Abstract
TonB is known to be a bacterial periplasmic protein that transduces proton from the inner membrane to the outer membrane receptor in complex with the ExbB and ExbD proteins. Actinobacillus pleuropneumoniae TonB2 protein is the second TonB protein that is important for iron acquisition and virulence. The TonB2 protein was verified to be immunogenic and could afford partial protection for animals from lethal infection. In the present study, the recombinant TonB2 (rTonB2) was overexpressed in Escherichia coli BL21(DE3) and purified. The rTonB2 was then used as antigen to immunize BALB/c mice for the production of monoclonal antibodies (MAb). Four clones of TonB2-specific MAb secretion hybridomas—2F2, 2G8, 3D2, and 6F10—were selected. The MAbs 2F2, 3D2, and 6F10 were classified as IgG1 isotype and 2G8 was of IgG2a isotype. Western blot and ELISA results indicated that MAbs had specific binding activity to rTonB2. The MAbs generated here will be used for further functional analyses of the TonB2 protein.
Introduction
Iron is an essential element in bacteria for a number of redox processes.(1) It is highly insoluble in its ferric (Fe3+) form at physiological pH. To ensure that there is sufficient iron for the requirement of cellular growth, host-adapted pathogens have evolved strategies for high-affinity Fe3+uptake. For example, they use host chelators or their self-synthesized small-molecular siderophores for iron acquisition.(1) However, transposition of iron from the outer membrane (OM) receptors to the cytoplasmic membrane (CM) is energy dependent. It is usually considered that the energy for this process is provided by TonB, which spans the periplasm and interacts with OM-embedded sideropher-binding receptors, and its accessory proteins ExbB and ExbD in the CM. TonB is charged by the passage of a proton through the ExbB/D complex and the energized TonB may propagate conformational changes to OM siderophore receptors, resulting in the release of siderophore into the periplasm.(2)
TonB homologues have been identified as widely distributed among Gram-negative bacteria, and except for their function as an energy transducer during iron-chelator transduction, they mediate drug and solvent tolerance(3,4) and are essential for virulence in pathogenic bacteria.(5,6)
A. pleuropneumoniae is the causative agent of porcine pleuropneumonia, a highly contagious and often fatal disease that leads to great economic losses in industrialized pig production worldwide.(7) Two TonB (TonB1 and TonB2) systems have been identified in A. pleuropneumoniae.(8,9) The TonB2 system is required for transferrin uptake and essential for virulence.(9) The TonB2 protein was confirmed as immunogenic and could provide partial protection for animals against lethal challenge.(10) However, molecular mechanisms of TonB2-mediated energy transduction for iron acquisition and the rationale of immunogenicity of TonB2 were undetermined. Therefore, in this article, the overexpressed A. pleuropneumoniae TonB2 protein in E. coli was used as antigen to immunize BALB/c mice for the production of monoclonal antibodies (MAb). Four hybridoma cell lines secreting MAbs against TonB2 were selected and then the MAbs were characterized.
Materials and Methods
Cell culture and preparation of rTonB2 protein
SP2/0 cells were maintained in RPMI-1640 with 10% fetal calf serum (FCS). The cell line and the hybridoma were cultured in humidified chamber with 5% CO2 at 37°C. The tonB2 gene was amplified by PCR method from the genomic DNA of A. pleuropneumoniae 4074 (serovar 1) with the following primers: forward: 5′GGG GGA TCC ATG AAG AAA AAA CAT TCT CG 3′, with BamHI site (underlined); reverse: 5′GGG AAG CTT TTA TTC AAT CGA GAA TTT CAC C 3′, with HindIII site (underlined). Then the amplicon was restricted by BamHI and HindIII and ligated into the prokaryotic expression vector pET-28a, resulting in plasmid pET-TonB2. E. coli BL21(DE3) carrying the recombinant expression plasmid pET-TonB2 was grown in LB media supplemented with appropriate antibiotics (kanamycin, 25 μg/mL) at 37°C to A600=0.6, and induced with 0.8 mmol/L isopropyl-1-thio-β-D-galactopyranoside (IPTG) at 37°C for 6 h. Recombinant rTonB2 fusion protein with 6×His-tag was expressed as soluble protein after induction. The protein was purified by Ni2+ affinity chromatography (Qiagen, Hilden, Germany) and then verified by sodium dodecyl sulfate polyacrylamide gel electropheresis (SDS-PAGE).
