Abstract
BACKGROUND
The arrhythmogenic potential of short QT intervals has recently been highlighted in patients with a short QT syndrome. Drug-induced QT-interval prolongation is a known risk factor for ventricular tachyarrhythmias. However, reports on drug-induced QT-interval shortening are rare and proarrhythmic effects remain unclear.
OBJECTIVE
Recently, rufinamide, a new antiepileptic drug for the add-on treatment of Lennox-Gastaut syndrome, was approved in the European Union and the United States. Initial trials showed drug-induced QT-interval shortening. The aim of our study was to evaluate the effects of rufinamide on QT intervals in patients with difficult-to-treat epilepsies.
METHODS
Nineteen consecutive patients with Lennox-Gastaut syndrome and other epilepsy syndromes were included (n = 12 men; mean age 41 ± 12 years). QRS, QT, and Tpeak-Tend intervals were analyzed before and during rufinamide treatment.
RESULTS
The mean QT interval shortened significantly following rufinamide administration (QT interval 349 ± 23 ms vs 327 ± 17 ms; corrected QT interval 402 ± 22 ms vs 382 ± 16 ms; P = .002). Tpeak-Tend intervals were 79 ± 17 ms before and 70 ± 20 ms on treatment (P = .07). The mean reduction of the corrected QT interval was 20 ± 18 ms. During follow-up (3.04 ± 1.09 years), no adverse events including symptomatic cardiac arrhythmias or sudden cardiac deaths were observed.
CONCLUSION
QTc-interval shortening following oral rufinamide administration in a small patient group was not associated with significant clinical adverse effects. These observations nothwithstanding, the ability of rufinamide to significantly shorten the QT interval portends a potential arrhythmogenic risk that may best be guarded against by periodic electrocardiographic recordings.
Keywords: Drug-induced QT-interval shortening, Short QT syndrome, SUDEP, sudden cardiac death, Proarrhythmia, Rufinamide
Introduction
The short QT syndrome (SQTS) represents a primary electrical disease characterized by a substantial shortening of the QT interval, atrial and ventricular effective refractory periods, and a risk for atrial and ventricular tachyarrhyth-mias.1,2 Up to now, gain-of-function mutations of cardiac potassium channels as well as loss-of-function mutations of cardiac L-type calcium channels have been described in SQTS with an increased risk for sudden cardiac death (SCD).3–5 However, the yield of genetic screening in familial SQTS is only 23% in index patients.6
Bellet et al7 reported for the first time drug-induced QT-interval prolongation in 1951 (quinidine). Meanwhile a variety of drugs have been identified to prolong the QT interval. The risk for proarrhythmia (torsades de pointes) significantly increases with a corrected QT (QTc) interval of >500 ms.8 The regulatory consequence of the potential life-threatening proarrhythmic side effects of drugs led to the implementation of a thorough QT/QTc analysis during the development of new drugs (International Conference on Harmonisation 2005, ICH E14).9 However, drug-induced QT-interval shortening is a rarely observed phenomenon.10 Furthermore, proarrhythmic effects of drug-induced QT-interval shortening have been shown only with digitalis.11
Recently, rufinamide, probably the first QT-interval-shortening drug in the post–ICH E14 period, was approved in the European Union by the European Medicines Agency (in 2007) and in the United States by the Food and Drug Administration (in 2008).10 Rufinamide is structurally distinct from other antiepileptic drugs (AEDs) and is used as an adjunctive treatment for seizures associated with Lennox-Gastaut syndrome (LGS) in children older than 4 years as well as in adults. This severe epileptic encephalopathy is characterized by multiple types of generalized seizures, especially drop attacks and tonic seizures, delayed psychomotor development, and behavioral and personality disorders. Clinical trials with rufinamide showed a concentration-dependent degree of QT-interval shortening.10,12 Although the underlying mechanism and safety relevance of this finding is not known, the European labeling advises clinicians to use their clinical judgment when prescribing rufinamide to patients at risk for further QT-interval shortening (SQTS).13 However, data on proarrhythmic side effects of drug-induced QT-interval shortening were not reported.10,12,13
The aim of our study was to assess the first clinical experience of the new drug rufinamide in the treatment of LGS in a tertiary epilepsy center in Germany with regard to changes in the QT interval and cardiovascular events during follow-up.
