Abstract
The involvement of granulocytes in immune response against cancer is not well understood. Depending on the cytokine milieu in which they act and on their oxidative burst, granulocytes may play either an inhibitory or stimulatory role in tumour growth. Unsaturated fatty acids, essential components of cellular membranes and storage lipids, are susceptible to granulocyte-derived reactive oxygen species (ROS). ROS can induce lipid peroxidation (LPO) resulting in the destruction of biomembranes. Thus, murine W256 tumour progressing and tumour regressing animal models were used to study the involvement of plasma inflammatory mediators and oxidative burst of circulating granulocytes in malignant destruction and detrimental tumour growth. The involvement of LPO-derived aldehydes (i.e. acrolein, 4-hydroxy-2-nonenal and malondialdehyde) and myeloperoxidase (MPO) appearance in the granulocyte anti-cancer response were further evaluated. The results obtained revealed a significant increase in neutrophil elastase in animals with regressing tumour. Furthermore, the presence of MPO in tumour microenvironment was accompanied by the formation of acrolein only 5 h after tumour transplantation and its presence increased during tumour regression. Later, at an early stage of tumour regression, the presence of other LPO-derived aldehydes were also observed. The results obtained suggest that elevated neutrophil elastase and initiation of LPO may play an important role in the tumour development leading to tumour regression.
Keywords: granulocytes, interleukin 17, lipid peroxidation, neutrophil elastase, tumour regression
Introduction
Granulocytes are the most abundant white blood cells, and play a fundamental role in the innate immune response [1]. Despite their known function as professional phagocytes, the role of granulocytes in tumour development has been controversial, and has received little attention compared with that of macrophages. Recently, it was reported that spontaneous regression/complete resistance to tumour cells is mediated by rapid infiltration of leucocytes, mainly as a consequence of innate immune response [2,3]. In addition, the administration of granulocytes at the site of solid tumours can lead to tumour regression or can slower tumour growth and extend the overall survival of animals [4].
It is known that granulocyte activation is accompanied by the intense production of reactive oxygen species (ROS) and an extended release of destructive hydrolytic enzymes. Granulocyte-derived ROS have been identified as effector molecules of the oxygen-dependent killing of cancer cells [5,6]. At low concentrations, ROS may function as physiological mediators of cellular responses to various stimuli [7]; however, over-production of ROS is cytotoxic. ROS react with macromolecules, causing DNA damage, protein oxidation and lipid peroxidation (LPO) [8]. LPO of polyunsaturated fatty acids results in the destruction of biomembranes. The final products of LPO are reactive aldehydes such as acrolein, 4-hydroxy-2-nonenal (HNE) and malondialdehyde (MDA) [8]. Unlike reactive free radicals, aldehydes are somewhat long-lived and can therefore diffuse from their original site (i.e. membranes) and attack targets intra- and extracellularly, which are distant from the initial radical event [8]. Moreover, activated granulocytes also employ myeloperoxidase (MPO) to generate reactive aldehydes such as acrolein that may play a role in tumour cell destruction [9].
Cytokines are well-known modulators of the immune response [10] controlling many cellular responses, such as expression of the innate proinflammatory immune response. Recently, the role of immune cells and cytokines in both the pathogenesis of tumorigenesis and the therapeutic response of tumours was reviewed [11]. Therefore, in the present paper we wanted to elucidate the difference in immune modulators [i.e. transforming growth factor beta (TGF-β), interleukin (IL)-17, growth-related oncogene 1 (GRO1), neutrophil elastase, cytokine-induced neutrophil chemoattractant-2 (CINC-2), vascular endothelial growth factor (VEGF), IL-6 and tumour necrosis factor-α (TNF-α)] present at an early stage of tumour progression or regression as well as the importance of lipid-derived aldehydes at an early stage of tumour regression.
Materials and methods
Animals
Experiments were performed on male 3-month-old Sprague–Dawley rats. Water and food were given ad libitum. All the experiments were performed in accordance with the ILAR Guide for the Care and Use of Laboratory Animals, EU Council Directive (86/609/EEC) and the Croatian Animal Protection Act (Official Gazette 135/06).
Treatment of animals
To study the role of immune modulators during tumour progression or regression 18 animals were injected intramuscularly in the right hindlimb with 250 µl of RPMI-1640 medium containing 107 W256 tumour cells harvested from Wistar W256-bearing donors. Nine animals were injected additionally with Sephadex (Sephadex G-150; Pharmacia Fine Chemicals, Sweden), which abolishes spontaneous W256 tumour regression in Sprague–Dawley rats, into the right hindlimb [3,4]. The Sephadex was prepared and used as described elsewhere [6].
