Abstract
To clarify the association between factors regulating DNA methylation and the prognosis of autoimmune thyroid diseases (AITDs), we genotyped single nucleotide polymorphisms in genes encoding DNA methyltransferase 1 (DNMT1), DNMT3A, DNMT3B, methylenetetrahydrofolate reductase (MTHFR) and methionine synthase reductase (MTRR), which are enzymes essential for DNA methylation. Subjects for this study included 125 patients with Hashimoto's disease (HD), including 48 patients with severe HD and 49 patients with mild HD; 176 patients with Graves’ disease (GD), including 79 patients with intractable GD and 47 patients with GD in remission; and 83 healthy volunteers (control subjects). The DNMT1+32204GG genotype was more frequent in patients with intractable GD than in patients with GD in remission. Genomic DNA showed significantly lower levels of global methylation in individuals with the DNMT1+32204GG genotype than in those with the AA genotype. The MTRR+66AA genotype was observed to be more frequent in patients with severe HD than in those with mild HD. The DNMT1+14395A/G, DNMT3B−579G/T, MTHFR+677C/T and +1298A/C polymorphisms were not correlated with the development or prognosis of AITD. Our study indicates that the DNMT1+32204GG genotype correlates with DNA hypomethylation and with the intractability of GD, and that the MTRR+66AA genotype may correlate with the severity of HD.
Keywords: DNMT1, intractability, methylation, single nucleotide polymorphism, severity
Introduction
Autoimmune thyroid diseases (AITDs), such as Graves’ disease (GD) and Hashimoto's disease (HD), are typical autoimmune diseases [1,2]. The severity of HD and the intractability (that is, inducibility to remission) of GD varies among patients. Some patients with HD develop hypothyroidism earlier in life, while some maintain a euthyroid state even up to old age. Some patients with GD achieve remission through medical treatment, whereas others do not [3,4]. However, the intractability of GD and the severity of HD are very difficult to predict at diagnosis.
DNA methylation occurs at cytosine residues in cytosine–phosphate–guanosine (CpG) dinucleotides and involves methylation of the fifth carbon of the pyrimidine ring leading to the formation of 5-methylcytosine (5-mC). The majority of CpG sites (70–80%) in human DNA are methylated and many of the non-methylated sites are found in so-called CpG islands, which are sites of transcription initiation [5]. Several studies have reported a strong correlation between DNA methylation and gene expression [6]. In addition, DNA methylation is one of the epigenetic processes regulating several biological events, including embryonic development, transcriptional regulation, X-chromosome inactivation, genomic imprinting and chromatin modification [7]. Altered DNA methylation patterns have been associated with tumorigenic events and development of autoimmune diseases [8].
DNA methylation is established and maintained by DNA methyltransferases (DNMTs). In humans, three enzymes, DNMT1, DNMT3A and DNMT3B, are known to have DNMT activity. DNMT3A and DMMT3B are responsible for de-novo methylation and modification of unmethylated DNA, whereas DNMT1 is required to maintain DNA methylation [9,10]. Previous studies have shown that mRNA levels of DNMT1 and DNMT3A are reduced in patients with atopic dermatitis [11], and that DNMT1 mRNA levels were also decreased in patients with systemic lupus erythematosus (SLE) [12]. There are several noteworthy polymorphisms in the genes encoding enzymes.
It has been reported that the A-allele of the DNMT1+14395A/G polymorphism (rs16999593) is present more frequently in patients with infiltrating ductal breast carcinoma than among controls [13]. The DNMT1+32204A/G (rs2228612) polymorphism is a non-synonymous substitution in which the frequency of the minor allele is 5% in the Japanese population, according to the National Center for Biotechnology Information (NCBI)-SNP (http://www.ncbi.nlm.nih.gov/snp/) and Japanese (J)-SNP databases (http://snp.ims.u-tokyo.ac.jp/). The A-allele of the DNMT3A−448A/G (rs1550157) polymorphism showed significantly higher promoter activity (>twofold) compared to the G-allele [14]. Carriers of the T-allele of the DNMT3B−283T/C polymorphism (rs6087990) showed significantly lower promoter activity compared to carriers of the C-allele [15]. However, unambiguous genotyping of the DNMT3B −283T/C polymorphism by restriction fragment length polymorphism (RFLP) analysis is complex.
Therefore, we examined The DNMT3B−579G/T polymorphism (rs1569686), which is in linkage disequilibrium (LD) with the −283T/C polymorphism. The two common haplotypes formed by these SNPs, −283T/−579G and −283C/−579T, account for 98% of the chromosome [15].
Methylenetetrahydrofolate reductase (MTHFR), which is involved in the supply of the methylation group, is an enzyme necessary for the folate metabolic pathway (Fig. 1) and is considered to result in hypermethylation of genomic DNA [16,17]. The MTHFR+677C/T polymorphism (rs1801133) results in an alanine (C)-to-valine (T) substitution and renders the enzyme less active [18,19]. The MTHFR+1298A/C polymorphism (rs1801131) results in a glutamic acid (A)-to-alanine (C) substitution and the CC genotype of the SNP results in a significant decrease of MTHFR activity [20].
Fig. 1.