Methods
Nineteen consecutive patients (n = 11 men; mean age 41 ± 12 years) with LGS and other epilepsy syndromes treated in the Bethel Epilepsy Center, Bielefeld, Germany, were included since the introduction of rufinamide in 2008. The mean duration of epilepsy in these patients was 40 ± 11 years. In 9 patients, the diagnosis was LGS. The other patients had other focal epilepsy (n = 5) or epilepsy with focal or generalized seizures (n = 5). They were treated off-label because of severe drug-refractoy epilepsies, mostly with drop attacks. Three patients had an implanted vagal nerve stimulator. At the time of electrocardiographic (ECG) recording, the neurostimulator was not active. Non-AED comedication with potential effects on the QT interval included digitalis (n = 1 patient) and promethazine (n = 2 patients). AEDs used were acetazolamide (n = 2), clobazam (n = 4), clonazepam (n = 3), carbamazepine (n = 4), diazepam (n = 1), ethosuximide (n = 1), felbamate (n = 1), lacosamide (n = 4), lamotrigine (n = 12), levetiracetam (n = 11), lorazepam (n = 1), methsuximide (n = 1), oxcarbazepine (n = 2), phenobarbital (n = 1), phenytoin (n = 2), pregrabalin (n = 1), topiramate (n = 3), valproic acid (n = 14), and zonisamide (n = 2). Thus, the most common baseline therapy of our patients included lamotrigine, levetiracetam, and valproic acid. Rufinamide was administered in all patients exclusively as an adjunctive treatment to a baseline therapy. Before the initiation of the drug in each patient, a 12-lead ECG was recorded and repeated during titration. Drug monitoring of all AEDs was performed in the fasting state and after no change in the dosage of the drug at least for >3 days.14
All ECGs were scanned and analyzed digitally (Adobe Acrobat Professional 8.0, Adobe Systems Inc, San Jose, CA). A standardized method was used to exactly measure the QT interval. The end of the T wave was defined as the intersection of a tangent to the steepest slope of the last limb of the T wave and the baseline preferably in lead II.15 The mean of the QT interval of 3 consecutive QT intervals was calculated. The QTc interval was defined as QT/√RR from the RR interval between the measured and the preceding complex (Bazett). Two cardiologists performed the ECG measurements without the knowledge of the underlying medication. ECGs with heart rates below 60 beats/min and above 100 beats/min were excluded from the QT/QTc analysis owing to imprecision of Bazett’s correction. Furthermore, Tpeak-Tend intervals were calculated as the difference of the interval of the Q wave to the peak of the T wave (QTpeak) and the interval of the Q wave to the end of the T wave (QTend), representing a surrogate marker of the transmural dispersion of repolarization (Tpeak-Tend interval).16 Finally, QRS width has been determined before and during treatment. Clinical information with respect to symptomatic cardiac arrhythmias and adverse events such as syncope and sudden unexpected death in epilepsy (SUDEP) was assessed during follow-up. All parameters were expressed as mean values ± SD. A Student t test was performed to test for statistical differences between 2 unpaired mean values. A P value of <.05 was considered to be statistically significant (Microsoft Excel 2008 for Macintosh, v.12.2.5, Redmond, WA).
Results
The follow-up was 3.6 ± 0.67 years, and on medication, 3.04 ± 1.09 years. In 7 patients, rufinamide was stopped owing to lack of clinical benefit. The final dosage of rufinamide in all patients was 2779 ± 410 mg (Table 1). The maximal serum concentration was 18.4 ± 8.9 μg/mL (therapeutic range 5–30 μg/mL; serum concentrations in adults according to data from routine therapeutic drug monitoring 15.9 ± 8.5 μg/mL).14 The most frequent comedications with the maximal dosage and blood level during follow-up were as follows: lamotrigine (n = 12; 344 ± 113 mg; 11 ± 2.1 μg/mL), levetiracetam (n = 11; 3091 ± 1136 mg; 25.4 ± 23.4 μg/mL), and valproic acid (n = 14; 1689 ± 1160 mg; 64.8 ± 23.4 μg/mL).
Table 1.
ECG parameters with corresponding rufinamide serum levels before and on final dosage
| Patient no. | Age (y) | Sex | QT interval (ms)
|
QTc interval (ms)
|
RUF (mg) | Serum level (μg/mL) | Lamotrigine (mg) | Serum level (μg/mL) | VPA (mg) | Serum level (μg/mL) | ΔQTc (ms) | ||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Before dose | On final dose | Before dose | On final dose | ||||||||||
| 1 | 33 | Male | 325 | 312 | 401 | 380 | 3.200 | 21.9 | 400 | 13.8 | 2.300 | 86.0 | 21 |
| 2 | 34 | Female | 342 | 315 | 390 | 393 | 2.400 | 18.7 | — | — | — | — | −3 |
| 3 | 68 | Female | 342 | 314 | 391 | 380 | 2.400 | 22.4 | — | — | — | — | 12 |
| 4 | 21 | Male | 324 | 323 | 391 | 389 | 2.400 | 9.9 | — | — | — | — | 1 |
| 5 | 53 | Female | 380 | 352 | 412 | 371 | 3.200 | 12.6 | — | — | — | — | 41 |
| 6 | 45 | Female | 357 | 327 | 411 | 382 | 3.200 | 13.2 | 300 | 8.0 | 900 | 48.6 | 29 |
| 7 | 45 | Male | 331 | 337 | 390 | 395 | 1.200 | 13.0 | — | — | — | — | −5 |
| 8 | 36 | Female | 330 | 314 | 415 | 385 | 3.200 | 17.7 | 250 | 10.6 | 1.200 | 69.3 | 30 |
| 9 | 48 | Male | 371 | 350 | 432 | 396 | 3.200 | 32.1 | 275 | 9.5 | 600 | 40.0 | 36 |
| 10 | 41 | Male | 344 | 333 | 410 | 396 | 2.400 | 20.5 | — | — | — | — | 14 |
| 11 | 44 | Male | 357 | 314 | 351 | 352 | 2.400 | 10.7 | — | — | — | — | −1 |
| 12 | 63 | Female | 376 | 349 | 417 | 372 | 2.400 | NA | 400 | 12.3 | — | — | 45 |
| 13 | 36 | Male | 350 | 334 | 418 | 396 | 2.400 | 9.9 | — | — | — | — | 22 |
| 14 | 32 | Female | 358 | 299 | 383 | 384 | 2.400 | 9.2 | — | — | — | — | −1 |
| 15 | 46 | Male | 407 | 356 | 446 | 408 | 3.200 | 31.6 | 400 | 10.4 | 3.000 | 54.2 | 39 |
| 16 | 40 | Male | 339 | 340 | 405 | 403 | 2.400 | 20.3 | — | — | — | — | 2 |
| 17 | 45 | Male | 350 | 325 | 365 | 354 | 2.400 | 12.8 | — | — | — | — | 12 |
| 18 | 22 | Male | 307 | 317 | 411 | 357 | 3.200 | 41.1 | 350 | 13.9 | 2.000 | 94.6 | 54 |
| 19 | 32 | Female | 334 | 303 | 395 | 366 | 3.200 | 12.9 | 300 | 11.4 | 600 | 35.9 | 28 |
— = no comedication with this substance; ΔQTc = difference of QTc interval before and during rufinamide treatment; NA = not available; RUF = rufinamide; VPA = valproic acid.
