Abstract
Maspin (SERPINB5) is a member of the Clade B subgroup of the large superfamily of serine protease inhibitors. It is proposed that maspin is a tumor suppressor; however, its molecular role remains to be elucidated. Here we report the characterization of a mouse monoclonal antibody directed against human maspin. This antibody, 16F7, recognizes maspin in both its native and denatured form, unlike several other commercial antibodies tested in this study. It will be a useful and versatile tool for future analyses of the biological function of maspin.
Introduction
Serine protease inhibitors (Serpins) primarily regulate proteases associated with a myriad of biochemical pathways to maintain tissue homeostasis. They have a conserved archetypal fold and employ a unique and extensive irreversible conformational change to inhibit proteases.(1–3) In humans, serpins can be divided into two groups: the extracellular serpins (clade A, C, D-I) and the intracellular serpins (clade B).(4–6) Maspin (SERPINB5) is a non-inhibitory member of the clade B subgroup of the serpin superfamily. It is a 42 kDa nucleocytoplasmic protein and was first identified as a potential tumor suppressor gene in human breast cancer cells.(7) Reintroduction of maspin in cells inhibits tumor growth, cell migration and invasion, and angiogenesis, and increases cell adhesion, all of which are hallmarks of a tumor suppressor. The expression of maspin has been associated with a good prognosis in clinical outcomes in patients with prostate or breast cancer, although this has been debated and it is suggested that the cellular localization of maspin may play a role in determining the prognosis.(8)
Despite the evidence for a pathophysiologically significant role, the molecular function of maspin is unknown. By analogy with most other non-inhibitory serpins, it is thought that maspin most likely interacts with intracellular proteins; a number of candidates have been suggested. Studies investigating maspin distribution and potential binding partners have employed various anti-maspin antibodies.(9–12) The commercial monoclonal antibody (clone G167–70) is most commonly used in these studies, with applications in immunoblotting, immunofluorescence, and immunohistochemistry. Another monoclonal antibody (clone EAW24) has also been used in immunohistochemistry.(13–15) However, most immunoprecipitation studies have used antibodies that are not available commercially.(12,16) Here we report the generation and characterization of a mouse monoclonal antibody that specifically recognizes human maspin and can be used in key analytical techniques. We show that the epitope recognized by this monoclonal antibody, 16F7, is accessible in native maspin (via immunofluorescence and immunoprecipitation), and is not denatured by SDS (via immunoblotting). 16F7 will be a useful tool in the search for proteins interacting with maspin.
Materials and Methods
Cell culture
COS-1 and MDA-MB-231 cells were maintained in Dulbecco's modified Eagle medium (DMEM) supplemented with 10% fetal calf serum and L-glutamine. MCF10A cells were maintained as described.(17) COS-1 cells were transfected using the DEAE-dextran/chloroquine method as described previously.(18)
Antibodies
Mouse maspin monoclonal antibody (clone designation G167–70) was purchased from BD Pharmingen (San Diego, CA), and purified mouse IgG2a monoclonal immunoglobulin isotype standard was purchased from R&D Systems (Minneapolis, MN). Mouse maspin monoclonal antibody (clone designation EAW24) was purchased from Lab Vision (Kalamazoo, MI), and mouse maspin monoclonal antibody (clone designation 3B8.2) was purchased from Chemicon (Billerica, MA). Secondary antibody used in immunoblotting was sheep anti-mouse IgG conjugated to horseradish peroxidise (Chemicon), and secondary antibody used in indirect immunofluorescence was goat anti-mouse IgG conjugated to Alexa 488 (Invitrogen, Carlsbad, CA).
Plasmids
For expression in COS-1 cells, the vector pEGFP-c2 (Clontech, Mountain View, CA) was used to generate a series of plasmids, each encoding a fusion protein comprising the human codon-enhanced green fluorescent protein (eGFP) fused to the N-terminus of a member of the 13 human clade B serpins used for expression in COS-1 cells. The construction of pEGFP/EI, -/PAI-2, -/PI-6, -/PI-8, and -/PI-9 has been described before(19) pEGFP/SCCA-1 was constructed by amplifying SCCA-1 cDNA with the oligonucleotide primers 5′-GGGATCCCATGAATTCACTCAGTG AAGGC-3′ and 5′-GCTCTAGACTACGGGGATGAGAAT CTGCC-3′ from the plasmid pET/SCCA-1 as a template. The resulting product was cloned into pZeroBlunt (Invitrogen), then released and purified as a BamHI and XbaI fragment. This was subcloned into pEGFP-c2, also digested with BamHI and XbaI to generate pEGFP/SCCA-1. SCCA-2 was amplified using the same oligonucleotide primers from pEFBOS/SCCA-2, cloned into pZeroBlunt (Invitrogen), then released and purified as a BamHI and XbaI fragment. This was subcloned into pEGFP-c3 vector digested with BglII and XbaI to generate pEGFP/SCCA-2. Other clade B serpin constructs were similarly generated.
