Abstract
T-cell immunodeficiency is a common feature in patients with chronic myeloid leukemia (CML), and deficiency in CD3 levels was detected in T cells from these patients, which may represent a characteristic that is related to a lower T cell activation. In this study, we explored the possibility that forced TCRζ gene expression may upreg-u-late T cell receptor (TCR) signaling activation and reverse interleukin-2 (IL-2) production in T cells from patients with CML. A recombinant eukaryotic vector expressing TCRζ was transfected into T cells by nucleofection. Phosphorylated TCRζ, phosphorylated NF-κB, and the IL-2 level in TCRζ-transfected CD3+T cells that were activated with anti-CD3 and anti-CD28 antibodies were measured by Western blot and enzyme-linked immunosorbent assay (ELISA). Significantly increased TCRζ levels were found in TCRζ-transfected CD3+T cells. After CD3 and CD28 antibody stimulation, a significantly higher phosphorylated TCRζ chain level was demonstrated, and an increased IL-2 production in TCRζ-upregulated T cells was associated with the increased expression of the phosphorylated NF-κB. In conclusion, TCRζ gene transfection could restore TCRζ chain deficiency and enhance IL-2 production in T cells from patients with CML. It is possible that TCRζ chain reconstitution in leukemia-specific, clonally expanded T cells will effectively increase their activation of antileukemia cytotoxicity.
One chain of the T cell receptor TCRζ was overexpressed in CD3+T cells and following activation of those cells by antibody, the gene product was observed to be phosphorylated. The Ab-stimulated T cells produced more IL-2 and also had more activated NF-κB.
Introduction
Chronic myeloid leukemia (CML) accounts for 15%–20% of newly diagnosed cases of leukemia in adults. CML is a clonal disease of hematopoietic stem cells that is characterized by the Philadelphia chromosome (Ph), which is generated by the reciprocal translocation t(9;22) (q34;q11) that results in the fusion of the c-abl oncogene 1 (ABL1) with the breakpoint cluster region gene (BCR) (Smahel, 2011). The constitutively upregulated tyrosine kinase activity of the chimeric BCR-ABL1 protein affects several intracellular signaling pathways that promote cell proliferation and survival and, thus, contribute to their malignant transformation (Quintas-Cardama and Cortes, 2009; Smahel, 2011). In most cases, the chronic phase (CP) CML can be suppressed by imatinib mesylate (IM), a BCR-ABL1 protein tyrosine kinase inhibitor. However, numerous patients with CML die due to an Abl mutation that is related to drug resistance and blast crisis. Because IM does not kill quiescent leukemia stem cells, these cells persist in a majority of patients and may cause disease relapse. Moreover, advanced-stage CML responds poorly to all therapies, including IM (Savona and Talpaz, 2008; Jabbour et al., 2009; Smahel, 2011). Further studies are needed to better understand this disease and improve patient outcome.
In patients with leukemia, T-cell function becomes suppressed as the disease progresses. Such immune dysfunction, which has been demonstrated in many patients with leukemia, may be due to a disorder in the thymic output function, and the abnormal expression of the T cell receptor (TCR) repertoire and may, in part, be due to abnormal TCR signal transduction, possibly through altered expression of the CD3 genes (Rezvany et al., 1999; Chen et al., 2000; Torelli et al., 2003; Li, 2008; Chen et al., 2009; Li et al., 2010; Li et al., 2011b). In CML, decreased levels of recent thymic emigrants in both CD4+ and CD8+T cells may underlie the persistent immunodeficiency found in patients. Restricted expression of the TCR Vβ repertoire indicates T cell immunodeficiency in patients, although clonally expanded T cells suggest a specific immune response to leukemia-associated antigens. Deficiency in CD3 gene expression levels may represent a characteristic of lower T cell activation (Chen et al., 2000; Li, 2008; Chen et al., 2009).
The TCR complex is an octameric receptor composed of two chains, αβ or γδ, that bind to specific ligands that are antigenic peptides presented on major histocompatibility complex molecules. These chains are noncovalently associated with CD3 subunits, which include four transmembrane proteins named CD3γ, CD3δ, CD3ɛ, and CD3ζ (TCRζ), that form three distinct dimers (i.e., CD3γɛ, CD3δɛ, and CD3ζζ) and mediate TCR signal transduction (Clevers et al., 1988; Call et al., 2004; Call and Wucherpfennig, 2005; Fischer et al., 2005).
The absence of the TCRζ chain not only influences TCR expression on the cell membrane and the number of single-positive (CD4+ or CD8+) circulating T cells, it also impairs the proliferative response and mature T cell activation level. T cells from patients with leukemia (Chen et al., 2000; Mozaffari et al., 2004; Li, 2008; Chen et al., 2009) are functionally impaired, and this is indicated by decreased TCRζ chain expression. Recently, our studies have shown the expression pattern of the four CD3 genes in patients with AML, CML, and MM (Li, 2008; Chen et al., 2009; Chen et al., 2011; Li et al., 2011a). Aberrant TCRζ expression in T-cells from patients with Systemic lupus erythematosus (SLE) may be associated with decreased stability and the translation of a TCRζ mRNA with an alternatively spliced 3′-untranslated region (Chowdhury et al., 2005). However, the mechanism of TCRζ deficiency in T-cells from tumors and patients with leukemia remains unclear.
TCRζ upregulation may improve T cell activation and recover their cytotocixity function. TCRζ chain upregulation was first investigated by Nambiar MP and colleagues who transferred a eukaryotic expression vector containing TCRζ chain cDNA into freshly isolated SLE T cells and indicated that reconstitution of the deficient TCRζ chain could reverse TCR/CD3-mediated signaling abnormalities and the defective interleukin-2 (IL-2) production in the T cells of patients with SLE (Nambiar et al., 2003). However, little is known about whether TCRζ upregulation in T cells from CML patients could improve T cell function, which may restore the immunodeficiency status of patients with CML to a certain degree. In this study, we investigated the T cell IL-2 production characteristics after the upregulation of TCRζ by gene transfer in the T cells of patients with CML.
