Abstract
The effect of light intensity on antioxidants, antioxidant enzymes, and chlorophyll content was studied in common bean (Phaseolus vulgaris L.) exposed to excess Mn. Leaves of bean genotypes contrasting in Mn tolerance were exposed to two different light intensities and to excess Mn; light was controlled by shading a leaflet with filter paper. After 5 d of Mn treatment ascorbate was depleted by 45% in leaves of the Mn-sensitive genotype ZPV-292 and by 20% in the Mn-tolerant genotype CALIMA. Nonprotein sulfhydryl groups and glutathione reductase were not affected by Mn or light treatment. Ten days of Mn-toxicity stress increased leaf ascorbate peroxidase activity of cv ZPV-292 by 78% in low light and by 235% in high light, and superoxide dismutase activity followed a similar trend. Increases of ascorbate peroxidase and superoxide dismutase activity observed in cv CALIMA were lower than those observed in the susceptible cv ZPV-292. The cv CALIMA had less ascorbate oxidation under excess Mn-toxicity stress. Depletion of ascorbate occurred before the onset of chlorosis in Mn-stressed plants, especially in cv ZPV-292. Lipid peroxidation was not detected in floating leaf discs of mature leaves exposed to excess Mn. Our results suggest that Mn toxicity may be mediated by oxidative stress, and that the tolerant genotype may maintain higher ascorbate levels under stress than the sensitive genotype.
Mn is an essential micronutrient for most living organisms. It is involved in redox reactions as a cofactor for different enzymes (for a detailed list of enzymes, see Burnell, 1988) such as the Mn-containing isozyme of Mn-SOD, which is one of the essential mechanisms in protection against oxidative stress in plants (for review, see Bowler et al., 1994). Mn is also important in the water-splitting system that provides electrons to PSII. Various soil conditions often present in acid and volcanic soils can lead to Mn reduction and create Mn toxicity in many natural and agricultural systems (Foy et al., 1978; Carver and Ownby, 1995).
In Mn-sensitive plant species, the deleterious effect of Mn toxicity is often observed in the shoots as stunted growth, chlorosis, crinkled leaves, and brown lesions or “speckles.” However, the response of plants to excess Mn is affected by leaf age (Horst, 1988), temperature (Heenan and Carter, 1977; Rufty et al., 1979), soil nutrient balance, soil pH, genotype, and light intensity. The effect of light intensity on Mn-toxicity symptoms was first reported in 1935, when McCool (1935) found that plants grown in low light displayed fewer symptoms of Mn toxicity than those grown in high light. Subsequent reports with different crops found a similar effect of low light (Sirkar and Amin, 1974; Elamin and Wilcox, 1986; Horiguchi, 1988; Nable et al., 1988). Wissemeier and Horst (1992) reported that symptoms of Mn toxicity (localized brown spots and callose formation) in mature leaves of cowpea were enhanced under low-light conditions, but did not rule out that chlorosis in immature leaves might be enhanced under high-light conditions. However, a factor that complicates the interpretation of most previous studies of the effect of light intensity on Mn-toxicity symptoms is the fact that plants grown in low light usually accumulate less foliar Mn that those grown at a higher light intensity, as found in maple trees (McCain and Markley, 1989).
Although the physiological mechanisms of Mn toxicity and tolerance are still unknown, several reports suggest a role for excess Mn in the induction of oxidative stress. Gerretsen (1950) proposed that Mn-induced chlorosis was not caused by inhibition of chlorophyll synthesis but rather by photooxidation of chlorophyll. Campbell and Nable (1988) suggested that low light intensity may also decrease the photodestruction of chlorophyll in plants suffering Mn toxicity. In pea del Rio et al. (1985) reported the induction of Mn-SOD in plants exposed to high levels of Zn and Mn. Also, Leidi et al. (1987a, 1987b) found high Mn-SOD activity in soybean genotypes grown under excess Mn. Panda et al. (1986) reported lipid peroxidation in aging, isolated chloroplasts treated with excess Mn.
The toxic effects of heavy metals, both essential and nonessential elements, have been linked to the production of free radicals (De Vos and Schat, 1991). Free radicals are usually formed as by-products of normal biological reactions, but their lifespan and diffusion into the cell space are closely controlled by the cell antioxidant system. Enzymes such as SOD, GR, ASPX, and DAS reductase, and compounds such as ascorbate and glutathione are actively involved in the ascorbate-glutathione scavenging cycle, controlling reactive oxygen species not only in the chloroplast but also in the cytosol (for reviews, see Alscher and Hess, 1993; Foyer and Mullineaux, 1994). The involvement and role of antioxidants in protection against oxidative stress have been demonstrated using transgenic plants (Foyer et al., 1994; Allen, 1995; Slooten et al., 1995), and genetic variability in the content of antioxidant elements has been reported in several species (Sevilla et al., 1988; Daza et al., 1993; Guzy and Heath, 1993). Tolerance of oxidative stresses such as the herbicide paraquat and NaCl were partially associated with a better antioxidant response in tolerant genotypes (Gossett et al., 1994; Hernández et al., 1995).