Mice and immunization
BALB/c mice (female) were purchased from the Centre for Disease Control and Prevention of Hubei Province. Mice were maintained under standard animal housing conditions, with a temperature of 22±1°C and a regular 12-h light/12-h dark cycle and allowed free access to food and water. The animal experiments were conducted in accordance with the Guide for the Care and Use of Laboratory Animals established by the Centre for Disease Control and Prevention of Hubei Province. Six-week-old BALB/c mice were injected subcutaneously three times with 75 μg rTonB2 at an interval of 2 weeks. Complete Freund's adjuvant and incomplete Freund's adjuvant (Sigma-Aldrich, St. Louis, MO) were used in the first immunization and the subsequent two booster shots, respectively. A final immunization with 75 μg rTonB2 was given intravenously 3 days before euthanasia.
Cell fusion
Cell preparation and fusion were performed as described previously.(11) Briefly, spleens from BALB/c mice immunized with rTonB2 were harvested, and splenocytes were prepared in Hank's salt solution (Invitrogen, Carlsbad, CA). For hybridoma preparation, splenocytes were fused with mouse myeloma cells SP2/0 at a ratio of 10:1 in RPMI-1640 medium at 37°C. The splenocytes and myeloma cell mixture were centrifuged and resuspended in 1 mL 50% PEG1450 solution (Sigma-Aldrich, St. Louis, MO) over 1 min, followed by serial addition of 3 mL RPMI-1640 over 3 min and 10 mL RPMI-1640 over 1 min, with gentle stirring. Fused cells were centrifuged at 1000 rpm for 5 min and resuspended in 200 mL of RPMI-1640 medium, which contained hypoxanthine, aminopterin, and thymidine (Sigma-Aldrich) and 10% fetal calf serum. Fused cells were then plated at 100 μL/well into 96-well cell culture plates (Falcon, BD Biosciences, Auckland, New Zealand). Cells were incubated at 37°C, with 5% CO2 for 14 days. Supernatant of positive wells were tested for antibody production by ELISA screening. Positive clones were cloned three times by limiting dilution using spleen feeders as filter cells.
ELISA screening
To select the positive hybridomas, 96-well plates were coated with 0.4 μg rTonB2 diluted in 100 μL of coating buffer (50 mM sodium carbonate buffer, pH 9.6) at 4°C overnight. The coated plates were washed three times with PBST (PBS with 0.05% Tween-20) and blocked for 1 h with blocking buffer (5% skimmed milk in PBST), then washed three times with PBST. Cell culture supernatants (100 μL aliquots) were added and incubated at 37°C for 30 min. After four washes, 100 μL of horseradish peroxidase-conjugated secondary antibody (Southern Biotechnology Associates, Birmingham, AL), diluted to 1:5000 in PBST, was added to each well and the plates were incubated in 37°C for 30 min. Next, plates were washed five times with PBST, and ELISAs were developed using 100 μL 3,3’,5,5’-Tetramethylbenzidine (TMB) color development solution (Biotime Institute of Biotechnology, Haimen, Jiangsu, China); the catalytic reaction was stopped by 50 μL 1% SDS and read at 630 nm.
Determination of MAb specificity
The specificity of the MAbs was evaluated by the ELISA methods described above with rTonB2 and Western blot (WB), while the same molar concentration of E. coli BL21(DE3) cell lysate containing pET-28a was used as a negative control. For WB analysis, the protein samples were dissolved in protein loading buffer, immediately boiled for 5 min, and then separated by 12% SDS-PAGE. After separation, the proteins were transferred to a nitrocellulose membrane. The membrane was washed once with TBST (10 mM Tris-HCl [pH 8.0], 150 mM NaCl, 0.05% Tween-20) for 10 min and blocked with 5% skimmed milk in TBST at 4°C overnight. Then the blot was rinsed twice with TBST and incubated with MAbs against rTonB2 at room temperature for 30 min, followed by secondary antibody for 30 min, and then washed. Finally, immunoreactive sites in the membrane were revealed by TMB reagent.