Materials and Methods
Construction of the TCRζ-pIRES2-EGFP recombinant plasmid
The plasmid used in this study is based on the pIRES2-EGFP vector (Clontech Laboratories, Inc.), which encodes an internal ribosome entry site followed by the enhanced green fluorescent protein (EGFP). The sequence encoding the full-length TCRζ gene was amplified from cDNA that was prepared from a healthy individual, and it was cloned between the NheI and EcoRI restriction sites. The recombinant plasmid was confirmed by double digestion using the NheI and EcoRI restriction enzymes, and the concentration was adjusted to 0.5 μg/μL (Chen et al., 2012).
Human CD3+ T cell isolation and culture
Peripheral blood mononuclear cells (PBMCs) that were obtained from 12 newly diagnosed, untreated patients with CP CML were isolated from heparinized venous blood by Ficoll-Paque gradient centrifugation. All procedures were conducted according to the guidelines of the Medical Ethics Committee of the Health Bureau of the Guangdong Province of China. The cells were collected, washed twice in the Hank's balanced salt solution, and resuspended at a final concentration of 2×106 cells/mL in a complete RPMI 1640 medium (Invitrogen) that was supplemented with 10% heat-inactivated fetal calf serum (HyClone), 100 U/mL penicillin, 100 μg/mL streptomycin, 2 mM L-glutamine, and 50 μM 2-mercaptoethanol. CD3+T cells were positively purified from freshly isolated PBMCs from four CML cases using CD3+ microbeads (Miltenyi Biotec) according to the manufacturer's protocol. The purity of the collected CD3+T cells was assessed by flow cytometry (FCM). More than 95% of the CD3+T cells were collected using this technique. The peripheral blood from 10 healthy individuals served as control.
TCRζ chain expression in T cells
TCRζ chain expression was measured in permeabilized cells by FCM using a phycoerythrin (PE)-conjugated mouse anti-human TCRζ (6B10.2) mAb (Santa Cruz Biotechnology). For each sample, 10,000 events were acquired, and all cells were included in the forward scatter/side scatter gate. The TCRζ cell surface density was expressed as the average fluorescence intensity of the analyzed cell populations (mean fluorescence intensity [MFI]). The data were processed using the FlowJo software (Tree Star, Inc.).
TCRζ gene transduction in T cells
Briefly, cells (5×106) were resuspended in 0.1 mL of supplemented Nucleofector solution (human T cell Nucleofector™ kit; Amaxa) at room temperature. TCRζ-IRES2-EGFP and IRES2-EGFP (5 μg) were mixed with 0.1 mL of cell suspension, transferred to a 2.0-mm electroporation cuvette, and nucleofected using an Amaxa Nucleofector II apparatus according to the manufacturer's guidelines. Storage of the cell suspension in the human T cell Nucleofector solution for longer than 20 min was avoided because this reduces cell viability and gene transfer efficiency. The cells were transfected using the U-014 program. The transfected T cells were immediately transferred to a prewarmed complete culture medium and cultured in 12-well plates in a humidified incubator at 37°C and 5% CO2 (Chen et al., 2012).
Transfection efficiency determination
The transfection efficiency was estimated in each experiment by scoring the number of double-positive CD3+ and GFP cells stained with a PE-conjugated mouse anti-human CD3 antibody (eBioscience) 18 h post-transfection using FCM analysis. For analysis, the relative fluorescence density (Log transformed) of the live cells was measured using a multicolor FACScan flow cytometer (Calibur; BD Biosciences). The data were analyzed using the FlowJo software (Tree Star).
Western blot analysis
CD3+T cells at 1×106/0.2 mL were stimulated with 10 μg/mL OKT3 for 2 min at 37°C 18 h after transfection. The reaction was stopped by the addition of 1 mL of ice-cold stop buffer (PBS, 5 mM EDTA, 10 mM NaF, 10 mM sodium pyrophosphate, and 0.4 mM sodium vanadate), and the cell pellets were solubilized in RIPA lysis buffer (1× PBS, 1% Nonidet P-40, 0.5% sodium deoxycholate, 0.1% sodium dodecyl sulfate, 10 mM phenylmethylsulfonyl fluoride, 1 μg/mL aprotinin, and 100 mM sodium orthovanadate) and incubated on ice for 30 min to isolate total protein. Proteins (20 μg) were separated using 12% SDS-PAGE and transferred to a PVDF membrane (Pall) using a damp-dry transfer device (Bio-rad). After blocking for 1 h in 5% defatted milk powder in PBS, the membrane was washed and probed with 1:200 mouse anti-human TCRζ or phosphorylated TCRζ (Santa Cruz) or 1:1000 rabbit anti-human phosphorylated NF-κB (p65 subunit) monoclonal antibody (CST). Similar studies were performed using 1:500 mouse anti-human β-actin (CST). The antibodies were detected using 1:10,000 horseradish peroxidase-conjugated goat anti-rabbit IgG and horseradish peroxidase-conjugated donkey anti-mouse IgG (Jackson ImmunoResearch). A Western blotting luminol reagent (Lianke) was used to visualize the bands corresponding to each antibody (Nambiar et al., 2003). Blot images were captured using an Alliance 4.7 Gel doc system (UVI), and densitometric analysis of the bands was performed using ImageJ software (http://rsb.info.nih.gov/ij).
Enzyme-linked immunosorbent assay
Cell supernatants were collected from the TCRζ-IRES2-EGFP or IRES2-EGFP groups 24 h after OKT3 (CD3) (10 μg/mL) and anti-human CD28 antibody (2.5 μg/mL) stimulation, and the IL-2 levels were measured using the human IL-2 enzyme-linked immunosorbent assay (ELISA) Kit (Raybiotech) according to the protocol of the manufacturer.
Statistical analysis
Statistical analyses were performed using the Independent Samples t-test for TCRζ, phosphorylated TCRζ, and phosphorylated NF-κB protein levels in the TCRζ-IRES2-EGFP or IRES2-EGFP groups. Differences with a p<0.05 were considered statistically significant.