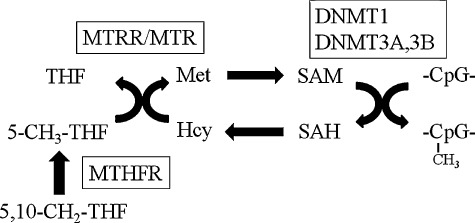
Summary of folate metabolic pathway. MTHFR: 5,10-methylenetetrahydrofolate reductase; MTR: methionine synthase; MTRR: methionine synthase reductase; THF: tetrahydrofolate; Met: methionine; 5,10-CH2-THF: 5,10-methylene-THF; 5-CH3-THF: 5-methyltetrahydrofolate; SAM: S-adenosylmethionine; Hcy: homocystein; SAH: S-adenosylhomocysteine.
Methionine synthase reductase (MTRR) plays a crucial role in maintaining the active state of methionine synthase (MTR), which is associated with an increase in the DNA methylation level [21,22]. Because the minor allele frequency of a functional polymorphism in the MTR gene (rs1805087) [23] was less than 5% in the Japanese population, we focused on another polymorphism in the MTRR gene as an alternative candidate. The most common polymorphism in the MTRR gene is the +66A/G polymorphism (rs1801394), which results in an isoleucine (A)-to-methionine (G) substitution at position 22; its minor allele frequency in the Japanese population is 30%. The MTRR+66AA genotype may induce hypomethylation of DNA by the regulation of homocysteine levels [21,24].
In this study, to elucidate the association of DNMT1, DNMT3A, DNMT3B, MTHFR and MTRR polymorphisms with the prognosis of AITDs and DNA methylation levels, we genotyped these polymorphisms and investigated global methylation levels of DNA.
Materials and methods
Subjects
We screened each polymorphism among 125 patients (17 men and 108 women) with HD, 176 patients (30 men and 146 women) with GD, and 83 healthy volunteers (control subjects; 29 men and 54 women). Patients with HD were positive for anti-thyroid microsomal antibody (McAb) or anti-thyroglobulin antibody (TgAb), and showed hypothyroidism or euthyroidism with palpable diffuse goitre. Patients with GD had a clinical history of thyrotoxicosis with a positive test for anti-thyrotrophin receptor antibody (TRAb). Healthy volunteers were euthyroid and negative for thyroid autoantibodies.
Forty-eight of these patients (seven men and 41 women) with HD developed moderate to severe hypothyroidism before 50 years of age, and were treated on a daily basis (subgroup with severe HD). Forty-nine untreated euthyroid HD patients (six men and 43 women) were more than 50 years of age (subgroup with mild HD). Seventy-nine euthyroid patients (15 men and 64 women) with GD had been treated with methimazole for at least 5 years and were still positive for TRAb (subgroup with intractable GD). Forty-seven patients (seven men and 40 women) with GD in remission had maintained a euthyroid state and were negative for TRAb for more than 2 years without medication (subgroup with GD in remission).
All patients and control subjects were Japanese and were unrelated to each other. All patients were followed-up closely for more than 5 years as out-patients at our thyroid clinic. Genomic DNA was isolated from ethylenediamine tetraacetic acid (EDTA)-treated peripheral blood mononuclear cells with a commercially available kit (Dr. GenTLE™, Takara Bio Inc., Shiga, Japan). Written informed consent was obtained from all patients and controls, and the study protocol was approved by the Ethics Committee of Osaka University. Clinical characteristics of the examined subjects are given in Table 1.
Table 1.
Clinical characteristics of the patients with autoimmune thyroid disease at the time of sampling
| Graves' disease | Hashimoto's disease | ||||
|---|---|---|---|---|---|
| Past clinical history of thyrotoxicosis with elevated TRAb | Diffuse goitre and positive TgAb and/or McAb | ||||
| Controls | Intractable | In remission | Severe | Mild | |
| n (female/male) | 83 (54/29) | 50 (42/8) | 47 (40/7) | 48 (41/7) | 49 (43/6) |
| Age of onset (years) (range) | 40·2 ± 15·9‡ (19–76) | 34·0 ± 13·5 (11–66) | 32·6 ± 13·3 (10–66) | 37·7 ± 10·5 (10–49) | 59·0 ± 10·3‡ (50–92) |
| Goitre size (cm) | n.d. | 5·0 ± 1·4* | 4·2 ± 0·6 | 4·2 ± 1·0** | 4·7 ± 1·3 |
| Free T4 (ng/dl) | n.d. | 1·2 ± 0·3 | 1·2 ± 0·2 | 1·4 ± 0·3 | 1·2 ± 0·2 |
| Free T3 (pg/ml) | n.d. | 2·7 ± 0·6 | 2·6 ± 0·3 | 2·6 ± 0·6 | 2·8 ± 0·4 |
| TSH (µU/ml) | n.d. | 1·6 ± 1·2 | 1·8 ± 1·2 | 1·8 ± 1·3 | 2·8 ± 1·7 |
| TRAb (IU/l) (range) | <1·0 | 7·9 ± 13·2 (1·1–71·0) | <1·0 | <1·0 | <1·0 |
| TgAb (2n × 100) | Negative | 2·8 ± 2·9 | 2·3 ± 2·7 | 7·1 ± 3·4** | 1·5 ± 2·2 |
| McAb (2n × 100) | Negative | 4·3 ± 2·7 | 5·3 ± 2·1 | 5·4 ± 3·1 | 3·9 ± 3·1 |
| Current treatment | None | Methimazole or PTU | None | L-thyroxine | None |
| Duration of treatment (years) | None | 11·7 ± 7·3 | 3·2 ± 1·3§ | 10·9 ± 8·5 | None |
| Current dose of anti-thyroid drug (mg/day)† (range) | None | 18·2 ± 32·2 (2·5–200) | None | None | None |
| Current dose of L-thyroxine (µg/day) (range) | None | None | None | 91·1 ± 36·8 (50–250) | None |
Data are expressed as mean ± standard deviation.