Nine tumour-bearing rats were used in order to determine the involvement of LPO during tumour regression. Rats were anaesthetized using intraperitoneal injection of chloralhydrate (150 mg/ml) and killed by bleeding from the heart [12] under ether anaesthesia 5 h, 2 and 6 days after tumour injection. Blood was collected in ethylenediamine tetraacetic acid (EDTA)-coated tubes and further used for MDA analyses. Upon death, tumour mass was removed surgically, fixed in 10% buffered formalin and embedded in paraffin.
Blood samples and functional activity of circulating granulocytes
On days 4 and 7 after W256 injection, blood was taken from the tail vein in EDTA-containing tubes for the differential counting of leucocytes and for plasma preparation [4]. Three healthy rats served as controls in all analyses. Then, 10 µl of blood was used to measure oxidative burst of granulocytes by chemiluminescence assay that was carried out at 37°C using a Berthold FB12 Luminometer, as described previously [13].
Determination of TGF-β, IL-17, GRO1 and elastase
The dot-blot immunostaining method [14] was used to analyse the presence of TGF-β1, IL-17, GRO1 and neutrophil elastase in plasma samples. One hundred microlitres of 5% plasma samples diluted in phosphate-buffered saline (PBS) were spotted onto nitrocellulose membranes (Amersham, UK). The membranes were incubated in blocking solution (1% non-fat milk powder in PBS) at room temperature for 60 min and subsequently incubated overnight with rabbit polyclonal antibodies directed against neutrophil elastase (Santa Cruz, Inc., Santa Cruz, CA, USA) and goat polyclonal antibodies directed against TGF-β (Santa Cruz, Inc.), IL-17 (Santa Cruz, Inc.) and GRO1 (Santa Cruz, Inc.). The labelled streptavidin–biotin (LSAB; Dako, Glostrup, Denmark) method was used for elastase immunoblotting, while the immunoperoxidase technique was used for immunoblotting of TGF-β1, IL-17 and GRO1. Immunocomplexes were visualized using 3,3′-diaminobenzidine tetrahydrochloride (DAB; Dako) staining and scanned for quantization of signals. Negative controls were included in all experiments in which the test antibody was omitted and replaced by control irrelevant diluents. Signals obtained by dot-blot were quantified with a Wersa Doc Imaging system (Biorad, Hercules, CA, USA). The signals were quantified with Quantity Software and expressed as percentage of control.
Determination of CINC-2, VGEF, IL-6 and TNF-α
A commercial rat 4-plex luminex kit (R&D Systems, Minneapolis, MN, USA) was used for determination of CINC-2, VEGF, IL-6 and TNF-α. The assay was performed as described previously [4], and plasma samples were diluted threefold according to the manufacturer's instructions. The assay was performed on a luminex-200™ instrument using AtheNA Analyzer LX200 (full) 2·3 software.
MDA determination by high-performance liquid chromatography (HPLC)
MDA concentration in EDTA plasma samples was analysed by an HPLC system (Shimadzu, Kyoto, Japan) with ultraviolet (UV) detection (533 nm), Midas Spark Holland auto sampler and Waters Spherisorb ODS2, 5 µm, 4·6 × 150 mm column, as described previously [15]. Concentration of MDA was expressed as nmol/ml in EDTA plasma samples.
HNE, acrolein and MPO imunohistochemistry
Sections made from paraffin blocks were stained with haematoxylin and eosin (H&E). Additional staining was carried out for MPO, HNE–protein adducts and acrolein–protein adducts. The sections were examined by a pathologist extremely experienced in tumour histopathology but without previous knowledge of the experimental groups.
For the immunohistochemical detection of acrolein–Lys and HNE–His conjugates, the LSAB method was used. Our genuine antibodies directed against acrolein–Lys and HNE–His conjugates were applied as described previously [16,17]. For the immunohistochemical detection of MPO, a rabbit polyclonal antibody directed against MPO (Dako) was performed using the immunoperoxidase technique. DAB was used as chromogen. The sections were counterstained with haematoxylin. Negative control slides, in which the test antibody was omitted and replaced by control irrelevant diluents, were included in all experiments.
Statistics
Descriptive statistics are shown as the mean ± standard error (s.e.). Significant differences between groups were assessed using Student's t-test and the χ2 test. When more than two groups were compared, we used one-sided analysis of variance (anova) with appropriate post-hoc testing. MedCalc software and spss version 11·01 for Mircosoft Windows were used. Differences with P less than 0·05 were considered statistically significant.
