Abstract
We have observed T helper type 2 (Th2) polarization of systemic immunity in patients with metastatic malignant melanoma. We hypothesized that similar changes in systemic immunity occur with ageing and may be permissive for the development of melanoma. We analysed the peripheral blood of 389 healthy blood donors. All subjects were profiled for peripheral blood T cell and B cell subsets, and 58 of these subjects were profiled for antigen-specific cytotoxic T cell subsets [cytomegalovirus (CMV), influenza and melanoma antigen recognized by T cells 1 (MART-1)]. Ninety-five separate healthy subjects underwent profiling of 42 plasma cytokines. Ageing was associated positively with CD4+CD294+ Th2 cells, and associated negatively with CD3+ T cells, cytotoxic T cells and T helper cells. Ageing was also associated negatively with CMV-, influenza- and MART-1-specific naive and CD8+ T cells. There were significant increases in plasma monocyte chemotactic protein 1 (MCP-1) (CCL1) and regulated upon activation normal T cell expressed and secreted (RANTES) (CCL5) with age. We observed differences in cytokine profiles between males and females; specifically, women had higher levels of sCD40L and PDGF-AA. In summary, we demonstrated in healthy blood donors that ageing was associated with an increase in cellular Th2 bias and a decline in total numbers of T cells. Additionally, there was an increase in MCP-1 and RANTES with ageing. Women had higher levels of sCD40L and PDGF-AA than men.
Keywords: ageing, immunity, MCP-1, RANTES, Th2 cells
Introduction
Ageing has a profound impact on human physiology. At a cellular level, in-vitro experiments have demonstrated the limited proliferative capacity of non-malignant cells [1,2]. On an organ level, age-associated changes influence clinical decision-making on a daily basis [3]. Age itself is one of the strongest predictors of clinical outcomes in human diseases [4].
As with any organ system, the human immune system is a complex, dynamic network of proteins, cells and organs. Although the primary role of the immune system is to prevent or fight infection, it also plays a role in many disease processes, including malignancies [5]. As many malignancies develop with advancing age, it has long been postulated that age-related changes in immunity may be permissive for the development of cancer (failure of immunosurveillance) [6]. Supporting this notion has been a number of findings describing various immune down-regulatory changes in patients with malignant disorders, including our work in human melanoma describing the dominance of T helper type 2 (Th2) systemic immune bias in patients with metastatic melanoma, as well as depletion of cytotoxic T lymphocytes (CTL) in sentinel lymph nodes downstream of invasive cutaneous melanoma [7,8]. Furthermore, other, smaller studies have associated ageing with an increase in Th2 cells with age in healthy volunteers and those with chronic allergic conditions [9].
Given the known associations between ageing, cancer and immunity, we hypothesized that normal ageing of the immune system may promote the development of specific changes in systemic immunity that would be permissive for cancer development, emphasizing the possibility of age-related evolution of systemic Th2 immune bias as a predecessor of such changes in metastatic melanoma. Additionally, considering the sex-based differences in melanoma outcomes with better outcomes in women [10], as well as sex hormone effects on immunity, we evaluated the influence of sex on age-related changes in systemic immunity. Herein we present the results of a cross-sectional laboratory study profiling parameters of systemic immunity, categorized by participant age and sex, in a cohort of almost 400 individual healthy blood donors.
Materials and methods
Patient selection and cell isolation
Peripheral blood mononuclear cells (PBMC) were isolated from buffy coats collected from 389 donors who donated blood at the Mayo Clinic Division of Transfusion Medicine for cell subset and tetramer analysis. Blood donors at Mayo Clinic are screened for infections (including bacterial, viral, spirochetal and parasitic), use of antibiotics, recent receipt of blood products, recent major illness, cardiac disease, severe respiratory disease, hepatic disease, use of medications (including any medications in the previous 4 weeks and etretinate, acitretin, isotretinoin, finasteride, bovine insulin, human pituitary-derived growth hormone and dutasteride), recent vaccinations, including rabies shots and hepatitis B immunoglobulin, pregnancy, travel outside the United States, receipt of blood products abroad or in the United Kingdom between 1980–1996, sick contacts, recent tattoos or piercings, exposure to individuals with Creutzfeldt–Jakob disease, illicit drug use, organ or tissue transplant or graft, prostitution, sexual contact with an illicit drug user, receipt of clotting factor concentrates, diagnosis of human immunodeficiency virus (HIV) or acquired immunodeficiency syndrome (AIDS), donation for the purpose of HIV/AIDS testing, recent incarceration, recent dental work or surgery, contact with body fluids, recent fevers or headache. Donors were de-identified with only age and gender information collected. PBMCs were isolated by density centrifugation over a Ficoll gradient, washed, viable frozen in cosmic calf serum (Hyclone, Logan, UT, USA) and 10% dimethylsulphoxide (DMSO) (Sigma, St Louis, MO, USA) and stored in liquid nitrogen. Normal donor plasma samples were purchased from Bioreclamation Inc (Westbury, NY, USA) for cytokine analysis.