Results
Transfection and expression of the TCRζ chain in cell lines
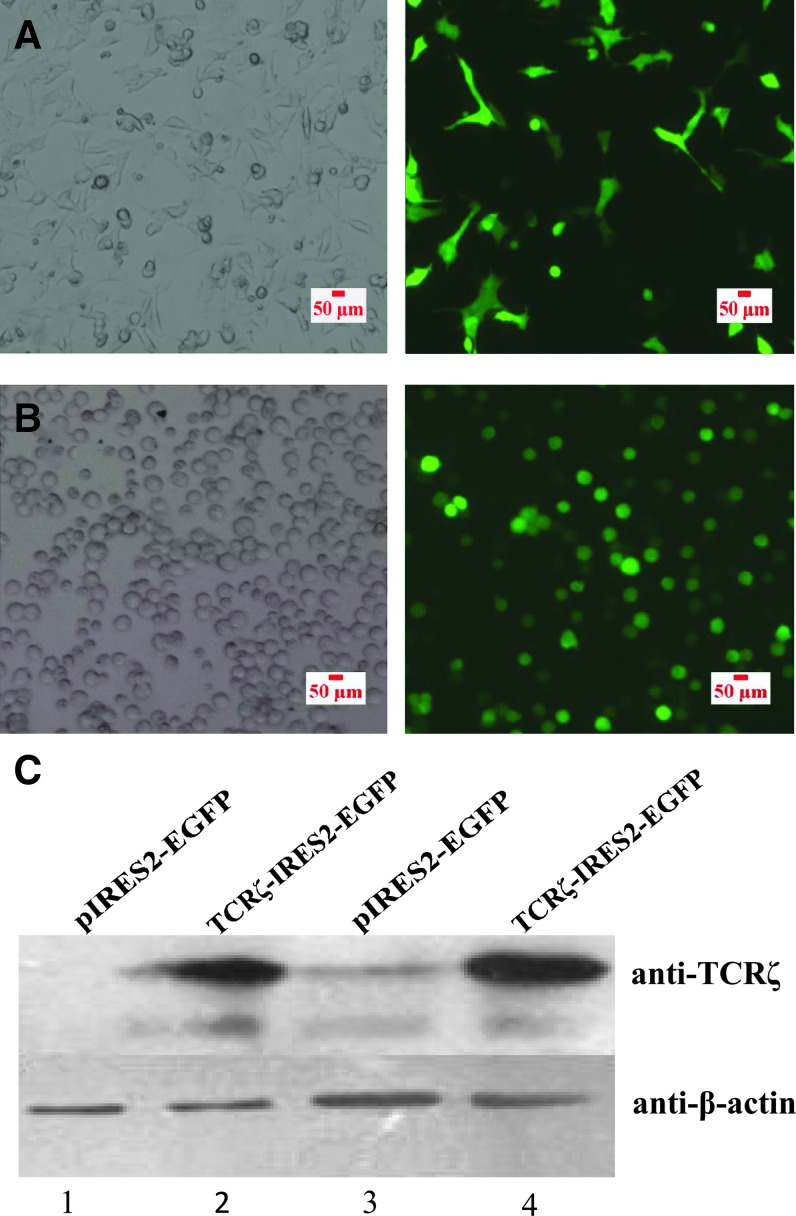
The full-length TCRζ gene was amplified, and its sequence was confirmed by restriction enzyme digestion analysis and sequencing. The TCRζ gene sequence (537 bp) was confirmed by comparison with the TCRζ gene sequence in the NCBI gene bank (data not shown). The pIRES plasmid, which contains the enhanced green fluorescent protein (IRES2-EGFP), was used as a positive control and to construct TCRζ recombinant plasmids (TCRζ-IRES2-EGFP). The TCRζ recombinant vector and IRES2-EGFP were first tranferred into K293 and Jurkat cell lines by Nucleofection. Indirect immunofluorescence and Western blot were used to verify the transfection efficiency and TCRζ protein expression, respectively, 24 h post-transfection (Fig. 1A, B). The results indicated that the TCRζ recombinant vector was successfully constructed and could be used to upregulate the TCRζ gene in primary T cells by gene transfer.
FIG. 1.
Immunofluorescence detection (200×) of enhanced green fluorescent protein (EGFP) expression to measure the TCRζ-IRES2-EGFP transfection efficiency in K293 (A) and Jurkat cells (B) and Western blot analysis of TCRζ protein expression in K293 (C, 1–2) and Jurkat cells (C, 3–4) 24 h post-transfection. Color images available online at www.liebertpub.com/dna
TCRζ overexpression in T cells from patients with CML
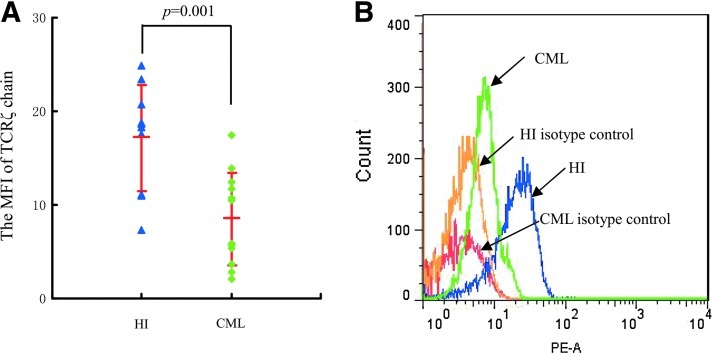
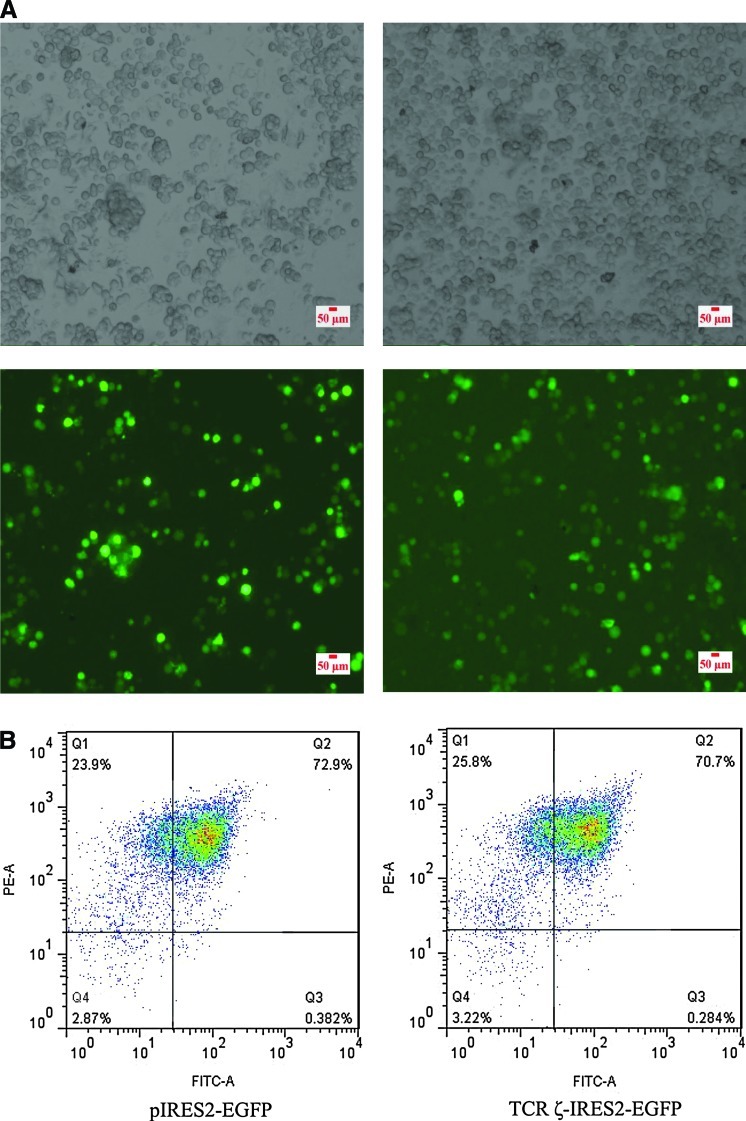
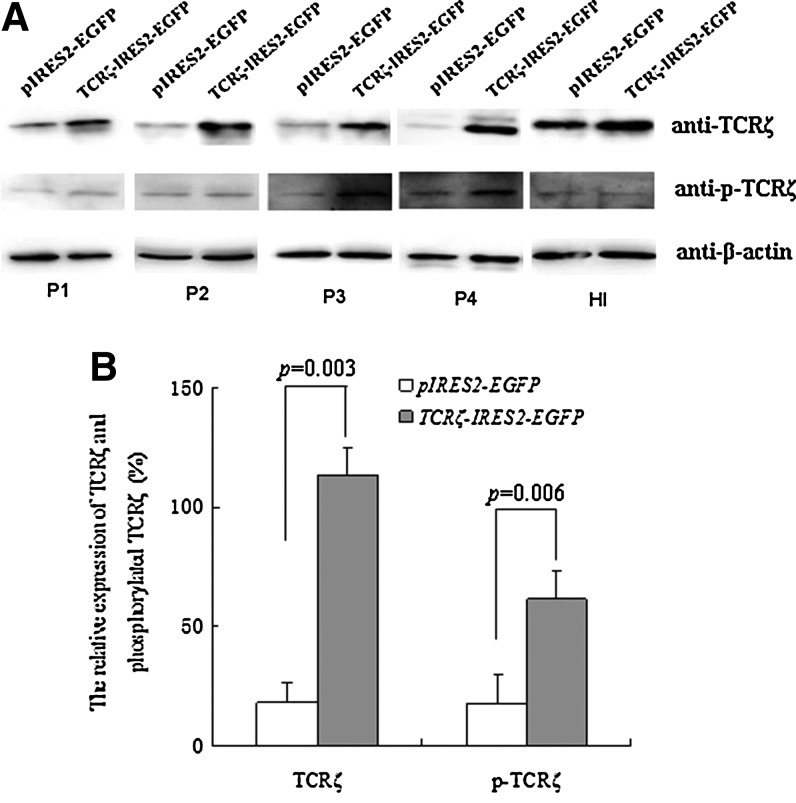