Results
Preparation of rTonB2
The recombinant TonB2 protein was expressed in E. coli BL21(DE3) after induction by IPTG and separated by SDS-PAGE. As shown in Figure 1, the rTonB2 exhibited an apparent molecular weight of 45 kDa, which was 10 kDa larger than the deduced molecular weight of rTonB2 (Fig. 1, lane 2). Since the molecular mass of rTonB2 was predicted to be 30 kDa, the molecular mass of fusion tag was about 4 kDa. The correctness of the band was confirmed by sequence analysis and WB using porcine anti-A. pleuropneumoniae polyclonal antibody (data not shown). The recombinant protein that existed in the supernatant of the E. coli cell lysate was purified with Ni2+ affinity chromatography, and the protein was then found to have a relatively high purity (Fig. 1, lane 3).
FIG. 1.
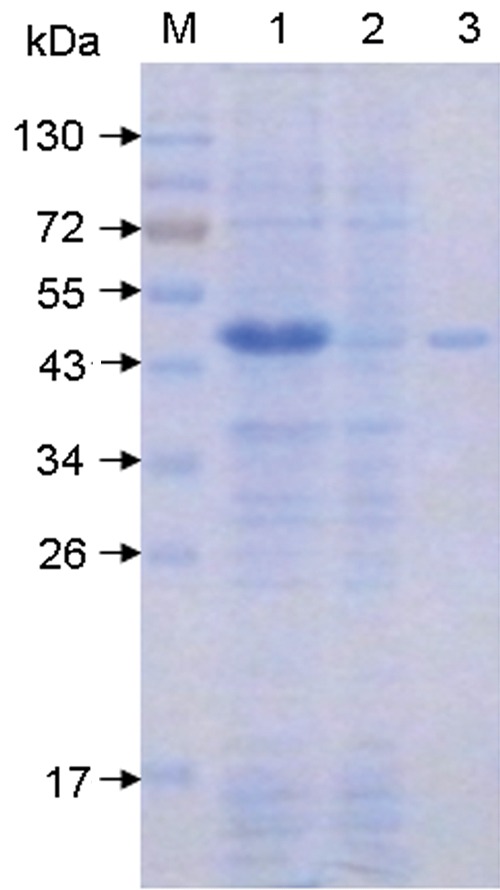
Expression and purification of the rTonB2 protein. Protein samples were separated by SDS-PAGE and stained with Coomassie brilliant blue. Lane M, pre-stained protein marker (Fermentas, Vilnius, Lithuania); lane 1, successful induction of rTonB2 in E. coli BL21(DE3) transformed by pET-TonB2; lane 2, lysate of E. coli BL21(DE3) containing plasmid pET-28a; lane 3, rTonB2 was purified by Ni2+ affinity chromatography column.
Immunization
75 μg purified rTonB2 was administrated to mice with and without adjuvant. Blood was collected from the tail vein before every immunization and before euthanasia, and serum samples were prepared. Antibody titers against the antigen were evaluated by indirect ELISA. Results indicated that all the immunized mice had high antibody titers (from 1:10000 to 1:100000) after three booster immunizations.
Preparation of hybridoma cell lines, screening, and MAb characterization
At the end of the immunization period, the splenocytes from the BALB/c mice with the highest levels of antibodies were prepared and used as fusion partner cells for SP2/0 myeloma cells through conventional hybridoma technology. Supernatants from the positive wells were detected by ELISA. Eventually, four hybridomas secreting the MAbs against rTonB2 were selected following limiting dilution analysis and designated as 2F2, 2G8, 3D2, and 6F10, respectively. Isotyping results indicated that MAbs 2F2, 3D2, and 6F10 were of subclass IgG1 and 2G8 was of subclass IgG2a.