Leaf antioxidant capacity is modified by long-term light acclimation (Grace and Logan, 1996) and might confer tolerance to oxidative stresses. Variability in antioxidant capacity within common bean genotypes exists (Guzy and Heath, 1993), but it is not known if tolerance to Mn toxicity is correlated with better antioxidant capacity of tolerant genotypes. Because Mn might promote oxidative stress, it is important to characterize the response and levels of antioxidants in leaves accumulating excess Mn. It is also important to explore how that response is modified by light intensity within and among contrasting genotypes suffering Mn-toxicity stress. In this study we used a system that permitted equivalent Mn accumulation in tissue at different irradiance levels to examine the combined effect of light intensity and Mn-toxicity stress on antioxidants and chlorophyll in leaves of two bean genotypes with contrasting sensitivity to Mn.
MATERIALS AND METHODS
Plant Material
The common bean genotypes included in this report (Phaseolus vulgaris L., cvs CALIMA [tolerant] and ZPV-292 [susceptible]) were significantly different in response to excess Mn when biomass accumulation and seed yield were evaluated in long-term experiments under diverse growing conditions (González, 1996). Also, in a study of the effect of excess Mn on CO2 assimilation of these two genotypes it was found that excess Mn decreased CO2 assimilation mainly in immature leaves by causing rapid degradation of chlorophyll. Mature leaves were less affected (González and Lynch, 1997), although both leaf types accumulated excess Mn at comparable rates.
Seedlings were grown in pots in the greenhouse until they were 21 d old. Pots (12 cm in height and 10.5 cm in internal diameter) were filled with a mixture (1:1, v/v) of silica sand (U.S. Silica, Ottawa, IL) and perlite (PVP Industries, Bloomfield, OH). Seedlings were irrigated six times per day with a nutrient solution containing (μmol/L): KCl (500), KNO3 (300), Ca(NO3)2 (2000), CaCl2 (2000), Fe-EDTA (20), MgSO4 (1000), K2SO4 (500), (NH4)SO4 (500), MnSO4 (5), ZnSO4 (5), CuSO4 (3), H3BO3 (3), NH4Mo7O24 (2), and KH2PO4 (200). Solution pH was adjusted to 5.5 with KOH. Thirty-two pots were moved to the growth chamber, where one-half of the plants were irrigated with a nutrient solution as described above and the other half received nutrient solution plus 200 μm MnSO4. The first trifoliate leaves were more than 80% expanded and the second trifoliate leaves were between 20% and 30% expanded when the plants were relocated to the growth chamber. To avoid self-shading the second trifoliate leaf was removed. Growing conditions were 23°C/20°C day/night temperature and 80%/55% day/night RH, with a 12-h photoperiod. From each first trifoliate leaf one lateral leaflet received 870 μmol m−2 s−1 (high light = 100% incident PAR) and the other received 220 μmol m−2 s−1 (low light = 25% of maximum PAR). Plants were harvested at 5 and 10 d after Mn treatment was initiated, and leaf tissue was split into three subsamples: (a) two leaf discs of 1.24 cm2, which were used for chlorophyll analysis; (b) a subsample, which was designated for nutrient analysis; and (c) the remaining tissue, which was frozen in liquid nitrogen and stored at −70°C for biochemical analysis.
Mn Analysis
Tissue was dried at 70°C, weighed, ashed at 500°C for 12 h, dissolved in 0.1 n HCl, and analyzed for Mn content by atomic absorption spectrophotometry.
Chlorophyll
Chlorophyll was extracted overnight in a dark, cold room by placing two leaf discs of 1.24 cm2 into 2 mL of N,N-dimethylformamide (Sigma). Aliquots were taken to measure the A664.5 and A667 in a spectrophotometer, and chlorophyll content was calculated according to the method of Inskeep and Bloom (1985).
Enzyme Assays
For enzyme activity measurements all operations were carried out at 4°C by keeping the samples in an ice bath until assays were completed.
SOD activity was assayed in nondenaturing gels (Beauchamp and Fridovich, 1971). Two leaf discs (1.24 cm2) were ground with 600 μL of a cold buffer containing Bicine (50 mm, pH 8.0), NaHCO3 (20 mm), MgCl2 (20 mm), Na-EDTA (1 mm), PVP (1%), and leupeptin sulfate (5 μm). Leaf extract was spun at 14,000g for 15 min and diluted 1:1 with a solution containing 62.5 mm Tris-HCl (pH 6.8) and 10% glycerol. Protein in the supernatant was determined using Bradford's reagent (Bio-Rad).
To estimate the level of protein needed to produce a linear relation between SOD activity and optical density, a gel loaded with different amounts of protein was assayed for SOD activity. White bands representing SOD were scanned and optical density was plotted against protein level. For subsequent assays we chose a protein level from the linear portion of the curve. Aliquots containing 18 μg of protein were loaded into non-SDS polyacrylamide gels (1.5 mm thick, consisting of 10% resolving gel and 4% stacking gels), and samples of contrasting treatments were always run in the same gel to minimize variation attributable to running and staining conditions. Electrophoresis steps were run at a constant voltage (200 V) for 30 min; running buffer (Tris-Gly, pH 8.3, 25 mm) was ice cold and the electrophoresis apparatus was placed in icy water. This method prevented the inhibition of cytoplasmic SOD observed when gels warmed because of high voltage, as reported by Burke and Oliver (1992).