Cell subset and tetramer studies
The following monoclonal antibodies were used for PBMC immunophenotyping by flow cytometry: anti-CD3 allophycocyanin (APC), fluorescein isothiocyanate (FITC) and phycoerythrin (PE), anti-CD4 FITC, anti-CD8 PC5, anti-CD16 PE and FITC, anti-CD56 PE, anti-62 L APC, anti-CD69 FITC, anti-CD11c PC5 and APC, anti-CD14 FITC, anti-CD197 PE, anti-CD206 APC, anti-CD19 FITC, anti-CD80 PE, anti-CD83 PE, anti-CD86 PE, anti-human leucocyte antigen D-related (HLA-DR) PC5, anti-CD40 APC, anti-CD123 PE, anti-CD294 AlexaFluor 647 (BD Pharmingen, San Jose, CA, USA) and anti-T cell immunoglobulin and mucin domain containing 3 (TIM-3) PE (R&D Systems, Minneapolis, MN, USA).
Previously frozen PBMC (0·5–1·0 × 106 cells/ml) were thawed and aliquoted into 96-well round-bottomed plates (100 µl/well). The desired antibody or antibody pool was added at 5 µl/well. The cells and antibodies were incubated for 30 min at 4°C and washed twice with 1 × phosphate-buffered saline (PBS) (Cellgro, Manassas, VA, USA), 0·1% bovine serum albumin (BSA) and 0·05% sodium azide (Sigma). Four-colour flow cytometry was performed on a LSRII flow cytometer (Becton Dickinson) and CellQuest software (Becton Dickenson) was utilized for data analysis. For dendritic cells, a gate was set on cells, which were HLA-DR+ and Lin- (CD3, CD14, CD16 and CD19). From this population, the percentage of cells, which were CD11c+ and positive for co-stimulatory molecules (CD80, CD83 and CD86), was determined. Samples from HLA-A201-positive individuals (10 per age group and eight in the over-70 group) were assessed for percentages of killer T cells, which were specific for melanoma antigen recognized by T cells-1 (MART-1)26–35, flu matrix protein58–66 and CMV495–503 by tetramers (Proimmune, Sarasota, FL, USA). For tetramer frequencies, a gate was set on lymphocytes, which were CD8+. Tetramer-positive CD8 cells were also assessed for memory phenotype using FITC anti-human CD45RA and PE-Cy7 anti-human CCR7 (CD197). Terminally differentiated cells were defined as the cells which were CD45RA+ and CCR–, effector memory cells were double-negative, naive cells were double-positive and central memory cells were CD45RA- and CCR+. Four-colour flow cytometry was performed on a LSRII flow cytometer (Becton Dickinson) and CellQuest software (Becton Dickinson) was utilized for data analysis.
Cytokine studies
Protein levels for 42 cytokines, chemokines and growth factors, including epidermal growth factor (EGF), eotaxin (CCL11), fibroblast growth factor (FGF)-2, Flt-3 ligand, fractalkine (CX3CL1), granulocyte–colony-stimulating factor (G-CSF), granulocyte–macrophage colony-stimulating factor (GM-CSF), growth-regulated oncogene (GRO) (CXCL1), interferon (IFN)-α2, IFN-γ, interleukin (IL)-1a, IL-1b, IL-1ra, IL-2, IL-3, IL-4, IL-5, IL-6, IL-7, IL-8, IL-9, IL-10, IL-12 (p40), IL-12 (p70), IL-13, IL-15, IL-17, IFN-γ-induced protein-10 (IP-10) (CXCL10), monocyte chemotactic protein 1 (MCP-1) (CCL2), MCP-3 (CCL7), macrophage-derived chemokine (MDC) (CCL22), macrophage inflammatory protein-1a (MIP-1a) (CCL3), MIP-1b (CCL4), platelet-derived growth factor (PDGF)-AA, PDGF AB/BB, regulated upon activation normal T cell expressed and secreted (RANTES) (CCL5), sCD40L, sIL-2Ra, transforming growth factor (TGF)-α, tumour necrosis factor (TNF)-α, TNF-β and vascular endothelial growth factor (VEGF) were measured using the Milliplex cytokine assay (Millipore, Billerica, MA, USA) as per the manufacturer's instructions. Normal donor plasma was added to washed, fluorescently dyed microspheres (beads) to which biomolecules of interest were bound. The beads and donor plasma were incubated for 1 h at room temperature with agitation. After the incubation the beads were washed in wash buffer and placed in 25 µl of detection antibody and incubated for 30′ as described above. After incubation, the beads were placed in streptavidin–PE, incubated and washed a final time. The bound beads were resuspended in 150 µl sheath fluid and read with the Luminex plate reader (Millipore). Protein concentrations were determined using a standard curve with sensitivity from 1·6–5000 pg/ml.
Principal components analysis
Principal components analysis (PCA) was performed using correlation matrix. A feature vector was constructed including three top principal components (PC). Subsequently, cytokines with highest correlation of their concentration profiles with each of the three PC were identified.