Full length recombinant protein expression and purification
Full length recombinant maspin was expressed and purified as described previously.(20) Briefly, maspin was expressed in Escherichia coli and purified using nickel-nitrilotriacetic acid-agarose, followed by tobacco etch virus (TEV) protease removal of the N-terminal hexahistidine tag. The tag-less recombinant maspin protein was further purified by gel filtration using Superdex 200 (GE Healthcare, Waukesha, WI), and stored in 50 mM Tris-HCl (pH 8.0), 150 mM NaCl, and 5 mM b-mercaptoethanol.
Immunization of mice and production of monoclonal antibody
Female Balb/c mice at 8–9 weeks of age were injected intraperitoneally with 400 mL of an emulsion containing 10 mg of full length recombinant maspin and monophosphoryl-lipid A+trehalose dicorynomycolate adjuvant (MPL+TDM emulsion, Sigma-Aldrich, St. Louis, MO). Mice received three boosts in total, and splenocytes of immunized animals were fused with mouse myeloma Sp2/0-Ag14 at a ratio of 1:5 (splenocyte-myeloma) in 50% PEG. Resulting hybridoma cells were plated on 96-well plates and cultured in AH selective media (DME supplemented with 20% FCS, 1% OPI, 2% AH). After 10 days post-fusion, the hybridoma supernatants were screened by enzyme-linked immunoadsorbent assay (ELISA) against full length recombinant maspin. Positive clones were subcloned and rescreened by ELISA, as well as immunofluorescence on MCF10A cells or COS-1 cells transfected with the vectors pSVTf/maspin or pSHT/maspin, which expresses maspin in its native fold or maspin in an unfolded state, respectively. Both vectors have been described previously.(21) 16F7 monoclonal antibody was typed using IsoStrip Mouse Monoclonal Antibody Isotyping Kit (Roche, Indianapolis, IN).
ELISA
Full length recombinant maspin (0.2 mg/well) was adsorbed to the surface of 96-well Nunc immunoplates (Rochester, NY) overnight at room temperature. To avoid non-specific binding, the plates were blocked with 3% bovine serum albumin (BSA) in phosphate-buffered saline (PBS; 154 mM NaCl, 2.7 mM Na2HPO4, and 1.54 mM KH2PO4) for 1 h at room temperature. The hybridoma supernatants were incubated for 1 h at room temperature, then washed three times with PBS-Tween (0.05% (v/v) Tween in PBS). The plates were incubated for 30 min at room temperature with alkaline-phosphatase-conjugated anti-mouse IgG antibody. After washing with PBS-Tween three times, immunoreactivity was visualized by means of a pNPP phosphate substrate system (Sigma-Aldrich).
Immunoblotting
Whole cell lysates of transfected COS-1 were separated by 12.5% SDS-PAGE and then electrophoretically transferred to nitrocellulose membrane (Pall, Ann Arbor, MI). The membrane was blocked for 1 h at room temperature with blotto (5% skim milk in Tris saline [20 mM Tris-HCl (pH 7.5) and 150 mM NaCl]), and then incubated overnight with 16F7 mouse monoclonal anti-maspin at 1 mg/mL concentration. After washing with Tris-Tween (0.1% (v/v) Tween-20 in Tris saline), the membrane was incubated for 1 h with HRP-conjugated anti-mouse IgG (Chemicon), followed by more washing with Tris-Tween and membrane developed with Western Lightning Plus-ECL enhanced chemiluminescence substrate (Perkin Elmer, Waltham, MA).
Indirect immunofluorescence
Cell monolayers grown on 10-well microscope slides were washed in PBS containing 0.1 mM CaCl2 and 1.0 mM MgCl2 (PBS+), fixed in 4% (w/v) paraformaldehyde in PBS+ for 20 min, and permeabilized by 0.5% (v/v) Triton X-100 in PBS+ for 5 min. For antigen retrieval, cells were either permeabilized by 0.5% SDS in PBS+ instead for 5 min after fixing, or fixed and permeabilized by pre-cooled 1:1 acetone/methanol mix for 2 min. Maspin was detected by incubation of the cells for 30 min with the various mouse monoclonal antibodies at final concentration of 1 mg/mL. After being washed with PBS+, cells were incubated with 1:800 dilution of Alexa 488-conjugated goat anti-mouse IgG (Invitrogen, Carlsbad, CA) for 30 min, then washed with PBS+ and mounted in Mowiol. Cells were examined using a Nikon TE-2000U Eclipse upright fluorescence microscope (Nikon, Melville, NY).