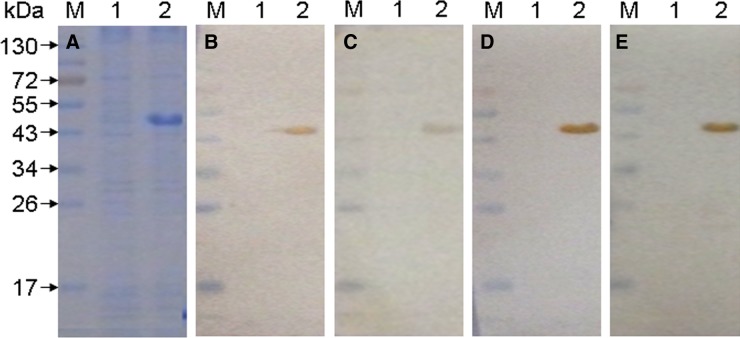
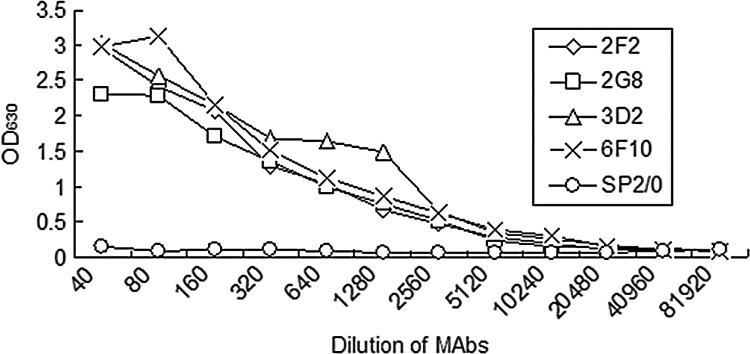
The specificity of the MAbs obtained here was further investigated by WB and ELISA analyses. Results showed that all four MAbs do not react with E. coli BL21(DE3) lysate containing plasmid pET-28a (Fig. 2, lane 1), but they were directed against the rTonB2 (Fig. 2, lane 2). Hybridization bands at approximately 45 kDa were observed, consistent with the expected site of the recombinant proteins (Fig. 2, lane 2). The affinity of MAbs to rTonB2 was measured by ELISA using serial dilutions of MAbs. The results indicated that these MAbs had high titers with the protein, and the supernatant from SP2/0 myeloma cell culture was negative in the ELISA analysis (Fig. 3).
FIG. 2.
Expression of rTonB2 (A) and WB analysis of MAbs (B–E). Protein samples were separated by SDS-PAGE and transferred to nitrocellulose membranes, then reacted with MAbs 2F2 (B), 2G8 (C), 3D2 (D), and 6F10 (E), separately. Lane M, pre-stained protein marker; lane 1, E. coli BL21(DE3) cell lysate containing plasmid pET-28a; lane 2, rTonB2.
FIG. 3.
MAb titers against rTonB2. MAbs were collected from the supernatants of hybridomas and serially diluted, then added into the rTonB2-coated 96-well ELISA plates, respectively. Supernatant from SP2/0 cells was used as negative control. The ELISA analyses were performed at least in triplicate.
Discussion
A. pleuropneumoniae causes porcine pleuropneumonia, which leads to severe economic losses for the worldwide pig industry. Increased antibiotic resistance of A. pleuropneumoniae presents a threat to the drug treatment of the disease.(12) Of late, more attention has been focused on vaccination prevention of bacterial infection. Understanding the pathogenesis of the bacterium is one of the most important aspects for vaccine development. To date, infection of A. pleuropneumoniae has been extensively studied. Data suggest that pathogenesis is a complex process involving various virulence factors of the bacterium.(13) However, many cellular mechanisms and pathways involved in the infection cycle of A. pleuropneumoniae are still unknown. Limited knowledge of the characterizations of A. pleuropneumoniae has been used to design new vaccines, which can offer partial protection for animals against all serotypes, such as killed bacterins, subunit vaccines, and live attenuated vaccines.(14,15)
As a wide existence of TonB proteins in Gram-negative bacteria and their important roles for growth and pathogenicity of many microorganisms, TonB family proteins have been extensively investigated,(16) including A. pleuropneumoniae TonB proteins. The TonB2 protein of A. pleuropneumoniae shares some common features of the TonB family proteins. It can be divided into three functional domains according to the amino acid sequence analysis. First is the N-terminal region (1 to 33 aa), which may be required for anchoring TonB in the CM, and which participates in energy transduction processes with ExbB/ExbD.(17) The N-terminal region is followed by a proline rich region (34 to 186 aa), which contains a series of Pro-Glu and Pro-Lys repeats, and is proposed to be essential for linking energy transduction to spatially distant receptors.(18) The C-terminal domain of TonB2 (187 to 285 aa) may interact directly with the TonB-dependent receptors, particularly with TonB box.(19) However, it appears that there are some differences between TonB2 and TonB1. For example, unlike tonB1, tonB2 is not positionally associated with any iron uptake genes, and TonB2 seems to be more important for A. pleuropneumoniae survival in vivo and cannot be substituted by TonB1, especially for the acquisition of heam, hemoglobin, and ferrichrome.(9) In addition, antigenic prediction by EMBOSS explorer indicates that many potential epitopes were included in TonB2, and that the TonB2 protein was demonstrated to be immunogenic and can be used as a potential vaccine candidate.(10)
