Abstract
Objective
The purpose of this study was to determine psychiatrists' barriers, attitudes, and practices regarding cardiac screening prior to initiating stimulants in children with attention-deficit/hyperactivity disorder.
Background
Professional and federal oversight organizations recently have debated the evidence regarding sudden cardiac death (SCD) risk with stimulants, and have published guidelines recommending cardiac screening. It is not known how psychiatrists have responded.
Methods
This study was a cross-sectional survey of 1,600 randomly-selected U.S. members of the American Academy of Child and Adolescent Psychiatry. Analyses included descriptive statistics and logistic regression.
Results
Response rate was 40%; 96% met eligibility criteria. Barriers to identifying cardiac disorders in general included ability to perform a routine physical examination (74%) and care coordination with primary care providers (35%). Only 27% agreed that SCD risk warranted cardiac assessment. Prior to starting a patient on stimulants, 95% of psychiatrists obtained a routine history. The majority either conducted (9%), or relied on primary care providers to conduct (67%) a physical examination; 26% did not obtain a physical examination. Nineteen percent of psychiatrists ordered an electrocardiogram (ECG), of those, non-mutually exclusive reasons for ordering an ECG included standard practice procedure (62%), clinical findings (27%), medicolegal considerations (25%), and guideline adherence (24%). On multivariate modeling, psychiatrists were less likely to conduct cardiac screening themselves if in private practice (referent: academic medical center), if >50% of their patients had private insurance, or if they believed their ability to perform a physical examination to be a barrier. When modeling cardiac screening performed by any healthcare professional (e.g., psychiatrist, primary care practitioner), screening was less likely if the psychiatrist was practicing in a community mental health center (referent: academic medical center), was male, or if >50% of that psychiatrist's patients had private insurance.
Conclusion
Findings suggest the tacit interplay between primary care and psychiatry for the assessment and management of medical risks associated with psychotropic medications should be improved, and solutions prioritized.
Introduction
Collaborative relationships between child and adolescent psychiatrists (hereafter “psychiatrists”) and primary care providers (PCPs) have been the focus of several recent policy statements by the American Academy of Child and Adolescent Psychiatry (AACAP) (Committee on Collaboration with Medical Professionals, 2010), the American Academy of Pediatrics (AAP) (AACAP Committee on Health Care Access and Economics 2009; Committee on Psychosocial Aspects of Child and Family Health and Task Force on Mental Health 2009), and the Institute of Medicine (Institute of Medicine [US] 2001). These statements emphasize the substantial mental health needs among children and adolescents (hereafter “children”) and barriers to meeting these needs, such as lack of insurance coverage for adequate mental health services and child mental health professional shortages. For the most part, proposed solutions have focused on early identification and provision of interventions in primary care settings to improve the identification and management of pediatric mental health needs. However, collaborative relationships between psychiatrists and PCPs also may be critical to ensure the early assessment and management of medical risks of mental health treatments, particularly psychotropic medications.
Recent concerns regarding possible cardiovascular risks associated with the use of stimulants among children with attention-deficit/hyperactivity disorder (ADHD) provide an opportunity to examine current processes for assessing and managing potential medical risks related to psychotropic medications. Following a 2004 post-marketing report citing a possible link between Adderall XR and increases in sudden cardiac death (SCD) among children with ADHD (U.S. Food and Drug Administration 2005), a series of conflicting guidelines were rapidly issued by the Food and Drug Administration (Rosack 2006; U.S. Food and Drug Administration 2005), the American Heart Association (AHA) (Vetter et al. 2008), and the AAP (Perrin et al. 2008). In response to this debate, the AAP and the AHA jointly published a statement in 2008, endorsed by the AACAP, stating that the evidence does not currently indicate the need for a routine electrocardiogram (ECG) prior to initiating stimulants but recommending that care providers “carefully assess children for heart conditions who need to receive treatment with drugs for ADHD”(American Academy of Pediatrics/American Heart Association 2008). Several recent studies have also examined the link between stimulants and SCD with mixed results; one case–control study suggested an association (Gould et al. 2009) whereas two retrospective cohorts from administrative data were either inconclusive (Schelleman et al. 2011), or failed to find an association (Cooper et al. 2011). Further, a recent cost-effective analysis found that the monetary cost of targeted cardiac screening of children initiating stimulants for ADHD was high compared with other potential life-saving interventions (Leslie et al. 2012a).
How psychiatrists have responded to this debate and defined their role and responsibilities with respect to other healthcare professionals is unknown. The 2007 AACAP practice parameter for the assessment and treatment of ADHD notes that stimulant package inserts recommend that these medications generally not be prescribed for children with symptoms suggestive of significant cardiac disease, and that such patients should be referred to a cardiologist for evaluation (Pliszka 2007). Both the AHA and the AAP recommend routine physical examinations for children with ADHD when stimulant medications are being considered (Perrin et al. 2008; Vetter et al. 2008). However, these organizations are silent on what components of an evaluation should be completed by a prescribing psychiatrist, and the interplay between psychiatry and primary care in terms of assessing these children for potential cardiac risks.
We undertook this study to elucidate psychiatrists' 1) barriers to identifying cardiac disorders among their patients in general, 2) attitudes about stimulants and cardiac risks, and 3) current cardiac screening practices for children with ADHD for whom stimulants are being considered, with a particular focus on the psychiatrist's practice setting. Over the past decade, psychiatrists have worked increasingly in academic medical centers rather than in private office practices. This professional trend has the potential to promote collaborative care with other healthcare professions (Ranz et al. 2006). We hypothesized that practice setting would be associated with cardiac screening practices. Specifically, we hypothesized that cardiac screening practices would more likely be completed by either prescribing psychiatrists, their office staff, or other healthcare providersfor those psychiatrists working in academic medical centers compared with psychiatrists in private practice, community mental health settings, or residential/inpatient settings. We anticipated that psychiatrists in academic medical centers would be more likely to conduct cardiac screening practices because of 1) their exposure to new information, either in the form of practice parameters, guidelines, or current research, and 2) co-location with other healthcare providers permitting collaborative models of care.
Methods
Participants and procedures
Sixteen hundred subjects were randomly selected from the AACAP's membership of >7,400. Eligible physicians provided direct patient care to children ages 5–18 years with ADHD. Retirees, trainees, fellows, and non U.S.-based physicians were excluded. It should be noted that although the AACAP provided a randomly selected sample from their membership, the mailing list did not permit rigorous comparisons between respondents and nonrespondents, except for training date.
Following institutional review board (IRB) approval (including a waiver of informed consent), a self-administered, cross-sectional survey was mailed in three rounds (United States Postal Service [USPS], surveymonkey.com, USPS) at 3-week intervals between March and May 2011. No incentive was provided, at the request of the AACAP.
