secreted by myeloma cells, has been the main target for immunotherapy in B cell malignancies, including MM, as it is the best-defined tumour-specific antigen [3,29]. Id-pulsed DC vaccine has been used for these malignancies. Unfortunately, the results have been somewhat disappointing, because Id protein has been weak in promoting myeloma-specific immune responses and maintaining tumour-specific responses with large amounts of tumour burden in vivo. Thus, it is necessary to search for other tumour antigens for immunotherapy in MM. Tumour cells can be a source of tumour-specific antigens for immunotherapy because it may contain a multitude of tumour antigens. Although the target proteins were undefined, the tumour-derived protein extracts may be used to increase the anti-tumour immunity in response to more than one tumour-associated antigen [30]. Tumour cell lysate-pulsed DCs as antigen-presenting cells have been used for immunotherapy in numerous cancers, such as melanoma, T cell lymphoma, leukaemia and renal cell carcinoma [31–34].
Although both types of vaccines, such as Id-pulsed DCs or myeloma tumour cell lysate-pulsed DCs, have been shown previously to be able to induce potent immunological and clinical responses in both preclinical and clinical studies, they have never been compared in terms of whether these two vaccines are equally efficient at inducing anti-myeloma immunity. Thus, in this study, we have used Id- and 5TGM1 myeloma cell lysate-pulsed DC vaccines to promote, accelerate and prolong tumour-specific immune responses, and we compared the capacity of these two DC vaccines to prevent and treat myeloma in the 5TGM1 myeloma mouse model, which manifests similarly to the human disease, including: monoclonal gammopathy, marrow replacement, osteolytic bone lesions and hypercalcaemia [35,36]. Our results showed clearly that Id-pulsed DC vaccine or tumour lysate-pulsed DC vaccine, but not unpulsed DCs, protected mice efficiently against developing myeloma and protected surviving mice from tumour rechallenge, and retarded tumour growth and induced tumour regression against established tumour. In prophylactic and therapeutic experiments of the vaccination, 30 and 10%, respectively, of mice receiving tumour lysate-pulsed DC vaccine survived myeloma, whereas 20 and 10%, respectively, of mice receiving Id-pulsed DC vaccine survived myeloma. These results showed clearly that the tumour lysate-pulsed DC vaccine is more effective than Id-pulsed DC vaccine for DC-based immunotherapy in myeloma. Our mechanistic studies showed that the tumour lysate-pulsed DC vaccine induced a significantly stronger anti-myeloma immunity than Id-pulsed DC vaccine. Id protein is a potential target for the immunotherapy of B cell lymphoma as well as myeloma. However, Id-based vaccination as a single antigen has disadvantages in MM, because Id-specific T cells can be tolerized to the protein by elevated Id proteins in patients with MM [37]. Conversely, tumour lysate-pulsed DCs may induce a polyclonal expansion of T cells, which contain multiple known and unknown antigen-specific epitopes, including both MHC class I-restricted CTLs and MHC class II-restricted T helper type 1 (Th1). Thus, these various myeloma-specific T cells resulted in a synergistic anti-myeloma response.
We also demonstrated that tumour lysate-pulsed DC vaccine induced significantly higher titres of tumour lysate- and KLH-specific antibodies than PBS or unpulsed DCs controls in tumour-free mice as well as in tumour-bearing mice. However, in mice receiving Id-KLH-pulsed DC vaccine, we noticed that the titres of anti-Id antibodies were lower in tumour-bearing mice compared with vaccinated tumour-free mice. This phenomenon can be explained by the presence of a large amount of circulating Id proteins in tumour-bearing mice that probably neutralize the antibodies [27]. The circulating Id proteins may block the Id-specific humoral immune responses against myeloma tumour cells, which usually do not express or express low levels of surface Id proteins. Therefore, the role of tumour antigen-specific antibodies in controlling myeloma growth in vivo is unclear. Nevertheless, the fact that we can still detect a large amount of anti-lysate antibodies in tumour-bearing mice suggests that these various tumour antigen-specific antibodies may play a role in mediating the killing of myeloma cells in vivo, directly or indirectly, with the help of killer cells and/or complements. Moreover, we showed that the efficacy of Id-KLH-pulsed DC vaccine or tumour lysate-KLH-pulsed DC vaccine to eradicate established myeloma was associated with an induction and expansion of potent tumour-specific CD4+ and CD8+ T cell responses, increasing capacity of tumour-specific CD8+ CTLs to lyse myeloma cells and generation of CD4+ and CD8+ memory T cells.
