Abstract
Common variable immunodeficiency disorders (CVID) are a group of heterogeneous conditions that have in common primary failure of B cell function, although numerous T cell abnormalities have been described, including reduced proliferative response and reduced regulatory T cells. This study compared the T cell phenotype of CVID patients subdivided into clinical phenotypes as well as patients with partial antibody deficiencies [immunoglobulin (Ig)G subclass deficiency and selective IgA deficiency], X-linked agammaglobulinaemia (XLA) and healthy and disease controls. Absolute numbers of T cell subpopulations were measured by four-colour flow cytometry: naive T cells, central and effector memory and terminally differentiated (TEM) T cells, using CD45RA and CCR7 expression. Early, intermediate and late differentiation status of T cells was measured by CD27/CD28 expression. Putative follicular T cells, recent thymic emigrants and regulatory T cells were also assessed. Significant reduction in naive CD4 T cells, with reduced total CD4 and recent thymic emigrant numbers, was observed in CVID patients, most pronounced in those with autoimmune cytopenias or polyclonal lymphoproliferation. These findings suggest a lack of replenishment by new thymically derived cells. CD8 naive T cells were reduced in CVID patients, most significantly in the autoimmune cytopenia subgroup. There was a reduction in early differentiated CD4 and CD8 T cells and increased CD8 TEM in the CVID patients, particularly autoimmune cytopenia and polyclonal lymphoproliferation subgroups, suggesting a more activated T cell phenotype, due perhaps to an antigen-driven process. XLA patients had significantly reduced putative follicular T cells, which may depend on B cells for survival, while no significant alterations were observed in the T cells of those with IgG subclass deficiency or selective IgA deficiency.
Keywords: CVID, memory cells, primary antibody deficiency, T cells, XLA
Introduction
Common variable immunodeficiency disorders (CVID) are heterogeneous conditions that make up the most common group of clinically significant primary antibody deficiency (PAD). Patients with CVID are characterized by increased susceptibility to recurrent bacterial infection, coupled with low serum immunoglobulin levels and reduced specific antibody production in response to vaccination [1]. Patients may also have numerous clinical complications, including enteropathy, lymphoid malignancy, granuloma and autoimmunity, which have been used recently to classify patients into clinical phenotypes with varying prognoses [2,3]. CVID probably represent a polygenic group of primary antibody deficiency disorders of unknown aetiology [4].
Other PADs include X-linked agammaglobulinaemia (XLA), immunoglobulin (Ig)G subclass deficiency and selective IgA deficiency. Patients with XLA are profoundly antibody and B cell-deficient, and therefore experience recurrent bacterial infections [5]. However, they do not encounter the other clinical complications, common to many CVID patients, which are thought to relate to the underlying immune dysregulation. There have been suggestions that partial antibody deficiencies, in particular selective IgA deficiency, may share a genetic basis with some types of CVID [6]. This is supported by reports of progression of selective IgA deficiency to CVID in rare patients [6].
Patients with CVID have a common feature in failure of B cell function, although a number of T cell abnormalities have been described, including reduced naive CD4 T cells [7], reduced proliferative responses to mitogens [8,9], reduced cytokine responses to mitogens and recall antigen [10,11] and reduced T regulatory cells (Tregs) [12–14] in selected patients. A subset of CVID patients are reported to have an increased susceptibility to recurrent viral infections or opportunistic infections that are more associated with T cell defects [7,15], particularly in those patients from consanguineous families [16], suggesting an unknown, autosomal recessive, combined immune deficiency.
Upon antigen encounter, naive T cells undergo a developmental pathway, resulting in the generation of central memory and effector memory T cells [17]. They can be measured in blood by use of the accepted markers, CCR7 and CD45RA [18]. In the early stages of differentiation, T cells express high levels of co-stimulatory molecules CD28 and CD27, which are lost sequentially upon differentiation [19,20]. CD31 and CD45RA co-expression is used to define recent thymic emigrants and correlates well with T cell receptor excision circle (TREC) levels [21]. Follicular T cells are a distinct CD4 T cell lineage that regulates the development of antigen-specific B cell responses in the germinal centre and are identified by CXCR5 expression; putative follicular T cells in the periphery are identified by CD45RO and CXCR5 expression [22]. Percentages of these putative follicular T cells reduced in inducible T cell co-stimulator (ICOS) deficiency – a germinal-centre defect [23]. A recent study in CVID patients demonstrated that use of CD127low CD25+ markers to discern Tregs correlated well with forkhead box protein 3 (FoxP3) expression [14]. These markers were utilized in this study.
T cell phenotypes have been investigated in a number of CVID cohorts, with reduction in CD4 naive T cells being the most consistent outcome [8,24,25]. However, the main limitation with most studies [24,26] was the heterogeneity of the CVID patient groups studied and the difficulties encountered in correlating laboratory phenotypes with clinically useful, defined clinical phenotypes. This study aimed to investigate a comprehensive range of T cell phenotypes in a large group of well-researched CVID patients in the context of their well-defined clinical phenotypes [2,3]. Also, for the first time, we have compared results from CVID patients with those from a disease control as well as a healthy control group. As a comparison, we also investigated the T cell phenotypes in other partial antibody deficiency groups and XLA. To our knowledge, this paper investigates the most comprehensive selection of T cell subsets of all papers published so far, including CD45RA, CCR7 to distinguish naive, effectors, central memory and terminally differentiated T cells; CD28/CD27 co-stimulation markers to determine differentiation state (not published in antibody deficiency groups to our knowledge); and recent thymic emigrants, putative follicular T cells and Tregs.
Materials and methods
Study population
Controls and patient groups were recruited to this study through the Clinical Immunology Department at the John Radcliffe Hospital, Oxford, UK under the ethical approval of the Central Oxfordshire Research Ethics Committee (05/Q1605/88). All subjects gave informed, written consent and the studies were performed according to the Declaration of Helsinki.