Gels were soaked in 100 mL of a solution containing riboflavin (28 μm), phosphate buffer (32 mm, pH 7.8), and N,N,N′,N′-tetramethylethylenediamine (28 mm) for 25 min; the previous buffer was replaced with 100 mL of a solution containing nitroblue tetrazolium (2 mm, Sigma) in buffer phosphate (36 mm, pH 7.8) and the gel was shaken for 30 min. Gels were illuminated for 15 min or until white bands appeared, the solution was replaced with phosphate buffer (36 mm, pH 7.8), and gels were placed in a light box to continue the reaction very slowly. Gels were fixed in a solution containing 30% methanol, 7.5% glycerol for 1 h, and dried in a gel-drying membrane (Bio-Rad). Dried gels were scanned and the integrated density calculated. To determine if peroxidase interfered with the SOD assay in the activity gel, samples were run and a gel was vertically split into two sections. One section was stained for SOD activity, and the other section was stained for peroxidase with 3,3′,5,5′-tetramethylbenzidine (Guikema and Sherman, 1980; as described by Vallejos, 1983).
ASPX and GPX scavenge H2O2 and both use ascorbate as an electron donor, but ASPX has higher specificity for ascorbate than GPX. The fact that both enzymes can use ascorbate as an electron donor offers some problems in interpreting data from the standard assay used for ASPX activity. The chemical pCMB was used to discriminate the contribution of GPX during assays for ASPX (Amako et al., 1994). pCMB inhibits the activity of ASPX, and by running the assay with and without pCMB one may determine the activity of ASPX in the leaf extract. The effect of pCMB on ASPX was confirmed by assaying ASPX in the activity gel (Mitler and Zilinskas, 1993) and in the spectrophotometer on randomly selected samples.
Tissue was extracted with 400 μL of extraction buffer (100 mm buffer phosphate, pH 7.8, 8% glycerol, 2% PVP, 2 mm EDTA, and 5 mm ascorbate added immediately before extraction). Samples were centrifuged at 14,000g for 10 min in a cold room and kept cold until activity was measured. Eighty-microliter aliquots of supernatant were incubated with 3 μL of pCMB (final concentration in the Eppendorf tube was 1 mm) for 10 min. Leaf extract with and without pCMB was used to measure changes in A290 (extinction coefficient = 2.8 mm−1 cm−1; Nakano and Asada, 1981) at 25°C in a reaction mixture containing KPO4 (50 mm, pH 7.0), EDTA (5 mm), ascorbate (0.5 mm), and H2O2 (0.1 mm) in a final volume of 1 mL. Each assay was run three times. ASPX activity was calculated by subtracting the change in absorbance measured in the presence of the inhibitor from the absorbance measured without the inhibitor.
The assay of GR using DTNB is based on the reactions:
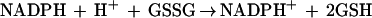 |
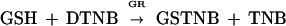 |
where GSTNB is glutathione-nitrobenzoic acid.
The activity of GR was tested in leaf supernatant (the same one as in the ASPX assay) using the method of Smith and Vierheller (1988) with slight modifications. The reaction mixture contained phosphate buffer (100 mm, pH 7.5), EDTA (2.5 mm), DTNB (0.75 mm), NADPH (0.1 mm), GSSG (1 mm), and leaf extract in a volume of 1 mL. The reaction was run at 30°C, activated by adding NADPH, and formation of TNB was followed at 412 nm for 1 min (Smith and Vierheller, 1988). Changes in absorbance were translated to nanomoles of GSSG from a standard curve prepared with commercial GR (Sigma) and known concentrations of GSSG.
Water-Soluble Compounds
Nonprotein SH groups were determined by a modification of the protocol of Tanaka et al. (1985), and in common bean are assumed to represent mainly GSH (Guzy and Heath, 1993). Four leaf discs were homogenized with a mortar and pestle in 2 mL of 10% (w/v) TCA containing 1.5 mm EDTA, and centrifuged for 15 min at 14,000g. The supernatant was split into two aliquots and stored at −70°C. For SH determination one aliquot was thawed and kept cold. From each sample 100 μL of supernatant was added to 790 μL of 150 mm K2HPO4 with 6.3 mm EDTA, added while vortex mixing, followed by 10 μL of 5 n NaOH to bring the pH close to 7.3, which is appropriate for color development. Then, 100 μL of 6 mm DTNB in 0.15 mm phosphate buffer was added and mixed vigorously. Tubes were incubated at room temperature for 10 min before A412 determination because of the formation of TNB. Absorbances were converted to SH concentration using a standard curve prepared in a similar way but including known amounts of GSH instead of leaf supernatant.