P < 0·01 (versus Graves’ disease in remission);
P < 0·01 (versus mild Hashimoto's disease).
Dose were expressed as the comparable dose of methimazole (50 mg of PTU was converted to 5 mg of methimazole).
Age at the time of sampling.
Duration of treatment with anti-thyroid drug before remission. T4: thyroxine; T3: triiodothyronine; TSH: thyrotrophin; TRAb: anti-thyrotrophin receptor antibody; McAb: anti-thyroid microsomal antibody; n.d.: not determined; TgAb: anti-thyroglobulin antibody; PTU: propylthiouracil.
Genotyping of polymorphisms
We used RFLP analysis for genotyping the DNMT1+32204A/G, DNMT1+14395A/G, DNMT3B−579G/T, MTHFR+677C/T and MTHFR+1298A/C polymorphisms. Target sequences of each gene were amplified using polymerase chain reaction (PCR), and the PCR product was digested by the addition of restriction enzyme. The sequences of forward and reverse primers, the PCR conditions and restriction enzymes used are summarized in Table 2. TaqMan SNP genotyping assays (Applied Biosystems, Tokyo, Japan) were used to genotype DNMT3A−448A/G and MTRR+66A/G polymorphisms.
Table 2.
The primers, polymerase chain reaction (PCR) conditions and restriction enzymes used in this study
| Primer pairs | PCR conditions | Restriction enzymes | ||
|---|---|---|---|---|
| DNMT1 | +32204A/G | 5′-AGAACCTGAAAAAGTAAATCCACCG-3′ | 96°C for 5 min | MspI |
| 5′-CATGTGATTCACCCGCTTCAG-3′ | (94°C for 30 s, 62°C for 30 s, 72°C for 30 s) × 35 cycles | |||
| 72°C for 7 min | ||||
| +14395A/G | 5′-TGTTTCCTTGTTCTCTGACAC-3′ | 94°C for 5 min | BstEII | |
| 5′-TTGGTTCCCGTTTTCTAGAC-3′ | (94°C for 30 s, 62°C for 30 s, 72°C for 30 s) × 30 cycles | |||
| 72°C for 5 min | ||||
| DNMT3B | −579G/T | 5′-GAGGTCTCATTATGCCTAGG-3′ | 96°C for 5 min | PvuII |
| 5′-GGGAGCTCACCTTCTAGAAA-3′ | (96°C for 30 s, 56°C for 30 s, 72°C for 30 s) × 30 cycles | |||
| 72°C for 5 min | ||||
| MTHFR | +677C/T | 5′-TGAAGGAGAAGGTGTCTGCGG-3′ | 94°C for 5 min | HinfI |
| 5′-AGGACGGTGCGGTGAGAGTG-3′ | (96°C for 30 s, 66°C for 30 s,72°C for 30 s) × 30 cycles | |||
| 72°C for 5 min | ||||
| +1298G/T | 5′-CTTTGGGGAGCTGAAGGACTACTAC-3′ | 96°C for 5 min | MboII | |
| 5′-CACTTTGTGACCATTCCGGTTTG-3′ | (96°C for 30 s, 61°C for 30 s, 72°C for 30 s) × 32 cycles | |||
| 72°C for 5 min |
Global methylation levels of DNA in peripheral whole blood cells
The global methylation levels of genomic DNA isolated from the whole blood were determined by a commercially available kit (MethylFlash™ Methylated DNA Quantification Kit; Epigentek, New York, USA). We examined 13 patients with GD in remission (three men and 10 women; 42·4 ± 15·5), 12 patients with mild HD (two men and 10 women; 60·8 ± 9·6) and 28 healthy volunteers (10 men and 18 women; 38·2 ± 12·3), because these subjects did not receive any medical treatment. The proportion of 5-methylcytosine in the whole DNA was expressed as a percentage of methylation.
Statistical analysis
We used the χ2 and Fisher's exact tests to evaluate the significance of differences between the genotype and allele frequencies among the subject's groups. Tukey's honestly significant difference test was used for multiple comparisons to analyse the differences between global DNA methylation levels among each genotype. Data were analysed with jmp8 software (SAS Institute Inc., Tokyo, Japan). Probability values of less than 0·05 were considered significant.
Results
DNMT1 polymorphisms
Although there was no significant difference in genotype and allele frequencies of the DNMT1+32204A/G polymorphisms between healthy controls and patients with AITD (Table 3), the G allele and GG genotype of this polymorphism was significantly more frequent in patients with intractable GD than in those with GD in remission (P = 0·033 and P = 0·005, respectively, Table 4).
Table 3.