QT intervals were 349 ± 23 ms (QTc interval 402 ± 22 ms) before the initiation of therapy and significantly decreased to 327 ± 17 ms (QTc interval 382 ± 16 ms) after steady state (P = .002; Figure 1). Tpeak-Tend intervals averaged 79 ± 17 ms before therapy and were nonsignificantly decreased to 70 ± 20 ms (P = .07). The mean reduction in the QTc interval was −20 ± 18 ms, and the change in the Tpeak-Tend intervals was −8 ± 16 ms. QRS duration did not change before and after treatment (95 ± 11 ms vs 94 ± 10 ms; P = .89). The heart rates were not different between baseline and rufinamide treatment (81 ± 11 beats/min vs 82 ± 8 beats/min; P = .54).
Figure 1.
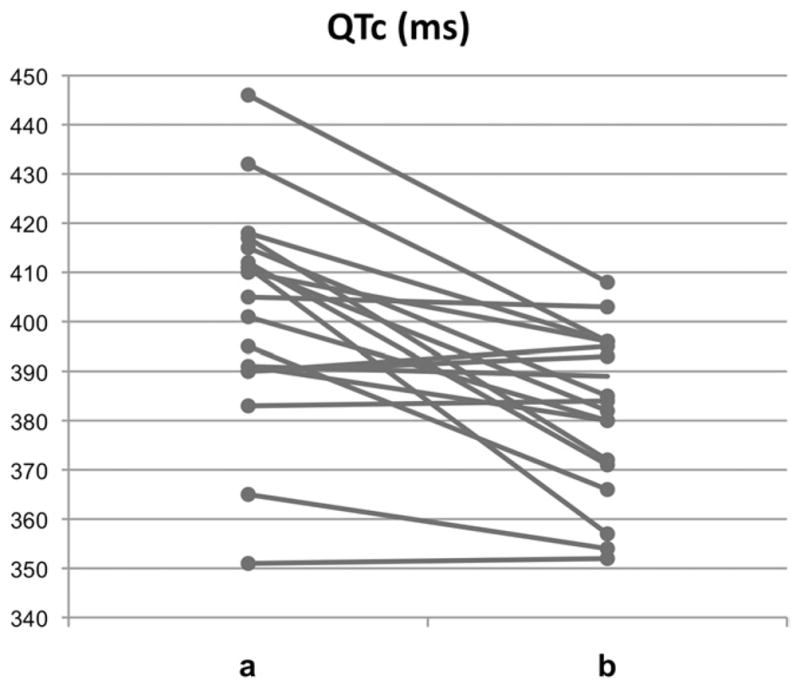
QTc intervals (a) before and (b) during rufinamide treatment of 19 patients. For better visualization, QTc-interval scale (left) starts with 340 ms.
The maximal individual difference in the QTc interval in a single patient between baseline and rufinamide treatment was −54 ms (QTc interval 411 ms vs 357 ms; 3200 mg of rufinamide; serum level 41.1 μg/mL). The QTc interval of <320 ms was not recorded in any patient, as observed in patients with SQTS (SQTS 1–3). However, drug-induced QT-interval shortening to QTc values of 350 ms occurred in 2 patients. A drug-induced reduction in the QTc interval to ≥30 ms (range 30–54 ms) was observed in 6 of the 19 (32%) patients (Figure 2). Furthermore, QTc-interval shortening was significantly more prominent if a certain comedication was present. Under the final dosage of rufinamide, comedication consisted of lamotrigine and valproic acid. The difference of QTc interval under comedication with lamotrigine (n = 8) was 33.9 ± 10.6 ms in comparison to rufinamide without lamotrigine (QTc interval 8.5 ± 13.7 ms; P <.005). The difference of QTc interval under comedication with valproic acid (n = 7) was 33.9 ± 10.6 ms in comparison to rufinamide without valproic acid (QTc interval 11.6 ± 16.8 ms; P < .005).