The TCRζ chain expression level was detected in PBMCs from patients with CP CML by counting the MFI using FCM, and a significantly lower MFI of TCRζ in PBMCs from patients with CML (8.5±4.9, n=12) in comparison with those of healthy individuals (17.2±5.7, n=10; p=0.001) was found (Fig. 2). Thus, CD3+T cells were sorted from the PBMCs of four patients with CML that had TCRζ deficiency. The CD3+T cell purity was evaluated by fluorescent microscope detection. More than 95% of the isolated cells were CD3-positive (data not shown). The CD3+T cells were transfected with TCRζ-IRES2-EGFP and IRES2-EGFP by nucleofection (Fig. 3A). The transfection efficiencies were determined by FCM analysis, and the results demonstrated that the cells double-positive for CD3+ and GFP were 58.3%±19.2% and 57.2%±19.4% for TCRζ-IRES2-EGFP and IRES2-EGFP transfected CD3+T cells, respectively, 18 h post-transfection (Fig. 3B). After 18 h, the cells were lysed, and the TCRζ level was measured by Western blot analysis. A significantly increased TCRζ level was found in the TCRζ-IRES2-EGFP transfected CD3+T cell group (gray value: 113.0±19.9) compared to that of the controls (gray value: 17.8±8.6, p=0.003) (Fig. 4).
FIG. 2.
The TCRζ expression level in peripheral blood mononuclear cells (PBMCs) from patients with chronic myeloid leukemia (CML) as determined by flow cytometry (FCM) analysis. (A) Comparison of the mean fluorescence intensity (MFI) of TCRζ in PBMCs between healthy individuals (HI) (17.2±5.7, n=10) and patients with CML (8.5±4.9, n=12), (B) Example of the shift in the MFI of TCRζ in PBMCs from a patient with CML relative to the MFI (PE-A) of TCRζ in PBMCs of a healthy individual. PE-A=fluorescence intensity of PE. Color images available online at www.liebertpub.com/dna
FIG. 3.