Statistics
The percentage coefficients of variation were determined for each marker in the circulating cell subsets and cytokines analyses by dividing the standard deviations by the means for randomly selected samples that were run multiple times for each cell subset marker and cytokine. Any marker or cytokine with a coefficient of variation greater than 30% was excluded from further analysis. For that reason we excluded CD14+197+, CD14+206+, CD11c+80+, CD11c+83+, CD11c+86+ and DR40 from our analysis. Linear regression using the least-squares method was then performed on all remaining markers and cytokines to determine if age or sex predicted changes in markers of cell subsets and cytokines among all subjects.
The cytokines that PCA identified as significant between sexes, and between sexes by decade, were then compared using the Wilcoxon rank-sum test or Mann–Whitney U-test. Cytokines and cell subsets not identified as PC were excluded from further analysis. jmp (Cary, NC, USA) was used for statistical analysis.
Results
Study subjects
Analysis of immune cell subsets was performed in all 389 samples from Mayo Clinic blood donors, and analysis of T cell antigen specificity was performed on 58 of the subjects for whom sufficient material was available for the assay. A separate set of 95 plasma samples was analysed for plasma cytokine concentrations (Table 1). The mean age of donors in our study was 49 years. The youngest donor was aged 16 years and the oldest donor was aged 81 years.
Table 1.
Patient characteristics
| All | Males | Females | |
|---|---|---|---|
| Cell subsets | |||
| Number | 389 | 227 (58) | 162 (42) |
| Age | 50 (38–61) | 49 (37–62) | 51 (39–60) |
| Cytokines | |||
| Number | 95 | 50 (53) | 45 (47) |
| Age | 46 (34–56) | 45 (31–57) | 47 (38–56) |
| Tetramers | |||
| Number | 58 | 30 (52) | 28 (48) |
| Age | 49 (33–61) | 50 (34–65) | 49 (32–61) |
Numbers are displayed as total (%) for the sexes. Age is displayed as median (interquartile range).
Immune cell subsets
Ageing is associated with an increase in CD4+CD294+ (Th2) cells among all subjects and both sexes (Table 2, Table S1). There were decreases in multiple cell subsets associated with ageing. Specifically, we observed negative correlations in the total number of T cells (CD3+), T helper cells (CD3+CD4+), cytotoxic T cells (CD3+CD8+) and naive T cells (CD3+CD62L+) with age. The decreases in T helper cells and cytotoxic T cells were seen primarily in males. There were also positive correlations of natural killer cells (NK) (CD16+CD56+) and a subset of monocytes (CD14+CD11c–) with age, but the increase in monocytes was seen primarily in females. There was a trend towards a lower Th1 : Th2 ratio with age among all subjects (r2 = 0·01, P = 0·03). For example, patients in their 20s had a Th1 : Th2 median ratio of 1·8 (interquartiles 1·1–2·7), whereas those in their 70s had a ratio of 1·3 (0·6–1·8). This trend was due primarily to a rise in Th2 cells, as there was no significant change in Th1 cells. PCA did not discriminate a set of identifiers between the cell subsets of our patients by sex or age (Fig. 1a).
Table 2.
Summary of cell subset analysis by linear regression
| All subjects | Increase | Decrease |
|---|---|---|
| P ≤ 0·01 | CD3–CD16+CD56+ | CD3+ |
| CD4+CD294+ (Th2) | CD3+CD4+ | |
| CD14+CD11c– | CD3+CD8+ | |
| CD3+CD62L+ | ||
| 0·01 < P < 0·05 | CD14+CD11c+ | CD4+TIM3+: CD4+294+(Th1 : Th2) |
| DR+CD123+ (DC2) | ||
| Females | ||
| P ≤ 0·01 | CD4+CD294+ (Th2) | CD3+ |
| CD14+CD11c– | CD3+CD62L+ | |
| 0·01 < P < 0·05 | CD3+4+ | |
| Males | ||
| P ≤ 0·01 | CD4+CD294+ (Th2) | CD3+ |
| CD3+CD4+ | ||
| CD3+CD8+ | ||
| CD3+CD62L+ | ||
| 0·01 < P < 0·05 | CD3–CD16+CD56+ | CD4+TIM3+ : CD4+294+(Th1 : Th2) |
| DR+CD123+ (DC2) | ||
The significant differences and trends among all subjects and both sexes are displayed.
Fig. 1.
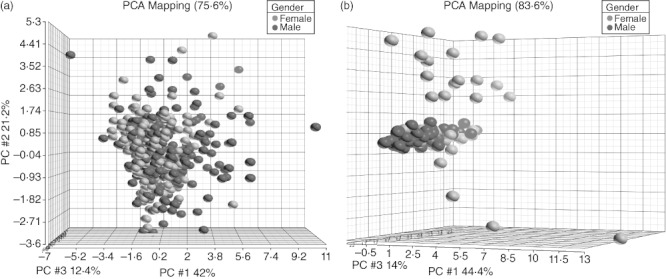
Principal components analysis (PCA) of cell subsets and cytokines between sexes. PCA did not identify any classifiers discriminating cell subsets between the sexes (a). PCA identified sCD40L, platelet-derived growth factor (PDGF)-AA, growth-regulated oncogene (GRO) (CXCL1), macrophage-derived chemokine (MDC) (CCL22), fibroblast growth factor (FGF)-2, interleukin (IL)-8 and interferon (IFN)-γ as a set of classifiers discriminating between male and female subjects (b). Females are represented with red dots and males with blue dots.