Results
Tumour growth dynamics of W256 tumour-bearing rats is summarized in Fig. 1. As observed, on day 4 there were no significant differences in the tumour volume while later tumours that had progressed were growing rapidly, and tumour volume differed significantly from regressing tumours (P < 0·05). In Fig. 2 histological differences are clearly visible in progressing tumour compared to tumour in regression. The number of peripheral blood granulocytes and their functional activity during tumour progression or regression are also shown in Fig. 2. The number and functional activity of peripheral blood granulocytes were increased significantly in tumour-bearing animals on day 4 after W256 transplantation (Fig. 2, P < 0·05). Tumour progression was associated with excessive oxidative burst and granulocyte numbers (Fig. 2, P < 0·05), whereas the number and activity of blood granulocytes was significantly lower during tumour regression when compared to tumour progression (Fig. 2, P < 0·05).
Fig. 1.
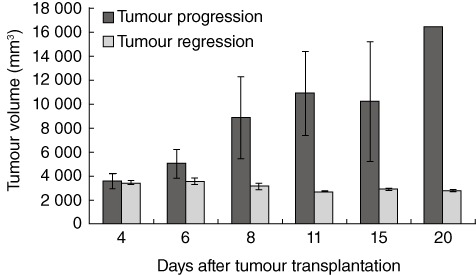
Changes in W256 tumour volume during tumour progression or regression for all animals. The results are presented as mean ± standard error during the 20-day time-period.
Fig. 2.
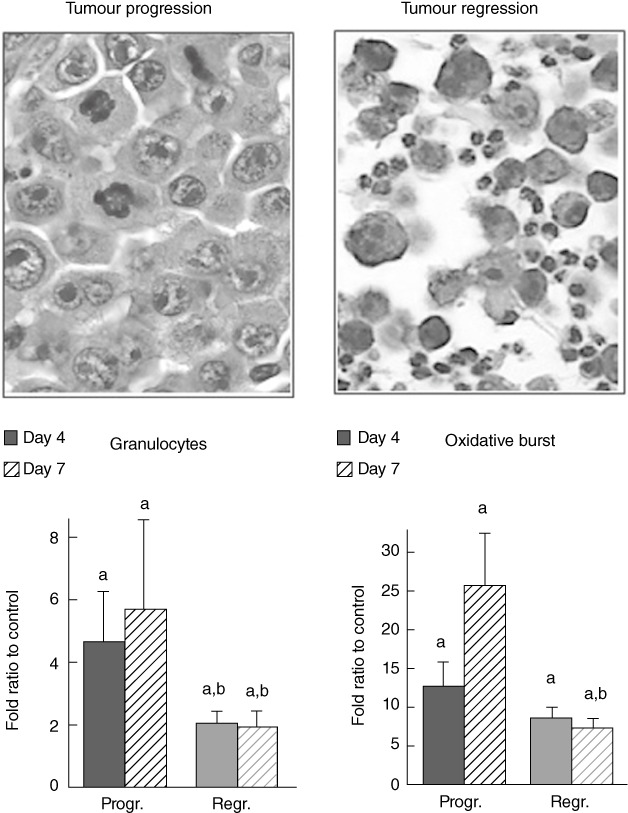
The involvement of granulocytes in tumour growth. Distribution of peripheral blood granulocytes [mean ± standard error (s.e.) per group], granulocyte oxidative burst (1O2 production measured by chemiluminescence assay) during tumour progression or regression (mean ± s.e. per group) and representative histological image of tumour transplantation site taken on day 4 in tumour progression or regression tissue samples (both ×400, haematoxylin and eosin) are shown. (a) Significance P < 0·05 in comparison to healthy controls, (b) significance P < 0·05 in comparison to samples obtained from tumour progressing animals.
Differences in plasma inflammatory mediators during tumour regression or progression are presented in Figs 3 and 4. The data obtained showed elevated plasma levels of TGF-β, IL-17 and GRO1 on day 4 of tumour progression (Fig. 3, P < 0·05), while neutrophil elastase level did not differ from controls (Fig. 3, P > 0·05). On day 7 levels of TGF-β and IL-17 remained increased when compared significantly to control plasma samples (Fig. 3, P < 0·05), while GRO1 and neutrophil elastase did not differ significantly when compared to the control (Fig. 3, P > 0·05). Interestingly, during tumour regression plasma levels of TGF-β were also elevated (Fig. 3, P < 0·05, both days 4 and 7). Although TGF-β level was increased on day 7 of tumour regression it was significantly lower compared to tumour progression samples (P < 0·05). Furthermore, the results revealed increased elastase levels on days 4 and 7 (Fig. 3, P < 0·05). Although IL-17 was unchanged on day 4, it was increased on day 7 (Fig. 3, P < 0·05). IL-17 and elastase plasma levels on day 4 of tumour regression were significantly different from those during tumour progression on the same (fourth) day (Fig. 3, P < 0·05), indicating their possible role at an early stage of tumour development.