Genotype and allele frequencies of polymorphisms genotyped in this study in patients with autoimmune thyroid diseases and control subjects
| Control | All patients with AITD | All patients with Graves’ disease | All patients with Hashimoto's disease | |||||
|---|---|---|---|---|---|---|---|---|
| DNMT1 | AA | 30 (45·5%) | 128 (46·5%) | n.s. | 83 (48·3%) | n.s. | 45 (43·7%) | n.s. |
| rs2228612 | AG | 26 (39·4%) | 118 (42·9%) | 70 (40·7%) | 48 (46·6%) | |||
| +32204A/G | GG | 10 (15·1%) | 29 (10·6%) | 19 (11·0%) | 10 (9·7%) | |||
| A allele | 86 (65·1%) | 374 (68·0%) | n.s. | 236 (68·6%) | n.s. | 138 (67·0%) | n.s. | |
| G allele | 46 (34·9%) | 176 (32·0%) | 108 (31·4%) | 68 (33·0%) | ||||
| DNMT1 | AA | 42 (61·8%) | 175 (64·0%) | n.s. | 101 (66·5%) | n.s. | 74 (66·1%) | n.s. |
| rs16999593 | AG | 24 (35·2%) | 77 (31·5%) | 44 (29·0%) | 33 (29·4%) | |||
| +14395A/G | GG | 2 (3·0%) | 12 (4·5%) | 7 (4·5%) | 5 (4·5%) | |||
| A allele | 108 (79·4%) | 427 (80·9%) | n.s. | 246 (80·9%) | n.s. | 181 (80·8%) | n.s. | |
| G allele | 28 (20·6%) | 101 (19·1%) | 58 (19·1%) | 43 (19·2%) | ||||
| DNMT3A | GG | 36 (54·6%) | 163 (56·8%) | n.s. | 98 (57·0%) | n.s. | 65 (56·5%) | n.s. |
| rs1550157 | GA | 25 (37·9%) | 111 (38·7%) | 68 (39·5%) | 43 (37·4%) | |||
| −448A/G | AA | 5 (7·5%) | 13 (4·5%) | 6 (3·5%) | 7 (6·1%) | |||
| G allele | 97 (73·5%) | 437 (76·1%) | n.s. | 264 (76·7%) | n.s. | 173 (72·1%) | n.s. | |
| A allele | 35 (26·5%) | 137 (23·9%) | 80 (23·3%) | 57 (27·9%) | ||||
| DNMT3B | TT | 68 (82·9%) | 216 (82·1%) | n.s. | 115 (81·0%) | n.s. | 101 (83·5%) | n.s. |
| rs1569686 | TG | 13 (15·9%) | 44 (16·7%) | 25 (17·6%) | 19 (15·7%) | |||
| −579 G/T | GG | 1 (1·2%) | 3 (1·2%) | 2 (1·4%) | 1 (0·8%) | |||
| T allele | 131 (91·0%) | 476 (90·5%) | n.s. | 255 (89·8%) | n.s. | 221 (91·3%) | n.s. | |
| G allele | 13 (9·0%) | 50 (9·5%) | 29 (10·2%) | 21 (8·7%) | ||||
| MTHFR | CC | 36 (42·9%) | 99 (35·5%) | n.s. | 54 (33·8%) | n.s. | 45 (37·8%) | n.s. |
| rs1801133 | CT | 35 (41·7%) | 142 (50·9%) | 79 (49·4%) | 63 (52·9%) | |||
| +677C/T | TT | 13 (15·4%) | 38 (13·6%) | 27 (16·8%) | 11 (9·3%) | |||
| C allele | 106 (63·9%) | 340 (60·9%) | n.s. | 187 (56·7%) | n.s. | 153 (64·3%) | n.s. | |
| T allele | 60 (36·1%) | 218 (39·1%) | 143 (43·3%) | 85 (35·7%) | ||||
| MTHFR | AA | 37 (57·8%) | 170 (62·7%) | n.s. | 95 (60·5%) | n.s. | 75 (65·8%) | n.s. |
| rs1801131 | AC | 26 (40·6%) | 96 (35·4%) | 60 (38·2%) | 36 (31·6%) | |||
| +1298A/C | CC | 1 (1·6%) | 5 (1·9%) | 2 (1·3%) | 3 (2·6%) | |||
| A allele | 84 (76·4%) | 436 (80·4%) | n.s. | 250 (79·6%) | n.s. | 186 (81·6%) | n.s. | |
| C allele | 26 (23·6%) | 106 (19·6%) | 64 (20·4%) | 42 (18·4%) | ||||
| MTRR | AA | 37 (52·9%) | 141 (49·1%) | n.s. | 80 (51·6%) | n.s. | 61 (46·2%) | n.s. |
| rs1801394 | AG | 27 (38·6%) | 117 (40·8%) | 58 (37·4%) | 59 (44·7%) | |||
| +66A/G | GG | 6 (8·5%) | 29 (10·1%) | 17 (11·0%) | 12 (9·1%) | |||
| A allele | 101 (72·1%) | 399 (70·0%) | n.s. | 218 (70·3%) | n.s. | 181 (68·6%) | n.s. | |
| G allele | 39 (27·9%) | 175 (30·0%) | 92 (29·7%) | 83 (31·4%) | ||||
AITD: autoimmune thyroid diseases; DNMT: DNA methyltransferase; MTRR: methionine synthase reductase; MTHFR: 5,10-methylenetetrahydrofolate reductase; n.s.: not significant.
Table 4.