Tetramers
There were 58 samples available for tetramer analysis from subjects aged 16–81 years, with a median age of 58 years (Table 1). Twenty-eight (48%) of these subjects were female. We observed decreases in antigen-specific naive and total CD8+ T cells with age, and an increase in central memory T cells with age (Table 3). There were negative correlations of naive T cells and CD8+ cells specific for CMV, influenza and MART-1 with ageing, but a positive correlation in central memory T cells for all tested antigens with ageing. There were no differences in terminally differentiated and effector T cells across subjects' age or sex.
Table 3.
Antigen-specific T cell changes with age
| CMV | Influenza | MART-1 | |
|---|---|---|---|
| Terminally differentiated | – (r2 = 0·02, P = 0·32) | – (r2 = 0·009, P = 0·49) | – (r2 = 0·003, P = 0·69) |
| Naive | ↓ (r2 = 0·15, P = 0·003) | ↓ (r2 = 0·15, P = 0·03) | ↓ (r2= 0·18, P = 0·001) |
| Effector | – (r2 = 0·001, P = 0·82) | – (r2 = 0·002, P = 0·77) | – (r2= 0·04, P = 0·38) |
| Central | ↑ (r2 = 0·08, P = 0·03) | ↑ (r2 = 0·09, P = 0·03) | ↑ (r2 = 0·08, P = 0·03) |
| CD8 | ↓ (r2 = 0·07, P = 0·05) | ↓ (r2 = 0·085, P = 0·03) | ↓ (r2 = 0·06, P = 0·07) |
The directions of changes with age that were significant, or trended towards significance, are denoted with arrows, whereas non-significant changes are denoted with a dash. Ageing is associated with negative decreases in naive and CD8+ T cells specific for cytomegalovirus (CMV), influenza and melanoma antigen recognized by T cells 1 (MART-1). Ageing was associated with positive increases in central memory T cells specific for CMV, influenza and MART-1.
Cytokines
Ageing among all subjects was associated with an increase in MCP-1 (CCL2) and RANTES (CCL5). Specifically, we observed positive correlations of ageing with MCP-1 (P < 0·0001, r2 = 0·19), and RANTES (P = 0·007, r2 = 0·08) (Table 4, Table S2). Females also demonstrated a positive correlation with MDC and ageing (P = 0·004, r2 = 0·18). No significant decreases in cytokines with age were observed in female subjects, but there was a trend in male subjects towards a negative correlation with ageing and IL-15 (P = 0·03, r2 = 0·10) and sCD-40L (P = 0·02, r2 = 0·11).
Table 4.
Summary of cytokine analysis by linear regression
| All Subjects | Increase | Decrease |
|---|---|---|
| P ≤ 0·01 | RANTES (CCL5) | |
| MCP-1 (CCL2) | ||
| 0·01 < P < 0·05 | IP-10 (CXCL10) | IL-12p70 |
| Eotaxin (CCL11) | FGF-2 | |
| Females | ||
| P ≤ 0·01 | MCP-1 (CCL2) | |
| MDC (CCL22) | ||
| RANTES (CCL5) | ||
| 0·01 < P < 0·05 | IL-10 | |
| IL-12p40 | ||
| sIL-2Ra | ||
| Males | ||
| P ≤ 0·01 | ||
| 0·01 < P < 0·05 | MCP-1 (CCL2) | IL-15 |
| PDGF-AA | sCD40L | |
| PDGF-AA/AB | ||
The significant differences and trends among all subjects and both sexes are displayed. IL: interleukin; IP-10: interferon gamma-induced protein-10; MDC: macrophage-derived chemokine; MCP-1: monocyte chemotactic protein 1; PDGF: platelet-derived growth factor; RANTES: regulated upon activation normal T cell expressed and secreted.
We found significant differences in the expression of cytokines between the sexes using PCA. PCA identified sCD40L, PDGF-AA, GRO (CXCL1), MDC (CCL22), FGF-2, IL-8 and IFN-γ as a set of classifiers discriminating between all male and female subjects (Fig. 1b). Females demonstrated greater expression of sCD40L (5·6-fold, P = 0·0001), PDGF-AA (2·4-fold, P = 0·0002), GRO (1·9-fold, P = 0·001) and MDC (1·1-fold, P = 0·002) than males. Although females had greater expression of FGF-2 (2·6-fold, P = 0·02), IL-8 (2·3-fold, P = 0·03) and IFN-γ (2·3-fold, P = 0·08) than males, these differences were not significant. PCA identified GRO, PDGF-AA, sCD40L, IL-8, VEGF and FGF-2 as a set of classifiers between women and men aged less than 50 years (Fig. 2a). Females aged less than 50 years demonstrated greater expression of GRO (2·2-fold, P = 0·003), PDGF-AA (3·0-fold, P = 0·004) and sCD40L (4·9-fold, P = 0·007) than males aged under 50. Although females aged under 50 years had greater expression of IL-8 (3·4-fold, P = 0·04), VEGF (2·3-fold, P = 0·05) and FGF-2 (3·1-fold, P = 0·05) than males under 50 years, these differences were not considered significant. PCA identified MDC, sCD40L, PDGF-AA and FGF-2 as a set of classifiers between women and men aged 50 years and older (Fig. 2b). Females aged 50 years and older demonstrated greater expression of MDC (1·3-fold, P = 0·001) and sCD40L (6·8-fold, P = 0·01) than males aged 50 years and older. Although females aged 50 years and older demonstrated greater expression of PDGF-AA (1·9-fold, P = 0·02) and FGF-2 (1·8-fold, P = 0·03) than males aged 50 years and older, these differences were not considered significant.
