Abstract
Dendritic cells (DC) in HIV-1-infected individuals are decreased and their dysfunction has been implicated in HIV-1 immunopathogenesis. The mechanism of their dysfunction remains unclear, thus we analysed the expression of membrane molecules associated with immune regulation and DC activation in myeloid (mDC) and plasmacytoid DC (pDC) in therapy-naive and highly active anti-retroviral therapy (HAART)-treated HIV-1+ patients. DC from healthy controls, untreated HIV-1+ and HAART-treated patients were assessed by flow cytometry for expression of: anergy and apoptosis inducing molecules [programmed death (PD)-1 and its ligands PD-L1 and PD-L2], inhibitory and regulatory T cell-inducing molecules [immunoglobulin-like transcript (ILT)-3 and ILT-4], interferon (IFN)-α inhibitory receptor (ILT-7) and co-stimulatory molecules (CD80, CD83, and CD86). pDC from untreated HIV-1+ patients expressed significantly lower levels of ILT-7 compared to healthy controls, while HAART-treated patients showed normal expression. pDC were also found to express moderately higher levels of PD-L1 and ILT-3 and lower levels of PD-L2 receptors in untreated patients compared to controls and HAART-treated patients. No significant changes were observed in mDC. There were no associations between the percentages and levels of expression of these molecules by pDC and viral load or CD4 T cell count. In conclusion, pDC but not mDC from HIV-1+ patients with active viraemia display higher levels of apoptosis and T regulatory-inducing molecules and may be predisposed to chronically produce IFN-α through down-regulation of ILT-7. HAART restored normal expression levels of these receptors.
Keywords: HIV-1, ILT-7, mDC, pDC, PD-L1
Introduction
Dendritic cells (DC) are antigen-presenting cells with a unique capacity to activate naive T cells. They are a heterogeneous population, with different localizations, phenotype, pattern recognition molecules, cytokine secretion profiles and functional properties [1–3]. DC are found in human blood and represent about 1% of peripheral blood mononuclear cells (PBMC) and are identified by expression of major histocompatibility complex (MHC) class II human leucocyte antigen D-related (HLA-DR) and the absence of leucocyte lineage markers. This population comprises two main subsets of DC, termed myeloid DC (mDC) and plasmacytoid DC (pDC), that can be differentiated by expression of CD11c and the interleukin (IL)-3 receptor (CD123), respectively [4,5]. mDC are thought to be the precursors of tissue DC facilitating Th1 polarization and induction of CD8 T cell immunity [1,6]. Conversely, pDC possess a lymphoid-like morphology and do not migrate to peripheral tissues unless inflamed [7], and are believed to migrate directly from blood to lymph nodes through high endothelial venules [8]. pDC produce large amounts of type I interferon (IFN) upon viral contact, well documented for HIV-1 and influenza [9,10]. Both pDC and mDC can either induce T cell immunity or tolerance depending on a number of factors, including degree of cell maturation, type of antigen, duration of stimulation and cytokine milieu [2,3,11].
DC are proposed to play a role in HIV-1-associated immunopathogenesis. Both mDC and pDC express the HIV-1 receptor CD4 and chemokine co-receptors, and are susceptible to HIV-1 infection [4,12]. HIV-1-infected pDC and mDC may facilitate HIV-1 transmission to antigen-specific CD4 T cells and hence suppress HIV-1 immunity specifically [13]. There is a progressive loss of blood mDC and pDC in HIV-1-infected individuals [14–18] and in simian immunodeficiency virus (SIV)-infected macaques [19], and their impaired ability to stimulate T cell proliferation reduces the efficacy of adaptive immunity [20]. Chronic immune activation is considered to be the main driving mechanism underlying HIV-1 immunopathogenesis and pDC, through dysregulated IFN-α production, may play a role [21]. Although a number of studies describe impaired DC function in HIV-1 infection, data on the potential mechanisms are scarce [14,22,23].
We thus analysed stimulatory, inhibitory, tolerogenic, anergy or apoptosis-inducing molecules expressed by blood mDC and pDC in HIV-1-infected patients. We grouped these receptors into three different families. First, because the levels of programmed death-1 (PD-1) receptor are up-regulated by T cells from HIV-1-infected individuals, suggesting an anergic or apoptotic predisposition [24–26], we analysed the levels of its natural ligands, PD-L1 and PD-L2, expressed by DC [27]. Secondly, a number of studies demonstrated elevated levels of regulatory T cells in HIV-1+ patients [28–30]; we therefore measured mDC and pDC expression of immunoglobulin-like transcript (ILT)-3 and ILT-4, receptors known to be expressed by tolerogenic DC and to mediate regulatory T cell generation [31–33]. In addition, a novel inhibitory receptor that is expressed exclusively by pDC, ILT-7, associated recently with suppression of IFN-α production [34,35], was also measured. To our knowledge, expression of the PD-1 ligands and ILT molecules has not been analysed previously on pDC from HIV-1-infected individuals. Thirdly, expression of co-stimulatory molecules associated with DC activation has been analysed, as activated DC may mediate chronic T cell activation, a possible driving factor of HIV-1 disease progression [21]. We also performed correlation analysis between the levels of surface molecule expression and the patients' CD4 T cell count and HIV-1 viraemia. Furthermore, we explored the effect of highly active anti-retroviral therapy (HAART) on the reversal of potential blood DC phenotypic dysregulation that may be associated with persistent viraemia.
Materials and methods
Study participants
Three cohorts of donors were recruited: healthy controls (HC; n = 11), untreated HIV-1+ patients (naive; n = 11) and HAART-treated HIV-1+ patients (HAART; n = 11) attending Chelsea and Westminster hospital (London, UK). Ethical approval and informed written consent were obtained from all participants.