Pulse-chase and immunoprecipitation
MCF10A cells at 80% confluence were labeled for 30 min, and chased for 2 h as described previously.(22) Cells were then either lysed in NP40 lysis buffer (50 mM Tris [pH 8.0], 10 mM EDTA, 1% (v/v) Nonidet P40), or 0.5% (v/v) SDS in 1x NETGEL (150mM NaCl, 5 mM EDTA, 50 mM Tris [pH 7.4], 0.05% (v/v) Nonidet P40, 0.25% (w/v) gelatine, 60 Bloom, 0.02% (w/v) NaN3). SDS lysates were further diluted in 1x NETGEL to a final concentration of 0.05% (v/v) SDS before the addition of Protein A Sepharose beads and antibodies, which were used at a final concentration of 1 mg/mL. Samples were incubated overnight at 4°C with gentle rocking, then analyzed by SDS-PAGE and fluorography.
Immunohistochemistry
Paraffin sections of human prostate isolated from prostate cancer patients with radical prostatectomy were obtained from The Australian Prostate Cancer BioResource in accordance with ethics approval by the Monash University Human Research Ethics Committee (ID: 2004/145 and 2005/442MC). The localization of maspin on human prostate cancer sections was performed by a Leica Bond-max Automated IHC staining system (Wetzlar, Germany), according to manufacturer's instructions, which include dewaxing, antigen unmasking, immunostaining, and haematoxylin counter-staining. Antigens were retrieved by Bond TM epitope retrieval solution 2 (Leica) with 20 min heating. 16F7 was used at a dilution of 1:200 (5 mg/mL), incubated on slides for 60 min, and immunoreactivity detected by Bond polymer refine detection kit (Leica). After immunostaining and counter-staining by the automated system, the slides were gradually dehydrated with alcohol, cleared with Histolene, and covered with DPX mounting solution and a coverslip.
Human breast tissue microarray slides comprising 5 mm sections of duplicates of DCIS, invasive and matched normal needle cores from eight individuals were obtained from the Victorian Cancer BioBank (Project ID: VCB 11011) in accordance with ethics approval from the Monash University Human Research Ethics Committee (ID: CF11/1340–2011000743). Sections were dewaxed and rehydrated in a gradient of ethanol/dH2O, and antigens retrieved by microwaving slides in TE buffer (Tris, EDTA [pH 9.0]). Slides were washed in PBS containing 1% (v/v) Tween (PBST), and blocked with 2% BSA (w/v) in PBS for 10 min. 16F7 was diluted 1:200 in 1% BSA (w/v) in PBS and incubated overnight on sections at room temperature. Bound antibody was detected by biotinylated rabbit anti-mouse IgG (Rockland, Gilbertsville, PA) at 1:200 dilution for 30 min, followed by HRP-conjugated streptavidin (Chemicon) at 1:200 dilution for 30 min, and visualized using the Liquid DAB Chromogen Substrate System (Dako, Glostrup, Denmark). Harris' hematoxylin solution (Sigma-Aldrich) was used as a counter-stain and slides were mounted in MOWIOL reagent. Slides were viewed under an Olympus CKX41 light microscope (Olympus, Center Valley, PA).
Results
To generate monoclonal antibodies against maspin, we first generated full length recombinant maspin protein, expressed and purified from E. coli. An 8- to 9-week-old Balb/c female mouse was then immunized with the recombinant protein by intraperitoneal injection. Hybridomas were obtained by fusing lymphocytes from the immunized mouse with mouse myeloma cells, and clones were tested for the production of monoclonal antibodies that reacted with the full length recombinant maspin by ELISA. ELISA-positive supernatants were further screened by immunofluorescence (IF) analysis of COS-1 cells either overexpressing maspin in its native fold, or expressing unfolded maspin (maspin with a HA signal sequence retained in the ER).(21) Supernatants were also tested on MCF10A cells, which naturally produce maspin. Clones were further tested for cross-reactivity with the other 12 human clade B serpins by indirect immunofluorescence of transfected COS-1 cells. Multiple sequence alignment of the 13 human clade B serpins shows many areas of conserved sequences,(1) and maspin adopts this typical native serpin fold,(20) raising the possibility that an antibody raised to maspin will recognize other serpins. None of the clones showed cross-reactivity. Clones were then subcloned, and tested by ELISA, immunofluorescence, immunoblotting, and immunoprecipitation. 16F7 was chosen for purification and concentration, as it was able to immunoprecipitate better than the other subclones. As 16F7 is an IgG2a monoclonal antibody, an IgG2a isotype control was included in experiments outlined below.