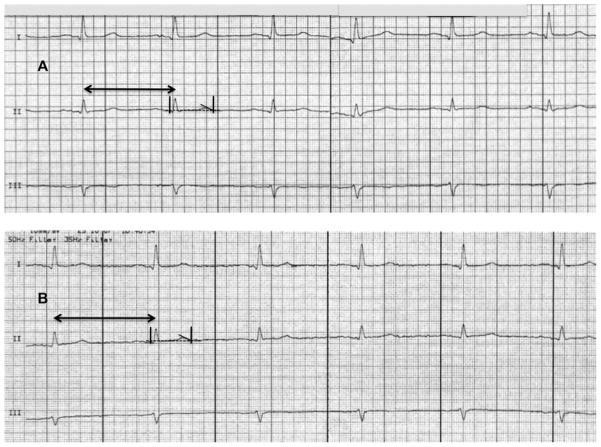
Figure 2.
Electrocardiogram (ECG) of drug-induced QTc-interval shortening during rufinamide treatment of a 63-year-old woman with drug-refractory focal epilepsy since 60 years. A: Before rufinamide treatment: heart rate of the preceding RR interval 74 beats/min (arrow); QT interval 381 ms; corrected QTc interval 424 ms; anti-epileptic comedication clonazepam (2 mg/d), zonisamide (500 mg/d), and lam-otrigine (400 mg/d). B: During rufinamide treatment: heart rate of the preceding RR interval 67 beats/min (arrow); QT interval 365 ms; QTc interval 385 ms, and thus QTc-interval shortening of −39 ms; rufinamide (2400 mg/d) with comedication lamotrigine (325 mg/d) and pregabalin (375 mg/d) (paper speed 50 mm/s).
During follow-up (outpatient visit or phone call) of 3.6 ± 0.67 years, no symptomatic cardiac arrhythmias occurred. Furthermore, no adverse events, such as syncope and SUDEP, were reported.
Discussion
Treatment of patients with LGS and other forms of epilepsy with the new antiepileptic drug rufinamide led to a significant shortening of the QTc interval as a drug side effect in our analysis of clinical application in a large referral epilepsy center. During long-term follow-up, no cardiovascular events such as symptomatic arrhythmias, syncope, SCD, and SUDEP have been reported.
Drug-induced QT-interval shortening under AED treatment
During AED treatment, drug-induced QT-interval shortening has been reported. Conflicting reports are available on carbamazepine and phenytoin, some indicating that they shorten QTc.17,18 Primidone can shorten the QTc interval.19 Rufinamide demonstrated a good cardiovascular tolerability and no increase in QT intervals in an initial safety study. Surprisingly, this agent produced the contrary: a small dose-dependent decrease in the QTc interval with a mean of −7.5 ms.12 In one further report, the maximal QTc-interval shortening effect observed was −27.8 ms.10 The efficacy of rufinamide as an adjunctive therapy for drug-resistant LGS has been evaluated in a multicenter study in 138 patients (age 4–37 years; mean age 14.1 years).20
The principal mechanism responsible for the antiepileptic activity of rufinamide is its ability to modulate neuronal sodium channels. In vitro examinations demonstrated reductions in high-frequency firing of neuronal action potentials over a broad range of concentrations.21 The reason for the QT-interval shortening still remains to be elucidated. In previous trials, a rufinamide-induced electrolyte imbalance has not been observed as a potential confounder on the QT interval.22 In preclinical studies, rufinamide had no effect on hERG current when investigated in human embryonic kidney cells.23
Proarrhythmia due to QT-interval shortening?
Congenital SQTS increases the risk for supraventricular and ventricular tachyarrhythmias and SCD.6 However, the clinical relevance of drug-induced QT-interval shortening remains unclear.24
Furthermore, it is still a matter of discussion whether QT-interval shortening should be taken as an additional safety surrogate parameter of drug development.25,26 QT-interval shortening occurs more often than expected. Holbrook et al24 conducted a survey in 2007 to assess the incidence and impact of drug-induced QT-interval shortening in drug-developing companies.24 A compound-related QT-interval-shortening effect was reported in 51% (27 of 53), whereas 22% (9 of 41) documented QT-interval shortening in their clinical studies. Furthermore, 13% (7 of 56) of the compounds were stopped prior to further clinical development because of QT-interval shortening.
In 2011, Raschi et al25 retrieved spontaneous reports of QT-interval shortening, which have been submitted to the Food and Drug Administration’s Adverse Event Reporting System. QT-interval shortening was reported in 42 cases in comparison to 5323 reports with QT-interval prolongation. In 35 cases (83%), QT-interval shortening was associated with arrhythmia-related events: atrial fibrillation (n = 9), ventricular fibrillation (n = 7), cardiac arrest (n = 5), and polymorphic ventricular tachycardia (n = 3). However, these findings have to be interpreted with great caution, because additional clinical information was not available, including the degree of QT-interval shortening, measurement method of the QT interval, comedication, and prevalence of cardiovascular disease.