Genotype and allele frequencies of polymorphisms genotyped in this study in patients with Graves’ disease and Hashimoto's disease
| Graves' disease | Hashimoto's disease | |||||||
|---|---|---|---|---|---|---|---|---|
| Intractable | In remmision | P value | Odds ratio (95% CI) | Severe | Mild | P value | ||
| DNMT1 | AA | 35 (44·3%) | 25 (53·2%) | 0·009* | 18 (40·0%) | 17 (44·7%) | n.s. | |
| rs2228612 | AG | 29 (36·7%) | 21 (44·7%) | 22 (48·9%) | 16 (42·1%) | |||
| +32204A/G | GG | 15 (19·0%) | 1 (2·1%) | 5 (11·1%) | 5 (13·2%) | |||
| A Allele | 99 (62·7%) | 71 (75·5%) | 0·033* | 1·8 | 58 (64·4%) | 50 (65·8%) | n.s. | |
| G Allele | 59 (37·3%) | 23 (24·5%) | (1·0∼3·3) | 32 (35·6%) | 26 (34·2%) | |||
| AA+AG | 64 (81·0%) | 46 (97·9%) | 0·005** | 10·8 | 40 (88·9%) | 33 (86·8%) | n.s. | |
| GG | 15 (19·0%) | 1 (2·1%) | (1·4∼84·5) | 5 (11·1%) | 5 (13·2%) | |||
| DNMT1 | AA | 46 (62·2%) | 25 (67·6%) | n.s. | 29 (60·4%) | 28 (68·3%) | n.s. | |
| rs16999593 | AG | 24 (32·4%) | 11 (29·7%) | 18 (37·5%) | 10 (24·4%) | |||
| +14395A/G | GG | 4 (5·4%) | 1 (2·7%) | 1 (2·1%) | 3 (7·3%) | |||
| A Allele | 116 (78·4%) | 61 (82·4%) | n.s. | 76 (79·2%) | 66 (80·5%) | n.s. | ||
| G Allele | 32 (21·6%) | 13 (17·6%) | 20 (20·8%) | 16 (19·5%) | ||||
| DNMT3A | GG | 38 (50·7%) | 25 (56·8%) | n.s. | 23 (60·5%) | 22 (44·9%) | n.s. | |
| rs1550157 | GA | 34 (45·3%) | 17 (38·6%) | 12 (31·6%) | 23 (46·9%) | |||
| −448A/G | AA | 3 (4·0%) | 2 (4·6%) | 3 (7·9%) | 4 (8·2%) | |||
| G Allele | 110 (73·3%) | 67 (76·1%) | n.s. | 58 (76·3%) | 67 (68·4%) | n.s. | ||
| A Allele | 40 (26·7%) | 21 (23·9%) | 18 (23·7%) | 31 (31·6%) | ||||
| DNMT3B | TT | 51 (79·7%) | 30(76·9%) | n.s. | 39 (86·7%) | 36 (78·3%) | n.s. | |
| rs1569686 | TG | 13 (20·3%) | 8 (20·5%) | 5 (11·1%) | 10 (21·7%) | |||
| −579 G/T | GG | 0 (0·0%) | 1 (2·6%) | 1 (2·2%) | 0 (0·0%) | |||
| T Allele | 91 (87·5%) | 65 (87·8%) | n.s. | 85 (92·4%) | 84 (89·4%) | n.s. | ||
| G Allele | 13 (12·5%) | 9 (12·2%) | 7 (7·6%) | 10 (10·6%) | ||||
| MTHFR | CC | 27 (36·5%) | 13 (27·7%) | n.s. | 15 (40·5%) | 25 (45·5%) | n.s. | |
| rs1801133 | CT | 36 (48·6%) | 25 (53·2%) | 18 (48·7%) | 28 (50·9%) | |||
| +677C/T | TT | 11 (14·9%) | 9 (19·1%) | 4 (10·8%) | 2 (3·6%) | |||
| C Allele | 90 (60·8%) | 45 (59·2%) | n.s. | 58 (61·7%) | 78 (71·0%) | n.s. | ||
| T Allele | 58 (39·2%) | 31 (40·8%) | 36 (38·3%) | 32 (29·0%) | ||||
| MTHFR | AA | 43 (55·1%) | 28 (62·2%) | n.s. | 26 (57·8%) | 29 (63·0%) | n.s. | |
| rs1801131 | AC | 34 (43·6%) | 17 (37·8%) | 18 (40·0%) | 15 (32·6%) | |||
| +1298A/C | CC | 1 (1·3%) | 0 (0·0%) | 1 (2·2%) | 2 (4·4%) | |||
| A Allele | 120 (76·9%) | 73 (75·7%) | n.s. | 48 (80·0%) | 73 (79·3%) | n.s. | ||
| C Allele | 36 (23·1%) | 17 (24·3%) | 12 (20·0%) | 19 (20·7%) | ||||
| MTRR | AA | 34 (50·0%) | 21 (53·9%) | n.s. | 26 (57·8%) | 22 (37·9%) | n.s. | |
| rs1801394 | AG | 28 (41·2%) | 16 (41·0%) | 16 (35·5%) | 30 (51·7%) | |||
| +66A/G | GG | 6 (8·8%) | 2 (5·1%) | 3 (6·7%) | 6 (10·4%) | |||
| A Allele | 96 (70·6%) | 58 (74·4%) | n.s. | 68 (75·6%) | 74 (63·8%) | n.s. | ||
| G Allele | 40 (29·4%) | 20 (25·6%) | 22 (24·4%) | 42 (36·2%) | ||||
| AA | 34 (50·0%) | 21 (53·8%) | n.s. | 26 (57·8%) | 22 (37·9%) | 0·045* | ||
| AG+GG | 34 (50·0%) | 18 (46·2%) | 19 (42·2%) | 36 (62·1%) | ||||
Value calculated by the χ2 test,
Value calculated by Fisher's exact test.
DNMT: DNA methyltransferase; MTRR: methionine synthase reductase; MTHFR: 5,10-methylenetetrahydrofolate reductase; n.s.: not significant.
The genotype and allele frequencies of the DNMT1+14395A/G polymorphisms showed no difference between healthy controls and patients with AITD (Table 3), and showed no evidence of being involved in the prognosis of AITD (Table 4).