Measures
The survey was developed by researchers at Tufts Medical Center. We based the content of the survey on a review of the literature, a previously conducted survey of pediatricians (Leslie et al. 2012b), and consultation with AACAP staff and the AACAP Department of Research, Training, and Education. We conducted retrospective cognitive debriefing interviews (Collins 2003) with five psychiatrists, and then pilot tested the instrument. The final 30-item questionnaire contained four sections: 1) demographic and setting characteristics, 2) barriers to identifying pediatric cardiac disorders in general in psychiatric practices, 3) attitudes about stimulants and perceived cardiac risks, and 4) current screening practices for a recent patient with ADHD for whom stimulant medications were considered. A copy of the questionnaire is available online. (Fig. S1) (www.liebertonline.com/cap).
Independent variables
The primary independent variable was practice setting, which had five categories: academic medical center, private practice, community mental health center, residential/inpatient facility, and other. These categories were developed in collaboration with the AACAP and pilot tested to assure their relevance to the respondents. The fifth category, other, was dropped for analytic purposes because of its small cell size (n=12), and primarily consisted of respondents who practiced in multiple settings.
Additional independent variables included as covariates were psychiatrists' responses to items about: 1) demographic characteristics (i.e., gender, race/ethnicity, years in practice, age, training date; all variables except for training date obtained from survey); 2) setting characteristics other than practice setting itself (i.e., practice location, patient insurance); 3) reported barriers to the identification of cardiac disorders in general; and 4) reported attitudes toward cardiac risks associated with stimulants (see Table 1). For race/ethnicity, physicians were classified as non-Hispanic/white versus all other ethnicities and races based on their responses to separate questions about ethnicity and race. Although “years in practice” was a continuous variable, it was categorized by quartile to facilitate interpretation. Provider age and training date were collinear with years in practice (r=0.7, p<0.001 and r=0.8, p<0.001, respectively); therefore, they were not included in any analyses, but are reported subsequentlyto describe the sample. As most (92%) participants selected either private or public insurance as response choices, a dichotomous insurance type variable was created: >50% of patients with private insurance versus ≥50% of patients with public insurance, other, or no insurance (hereafter, “mostly privately insured patients” and “mostly publicly insured patients”). Because of the distribution of the responses, the three-level choices for the barrier questions was collapsed into “barrier” (“minor barrier” and “major barrier”) or “not a barrier” and the four-level response set for the attitude questions was collapsed into “agree” (“strongly agree” and “agree”) or “disagree” (“strongly disagree” and “disagree”).
Table 1.
| |
|
Practice settingc |
|||
|---|---|---|---|---|---|
| Variable Overall, n (row %) | Overall, n (column %) 615 (100%) | Academic medical center 89 (15.2%) | Community mental health center 126 (21.5%) | Private 249 (42.5%) | Inpatient or residential 122 (20.8%) |
| Psychiatrist gender | |||||
| Male | 277 (46.5%) | 41 | 55 | 111 | 64 |
| 15.1% | 20.3% | 41.0% | 23.6% | ||
| 46.6% | 44.0% | 44.8% | 52.5% | ||
| Female | 319 (53.5%) | 47 | 70 | 137 | 58 |
| 15.1% | 22.4% | 43.9% | 18.6% | ||
| 53.4% | 56.0% | 55.2% | 47.5% | ||
| Race/Ethnicity | |||||
| Non-Hispanic/White | 423 (73.7%) | 69 | 84 | 181 | 84 |
| 16.5% | 20.1% | 43.3% | 20.1% | ||
| 79.3% | 71.8% | 75.1% | 72.4% | ||
| Other | 151 (26.3%) | 18 | 33 | 60 | 32 |
| 12.6% | 23.1% | 42.0% | 22.4% | ||
| 20.7% | 28.2% | 24.9% | 27.6% | ||
| Years in practice | |||||
| <8 years | 123 (20.7%) | 19 | 31 | 46 | 27 |
| 15.5% | 25.2% | 37.4% | 22.0% | ||
| 21.4% | 24.8% | 18.6% | 22.1% | ||
| 8–13 years | 153 (25.7%) | 20 | 36 | 66 | 24 |
| 13.7% | 24.7% | 45.2% | 16.4% | ||
| 22.5% | 28.8% | 26.7% | 19.7% | ||
| 13–18 years | 168 (28.2%) | 22 | 30 | 74 | 37 |
| 13.5% | 18.4% | 45.4% | 22.7% | ||
| 24.7% | 24.0% | 30.0% | 30.3% | ||
| >18 years | 151 (25.4%) | 28 | 28 | 61 | 34 |
| 18.5% | 18.5% | 40.4% | 22.5% | ||
| 31.5% | 22.4% | 24.7% | 27.9% | ||
| Practice location | |||||
| Urban | 288 (49.7%) | 64 | 65 | 91 | 63 |
| 22.6% | 23.0% | 32.2% | 22.3% | ||
| 74.4% | 52.9% | 37.1% | 53.9% | ||
| Suburban | 226 (39.0%) | 18 | 30 | 140 | 35 |
| 8.1% | 13.5% | 62.8% | 15.7% | ||
| 20.9% | 24.4% | 57.1% | 29.9% | ||
| Rural | 65 (11.2%) | 4 | 28 | 14 | 19 |
| 6.2% | 43.1% | 21.5% | 29.2% | ||
| 4.7% | 22.8% | 5.7% | 16.2% | ||
| Patient insurance | |||||
| >50% with private insurance | 238 (42.7%) | 35 | 2 | 172 | 26 |
| 14.9% | 0.9% | 73.2% | 11.1% | ||
| 42.2% | 1.6% | 77.1% | 22.2% | ||
| ≥50% with public, other, no insurance | 319 (57.3%) | 48 | 120 | 51 | 91 |
| 15.5% | 38.7% | 16.5% | 29.4% | ||
| 57.8% | 98.4% | 22.9% | 77.8% | ||
Bolding indicates p-values ≤0.01 from χ2 test; italics indicates p-value ≤0.05.
Frequencies may not sum to 615 because of missing data.
Respondents who reported working in other settings were not included because of small cell size (n=12).
Dependent variables
Dependent (i.e., outcome) variables in the survey included reported completion of the following cardiac screening practices for a recent patient with ADHD: routine history, vital signs, routine physical examination, and ECG. These variables were based on psychiatrists' recall of their most recent patient seen with ADHD for whom stimulants were considered. This “most recent patient” method has been successfully used to anchor respondents to a particular patient rather than self-reports regarding general practice (Leslie et al. 2012b; Parsons et al. 2007).
To better understand cardiac screening practices performed for the most recent patient and by whom, we created two binary, composite dependent variables. The first variable was assigned a value of “yes” if the psychiatrist or his/her staff conducted any screening practices (i.e., vital signs and/or routine physical examination and/or ECG) and “no” otherwise. Using this dependent variable, we examined the association between practice setting and cardiac screening practices performed by the psychiatrist or his/her staff. The second variable was assigned a value of “yes” if any physician (the psychiatrist or his/her staff, or an off-site physician, including a PCP, emergency room/urgent care physician, or subspecialist) conducted any screening practices (i.e., vital signs and/or routine physical examination and/or ECG), and “no” otherwise. Using this second dependent variable, we examined the association between practice setting and cardiac screening practices performed by any provider at that setting or at another setting.