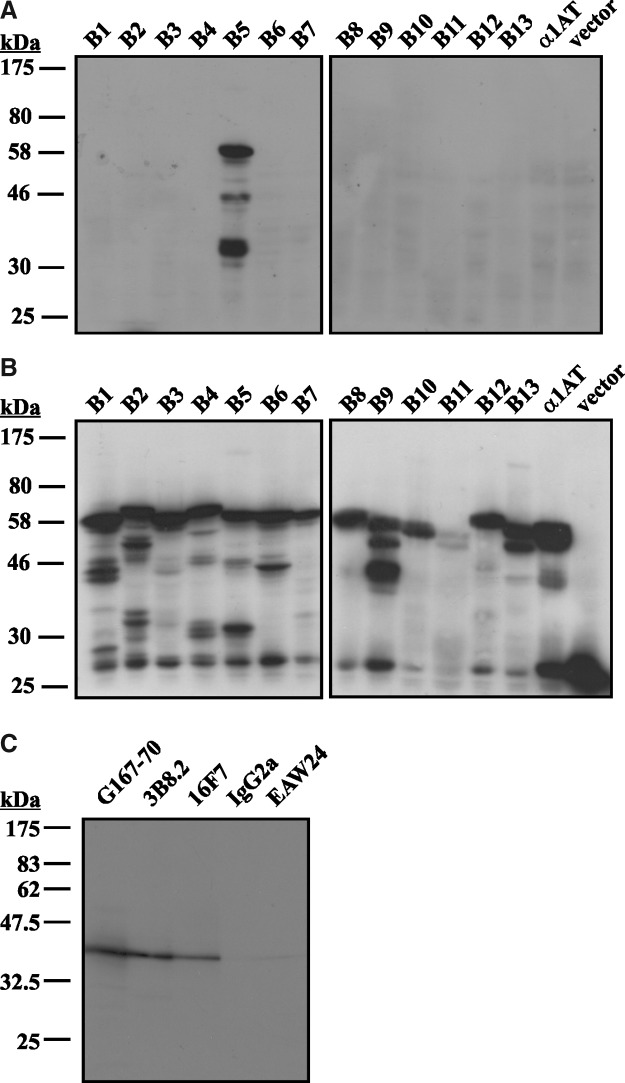
We illustrate here the characterization of 16F7. To confirm that 16F7 only recognizes maspin, we transfected COS-1 cells with eGFP fusions of all 13 human members of the clade B serpins, and immunoblotted cell lysates with 16F7. As shown in Figure 1A (top panel), 16F7 is specific for maspin. While maspin is a 42 kDa protein, the addition of the eGFP tag produces a protein at 67 kDa. The smaller molecular weight species represent breakdown of the protein due to overexpression in COS-1 cells. The blot was stripped and reprobed for GFP to show that all 13 members of the clade B serpins were transfected and that similar amounts of protein were loaded on the SDS-PAGE (Fig. 1B, bottom panel). The construct that encoded serpin B11 did not produce as much fusion protein as the other constructs, but longer exposures of the blot show the presence of serpin B11 (data not shown).
FIG. 1.
16F7 specifically detects maspin. (A) Lysate samples of COS-1 cells transiently expressing eGFP fusions of all 13 human clade B serpins or vector alone control were equally loaded onto a 12.5% SDS-PAGE and immunoblotted with 16F7. Maspin appears as a ∼60 kDa product due to eGFP tag. (B) The same blots were stripped and reprobed for GFP as a loading control. (C) Lysate samples of MCF10A cells were separated via 12.5% SDS-PAGE and immunoblotted with the indicated monoclonals or isotype control.
Once we ascertained that 16F7 is specific for maspin, we next tested the ability of 16F7 to detect endogenous maspin in MCF10A cells compared to other commercially available mouse monoclonals. As shown in Figure 1C, 16F7 is comparable to mouse monoclonal clone G167–70 (BD Pharmingen) and 3B8.2 (Chemicon) at detecting endogenous maspin by immunoblotting. Mouse monoclonal clone EAW24 (Lab Vision) could only detect maspin very weakly; we did not test this antibody in further applications.