All patients used met international diagnostic criteria [Pan-American Group for Immunodeficiency (PAGID) and European Society for Immunodeficiencies (ESID)], and included 58 CVID patients, 15 IgG subclass with IgA-deficient patients (Gsub), 14 IgA-deficient patients (IgA) and nine XLA patients. Healthy controls were recruited from hospital staff to match the age range and gender bias of the total CVID group (see Table 1 for study group demographics). Healthy controls were individuals aged 18 years or over willing to donate blood who passed our exclusion criteria. Exclusion criteria included pregnant women, any medical condition which affects the immune system (for example, autoimmunity, recurrent infections, immunodeficiency, lymphoproliferative disease) or treatment with a drug known to affect the immune system.
Table 1.
Demographics of the study groups
| Number | Male (% male) | Age (years), median and range | P-value | Age of presentation (years), median and range | |
|---|---|---|---|---|---|
| Healthy controls | 48 | 17 (35·4%) | 43·4 (21·4–84·6) | n.s. | – |
| Disease controls | 31 | 10 (32·3%) | 42·7 (18·5–73·8) | n.s. | – |
| IgG subclass deficiency | 15 | 8 (53·3%) | 36·4 (15·6–66·8) | n.s. | 14·5 (2–58) |
| Selective IgA deficiency | 14 | 5 (35·7%) | 32·9 (13·5–63·7) | n.s. | 37 (13–63) |
| XLA | 9 | 9 (100%) | 32·0 (0·5–56·17) | P < 0·05 | 1 (0·5–5) |
| CVID total | 58 | 28 (48·3%) | 46·4 (17·3–75·2) | n.s. | 25 (0·75–63) |
| Infection only | 29 | 14 (48·3% | 39·9 (17·3–75·2) | n.s. | 18 (1–55) |
| Polyclonal lymphoproliferation | 9 | 5 (55·6%) | 54·2 (34·5–67·3) | n.s. | 31 (13–54) |
| Cytopenias | 10 | 4 (40%) | 50·0 (20·0–75·1) | n.s. | 28 (0·75–62) |
| Organ-specific autoimmunity | 20 | 11 (55%) | 51·4 (20·0–75·1) | n.s. | 31 (0·75–63) |
| Lymphoid malignancy | 1 | 1 (100%) | 55·4 | – | 28 |
| Enteropathy | 3 | 2 (66·7%) | 54·5 (44·4–55·4) | – | 27 (20–28) |
Age distribution of groups was compared using one-way analysis of variance with Tukey's multiple comparison test as a post-hoc test. CVID: common variable immunodeficiency disorder; Ig: immunoglobulin; n.s.: not significant; XLA: X-linked agammaglobulinaemia.
In order to control for the effect of infection on the T cell subpopulations, disease controls were recruited from the immunodeficiency clinic. These were immune-competent patients who had an increased infection burden, in whom no clinical or laboratory evidence for immunodeficiency was found. Results from this group were included only once a period of 1 year had elapsed since discharge from the clinic, to rule out an evolving immunodeficiency. The immune tests undertaken were guided by clinical and family histories. The typical panel of tests performed included: IgG, IgA and IgM, and serum and urine electrophoresis with immunofixation if indicated. Specific antibody responses to the vaccines tetanus, pneumococcal and Haemophilius influenza B were performed, and if absent/low responses were noted the patient was vaccinated and these retested after 1 month. Lymphocyte subsets, both percentage and absolute count, were also performed, including measurement of B cells, CD4 and CD8 T cells and natural killer (NK) cells [3,27].
At the time of analysis, all XLA and 55 of 58 CVID patients were on immunoglobulin replacement, but not on immunosuppressive therapy. Those with autoimmune cytopenia or lymphoid interstitial pneumonia had not received corticosteroid therapy within 6 months, and only at prior doses <25 mg/kg. No patient had an affected parent, sibling or child.
CVID patients were categorized into the following clinical phenotypes, as described in Chapel et al. [2,3]: infection only (IO), enteropathy, lymphoid malignancy, polyclonal lymphoproliferation (PL), organ-specific autoimmune disease (OSAI) or autoimmune cytopenias (AC) which included immune thrombocytopenia (ITP). ITP is defined as platelets <100 × 109/l, persistent (>6 months), one episode treated with steroids [3]. The autoimmune diseases in patients in the OSAI group included: autoimmune thyroid disease (n = 5), psoriasis (n = 6), uveitis (n = 2), vitiligo (n = 2), pernicious anaemia (n = 3), ulcerative colitis (n = 4) and type 1 diabetes (n = 2). Only one patient had a subsequent lymphoid malignancy and only three had an enteropathy, so these categories were not utilized in the analysis; these patients were included in the CVID total group. Figure 1 demonstrates the distribution of clinical phenotypes of the CVID patient group.
Fig. 1.
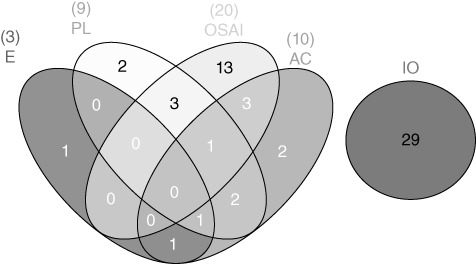
Venn diagram illustrating the distribution of common variable immunodeficiency disorder (CVID) patients into clinical phenotypes [2,3]. Numbers in brackets indicate numbers in each division; patients may appear in more than one group, as indicated. E: enteropathy; PL: polyclonal lymphoproliferation; AC: autoimmune cytopenias; OSAI: organ-specific autoimmune disease; IO: infection only.
The number of patients stated in each group in Table 1 is the maximum number of patients analysed for a T cell subpopulation. However, for some of the T cell subpopulations smaller numbers were analysed due to either technical difficulties with a particular tube or limited sample availability.
Flow cytometry
All flow cytometric analysis was performed on ethylenediamine tetraacetic acid (EDTA) blood samples within 48 h of venepuncture. Lymphocyte subset analysis and absolute counts of total lymphocytes, total T cells, CD4 and CD8 T, B and NK cells were performed using BD Multitest™ CD3/CD16+CD56/CD45/CD19 and CD3/CD8/CD45/CD4 with BD Trucount™ Tubes (Becton-Dickinson, San Jose, CA, USA) and acquired on a BD fluorescence activated cell sorter (FACS)Calibur (Becton Dickinson), as per the manufacturer's instructions.