Fig. 3.
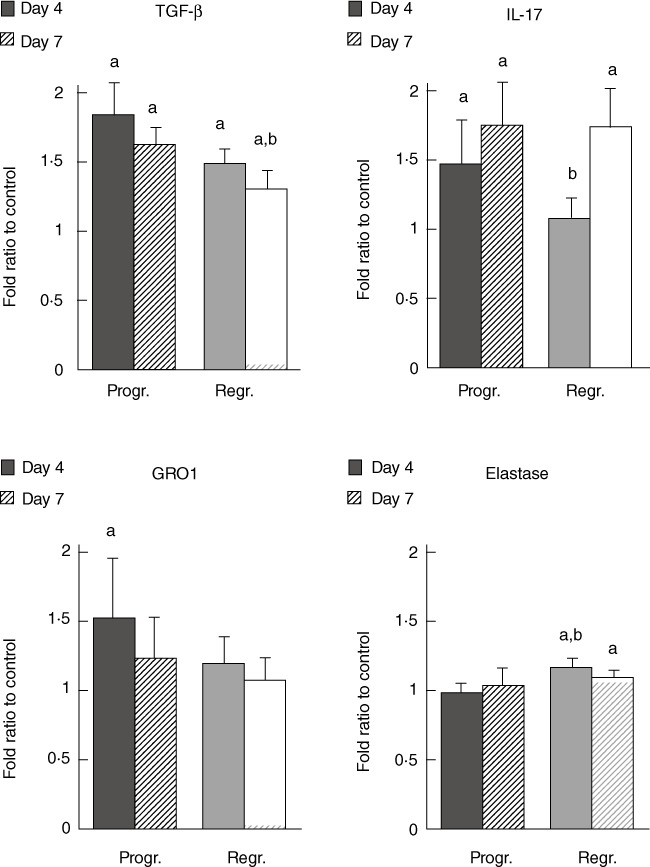
Plasma levels of transforming growth factor (TGF)-β, interleukin (IL)-17, growth-regulated oncogene 1 (GRO1) and elastase assessed by the dot-blot immunostaining method. The results obtained from plasma samples of animals during tumour progression or regression are presented as mean ± standard error per group. (a) Significance P < 0·05 in comparison to healthy controls, (b) significance P < 0·05 in comparison to samples obtained from tumour progressing animals.
Fig. 4.
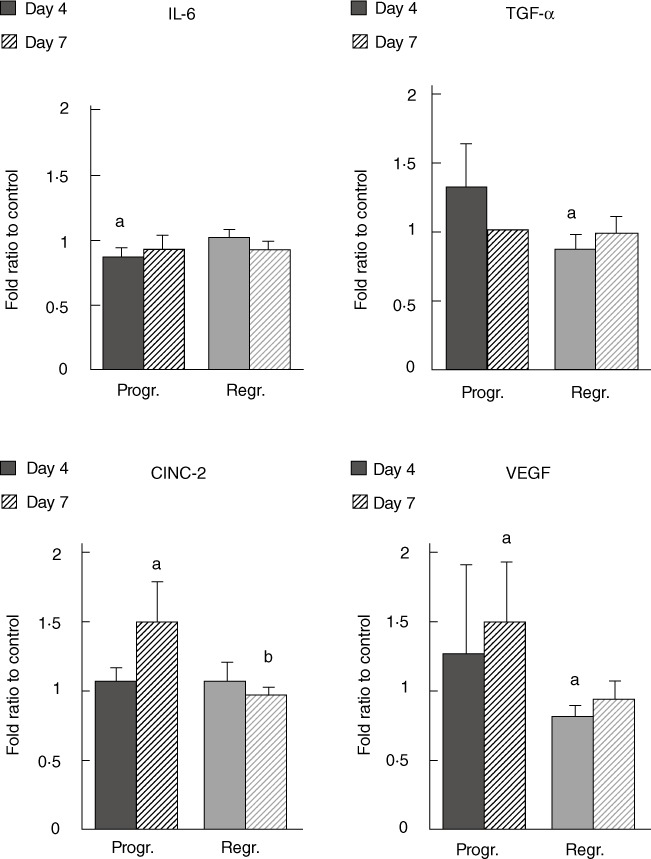
Plasma levels of cytokine-induced neutrophil chemoattractant 2 (CINC-2), vascular endothelial growth factor (VEGF), interleukin (IL)-6 and tumour necrosis factor (TNF)-α determined by xMAP technology. The results obtained from plasma samples of animals during tumour progression or regression are presented as mean ± standard error per group. (a) Significance P < 0·05 in comparison to healthy controls, (b) significance P < 0·05 in comparison to samples obtained from tumour progressing animals.