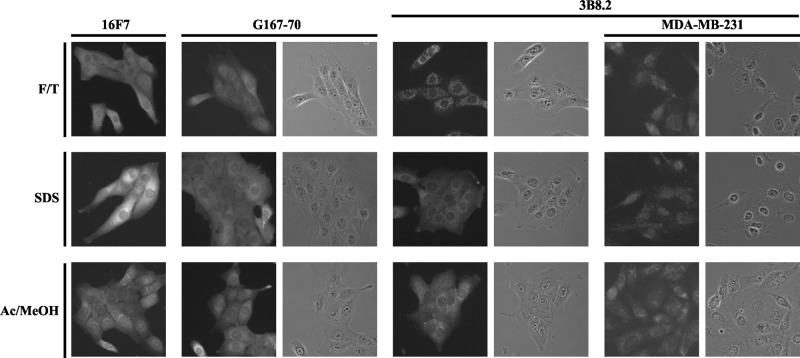
We then tested 16F7 for its ability to detect maspin via indirect immunofluorescence. To do this, we used MCF10A cells, which express maspin endogenously, and compared 16F7 to the other commercially available mouse monoclonals tested on immunoblotting. Cells were fixed and permeabilized using three different methods: paraformaldehyde-triton; paraformaldehyde-triton with SDS antigen retrieval; and acetone-methanol. We showed that 16F7 detects endogenous maspin in MCF10A cells with better clarity than G167–70 in cells fixed and permeabilized by paraformaldehyde-triton, as well as in cells treated with SDS, showing the expected nucleocytoplasmic staining (Fig. 2, top and middle rows).
FIG. 2.
16F7 detects intracellular, endogenous maspin via indirect immunofluorescence. MCF10A cells were fixed with 4% paraformaldehyde and permeabilized with 10% (v/v) Triton X-100 alone (F/T; top row) or with 0.5% (v/v) SDS (middle row), or treated with 1:1 acetone-methanol (Ac/MeOH; bottom row), then probed for maspin with G167–70, 3B8.2, or 16F7. Staining by 3B8.2 is non-specific in F/T and SDS antigen retrieval methods, as shown by staining patterns in MDA-MB-231 cells, a line that does not endogenously express maspin.
The commercially available 3B8.2 detected maspin poorly in paraformaldehyde-fixed cells, with SDS only marginally improving the labeling (Fig. 2). Although it improved when cells were fixed with acetone-methanol, it did not perform better than 16F7 or G167–70 (Fig. 2). In addition, the punctate pattern of labeling seen with 3B8.2, especially in cells fixed and permeabilized with paraformaldehyde-triton, with or without SDS antigen retrieval treatment, is due to non-specific binding, as shown in the similar pattern of staining in MDA-MB-231 cells, which do not express maspin(23) (Fig. 2, final two columns). Altogether, these results show that 16F7 is better at detecting maspin than these two commercially available mouse monoclonal antibodies, and that 3B8.2 is not specific for maspin.
Immunoprecipitation (IP) is routinely used to study protein-protein interactions and identify binding partners of a protein. To keep a protein-protein complex intact, most IPs are performed in buffers that do not contain SDS. As we have seen in Figure 2, the two commercially available monoclonals we tested require at least SDS-based antigen retrieval or the denaturation of maspin for successful binding, which would not be useful in an IP assay. We tested 16F7 in IP of maspin from NP40 and SDS lysates of MCF10A cells and compared it to G167–70 and 3B8.2. Only 16F7 is able to IP maspin in native (non-SDS) conditions (Fig. 3, left panel). However, it is unable to do so in IP buffers containing SDS (Fig. 3, right panel). This is probably due to denaturation of antibody molecules by SDS. In contrast, G167–70 was only able to IP maspin from SDS lysates, although with reduced efficacy (Fig. 3). We could not successfully apply 3B8.2 in this technique (Fig. 3).
FIG. 3.