The transfection efficiencies of TCRζ-IRES2-EGFP or IRES2-EGFP transfected CD3+T cells as determined by fluorescence microscopy (A) and FCM detection (B) 18 h post-transfection. FITC-A=the fluorescence intensity of FITC. Color images available online at www.liebertpub.com/dna
FIG. 4.
The expression levels of TCRζ and phosphorylated TCRζ as determined by Western blot analysis. (A) Western blot results, P1–P4: CML samples, HI: healthy individual control, (B) Comparison of the expression levels of TCRζ and phosphorylated TCRζ between the TCRζ-IRES2-EGFP and IRES2-EGFP transfected CD3+T cell groups (n=4).
IL-2 production in TCRζ chain upregulating T cells from patients with CML
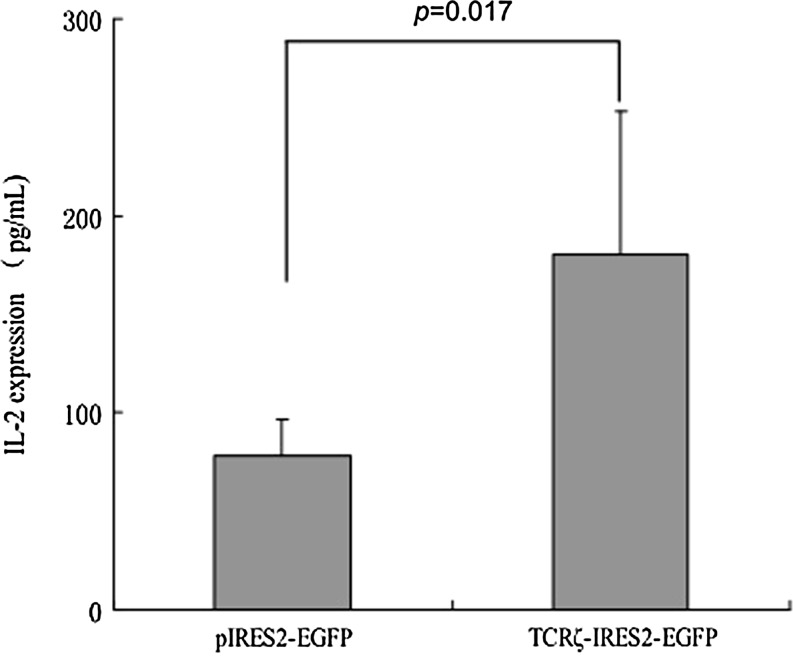
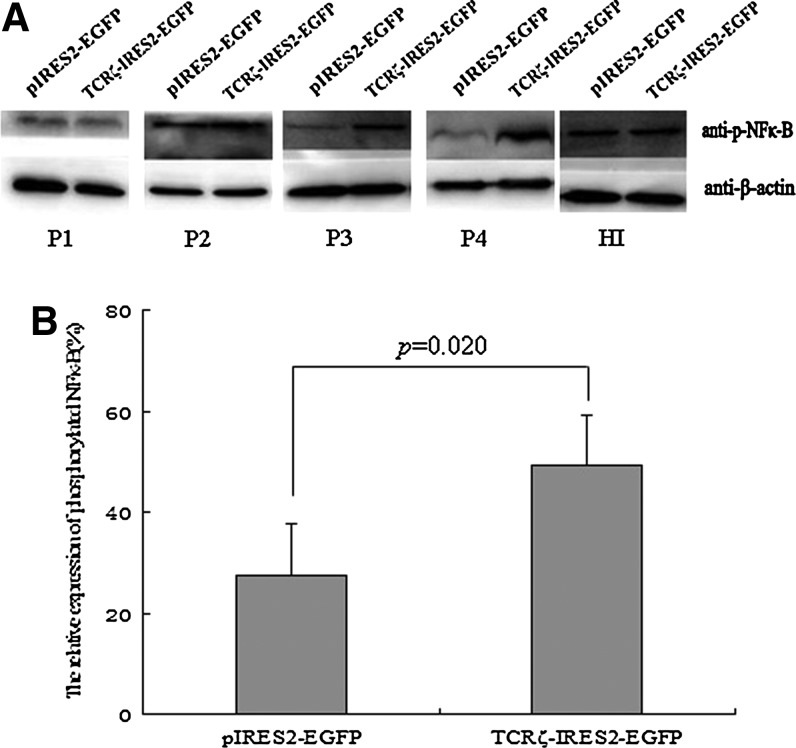
After stimulation with anti-CD3 antibodies to activate the TCRζ-transfected T cells, the phosphorylated TCRζ chain expression level was measured by Western blot analysis. A significantly higher phosphorylated TCRζ chain level was observed in the TCRζ-transfected CD3+T cell group (gray value: 61.5±11.7) than in the control group (gray value: 17.2±12.9; p=0.006) (Fig. 4). A lower T cell activation level has been described in CML T cells, and to investigate whether upregulating TCRζ in CML T cells could reconstitute IL-2 production, we measured the IL-2 levels in TCRζ-transfected CD3+T cells that were activated with anti-CD3 and anti-CD28 antibodies using an ELISA. As shown in Figure 5, the amount of IL-2 secretion was significantly higher in the TCRζ-transfected CD3+T cell group (180.4±73.9 pg/mL) compared with that of the control group (78.4±18.2 pg/mL) (p=0.017) (Fig. 5). To determine whether an increased IL-2 production was associated with restoration of the NF-κB level, we further analyzed the phosphorylated NF-κB level. In comparison with the control group (gray value: 27.57±16.83), a significantly higher phosphorylated NF-κB level was found in the TCRζ-IRES2-EGFP-transfected CD3+T cell group (gray value: 49.21±20.55) (p=0.02) (Fig. 6). Thus, the increased IL-2 production in TCRζ upregulated T cells may associate with increased phosphorylated NF-κB (p65 subunit) in these T cells.
FIG. 5.
The interleukin-2 (IL-2) levels in TCRζ-IRES2-EGFP or IRES2-EGFP transfected CD3+T cell groups (n=4).
FIG. 6.
The expression level of phosphorylated NF-κB by Western blot analysis. (A) Western blot results, P1–P4: CML samples, HI: healthy individual control, (B) Comparison of the expression levels of phosphorylated NF-κB between the TCRζ-IRES2-EGFP and IRES2-EGFP transfected CD3+T cell groups (n=4).