In conclusion, our study shows that tumour lysate-pulsed DC vaccine protected mice efficiently and eradicated tumour in the myeloma mouse model. The therapeutic immunity against myeloma induced by the vaccines was associated with an induction of humoral immune response, such as tumour lysate-specific antibodies and cellular immune responses, including myeloma-specific CTLs and Th1 cells. In addition, the vaccines induced specific immune memory responses that were able to protect surviving mice from tumour rechallenge. Thus, this study shows clearly that tumour lysate-pulsed DC vaccine is much better than Id-pulsed DC vaccine for DC-based immunotherapy in MM. This information is important for improving the efficacy of DC-based immunotherapy for patients with MM, and possibly other B cell tumours.
Acknowledgments
We thank Ms Angelique Harkins for providing editorial assistance. This study was supported by The University of Texas MD Anderson Cancer Center, National Cancer Institute grants R01s (CA96569, CA103978, CA138402 and CA138398) and P50 CA142509, the Leukemia and Lymphoma Society Translational Research Grants, Multiple Myeloma Research Foundation and Commonwealth Foundation for Cancer Research.
Disclosure
No potential conflicts of interest were disclosed.
References
- 1.Palumbo A, Anderson K. Multiple myeloma. N Engl J Med. 2011;364:1046–60. doi: 10.1056/NEJMra1011442. [DOI] [PubMed] [Google Scholar]
- 2.Kyle RA, Rajkumar SV. Multiple myeloma. Blood. 2008;111:2962–72. doi: 10.1182/blood-2007-10-078022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Yi Q. Immunotherapy in multiple myeloma: current strategies and future prospects. Exp Rev Vaccines. 2003;2:391–8. doi: 10.1586/14760584.2.3.391. [DOI] [PubMed] [Google Scholar]
- 4.Rosenberg SA, Yang JC, Restifo NP. Cancer immunotherapy: moving beyond current vaccines. Nat Med. 2004;10:909–15. doi: 10.1038/nm1100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Yi Q. Novel immunotherapies. Cancer J. 2009;15:502–10. doi: 10.1097/PPO.0b013e3181c51f0d. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Banchereau J, Steinman RM. Dendritic cells and the control of immunity. Nature. 1998;392:245–52. doi: 10.1038/32588. [DOI] [PubMed] [Google Scholar]
- 7.Young JW, Inaba K. Dendritic cells as adjuvants for class I major histocompatibility complex-restricted antitumor immunity. J Exp Med. 1996;183:7–11. doi: 10.1084/jem.183.1.7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Grabbe S, Beissert S, Schwarz T, Granstein RD. Dendritic cells as initiators of tumor immune responses: a possible strategy for tumor immunotherapy? Immunol Today. 1995;16:117–21. doi: 10.1016/0167-5699(95)80125-1. [DOI] [PubMed] [Google Scholar]
- 9.Girolomoni G, Ricciardi-Castagnoli P. Dendritic cells hold promise for immunotherapy. Immunol Today. 1997;18:102–4. doi: 10.1016/s0167-5699(97)01030-x. [DOI] [PubMed] [Google Scholar]
- 10.Steinman RM, Dhodapkar M. Active immunization against cancer with dendritic cells: the near future. Int J Cancer. 2001;94:459–73. doi: 10.1002/ijc.1503. [DOI] [PubMed] [Google Scholar]
- 11.Wen YJ, Barlogie B, Yi Q. Idiotype-specific cytotoxic T lymphocytes in multiple myeloma: evidence for their capacity to lyse autologous primary tumor cells. Blood. 2001;97:1750–5. doi: 10.1182/blood.v97.6.1750. [DOI] [PubMed] [Google Scholar]