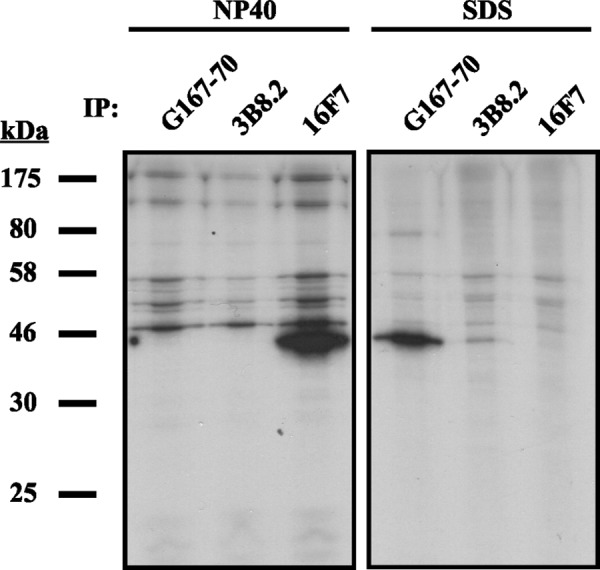
16F7 immunoprecipitates native maspin. MCF10A cells were radiolabeled then lysed by either 1% NP40 (v/v) or 0.5% (v/v) SDS, and maspin immunoprecipitated from the lysates with 16F7 or other commercially available monoclonals.
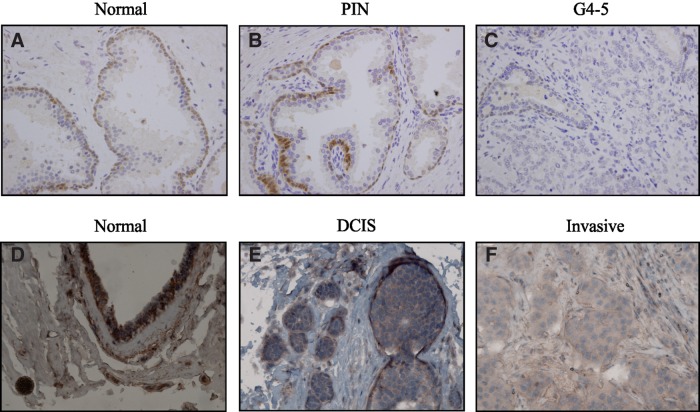
Immunohistochemistry is used to study the distribution and localization of proteins in tissues and has been widely used to study alterations in maspin distribution and subcellular localization in neoplastic disease. Most studies on maspin have been performed on normal and cancer cell lines from breast and prostate, and maspin was originally identified as a potential tumor suppressor in breast cancer.(7) We therefore tested the ability of 16F7 to identify maspin via immunohistochemistry of normal breast and prostate sections, as well as on different grades of cancer of these tissues. Staining with 16F7 showed that maspin localizes to basal cells in normal tissues of the prostate (Fig. 4A) and to myoepithelial cells of the breast (Fig. 4D). Maspin is gradually lost as cancer progresses from prostate intraepithelial neoplasia (PIN) to high-grade tumor in the prostate, and from ductal carcinoma in situ (DCIS) to invasive cancer in the breast (Fig. 4B, C, E, F). The decline in maspin expression is accompanied by the loss of basal and myoepithelial cells in the prostate and breast, respectively. It is important to note that the difference in the intensity of staining between the prostate and breast tissue sections is not indicative of the level of maspin expression in each organ, but a result of differing staining methods. The localization of maspin seen here in the breast and the prostate agrees with previous reports,(14,24,25) showing that 16F7 performs comparably in this application to other antibodies.
Fig. 4.
16F7 detects maspin in prostate and breast tissue sections via immunohistochemistry. Maspin localizes to basal cells of normal prostate (A) and prostate intraepithelial neoplasia (PIN) (B). In normal breast (D) and ductal carcinoma in situ (DCIS) (E), maspin localizes to myoepithelial cells, and slightly in carcinoma cells. Maspin expression is decreased in grade 4–5 prostate cancer (C) and invasive breast cancer (F).
Discussion
An antibody that can detect a protein either in its native or denatured state, and thus applicable to a wide range of analytical techniques, is a versatile tool for examining biological function and molecular mechanism. Here we have characterized a new monoclonal antibody to maspin, and have shown that it performs well in immunoblotting, indirect immunofluorescence, immunoprecipitation, and immunohistochemistry.
Despite the availability of many commercial antibodies raised against maspin, many fail to detect maspin in its native fold in cells. As we have shown here (Fig. 3), 0.05% (v/v) SDS is required to enable G167–70 and 3B8.2 to detect maspin, suggesting that they are unlikely to be useful for detecting native maspin bound to interacting proteins. By contrast, 16F7 recognizes both native and SDS-denatured maspin, suggesting it binds a linear surface epitope. It may, therefore, serve as a useful tool for elucidating the function of maspin through identification of its intracellular partners.