Statistical analysis
Descriptive statistics (mean, standard deviation [SD], frequency, percent) were computed for demographic and setting characteristics, barriers, attitudes, and practices. Chi-square tests were done to evaluate associations between demographics and setting characteristics and practice setting. Bivariate analyses using logistic regression examined relationships between demographic and setting characteristics and barriers, attitudes, and screening practices.
Separate multivariate logistic regression models were created for the two composite dependent variables. Independent variables that were associated with the two dependent variables at an alpha level of 0.05 on bivariate analyses were included in the multivariate model. Depending upon which main effects were included in the multivariate models, we explored possible two-way interactions between practice setting, gender, patient insurance, and practice location. Variance inflation factors (VIF) were calculated for the final models to assess for multicollinearity with a cutoff of 2.5 (Allison 1999). Odds ratios (OR) and 99% confidence intervals (CI) were reported from bivariate and multivariate logistic regression. We elected to use 99% CIs because of the multiple comparisons conducted. SAS software version 9.2 (Cary, NC) was used to perform all analyses.
Results
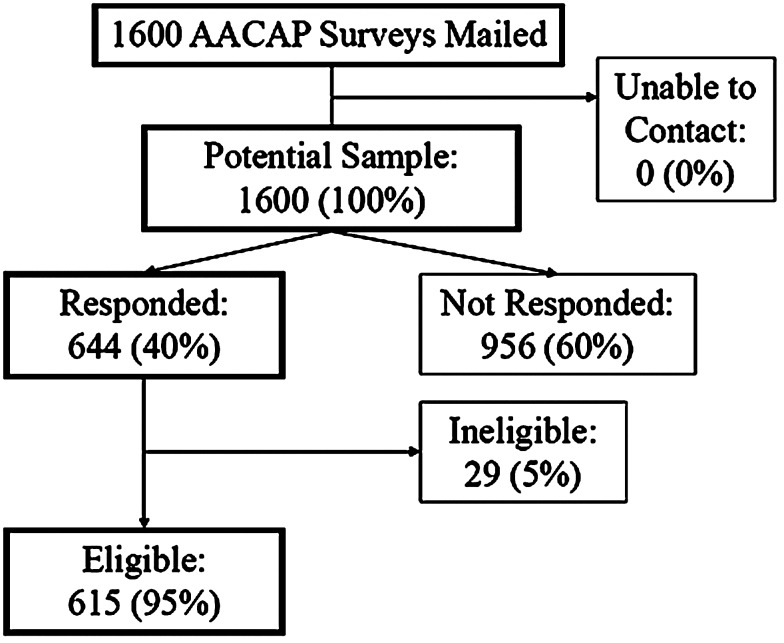
Of the 1,600 subjects, 644 (40%) responded to the survey, the majority by USPS mail. Of the respondents, 615 (95%) met eligibility criteria (Fig. 1). No information was available from the AACAP regarding demographic characteristics of the random sample generated (e.g., gender, age, practice setting, years in practice) except for training date. Respondents and nonrespondents did not differ significantly in time since training, using data provided by the AACAP (results not shown). All analyses discussed subsequently focused on eligible respondents and survey responses.
FIG. 1.
Survey response. AACAP=American Academy of Child and Adolescent Psychiatry.
As Table 1 illustrates, half (53%) of psychiatrists were female and three quarters (74%) were non-Hispanic/white. Respondents had been practicing for a mean of 13.4 years (SD=6.3) and had a mean age of 48.5 (SD=7.3) years. The majority (88%) reported their clinical area as child and adolescent psychiatry; the remainder reported general psychiatry. Private practice (43%) was the most common practice setting. Half (50%) practiced in urban areas. Less than half (43%) reported that their patients were mostly privately insured.
Practice setting was associated with both practice location (i.e., urban, suburban, rural) and patient insurance type (see Table 1). The majority of psychiatrists in private practice (57%) were located in a suburban area where as respondents in community mental health centers (53%), academic medical centers (74%), and residential/inpatient facilities (54%) were located in urban settings (χ2[df=6]=83, p<0.0001). Those in private practice were more likely to report that their patients were mostly privately insured (77%) than those in community mental health centers (2%), academic medical centers (42%), or residential/ inpatient facilities (22%) (χ2[df=3]=212, p<0.0001). There was no difference in practice setting by gender (χ2[df=3]=2.4, p=0.50), race/ethnicity (χ2[df=3]=1.8, p=0.61), or years in practice (χ2[df=9]=7.8, p=0.55). In addition, there was no statistically significant relationship between gender and insurance (χ2[df=1]=2.6, p=0.11) or gender and practice location (χ2[df=2]=1.0, p=0.62). We did find that more physicians in rural settings had mostly publically insured patients (87%), whereas more physicians in suburban settings had mostly privately insured patients (60%) (χ2[df=2]=49.9, p<0.0001).
Barriers to identification of cardiac disorders and attitudes about stimulants and cardiac risks
Few respondents (12%) reported their lack of ability to perform a routine history as a barrier, but approximately three-quarters (74%) did report as a barrier their lack of ability to perform a routine physical examination and over three-quarters reported as a barrier their ability to interpret an ECG (85%) (see Table 2). One-third (35%) reported that coordinating care with a PCP was a barrier. Only 27% agreed that SCD risk was sufficiently high to warrant a cardiac assessment, and 31% agreed that the risk of potential legal liability warranted a cardiac assessment. With regard to the role of an ECG in a cardiac assessment prior to starting stimulants, many (73%) agreed that an ECG did not provide sufficient information to rule out SCD.
Table 2.