- 12.Hong S, Qian J, Yang J, Li H, Kwak LW, Yi Q. Roles of idiotype-specific t cells in myeloma cell growth and survival: Th1 and CTL cells are tumoricidal while Th2 cells promote tumor growth. Cancer Res. 2008;68:8456–64. doi: 10.1158/0008-5472.CAN-08-2213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Titzer S, Christensen O, Manzke O, et al. Vaccination of multiple myeloma patients with idiotype-pulsed dendritic cells: immunological and clinical aspects. Br J Haematol. 2000;108:805–16. doi: 10.1046/j.1365-2141.2000.01958.x. [DOI] [PubMed] [Google Scholar]
- 14.Yi Q, Desikan R, Barlogie B, Munshi N. Optimizing dendritic cell-based immunotherapy in multiple myeloma. Br J Haematol. 2002;117:297–305. doi: 10.1046/j.1365-2141.2002.03411.x. [DOI] [PubMed] [Google Scholar]
- 15.Curti A, Tosi P, Comoli P, et al. Phase I/II clinical trial of sequential subcutaneous and intravenous delivery of dendritic cell vaccination for refractory multiple myeloma using patient-specific tumour idiotype protein or idiotype (VDJ)-derived class I-restricted peptides. Br J Haematol. 2007;139:415–24. doi: 10.1111/j.1365-2141.2007.06832.x. [DOI] [PubMed] [Google Scholar]
- 16.Yi Q, Szmania S, Freeman J, et al. Optimizing dendritic cell-based immunotherapy in multiple myeloma: intranodal injections of idiotype-pulsed CD40 ligand-matured vaccines led to induction of type-1 and cytotoxic T-cell immune responses in patients. Br J Haematol. 2010;150:554–64. doi: 10.1111/j.1365-2141.2010.08286.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Wen YJ, Min R, Tricot G, Barlogie B, Yi Q. Tumor lysate-specific cytotoxic T lymphocytes in multiple myeloma: promising effector cells for immunotherapy. Blood. 2002;99:3280–5. doi: 10.1182/blood.v99.9.3280. [DOI] [PubMed] [Google Scholar]
- 18.Raje N, Hideshima T, Davies FE, et al. Tumour cell/dendritic cell fusions as a vaccination strategy for multiple myeloma. Br J Haematol. 2004;125:343–52. doi: 10.1111/j.1365-2141.2004.04929.x. [DOI] [PubMed] [Google Scholar]
- 19.Hao S, Bi X, Xu S, et al. Enhanced antitumor immunity derived from a novel vaccine of fusion hybrid between dendritic and engineered myeloma cells. Exp Oncol. 2004;26:300–6. [PubMed] [Google Scholar]
- 20.Walewska R, Teobald I, Dunnion D, et al. Preclinical development of hybrid cell vaccines for multiple myeloma. Eur J Haematol. 2007;78:11–20. doi: 10.1111/j.1600-0609.2006.00769.x. [DOI] [PubMed] [Google Scholar]
- 21.Garrett IR, Dallas S, Radl J, Mundy GR. A murine model of human myeloma bone disease. Bone. 1997;20:515–20. doi: 10.1016/s8756-3282(97)00056-2. [DOI] [PubMed] [Google Scholar]
- 22.Mundy G. Preclinical models of bone metastases. Semin Oncol. 2001;28:2–8. doi: 10.1016/s0093-7754(01)90225-8. [DOI] [PubMed] [Google Scholar]
- 23.Liso A, Stockerl-Goldstein KE, Auffermann-Gretzinger S, et al. Idiotype vaccination using dendritic cells after autologous peripheral blood progenitor cell transplantation for multiple myeloma. Biol Blood Marrow Transplant. 2000;6:621–7. doi: 10.1016/s1083-8791(00)70027-9. [DOI] [PubMed] [Google Scholar]