Fig. 2.
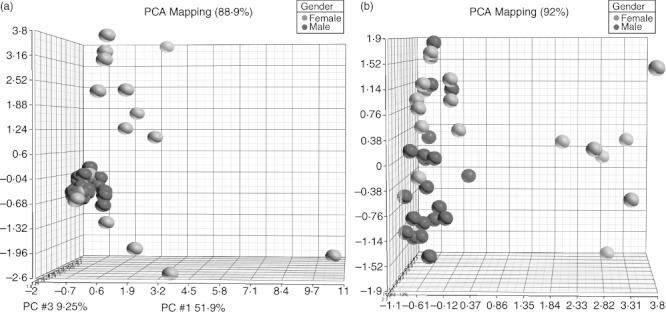
Principal components analysis (PCA) of cytokines between sexes by age. PCA identified growth-regulated oncogene (GRO) (CXCL1), platelet-derived growth factor (PDGF)-AA, sCD40L, interleukin (IL)-8, vascular endothelial growth factor (VEGF) and fibroblast growth factor (FGF)-2 as a set of classifiers discriminating between female and male subjects under 50 years of age (a). PCA identified macrophage-derived chemokine (MDC) (CCL22), sCD40L, PDGF-AA and FGF-2 as a set of classifiers discriminating between female and male subjects aged 50 years and older (b). Females are represented with red dots and males with blue dots.
Discussion
We observed that ageing was associated with numerous changes in immune cell subsets, antigen-specific cells and cytokines, consistent with an increasing acquisition of a Th2 bias. Most significantly, our data suggest that there was an increase with age in CD4+CD294+ (Th2) cells, and a trend towards a lower Th1 : Th2 ratio. We hypothesized that ageing was associated with a systemic Th2 immune bias. Although this study does not prove that an observed Th2 bias with age would be permissive for the development of cancer, it raises significant concerns as to the effectiveness of immunosurveillance with ageing. Cell subset analysis consistently demonstrated an increase in Th2 cells in both sexes with age. Among all subjects there was also an increase with age in NK cells. We observed decreases in the total number of T cells and naive T cells in males and females in addition to decreases in T helper cells and cytotoxic T cells in males.
We observed significant changes in cytokines with age. There was an increase in MCP-1 with age. MCP-1 is known as monocyte chemoattractant protein 1, or chemokine ligand 2 (CCL2). MCP-1 attracts monocytes and memory T cells to sites of antigen-induced inflammation [11]. The increase in MCP-1 with age is consistent with the preservation of the memory T cell population in elderly people [12]. We observed an increase with age in RANTES, which is also known as chemokine ligand 5 (CCL5). RANTES has been shown to promote the release of TNF-α from macrophages [13], but we did not find a concurrent increase in TNF-α in our subjects. RANTES has been shown to induce the proliferation of NK cells [14]; hence, the increase in RANTES with age we observed is consistent with the increase in NK cells we observed with age. Both MCP-1 and RANTES have been shown to have a role in T cell activation [15], and may enhance T cell proliferation and cytotoxicity [15]. In women we observed an increase in MDC, which is also known as CCL22. This rise in MDC in women aged 50 years and older was significantly greater than in men aged 50 years and older. MDC is a chemoattractant for monocytes, dendritic cells and NK cells [16], and has been shown to specifically stimulate the chemotaxis of Th2 cells [17]. This rise in MDC is consistent with the concurrent changes we observed in Th2 cells and NK cells in our study.
There were significant differences in cytokine expression between sexes. Women were found to have more sCD40L than men. sCD40L is released from platelets upon activation and has roles in dendritic cell maturation [18] and dendritic cell cytotoxicity [19]. People with increased levels of sCD40L are thought to be at higher risk of cardiovascular disease [20], and higher levels of sCD40L have been found in patients with lung cancer [21]. In our study there were higher levels of sCD40L in women compared to men, and there were no significant changes with ageing. Women were also found to have a higher expression of PDGF-AA than were men. PDGF-AA is a pro-angiogenic factor that promotes development of tumour stroma in melanoma and other cancers [22].