Analysis of IL-6 plasma levels revealed that it was below control values on day 4 of tumour progression (Fig. 4, P < 0·05), while afterwards and also during tumour regression it did not differ significantly from the control (Fig. 4, P > 0·05). Furthermore, we also observed elevated CINC-2 and VEGF values on day 7 of tumour progression (Fig. 4, P < 0·05) and only a slight increase in TNF-α plasma level on day 4 of tumour progression (Fig. 4, P > 0·05). Conversely, the results revealed decreased VEGF and TNF-α levels on day 4 of tumour regression (Fig. 4, P < 0·05), while CINC-2 did not differ significantly from the control.
The involvement of LPO during tumour regression is shown in Fig. 5. Rapid granulocyte response to the W256 transplantation was revealed by H&E staining (Fig. 5a). Just 5 h after tumour transplantation, granulocytes had infiltrated the injection site. On day 2 after tumour injection, granulocytes were present inside the developing tumour tissue and the limb muscles. The inflammation associated with the healing of the affected limb tissues was present on day 6, but there were no tumour cells. An increased MPO level was observed at all stages during tumour regression (Fig. 5a). However, the presence of reactive aldehydes changed during tumour regression. Tumour cell membranes had already stained positive for acrolein protein conjugates 5 h after tumour transplantation and its presence increased during tumour regression (Fig. 5a). Furthermore, at an early stage of tumour development (days 0 and 2) HNE protein conjugates were not observed, while the presence of HNE was observed later (day 6, Fig. 5a). Another reactive aldehyde, MDA, was monitored by the HPLC method in plasma samples. The results obtained showed increased MDA levels (Fig. 5b, P < 0·05) and blood granulocyte number (Fig. 5c, P < 0·05) on days 2 and 6.
Fig. 5.
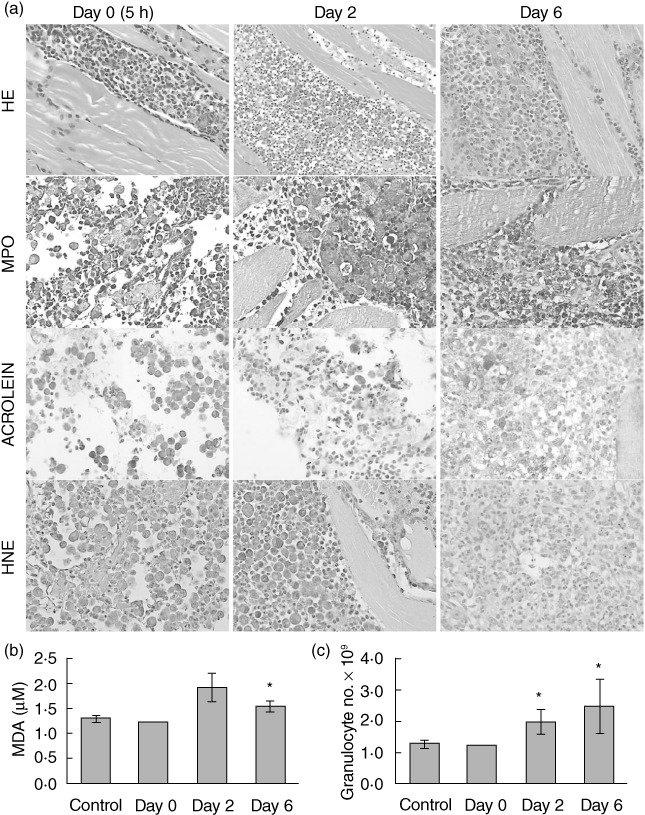
Lipid peroxidation (LPO) during tumour regression. (a) Tumour tissue samples stained with haematoxylin and eosin (H&E), myeloperoxidase (MPO), acrolein and 4-hydroxy-2-nonenal (HNE) on days 0 (5 h), 2 and 6 after tumour transplantation. (b) Changes in MDA concentration and (c) whole blood granulocyte number during tumour regression (mean ± standard error per group). Asterisks indicate statistically significant differences with respect to the values of healthy controls (P < 0·05).