Dixon et al27 recently showed that lamotrigine induced small reductions of −7.48 ms in QTc intervals in healthy subjects. Lamotrigine inhibits the rapidly activating delayed rectifier potassium current (IKr) of cardiac cells. IKr is the key current responsible for phase 3 repolarization of the cardiac action potential, and IKr-blocking drugs may increase the risk for cardiac arrhythmia and SCD.28 However, the reason for drug-induced QT-interval shortening of lamotrigine is unclear, as inhibition of IKr is the most common mechanism of drug-induced QT-interval prolongation. In the present study, QTc-interval shortening was significantly more prominent in patients on comedication of lamotrigine and valproic acid. Whether lamotrigine was the essential contributor to the QTc-interval-shortening effect of rufinamide remains unclear.
Blockers of neuronal sodium channels have also been documented to block cardiac sodium channels.29,30 The agents that block peak sodium channel current (INa) generally block late INa, which can contribute to the action potential duration and QT-interval shortening. This mechanism may underlie the effect of lamotrigine and valproic acid to exacerbate the action of rufinamide to shorten the QT interval. Sipatrigine, a blocker of neuronal Na+ and Ca2+ channels that is structurally related to lamotrigine, has been reported to shorten the cardiac action potential in isolated guinea pig hearts secondary to the inhibition of L-type calcium and sodium channel currents.31 An effect of lamotrigine to block ICa may therefore also contribute to its effect to shorten QT and to exacerbate the effect of rufinamide.
In our patients, no QTc-interval shortening of <320 ms was observed. However, 2 patients had a drug-induced QTc-interval shortening of 354 and 352 ms. These QTc values have been documented in patients with an overlap syndrome of Brugada syndrome and SQTS and in a recent publication in a patient with SQTS (QTc interval 362 ms).4,5 Furthermore, only very few healthy individuals have a QTc interval of <360 ms when patients with heart rates of <60 beats/min are excluded.32,33 Finally, proarrhythmic effects on noncardiac drugs are not limited to QT-interval prolongation, but they also include Brugadogenic effects and QT-interval shortening.34 However, changes in repolarization (T-wave morphology, Tpeak-Tend-interval prolongation, and pathologic U waves) or increase in QRS duration as common markers of a proarrhythmia were not observed in the present study.
SUDEP and antiepileptic drug treatment
SUDEP is the most frequent epilepsy-related cause of death. It is defined as the sudden, unexpected, nontraumatic, and nondrowning death in patients with epilepsy with or without evidence for a seizure, excluding status epilepticus and structural or toxicological causes of death. In patients with epilepsy, the risk for sudden death is increased 20-fold as compared with that in the general population. The risk further increases in patients with chronic refractory epilepsy and frequent generalized tonic-clonic seizures, early onset of epilepsy, male sex, and AED polytherapy.35 Periictal cardiorespiratory alterations are likely to be involved in SUDEP, including tachyarrhythmias, bradyarrhythmias, and central or obstructive hypoventilation with neurogenic pulmonary edema. Ictal asystole is a rare serious complication. One of the causes responsible for SUDEP may be a sudden electrical shutdown of the central nervous system associated with a seizure and final cardiore-spiratory failure.
However, some cases of SUDEP are pointing to complex abnormalities of the cardiac and respiratory systems. In recent years, a potential mechanistic link between SUDEP and cardiac arrhythmias has been discussed.
Increased QT-interval dispersion has been demonstrated in children with epilepsy compared with controls, potentially predisposing them to cardiac arrhythmias. However, antiepileptic treatment did not affect QT-interval dispersion as contribution to SUDEP in an analysis by Kwon and coworkers.36,37 AED-induced QT-interval prolongation was not documented in our patients before adding rufinamide. Postmortem genetic analysis of 68 SUDEP cases revealed in 13% variants in the KCNH2 and SCN5A genes, which have been previously reported in patients with long QT syndrome.38 The same group screened 48 SUDEP cases for gene variants encoding the hyperpolarization-activated cyclic nucleotide-gated cation ion channels, which are involved in idiopathic and acquired epilepsies. Hyperpolarization-activated cyclic nucleotide-gated cation ion channels are generating spontaneous rhythmic acitivity in cardiac pacemaker and neuronal cells. A link of mutations of the HCN4 gene to arrhythmias could be demonstrated in 1 patient suffering from both sinus node dysfunction with recurrent syncope and QT-interval prolongation with polymorphic ventricular tachycardia.39,40 Finally, a postmortem molecular autopsy of a SUDEP case revealed a cardiac ryanodine receptor mutation. As the cardiac ryanodine receptor is also neuronally expressed, it may predispose to a phenotype with exercise-induced catecholaminergic polymorphic ventricular tachycardia and abnormal electroencephalogram.41,42
However, some of these data postulating potential neurocardiac mechanisms associated with cardiac arrhythmias leading to death in epilepsy patients have the limitation that ECG data were not reported.39,43
QT-interval shortening in epilepsy and SUDEP has been described in 2 clinical situations. Lamotrigine treatment was the only denominator associated with 5 SUDEP cases.28 In our study, 13 patients with lamotrigine treatment showed no fatal complication. Interestingly, all our patients with significant drug-induced QTc-interval shortening were on comedication with lamotrigine, whereas patients without lamotrigine comedication showed much less prominent QT-interval shortening.