Untreated HIV-1+ patients were aged 18 years and over and had no previous history of ART. Their CD4 T cell count ranged between 143 and 508 cells/µl blood, with plasma viral load ranging from 24 374 to 568 080 copies/ml (Table 1). ART-treated patients had the following eligibility criteria: patients aged 18 and over who were stable on ritonavir-boosted protease inhibitor/r (PI/r) [atazanovir (ATV), lopinavir (LPV) or darunavir (DRV)] plus Truvada or Kivexa for more than 24 weeks, with viral load < 50 HIV-1 RNA copies/ml plasma on two occasions in the last 4 weeks prior to blood collection with known CD4 nadir count. Thirty ml of blood from healthy HIV-1-seronegative controls was also collected.
Table 1.
Clinical characteristics of HIV-1+ patient cohorts
| Patient ID | Treatment history | Age (years) | Gender | CD4 T cell count (cell/µl blood) | Plasma viral load (copies/ml plasma) | Treatment regimen |
|---|---|---|---|---|---|---|
| Control group | Healthy controls (n = 11) | Median (29) range (24–62) | 6 M, 5 F | n.t. | n.a. | n.a. |
| N1 | Naive | 54 | M | 508 | 24 374 | n.a. |
| N2 | Naive | 24 | M | 196 | 26 375 | n.a. |
| N3 | Naive | 44 | M | 274 | 53 916 | n.a. |
| N4 | Naive | 39 | M | 313 | 158 862 | n.a. |
| N5 | Naive | 36 | M | 294 | 136 943 | n.a. |
| N6 | Naive | 52 | M | 461 | 117 738 | n.a. |
| N7 | Naive | 33 | M | 342 | 449 558 | n.a. |
| N8 | Naive | 25 | F | 143 | 67 749 | n.a. |
| N9 | Naive | 39 | M | 283 | 568 080 | n.a. |
| N10 | Naive | 43 | M | 337 | 62 445 | n.a. |
| N11 | Naive | 30 | M | 335 | 48 148 | n.a. |
| Median (range) | – | 39 (24–54) | – | 313 (143–508) | 67 749 (24 374–568 080) | – |
| H1 | HAART | 41 | M | 757 | < 50 | TFV, FTC, DRV, RTV |
| H2 | HAART | 41 | M | 942 | < 50 | ABC, 3TC, EFV |
| H3 | HAART | 47 | M | 293 | < 50 | TFV, FTC, AZT, RTV |
| H4 | HAART | 47 | M | 726 | < 50 | ABC, 3TC, AZT, RTV |
| H5 | HAART | 46 | M | 251 | < 50 | DRV, RTV, TFV, FTC |
| H6 | HAART | 61 | M | 1500 | < 50 | TFV, FTC, EFV |
| H7 | HAART | 41 | M | 475 | < 50 | TFV, FTC, AZT, RTV |
| H8 | HAART | 50 | M | 419 | < 50 | TFV, FTC, DRV, RTV |
| H9 | HAART | 66 | M | 454 | < 50 | TFV, FTC, AZT, RTV |
| H10 | HAART | 35 | M | 889 | < 50 | TFV, FTC, EFV |
| H11 | HAART | 51 | M | 391 | < 50 | TFV, FTC, AZT, RTV |
| Median (range) | – | 47 (35–66) | – | 475 (251–1500) | < 50 | – |
n.a., not applicable; n.t., not tested; 3TC, lamivudine; ABC, abacavir; AZT, zidovudine; F, female; M, male; DRV, darunavir; FTC, emtricitabine; RTV, ritonavir; TFV, tenofovir; HAART, highly active anti-retroviral therapy.
Peripheral blood collection, cell separation, virus load assay and lymphocyte subsets
Blood (30–60 ml) was collected, PBMC separated, plasma viral load measured and lymphocyte subsets quantified, all as described previously [15][36]; 10–20 million PBMC from fresh blood were used for this study.
Flow cytometry
Six-colour flow cytometry was performed to determine the levels of inhibitory and stimulatory receptor expression by pDC and mDC within PBMC. The following antibodies were used: anti-ILT-3-fluorescein isothiocyanate (FITC), -ILT-4-phycoerythrin (PE), -immunoglobulin (IgG)2a-isotype-FITC, -IgG2a-isotype-PE (R&D Systems, Abingdon, UK), -PD-1-FITC, -PD-L1 (CD279)-PE, -PD-L2 (CD278)-FITC, -CD80-FITC, -CD83-FITC, -CD86-PE, -lineage (CD3, CD14, CD16, CD19, CD34)-PE-cyanin-5 (Cy5), -CD11c-Alexa647, -IgG1-isotype-FITC, -IgG1-isotype-PE (Serotec Ab, Oxford, UK), -CD123-PE-Cy7 and -ILT-7-PE (eBiosciences, Hatfield, UK); 106 cells per tube were stained at 4°C with the appropriate antibodies for 20 min, washed with fluorescence activated cell sorter (FACS) buffer and then fixed with BD stabilizing fixative (BD Biosciences, Hatfield, UK). Cells were acquired using a three-laser-configuration LSR II machine (BD Biosciences). Gates were drawn on the basis of 0·01 isotype expression and data analysis performed using FlowJo version 7·6 (Tree Star Inc., Ashland, OR, USA).
To analyse pDC and mDC, a minimum of 2 × 105 live cells were collected and identified as shown in supplementary Fig. S1. Briefly, within the live gate, based on cell size and granularity (supplementary Fig. S1a), single cells were identified based on forward-scatter area and height (supplementary Fig. S1b). Total DC were then gated based on lack of expression of lineage markers and expression of HLA-DR (supplementary Fig. S1c). Finally, pDC and mDC were distinguished on the basis of CD123 and CD11c expression, respectively (supplementary Fig. S1d). Surface expression of PD-1, PD-L1, PD-L2, ILT-3, ILT-4, ILT-7, CD80, CD83 and CD86 by pDC and mDC were determined using appropriate isotype controls (supplementary Fig. S1e).
Statistical analysis
Data are expressed as median (25th–75th percentile). Non-parametric tests were used throughout, as normality of data distribution could not be tested. The Mann–Whitney U-test was employed to determine significance between two unpaired groups. Spearman's r test was used to determine correlations between two variables. All statistical tests were two-sided and performed using GraphPad Prism 5 (GraphPad Software, San Diego, CA, USA). P-values below 0·05 were considered statistically significant.