For T cell subpopulations, 100 µl of whole blood was incubated with directly conjugated fluorescent antibodies for 30 min in the dark at room temperature, then red cells were lysed using FACSlyse (Becton-Dickinson), washed in phosphate-buffered saline (PBS) and fixed in PBS with 1% formaldehyde. Samples were acquired using four-colour acquisition on a FACSCalibur and data analysed using CellquestPro software (Becton-Dickinson). Fluorescence minus one gating techniques were employed to evaluated thresholds for positivity of individual antibodies and aid gating of T cell subpopulations. The following CD3+ T cell subpopulations were analysed on CD4 and CD8 cells: naive, central memory (CM), effector memory (EM) and terminally differentiated determined (TEM) by CCR7 and CD45RA expression; early, intermediate and late differentiation status was determined by CD28/CD27 expression. Other CD4 T cell populations included recent thymic emigrant (defined by CD45RA/CD31), putative follicular T cells (defined by CXCR5/CD45RO) and Tregs (defined by CD25+CD127-). Absolute cell counts were calculated using the CD4 or CD8 T cell counts from the lymphocyte subset analysis. Subpopulations were added together to ensure that the total number of CD4 or CD8 matched those from the lymphocyte subset analysis.
Statistical analyses
All statistical analyses were performed using GraphPad Prism version 4 (GraphPad Software, San Diego, CA, USA). All data were analysed using non-parametric one-way analysis of variance (anova) Kruskal–Wallis with Dunn's multiple comparison test as a post-hoc test or one-way anova with Tukey's multiple comparison test as a post-hoc test. T cell subpopulation correlations with age were analysed by Spearman's correlation. The Venn diagram (Fig. 1) was made using the J.C. Oliveros (2007) VENNY tool from http://bioinfogp.cnb.csic.es/tools/venny/index.html
Results
Comparison of T cell populations with age
It was important to age- and gender-match the healthy controls and patient groups, as an effect of age on some T cell subpopulations has been described [21]. This was performed successfully, except for the XLA patients, who were significantly younger and all male (Table 1).
There were no significant differences observed between the disease controls (n = 31) and healthy controls (n = 48) in any of the T cell subpopulations studied (see Figs 3 and 4), so the two control groups were combined to determine whether or not there were any relationships between T cell subpopulations and age. No significant correlations between age and any T cell subpopulation were observed in this age range (18·5–84·6years at the time of study) (all r2 values were less than 0·5 or −0·5).
Fig. 3.
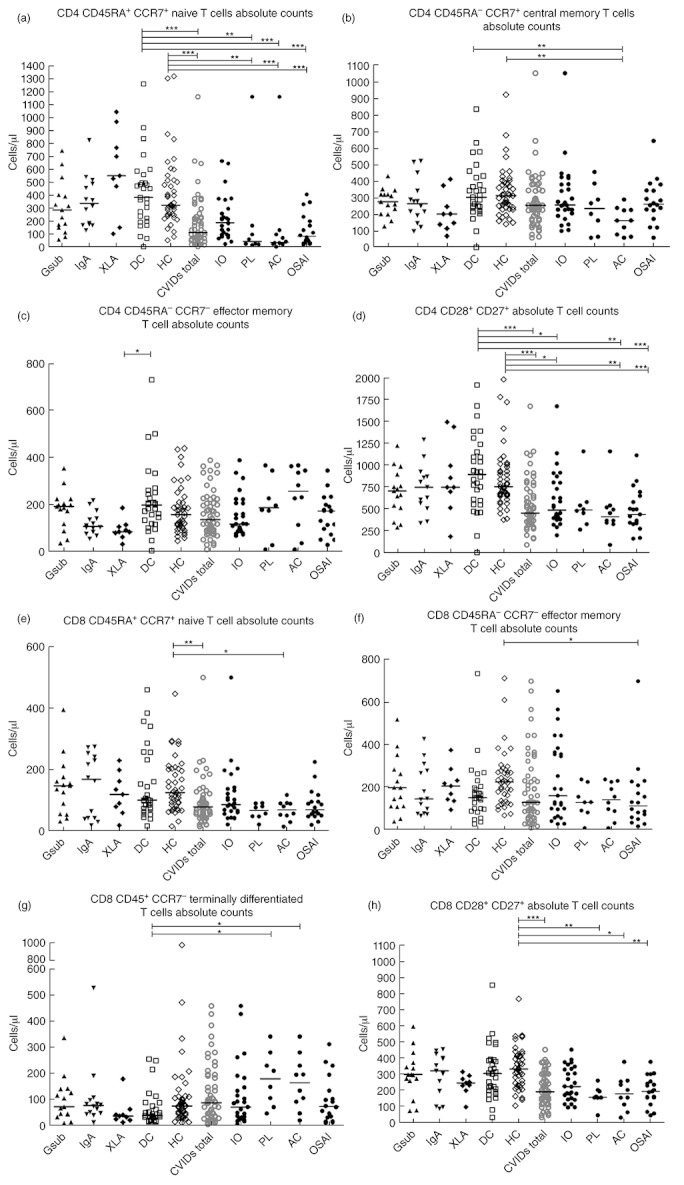
Comparison of T cell naive and memory subset absolute counts (cells/µl) (a) CD4 naive T cells, (b) CD4 central memory, (c) CD4 effector memory, (d) CD4 early differentiation, (e) CD8 naive T cells, (f) CD8 effector memory, (g) CD8 terminally differentiated, (h) CD8 early differentiation. Common variable immunodeficiency disorder (CVID) patients may appear in more than one group, as indicated in Fig. 1. Gsub: immunoglobulin (Ig)G subclass-deficient; IgA: IgA-deficient; XLA: X-linked agammaglobulinaemia; DC: disease control; HC: healthy control; CVID all subjects with CVID; IO: infection only; PL: polyclonal lymphoproliferation; AC: autoimmune cytopenias; OSAI: organ-specific autoimmune disease. Statistics performed with one-way analysis of variance (anova) Kruskal–Wallis, with Dunn's multiple comparison test as a post-hoc test. *P < 0·05; **P < 0·01; ***P < 0·001.
Fig. 4.