AS was assayed according to the method of Law et al. (1983). The assay is based on the reduction of Fe3+ to Fe2+ by ascorbic acid in acidic solution. The Fe2+ then forms complexes with bipyridyl, producing a pink color that absorbs at 525 nm. Total ascorbate (AS plus DAS) is determined through a reduction of DAS to AS by DTT. For the concentrations cited, 100 μL of the frozen aliquot was assayed for total ascorbate content, and the other half was assayed for AS only. DAS concentrations were then deduced from the difference. To determine if the Mn that was present in high-Mn tissue interfered with the development of color, we added several concentrations of MnSO4 to known amounts of AS and read the A525.
TCA-extracted leaf tissue was thawed and 200 μL was mixed with 5 μL of 5 n NaOH. To 100 μL of this mixture was added 100 μL of potassium phosphate buffer (150 mm, pH 7.4) and 100 μL of water to measure ascorbate. To another 100 μL of the sample was added 100 μL of potassium phosphate buffer, 50 μL of 20 mm DTT, and, after mixing and being left at room temperature for 15 min, 50 μL of 0.5% (v/v) N-ethylmaleimide. Both samples were stirred and incubated at room temperature for 5 min. To each was then added 200 μL of 10% TCA, 200 μL of 44% H3PO4, 200 μL of 4% bipyridil, and 100 μL of freshly made 3% FeCl3. Samples were stirred and incubated at 37°C in a water bath for 60 min. A525 was recorded and amounts of ascorbate were calculated from a standard curve (0–40 nmol) of pure l-ascorbic acid (Sigma) that underwent the same treatment.
Lipid Peroxidation
Lipid peroxidation was assayed by the reaction of MDA with thiobarbituric acid, which produces a pinkish chromagen (Du and Bramlage, 1992). The thiobarbituric acid reagent consisted of 20% (w/v) TCA, 0.65% (w/v) thiobarbituric acid, and 0.01% (w/v) butylated hydroxy toluene. From an independent set of plants grown in the greenhouse, leaf discs (23 mm in diameter) from fully expanded leaves were floated in nutrient solution containing 0.01, 0.8 and 3.2 mm MnSO4 in Petri dishes in the growth chamber for a 12-h photoperiod and with high light intensity (670 μmol m−2 s−1). Leaf discs were placed in the Petri dishes at the end of the photoperiod and kept there for at least 120 h. Each day four discs from each genotype and each Mn treatment were sampled. For Mn determination we harvested discs at 0, 3, and 6 h after they were placed in the nutrient solution. Discs were vigorously stirred in cold CaCl2 (10 mm) for 10 min to remove excess Mn. Two discs were used for Mn content and two discs were frozen in liquid nitrogen and assayed for chlorophyll determination and lipid-peroxidation products. Discs were extracted with 1 mL of anhydrous ethanol, mixed with the same volume of thiobarbituric acid reagent, and heated for 25 min at 90°C. Samples were centrifuged for 15 min at 14,000g and supernatant was measured spectrophotometrically at 532 and 600 nm. Data were calculated as nanomoles of MDA per milligram of dry tissue using the extinction coefficient of 156,000 m−1 cm−1.
RESULTS
All of the variables measured in this report were affected by light intensity regardless of Mn treatment or genotype. Consequently, the effect of Mn-toxicity stress on a given variable within a light treatment is expressed as a percentage by comparing the value observed in Mn control samples with the value in samples treated with high Mn. Changes observed in a response variable are compared across light treatments.
Chlorophyll and Mn Content
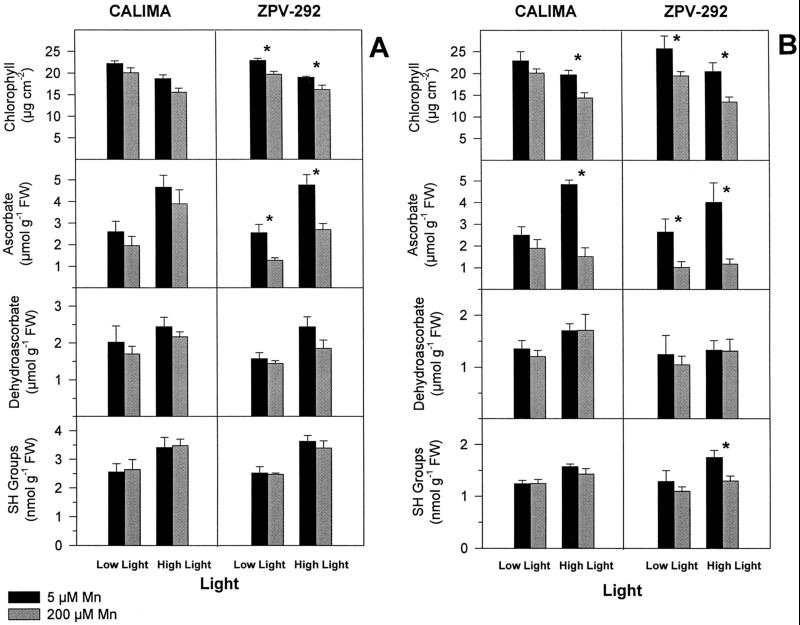
High-light leaves had less chlorophyll content than low-light leaves regardless of Mn treatment. Excess foliar Mn caused chlorosis at both light treatments and in both genotypes; however, chlorosis was significantly greater in high-light leaves of the susceptible genotype ZPV-292. Chlorosis among light treatments was not significantly different at 5 d, but at 10 d high-light leaves suffered more chlorosis than low-light leaves. In low light we observed a 12% loss of chlorophyll in the tolerant cv CALIMA and a 24.2% loss in the susceptible cv ZPV-292. In high light, cv CALIMA suffered a 26.7% decrease in chlorophyll compared with 34.4% in cv ZPV-292 (Fig. 1B). In the susceptible genotype ZPV-292, loss of chlorophyll among light treatments occurred at similar foliar Mn concentrations; however, leaflets of the genotype CALIMA accumulated slightly less Mn at low light (Fig. 2B).