Barriers and Attitudes of Identifying Cardiac Disorders by Demographic and Setting Characteristics, OR (99% CI)a
| |
My ability to:b |
Respondents agreeing that:c,d |
|||||
|---|---|---|---|---|---|---|---|
| Variable Overall, n (%) | Perform a routine history is barrier 71 (11.8%) | Perform a routine physical is barrier 445 (73.8%) | Interpret an ECG is barrier 512 (84.8%) | Coordinate care with PCP is barrier 209 (34.6%) | Sufficient SCD risk 161 (26.8%) | Legal liability 184 (30.5%) | ECG does not provide info 441 (73.0%) |
| Practice settinge | |||||||
| Private practice | 0.7 (0.2, 1.9) | 1.1 (0.5, 2.3) | 1.2 (0.5, 2.8) | 0.4 (0.2, 0.7) | 0.9 (0.4, 1.9) | 0.8 (0.4, 1.7) | 0.8 (0.4, 1.7) |
| Community mental health center | 1.2 (0.4, 3.4) | 1.4 (0.6, 3.2) | 0.8 (0.3, 2.2) | 1.3 (0.6, 2.7) | 0.7 (0.3, 1.5) | 0.6 (0.3, 1.3) | 0.9 (0.4, 2.0) |
| Inpatient or residential | 1.1 (0.4, 3.2) | 0.9 (0.4, 2.0) | 1.2 (0.4, 3.2) | 0.6 (0.3, 1.2) | 1.3 (0.6, 3.2) | 0.8 (0.3, 1.7) | 0.6 (0.3, 1.4) |
| Psychiatrist genderf | |||||||
| Male | 1.2 (0.6, 2.4) | 0.7 (0.4, 1.1) | 0.6 (0.3, 1.1) | 1.0 (0.7, 1.6) | 2.5 (1.5, 4.2) | 1.9 (1.2, 3.1) | 0.8 (0.5, 1.3) |
| Race/Ethnicityg | |||||||
| Non-Hispanic/White | 1.2 (0.5, 2.6) | 0.9 (0.5, 1.6) | 0.6 (0.3, 1.3) | 0.7 (0.4, 1.1) | 1.8 (1.0, 3.1) | 2.0 (1.2, 3.2) | 0.8 (0.5, 1.5) |
| Years in practiceh | |||||||
| 8–13 years | 1.8 (0.6, 5.1) | 1.4 (0.7, 2.9) | 0.9 (0.3, 2.2) | 1.1 (0.5, 1.8) | 1.9 (0.9, 3.7) | 1.2 (0.6, 2.3) | 1.2 (0.6, 2.3) |
| 13–18 years | 1.9 (0.7, 5.4) | 1.8 (0.9, 3.8) | 0.8 (0.3, 2.1) | 0.6 (0.3, 1.1) | 1.8 (0.9, 3.5) | 1.3 (0.7, 2.6) | 0.9 (0.5, 1.8) |
| >18 years | 1.2 (0.4, 3.5) | 0.7 (0.4, 1.4) | 0.5 (0.2, 1.2) | 0.6 (0.3, 1.1) | 2.6 (1.3, 5.3) | 1.9 (1.0, 3.9) | 0.8 (0.4, 1.7) |
| Practice locationi | |||||||
| Suburban | 0.8 (0.4, 1.7) | 1.3 (0.8, 2.2) | 1.2 (0.6, 2.3) | 1.0 (0.6, 1.7) | 1.0 (0.6, 1.8) | 0.9 (0.5, 1.4) | 1.2 (0.7, 1.9) |
| Rural | 1.0 (0.3, 3.0) | 1.8 (0.7, 4.3) | 2.0 (0.6, 6.6) | 1.8 (0.9, 3.8) | 0.9 (0.4, 1.9) | 1.0 (0.4, 2.1) | 0.8 (0.3, 1.8) |
| Patient insurancej | |||||||
| >50% with private | 0.6 (0.3, 1.3) | 1.1 (0.7, 1.8) | 1.0 (0.6, 1.9) | 0.6 (0.4, 0.9) | 1.7 (1.0, 2.9) | 1.5 (0.9, 2.5) | 0.9 (0.5, 1.4) |
Bolding indicates p-values ≤0.01; italics indicates p-value ≤0.05.
Barrier includes “minor” and “major” barrier.
Agree includes both “agree” and “strongly agree.”
Variable abbreviations: sufficient SCD risk=the risk for SCD in children is sufficiently high to warrant cardiac assessment before initiating stimulant treatment; legal liability=the risk for potential legal liability is sufficiently high to warrant cardiac assessment in children before initiating stimulant treatment; and ECG does not provide info=an ECG does not provide sufficient information to rule out undetected cardiac disorders in children.
Academic medical center is reference category.
Female is reference category.
Other race/ethnicity is reference category.
<8 years is reference category.
Urban is reference category.
≥50% patients with public, other, no insurance is reference category.
ECG=electrocardiogram; SCD=sudden cardiac death; PCP=primary care physician; OR=odds ratio; CI=confidence interval.
Table 2 also presents bivariate relationships, examined using logistic regression, between practice setting and the other independent variables included as covariates (i.e., demographic and setting characteristics, barriers, and attitudes). Respondents were less likely to report coordinating with the PCP as a barrier if they were in private practice (OR=0.4, 99% CI: 0.2, 0.7, p=0.0002; comparator: academic medical center) or had mostly privately insured patients (OR=0.6, 99% CI: 0.4, 0.9, p=0.003; comparator: mostly publicly insured patients). Respondents more likely to agree that there was sufficient SCD risk to warrant cardiac assessment included males (OR=2.5, 99% CI: 1.5, 4.2, p<0.0001; comparator: females), non-Hispanic/whites (OR=1.8, 99% CI: 1.0, 3.1, p=0.005); comparator: all other races and ethnicities), those practicing for >18 years (OR=2.6, 99% CI: 1.3, 5.3, p=0.0007; comparator: those practicing <8 years), and those with mostly privately insured patients (OR=1.7, 99% CI: 1.0, 2.9, p=0.008). Males (OR=1.9, 99% CI: 1.2, 3.1, p=0.0004), and non-Hispanic/whites (OR=2.0, 99% CI: 1.2, 3.2, p=0.0007) were more likely to agree that legal liability warranted cardiac assessment.
Screening practices for a recent patient with ADHD
We next examined screening practices based on the most recent patient seen for whom ADHD stimulant medications were considered (Table 3). The mean age of the most recent patient was 9.7 (SD=3.1) years and 79% were boys. The majority (74%) of respondents reported having seen this recent patient in the last 2 weeks.
Table 3.
Type and Source of Most Recent Patient Information Overall and by Practice Setting
| Type of patient information | Psychiatrist /staff, n (%) | PCP, n (%) | Other (ED or cardiologist), n (%) | No information collected, n (%) |
|---|---|---|---|---|
| Overall | ||||
| Routine history, n=615a | 591 (96.1%) | 83 (13.5%) | 15 (2.4%) | 19 (3.1%) |
| Vital signs, n=615a | 438 (71.2%) | 142 (23.1%) | 13 (2.1%) | 72 (11.7%) |
| Routine physical examination, n=615a | 53 (8.6%) | 409 (66.5%) | 17 (2.8%) | 161 (26.2%) |
| ECG, n=609b | 39 (6.4%) | 46 (7.6%) | 27 (4.4%) | 497 (81.6%) |
Respondents were allowed to select more than one response; therefore variables are not mutually exclusive.
Respondents were allowed to select only one response; 6 respondents did not specify how ECG was obtained.
PCP=primary care physician; ED=emergency department; ECG=electrocardiogram.
Most respondents reported conducting a routine history (96%) and obtaining vital signs (71%) within their office (conducted by themselves or a staff member). In contrast to obtaining vital signs, only 9% performed a routine physical examination in their office whereas 67% relied on physical examinations completed by a PCP. One-quarter (26%) reported not obtaining a routine physical examination, either conducted by themselves or their staff or by another healthcare provider, before initiating treatment. It should be noted that respondents could check more than one approach to this question and responses therefore do not add to 100%.