Discussion
The role of granulocytes in tumour development is controversial. The presence of granulocytes may sometimes be detrimental by degrading the extracellular matrix and enhancing angiogenesis, thus favouring malignant growth and progression [18]. Conversely, granulocytes can act as direct effector cells in the host defence against tumour [3,4,19]. Di Carlo and colleagues suggested that these controversial effects are the result of the interplay between the type and amount of tumour-derived cytokines and the degree of recruitment and activation of the intermingled granulocytes [18]. The involvement of IL-17 in tumour development was reviewed recently by Murugaiyan and Saha [20]. IL-17 may promote an anti-tumour response via T cells leading to tumour regression, but can also have a pro-tumour effect by facilitating angiogenesis and egress of tumour cells from the primary focus. Our results support an IL-17 tumour-promoting role; IL-17 was increased significantly at an early stage of tumour progression, while it was unchanged in tumour regressing animals. Furthermore, IL-17 was found to promote angiogenesis [21]. These effects were suggested to be achieved both by direct action of IL-17 and by its co-operation with TNF-α[20]. Our results support this hypothesis, revealing that elevated IL-17 and TNF-α at an early stage of tumour development are associated with elevated VEGF levels and tumour progression. Conversely, we have found that IL-17, TNF-α and VEGF were in the range of control values or even below at an early stage of tumour regression. Moreover, the IL-17–VEGF loop that modulates angiogenesis also includes TGF-β, another angiogenic factor. IL-17-induced VEGF further induces TGF-β, that may then lead to enhanced endothelial cell VEGF receptivity [22]. We have observed a strong increase in the TGF-β level during tumour progression, supporting its important role in tumour development.
Furthermore, during tumour regression we did not observe elevated levels of standard proinflammatory cytokines IL-6 and TNF-α, indicating that tumour regression is not mediated by the ‘standard’ immune response. There is now convincing evidence that IL-6 and TNF-α play a role in carcinogenesis by promoting the survival and proliferation of malignant cells [23,24]. The observed elevated levels of elastase in tumour regression could induce the release of inflammatory mediators and influence granulocyte chemotaxis and cell adhesion [25], thus facilitating tumour regression.
Of particular importance for the host defence against cancer is a strong oxidative burst of granulocytes observed in tumour-bearing animals [3,6,13]. We have shown previously that spontaneous W256 tumour regression in Sprague–Dawley rats can be abolished by distraction of functional granulocytes from the blood [3,4]. The restoration of the functional granulocytes obtained by granulocyte administration at the site of tumour [4] can lead to either tumour regression or slower tumour growth, extending the overall survival. ROS are thought to have both a tumour-promoting and -suppressing role. The manner in which they will act depends upon the cell and tissue type, the location of production and the concentration of individual ROS. Our results suggest that elevated functional activity of circulating granulocytes may facilitate tumour regression, while an additional increment in granulocyte oxidative burst could be tumour promoting; excessive granulocyte-derived ROS can induce LPO, which acts as a double-edged sword in carcinogenesis exhibiting an either pro- or anti-tumour effect. For this reason, lipid-derived reactive aldehydes also acting as ‘second messengers of free radicals’ are of high importance. Among these aldehydes are HNE, MDA and acrolein which, until now, have not been studied in this light.
In the current study we also analysed the appearance of MPO which, in the presence of adequate stimulus granulocytes, beside various cytokines, also secrete MPO [26], which is required for neutrophil extracellular trap (NET) formation to trap and kill pathogen targets, as are cancer cells extracellularly [27]. Thus, granulocytes employ the MPO-hydrogen peroxide–chloride system to convert hydroxy-amino acids into acrolein [9]. In the present work we have observed rapid infiltration of granulocytes at the site of tumour transplantation [3] and a pronounced MPO presence. The presence of MPO was accompanied by acrolein formation at an early stage of tumour regression. Acrolein can induce ROS formation, which has been suggested to be responsible for the induction of LPO [28], and may thus lead subsequently to HNE formation. Our results support this hypothesis, revealing the presence of HNE several days after acrolein formation. Due to our results we could assume that acrolein may serve as a positive feedback signalling molecule acting in regulation of the MPO activity. Namely, acrolein may lead to elevated MPO exerting its function either directly or endogenously by inducing ROS formation. Tumour regression described in this study could, at least in part, be a result of elevated acrolein observed at the site of tumour transplantation.