Acknowledgments
This work was supported by the National Health and Medical Research Council of Australia (program grant 490900). We thank Alan Sawyer and the Monash University Monoclonal Antibody Technology Facility for assistance with the production of 16F7.
Author Disclosure Statement
The authors declare that there is no conflict of interest in the publication of this article.
References
- 1.Silverman GA. Whisstock JC. Askew DJ. Pak SC. Luke CJ. Cataltepe S. Irving JA. Bird PI. Human clade B serpins (ov-serpins) belong to a cohort of evolutionarily dispersed intracellular proteinase inhibitor clades that protect cells from promiscuous proteolysis. Cell Mol Life Sci. 2004;61:301–325. doi: 10.1007/s00018-003-3240-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Silverman GA. Whisstock JC. Bottomley SP. Huntington JA. Kaiserman D. Luke CJ. Pak SC. Reichhart JM. Bird PI. Serpins flex their muscle: I. Putting the clamps on proteolysis in diverse biological systems. J Biol Chem. 2010;285:24299–24305. doi: 10.1074/jbc.R110.112771. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Whisstock JC. Silverman GA. Bird PI. Bottomley SP. Kaiserman D. Luke CJ. Pak SC. Reichhart JM. Huntington JA. Serpins flex their muscle: II. Structural insights into target peptidase recognition, polymerization, and transport functions. J Biol Chem. 2010;285:24307–24312. doi: 10.1074/jbc.R110.141408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Law RH. Zhang Q. McGowan S. Buckle AM. Silverman GA. Wong W. Rosado CJ. Langendorf CG. Pike RN. Bird PI. Whisstock JC. An overview of the serpin superfamily. Genome Biol. 2006;7:216. doi: 10.1186/gb-2006-7-5-216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Irving JA. Pike RN. Lesk AM. Whisstock JC. Phylogeny of the serpin superfamily: implications of patterns of amino acid conservation for structure and function. Genome Res. 2000;10:1845–1864. doi: 10.1101/gr.gr-1478r. [DOI] [PubMed] [Google Scholar]
- 6.Silverman GA. Bird PI. Carrell RW. Church FC. Coughlin PB. Gettins PG. Irving JA. Lomas DA. Luke CJ. Moyer RW. Pemberton PA. Remold-O'Donnell E. Salvesen GS. Travis J. Whisstock JC. The serpins are an expanding superfamily of structurally similar but functionally diverse proteins. Evolution, mechanism of inhibition, novel functions, and a revised nomenclature. J Biol Chem. 2001;276:33293–33296. doi: 10.1074/jbc.R100016200. [DOI] [PubMed] [Google Scholar]
- 7.Zou Z. Anisowicz A. Hendrix MJ. Thor A. Neveu M. Sheng S. Rafidi K. Seftor E. Sager R. Maspin, a serpin with tumor-suppressing activity in human mammary epithelial cells. Science. 1994;263:526–529. doi: 10.1126/science.8290962. [DOI] [PubMed] [Google Scholar]
- 8.Goulet B. Chan G. Chambers AF. Lewis JD. An emerging role for the nuclear localization of maspin in the suppression of tumor progression and metastasis. [Special issue on asilomar chromatin.] Biochem Cell Biol. 2011;90(1):22–38. doi: 10.1139/o11-053. [DOI] [PubMed] [Google Scholar]
- 9.Bailey CM. Khalkhali-Ellis Z. Kondo S. Margaryan NV. Seftor RE. Wheaton WW. Amir S. Pins MR. Schutte BC. Hendrix MJ. Mammary serine protease inhibitor (Maspin) binds directly to interferon regulatory factor 6: identification of a novel serpin partnership. J Biol Chem. 2005;280:34210–34217. doi: 10.1074/jbc.M503523200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Yin S. Li X. Meng Y. Finley RL., Jr Sakr W. Yang H. Reddy N. Sheng S. Tumor-suppressive maspin regulates cell response to oxidative stress by direct interaction with glutathione S-transferase. J Biol Chem. 2005;280:34985–34996. doi: 10.1074/jbc.M503522200. [DOI] [PubMed] [Google Scholar]
- 11.Li X. Yin S. Meng Y. Sakr W. Sheng S. Endogenous inhibition of histone deacetylase 1 by tumor-suppressive maspin. Cancer Res. 2006;66:9323–9329. doi: 10.1158/0008-5472.CAN-06-1578. [DOI] [PubMed] [Google Scholar]