Fewer than one-fifth of respondents ordered an ECG (19%). Non-mutually exclusive reasons for ordering an ECG among respondents in this subsample included adherence to standard practice procedures (62%), clinical findings (27%), medicolegal considerations (25%), guidelines and recommendations (24%), and other reasons (24%). Among those ordering an ECG, most relied on a PCP, cardiologist, or emergency department/urgent care clinician to obtain (65%) and read (83%) the ECG, whereas 77% of respondents reported sharing the results with the patient and family themselves (data not shown).
More than half of all respondents (61%) reported discussing SCD risks associated with stimulants with families (data not shown). By comparison, nearly all respondents (97%) warned of weight loss/appetite suppression, 95% mentioned sleep disturbance, and 88% discussed affective symptoms (e.g., moodiness, irritability, suicidality), which were all statistically higher than the percentage discussing SCD (χ2[df=1]=23, p<0.0001; χ2[df=1]=21, p<0.0001; χ2[df=1]=13, p=0.0003, respectively). Other commonly mentioned side effects were exacerbation or precipitation of tics (67%) and delays in linear growth (61%).
Bivariate and multivariate modeling of screening practices and practice setting
We next examined the association between practice setting, our primary independent variable, and the two dependent variables of interest. We also identified any covariates at an alpha level of 0.05 on bivariate analyses for inclusion in multivariate modeling. For the composite dependent variable examining if the respondent or his/her staff performed vital signs and/or a routine physical examination and/or an ECG, the following independent variables were significant (p≤0.05) in bivariate logistic analyses: practice setting, patient insurance, and belief that conducting a physical examination was a barrier (see Table 4). Practice setting remained significant in the multivariate model; specifically, those in private practice were less likely to perform the screening practices themselves than were those in academic medical centers (OR=0.4, 99% CI: 0.2, 1.0, p=0.008). With respect to covariates, respondents with mostly privately insured patients (OR=0.6, 99% CI: 0.3, 1.1, p=0.04) and those who identified conducting a physical as a barrier (OR=0.6, 99% CI: 0.3, 1.1, p=0.02)were less likely to perform screening practices themselves, controlling for the other variables in the model. The interaction term between practice setting and insurance (p=0.61) was not statistically significant. All VIFs were <2.5, indicating no evidence of multicollinearity.
Table 4.
Bivariate and Multivariate Models for Screening Practices Performed by Practice and Demographic Characteristics and Barriers, OR (99% CI)a,b
| |
Model 1: Vital signs, physical, or ECG done by psychiatrist or office staff, n=442/615 (71.9%)c |
Model 2: Vital signs, physical, or ECG done by any physician,n=558/615 (90.7%)d |
||
|---|---|---|---|---|
| Variable | Bivariate | Multivariate | Bivariate | Multivariate |
| Practice settinge | ||||
| Private practice | 0.3 (0.1, 0.7) | 0.4 (0.2, 1.0) | 0.4 (0.1, 1.8) | 0.5 (0.1, 2.6) |
| Community MH center | 0.8 (0.3, 2.1) | 0.7 (0.2, 2.0) | 0.5 (0.1, 2.3) | 0.2 (0.0, 1.5) |
| Residential/inpatient | 0.9 (0.4, 2.4) | 0.8 (0.3, 2.2) | 1.4 (0.2, 8.9) | 1.2 (0.1, 10.8) |
| Psychiatrist genderf | ||||
| Male | 0.9 (0.5, 1.4) | Not included | 0.4 (0.2, 1.0) | 0.4 (0.2, 1.0) |
| Race/Ethnicityg | ||||
| Non-Hispanic/White | 1.0 (0.6, 1.7) | Not included | 0.8 (0.3, 2.1) | Not included |
| Years in practiceh | ||||
| 8–13 years | 0.7 (0.4, 1.5) | Not included | 0.6 (0.1, 2.1) | Not included |
| 13–18 years | 0.9 (0.4, 1.8) | Not included | 0.5 (0.1, 1.9) | Not included |
| >18 years | 0.8 (0.4, 1.7) | Not included | 0.7 (0.2, 2.8) | Not included |
| Practice locationi | ||||
| Suburban | 0.8 (0.5, 1.3) | Not included | 1.6 (0.6, 3.9) | Not included |
| Rural | 1.7 (0.7, 4.3) | Not included | 1.1 (0.3, 4.3) | Not included |
| Patient insurancej | ||||
| >50% with private insurance | 0.4 (0.3, 0.7) | 0.6 (0.3, 1.1) | 0.4 (0.2, 1.0) | 0.3 (0.1, 1.1) |
| Barriersk | ||||
| Perform routine history | 0.9 (0.5, 1.9) | Not included | 0.5 (0.2, 1.5) | Not included |
| Perform routine physical | 0.6 (0.3, 1.1) | 0.6 (0.3, 1.1) | 0.7 (0.3, 1.9) | Not included |
| Interpret ECG | 1.2 (0.7, 2.3) | Notincluded | 1.5 (0.6, 3.9) | Not included |
| Coordinate care with PCP | 1.3 (0.8, 2.1) | Not included | 1.6 (0.7, 4.0) | Not included |
| Attitudesl,m | ||||
| Sufficient SCD risk | 1.1 (0.6, 1.9) | Not included | 1.3 (0.5, 3.3) | Not included |
| Legal liability | 1.1 (0.7, 1.8) | Not included | 1.4 (0.6, 3.5) | Not included |
| ECG does not provide info | 1.3 (0.8, 2.3) | Not included | 0.8 (0.3, 2.1) | Not included |
Only variables that were significant at the 0.05-level for bivariate analyses were included in multivariate analyses.
Bolding indicates p-values ≤to 0.01; italics indicates p-value ≤0.05.
Reference for Model 1 is comparison to an off-site physician(s) or not at all.
Reference for Model 2 is none of the three (vital signs, physical, or ECG) performed by any physician.
Academic medical center is reference category.
Female is reference category.
Other race/ethnicity is reference category.
<8 years is reference category.
Urban is reference category.
≥50% of patients with public, other, no insurance is reference category.
Barrier includes “minor” and “major” barrier.
Agree includes both “agree” and “strongly agree.”
Variable abbreviations: sufficient SCD risk=the risk for SCD in children is sufficiently high to warrant cardiac assessment before initiating stimulant treatment; legal liability=the risk for potential legal liability is sufficiently high to warrant cardiac assessment in children before initiating stimulant treatment; and ECG does not provide info=an ECG does not provide sufficient information to rule out undetected cardiac disorders in children.
ECG=electrocardiogram; MH=mental health; SCD=sudden cardiac death; PCP=primary care physician; OR=odds ratio; CI=confidence interval.