There have been many previous investigations into the effects of ageing on the immune system which have demonstrated a quantitative and functional remodelling of its many components [23]. One study of 47 patients aged 21–99 years evaluated stimulated CD4+ T cell subsets in vitro, and found that with ageing there were decreases in intracellular Th1 cytokines IFN-γ and TNF-α among activated memory cells (CD95+CD28+), a decrease in IFN-γ in virgin cells (CD95–CD28+), but an increase in the Th2 cytokine IL-4 in activated memory T cells. There were no significant changes in effector memory cells (CD95+CD28–) [24]. In contrast, a study that analysed intracellular cytokine production of stimulated CD8+ T cell subsets showed increases in Th1 cytokines (IFN-γ, TNF-α, IL-2) in naive (CD95–CD28+), effector cytotoxic (CD95+CD28–) and memory (CD95+CD28+) cells, but an increase in Th2 cytokines (IL-4, IL-6, IL-10) only in memory cells [25].
There have been other studies on the effects of ageing on cytokines. One of the largest population studies to date measured IL-1ra, IL-6, IL-10, C-reactive protein (CRP) and TNFr-1 and found a significant increase in IL-6 and TNF-r1 with ageing [26]. One study compared 22 cytokines between 55 Korean patients aged under 45 years aged and 55 patients aged over 65 years. This study included 23 males and 32 females in each group. Significant increases in sCD40L and TGF-α were associated with age, but there were significant decreases in G-CSF, GM-CSF and MCP-1 with age [27]. In contrast, we found an increase in MCP-1 with age. Our study, and that by Kim et al., had roughly the same number of patients for cytokine analysis; however, our study included patients aged between 45 and 65 years, and we used linear regression for statistical analysis instead of a standard t-test. Furthermore, our results are consistent with the results of other studies [28,29] in identifying an increase in MCP-1 and memory T cells with ageing. One study showed that lipopolysaccharide (LPS)-stimulated monocytes from nonagenarians produced more RANTES than younger controls, but IL-2-stimulated NK cells produced less RANTES than controls [30]. We were only able to measure circulating RANTES in our study, and found an increase with ageing. Another group in Japan investigated changes in the serum of cytokines among 122 pre-, peri- and postmenopausal women. They reported a positive correlation of IL-6 with age, in addition to significant increases in IL-2, GM-CSF, G-CSF and IL-4 in postmenopausal women [31]. We observed a trend towards an increase in IL-4 with age, but this was non-significant.
Previous studies have tried to determine the effects of menopause on immunity. One study showed that ageing was associated with a gradual increase in IL-10 in women, and that late menopause (>30 years after menopause) was associated with decreased IFN-γ production in LPS-stimulated PBMCs. Those subjects on menopausal hormone therapy (conjugated equine oestrogen 0·625 mg and medroxyprogesterone acetate 2·5 mg) had reduced levels of IL-10 compared to untreated women, and there were no significant differences in IFN-γ between these groups [32]. Similarly, menopause has been associated with a decrease in cells that secrete IFN-γ and TNF-α spontaneously when compared to premenopausal women [33]. Another study showed that 6 months of menopausal hormone therapy (oestradiol valerate 2 mg/day and medroxyprogesterone acetate 5 mg/day) restored NK cytotoxicity, but did not restore the decline in T cell populations or IFN-γ production [34]. One other study, which evaluated early postmenopausal women, found no differences between those who were treated with menopausal hormone therapy (oestradiol 0·1 mg twice per week transdermally) compared to those who were not treated in IL-1α, IL-1β, IL-6, IL-1ra or soluble IL-6 receptor production by stimulated bone marrow mononuclear cells collected by aspiration [35]. We did not have sufficient samples to determine if the female donors in this study were in menopause, but we used the age of 50 years as a rough estimate of menopause. Regardless, PCA did not identify IL-6, IFN-γ or TNF-α as discriminators.
One drawback to our study is that we were not able to profile each of the donors, although they were screened extensively for infections, certain medications, recent vaccinations and concerning exposures, as described in the Methods. Although it is possible that donors had diseases that do not exclude blood donation, most blood donors do not have significant co-morbidities. Accordingly, we have attributed the observed changes to age, rather than intercurrent illnesses or significant co-morbidities. Furthermore, as more than half our patients were aged 30–60 years, extremes of age are not well represented in this study.
In summary, we have demonstrated that ageing is associated with an increasing acquisition of a cellular Th2 bias, and a decline in total number of T cells, cytotoxic T cells and T helper cells. Additionally, there is an increase in MCP-1 and RANTES with ageing. A notable sex difference was detected, with women found to express more sCD40L and PDGF-AA than men.
Disclosure
The authors have no disclosures to make.
Supporting information
Additional Supporting Information may be found in the online version of this article:
Table S1. Cell subset changes with age by linear regression. The table above has an arrow to demonstrate the overall direction of change, or a dash if there was no clear change with ageing. The r2 and P values are displayed in order, parenthetically.
Table S2. Cytokine changes with age by linear regression. The table above has an arrow to demonstrate the overall direction of change, or a dash if there was no clear change with aging. The r2 and P values are displayed in order, parenthetically.
Please note: Wiley-Blackwell are not responsible for the content or functionality of any supporting materials supplied by the authors. Any queries (other than missing material) should be directed to the corresponding author for the article.