Discussion
T cell modification by the transfer of different target genes that enhance or suppress primary T cell function has been described in previous studies. The first TCR gene transfer into primary human T lymphocytes was accomplished in a study of a melanoma antigen, and CD8+T cells transduced with a TCR that is specific for MART-1 were able to lyse an HLA-A2+ melanoma cell line in vitro (Clay et al., 1999). The successful transfer of genes encoding TCRαβ chains, which recognize a variety of virus-specific and tumor-associated antigens, into primary T cells has been demonstrated (Xue et al., 2005; Engels and Uckert, 2007; Heemskerk, 2010; Yin et al., 2011; Stroncek et al., 2012). In comparison with the transfer of both the TCRα and TCRβ genes, which leads to the expression of the TCRαβ heterodimer in T-cells, the transfer of the TCRζ gene into primary T cells appeared to be easier. Using nucleoporation, successful upregulated TCRζ expression was achieved in SLE T cells that were transfected with a TCRζ chain containing eukaryotic expression vector at high efficiency (Nambiar et al., 2003). In this study, after the successful construction of a recombinant eukaryotic vector, we attempted to deliver the TCRζ gene into freshly sorted CD3+T cells from patients with CP CML.
Previous studies have shown decreased expression in the level of the TCRζ gene and impaired TCRζ expression in patients with CP CML (Chen et al., 2000; Chen et al., 2009), and we further confirmed the surface expression of the TCRζ chain in T cells from patients with CML by FCM, which is similar to the decreased TCRζ chain expression result that was found in T-cells in 12 cases with CP CML. After TCRζ transfection, TCRζ expression was increased, and phosphorylated TCRζ was increased in modified T cells after CD3 and CD28 monoclonal antibody stimulation. These data indicate that TCR/CD3 signaling may be reversed in CML T cells after upregulating TCRζ.
It has been demonstrated that forced TCRζ chain expression could reverse TCR/CD3-mediated signaling abnormalities and defective IL-2 production in SLE T cells (Nambiar et al., 2003). In this study, similar results were found in T cells from patients with CP CML who had different immunodeficiency statuses. When TCRζ was upregulated in T cells from patients with CML, their activation function in IL-2 production was enhanced, and this was associated with an increase in phosphorylated NF-κB. NF-κB plays a central role in regulating inflammatory and immune responses (Sakurai et al., 2003). NF-κB activated by T cell costimulation can induce the expression of IL-2 in T cells (Schmitz et al., 2003). The low level of NF-κB (p65 subunit) was associated with a decreased production of IL-2 in SLE T cells, and reconstitution of p65 NF-κB can restore CD3-mediated IL-2 production in SLE T cells (Herndon et al., 2002). In this study, we tried to prove that the reconstitution of TCRζ chain could increase phosphorylated NF-κB (p65 subunit), and then promote the IL-2 production, and it is possible that restoration of the TCRζ chain in T cells from CML will increase their antileukemia cytotoxic ability. Further investigation is needed to evaluate the anti-CML cytotoxicity of TCRζ-transfected T cells.
The upregulation of TCRζ expression could be stimulated in different ways using different active factors, including cytokines, such as IL-2 and IFN-γ (Chen et al., 2000; Frydecka et al., 2001), and anti-CD3 and anti-CD28 monoclonal antibodies (Renner et al., 1996). It has been reported that dexamethasone primarily acts at the transcriptional level, and it differentially modulates TCRζ chain expression in human T cells by the increased anti-CD3 antibody-induced tyrosine phosphorylation of the TCRζ chain and the downstream signaling intermediates ZAP-70 and PLCγ, which increase and sustain the TCR/CD3-mediated [Ca(2+)] (i) response (Nambiar et al., 2001). However, decreased TCRζ chain expression in patients with T-cell acute lymphoblastic leukemia was not reversed by immunotherapy using IL-2 and anti-CD3 alone or in combination (Torelli et al., 2003). Our previous study has shown that TCRζ expression in T cells from patients with CML-CP could not be completely upregulated after IL-2 or PHA stimulation (Chen et al., 2009). These findings suggested that stimulation plays a minor role in restoring this impaired T cell signal transduction in CML. Therefore, the establishment of a TCRζ gene transfer technique is required for deficient TCRζ chain reconstitution in T cells from patients with CML, and it may also be useful for restoring T cell function in different patients with cancer. Further studies are needed to investigate the mechanisms responsible for upregulating the TCRζ chain in T cells from patients with CML.
Adoptive immunotherapy of malignant diseases using tumor-specific cytotoxic T cells showed remarkable efficacy in recent trials. Such cytotoxic T cells possess not only specific TCRs that recognize the tumor-associated antigens expressed on tumor cells, they also have active TCR signaling, which transduces the immune response and performs cytotoxic functions (Yin et al., 2011; Stroncek et al., 2012). The combined transfection of specific TCR and TCRζ genes in T cells may be an ideal method to manufacture redirected T cells for immunotherapy. For example, the transgenic expression of the CD3ζ signaling molecule chimeric antigen receptor, which is specific for CMV, recovered hypo-responsive T cells to full effector functions, released cytokines and mediated redirected cytotoxicity as efficiently as younger effector T cells (Rappl et al., 2012). Clonally expanded T cells from patients with leukemia possess a leukemia-associated, antigen-specific TCR (Zha et al., 2011), and these T cells have a specific cytotoxic ability against antileukemia cells; however, their activation was poor due to deficiencies in TCR signaling, including decreased TCRζ expression (Chen et al., 2000; Li, 2008; Chen et al., 2009). Thus, restoring their activation function can enhance their antileukemia function. Transfecting the TCRζ gene into T cells from such patients with leukemia may be an alternative approach to establish an ideal immunotherapeutic strategy.
In conclusion, this study described the construction of a TCRζ recombinant vector that was successfully delivered and expressed in primary T cells from patients with CP CML. TCRζ gene transfection could restore TCRζ chain expression and enhance IL-2 production in T cells from patients with CML. It is possible that reconstitution of the TCRζ chain in leukemia-specific, clonally expanded T cells will effectively increase their antileukemia cytotoxic ability.