Surges et al44 observed abnormal QTc-interval shortening in 17 patients during the early postictal phase not during ictal heart rate peaks. The emerging profile of patients at risk for SUDEP is the person with early-onset refractory symptomatic epilepsy with frequent generalized tonic-clonic seizures and AED polytherapy.45 Whether drug-induced QT-interval shortening facilitates events remains speculative and further studies are warranted.
The following limitations of the study have to be acknowledged. The study population is very small and a substantial amount of potential confounding factors of the QT interval exist, such as hormonal influence, electrolyte imbalance (not available), intra- and interindividual variability of the QT interval, AED polytherapy with unknown potential interactions, as well as a lack of a placebo group. Echocardiography was not available. However, none of the patients had symptomatic cardiac disease.
In patients with epileptic seizures, it is sometimes very difficult to differentiate between seizures or drop attacks from cardiogenic syncope. However, all the patients had been thoroughly examined as inpatients establishing the diagnosis of epilepsy. At least no change in the clinical presentation of seizures with a mean history of 40 ± 11 years has been reported.
Conclusion
Our findings confirm previous data concerning QT-interval shortening during rufinamide treatment. We came up with 2 novel findings: First, QT-interval shortening was more marked than in the previous studies, which might be due to the baseline comedication with lamotrigine and/or valproic acid. Second, no clinical safety issues have been reported. The small number of cases constitutes a limitation. It is, however, not unusual with regard to the limited approval status of rufinamide.
The implementation of ECG testing in patients with epilepsy as safety control under AED therapy (QT-interval prolongation and PQ-interval prolongation) is advisable, especially in the case of comedication with neuroleptic agents or antidepressants. Whether new drugs with potential drug-induced QT-interval shortening, such as rufinamide, could generate a proarrhythmic effect for SCD and/or SUDEP remains presently speculative. However, because of the emergence of the arrhythmogenic effect of short QT intervals (SQTS), periodic ECG recording under single or combined drug therapy to exclude both drug-induced QTc-interval prolongation and drug-induced QTc-interval shortening appears to be a reasonable precaution.
ABBREVIATIONS
- AED
antiepileptic drugs
- ECG
electrocardiogram/electrocardiographic
- IKr
delayed rectifier potassium current
- INa
sodium channel current
- LGS
Lennox-Gastaut syndrome
- SCD
sudden cardiac death
- SQTS
short QT syndrome
- SUDEP
sudden unexpected death in epilepsy
References
- 1.Gussak I, Brugada P, Brugada J, et al. Idiopathic short QT interval: a new clinical syndrome? Cardiology. 2000;94:99–102. doi: 10.1159/000047299. [DOI] [PubMed] [Google Scholar]
- 2.Gaita F, Giustetto C, Bianchi F, et al. Short QT syndrome: a familial cause of sudden death. Circulation. 2003;108:965–970. doi: 10.1161/01.CIR.0000085071.28695.C4. [DOI] [PubMed] [Google Scholar]
- 3.Brugada R, Hong K, Dumaine R, et al. Sudden death associated with short-QT syndrome linked to mutations in HERG. Circulation. 2004;109:30–35. doi: 10.1161/01.CIR.0000109482.92774.3A. [DOI] [PubMed] [Google Scholar]
- 4.Antzelevitch C, Pollevick GD, Cordeiro JM, et al. Loss-of-function mutations in the cardiac calcium channel underlie a new clinical entity characterized by ST-segment elevation, short QT intervals, and sudden cardiac death. Circulation. 2007;115:442–449. doi: 10.1161/CIRCULATIONAHA.106.668392. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Templin C, Ghadri JR, Rougier JS, et al. Identification of a novel loss-of-function calcium channel gene mutation in short QT syndrome (SQTS6) Eur Heart J. 2011;32:1077–1088. doi: 10.1093/eurheartj/ehr076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Giustetto C, Schimpf R, Mazzanti A, et al. Long-term follow-up of patients with short QT syndrome. J Am Coll Cardiol. 2011;58:587–595. doi: 10.1016/j.jacc.2011.03.038. [DOI] [PubMed] [Google Scholar]
- 7.Bellet S, Finkelstein D. Significance of QT prolongation in the electrocardiogram: based on the study of 168 cases. Am J Med Sci. 1951;222:263–278. doi: 10.1097/00000441-195109000-00004. [DOI] [PubMed] [Google Scholar]
- 8.Priori SG, Schwartz PJ, Napolitano C, et al. Risk stratification in the long-QT syndrome. N Engl J Med. 2003;348:1866–1874. doi: 10.1056/NEJMoa022147. [DOI] [PubMed] [Google Scholar]
- 9.Food and Drug Administration, HHS. International Conference on Harmonisation; guidance on E14 Clinical Evaluation of QT/QTc Interval Prolongation and Proarrhythmic Potential for Non-Antiarrhythmic Drugs; availability. Notice Fed Regist. 2005;70:61134–61135. [PubMed] [Google Scholar]
- 10.Shah RR. Drug-induced QT interval shortening: potential harbinger of proarrhythmia and regulatory perspectives. Br J Pharmacol. 2010;159:58–69. doi: 10.1111/j.1476-5381.2009.00191.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Garberoglio L, Giustetto C, Wolpert C, Gaita F. Is acquired short QT due to digitalis intoxication responsible for malignant ventricular arrhythmias? J Electrocardiol. 2007;40:43–46. doi: 10.1016/j.jelectrocard.2006.07.003. [DOI] [PubMed] [Google Scholar]
- 12.Marchand M, Critchley D, Nagy C, Fuseau E. The effect of rufinamide concentration on the QT interval in healthy subjects treated during 18 days with multiple ascending doses: a population PKPD analysis. Annual Meeting of the Population Approach Group in Europe; 2005. p. Abstract A785. [Google Scholar]
- 13.European Medicines Agency. [Accessed August 15, 2011];Inovelon® (rufinamide) product information. 2009 Available at: http://wwwemaeuropaeu/ema/indexjsp?curl=pages/medicines/human/medicines/000660/human_med_000837jsp&murl=menus/medicines/medicinesjsp&mid=WC0b01ac058001d124.