Results
Expression of PD receptors by pDC and mDC
PD-1 is expressed mainly by T cells, B cells and natural killer (NK) cells [37]. pDC and mDC from healthy controls expressed minimal levels of this receptor [medians of 0·24%, interquartile range (IQR) 0·00–0·55%, Fig. 1a]; and 0·68%, IQR 0·05–1·76%, Fig. 1b, respectively]. Similarly, blood DC in HIV-1-infected individuals also lacked expression of PD-1 (Fig. 1a,b). Because PD-1 is reported to be up-regulated by T cells during HIV-1 infection [24–26], we investigated the expression levels of its two known ligands, PD-L1 and PD-L2, by both blood DC subsets. In healthy controls, pDC expressed low levels of PD-L1 and PD-L2 (medians of 2·22%, IQR 1·52–2·74%, Fig. 1c; and 0·08%, IQR 0·0–0·62%, Fig. 1e, respectively). There was a small but statistically significant increase in the percentages of pDC expressing PD-L1 in viraemic untreated HIV-1+ patients (median of 3·73%, IQR 2·37–5·61%, Fig. 1c). Surprisingly, this was coupled with a significant decrease in PD-L2 expression levels both in terms of percentages and intensity of expression in comparison to pDC from healthy controls (Fig. 1e).
Fig. 1.
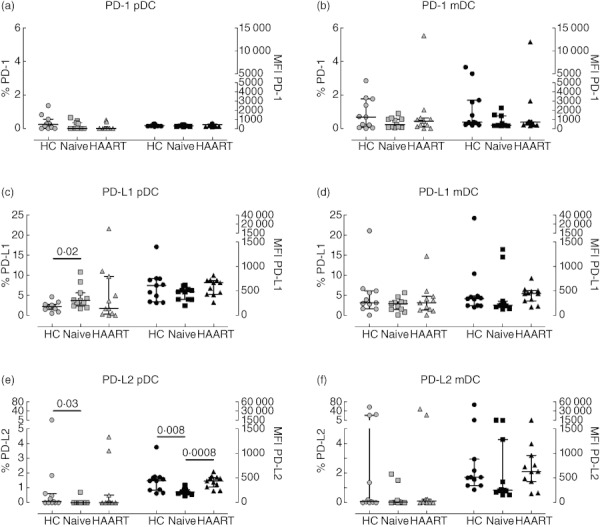
Expression of programmed death (PD) receptors by plasmacytoid dendritic cells (pDC) and myeloid DC (mDC) from HIV-1+ patients. Peripheral blood mononuclear cells (PBMC) from healthy controls (circles), therapy-naive (squares) and therapy-treated patients (triangles) were collected, stained and analysed by flow cytometry. Expression of PD-1 (top plots), PD-L1 (middle plots) and PD-L2 (bottom plots) by pDC (left plots) and mDC (right plots) are shown. Light symbols indicate the percentages of cells expressing the specific markers (left y-axes), whereas dark symbols represent the mean fluorescence intensity of each receptor (right y-axes). The lines in the middle show the median value, whereas error bars represent the 25th and 75th percentiles. Statistically significant P-values are shown (Mann–Whitney U-test).
In contrast to a previous report [23], we found no significant difference in the percentages of PD-L1 expressing mDCs or in expression levels between groups (Fig. 1c,d). PD-L2 expression by mDC was minimal in most samples analysed except for three healthy controls and two HAART-treated HIV-1+ patients (Fig. 1f). There were no statistically significant differences between the three cohorts.
For HIV-1+ patients receiving HAART, the percentage of pDC expressing PDL-1 and PD-L2 returned to those of healthy controls (Fig. 1c–f).
Immunoglobulin-like transcript receptor expression by pDC and mDC
Figure 2 shows the percentages and intensity of expression of ILT-3, -4 and -7 by pDC and mDC in the different cohorts. ILT-3 and ILT-4 are receptors involved in the induction of regulatory T cells and are highly expressed by immature DC [31,38]. The majority of pDC from healthy donors expressed ILT-3 and ILT-4 (medians of 98·28%, IQR 82·14–99·06%, Fig. 2a; and 85·40%, IQR 69·15–96·10%, Fig. 2c, respectively). The percentages of pDC expressing ILT-3 was higher in therapy-naive patients (median of 99·29%, IQR 98·36–100%, P = 0·03) in comparison to healthy controls, but not statistically different (Fig. 2a); mDC did not display any statistically significant differences across the three cohorts with regard to ILT-3 and ILT-4 (Fig. 2b,d).
Fig. 2.
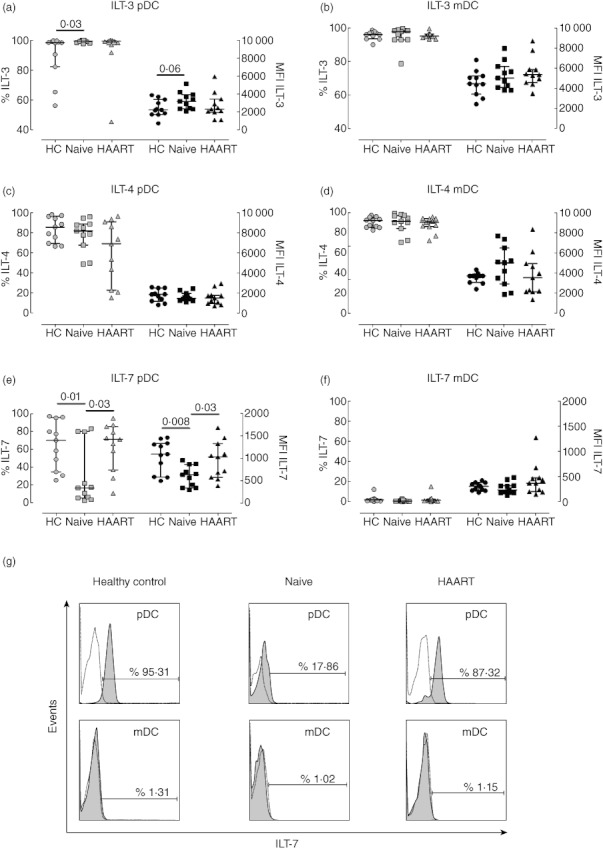
Expression of immunoglobulin-like transcript receptors by plasmacytoid dendritic cells (pDC) and myeloid DC (mDC) from HIV-1+ patients. Peripheral blood mononuclear cells (PBMC) from healthy controls (circles), therapy-naive (squares) and therapy-treated patients (triangles) were collected, stained and analysed by flow cytometry. Expression of immunoglobulin-like transcript (ILT)-3 (top plots), ILT-4 (middle plots), and ILT-7 (bottom plots) by pDC (left plots) and mDC (right plots) are shown. Light symbols indicate the percentages of cells expressing the specific markers (left y-axes), whereas dark symbols represent the mean fluorescence intensity of each receptor (right y-axes). The lines in the middle show the median value, whereas error bars represent the 25th and 75th percentiles. Statistically significant P-values are shown (Mann–Whitney U-test). (g) Representative histograms are shown for ILT-7 expression by pDC (top panels) and mDC (bottom panels) from one healthy control (left panels), therapy-naive (middle panels) and highly active anti-retroviral therapy (HAART)-treated HIV-1 patient (right panels). Dotted lines represent isotype controls, while filled histograms show ILT-7 expression.