For the composite dependent variable examining if any physician completed vital signs and/or a routine physical examination and/or an ECG, bivariate logistic analyses identified the following as significant (p≤0.05): practice setting, gender, and patient insurance (see Table 4). Multivariate modeling showed community mental health centers were less likely to report having any physician complete these screening practices than were academic medical centers (OR=0.2, 99% CI: 0.0, 1.5, p=0.04). Males (OR=0.4, 99% CI: 0.2, 1.0, p=0.01) and those with mostly privately insured patients (OR=0.3, 99% CI: 0.1, 1.1, p=0.02) were less likely to report having any physician complete these screening practices. The interactions between practice setting and insurance (p=0.77), practice setting and gender (p=0.28), and insurance and gender (p=0.82) were not significant. All VIFs were <2.5, indicating no evidence of multicollinearity.
Discussion
Our findings demonstrate variation in barriers and attitudes with respect to risk assessment and management of cardiac complications associated with stimulant medication. With respect to attitudes, only a little over one-quarter of respondents thought there was sufficient SCD risk to warrant a cardiac assessment; similarly, 27% agreed there was sufficient risk of legal liability. However, psychiatrists' attitudes were comparable to those found in a similar survey fielded with pediatricians, who agreed that the risk for SCD (24%) and legal liability (30%) were sufficiently high to warrant cardiac assessment. Psychiatrists who responded to the current survey also identified their ability to perform a routine physical examination (74%) and interpret an ECG (85%) as barriers to the identification of cardiac disorders in general. Contrastingly, few pediatricians (18%) considered performing an in-depth cardiac history and physical examination as a barrier; however, 71% considered interpreting a pediatric ECG as a barrier (Leslie et al. 2012b).
With regard to cardiac screening practices, >75% of psychiatrists did not obtain a routine physical examination, either conducted by themselves, their office staff, or a PCP or other provider, prior to beginning a patient on stimulants. Of those respondents obtaining a routine physical examination, two thirds relied on PCPs or other providers as part of their current cardiac screening practices prior to starting patient on stimulants. Contrastingly, almost all pediatricians in the previously mentioned study performed a physical examination, although<50% stated that they had completed an in-depth cardiac examination. Fewer than 20% of psychiatrists completed an ECG; which was similar to the rate reported by pediatricians (Leslie et al. 2012b). Those psychiatrists who requested an ECG, however, did see that it was their role to discuss SCD risk and share results of an ECG with families, demonstrating an overall sense of shared responsibility for managing potential medical complications of stimulant treatment. On multivariate modeling, respondents in private practice were less likely to perform cardiac screening practice themselves than were those in academic medical centers (Model 1). However, those in community mental health centers were less likely than those in academic medical centers to report that screening was performed by any provider (Model 2). Respondents with mostly privately insured patients were less likely to perform a screening themselves (Model 1), or to report that screening was performed by any provider (Model 2). In addition, those who identified their ability to conduct a physical as a barrier were less likely to conduct cardiac screening practices themselves (Model 1). Male gender was the only demographic characteristic associated with no provider reportedly completing any screening practice.
Our findings with respect to practice setting do suggest an association between academic medical centers and cardiac screening by the prescribing psychiatrist or another staff member on our first multivariate model as hypothesized. Interestingly, psychiatrists at academic medical centers compared with those in private practice indicated barriers to collaborating with PCPs, suggesting that, at least in some academic medical centers, collaborative care may be the exception rather than the rule despite co-location within the same institution. Psychiatrists with more privately insured patients and male psychiatrists were less likely to obtain cardiac screening by any provider, but our data do not permit further data-driven explanation of these findings. Surprisingly, respondent attitudes about SCD risk were not associated with these cardiac screening practices on multivariate modeling.
Our purpose in this article was not to discuss the appropriateness of cardiac screening prior to starting stimulants but to use this ongoing debate as a mechanism for investigating current prescriber practices with respect to the identification of potential medical complications related to psychotropic medication use. Our findings suggest that that many of the barriers and potential opportunities discussed in the joint AAP/AACAP statement on Mental Health Services in Primary Care (AACAP Committee on Health Care Access and Economics 2009) that focus on the identification and treatment of mental health disorders in primary care also may be particularly salient with respect to risk assessment and management of potential medical complications of psychotropic medications. Collaboration between psychiatrists and PCPs who care for children remains hindered by a number of factors not explored in this survey. These include an overall shortage of child and adolescent psychiatrists to meet the mental health needs of U.S. children (∼7,400 practicing child and adolescent psychiatrists for 15,000,000 U.S. children) (Kim 2003; Center for Workforce Studies 2008) as well as geographic maldistribution in rural areas and urban areas of low socioeconomic status (Thomas and Holzer 2006). In addition, fiscal, structural, and cultural barriers may affect not only the identification and management of mental health needs but the assessment and management of medical complications related to psychotropic medications (Sarvetand Wegner 2010). Last, collaborative care as currently practiced relies heavily on the family as care coordinator and assumes a child's medical health is being adequately monitored (Thomas and Holzer 2006). A recent survey of pediatricians caring for children with ADHD, depression, and anxiety suggested that families often serve as the de facto “primary avenue of communication with the psychiatrist,” however, few pediatricians felt that families were a “dependable means” of communication (Ross et al. 2011). Families and providers also have been found to hold differing opinions with respect to the necessity of a referral and the seriousness of a child's health condition in general (Zuckerman et al. 2011). This reliance on the family as care coordinator may lead to incomplete referrals and potentially missed or delayed identification of medical risks.
Recommendations in the AAP/AACAP statement to improve models of collaborative care include developing communication tools that clarify responsibilities and reasons for consultation, providing reimbursement for collaborative care, developing electronic communication tools, and taking advantage of the federal government's encouragement of computerized medical records under the Affordable Care Act (DeMaso and Martini 2010; U.S. Department of Health & Human Services 2011). For example, in our previous study, both pediatricians and psychiatrists stated that their ability to read an ECG was a barrier to identifying cardiac disorders. As new models of care are developed, it will be important to carefully delineate what practices are completed by which provider. If psychiatrists are relying on PCPs, PCPs may need additional training to identify cardiac disorders or may need to maintain close collaboration with pediatric cardiologists. Proactively planning and implementing these recommendations during this period of enormous change in healthcare delivery may improve coordination of care concerning stimulant medications as well as other psychotropic medications that have potential medical complications. In particular, as electronic medical records are promoted in national policy, we must assure that they promote collaboration between pediatric physical and mental health providers in the comanagement of medical risks of medications.
Other solutions proposed to improve collaboration include implementing community care systems that take a team-based, comprehensive approach and actively involve the patient, family, and community in assessing the physical and mental health needs of a child (Chenven 2010). Fostering professional partnerships between psychiatrists and medical centers, schools, community groups, and state agencies would leverage psychiatrists' expertise, extending comprehensive care and services to all youth in those settings (Zachik et al. 2010). This administrative partnership could also have a policy-level impact with the ultimate goal of meeting children's mental health needs and associated medical needs within an inclusive, standard care program.