References
- 1.Hayflick L. The limited in vitro lifetime of human diploid cell strains. Exp Cell Res. 1965;37:614–36. doi: 10.1016/0014-4827(65)90211-9. [DOI] [PubMed] [Google Scholar]
- 2.Hayflick L, Moorhead PS. The serial cultivation of human diploid cell strains. Exp Cell Res. 1961;25:585–621. doi: 10.1016/0014-4827(61)90192-6. [DOI] [PubMed] [Google Scholar]
- 3.Cefalu CA. Theories and mechanisms of aging. Clin Geriatr Med. 2011;27:491–506. doi: 10.1016/j.cger.2011.07.001. [DOI] [PubMed] [Google Scholar]
- 4.Harman D. The aging process: major risk factor for disease and death. Proc Natl Acad Sci USA. 1991;88:5360–3. doi: 10.1073/pnas.88.12.5360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Willimsky G, Blankenstein T. Sporadic immunogenic tumours avoid destruction by inducing T-cell tolerance. Nature. 2005;437:141–6. doi: 10.1038/nature03954. [DOI] [PubMed] [Google Scholar]
- 6.Fulop T, Kotb R, Fortin CF, Pawelec G, de Angelis F, Larbi A. Potential role of immunosenescence in cancer development. Ann NY Acad Sci. 2010;1197:158–65. doi: 10.1111/j.1749-6632.2009.05370.x. [DOI] [PubMed] [Google Scholar]
- 7.Nevala WK, Vachon CM, Leontovich AA, Scott CG, Thompson MA, Markovic SN. Evidence of systemic Th2-driven chronic inflammation in patients with metastatic melanoma. Clin Cancer Res. 2009;15:1931–9. doi: 10.1158/1078-0432.CCR-08-1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Mansfield AS, Holtan SG, Grotz TE, et al. Regional immunity in melanoma: immunosuppressive changes precede nodal metastasis. Mod Pathol. 2011;24:487–94. doi: 10.1038/modpathol.2010.227. [DOI] [PubMed] [Google Scholar]
- 9.Kawamoto N, Kaneko H, Takemura M, et al. Age-related changes in intracellular cytokine profiles and Th2 dominance in allergic children. Pediatr Allergy Immunol. 2006;17:125–33. doi: 10.1111/j.1399-3038.2005.00363.x. [DOI] [PubMed] [Google Scholar]
- 10.Joosse A, de Vries E, Eckel R, et al. Gender differences in melanoma survival: female patients have a decreased risk of metastasis. J Invest Dermatol. 2011;131:719–26. doi: 10.1038/jid.2010.354. [DOI] [PubMed] [Google Scholar]
- 11.Carr MW, Roth SJ, Luther E, Rose SS, Springer TA. Monocyte chemoattractant protein 1 acts as a T-lymphocyte chemoattractant. Proc Natl Acad Sci USA. 1994;91:3652–6. doi: 10.1073/pnas.91.9.3652. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Buchholz VR, Neuenhahn M, Busch DH. CD8+ T cell differentiation in the aging immune system: until the last clone standing. Curr Opin Immunol. 2011;23:549–54. doi: 10.1016/j.coi.2011.05.002. [DOI] [PubMed] [Google Scholar]
- 13.Qiu L, Ding L, Huang J, Wang D, Zhang J, Guo B. Induction of copper/zinc-superoxide dismutase by CCL5/CCR5 activation causes tumour necrosis factor-alpha and reactive oxygen species production in macrophages. Immunology. 2009;128:e325–34. doi: 10.1111/j.1365-2567.2008.02966.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Maghazachi AA, Al-Aoukaty A, Schall TJ. CC chemokines induce the generation of killer cells from CD56+ cells. Eur J Immunol. 1996;26:315–9. doi: 10.1002/eji.1830260207. [DOI] [PubMed] [Google Scholar]
- 15.Taub DD, Turcovski-Corrales SM, Key ML, Longo DL, Murphy WJ. Chemokines and T lymphocyte activation: I. Beta chemokines costimulate human T lymphocyte activation in vitro. J Immunol. 1996;156:2095–103. [PubMed] [Google Scholar]
- 16.Godiska R, Chantry D, Raport CJ, et al. Human macrophage-derived chemokine (MDC), a novel chemoattractant for monocytes, monocyte-derived dendritic cells, and natural killer cells. J Exp Med. 1997;185:1595–604. doi: 10.1084/jem.185.9.1595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Andrew DP, Chang MS, McNinch J, et al. STCP-1 (MDC) CC chemokine acts specifically on chronically activated Th2 lymphocytes and is produced by monocytes on stimulation with Th2 cytokines IL-4 and IL-13. J Immunol. 1998;161:5027–38. [PubMed] [Google Scholar]
- 18.Cella M, Scheidegger D, Palmer-Lehmann K, Lane P, Lanzavecchia A, Alber G. Ligation of CD40 on dendritic cells triggers production of high levels of interleukin-12 and enhances T cell stimulatory capacity: T-T help via APC activation. J Exp Med. 1996;184:747–52. doi: 10.1084/jem.184.2.747. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Vidalain PO, Azocar O, Yagita H, Rabourdin-Combe C, Servet-Delprat C. Cytotoxic activity of human dendritic cells is differentially regulated by double-stranded RNA and CD40 ligand. J Immunol. 2001;167:3765–72. doi: 10.4049/jimmunol.167.7.3765. [DOI] [PubMed] [Google Scholar]