MDA does not appear to have an important role during tumour regression.
Finally, we can conclude that in the presence of tumour cells intense oxidative burst of granulocytes occurs, during which granulocytes employ MPO and release excessive ROS. ROS induce LPO of tumour cell membranes and thus lead to the formation of reactive aldehydes (e.g. HNE and acrolein). Furthermore, changes in cytokine levels during tumour progression and regression could be the result of the mutually dependent interplay of cytokines and granulocyte-induced LPO that may determine the tumour : host relationship in the case of cancer regression and should therefore be studied further in other cancer models and in clinical settings.
Acknowledgments
This study was supported by the Croatian Ministry of Science, Education and Sports and by the COST B35 Action of the European Union. The authors thank Mrs Nevenka Hirsl for her excellent technical assistance.
Disclosures
The authors have declared that no conflicts of interest exist.
References
- 1.Babior BM. Phagocytes and oxidative stress. Am J Med. 2000;109:33–44. doi: 10.1016/s0002-9343(00)00481-2. [DOI] [PubMed] [Google Scholar]
- 2.Hicks AM, Riedlinger G, Willingham MC, et al. Transferable anticancer innate immunity in spontaneous regression/complete resistance mice. Proc Natl Acad Sci USA. 2006;103:7753–8. doi: 10.1073/pnas.0602382103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Jaganjac M, Poljak-Blazi M, Zarkovic K, Schaur RJ, Zarkovic N. The involvement of granulocytes in spontaneous regression of Walker 256 carcinoma. Cancer Lett. 2008;260:180–6. doi: 10.1016/j.canlet.2007.10.039. [DOI] [PubMed] [Google Scholar]
- 4.Jaganjac M, Poljak-Blazi M, Kirac I, Borovic S, Joerg Schaur R, Zarkovic N. Granulocytes as effective anticancer agent in experimental solid tumor models. Immunobiology. 2010;215:1015–20. doi: 10.1016/j.imbio.2010.01.002. [DOI] [PubMed] [Google Scholar]
- 5.Dallegri F, Ottonello L, Ballestrero A, et al. Tumor cell lysis by activated humtaan neutrophils: analysis of neutrophil-delivered oxidative attack and role of leukocyte function-associated antigen 1. Inflammation. 1991;15:15–30. doi: 10.1007/BF00917906. [DOI] [PubMed] [Google Scholar]
- 6.Zivkovic M, Poljak-Blazi M, Egger G, Sunjic SB, Schaur RJ, Zarkovic N. Oxidative burst and anticancer activities of rat neutrophils. Biofactors. 2005;24:305–12. doi: 10.1002/biof.5520240136. [DOI] [PubMed] [Google Scholar]
- 7.Jaganjac M. Possible involvement of granulocyte oxidative burst in Nrf2 signaling in cancer. Indian J Med Res. 2010;131:609–16. [PubMed] [Google Scholar]
- 8.Esterbauer H, Schaur RJ, Zollner H. Chemistry and biochemistry of 4-hydroxynonenal, malonaldehyde and related aldehydes. Free Radic Biol Med. 1991;11:81–128. doi: 10.1016/0891-5849(91)90192-6. [DOI] [PubMed] [Google Scholar]
- 9.Anderson MM, Hazen SL, Hsu FF, Heinecke JW. Human neutrophils employ the myeloperoxidase-hydrogen peroxide-chloride system to convert hydroxy-amino acids into glycolaldehyde, 2-hydroxypropanal, and acrolein. A mechanism for the generation of highly reactive alpha-hydroxy and alpha,beta-unsaturated aldehydes by phagocytes at sites of inflammation. J Clin Invest. 1997;99:424–32. doi: 10.1172/JCI119176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Kim R, Emi M, Tanabe K. Cancer immunoediting from immune surveillance to immune escape. Immunology. 2007;121:1–14. doi: 10.1111/j.1365-2567.2007.02587.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Bachireddy P, Rakhra K, Felsher DW. Immunology in the clinic review series; focus on cancer: multiple roles for the immune system in oncogene addiction. Clin Exp Immunol. 2012;167:188–94. doi: 10.1111/j.1365-2249.2011.04514.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Zivkovic M, Zarkovic K, Skrinjar L, et al. A new method for detection of HNE-histidine conjugates in rat inflammatory cells. Croat Chem Acta. 2005;78:91–8. [Google Scholar]