Figure 1.
Effect of light intensity and excess Mn on total chlorophyll, ascorbate, DAS, and SH groups in mature leaves of two different bean genotypes grown in silica sand irrigated with nutrient solution containing different doses of Mn (black bars = 5 μm; gray bars = 200 μm Mn). Light intensity was controlled by shading one leaflet with Whatman No. 1 filter paper (Low Light = 220 μmol m−2 s−1; High Light = 870 μmol m−2 s−1). Asterisks represent means (n = 4) that were significantly different (lsd = 5%) from control plants within a light treatment. Bars represent se. Plants were harvested at 5 (A) and 10 (B) d. FW, Fresh weight.
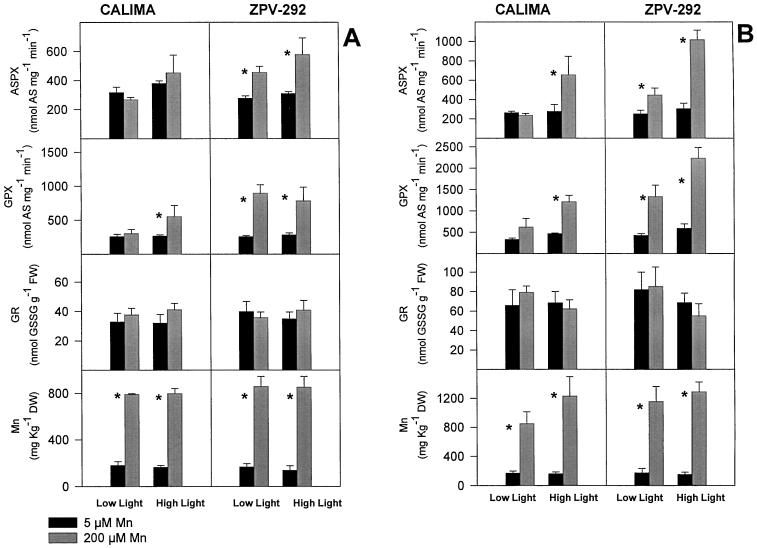
Figure 2.
ASPX, GPX, GR activity, and Mn content of mature leaves of two different bean genotypes grown in silica sand irrigated with nutrient solution containing different doses of Mn (black bars = 5 μm; gray bars = 200 μm Mn). Light intensity was controlled by shading one leaflet with Whatman No. 1 filter paper (Low Light = 220 μmol m−2 s−1; High Light = 870 μmol m−2 s−1). Asterisks represent means (n = 4) that were significantly different (lsd = 5%) from control plants within a light treatment. Bars represent se. Plants were harvested at 5 (A) and 10 (B) d. DW, Dry weight; FW, fresh weight.
Antioxidant Enzymes
The effect of pCMB allowed us to discriminate the reduction of ascorbate attributable to ASPX from that attributable to the less-specific GPX. When leaf extracts were incubated with pCMB, the white bands indicating the activity of ASPX in the activity gel disappeared almost completely. When a leaf extract had high ASPX activity in the spectrophotometric assay, it also displayed wide bands in the activity gel (data not shown). After 5 d of Mn treatment (Fig. 2A), the activity of ASPX increased by 87% and 64% in leaflets of the susceptible genotype ZPV-292 receiving high and low light, respectively; in cv CALIMA, the activity of ASPX in high-Mn plants was not significantly different from that in the control plants at any light treatment (Fig. 2A). A similar response pattern was observed after 10 d (Fig. 2B), but the effect of light intensity was more evident: in leaflets receiving high light the activity of ASPX increased by 270% in the susceptible cv ZPV-292 and by 130% in the tolerant cv CALIMA. At low light, even though there was an increase in the activity of ASPX, the magnitude was substantially lower; nonetheless, genotypic differences were conserved (Fig. 2B). It is important to note that at least in the susceptible genotype ZPV-292, the differential increase in activity of ASPX between high and low light occurred at identical foliar Mn.
GR did not respond to either Mn treatment or light intensity in either genotype during the course of this study (Fig. 2); activities measured in high-Mn treatment were not significantly different from those in corresponding control treatments.