- 12.Cella N. Contreras A. Latha K. Rosen JM. Zhang M. Maspin is physically associated with [beta]1 integrin regulating cell adhesion in mammary epithelial cells. Faseb J. 2006;20:1510–1512. doi: 10.1096/fj.05-5500fje. [DOI] [PubMed] [Google Scholar]
- 13.Umekita Y. Ohi Y. Sagara Y. Yoshida H. Expression of maspin predicts poor prognosis in breast-cancer patients. J Intl Cancer. 2002;100:452–455. doi: 10.1002/ijc.10500. [DOI] [PubMed] [Google Scholar]
- 14.Umekita Y. Yoshida H. Expression of maspin is up-regulated during the progression of mammary ductal carcinoma. Histopathology. 2003;42:541–545. doi: 10.1046/j.1365-2559.2003.01620.x. [DOI] [PubMed] [Google Scholar]
- 15.Umekita YD. Ohi YD. Souda MD. Rai YD. Sagara YD. Tamada SD. Tanimoto AD. Maspin expression is frequent and correlates with basal markers in triple-negative breast cancer. Diagn Pathol. 2011;6:36. doi: 10.1186/1746-1596-6-36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Li X. Kaplun A. Lonardo F. Heath E. Sarkar FH. Irish J. Sakr W. Sheng S. HDAC1 inhibition by maspin abrogates epigenetic silencing of glutathione S-transferase pi in prostate carcinoma cells. Mol Cancer Res. 2011;9:733–745. doi: 10.1158/1541-7786.MCR-10-0505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Debnath J. Muthuswamy SK. Brugge JS. Morphogenesis and oncogenesis of MCF-10A mammary epithelial acini grown in three-dimensional basement membrane cultures. Methods. 2003;30:256–268. doi: 10.1016/s1046-2023(03)00032-x. [DOI] [PubMed] [Google Scholar]
- 18.Chu M. Bird CH. Teasdale M. Bird PI. Turnover of thrombomodulin at the cell surface occurs at a similar rate to receptors that are not actively internalized. Thromb Haemost. 1998;80:119–127. [PubMed] [Google Scholar]
- 19.Bird CH. Blink EJ. Hirst CE. Buzza MS. Steele PM. Sun J. Jans DA. Bird PI. Nucleocytoplasmic distribution of the ovalbumin serpin PI-9 requires a nonconventional nuclear import pathway and the export factor Crm1. Mol Cell Biol. 2001;21:5396–5407. doi: 10.1128/MCB.21.16.5396-5407.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Law RH. Irving JA. Buckle AM. Ruzyla K. Buzza M. Bashtannyk-Puhalovich TA. Beddoe TC. Nguyen K. Worrall DM. Bottomley SP. Bird PI. Rossjohn J. Whisstock JC. The high resolution crystal structure of the human tumor suppressor maspin reveals a novel conformational switch in the G-helix. J Biol Chem. 2005;280:22356–22364. doi: 10.1074/jbc.M412043200. [DOI] [PubMed] [Google Scholar]
- 21.Teoh SS. Whisstock JC. Bird PI. Maspin (SERPINB5) is an obligate intracellular serpin. J Biol Chem. 2010;285:10862–10869. doi: 10.1074/jbc.M109.073171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Teoh SS. Whisstock JC. Bird PI. Maspin (SERPINB5) is an obligate intracellular serpin. J Biol Chem. 2010;285:10862–10869. doi: 10.1074/jbc.M109.073171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Sheng S. Carey J. Seftor EA. Dias L. Hendrix MJ. Sager R. Maspin acts at the cell membrane to inhibit invasion and motility of mammary and prostatic cancer cells. Proc Natl Acad Sci USA. 1996;93:11669–11674. doi: 10.1073/pnas.93.21.11669. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Lovric E. Gatalica Z. Eyzaguirre E. Kruslin B. Expression of maspin and glutathionine-S-transferase-pi in normal human prostate and prostatic carcinomas. Appl Immunohistochem Mol Morphol. 2010;18:429–432. doi: 10.1097/PAI.0b013e3181dbc77e. [DOI] [PubMed] [Google Scholar]
- 25.Maass N. Teffner M. Rosel F. Pawaresch R. Jonat W. Nagasaki K. Rudolph P. Decline in the expression of the serine proteinase inhibitor maspin is associated with tumour progression in ductal carcinomas of the breast. J Pathol. 2001;195:321–326. doi: 10.1002/path.948. [DOI] [PubMed] [Google Scholar]