- 14.May TW, Boor R, Rambeck B, et al. Serum concentrations of rufinamide in children and adults with epilepsy: the influence of dose, age, and comedication. Ther Drug Monit. 2011;33:214–221. doi: 10.1097/FTD.0b013e31820fa9ad. [DOI] [PubMed] [Google Scholar]
- 15.Postema PG, De Jong JS, Van der Bilt IA, Wilde AA. Accurate electrocardiographic assessment of the QT interval: teach the tangent. Heart Rhythm. 2008;5:1015–1018. doi: 10.1016/j.hrthm.2008.03.037. [DOI] [PubMed] [Google Scholar]
- 16.Antzelevitch C, Yan GX, Shimizu W. Transmural dispersion of repolarization and arrhythmogenicity: the Brugada syndrome versus the long QT syndrome. J Electrocardiol. 1999;32:158–165. doi: 10.1016/s0022-0736(99)90074-2. [DOI] [PubMed] [Google Scholar]
- 17.Kenneback G, Bergfeldt L, Vallin H, Tomson T, Edhag O. Electrophysiologic effects and clinical hazards of carbamazepine treatment for neurologic disorders in patients with abnormalities of the cardiac conduction system. Am Heart J. 1991;121:1421–1429. doi: 10.1016/0002-8703(91)90148-b. [DOI] [PubMed] [Google Scholar]
- 18.Wyte CD, Berk WA. Severe oral phenytoin overdose does not cause cardiovascular morbidity. Ann Emerg Med. 1991;20:508–512. doi: 10.1016/s0196-0644(05)81604-x. [DOI] [PubMed] [Google Scholar]
- 19.DeSilvey DL, Moss AJ. Primidone in the treatment of the long QT syndrome: QT shortening and ventricular arrhythmia suppression. Ann Intern Med. 1980;93:53–54. doi: 10.7326/0003-4819-93-1-53. [DOI] [PubMed] [Google Scholar]
- 20.Kluger G, Glauser T, Krauss G, et al. Adjunctive rufinamide in Lennox-Gastaut syndrome: a long-term, open-label extension study. Acta Neurol Scand. 2010;122:202–208. doi: 10.1111/j.1600-0404.2010.01334.x. [DOI] [PubMed] [Google Scholar]
- 21.Cheng-Hakimian A, Anderson GD, Miller JW. Rufinamide: pharmacology, clinical trials, and role in clinical practice. Int J Clin Pract. 2006;60:1497–1501. doi: 10.1111/j.1742-1241.2006.01173.x. [DOI] [PubMed] [Google Scholar]
- 22.Wheless JW, Vazquez B. Rufinamide: a novel broad-spectrum antiepileptic drug. Epilepsy Curr. 2010;10:1–106. doi: 10.1111/j.1535-7511.2009.01336.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.European Medicines Agency. [Accessed December 18, 2011];Inovelon® (rufinamide) scientific information. 2007 Available at: http://wwwemaeuropaeu/docs/en_GB/document_library/EPARScientific_Discussion/human/000660/WC500032940pdf.