To further elucidate the mechanisms of TonB2 in cellular processes and infection, MAbs against rTonB2 were produced in this study. WB and ELISA results showed that the MAbs have high affinity, stability, and specificity. Therefore, the MAbs obtained here will provide useful tools in the functional studies of TonB2. Since there is little information about the structural features of TonB2 protein, except for a C-terminal domain of the TonB2 protein (residues 106–206) of Vibrio anguillarum,(20) so the structural analysis of the TonB2 protein of A. pleuropneumoniae was carried out. However, we found that the crystal of the protein was difficult to obtain. Then the MAbs could be used as stable and specific ligands to co-crystallization and to obtain the crystal of TonB2 for structural analysis. The resolution of the TonB2 structure will be useful for function elucidation and structure-based drug design in the future, as well as to explain the molecular basis of the differences between TonB2 and other TonB proteins. In addition, generation of these four MAbs enables us to map the epitopes of TonB2 and obtain an insight into the immunogenicity of TonB2. Relevant additional work is now underway.
Acknowledgments
We would like to thank Yan Chen and Min Xiang (College of Veterinary Medicine, Huazhong Agricultural University) for screening hybridomas, and Huizi Yang and Shuyi Feng (College of Life Science, Central China Normal University) for assistance with WB analysis of MAbs. This research is supported by grants from the National Nature Science Foundation of China (nos. 31101820, 31172352 and 30970109), the Hubei Province Technology Program (no. 2011BBB082), and the Fundamental Research Funds for the Central Universities (no. CCNU11A01022).
Author Disclosure Statement
The authors have no financial interests to disclose.
References
- 1.Wandersman C. Delepelaire P. Bacterial iron sources: from siderophores to hemophores. Annu Rev Microbiol. 2004;58:611–647. doi: 10.1146/annurev.micro.58.030603.123811. [DOI] [PubMed] [Google Scholar]
- 2.Ferguson AD. Deisenhofer J. Metal import through microbial membranes. Cell. 2004;116:15–24. doi: 10.1016/s0092-8674(03)01030-4. [DOI] [PubMed] [Google Scholar]
- 3.Godoy P. Ramos-González MI. Ramos JL. Involvement of the TonB system in tolerance to solvents and drugs in Pseudomonas putida DOT-T1E. J Bacteriol. 2001;183:5285–5292. doi: 10.1128/JB.183.18.5285-5292.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Zhao Q. Poole K. A second tonB gene in Pseudomonas aeruginosa is linked to the exbB and exbD genes. FEMS Microbiol Lett. 2000;184:127–132. doi: 10.1111/j.1574-6968.2000.tb09002.x. [DOI] [PubMed] [Google Scholar]
- 5.Torres AG. Redford P. Welch RA. Payne SM. TonB-dependent systems of uropathogenic Escherichia coli: aerobactin and heme transport and TonB are required for virulence in the mouse. Infect Immun. 2001;69:6179–6185. doi: 10.1128/IAI.69.10.6179-6185.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Stork M. DiLorenzo M. Mouriño S. Osorio CR. Lemos ML. Crosa JH. Two tonB systems function in iron transport in Vibrio anguillarum. Infect Immun. 2004;72:7326–7329. doi: 10.1128/IAI.72.12.7326-7329.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Fenwick B. Henry S. Porcine pleuropneumonia. J Am Vet Med Assoc. 1994;204:1334–1340. [PubMed] [Google Scholar]