Authors' Contributions
Y.Q.L. contributed to the concept development and study design. X.F.Z., S.H.C., L.J.Y., S.L., and B.L. performed the laboratory studies. X.L.W. and Y.H.L. were responsible for collection of clinical data. Y.Q.L. and X.F.Z. coordinated the study and helped draft the manuscript. All authors read and approved the final manuscript.
Acknowledgments
The study was supported by grants from the National Natural Science Foundation of China (No. 81100353 and 81270604), the Natural Science Foundation of Guangdong Province, China (No. 9251063201000001), the Fundamental Research Funds for the Central Universities (No. 21611447, 21612116), and the Medical Science Foundation of Guangdong Province (A2011325).
Disclosure Statement
The authors declare that they have no competing interests.
References
- Call M.E. Pyrdol J. Wucherpfennig K.W. Stoichiometry of the T-cell receptor-CD3 complex and key intermediates assembled in the endoplasmic reticulum. EMBO J. 2004;23:2348–2357. doi: 10.1038/sj.emboj.7600245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Call M.E. Wucherpfennig K.W. The T cell receptor: critical role of the membrane environment in receptor assembly and function. Annu Rev Immunol. 2005;23:101–125. doi: 10.1146/annurev.immunol.23.021704.115625. [DOI] [PubMed] [Google Scholar]
- Chen S. Huang X. Chen S. Yang L. Shen Q. Zheng H. Li B. Grabarczyk P. Przybylski G.K. Schmidt C.A. Li Y. The role of BCL11B in regulating the proliferation of human naive T cells. Hum Immunol. 2012;73:456–464. doi: 10.1016/j.humimm.2012.02.018. [DOI] [PubMed] [Google Scholar]
- Chen S. Yang L. Chen S. Li Y. TCR zeta chain expression in T cells from patients with CML. Hematology. 2009;14:95–100. doi: 10.1179/102453309X385241. [DOI] [PubMed] [Google Scholar]
- Chen S. Zha X. Yang L. Li B. Liye Z. Li Y. Deficiency of CD3gamma, delta, epsilon, and zeta expression in T cells from AML patients. Hematology. 2011;16:31–36. doi: 10.1179/102453311X12902908411832. [DOI] [PubMed] [Google Scholar]
- Chen X. Woiciechowsky A. Raffegerst S. Schendel D. Kolb H.J. Roskrow M. Impaired expression of the CD3-zeta chain in peripheral blood T cells of patients with chronic myeloid leukaemia results in an increased susceptibility to apoptosis. Br J Haematol. 2000;111:817–825. [PubMed] [Google Scholar]
- Chowdhury B. Tsokos C.G. Krishnan S. Robertson J. Fisher C.U. Warke R.G. Warke V.G. Nambiar M.P. Tsokos G.C. Decreased stability and translation of T cell receptor zeta mRNA with an alternatively spliced 3′-untranslated region contribute to zeta chain down-regulation in patients with systemic lupus erythematosus. J Biol Chem. 2005;280:18959–18966. doi: 10.1074/jbc.M501048200. [DOI] [PubMed] [Google Scholar]
- Clay T.M. Custer M.C. Sachs J. Hwu P. Rosenberg S.A. Nishimura M.I. Efficient transfer of a tumor antigen-reactive TCR to human peripheral blood lymphocytes confers anti-tumor reactivity. J Immunol. 1999;163:507–513. [PubMed] [Google Scholar]
- Clevers H. Alarcon B. Wileman T. Terhorst C. The T cell receptor/CD3 complex: a dynamic protein ensemble. Annu Rev Immunol. 1988;6:629–662. doi: 10.1146/annurev.iy.06.040188.003213. [DOI] [PubMed] [Google Scholar]
- Engels B. Uckert W. Redirecting T lymphocyte specificity by T cell receptor gene transfer—a new era for immunotherapy. Mol Aspects Med. 2007;28:115–142. doi: 10.1016/j.mam.2006.12.006. [DOI] [PubMed] [Google Scholar]
- Fischer A. de Saint Basile G. Le Deist F. CD3 deficiencies. Curr Opin Allergy Clin Immunol. 2005;5:491–495. doi: 10.1097/01.all.0000191886.12645.79. [DOI] [PubMed] [Google Scholar]
- Frydecka I. Bocko D. Kosmaczewska A. Ciszak L. Morilla R. The effect of peripheral blood lymphocyte stimulation on zeta chain expression and IL-2 production in Hodgkin's disease. Br J Cancer. 2001;84:1339–1343. doi: 10.1054/bjoc.2001.1792. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heemskerk M.H. T-cell receptor gene transfer for the treatment of leukemia and other tumors. Haematologica. 2010;95:15–19. doi: 10.3324/haematol.2009.016022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Herndon T.M. Juang Y.T. Solomou E.E. Rothwell S.W. Gourley M.F. Tsokos G.C. Direct transfer of p65 into T lymphocytes from systemic lupus erythematosus patients leads to increased levels of interleukin-2 promoter activity. Clin Immunol. 2002;103:145–153. doi: 10.1006/clim.2002.5192. [DOI] [PubMed] [Google Scholar]
- Jabbour E. Cortes J. Kantarjian H. Treatment selection after imatinib resistance in chronic myeloid leukemia. Target Oncol. 2009;4:3–10. doi: 10.1007/s11523-008-0100-y. [DOI] [PubMed] [Google Scholar]
- Li Y. Alterations in the expression pattern of TCR zeta chain in T cells from patients with hematological diseases. Hematology. 2008;13:267–275. doi: 10.1179/102453308X343482. [DOI] [PubMed] [Google Scholar]
- Li Y. Chen S. Yang L. Chen S. Lin C. Wang L. Lu Y. Geng S. Du X. Schmidt C.A. Change in expression pattern of TCR-CD3 complex in patients with multiple myeloma. Hematology. 2011a;16:143–150. doi: 10.1179/102453311X12953015767491. [DOI] [PubMed] [Google Scholar]