Unlike other ILT receptors, ILT-7 is expressed exclusively by pDC [35,38,39]. Expression and signalling through this receptor has been reported to inhibit secretion of type I IFN [39], and ILT-7 down-regulation is associated with pDC activation [38]. pDC from healthy controls were found to express high levels of ILT-7 (median percentage of 70·17, IQR 34·83–95·31, Fig. 2e,g), suggesting a resting phenotype. pDC from untreated viraemic patients were found to express significantly less ILT-7 compared to healthy controls. This was true for both the percentages of pDC expressing ILT-7 (median of 16·86%, IQR 5·88–79·84%, P = 0·01, Fig. 2e) and the intensity of ILT-7 expression per cell [median mean fluorescence intensity (MFI) of 632 (340–867)] compared to a median MFI of 1094 (586–1335) in healthy controls (P = 0·008; Fig. 2e). ILT-7 expression by pDC from HAART-treated HIV-1+ patients was comparable to that in healthy individuals (median percentage of 71·29, IQR 37·10–85·55%), indicating that HAART may restore pDC to a resting state with regard to ILT-7 expression (Fig. 2e,g). In contrast, mDC from all three cohorts did not express ILT-7 (Fig. 2f), as shown previously [38,39].
Expression of co-stimulatory and activation markers by pDC and mDC
Both DC subsets up-regulate CD80, CD86 and CD83 upon maturation. Here we analysed their expression on pDC and mDC from the different cohorts (Fig. 3). None of these molecules were up-regulated to levels expected on stimulated DC. pDC expressed minimal levels of all three receptors across the groups analysed, suggesting an immature phenotype (Fig. 3a,c,e). No statistical differences were observed across the cohorts except for the percentages of CD83-expressing pDC from HAART-treated patients, which were lower than those in healthy controls and untreated HIV-1+ patients (Fig. 3e). There may be no biological relevance for this difference, as in most samples fewer than 1% of pDC expressed CD83. There was no significant difference between the three groups in percentages of mDC expressing co-stimulatory molecules (Fig. 3b,d,f). However, mDC showed some differences in levels of CD80 expression per cell which were lower in viraemic patients [median MFI of 491·8 (372·8–641·5)] compared to HAART patients [median MFI of 738 (626·4–937·2), P = 0·008]. Similarly, HAART patients showed higher levels of CD83 than controls and untreated patients (Fig. 3f); however, the differences in MFI for CD80 and CD83 were relatively small for mDC and may not be functionally significant.
Fig. 3.
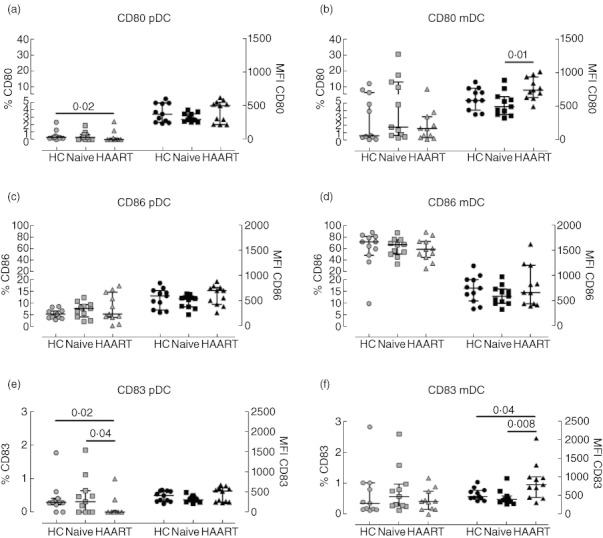
Expression of activation and co-stimulatory molecules by plasmacytoid dendritic cells (pDC) and myeloid DC (mDC) from HIV-1+ patients. Peripheral blood mononuclear cells (PBMC) from healthy controls (circles), therapy-naive (squares) and therapy-treated patients (triangles) were collected, stained and analysed by flow cytometry. Expression of CD80 (top plots), CD86 (middle plots) and CD83 (bottom plots) by pDC (left plots) and mDC (right plots) are shown. Light symbols indicate the percentages of cells expressing the specific markers (left y-axes), whereas dark symbols represent the mean fluorescence intensity of each receptor (right y-axes). The lines in the middle show the median value, whereas error bars represent the 25th and 75th percentiles. Statistically significant P-values are shown (Mann–Whitney U-test).
pDC dysregulation does not correlate with HIV-1 disease progression
In viraemic HIV-1+ patients, we observed a reduced proportion of pDC expressing ILT-7 and PD-L2, accompanied by higher percentages of pDC expressing PD-L1 and elevated levels of ILT-3. Thus, we investigated whether these observations were linked to the patients' disease status. As shown in Fig. 4, there were no statistically significant correlations between the percentage of pDC expressing PD-L1, PD-L2, ILT-3 or ILT-7 and the patients' CD4 count (Fig. 4a) or plasma viral load (Fig. 4c). Similarly, the mean fluorescence intensities of these receptors did not correlate with CD4 count or plasma viraemia (Fig. 4b,d, respectively).