DNMT3A and DNMT3B polymorphisms
Genotype and allele frequencies of the DNMT3A−448A/G and DNMT3B−579G/T polymorphisms showed no significant differences between healthy controls and patients with AITD (Table 3), and were not involved in the prognosis of AITD (Table 4).
MTHFR polymorphisms
Genotype and allele frequencies of the MTHFR+677C/T and +1298A/C polymorphisms showed no significant differences between healthy controls and patients with AITD (Table 3); these genotype and allele frequencies did not influence prognosis of AITD (Table 4).
MTRR polymorphism
The MTRR+66AA genotype was observed to be more frequent in patients with severe HD than in patients with mild HD (P = 0·045, Table 4), although we found no significant differences in genotype or allele frequencies of this polymorphism between patients with AITD and healthy control subjects (Table 3).
DNA methylation levels and polymorphisms in methylation-related genes
DNA methylation levels in individuals with DNMT1+32204GG genotype were significantly lower than those in carriers of the AA genotype (P = 0·025, Fig. 2). We were unable to obtain any evidence of a significant relationship between each of the polymorphisms and DNA methylation levels (data not shown).
Fig. 2.
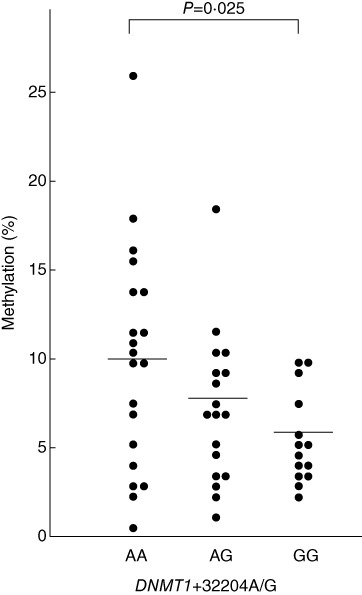
Methylation levels in individuals with each genotype of DNMT1+32204 A/G polymorphism.
Conversely, MTRR+66AG polymorphisms were not associated with DNA methylation levels (Fig. 3).
Fig. 3.
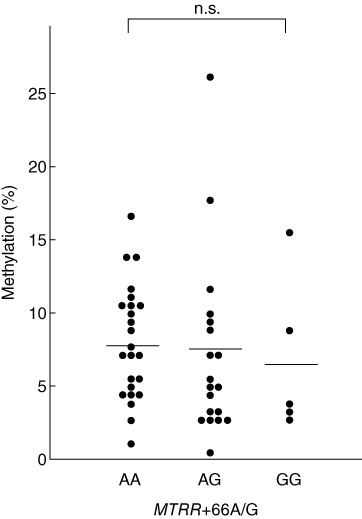
Methylation levels in individuals with each genotype of methionine synthase reductase (MTRR) +66A/G polymorphism.
Clinical characteristics and polymorphisms in methylation-related genes
No association was observed between each allele or genotype of the examined polymorphisms and the levels of TRAb, McAb or TgAb, or goitre size (data not shown).
Discussion
In the present study, we observed that the DNMT1+32204GG genotype was correlated with lower levels of global DNA methylation (Fig. 2). Because this polymorphism is located in the region of the DNMT1 gene encoding the nuclear localization sequence (NLS)-containing domain (NlsD) of the enzyme, which plays an important role in binding to CpG sites [25], we anticipate that the substitution of Ile (+32204A allele) to Val (+32204G allele) may transform the structure of the NlsD and decrease the DNA-binding ability of the enzyme; therefore, DNA methylation levels may be lower in individuals with the +32204GG genotype. To the best of our knowledge, this is the first report demonstrating that a functional polymorphism in the DNMT1 gene is related to methylation levels of DNA. In addition, The DNMT1+32204GG genotype was observed to be more frequent in patients with intractable GD than in those with GD in remission (Table 3). This indicates that the +32204GG genotype, which is related to a lower level of global DNA methylation, is associated with the intractability of GD.
In addition, we have reported previously that genetic programming for production of higher interleukin (IL)-1β and transforming growth factor (TGF)-β levels are associated with intractability of GD [26,27]. It is well known that demethylation of the CpG site in the promoter regions enhance gene expression [28,29]. Therefore, lowered global DNA methylation ability in individuals with the +32204GG genotype may decrease the methylation levels of IL1B and TGFB promoter regions and enhance the production of IL-1β and TGF-β. This may be a potential candidate mechanism to impede induction remission in GD patients with DNMT1+32204GG genotype.
In addition, we clarified that the MTRR+66AA genotype was more frequent in patients with severe HD than in those with mild HD. Previous reports showed that homocysteine levels were increased in plasma obtained from individuals with the MTRR+66AA genotype [21]. There are two possible mechanisms that may explain how the MTRR+66AA genotype is related to the severity of HD. One possibility is that increased homocysteine levels may cause an insufficient supply of methyl groups and result in a decrease in global DNA methylation [24]. In this study, however, global methylation levels of DNA were not associated with the genotypes of the MTRR+66A/G polymorphism (Fig. 3). Another possibility is that an increase in homocysteine levels may increase caspase-3 activity and, thus, apoptosis in thyroid epithelial cells in HD patients with the MTRR+66AA genotype, as it has been reported that homocysteine induces caspase-3 activity and apoptosis in various cells [30–33]. Previous reports also suggest that caspase-3 is highly expressed in thyroid epithelial cells in HD patients [34].
In conclusion, the DNMT1+32204GG genotype is associated with hypomethylation of DNA and is related to the intractability of GD, while the MTRR+66A/G polymorphism is associated with the severity of HD.