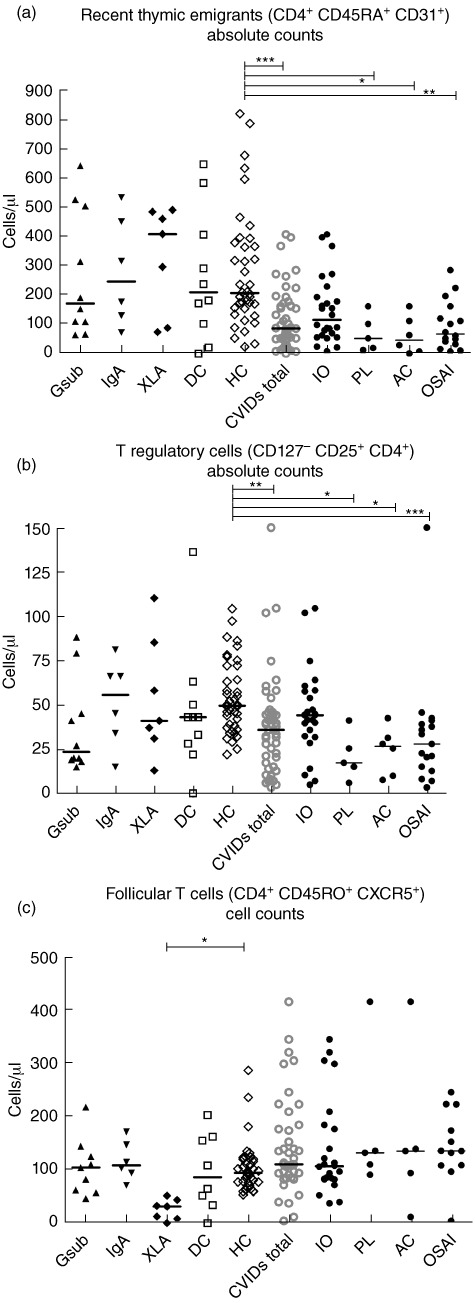
Comparison of putative follicular T cell, recent thymic emigrants and regulatory T cells (Tregs) as absolute values (cells/µl) (a) CD4 T cell recent thymic emigrants, (b) Tregs, (c) CD4 putative follicular T cells. Common variable immunodeficiency disorder (CVID) patients may appear in more than one group as indicated in Fig. 1. Gsub: immunoglobulin (Ig)G subclass-deficient; IgA: IgA-deficient; XLA: X-linked agammaglobulinaemia; DC: disease control; HC: healthy control; CVID all subjects with CVID; IO: infection only; PL: polyclonal lymphoproliferation; AC: autoimmune cytopenias; OSAI: organ-specific autoimmune disease. Statistics performed with one-way analysis of variance (anova) Kruskal–Wallis, with Dunn's multiple comparison test as a post-hoc test. *P < 0·05; **P < 0·01; ***P < 0·001.
Patient demographics and clinical phenotypes of the CVID group
Table 1 describes the demographics of the subjects studied and controls, including age of presentation. Partial antibody deficiency groups IgG subclass (n = 15) and selective IgA deficiency (n = 14), as well as XLA (n = 9) were included for comparison with CVID patients (n = 58). CVID patients were not included if they had suffered opportunistic infections. Figure 1 demonstrates the clinical phenotypes of the CVID patient group. Of the 58 CVID patients studied, 50% had infections only, with no other disease-related complications, while 34% had OSAI, 17% had AC, 16% had PL and 5% had enteropathy. Sixty-two per cent of CVID patients with complications had only one complication; Figure 1 indicates the overlap of complications within the patient group. Patients with more than one complication appear in all relevant subgroups in the figures.
Comparison of T cell subpopulation absolute counts in PAD
Lymphocyte subset analysis demonstrated that patients with CVID overall have significantly lower total CD4 T cells numbers compared with both control groups (P < 0·001; Fig. 2), while there was no significant difference in CD8 T cell numbers (data not shown).
Fig. 2.
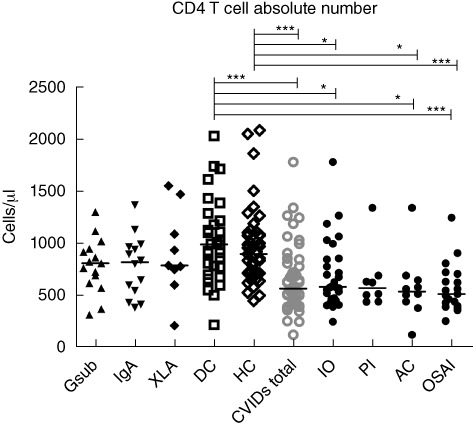
CD4 T cell counts (cells/µl). Common variable immunodeficiency disorder (CVID) patients may appear in more than one group, as indicated in Fig. 1. Gsub: immunoglobulin (Ig)G subclass-deficient; IgA: IgA-deficient; XLA: X-linked agammaglobulinaemia; DC: disease control; HC: healthy control; CVID all subjects with CVID; IO: infection only; PL: polyclonal lymphoproliferation; AC: autoimmune cytopenias; OSAI: organ-specific autoimmune disease. Statistics were performed with one-way analysis of variance (anova) Kruskal–Wallis, with Dunn's multiple comparison test as a post-hoc test. *P < 0·05; ***P < 0·001.
CD4 T cell subpopulation absolute counts
Table 2 summarizes the T cell subpopulation absolute counts in the PAD groups and controls. Figure 3a shows significantly lower CD4 naive T cell absolute numbers in the CVID total group compared to the disease and healthy controls groups (P < 0·001). When the CVID patients were subdivided into clinical phenotypes, the AC and OSAI groups had the most significantly reduced number of CD4 naive T cells (P < 0·001), followed by the PL group (P < 0·01), when compared to both control groups (see Fig. 3a). Within CD4 memory subpopulations CD4 CM and the CD4 EM cells demonstrated a significant difference between groups (Fig. 3b,c). The CD4 CM cells were reduced in the AC group compared to both control groups (Fig. 3b, P < 0·01).
Table 2.