Fig. 4.
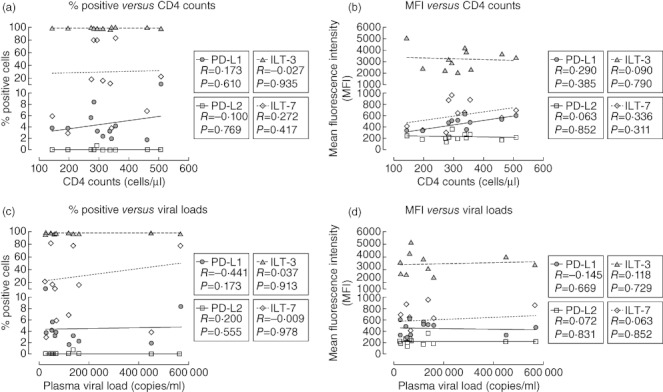
Plasmacytoid dendritic cells (pDC) phenotypic dysregulation does not correlate with disease progression. The percentages of pDC from HIV-1+ viraemic patients expressing programmed death (PD)-L1 (circles), PD-L2 (squares), ILT-3 (triangles) or ILT-7 (diamonds) were plotted against the patients' CD4 T cell count (a) or their plasma viral load (c). Similarly, the mean fluorescence intensities of PD-L1, PD-L2, immunoglobulin-like transcript (ILT)-3 and ILT-7 were plotted against CD4 T cell count (b) and viral load (d). Spearman's r and P-values are given for each correlation.
Discussion
AS DC are major regulators of T cell functions, we asked whether T cell anergy [24–26], increased T regulatory activity [28–30] and hyperactivation of T cells [40–42] in HIV-1 infection could, in part, be attributed to abnormalities in DC subsets by analysing expression of membrane molecules associated with these activities. No marked alterations in expression of any of these molecules were observed in mDC from infected patients, indicating that they may not be involved in mediating their dysfunction. Conversely, significant changes were observed in expression of some of these molecules in pDC in therapy-naive patients who were restored by HAART, findings that infer that they may play a role in pDC dysfunction.
In untreated viraemic HIV-1+ patients, a higher proportion of pDC expressed elevated levels of PD-L1 and ILT-3 and lower levels of ILT-7 in comparison to healthy controls, but showed an immature phenotype based on expression levels of co-stimulatory molecules. The increased percentages of pDC expressing PD-L1 in viraemic HIV-1+ patients was unrelated to plasma viraemia or CD4 T cell counts, suggesting that PD-L1 up-regulation by pDC does not increase progressively with developing disease. However, Trabattoni et al. showed that PD-L1 expression was increased on monocytes, T and B cells in viraemic HIV-1+ patients and, in contrast to pDC, correlated positively with virus load [43]. pDC from HIV-1-infected individuals show a reduced ability to stimulate T cells [19], and this dysfunction may be related to increased PD-L1 expression, because blocking PD-L1 but not PD-1 was shown to restore HIV-1-specific T cell proliferation in vitro[25][44,45]. In addition, exposure of pDC from healthy individuals to infectious and non-infectious HIV-1 was shown to induce up-regulation of PD-L1 in vitro[46]. The lower percentages and levels of PD-L2 expressed by pDC in viraemic HIV-1+ patients may be related to the observations of Youngnak and colleagues that PD-L1 and PD-L2 possess different binding affinities to PD-1 [47], and may reflect different functions when they engage PD-1 on T cells. Addressing this issue may shed light on why pDC from viraemic untreated HIV-1+ patients show a unidirectional bias towards PD-L1 expression and not PD-L2. Wang et al. demonstrated higher levels of PD-L1 expression by mDC from HIV-1+ untreated progressors compared to healthy controls and a positive correlation with viraemia [23]. The levels were highest in patients with CD4 counts below 250 cells/µl blood [23], and is a possible explanation for this discrepancy with our study in which only two HIV-1+ patients had CD4 counts below 250 cells/µl blood. In SIV-infected macaques, both pDC and mDC from peripheral blood, mesenteric lymph nodes and jejunum lamina propria lymphocytes (LPL) express significantly higher levels of PD-L1. This correlated positively with PD-1 expression by T cells with plasma viraemia and negatively with CD4 counts [48].
Ju et al. demonstrated that ILT-3 and ILT-7 expression by pDC is down-regulated upon stimulation with cytosine–phosphate–guanosine (CpG) DNA [38]. Similarly, levels of ILT-3 expression on in-vitro-derived myeloid DC are down-modulated upon stimulation with proinflammatory cytokines and CD40L [38]. Thus, the observation that pDC from therapy-naive patients exhibit significantly lower levels of ILT-7 suggests at least some degree of pDC activation. Full maturation should be accompanied by down-regulation of ILT-3 and up-regulation of CD80, CD83 and CD86, and this was not observed in pDC from untreated HIV-1+ patients (Figs 2 and 3). In fact, ILT-3 expression by pDC from therapy-naive patients was found to be increased in comparison to healthy controls, which may suggest that HIV-1 regulates ILT-3 expression differently to stimulation with CpG DNA as shown by Ju et al. [38]. ILT-7 down-regulation by pDC did not correlate with plasma viraemia or the patients' CD4 counts, indicating that this phenomenon may occur irrespective of disease progression. However, the observation that HAART restores ILT-7 levels may suggest that virus stimulation drives down ILT-7 expression. As ILT-7 functions as a negative regulator of IFN-α production [39], pDC from viraemic HIV-1+ patients may produce higher levels or prolonged secretion of IFN-α, contributing to HIV-1-associated immune hyperactivation [21]. In agreement with this hypothesis is the observation by Lehmann et al. showing accumulation of pDC in lymph nodes of treatment-naive HIV-1+ patients coupled with elevated expression of IFN- α[49]. However, it is also plausible that higher IFN-α may drive down-regulation of ILT-7 expression, and thus functional studies are required in the future to address these hypotheses.