Limitations
This cross-sectional survey is not without its limitations. The major limitation of this article is that we recruited respondents using a professional organization's membership mailing list but did not have access to demographic information except for training date. We therefore were able to compare only the training date of our respondents to that of nonrespondents. Respondent gender and ethnicity, requested on the survey itself, were comparable to an available survey of early-career members of the AACAP membership body (Stubbe and Thomas 2002). Respondent gender (46% female) was also comparable to the American Association of Medical Colleges' Physician Specialty Data report, drawing on data from the American Medical Association (AMA) 2008 Masterfile (Center for Workforce Studies 2008). However, the age of our respondents differed from the AMA data; 80% of our sample was <55 years of age compared to the 59% in the AMA sample. A large proportion of our sample included psychiatrists in private practices in suburban areas and therefore may not reflect the perspectives of psychiatrists in rural or underserved urban areas. We would anticipate, however, that given identified shortages of active psychiatrists in these areas, screening practices may be even more unlikely to occur, except if conducted in collaboration with PCPs (Thomas and Holzer 2006). Drawing a random sample from the AACAP membership as a whole should minimize threats to generalizability of our sample to the larger sample of practicing child and adolescent psychiatrists with membership in the AACAP.
There are also several issues regarding our survey and analyses. Although our response rate was only 40%, it is within the range of that for other surveys conducted with physicians (Cummings et al. 2001). The literature suggests that additional mailings might not have increased our response rate but that small incentives might have (Berry and Kanouse 1987; Kellerman and Harold 2001). In addition, although the “most recent patient” method should have reduced physician recall bias, respondents may still have overestimated their screening behavior or the most recent patient seen may not have been representative of the respondent's patient population as a whole. Last, some of our findings were not addressed in the questions included in the survey. For example, we did not ask why psychiatrists did not obtain a routine physical examination before starting a patient stimulants or probe how gender might impact screening practices. Questions such as these, however, may be more suited for qualitative approaches that permit exploration and development of possible hypotheses to be examined in future research (Sofaer 1999).
Conclusion
Our results demonstrate that potential collaborations between psychiatrists and PCPs with respect to the management of medical complications of psychotropic medication use deserve further study. Processes to assure the identification and treatment of medical complications of stimulant medication use as well as other medications such as second generation antipsychotics have not been investigated. Researchis needed to understand psychiatrists' and PCPs' knowledge, attitudes, and practices about medical complications of psychotropic medications in general and to identify strategies to improve collaborative care regarding the identification and management of these complications.
Clinical significance
Recent efforts regarding collaborative care between pediatric primary care and mental health providers have focused on improving early identification of mental health problems and implementing interventions in primary care settings. However, many psychotropic medications have physical health implications that also require collaborative approaches. As a salient example, we examined child and adolescent psychiatrists' attitudes, barriers, and practice patterns with respect to screening for cardiac disorders associated with SCD, prior to beginning stimulant medications. Results suggest substantial variation across respondents in terms of the completion of a history and physical examination, with some respondents completing these themselves, a larger proportion relying on primary care providers, and approximately one fifth not completing a history and physical examination. Collaborative models of care that directly address potential medical implications of psychopharmacological treatment should be prioritized.
Supplementary Material
Acknowledgments
We thank Jennifer Bakan, Doris Hernandez, and Brian Pennarola from the Institute of Clinical Research and Health Policy Studies at Tufts Medical Center for their assistance with data management; Yoshie Davison and Rob Grant from the AACAP Department of Research, Training and Education, for their collaboration; Robin Ruthazer at the Tufts CTSI for statistical support; physicians at the Floating Hospital for Children at Tufts Medical Center for survey cognitive interviewing and piloting; and all the participating psychiatrists for their time and efforts in supporting this research.
Disclosures
No competing financial interests exist
References
- American Academy of Child and Adolescent Psychiatry Committee on Health Care Access and Economics Task Force on Mental Health. Improving mental health services in primary care: Reducing administrative and financial barriers to access and collaboration. Pediatrics. 2009;123:1248–1251. doi: 10.1542/peds.2009-0048. [DOI] [PubMed] [Google Scholar]
- Allison PD. Logistic Regression Using SAS: Theory, Application. Cary, NC: SAS Institute, Inc.; 1999. [Google Scholar]
- American Academy of Pediatrics/American Heart Association: American Academy of Pediatrics/American Heart Association Clarification of Statement on Cardiovascular Evaluation and Monitoring of Children and Adolescents With Heart Disease Receiving Medications for ADHD. 2008. http://www2.aap.org/pressroom/aap-ahastatement.htm. [Jun 15;2011 ]. http://www2.aap.org/pressroom/aap-ahastatement.htm [DOI] [PubMed]
- Berry SH. Kanouse DE. Physician response to a mailed survey: An experiment in timing of payment. Public Opin Q. 1987;51:102–114. [Google Scholar]
- Center for Workforce Studies: 2008 Physician Specialty Data. Washington, DC: Association of American Medical Colleges; 2008. [Google Scholar]
- Chenven M. Community systems of care for children's mental health. Child Adolescent Psychiatr Clin N Am. 2010;19:163–174. doi: 10.1016/j.chc.2009.08.006. [DOI] [PubMed] [Google Scholar]
- Collins D. Pretesting survey instruments: An overview of cognitive methods. Qual Life Res. 2003;12:229–238. doi: 10.1023/a:1023254226592. [DOI] [PubMed] [Google Scholar]
- Committee on Collaboration with Medical Professionals: A Guide to Building Collaborative Mental Health Care Partnerships in Pediatric Primary Care. American Academy of Child and Adolescent Psychiatry; 2010. [Google Scholar]