T cell subpopulations expressed as absolute cell counts (cells/µl)
| IgG subclass | IgA | XLA | DC | HC | CVID total | IO | OSAI | AC | PL | anova result | |
|---|---|---|---|---|---|---|---|---|---|---|---|
| Cells/µl | Cells/µl | Cells/µl | Cells/µl | Cells/µl | Cells/µl | Cells/µl | Cells/µl | Cells/µl | Cells/µl | ||
| CD4 CD45RA+ CCR7+ naive | 58–748 | 130–827 | 103–1046 | 1–1262 | 54–1322 | 3–1162 | 24–667 | 20–408 | 3–1162 | 16–1162 | <0·0001 |
| 287 | 340 | 553 | 385 | 325 | 113 | 189 | 84 | 38 | 44 | ||
| CD4 CD45RA– CCR7+ central memory | 127–430 | 96–520 | 68–411 | 0–832 | 140–920 | 58–1049 | 64–1049 | 58–640 | 58–289 | 58–454 | 0·003 |
| 275 | 263 | 201 | 302 | 312 | 254 | 256 | 261 | 161 | 234 | ||
| CD4 CD45RA– CCR7– effector memory | 32–352 | 51–215 | 28–183 | 0–727 | 42–436 | 6–385 | 64–385 | 25–342 | 6–364 | 6–364 | 0·046 |
| 189 | 104 | 81 | 195 | 154 | 133 | 114 | 170 | 253 | 184 | ||
| CD4 CD45RA+ CCR7– terminally differentiated | 1–73 | 4–37 | 0–20 | 0–63 | 1–134 | 0–101 | 1–101 | 0–26 | 0–100 | 1–100 | n.s. |
| 10 | 10 | 7 | 12 | 9 | 7 | 7 | 5 | 7 | 9 | ||
| CD8 CD45RA+ CCR7+ naive | 31–395 | 18–274 | 18–230 | 16–460 | 15–447 | 14–500 | 21–586 | 20–225 | 14–117 | 21–91 | 0·0001 |
| 147 | 169 | 119 | 100 | 125 | 78 | 86 | 69 | 68 | 66 | ||
| CD8 CD45RA– CCR7+ central memory | 6–62 | 9–112 | 15–47 | 3–149 | 12–128 | 2–306 | 8–174 | 2–306 | 2–306 | 2–57 | 0·011 |
| 24 | 23 | 20 | 46 | 37 | 44 | 44 | 53 | 23 | 22 | ||
| CD8 CD45RA– CCR7- effector memory | 40–520 | 67–428 | 95–376 | 28–736 | 69–714 | 9–700 | 28–654 | 18–700 | 9–239 | 9–239 | 0·026 |
| 199 | 146 | 207 | 152 | 226 | 131 | 161 | 114 | 143 | 131 | ||
| CD8 CD45RA+ CCR7– terminally differentiated | 11–339 | 13–530 | 14–181 | 16–257 | 12–986 | 6–461 | 6–461 | 12–314 | 21–344 | 48–344 | 0·0293 |
| 74 | 79 | 38 | 40 | 77 | 89 | 73 | 74 | 166 | 181 | ||
| CD8 CD28+ CD27+ early differentiation | 75–599 | 88–458 | 98–318 | 32–857 | 107–772 | 32–1081 | 95–1081 | 46–380 | 32–380 | 46–263 | <0·0001 |
| 301 | 324 | 249 | 308 | 335 | 194 | 226 | 196 | 181 | 160 | ||
| CD8 CD28– CD27+ intermediate differentiation | 15–114 | 12–215 | 15–146 | 2–272 | 11–271 | 7–178 | 9–169 | 7–178 | 19–121 | 21–121 | n.s. |
| 37 | 42 | 58 | 33 | 44 | 45 | 51 | 31 | 57 | 60 | ||
| CD8 CD28– CD27– late differentiation | 5–402 | 8–349 | 13–321 | 2–284 | 7–557 | 2–703 | 5–556 | 2–703 | 29–329 | 20–329 | n.s. |
| 63 | 46 | 40 | 40 | 77 | 90 | 80 | 77 | 130 | 153 | ||
| CD4 CD28+ CD27+ early differentiation | 287–1220 | 338–1291 | 184–1493 | 1–1916 | 376–1980 | 87–1673 | 198–1673 | 160–111 | 87–1156 | 263–1156 | <0·0001 |
| 706 | 750 | 749 | 894 | 756 | 453 | 487 | 438 | 410 | 488 | ||
| CD4 CD28+ CD27– intermediate differentiation | 14–90 | 19–157 | 16–78 | 0–211 | 25–174 | 11–201 | 16–151 | 11–123 | 11–201 | 19–148 | n.s. |
| 40 | 30 | 37 | 46 | 53 | 47 | 50 | 34 | 45 | 89 | ||
| CD4 CD28– CD27– late differentiation | 0–294 | 0–74 | 0–16 | 0–133 | 0–208 | 0–228 | 0–228 | 0–209 | 1–51 | 0–51 | n.s. |
| 2 | 4 | 0 | 1 | 2 | 3 | 2 | 0 | 6 | 11 | ||
| Tregs CD4+ CD25hi CD127– | 15–88 | 15–81 | 13–110 | 0–136 | 22–104 | 3–150 | 5–104 | 3–150 | 8–42 | 6–41 | <0·0001 |
| 24 | 56 | 41 | 43 | 50 | 36 | 44 | 30 | 27 | 17 | ||
| Thymic emigrant CD45RA CD31+ | 65–645 | 73–537 | 74–494 | 0–651 | 24–824 | 1–409 | 9–409 | 8–287 | 1–163 | 13–163 | <0·0001 |
| 173 | 248 | 411 | 211 | 208 | 87 | 116 | 68 | 47 | 53 | ||
| Putative follicular T cells CXCR5+ CD45RO+ | 47–219 | 72–172 | 0–52 | 0–204 | 54–288 | 5–417 | 38–346 | 5–247 | 12–417 | 92–417 | <0·0001 |
| 105 | 110 | 32 | 87 | 95 | 111 | 108 | 136 | 136 | 133 |
Median and range are indicated for all groups, as well as result of Kruskal–Wallis one-way analysis of variance (anova). AC: autoimmune cytopenias; CVID: common variable immunodeficiency disorder; DC: disease control; HC: healthy control; Ig: immunoglobulin; IO: infection only; OSAI: organ-specific autoimmune disease; PL: polyclonal lymphoproliferation; Treg: regulatory T cells; XLA: X-linked agammaglobulinaemia.