We also observed that the percentages of pDC expressing ILT-3, as well as the levels of ILT-3 expression per cell, were significantly higher in viraemic patients compared to healthy controls and HAART-treated patients, although this difference was not as striking as that observed for ILT-7. Chang et al. showed that induction of ILT-3 expression by monocytes and dendritic cells renders these cells tolerogenic in function, express lower levels of co-stimulatory molecules and induce antigen-specific unresponsiveness in CD4 T helper cells [31]. Thus, HIV-1 infection may render pDC tolerogenic by up-regulating ILT-3 expression, a phenomenon that is abrogated by treatment (Fig. 2). The tolerogenic receptor, ILT-4 [31], was highly expressed by both pDC and mDC and unaltered during HIV-1 infection, and thus may not play a role in impaired DC function.
In a cohort of HIV-1 subtype C-infected Indians, Majumdar et al. showed that mDC and pDC from HIV-1+ patients express significantly higher levels of CD86 compared to healthy controls, which correlated positively with viraemia [22]. Similarly, Barron and co-workers reported that CD86 expression by blood DC correlated with HIV-1 RNA load [14]. We observed no differences in the levels of CD86, CD80 and CD83 between pDC and mDC from healthy controls, viraemic and aviraemic HIV-1+ patients. This is in agreement with the study by Lehmann et al., where pDC from lymph nodes of HIV-1 patients were found to express similar levels of CD83 and CD86 compared to healthy controls [49]. However, it is not clear why, in our study, blood DC exhibited a resting state with regard to these receptors when compared to the cohorts of Majumdar et al. and Barron et al. However, in the latter study, pDC and mDC were combined in one population and statistical correlations were performed on a mixture of anti-retroviral-treated and -untreated patients [14]. The likelihood that absence of these markers in our study may have been due to technical issues was ruled out by testing the antibodies used on a positive control – monocyte-derived DC stimulated with lipopolysaccharide, a strong inducer of CD80, CD83 and CD86 (data not shown). In addition, levels of HLA-DR, which is also up-regulated upon maturation of pDC and mDC, were found to be comparable across the three cohorts (data not shown). Although we have observed that circulating blood DC exhibited a resting state in all cohorts, our data provide information only about their baseline activation status. Thus, future studies should address the capacity of pDC and mDC from HIV-1 patients to mature and up-regulate these receptors in response to exogenous stimulation through Toll-like receptors in in-vitro assays. Similarly, our data provide promise for future investigations addressing the ability of pDC and mDC to regulate expression of PD and ILT receptors following maturation and their impact on T cell modulation during treated and untreated HIV-1 infection.
In conclusion, this study shows that pDC but not mDC from HIV-1+ viraemic patients exhibit changes in expression of immunoregulatory molecules. Reduced PD-L2 and ILT-7 expression and elevated levels of PD-L1 and ILT-3 may play a role in pDC dysfunction but, importantly, HAART restores normal expression levels.
Acknowledgments
This research was conducted with support from EUROPRISE (contract number 037611).
Disclosure
None of the authors have conflicts of interest to declare.
Supporting information
Additional Supporting Information may be found in the online version of this article:
Fig. S1. Six-colour gating strategy for the identification and phenotypic characterization of plasmacytoid dendritic cells (pDC) and myeloid DC (mDC). Peripheral blood mononuclear cells (PBMC) were collected from healthy controls, therapy-naive and highly active anti-retroviral therapy (HAART)-treated patients. Cells were surface-stained with appropriate antibodies and analysed by flow cytometry; 105 live events were collected and identified on the basis of forward- and side-scatter plots (a). Doublets were excluded on the basis of forward-scatter height versus area (b). Total DC were identified on the basis of expression of human leucocyte antigen D-related (HLA-DR) and lack of expression of lineage-associated markers (c). pDC and mDC were then distinguished on the basis of CD123 and CD11c expression, respectively (d). Surface expression of specific markers was determined using appropriate isotype controls (e, f).
Please note: Wiley-Blackwell are not responsible for the content or functionality of any supporting materials supplied by the authors. Any queries (other than missing material) should be directed to the corresponding author for the article.
Reference
- 1.Banchereau J, Briere F, Caux C, et al. Immunobiology of dendritic cells. Annu Rev Immunol. 2000;18:767–811. doi: 10.1146/annurev.immunol.18.1.767. [DOI] [PubMed] [Google Scholar]
- 2.Palucka K, Banchereau J, Mellman I. Designing vaccines based on biology of human dendritic cell subsets. Immunity. 2010;33:464–78. doi: 10.1016/j.immuni.2010.10.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Steinman RM. Lasker Basic Medical Research Award. Dendritic cells: versatile controllers of the immune system. Nat Med. 2007;13:1155–9. doi: 10.1038/nm1643. [DOI] [PubMed] [Google Scholar]
- 4.Grouard G, Rissoan MC, Filgueira L, et al. The enigmatic plasmacytoid T cells develop into dendritic cells with interleukin (IL)-3 and CD40-ligand. J Exp Med. 1997;185:1101–11. doi: 10.1084/jem.185.6.1101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Robinson SP, Patterson S, English N, et al. Human peripheral blood contains two distinct lineages of dendritic cells. Eur J Immunol. 1999;29:2769–78. doi: 10.1002/(SICI)1521-4141(199909)29:09<2769::AID-IMMU2769>3.0.CO;2-2. [DOI] [PubMed] [Google Scholar]