Declarations of interest
No potential conflicts of interest were disclosed.
References
- 1.Menconi F, Oppenheim Y, Tomer Y. Grave's disease. In: Shoenfeld Y, Cervera R, Gershwin M, editors. Diagnostic criteria in autoimmune diseases. Totowa: Humana Press; 2008. pp. 231–5. [Google Scholar]
- 2.Weetman A. Chronic autoimmune thyroiditis. In: Braverman L, Utiger R, editors. The thyroid: a fundamental and clinical text. Philadelphia: Lippincott Williams & Wilkins; 2000. pp. 721–32. [Google Scholar]
- 3.Amino N, Hagen SR, Yamada N, Refetoff S. Measurement of circulating thyroid microsomal antibodies by the tanned red cell haemagglutination technique: its usefulness in the diagnosis of autoimmune thyroid diseases. Clin Endocrinol (Oxf) 1976;5:115–25. doi: 10.1111/j.1365-2265.1976.tb02822.x. [DOI] [PubMed] [Google Scholar]
- 4.Yoshida H, Amino N, Yagawa K, et al. Association of serum antithyroid antibodies with lymphocytic infiltration of the thyroid gland: studies of seventy autopsied cases. J Clin Endocrinol Metab. 1978;46:859–62. doi: 10.1210/jcem-46-6-859. [DOI] [PubMed] [Google Scholar]
- 5.Zhang X, Yazaki J, Sundaresan A, et al. Genome-wide high-resolution mapping and functional analysis of DNA methylation in arabidopsis. Cell. 2006;126:1189–201. doi: 10.1016/j.cell.2006.08.003. [DOI] [PubMed] [Google Scholar]
- 6.Razin A, Riggs AD. DNA methylation and gene function. Science. 1980;210:604–10. doi: 10.1126/science.6254144. [DOI] [PubMed] [Google Scholar]
- 7.Mostoslavsky R, Bergman Y. DNA methylation: regulation of gene expression and role in the immune system. Biochim Biophys Acta. 1997;1333:F29–50. doi: 10.1016/s0304-419x(97)00010-3. [DOI] [PubMed] [Google Scholar]
- 8.Haluskova J. Epigenetic studies in human diseases. Folia Biol (Praha) 2010;56:83–96. [PubMed] [Google Scholar]
- 9.Rountree MR, Bachman KE, Herman JG, Baylin SB. DNA methylation, chromatin inheritance, and cancer. Oncogene. 2001;20:3156–65. doi: 10.1038/sj.onc.1204339. [DOI] [PubMed] [Google Scholar]
- 10.Pradhan S, Bacolla A, Wells RD, Roberts RJ. Recombinant human DNA (cytosine-5) methyltransferase. I. Expression, purification, and comparison of de novo and maintenance methylation. J Biol Chem. 1999;274:33002–10. doi: 10.1074/jbc.274.46.33002. [DOI] [PubMed] [Google Scholar]
- 11.Nakamura T, Sekigawa I, Ogasawara H, et al. Expression of DNMT-1 in patients with atopic dermatitis. Arch Dermatol Res. 2006;298:253–6. doi: 10.1007/s00403-006-0682-0. [DOI] [PubMed] [Google Scholar]
- 12.Park BL, Kim LH, Shin HD, Park YW, Uhm WS, Bae SC. Association analyses of DNA methyltransferase-1 (DNMT1) polymorphisms with systemic lupus erythematosus. J Hum Genet. 2004;49:642–6. doi: 10.1007/s10038-004-0192-x. [DOI] [PubMed] [Google Scholar]
- 13.Xiang G, Zhenkun F, Shuang C, et al. Association of DNMT1 gene polymorphisms in exons with sporadic infiltrating ductal breast carcinoma among Chinese Han women in the Heilongjiang Province. Clin Breast Cancer. 2010;10:373–7. doi: 10.3816/CBC.2010.n.049. [DOI] [PubMed] [Google Scholar]
- 14.Fan H, Liu D, Qiu X, et al. A functional polymorphism in the DNA methyltransferase-3A promoter modifies the susceptibility in gastric cancer but not in esophageal carcinoma. BMC Med. 2010;8:12. doi: 10.1186/1741-7015-8-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Lee SJ, Jeon HS, Jang JS, et al. DNMT3B polymorphisms and risk of primary lung cancer. Carcinogenesis. 2005;26:403–9. doi: 10.1093/carcin/bgh307. [DOI] [PubMed] [Google Scholar]
- 16.Stern LL, Bagley PJ, Rosenberg IH, Selhub J. Conversion of 5-formyltetrahydrofolic acid to 5-methyltetrahydrofolic acid is unimpaired in folate-adequate persons homozygous for the C677T mutation in the methylenetetrahydrofolate reductase gene. J Nutr. 2000;130:2238–42. doi: 10.1093/jn/130.9.2238. [DOI] [PubMed] [Google Scholar]
- 17.Chen Z, Karaplis AC, Ackerman SL, et al. Mice deficient in methylenetetrahydrofolate reductase exhibit hyperhomocysteinemia and decreased methylation capacity, with neuropathology and aortic lipid deposition. Hum Mol Genet. 2001;10:433–43. doi: 10.1093/hmg/10.5.433. [DOI] [PubMed] [Google Scholar]
- 18.Frosst P, Blom HJ, Milos R, et al. A candidate genetic risk factor for vascular disease: a common mutation in methylenetetrahydrofolate reductase. Nat Genet. 1995;10:111–13. doi: 10.1038/ng0595-111. [DOI] [PubMed] [Google Scholar]