The CVID total group, and most markedly the OSAI group, demonstrated significantly lower numbers of CD4 T cells at an early differentiation stage expressing both the co-stimulatory molecules CD28/27, compared to both control groups (P < 0·001) (Fig. 3d). The IO (P < 0·05) and AC groups (P < 0·01) also demonstrated significantly lower numbers of CD4 T cells expressing both the co-stimulatory molecules CD28/27 compared to both control groups. There was no compensatory increase in the numbers of CD4 T cells losing expression of either CD27 only or CD27/28 in the CVID subgroups (Table 2).
CD8 T cell subpopulation absolute counts
Significantly lower numbers of CD8 naive T cells were observed in the CVID total and AC groups compared to the healthy controls (P < 0·01 P < 0·05, respectively, Fig. 3e). Within the CD8 memory subpopulations, CD8 EM were significantly lower in number in OSAI compared to healthy controls (P < 0·05, Fig. 3f) and CD8 TEM were significantly higher in the PL and AC groups compared to disease controls (P < 0·05, Fig. 3g). This was accompanied by a significantly lower number of CD8s at an early differentiation stage co-expressing CD28 and CD27 compared to the healthy control group in the overall CVID group (P < 0·001), the PL and OSAI subgroups (P < 0·01) and the AC subgroup (P < 0·05) (Fig. 3h). There was no compensatory increase in CD8 T cells losing expression of either CD27 only or CD27/28 in the CVID subgroups (Table 2).
Recent thymic emigrants, Tregs and putative follicular T cells
Absolute numbers of recent thymic emigrants were decreased significantly in the CVID total group (P < 0·001) compared to the healthy control group, and were particularly decreased in the OSAI (P < 0·01), PL and AC subgroups (P < 0·05, Fig. 4a). The number of Tregs was significantly lower in CVID total group (P < 0·01) and in the OSAI, AC and PL subgroups (P < 0·001, P < 0·05 and P < 0·05, respectively) compared to healthy controls (Fig. 4b). The numbers of putative follicular T cells were altered significantly only in the XLA group (Fig. 4c), which were significantly lower than the healthy control group (P < 0·05).
T cell subpopulation absolute counts in partial antibody deficiencies and XLA patients
There were no significant differences in absolute cell counts between either the IgG subclass deficiency or IgA deficiency groups and either control groups in any of the CD4 or CD8 T cell subpopulations (Figs 3 and 4). However, there were significant differences in the XLA group compared to the healthy control group, including significantly lower numbers of CD4 effector T cells (P < 0·05, Fig. 3c), accompanied by a trend for higher numbers (Fig. 3a) of CD4 naive T cells and recent thymic emigrants (Fig. 4a). There was a significant decrease in numbers of putative follicular T cells in the XLA group compared to healthy controls (P < 0·05, Fig. 4c).
Discussion
This was a large one-centre study comparing absolute numbers of a comprehensive range of T cell subpopulation phenotypes in a well-defined group of patients with validated diagnoses of CVID and well-documented complications. The results were compared with those from 38 patients with XLA or partial antibody deficiencies, and with age-matched healthy or disease controls. We have found that a number of T cell subpopulations are altered in patients with CVID or XLA, compared to partial antibody deficiencies and both control groups.
The total CD4 numbers in CVID patients were reduced significantly compared to controls, as in other reported cohorts. This probably accounts for the reduction in CD4/8 ratio and increased CD8 percentages observed in a proportion of CVID patients [7,12,24], particularly in the subgroup with opportunistic infections [16]. The primary purpose of this study was to identify the changes in the absolute numbers of T cell subpopulations associated with different clinical CVID phenotypes. Naive CD4 T cell numbers were reduced significantly in CVID, specifically in the PL, AC and OSAI subgroups. This supports other reports [7,24], particularly from Mouillot et al. [25], who reported that CVID patients with lymphoproliferation or autoimmunity demonstrated the most profound reduction in CD4 naive T cells. Thymic output of new T cells is known to correlate negatively with age [21], and therefore age-matching of the control groups was important to minimize the impact. In addition, thymic cells are particularly sensitive to corticosteroids, so it was important to use a disease control group of immune-competent patients referred to the clinic with recurrent infections.
Recent thymic emigrant numbers were also reduced significantly in CVID patients, specifically in the PL, AC and OSAI subgroups; CVID patients with such complications treated with corticosteroids were excluded if they had received such therapy within 6 months of analysis. Together with the reduced CD4 naive T cells, reduced thymic emigrants suggest a lack of replenishment of the CD4 T cell pool by new thymically derived cells in CVID patients. Giovannetti et al. [24] also found that thymic output was reduced significantly in CVID patients, and associated this with a reduction in class-switch memory B cells, expansion of CD21lo B cells, splenomegaly and granuloma. They also showed increased cell turnover as measured by Ki-67, particularly in the CD4 naive subset and increased apoptosis [24]. We did not find such an association with CD21low B cells, although we found an association with PL for which granuloma is a criterion. Mouilott et al. [25] found a decrease in CD4 naive T cells which was accompanied by increased CD95+ expression, most pronounced in the PL and AC groups, while Iglesias et al. [28] found that CD4+CD45RA+ T cells, which contain predominantly naive CD4 T cells, had increased spontaneous apoptosis and CD95 expression in CVID patients. Therefore, the reduction in naive CD4 T cells may, in part, be due to both reduced thymic output and increased cell turnover.
Significant reductions in CD8 naive T cell numbers were seen in CVID patients compared to controls, particularly in the AC group. This has not been reported previously, and is likely to reflect the increases in terminally differentiated CD8 cells observed in the PL and AC groups.
Both CD4 and CD8 T cells in CVID patients, and most significantly in the AC, OSAI and PL groups, demonstrated a loss of the co-stimulatory molecules CD28 or CD27. This suggests T cell differentiation along an activation pathway. Other groups have observed increased activation in T cells of all CVID patients [25], as measured by CD38 and human leucocyte antigen D-related (HLA-DR) [24], particularly in patients with splenomegaly [26]. The possibility of an infectious agent driving the clinical manifestations of lymphoproliferation observed in the PL subset of CVID patients has been suggested, but not established – a hypothesis supported by these T cell phenotypes. It has been suggested that cytomegalovirus (CMV) may play a role in the T cell abnormalities seen in CVID, as patients in one study had a 13-fold increased proportion of CMV-specific, functional T cells compared to aged-matched controls [29]. CMV-specific CD8 T cells have the phenotype of CD45RA+CCR7-CD27- and the increase in CD8 T cells of this phenotype in the PL and AC subgroups of the CVID suggests that CMV or another similar infectious agent may be important [17,30].