- 6.Ito T, Inaba M, Inaba K, et al. A CD1a+/CD11c+ subset of human blood dendritic cells is a direct precursor of Langerhans cells. J Immunol. 1999;163:1409–19. [PubMed] [Google Scholar]
- 7.Li Q, Estes JD, Schlievert PM, et al. Glycerol monolaurate prevents mucosal SIV transmission. Nature. 2009;458:1034–8. doi: 10.1038/nature07831. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Cella M, Jarrossay D, Facchetti F, et al. Plasmacytoid monocytes migrate to inflamed lymph nodes and produce large amounts of type I interferon. Nat Med. 1999;5:919–23. doi: 10.1038/11360. [DOI] [PubMed] [Google Scholar]
- 9.Fitzgerald-Bocarsly P, Jacobs ES. Plasmacytoid dendritic cells in HIV infection: striking a delicate balance. J Leukoc Biol. 2010;87:609–20. doi: 10.1189/jlb.0909635. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Reizis B, Bunin A, Ghosh HS, Lewis KL, Sisirak V. Plasmacytoid dendritic cells: recent progress and open questions. Annu Rev Immunol. 2011;23:163–83. doi: 10.1146/annurev-immunol-031210-101345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Liu YJ, Kanzler H, Soumelis V, Gilliet M. Dendritic cell lineage, plasticity and cross-regulation. Nat Immunol. 2001;2:585–9. doi: 10.1038/89726. [DOI] [PubMed] [Google Scholar]
- 12.Patterson S, Rae A, Hockey N, Gilmour J, Gotch F. Plasmacytoid dendritic cells are highly susceptible to human immunodeficiency virus type 1 infection and release infectious virus. J Virol. 2001;75:6710–3. doi: 10.1128/JVI.75.14.6710-6713.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Lore K, Smed-Sorensen A, Vasudevan J, Mascola JR, Koup RA. Myeloid and plasmacytoid dendritic cells transfer HIV-1 preferentially to antigen-specific CD4+ T cells. J Exp Med. 2005;201:2023–33. doi: 10.1084/jem.20042413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Barron MA, Blyveis N, Palmer BE, MaWhinney S, Wilson CC. Influence of plasma viremia on defects in number and immunophenotype of blood dendritic cell subsets in human immunodeficiency virus 1-infected individuals. J Infect Dis. 2003;187:26–37. doi: 10.1086/345957. [DOI] [PubMed] [Google Scholar]
- 15.Benlahrech A, Gotch F, Kelleher P, Patterson S. Loss of NK stimulatory capacity by plasmacytoid and monocyte-derived DC but not myeloid DC in HIV-1 infected patients. PLoS ONE. 2011;6:e17525. doi: 10.1371/journal.pone.0017525. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Chehimi J, Azzoni L, Farabaugh M, et al. Baseline viral load and immune activation determine the extent of reconstitution of innate immune effectors in HIV-1-infected subjects undergoing antiretroviral treatment. J Immunol. 2007;179:2642–50. doi: 10.4049/jimmunol.179.4.2642. [DOI] [PubMed] [Google Scholar]
- 17.Donaghy H, Pozniak A, Gazzard B, et al. Loss of blood CD11c(+) myeloid and CD11c(–) plasmacytoid dendritic cells in patients with HIV-1 infection correlates with HIV-1 RNA virus load. Blood. 2001;98:2574–6. doi: 10.1182/blood.v98.8.2574. [DOI] [PubMed] [Google Scholar]
- 18.Lichtner M, Rossi R, Rizza MC, et al. Plasmacytoid dendritic cells count in antiretroviral-treated patients is predictive of HIV load control independent of CD4+ T-cell count. Curr HIV Res. 2008;6:19–27. doi: 10.2174/157016208783571937. [DOI] [PubMed] [Google Scholar]
- 19.Brown KN, Trichel A, Barratt-Boyes SM. Parallel loss of myeloid and plasmacytoid dendritic cells from blood and lymphoid tissue in simian AIDS. J Immunol. 2007;178:6958–67. doi: 10.4049/jimmunol.178.11.6958. [DOI] [PubMed] [Google Scholar]
- 20.Donaghy H, Gazzard B, Gotch F, Patterson S. Dysfunction and infection of freshly isolated blood myeloid and plasmacytoid dendritic cells in patients infected with HIV-1. Blood. 2003;101:4505–11. doi: 10.1182/blood-2002-10-3189. [DOI] [PubMed] [Google Scholar]
- 21.Boasso A, Shearer GM. Chronic innate immune activation as a cause of HIV-1 immunopathogenesis. Clin Immunol. 2008;126:235–42. doi: 10.1016/j.clim.2007.08.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Mojumdar K, Vajpayee M, Chauhan NK, Mendiratta S, Wig N. Defects in blood dendritic cell subsets in HIV-1 subtype c infected Indians. Indian J Med Res. 2010;132:318–27. [PubMed] [Google Scholar]
- 23.Wang X, Zhang Z, Zhang S, et al. B7-H1 up-regulation impairs myeloid DC and correlates with disease progression in chronic HIV-1 infection. Eur J Immunol. 2008;38:3226–36. doi: 10.1002/eji.200838285. [DOI] [PubMed] [Google Scholar]
- 24.Day CL, Kaufmann DE, Kiepiela P, et al. PD-1 expression on HIV-specific T cells is associated with T-cell exhaustion and disease progression. Nature. 2006;443:350–4. doi: 10.1038/nature05115. [DOI] [PubMed] [Google Scholar]
- 25.Rosignoli G, Lim CH, Bower M, Gotch F, Imami N. Programmed death (PD)-1 molecule and its ligand PD-L1 distribution among memory CD4 and CD8 T cell subsets in human immunodeficiency virus-1-infected individuals. Clin Exp Immunol. 2009;157:90–7. doi: 10.1111/j.1365-2249.2009.03960.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Trautmann L, Janbazian L, Chomont N, et al. Upregulation of PD-1 expression on HIV-specific CD8+ T cells leads to reversible immune dysfunction. Nat Med. 2006;12:1198–202. doi: 10.1038/nm1482. [DOI] [PubMed] [Google Scholar]