GPX activity measured in these samples corresponded to changes in absorbance observed in the assay of ASPX when it was run in the presence of the inhibitor pCMB. Presumably, pCMB inhibits ASPX and the remaining activity is the contribution of GPX (Amako et al., 1994). At 5 d GPX activity increased by almost 250% in the susceptible genotype ZPV-292, whereas in the tolerant genotype CALIMA the activity was not significantly different from that in the control plants with corresponding light treatments (Fig. 2A). At 10 d the effect of light intensity and the differences between genotypes were more striking. GPX activity in cv CALIMA with low light and high Mn was 87% higher than in the control, but in high light it was 160% higher than in the corresponding control. Although in the susceptible genotype ZPV-292 light intensity had less effect, GPX activity was considerably higher than in the tolerant cv CALIMA with similar light and Mn treatments (Fig. 2B).
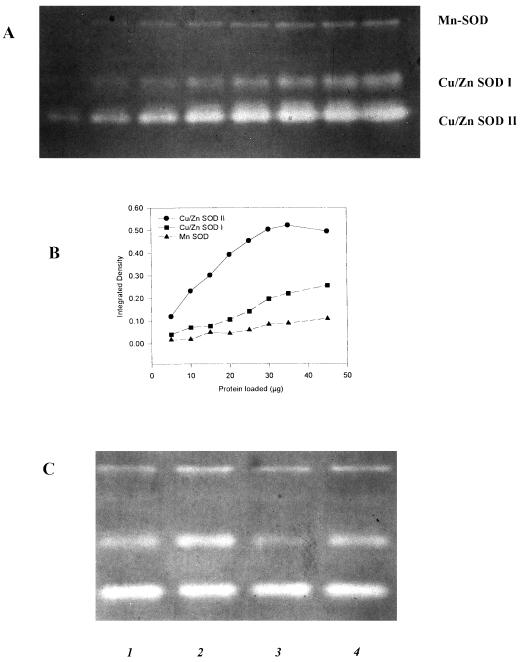
Three white bands representing the activity of SOD were detected in the activity gels (Fig. 3). Gels were loaded with 18 μg of protein, because this amount was within the linear range of the curve of loaded protein versus integrated density of the bands (data not shown). Gels treated with KCN (2 mm) before staining with nitroblue tetrazolium allowed us to differentiate the bands as containing the isozymes Mn-SOD and Cu/Zn-SOD. In KCN-treated gels the middle and bottom bands disappeared, indicating the Cu/Zn nature of these isozymes; the top band was not inhibited by KCN, which is characteristic of Mn-SOD (Bowler et al., 1992, 1994). Occasionally, a faint band appeared between Mn-SOD and Cu/Zn that might represent Fe-SOD, because it was tolerant of KCN but inhibited by H2O2.
Figure 3.
Typical pattern of SOD isozymes observed in bean leaves. A, SOD activity gel loaded with different amounts of protein required to produce an equivalent change in integrated density (B) of SOD bands. C, SOD activity observed in 1, ZPV-292 Control Mn; 2, ZPV-292 High Mn; 3, CALIMA Control Mn; 4, CALIMA High Mn. A nondenaturing gel was used to measure the activity of SOD. Gels were soaked in 100 mL of a solution containing riboflavin 28 μm, KPO4 (36 mm, pH 7.8) and temed (28 mm) for 25 min. This buffer was replaced with 100 mL of NBT (nitroblue tetrazolium) (2 mm) in KPO4 (36 mm, pH 7.8) and shaken for 30 min. Gels were illuminated for 15 min until white bands were detected. The top band was H2O2- and KCN-tolerant which is typical of Mn-SOD. The middle and lower bands disappeared when gels were soaked in KCN, a typical reaction of Cu/Zn-SOD isozymes.
del Rio et al. (1985) reported that excess Zn and Mn induced a Mn-containing SOD in pea. In our study the presence of this occasional band was not associated with a given treatment or genotype, and was not quantified. Peroxidase stained as a blue band at the upper part of the gel and did not migrate to the same distance as SOD (data not shown). Thus, we ruled out the interference of peroxidase with the bands of SOD in the activity gels. The pattern of SOD isozymes observed here has been reported for bean (Corpas et al., 1991) and pea (Burke and Oliver, 1992). For instance, the Mn-SOD, which is resistant to H2O2, appears to be found in the mitochondrial matrix (Bowler et al., 1994, and refs. therein). The Cu/Zn-SODs, which are KCN sensitive, have been studied extensively in many species and exist in more than one cell compartment, but at least one isozyme is always present in the cytosol and is referred to as Cu/Zn-SOD I (Bowler et al., 1994). Another isoform, the Cu/Zn-SOD II, has been found associated with the chloroplast in bean (Corpas et al., 1991), as well as in other species (Bowler et al., 1992, 1994, and refs. therein).