We found significantly increased percentages of putative follicular T cells in CVID patients (data not shown) although, interestingly, not increased absolute counts. This may reflect the lack of naive T cells altering the proportion of CD4 T cells, and suggests that the most accurate method of assessing lymphocyte phenotypes is by cell number, not percentage. There was a significant reduction in number of putative follicular T cells in XLA. Bossaller et al. [23] found reduced percentages of these putative follicular T cells in ICOS deficiency and suggested that such cells could be a marker for a functional GC in humans. Martini et al. [5] found CD4+CD45RO+ memory T cells and CD4+CD45RO+CXCR5+ putative follicular T cells to be reduced significantly in XLA patients, regardless of age. They also found these putative follicular T cells to be reduced significantly in CVID patients with <2% B cells, supporting the theory that the presence of B cells but not Btk is required for generation of these putative follicular T cells [5]. There was a larger range of putative follicular T cell number in patients with CVID compared to controls, suggesting that patients outside the normal range for these putative follicular T cells may warrant investigation for defects resulting in poor germinal-centre formation.
Tregs were reduced significantly in number in CVID patients, most profoundly in PL, AC and OSAI patients, confirming previous work [13,14,25,31]. Arumugakani et al. [12] found reduced FoxP3+ Treg numbers and percentages in CVID patients with autoimmunity and splenomegaly, and it was associated with an expansion of CD21lo B cells.
We found no significant differences in any T cell subpopulations in the partial antibody deficiency groups, namely IgG subclass or selective IgA-deficient. This supports the findings of Litzman et al. [32], who found no significant differences in a small range of T cell memory markers in selective IgA-deficiency patients compared to healthy controls. Our findings suggest no gross defect in T cell differentiation in these partial antibody deficiency groups. CVID patients with infections only demonstrated no significant differences in T cell subpopulations, except reduction in absolute numbers of CD4 T cells in the early differentiation stage (expressing CD28/27), suggesting that abnormalities in T cell subpopulations correlate with other complications such as autoimmunity, especially cytopenias and polyclonal lymphoproliferation, rather than being crucial for the pathogenesis of primary antibody failure.
In conclusion, there was a significant reduction in numbers of naive CD4 T cells in CVID patients, accompanied by a significant reduction in numbers of recent thymic emigrants, suggesting lack of replenishment of the CD4 T cell pool by new thymic-derived cells. CD8 naive T cells were also reduced, specifically in the AC subgroup, and were accompanied by an increase in terminally differentiated CD8s. There was a reduction in both early differentiated CD4 and CD8 T cells in the AC, OSAI and PL subgroups, demonstrating a more activated T cell phenotype, suggestive of an antigen-driven process.
Acknowledgments
We are most grateful to the patients and controls who generously donated blood samples and to Dr Misbah, Dr Lorton and Dr Patel, who care for these patients. We are also grateful to the staff at the Department of Clinical Laboratory Immunology at the Churchill Hospital, Oxford for their support and performing the lymphocyte subset analyses.
Disclosure
Authors' conflicts of interest: None declared.
References
- 1.Conley ME, Notarangelo LD, Etzioni A. Diagnostic criteria for primary immunodeficiencies. Representing PAGID (Pan-American Group for Immunodeficiency) and ESID (European Society for Immunodeficiencies) Clin Immunol. 1999;93:190–7. doi: 10.1006/clim.1999.4799. [DOI] [PubMed] [Google Scholar]
- 2.Chapel H, Lucas M, Lee M, et al. Common variable immunodeficiency disorders: division into distinct clinical phenotypes. Blood. 2008;112:277–86. doi: 10.1182/blood-2007-11-124545. [DOI] [PubMed] [Google Scholar]
- 3.Chapel H, Lucas M, Patel S, et al. Confirmation and improvement of criteria for clinical phenotyping in common variable immunodeficiency disorders in replicate cohorts. J Allergy Clin Immunol. 2012 doi: 10.1016/j.jaci.2012.05.046. doi: 10.1016/j.jaci.2012.05.046. [DOI] [PubMed] [Google Scholar]
- 4.Orange JS, Glessner JT, Resnick E, et al. Genome-wide association identifies diverse causes of common variable immunodeficiency. J Allergy Clin Immunol. 2011;127:1360–7. doi: 10.1016/j.jaci.2011.02.039. e6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Martini H, Enright V, Perro M, et al. Importance of B cell co-stimulation in CD4(+) T cell differentiation: X-linked agammaglobulinaemia, a human model. Clin Exp Immunol. 2011;164:381–7. doi: 10.1111/j.1365-2249.2011.04377.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Aghamohammadi A, Mohammadi J, Parvaneh N, et al. Progression of selective IgA deficiency to common variable immunodeficiency. Int Arch Allergy Immunol. 2008;147:87–92. doi: 10.1159/000135694. [DOI] [PubMed] [Google Scholar]
- 7.Farrant J, Spickett G, Matamoros N, et al. Study of B and T cell phenotypes in blood from patients with common variable immunodeficiency (CVID) Immunodeficiency. 1994;5:159–69. [PubMed] [Google Scholar]
- 8.North ME, Webster AD, Farrant J. Defects in proliferative responses of T cells from patients with common variable immunodeficiency on direct activation of protein kinase C. Clin Exp Immunol. 1991;85:198–201. doi: 10.1111/j.1365-2249.1991.tb05704.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Cunningham-Rundles C, Bodian C. Common variable immunodeficiency: clinical and immunological features of 248 patients. Clin Immunol. 1999;92:34–48. doi: 10.1006/clim.1999.4725. [DOI] [PubMed] [Google Scholar]