- 27.Keir ME, Butte MJ, Freeman GJ, Sharpe AH. PD-1 and its ligands in tolerance and immunity. Annu Rev Immunol. 2008;26:677–704. doi: 10.1146/annurev.immunol.26.021607.090331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Krathwohl MD, Schacker TW, Anderson JL. Abnormal presence of semimature dendritic cells that induce regulatory T cells in HIV-infected subjects. J Infect Dis. 2006;193:494–504. doi: 10.1086/499597. [DOI] [PubMed] [Google Scholar]
- 29.Lim A, Tan D, Price P, et al. Proportions of circulating T cells with a regulatory cell phenotype increase with HIV-associated immune activation and remain high on antiretroviral therapy. AIDS. 2007;21:1525–34. doi: 10.1097/QAD.0b013e32825eab8b. [DOI] [PubMed] [Google Scholar]
- 30.Tsunemi S, Iwasaki T, Imado T, et al. Relationship of CD4+CD25+ regulatory T cells to immune status in HIV-infected patients. AIDS. 2005;19:879–86. doi: 10.1097/01.aids.0000171401.23243.56. [DOI] [PubMed] [Google Scholar]
- 31.Chang CC, Ciubotariu R, Manavalan JS, et al. Tolerization of dendritic cells by T(S) cells: the crucial role of inhibitory receptors ILT3 and ILT4. Nat Immunol. 2002;3:237–43. doi: 10.1038/ni760. [DOI] [PubMed] [Google Scholar]
- 32.Manavalan JS, Rossi PC, Vlad G, et al. High expression of ILT3 and ILT4 is a general feature of tolerogenic dendritic cells. Transpl Immunol. 2003;11:245–58. doi: 10.1016/S0966-3274(03)00058-3. [DOI] [PubMed] [Google Scholar]
- 33.Vlad G, Chang CC, Colovai AI, et al. Immunoglobulin-like transcript 3: a crucial regulator of dendritic cell function. Hum Immunol. 2009;70:340–4. doi: 10.1016/j.humimm.2009.03.004. [DOI] [PubMed] [Google Scholar]
- 34.Cao W, Bover L, Cho M, et al. Regulation of TLR7/9 responses in plasmacytoid dendritic cells by BST2 and ILT7 receptor interaction. J Exp Med. 2009;206:1603–14. doi: 10.1084/jem.20090547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Cao W, Bover L. Signaling and ligand interaction of ILT7: receptor-mediated regulatory mechanisms for plasmacytoid dendritic cells. Immunol Rev. 2010;234:163–76. doi: 10.1111/j.0105-2896.2009.00867.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Imami N, Pires A, Hardy G, et al. A balanced type 1/type 2 response is associated with long-term nonprogressive human immunodeficiency virus type 1 infection. J Virol. 2002;76:9011–23. doi: 10.1128/JVI.76.18.9011-9023.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Francisco LM, Sage PT, Sharpe AH. The PD-1 pathway in tolerance and autoimmunity. Immunol Rev. 2010;236:219–42. doi: 10.1111/j.1600-065X.2010.00923.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Ju XS, Hacker C, Scherer B, et al. Immunoglobulin-like transcripts ILT2, ILT3 and ILT7 are expressed by human dendritic cells and down-regulated following activation. Gene. 2004;28:159–64. doi: 10.1016/j.gene.2004.02.018. [DOI] [PubMed] [Google Scholar]
- 39.Cao W, Rosen DB, Ito T, et al. Plasmacytoid dendritic cell-specific receptor ILT7-Fc epsilonRI gamma inhibits Toll-like receptor-induced interferon production. J Exp Med. 2006;203:1399–405. doi: 10.1084/jem.20052454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Gougeon ML, Lecoeur H, Dulioust A, et al. Programmed cell death in peripheral lymphocytes from HIV-infected persons: increased susceptibility to apoptosis of CD4 and CD8 T cells correlates with lymphocyte activation and with disease progression. J Immunol. 1996;156:3509–20. [PubMed] [Google Scholar]
- 41.Massanella M, Negredo E, Perez-Alvarez N, et al. CD4 T-cell hyperactivation and susceptibility to cell death determine poor CD4 T-cell recovery during suppressive HAART. AIDS. 2010;24:959–68. doi: 10.1097/QAD.0b013e328337b957. [DOI] [PubMed] [Google Scholar]
- 42.Pantaleo G, Fauci AS. New concepts in the immunopathogenesis of HIV infection. Annu Rev Immunol. 1995;13:487–512. doi: 10.1146/annurev.iy.13.040195.002415. [DOI] [PubMed] [Google Scholar]
- 43.Trabattoni D, Saresella M, Biasin M, et al. B7-H1 is up-regulated in HIV infection and is a novel surrogate marker of disease progression. Blood. 2003;101:2514–20. doi: 10.1182/blood-2002-10-3065. [DOI] [PubMed] [Google Scholar]
- 44.Benedict CA, Loewendorf A, Garcia Z, Blazar BR, Janssen EM. Dendritic cell programming by cytomegalovirus stunts naive T cell responses via the PD-L1/PD-1 pathway. J Immunol. 2008;180:4836–47. doi: 10.4049/jimmunol.180.7.4836. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Goldberg MV, Maris CH, Hipkiss EL, et al. Role of PD-1 and its ligand, B7-H1, in early fate decisions of CD8 T cells. Blood. 2007;110:186–92. doi: 10.1182/blood-2006-12-062422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Boasso A, Royle CM, Doumazos S, et al. Overactivation of plasmacytoid dendritic cells inhibits antiviral T-cell responses: a model for HIV immunopathogenesis. Blood. 2011;118:5152–62. doi: 10.1182/blood-2011-03-344218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Youngnak P, Kozono Y, Kozono H, et al. Differential binding properties of B7-H1 and B7-DC to programmed death-1. Biochem Biophys Res Commun. 2003;307:672–7. doi: 10.1016/s0006-291x(03)01257-9. [DOI] [PubMed] [Google Scholar]
- 48.Xu H, Wang X, Pahar B, et al. Increased B7-H1 expression on dendritic cells correlates with programmed death 1 expression on T cells in simian immunodeficiency virus-infected macaques and may contribute to T cell dysfunction and disease progression. J Immunol. 2010;185:7340–8. doi: 10.4049/jimmunol.1001642. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Lehmann C, Lafferty M, Garzino-Demo A, et al. Plasmacytoid dendritic cells accumulate and secrete interferon alpha in lymph nodes of HIV-1 patients. PLoS ONE. 2010;5:e11110. doi: 10.1371/journal.pone.0011110. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