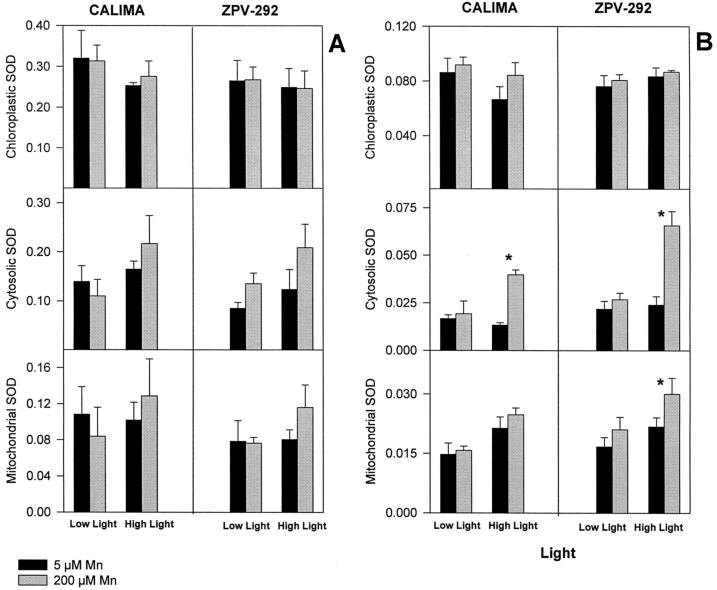
SOD activity responded to Mn and light treatments, but the response was distinct for some isozymes and was different among the two bean genotypes (Fig. 4). At 5 d the activity of Mn-SOD and Cu/Zn-SOD I increased by 43% and 68%, respectively, in leaves receiving high light and high Mn in the susceptible genotype ZPV-292 (Fig. 4). A lower increment in activity of these two isozymes was detected in the tolerant genotype CALIMA. The isozyme Cu/Zn-SOD I (cytosol) increased its activity by approximately 60% in the susceptible genotype regardless of the light treatment; in the genotype CALIMA the activity of this isozyme increased by 31% only in leaves receiving high light and high Mn. No increased activity of Cu/Zn-SOD II (chloroplast) was detected in any of the genotypes or during any of the light treatments at either 5 or 10 d.
Figure 4.
Relative activity of SOD isozymes in mature leaves of two different bean genotypes grown in silica sand irrigated with nutrient solution containing different doses of Mn (black bars = 5 μm; gray bars = 200 μm Mn) for 10 d (experiment 3). Light intensity was controlled by shading one leaflet with Whatman No. 1 filter paper (Low Light = 220 μmol m−2 s−1; High Light = 870 μmol m−2 s−1). Asterisks represent means (n = 3; three leaflets assayed once each) that were significantly different (lsd = 5%) from control plants within a light treatment. See Methods for details about SOD activity and scanning. Plants were harvested at 5 (A) and 10 (B) d.
After 10 d changes in SOD activity were more conspicuous, but differences among both light and genotypes were similar to those observed at 5 d (Fig. 4B). The most notable increment in SOD activity occurred in the cytosolic isozyme in leaves receiving high light. In both genotypes the leaves receiving high Mn and high light had 270% more activity of the Cu/Zn-SOD I isozyme than the control plants at a similar light level.
Water-Soluble Compounds
Ascorbate content declined in samples receiving high Mn, but the extent of the decline depended mainly on the tolerance of the genotypes to Mn-toxicity stress. Within a light treatment control plants of the two genotypes had similar levels of ascorbate. However, more depletion of total ascorbate occurred in the susceptible genotype ZPV-292 at any light treatment. The decline in ascorbate was not followed by an increase in DAS, the oxidized form of ascorbic acid, in any genotype or light level (Fig. 1). At 5 d the excess Mn-stressed plants of the susceptible cv ZPV-292 had 45% less ascorbate than control plants at any light treatment (Fig. 1A). In the genotype CALIMA, although depletion of ascorbate occurred in Mn-stressed plants, it was not significantly different from that in controls at any light level. At 10 d the concentration of AS in the genotype ZPV-292 was about 65% of that in the control, regardless of the light treatment. Leaves of the tolerant genotype CALIMA receiving high Mn and high light had 70% less ascorbate than control leaves receiving high light (Fig. 1), but this difference was not detected at low light.
Lipid Peroxidation
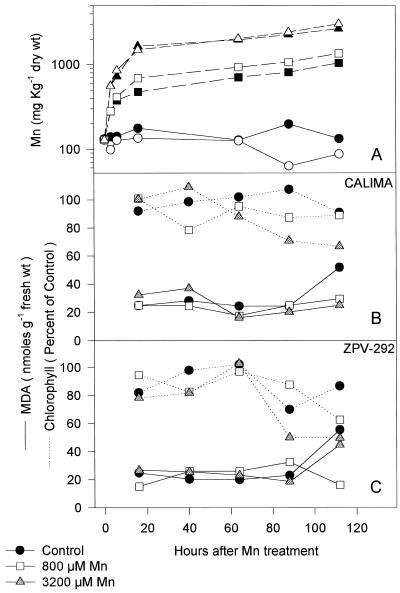
Lipid peroxidation was not detected in leaf discs floating in MnSO4 solution. MDA accumulation, an indication of lipid peroxidation, was not correlated with Mn treatment in leaf discs of mature leaves exposed to high Mn in solution (Fig. 5). Symptoms observed in the leaf discs of the two different genotypes followed very closely the symptoms of Mn toxicity observed in attached leaves. Leaf discs displayed the characteristic brown speckles and chlorosis; discs from the susceptible genotype ZPV-292 were more affected than discs from the genotype CALIMA. The increased MDA content observed after 100 h was also detected in control leaf discs, indicating that the discs were senescing at this time but that there was no effect of excess Mn, despite Mn accumulation in tissue up to 2200 mg kg−1 dry weight by that time (Fig. 5).