- 19.Kang SS, Zhou J, Wong PW, Kowalisyn J, Strokosch G. Intermediate homocysteinemia: a thermolabile variant of methylenetetrahydrofolate reductase. Am J Hum Genet. 1988;43:414–21. [PMC free article] [PubMed] [Google Scholar]
- 20.Lievers KJ, Boers GH, Verhoef P, et al. A second common variant in the methylenetetrahydrofolate reductase (MTHFR) gene and its relationship to MTHFR enzyme activity, homocysteine, and cardiovascular disease risk. J Mol Med. 2001;79:522–8. doi: 10.1007/s001090100253. [DOI] [PubMed] [Google Scholar]
- 21.Gaughan DJ, Kluijtmans LA, Barbaux S, et al. The methionine synthase reductase (MTRR) A66G polymorphism is a novel genetic determinant of plasma homocysteine concentrations. Atherosclerosis. 2001;157:451–6. doi: 10.1016/s0021-9150(00)00739-5. [DOI] [PubMed] [Google Scholar]
- 22.Paz MF, Avila S, Fraga MF, et al. Germ-line variants in methyl-group metabolism genes and susceptibility to DNA methylation in normal tissues and human primary tumors. Cancer Res. 2002;62:4519–24. [PubMed] [Google Scholar]
- 23.Chen J, Stampfer MJ, Ma J, et al. Influence of a methionine synthase (D919G) polymorphism on plasma homocysteine and folate levels and relation to risk of myocardial infarction. Atherosclerosis. 2001;154:667–72. doi: 10.1016/s0021-9150(00)00469-x. [DOI] [PubMed] [Google Scholar]
- 24.Yi P, Melnyk S, Pogribna M, Pogribny IP, Hine RJ, James SJ. Increase in plasma homocysteine associated with parallel increases in plasma S-adenosylhomocysteine and lymphocyte DNA hypomethylation. J Biol Chem. 2000;275:29318–23. doi: 10.1074/jbc.M002725200. [DOI] [PubMed] [Google Scholar]
- 25.Fatemi M, Hermann A, Pradhan S, Jeltsch A. The activity of the murine DNA methyltransferase Dnmt1 is controlled by interaction of the catalytic domain with the N-terminal part of the enzyme leading to an allosteric activation of the enzyme after binding to methylated DNA. J Mol Biol. 2001;309:1189–99. doi: 10.1006/jmbi.2001.4709. [DOI] [PubMed] [Google Scholar]
- 26.Hayashi F, Watanabe M, Nanba T, Inoue N, Akamizu T, Iwatani Y. Association of the –31C/T functional polymorphism in the interleukin-1beta gene with the intractability of Graves’ disease and the proportion of T helper type 17 cells. Clin Exp Immunol. 2009;158:281–6. doi: 10.1111/j.1365-2249.2009.04034.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Yamada H, Watanabe M, Nanba T, Akamizu T, Iwatani Y. The +869T/C polymorphism in the transforming growth factor-beta1 gene is associated with the severity and intractability of autoimmune thyroid disease. Clin Exp Immunol. 2008;151:379–82. doi: 10.1111/j.1365-2249.2007.03575.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Rodriguez-Cortez VC, Hernando H, de la Rica L, Vento R, Ballestar E. Epigenomic deregulation in the immune system. Epigenomics. 2011;3:697–713. doi: 10.2217/epi.11.99. [DOI] [PubMed] [Google Scholar]
- 29.Hashimoto K, Oreffo RO, Gibson MB, Goldring MB, Roach HI. DNA demethylation at specific CpG sites in the IL1B promoter in response to inflammatory cytokines in human articular chondrocytes. Arthritis Rheum. 2009;60:3303–13. doi: 10.1002/art.24882. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Park SJ, Kim KJ, Kim WU, Oh IH, Cho CS. Involvement of endoplasmic reticulum stress in homocysteine-induced apoptosis of osteoblastic cells. J Bone Miner Metab. 2012;30:474–84. doi: 10.1007/s00774-011-0346-9. [DOI] [PubMed] [Google Scholar]
- 31.Shastry S, Ingram AJ, Scholey JW, James LR. Homocysteine induces mesangial cell apoptosis via activation of p38-mitogen-activated protein kinase. Kidney Int. 2007;71:304–11. doi: 10.1038/sj.ki.5002031. [DOI] [PubMed] [Google Scholar]
- 32.Zbidi H, Redondo PC, Lopez JJ, Bartegi A, Salido GM, Rosado JA. Homocysteine induces caspase activation by endoplasmic reticulum stress in platelets from type 2 diabetics and healthy donors. Thromb Haemost. 2010;103:1022–32. doi: 10.1160/TH09-08-0552. [DOI] [PubMed] [Google Scholar]
- 33.Hirashima Y, Seshimo S, Fujiki Y, et al. Homocysteine and copper induce cellular apoptosis via caspase activation and nuclear translocation of apoptosis-inducing factor in neuronal cell line SH-SY5Y. Neurosci Res. 2010;67:300–6. doi: 10.1016/j.neures.2010.04.013. [DOI] [PubMed] [Google Scholar]
- 34.Kaczmarek E, Lacka K, Jarmolowska-Jurczyszyn D, Sidor A, Majewski P. Changes of B and T lymphocytes and selected apopotosis markers in Hashimoto's thyroiditis. J Clin Pathol. 2011;64:626–30. doi: 10.1136/jcp.2010.086553. [DOI] [PubMed] [Google Scholar]