- 20.Heeschen C, Dimmeler S, Hamm CW, et al. Soluble CD40 ligand in acute coronary syndromes. N Engl J Med. 2003;348:1104–11. doi: 10.1056/NEJMoa022600. [DOI] [PubMed] [Google Scholar]
- 21.Roselli M, Mineo TC, Basili S, et al. Soluble CD40 ligand plasma levels in lung cancer. Clin Cancer Res. 2004;10:610–4. doi: 10.1158/1078-0432.ccr-0348-03. [DOI] [PubMed] [Google Scholar]
- 22.Barnhill RL, Xiao M, Graves D, Antoniades HN. Expression of platelet-derived growth factor (PDGF)-A, PDGF-B and the PDGF-alpha receptor, but not the PDGF-beta receptor, in human malignant melanoma in vivo. Br J Dermatol. 1996;135:898–904. doi: 10.1046/j.1365-2133.1996.d01-1092.x. [DOI] [PubMed] [Google Scholar]
- 23.Sansoni P, Vescovini R, Fagnoni F, et al. The immune system in extreme longevity. Exp Gerontol. 2008;43:61–5. doi: 10.1016/j.exger.2007.06.008. [DOI] [PubMed] [Google Scholar]
- 24.Alberti S, Cevenini E, Ostan R, et al. Age-dependent modifications of Type 1 and Type 2 cytokines within virgin and memory CD4+ T cells in humans. Mech Ageing Dev. 2006;127:560–6. doi: 10.1016/j.mad.2006.01.014. [DOI] [PubMed] [Google Scholar]
- 25.Zanni F, Vescovini R, Biasini C, et al. Marked increase with age of type 1 cytokines within memory and effector/cytotoxic CD8+ T cells in humans: a contribution to understand the relationship between inflammation and immunosenescence. Exp Gerontol. 2003;38:981–7. doi: 10.1016/s0531-5565(03)00160-8. [DOI] [PubMed] [Google Scholar]
- 26.Stowe RP, Peek MK, Cutchin MP, Goodwin JS. Plasma cytokine levels in a population-based study: relation to age and ethnicity. J Gerontol A Biol Sci Med Sci. 2010;65:429–33. doi: 10.1093/gerona/glp198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Kim HO, Kim HS, Youn JC, Shin EC, Park S. Serum cytokine profiles in healthy young and elderly population assessed using multiplexed bead-based immunoassays. J Transl Med. 2011;9:113. doi: 10.1186/1479-5876-9-113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Gerli R, Monti D, Bistoni O, et al. Chemokines, sTNF-Rs and sCD30 serum levels in healthy aged people and centenarians. Mech Ageing Dev. 2000;121:37–46. doi: 10.1016/s0047-6374(00)00195-0. [DOI] [PubMed] [Google Scholar]
- 29.Inadera H, Egashira K, Takemoto M, Ouchi Y, Matsushima K. Increase in circulating levels of monocyte chemoattractant protein-1 with aging. J Interferon Cytokine Res. 1999;19:1179–82. doi: 10.1089/107999099313127. [DOI] [PubMed] [Google Scholar]
- 30.Mariani E, Pulsatelli L, Neri S, et al. RANTES and MIP-1alpha production by T lymphocytes, monocytes and NK cells from nonagenarian subjects. Exp Gerontol. 2002;37:219–26. doi: 10.1016/s0531-5565(01)00187-5. [DOI] [PubMed] [Google Scholar]
- 31.Yasui T, Maegawa M, Tomita J, et al. Changes in serum cytokine concentrations during the menopausal transition. Maturitas. 2007;56:396–403. doi: 10.1016/j.maturitas.2006.11.002. [DOI] [PubMed] [Google Scholar]
- 32.Deguchi K, Kamada M, Irahara M, et al. Postmenopausal changes in production of type 1 and type 2 cytokines and the effects of hormone replacement therapy. Menopause. 2001;8:266–73. doi: 10.1097/00042192-200107000-00008. [DOI] [PubMed] [Google Scholar]
- 33.Verthelyi D, Klinman DM. Sex hormone levels correlate with the activity of cytokine-secreting cells in vivo. Immunology. 2000;100:384–90. doi: 10.1046/j.1365-2567.2000.00047.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Yang JH, Chen CD, Wu MY, Chao KH, Yang YS, Ho HN. Hormone replacement therapy reverses the decrease in natural killer cytotoxicity but does not reverse the decreases in the T-cell subpopulation or interferon-gamma production in postmenopausal women. Fertil Steril. 2000;74:261–7. doi: 10.1016/s0015-0282(00)00622-1. [DOI] [PubMed] [Google Scholar]
- 35.Kassem M, Khosla S, Spelsberg TC, Riggs BL. Cytokine production in the bone marrow microenvironment: failure to demonstrate estrogen regulation in early postmenopausal women. J Clin Endocrinol Metab. 1996;81:513–8. doi: 10.1210/jcem.81.2.8636260. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