- 24.Holbrook M, Malik M, Shah RR, Valentin JP. Drug induced shortening of the QT/QTc interval: an emerging safety issue warranting further modelling and evaluation in drug research and development? J Pharmacol Toxicol Methods. 2009;59:21–28. doi: 10.1016/j.vascn.2008.09.001. [DOI] [PubMed] [Google Scholar]
- 25.Raschi E, Poluzzi E, Koci A, Boriani G, De Ponti F. QT-interval shortening in spontaneous reports submitted to the FDA: the need for consensus. Br J Clin Pharmacol. 2011;72:839–841. doi: 10.1111/j.1365-2125.2011.04065.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Shah RR, Bjerregaard P, Gussak I. Drug-induced QT interval shortening: an emerging component in integrated assessment of cardiac safety of drugs. J Electrocardiol. 2010;43:386–389. doi: 10.1016/j.jelectrocard.2010.06.008. [DOI] [PubMed] [Google Scholar]
- 27.Dixon R, Job S, Oliver R, et al. Lamotrigine does not prolong QTc in a thorough QT/QTc study in healthy subjects. Br J Clin Pharmacol. 2008;66:396–404. doi: 10.1111/j.1365-2125.2008.03250.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Aurlien D, Tauboll E, Gjerstad L. Lamotrigine in idiopathic epilepsy—increased risk of cardiac death? Acta Neurol Scand. 2007;115:199–203. doi: 10.1111/j.1600-0404.2006.00730.x. [DOI] [PubMed] [Google Scholar]
- 29.Sicouri S, Antzelevitch C. Sudden cardiac death secondary to antidepressant and antipsychotic drugs. Expert Opin Drug Saf. 2008;7:181–194. doi: 10.1517/14740338.7.2.181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Minoura Y, JMDID, Barajas-Martinez H, et al. Ionic and cellular mechanisms underlying the development of acquired Brugada syndrome in patients treated with antidepressants. J Cardiovasc Electrophysiol. 2011 doi: 10.1111/j.1540-8167.2011.02196.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Gao Z, Milnes JT, Choisy SC, et al. The neuroprotective agent sipatrigine blocks multiple cardiac ion channels and causes triangulation of the ventricular action potential. Clin Exp Pharmacol Physiol. 2005;32:1088–1096. doi: 10.1111/j.1440-1681.2005.04312.x. [DOI] [PubMed] [Google Scholar]
- 32.Kobza R, Roos M, Niggli B, et al. Prevalence of long and short QT in a young population of 41,767 predominantly male Swiss conscripts. Heart Rhythm. 2009;6:652–657. doi: 10.1016/j.hrthm.2009.01.009. [DOI] [PubMed] [Google Scholar]
- 33.Viskin S. The QT interval: too long, too short or just right. Heart Rhythm. 2009;6:711–715. doi: 10.1016/j.hrthm.2009.02.044. [DOI] [PubMed] [Google Scholar]
- 34.Viskin S, Rosso R, Marquez MF, Antzelevitch C. The acquired Brugada syndrome and the paradox of choice. Heart Rhythm. 2009;6:1342–1344. doi: 10.1016/j.hrthm.2009.07.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Shorvon S, Tomson T. Sudden unexpected death in epilepsy. Lancet. 2011 doi: 10.1016/S0140-6736(11)60176-1. [DOI] [PubMed] [Google Scholar]
- 36.Akalin F, Tirtir A, Yilmaz Y. Increased QT dispersion in epileptic children. Acta Paediatr. 2003;92:916–920. doi: 10.1080/08035250310003550. [DOI] [PubMed] [Google Scholar]
- 37.Kwon S, Lee S, Hyun M, et al. The potential for QT prolongation by antiepileptic drugs in children. Pediatr Neurol. 2004;30:99–101. doi: 10.1016/S0887-8994(03)00405-3. [DOI] [PubMed] [Google Scholar]
- 38.Tu E, Bagnall RD, Duflou J, Semsarian C. Post-mortem review and genetic analysis of sudden unexpected death in epilepsy (SUDEP) cases. Brain Pathol. 2011;21:201–208. doi: 10.1111/j.1750-3639.2010.00438.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Tu E, Waterhouse L, Duflou J, Bagnall RD, Semsarian C. Genetic analysis of hyperpolarization-activated cyclic nucleotide-gated cation channels in sudden unexpected death in epilepsy cases. Brain Pathol. 2011;21:692–698. doi: 10.1111/j.1750-3639.2011.00500.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Ueda K, Nakamura K, Hayashi T, et al. Functional characterization of a trafficking-defective HCN4 mutation, D553N, associated with cardiac arrhythmia. J Biol Chem. 2004;279:27194–27198. doi: 10.1074/jbc.M311953200. [DOI] [PubMed] [Google Scholar]
- 41.Johnson JN, Tester DJ, Bass NE, Ackerman MJ. Cardiac channel molecular autopsy for sudden unexpected death in epilepsy. J Child Neurol. 2010;25:916–921. doi: 10.1177/0883073809343722. [DOI] [PubMed] [Google Scholar]
- 42.Lehnart SE, Mongillo M, Bellinger A, et al. Leaky Ca2+ release channel/ryanodine receptor 2 causes seizures and sudden cardiac death in mice. J Clin Invest. 2008;118:2230–2245. doi: 10.1172/JCI35346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Aurlien D, Leren TP, Tauboll E, Gjerstad L. New SCN5A mutation in a SUDEP victim with idiopathic epilepsy. Seizure. 2009;18:158–160. doi: 10.1016/j.seizure.2008.07.008. [DOI] [PubMed] [Google Scholar]
- 44.Surges R, Scott CA, Walker MC. Enhanced QT shortening and persistent tachycardia after generalized seizures. Neurology. 2010;74:421–426. doi: 10.1212/WNL.0b013e3181ccc706. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Hesdorffer DC, Tomson T, Benn E, et al. Combined analysis of risk factors for SUDEP. Epilepsia. 2011;52:1150–1159. doi: 10.1111/j.1528-1167.2010.02952.x. [DOI] [PubMed] [Google Scholar]