- 10.Fischer MB, Hauber I, Eggenbauer H, et al. A defect in the early phase of T-cell receptor-mediated T-cell activation in patients with common variable immunodeficiency. Blood. 1994;84:4234–41. [PubMed] [Google Scholar]
- 11.Rezaei N, Aghamohammadi A, Nourizadeh M, et al. Cytokine production by activated T cells in common variable immunodeficiency. J Invest Allergol Clin Immunol. 2010;20:244–51. [PubMed] [Google Scholar]
- 12.Arumugakani G, Wood PM, Carter CR. Frequency of Treg cells is reduced in CVID patients with autoimmunity and splenomegaly and is associated with expanded CD21lo B lymphocytes. J Clin Immunol. 2010;30:292–300. doi: 10.1007/s10875-009-9351-3. [DOI] [PubMed] [Google Scholar]
- 13.Fevang B, Yndestad A, Sandberg WJ, et al. Low numbers of regulatory T cells in common variable immunodeficiency: association with chronic inflammation in vivo. Clin Exp Immunol. 2007;147:521–5. doi: 10.1111/j.1365-2249.2006.03314.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Horn J, Manguiat A, Berglund LJ, et al. Decrease in phenotypic regulatory T cells in subsets of patients with common variable immunodeficiency. Clin Exp Immunol. 2009;156:446–54. doi: 10.1111/j.1365-2249.2009.03913.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Oksenhendler E, Gerard L, Fieschi C, et al. Infections in 252 patients with common variable immunodeficiency. Clin Infect Dis. 2008;46:1547–54. doi: 10.1086/587669. [DOI] [PubMed] [Google Scholar]
- 16.Malphettes M, Gerard L, Carmagnat M, et al. Late-onset combined immune deficiency: a subset of common variable immunodeficiency with severe T cell defect. Clin Infect Dis. 2009;49:1329–38. doi: 10.1086/606059. [DOI] [PubMed] [Google Scholar]
- 17.Halwani R, Doroudchi M, Yassine-Diab B, et al. Generation and maintenance of human memory cells during viral infection. Springer Semin Immunopathol. 2006;28:197–208. doi: 10.1007/s00281-006-0027-2. [DOI] [PubMed] [Google Scholar]
- 18.Sallusto F, Lenig D, Forster R, Lipp M, Lanzavecchia A. Two subsets of memory T lymphocytes with distinct homing potentials and effector functions. Nature. 1999;401:708–12. doi: 10.1038/44385. [DOI] [PubMed] [Google Scholar]
- 19.Amyes E, Hatton C, Montamat-Sicotte D, et al. Characterization of the CD4+ T cell response to Epstein–Barr virus during primary and persistent infection. J Exp Med. 2003;198:903–11. doi: 10.1084/jem.20022058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Appay V, Rowland-Jones SL. Lessons from the study of T-cell differentiation in persistent human virus infection. Semin Immunol. 2004;16:205–12. doi: 10.1016/j.smim.2004.02.007. [DOI] [PubMed] [Google Scholar]
- 21.Junge S, Kloeckener-Gruissem B, Zufferey R, et al. Correlation between recent thymic emigrants and CD31+ (PECAM-1) CD4+ T cells in normal individuals during aging and in lymphopenic children. Eur J Immunol. 2007;37:3270–80. doi: 10.1002/eji.200636976. [DOI] [PubMed] [Google Scholar]
- 22.Fazilleau N, Mark L, McHeyzer-Williams LJ, McHeyzer-Williams MG. Follicular helper T cells: lineage and location. Immunity. 2009;30:324–35. doi: 10.1016/j.immuni.2009.03.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Bossaller L, Burger J, Draeger R, et al. ICOS deficiency is associated with a severe reduction of CXCR5+CD4 germinal center Th cells. J Immunol. 2006;177:4927–32. doi: 10.4049/jimmunol.177.7.4927. [DOI] [PubMed] [Google Scholar]
- 24.Giovannetti A, Pierdominici M, Mazzetta F, et al. Unravelling the complexity of T cell abnormalities in common variable immunodeficiency. J Immunol. 2007;178:3932–43. doi: 10.4049/jimmunol.178.6.3932. [DOI] [PubMed] [Google Scholar]
- 25.Mouillot G, Carmagnat M, Gerard L, et al. B-cell and T-cell phenotypes in CVID patients correlate with the clinical phenotype of the disease. J Clin Immunol. 2010;30:746–55. doi: 10.1007/s10875-010-9424-3. [DOI] [PubMed] [Google Scholar]
- 26.Lanio N, Sarmiento E, Gallego A, Carbone J. Immunophenotypic profile of T cells in common variable immunodeficiency: is there an association with different clinical findings? Allergol Immunopathol (Madr) 2009;37:14–20. doi: 10.1016/s0301-0546(09)70246-0. [DOI] [PubMed] [Google Scholar]
- 27.Spickett G. Oxford handbook of clinical immunology and allergy. 2nd edn. Oxford: Oxford University Press; 2006. [Google Scholar]
- 28.Iglesias J, Matamoros N, Raga S, Ferrer JM, Mila J. CD95 expression and function on lymphocyte subpopulations in common variable immunodeficiency (CVID); related to increased apoptosis. Clin Exp Immunol. 1999;117:138–46. doi: 10.1046/j.1365-2249.1999.00946.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Raeiszadeh M, Kopycinski J, Paston SJ, et al. The T cell response to persistent herpes virus infections in common variable immunodeficiency. Clin Exp Immunol. 2006;146:234–42. doi: 10.1111/j.1365-2249.2006.03209.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Champagne P, Ogg GS, King AS, et al. Skewed maturation of memory HIV-specific CD8 T lymphocytes. Nature. 2001;410:106–11. doi: 10.1038/35065118. [DOI] [PubMed] [Google Scholar]
- 31.Yu GP, Chiang D, Song SJ, et al. Regulatory T cell dysfunction in subjects with common variable immunodeficiency complicated by autoimmune disease. Clin Immunol. 2009;131:240–53. doi: 10.1016/j.clim.2008.12.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Litzman J, Vlkova M, Pikulova Z, Stikarovska D, Lokaj J. T and B lymphocyte subpopulations and activation/differentiation markers in patients with selective IgA deficiency. Clin Exp Immunol. 2007;147:249–54. doi: 10.1111/j.1365-2249.2006.03274.x. [DOI] [PMC free article] [PubMed] [Google Scholar]