- Li Y. Geng S. Du X. Chen S. Yang L. Wu X. Li B. Schmidt C.A. Przybylski G.K. Restricted TRBV repertoire in CD4+ and CD8+ T-cell subsets from CML patients. Hematology. 2011b;16:43–49. doi: 10.1179/102453311X12902908411634. [DOI] [PubMed] [Google Scholar]
- Li Y. Geng S. Yin Q. Chen S. Yang L. Wu X. Li B. Du X. Schmidt C.A. Przybylski G.K. Decreased level of recent thymic emigrants in CD4+ and CD8+T cells from CML patients. J Transl Med. 2010;8:47. doi: 10.1186/1479-5876-8-47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mozaffari F. Hansson L. Kiaii S. Ju X. Rossmann E.D. Rabbani H. Mellstedt H. Osterborg A. Signalling molecules and cytokine production in T cells of multiple myeloma-increased abnormalities with advancing stage. Br J Haematol. 2004;124:315–324. doi: 10.1046/j.1365-2141.2003.04789.x. [DOI] [PubMed] [Google Scholar]
- Nambiar M.P. Enyedy E.J. Fisher C.U. Warke V.G. Juang Y.T. Tsokos G.C. Dexamethasone modulates TCR zeta chain expression and antigen receptor-mediated early signaling events in human T lymphocytes. Cell Immunol. 2001;208:62–71. doi: 10.1006/cimm.2001.1761. [DOI] [PubMed] [Google Scholar]
- Nambiar M.P. Fisher C.U. Warke V.G. Krishnan S. Mitchell J.P. Delaney N. Tsokos G.C. Reconstitution of deficient T cell receptor zeta chain restores T cell signaling and augments T cell receptor/CD3-induced interleukin-2 production in patients with systemic lupus erythematosus. Arthritis Rheum. 2003;48:1948–1955. doi: 10.1002/art.11072. [DOI] [PubMed] [Google Scholar]
- Quintas-Cardama A. Cortes J. Molecular biology of bcr-abl1-positive chronic myeloid leukemia. Blood. 2009;113:1619–1630. doi: 10.1182/blood-2008-03-144790. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rappl G. Riet T. Awerkiew S. Schmidt A. Hombach A.A. Pfister H. Abken H. The CD3-zeta chimeric antigen receptor overcomes TCR Hypo-responsiveness of human terminal late-stage T cells. PLoS One. 2012;7:e30713. doi: 10.1371/journal.pone.0030713. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Renner C. Ohnesorge S. Held G. Bauer S. Jung W. Pfitzenmeier J.P. Pfreundschuh M. T cells from patients with Hodgkin's disease have a defective T-cell receptor zeta chain expression that is reversible by T-cell stimulation with CD3 and CD28. Blood. 1996;88:236–241. [PubMed] [Google Scholar]
- Rezvany M.R. Jeddi-Tehrani M. Osterborg A. Kimby E. Wigzell H. Mellstedt H. Oligoclonal TCRBV gene usage in B-cell chronic lymphocytic leukemia: major perturbations are preferentially seen within the CD4 T-cell subset. Blood. 1999;94:1063–1069. [PubMed] [Google Scholar]
- Sakurai H. Suzuki S. Kawasaki N. Nakano H. Okazaki T. Chino A. Doi T. Saiki I. Tumor necrosis factor-alpha-induced IKK phosphorylation of NF-kappaB p65 on serine 536 is mediated through the TRAF2, TRAF5, and TAK1 signaling pathway. J Biol Chem. 2003;278:36916–36923. doi: 10.1074/jbc.M301598200. [DOI] [PubMed] [Google Scholar]
- Savona M. Talpaz M. Getting to the stem of chronic myeloid leukaemia. Nat Rev Cancer. 2008;8:341–350. doi: 10.1038/nrc2368. [DOI] [PubMed] [Google Scholar]
- Schmitz M.L. Bacher S. Dienz O. NF-kappaB activation pathways induced by T cell costimulation. FASEB J. 2003;17:2187–2193. doi: 10.1096/fj.02-1100rev. [DOI] [PubMed] [Google Scholar]
- Smahel M. Antigens in chronic myeloid leukemia: implications for vaccine development. Cancer Immunol Immunother. 2011;60:1655–1668. doi: 10.1007/s00262-011-1126-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stroncek D.F. Berger C. Cheever M.A. Childs R.W. Dudley M.E. Flynn P. Gattinoni L. Heath J.R. Kalos M. Marincola F.M. Miller J.S. Mostoslavsky G. Powell D.J., Jr. Rao M. Restifo N.P. Rosenberg S.A. O'Shea J. Melief C.J. New directions in cellular therapy of cancer: a summary of the summit on cellular therapy for cancer. J Transl Med. 2012;10:48. doi: 10.1186/1479-5876-10-48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Torelli G.F. Paolini R. Tatarelli C. Soriani A. Vitale A. Guarini A. Santoni A. Foa R. Defective expression of the T-cell receptor-CD3 zeta chain in T-cell acute lymphoblastic leukaemia. Br J Haematol. 2003;120:201–208. doi: 10.1046/j.1365-2141.2003.04044.x. [DOI] [PubMed] [Google Scholar]
- Xue S. Gillmore R. Downs A. Tsallios A. Holler A. Gao L. Wong V. Morris E. Stauss H.J. Exploiting T cell receptor genes for cancer immunotherapy. Clin Exp Immunol. 2005;139:167–172. doi: 10.1111/j.1365-2249.2005.02715.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yin Q. Zha X. Yang L. Chen S. Zhou Y. Wu X. Li Y. Generation of diffuse large B cell lymphoma-associated antigen-specific Valpha6/Vbeta13+T cells by TCR gene transfer. J Hematol Oncol. 2011;4:2. doi: 10.1186/1756-8722-4-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zha X. Chen S. Yang L. Li B. Chen Y. Yan X. Li Y. Characterization of the CDR3 structure of the Vbeta21 T cell clone in patients with P210(BCR-ABL)-positive chronic myeloid leukemia and B-cell acute lymphoblastic leukemia. Hum Immunol. 2011;72:798–804. doi: 10.1016/j.humimm.2011.06.015. [DOI] [PubMed] [Google Scholar]