- 24.Kwak LW, Young HA, Pennington RW, Weeks SD. Vaccination with syngeneic, lymphoma-derived immunoglobulin idiotype combined with granulocyte/macrophage colony-stimulating factor primes mice for a protective T-cell response. Proc Natl Acad Sci USA. 1996;93:10972–7. doi: 10.1073/pnas.93.20.10972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Lutz MB, Kukutsch N, Ogilvie AL, et al. An advanced culture method for generating large quantities of highly pure dendritic cells from mouse bone marrow. J Immunol Methods. 1999;223:77–92. doi: 10.1016/s0022-1759(98)00204-x. [DOI] [PubMed] [Google Scholar]
- 26.Qian J, Wang S, Yang J, et al. Targeting heat shock proteins for immunotherapy in multiple myeloma: generation of myeloma-specific CTLs using dendritic cells pulsed with tumor-derived gp96. Clin Cancer Res. 2005;11:8808–15. doi: 10.1158/1078-0432.CCR-05-1553. [DOI] [PubMed] [Google Scholar]
- 27.Wang S, Hong S, Wezeman M, Qian J, Yang J, Yi Q. Dendritic cell vaccine but not idiotype-KLH protein vaccine primes therapeutic tumor-specific immunity against multiple myeloma. Front Biosci. 2007;12:3566–75. doi: 10.2741/2335. [DOI] [PubMed] [Google Scholar]
- 28.Qian J, Xie J, Hong S, et al. Dickkopf-1 (DKK1) is a widely expressed and potent tumor-associated antigen in multiple myeloma. Blood. 2007;110:1587–94. doi: 10.1182/blood-2007-03-082529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Houot R, Levy R. Vaccines for lymphomas: idiotype vaccines and beyond. Blood Rev. 2009;23:137–42. doi: 10.1016/j.blre.2008.09.001. [DOI] [PubMed] [Google Scholar]
- 30.Boon T, Cerottini JC, Van den Eynde B, van der Bruggen P, Van Pel A. Tumor antigens recognized by T lymphocytes. Annu Rev Immunol. 1994;12:337–65. doi: 10.1146/annurev.iy.12.040194.002005. [DOI] [PubMed] [Google Scholar]
- 31.Nestle FO, Alijagic S, Gilliet M, et al. Vaccination of melanoma patients with peptide- or tumor lysate-pulsed dendritic cells. Nat Med. 1998;4:328–32. doi: 10.1038/nm0398-328. [DOI] [PubMed] [Google Scholar]
- 32.Maier T, Tun-Kyi A, Tassis A, et al. Vaccination of patients with cutaneous T-cell lymphoma using intranodal injection of autologous tumor-lysate-pulsed dendritic cells. Blood. 2003;102:2338–44. doi: 10.1182/blood-2002-08-2455. [DOI] [PubMed] [Google Scholar]
- 33.Holtl L, Zelle-Rieser C, Gander H, et al. Immunotherapy of metastatic renal cell carcinoma with tumor lysate-pulsed autologous dendritic cells. Clin Cancer Res. 2002;8:3369–76. [PubMed] [Google Scholar]
- 34.Hus I, Rolinski J, Tabarkiewicz J, et al. Allogeneic dendritic cells pulsed with tumor lysates or apoptotic bodies as immunotherapy for patients with early-stage B-cell chronic lymphocytic leukemia. Leukemia. 2005;19:1621–7. doi: 10.1038/sj.leu.2403860. [DOI] [PubMed] [Google Scholar]
- 35.Asosingh K, Radl J, Van Riet I, Van Camp B, Vanderkerken K. The 5TMM series: a useful in vivo mouse model of human multiple myeloma. Hematol J. 2000;1:351–6. doi: 10.1038/sj.thj.6200052. [DOI] [PubMed] [Google Scholar]
- 36.Radl J, De Glopper ED, Schuit HR, Zurcher C. Idiopathic paraproteinemia. II. Transplantation of the paraprotein-producing clone from old to young C57BL/KaLwRij mice. J Immunol. 1979;122:609–13. [PubMed] [Google Scholar]
- 37.Bogen B, Schenck K, Munthe LA, Dembic Z. Deletion of idiotype (Id)-specific T cells in multiple myeloma. Acta Oncol. 2000;39:783–8. doi: 10.1080/028418600750063505. [DOI] [PubMed] [Google Scholar]