- 8.Tonpitak W. Thiede S. Oswald W. Baltes N. Gerlach GF. Actinobacillus pleuropneumoniae iron transport: a set of exbBD genes in transcriptionally linked to the tbpB gene and required for utilization of transferrin-bound iron. Infect Immun. 2000;68:1164–1170. doi: 10.1128/iai.68.3.1164-1170.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Beddek AJ. Sheehan BJ. Bossé JT. Rycroft AN. Kroll JS. Langford PR. Two TonB systems in Actinobacillus pleuropneumoniae: their roles in iron acquisition and virulence. Infect Immun. 2004;72:701–708. doi: 10.1128/IAI.72.2.701-708.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Liu J. Chen Y. Yuan F. Hu L. Bei W. Chen H. Cloning, expression, and characterization of TonB2 from Actinobacillus pleuropneumoniae and potential use as an antigenic vaccine candidate and diagnostic marker. Can J Vet Res. 2011;75:183–190. [PMC free article] [PubMed] [Google Scholar]
- 11.Deng Y. Lei M. Zhang W. Li W. Zhou E. Liu Z. Qi C. Monoclonal antibodies directed against Fpr3 protein as molecular chaperones. Hybridoma. 2011;30:491–493. doi: 10.1089/hyb.2011.0053. [DOI] [PubMed] [Google Scholar]
- 12.Kang M. Zhou R. Liu L. Langford PR. Chen H. Analysis of an Actinobacillus pleuropneumoniae multi-resistance plasmid, pHB0503. Plasmid. 2008;61:135–139. doi: 10.1016/j.plasmid.2008.11.001. [DOI] [PubMed] [Google Scholar]
- 13.Chiers K. De Waele T. Pasmans F. Ducatelle R. Haesebrouck F. Virulence factors of Actinobacillus pleuropneumoniae involved in colonization, persistence and induction of lesions in its porcine host. Vet Res. 2010;41:65. doi: 10.1051/vetres/2010037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Haesebrouck F. Pasmans F. Chiers K. Maes D. Ducatelle R. Decostere A. Efficacy of vaccines against bacterial diseases in swine: what can we expect? Vet Microbiol. 2004;100:255–268. doi: 10.1016/j.vetmic.2004.03.002. [DOI] [PubMed] [Google Scholar]
- 15.Ramjeet M. Deslandes V. Gouré J. Jacques M. Actinobacillus pleuropneumoniae vaccines: from bacterins to new insights into vaccination strategies. Anim Health Res Rev. 2008;9:25–45. doi: 10.1017/S1466252307001338. [DOI] [PubMed] [Google Scholar]
- 16.Noinaj N. Guillier M. Barnard TJ. Buchanan SK. TonB-dependent transporters: regulation, structure, and function. Annu Rev Microbiol. 2010;64:43–60. doi: 10.1146/annurev.micro.112408.134247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Larsen RA. Postle K. Conserved residues Ser(16) and His(20) and their relative positioning are essential for TonB activity, cross-linking of TonB with ExbB, and the ability of TonB to respond to proton motive force. J Biol Chem. 2001;276:8111–8117. doi: 10.1074/jbc.M007479200. [DOI] [PubMed] [Google Scholar]
- 18.Evans JS. Levine BA. Trayer IP. Dorman CJ. Higgins CF. Sequence-imposed structural constraints in the TonB protein of E. coli. FEBS Lett. 1986;208:211–216. doi: 10.1016/0014-5793(86)81020-1. [DOI] [PubMed] [Google Scholar]
- 19.Gunter K. Braun V. In vivo evidence for FhuA outer membrane receptor interaction with the TonB inner membrane protein of Escherichia coli. FEBS Lett. 1990;274:85–88. doi: 10.1016/0014-5793(90)81335-l. [DOI] [PubMed] [Google Scholar]
- 20.López CS. Peacock RS. Crosa JH. Vogel HJ. Molecular characterization of the TonB2 protein from the fish pathogen Vibrio anguillarum. Biochem J. 2009;418:49–59. doi: 10.1042/BJ20081462. [DOI] [PMC free article] [PubMed] [Google Scholar]