- 13.Zivkovic M, Poljak-Blazi M, Zarkovic K, Mihaljevic D, Schaur RJ, Zarkovic N. Oxidative burst of neutrophils against melanoma B16-F10. Cancer Lett. 2007;246:100–8. doi: 10.1016/j.canlet.2006.02.002. [DOI] [PubMed] [Google Scholar]
- 14.Poljak-Blazi M, Jaganjac M, Mustapic M, Pivac N, Muck-Seler D. Acute immunomodulatory effects of iron polyisomaltosate in rats. Immunobiology. 2009;214:121–8. doi: 10.1016/j.imbio.2008.07.005. [DOI] [PubMed] [Google Scholar]
- 15.Gveric-Ahmetasevic S, Sunjic SB, Skala H, et al. Oxidative stress in small-for-gestational age (SGA) term newborns and their mothers. Free Radic Res. 2009;43:376–84. doi: 10.1080/10715760902783285. [DOI] [PubMed] [Google Scholar]
- 16.Uchida K, Kanematsu M, Sakai K, et al. Protein-bound acrolein: potential markers for oxidative stress. Proc Natl Acad Sci USA. 1998;95:4882–7. doi: 10.1073/pnas.95.9.4882. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Waeg G, Dimsity G, Esterbauer H. Monoclonal antibodies for detection of 4-hydroxynonenal modified proteins. Free Radic Res. 1996;25:149–59. doi: 10.3109/10715769609149920. [DOI] [PubMed] [Google Scholar]
- 18.Coussens LM, Werb Z. Inflammatory cells and cancer: think different! J Exp Med. 2001;193:F23–6. doi: 10.1084/jem.193.6.f23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Hampson P, Kavanagh D, Smith E, Wang K, Lord JM, Ed Rainger G. The anti-tumor agent, ingenol-3-angelate (PEP005), promotes the recruitment of cytotoxic neutrophils by activation of vascular endothelial cells in a PKC-delta dependent manner. Cancer Immunol Immunother. 2008;57:1241–51. doi: 10.1007/s00262-008-0458-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Murugaiyan G, Saha B. Protumor vs antitumor functions of IL-17. J Immunol. 2009;183:4169–75. doi: 10.4049/jimmunol.0901017. [DOI] [PubMed] [Google Scholar]
- 21.Pickens SR, Volin MV, Mandelin AM, II, Kolls JK, Pope RM, Shahrara S. IL-17 contributes to angiogenesis in rheumatoid arthritis. J Immunol. 2010;184:3233–41. doi: 10.4049/jimmunol.0903271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Huang X, Lee C. Regulation of stromal proliferation, growth arrest, differentiation and apoptosis in benign prostatic hyperplasia by TGF-β. Front Biosci. 2003;8:740–9. doi: 10.2741/1093. [DOI] [PubMed] [Google Scholar]
- 23.Rodriguez-Vita J, Lawrence T. The resolution of inflammation and cancer. Cytokine Growth Factor Rev. 2010;21:61–5. doi: 10.1016/j.cytogfr.2009.11.006. [DOI] [PubMed] [Google Scholar]
- 24.Teruel A, Romero M, Cacalano NA, Head C, Jewett A. Potential contribution of naïve immune effectors to oral tumor resistance: role in synergistic induction of VEGF, IL-6, and IL-8 secretion. Cancer Immunol Immunother. 2008;57:359–66. doi: 10.1007/s00262-007-0375-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Kawabata K, Hagio T, Matsuoka S. The role of neutrophil elastase in acute lung injury. Eur J Pharmacol. 2002;451:1–10. doi: 10.1016/s0014-2999(02)02182-9. [DOI] [PubMed] [Google Scholar]
- 26.Lefkowitz DL, Lefkowitz SS. Macrophage–neutrophil interaction: a paradigm for chronic inflammation revisited. Immunol Cell Biol. 2001;79:502–6. doi: 10.1046/j.1440-1711.2001.01020.x. [DOI] [PubMed] [Google Scholar]
- 27.Metzler KD, Fuchs TA, Nauseef WM, et al. Myeloperoxidase is required for neutrophil extracellular trap formation: implications for innate immunity. Blood. 2011;117:953–9. doi: 10.1182/blood-2010-06-290171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Adams JD, Jr, Klaidman LK. Acrolein-induced oxygen radical formation. Free Radic Biol Med. 1993;15:187–93. doi: 10.1016/0891-5849(93)90058-3. [DOI] [PubMed] [Google Scholar]