- Committee on Psychosocial Aspects of Child and Family Health and Task Force on Mental Health: The future of pediatrics: Mental health competencies for pediatric primary care. Pediatrics. 2009;124:410–421. doi: 10.1542/peds.2009-1061. [DOI] [PubMed] [Google Scholar]
- Cooper WO. Habel LA. Sox CM. Chan KA. Arbogast PG. Cheetham TC. Murray KT. Quinn VP. Stein CM. Callahan ST. Fireman BH. Fish FA. Kirshner HS. O'Duffy A. Connell FA. Ray WA. ADHD drugs and serious cardiovascular events in children and young adults. N Engl J Med. 2011;365:1896–1904. doi: 10.1056/NEJMoa1110212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cummings SM. Savitz LA. Konrad TR. Reported response rates to mailed physician questionnaires. Health Serve Res. 2001;35:1347–1355. [PMC free article] [PubMed] [Google Scholar]
- Gould MS. Walsh BT. Munfakh JL. Kleinman M. Duan N. Olfson M. Greenhill L. Cooper T. Sudden death and use of stimulant medications in youths. Am J Psychiatry. 2009;166:992–1001. doi: 10.1176/appi.ajp.2009.09040472. [DOI] [PubMed] [Google Scholar]
- Institute of Medicine (US): Crossing the Quality Chasm: A New Health System for the 21st Century. Washington, DC: National Academies Press; 2001. [PubMed] [Google Scholar]
- Kim WJ. Child and adolescent psychiatry workforce: A critical shortage and national challenge. Acad Psychiatry. 2003;27:277–282. doi: 10.1176/appi.ap.27.4.277. [DOI] [PubMed] [Google Scholar]
- Leslie LK. Cohen JT. Newburger JW. Alexander ME. Wong JW. Sherwin ED. Rodday AM. Parsons SK. Triedman JT. Costs and benefits of targeted screening for causes of sudden cardiac death in children and adolescents. Circulation. 2012;125:2621–2629. doi: 10.1161/CIRCULATIONAHA.111.087940. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leslie LK. Rodday A. Saunders TS. Cohen JT. Wong JB. Parsons SK. Cardiac screening prior to stimulant treatment for ADHD: A survey of US-based pediatricians. Pediatrics. 2012b;129:222–230. doi: 10.1542/peds.2011-1574. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parsons SK. Saiki–Craighill S. Mayer DK. Sullivan AM. Jeruss S. Terrin N. Tighiouart H. Nakagawa K. Iwata Y. Hara J. Grier HE. Block S. Telling children and adolescents about their cancer diagnosis: Cross-cultural comparisons between pediatric oncologists in the US and Japan. Psychooncology. 2007;16:60–68. doi: 10.1002/pon.1048. [DOI] [PubMed] [Google Scholar]
- Perrin JM. Friedman RA. Knilans TK the Black Box Working Group. the Section on Cardiology and Cardiac Surgery: Cardiovascular monitoring and stimulant drugs for attention-deficit/hyperactivity disorder. Pediatrics. 2008;122:451–453. doi: 10.1542/peds.2008-1573. [DOI] [PubMed] [Google Scholar]
- Pliszka S. Practice parameter for the assessment and treatment of children and adolescents with attention-deficit/hyperactivity disorder. J Am Acad Child Adolesc Psychiatry. 2007;46:894–921. doi: 10.1097/chi.0b013e318054e724. [DOI] [PubMed] [Google Scholar]
- Ranz JM. Vergare MJ. Wilk JE. Ackerman SH. Lippincott RC. Menninger WW. Sharfstein SS. Sullivan A. The tipping point from private practice to publicly funded settings for early-and mid-career psychiatrists. Psychiatr Serv. 2006;57:1640–1643. doi: 10.1176/ps.2006.57.11.1640. [DOI] [PubMed] [Google Scholar]
- Rosack J. FDA panel wants warnings on ADHD medications. Psychiatr News. 2006;41:1–36. [Google Scholar]
- Ross WJ. Chan E. Harris SK. Goldman SJ. Rappaport LA. Pediatrician–psychiatrist collaboration to care for children with attention deficit hyperactivity disorder, depression, and anxiety. Clin Pediatr. 2011;50:37–43. doi: 10.1177/0009922810379499. [DOI] [PubMed] [Google Scholar]
- Sarvet BD. Wegner L. Developing effective child psychiatry collaboration with primary care: leadership and management strategies. Child Adolescent Psychiatr Clin N Am. 2010;19:139–148. doi: 10.1016/j.chc.2009.08.004. [DOI] [PubMed] [Google Scholar]
- Schelleman H. Bilker WB. Strom BL. Kimmel SE. Newcomb C. Guevara JP. Daniel GW. Cziraky MJ. Hennessy S. Cardiovascular events and death in children exposed and unexposed to ADHD agents. Pediatrics. 2011;127:1102–1110. doi: 10.1542/peds.2010-3371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sofaer S. Qualitative methods: What are they and why use them? Health Serv Res. 1999;34:1101–1118. [PMC free article] [PubMed] [Google Scholar]
- Stubbe DE. Thomas W. A survey of early-career child and adolescent psychiatrists: Professional activities and perceptions. J Am Acad Child Adolesc Psychiatry. 2002;41:123–130. doi: 10.1097/00004583-200202000-00005. [DOI] [PubMed] [Google Scholar]
- Thomas C. Holzer CE., III The continuing shortage of child and adolescent psychiatrists. J Am Acad Child Adolesc Psychiatry. 2006;45:1023–1031. doi: 10.1097/01.chi.0000225353.16831.5d. [DOI] [PubMed] [Google Scholar]
- U.S. Department of Health & Human Services: HITECH Programs. The Office of the National Coordinator for Health Information Technologies 2011. http://healthit.hhs.gov/portal/server.pt/community/healthit_hhs_gov__hitech_programs/1487. [Aug 5;2011 ]. http://healthit.hhs.gov/portal/server.pt/community/healthit_hhs_gov__hitech_programs/1487
- U.S. Food and Drug Administration. Public Health Advisory for Adderall and Adderall XR. Feb 9, 2005. http://www.fda.gov/Drugs/DrugSafety/PostmarketDrugSafetyInformationforPatientsandProviders/DrugSafetyInformationforHeathcareProfessionals/PublicHealthAdvisories/ucm051672.htm. [Jun 15;2011 ]. http://www.fda.gov/Drugs/DrugSafety/PostmarketDrugSafetyInformationforPatientsandProviders/DrugSafetyInformationforHeathcareProfessionals/PublicHealthAdvisories/ucm051672.htm
- U.S. Food and Drug Administration. Statement on Adderall. 2005. http://www.fda.gov/NewsEvents/Newsroom/PressAnnouncements/2005/ucm108411.htm. [Jun 15;2011 ]. http://www.fda.gov/NewsEvents/Newsroom/PressAnnouncements/2005/ucm108411.htm
- Vetter VL. Elia J. Erickson C. Berger S. Blum N. Uzark K. Webb CL. Cardiovascular monitoring of children and adolescents with heart disease receiving medications for attention deficit/hyperactivity disorder [corrected]: A scientific statement from the American Heart Association Council on Cardiovascular Disease in the Young Congenital Cardiac Defects Committee and the Council on Cardiovascular Nursing. Circulation. 2008;117:2407–2423. doi: 10.1161/CIRCULATIONAHA.107.189473. [DOI] [PubMed] [Google Scholar]
- Zachik AA. Naylor MW. Klaehn RL. Child and adolescent psychiatry leadership in public mental health, child welfare, and developmental disabilities agencies. Child Adolescent Psychiatr Clin N Am. 2010;19:47–61. doi: 10.1016/j.chc.2009.08.007. [DOI] [PubMed] [Google Scholar]
- Zuckerman KE. Nelson K. Bryant TK. Hobrecker K. Perrin JM. Donelan K. Specialty referral communication and completion in the community health center setting. Acad Pediatr. 2011;11:288–296. doi: 10.1016/j.acap.2011.03.002. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.