Figure 5.
Mn concentration (A), chlorophyll content, and MDA concentration in leaf discs of mature leaves of two different genotypes (CALIMA [tolerant, B] and ZPV-292 [susceptible, C]). Leaf discs were floated in Petri dishes holding a nutrient solution containing different levels of Mn (0.01, 0.8, and 3.2 mm Mn as MnSO4). Leaf discs received 670 μmol photons PAR m−2 s−1 for 12 h daily. The composition of the nutrient solution is described in Methods. Each point represents the average of two observations. In A, closed symbols represent Mn concentration in the discs of the tolerant genotype CALIMA, and open symbols represent Mn concentration in the discs of the susceptible genotype ZPV-292.
DISCUSSION
In this report we examined the combined effect of light intensity and excess Mn on both chlorophyll content and level and activity of antioxidants in leaves of two common bean genotypes. Although previous studies have suggested that low illumination delayed (McCool, 1935; Sirkar and Amin, 1974; Horiguchi, 1988; Nable et al., 1988) or even increased (Wissemeier and Horst, 1992) the symptoms of Mn toxicity, only one study focused specifically on the effect of light intensity on chlorophyll content of Mn-stressed plants, and to our knowledge no previous reports have addressed the combined effect of light intensity and Mn toxicity on leaf antioxidants. Horiguchi (1988) grew common bean and maize under four different light regimes (100%, 80%, 40%, and 5% of the total radiation) and several Mn levels. Leaf chlorophyll content for all three light levels were compared in plants grown at 32 ppm Mn and control plants (0.32 ppm Mn). At high light there was a reduction in leaf chlorophyll content of 48%, followed by 40% at medium light and 0% at low-light treatment; however, there was a 4.6-fold difference in foliar Mn between leaves grown in high light (1820 ppm Mn) and low light (396 ppm Mn), which confounds the effect of light intensity with the effect of foliar Mn concentration. Nevertheless, although quantitative data were not presented, the author claimed that bean leaves having 340 ppm Mn and grown at high light displayed severe chlorosis.
By shading one lateral leaflet from a trifoliate leaf we obtained equal accumulation of Mn in leaves receiving different light intensities; therefore, by having equal tissue Mn we were able to separate the effect of light intensity from the contribution of excess foliar Mn on chlorophyll and antioxidant levels. Our results demonstrated that, at similar foliar Mn content, leaves exposed to high light suffered more chlorosis than those receiving less light. We previously reported that within the same plant and at similar Mn content, young leaves suffered more chlorosis than mature leaves (González and Lynch 1997), suggesting that different mechanisms of Mn toxicity might occur at different leaf developmental stages.
Leaf antioxidant content varies with growing conditions, and the effect of light intensity has been addressed recently (Slooten, et al., 1995; Grace and Logan, 1996). Plants growing under high light are more frequently exposed to reactive oxygen species (the superoxide radical, H2O2, and singlet oxygen) generated in the plastids than plants growing at low light intensity, and consequently might respond by accumulating higher amounts of antioxidants in leaf tissue. The higher accumulation of antioxidants might protect the tissue against other events that result in oxidative stress. For instance, the extra accumulation of antioxidants in pea plants grown at a relatively high light intensity, compared with plants grown in low light seemed to confer more tolerance to paraquat (Gillham and Dodge, 1987). In our experiments control leaflets of both genotypes receiving high light contained greater amounts of antioxidants than leaflets receiving low light, except for GR and GPX activity. This scenario changed when Mn toxicity was imposed, and the antioxidant systems of the two genotypes responded differently.
SOD is responsible for the dismutation of superoxide radicals, with the subsequent production of H2O2. Because H2O2 is also highly toxic, any increase of SOD activity must be followed by a mechanism that controls H2O2, such as ASPX. Two SOD isozymes increased their activity in excess-Mn-stressed leaves: Cu/Zn-SOD I and Mn-SOD, located in the cytosol and in the mitochondria, respectively. Up-regulation of these enzymes by Mn-toxicity stress occurred to a greater extent in leaves of the susceptible genotype ZPV-292 and was enhanced under high-light conditions. Because one of the toxicity effects of excess Mn is chlorosis, we expected to see the effects of Mn-toxicity stress and genotype on the activity of chloroplastic SOD. Surprisingly, the chloroplast-located isozyme (Cu/Zn-SOD II) did not respond to Mn treatment in any of the genotypes or at any light treatment, not even at 10 d after 34% chlorosis was observed in leaves of the susceptible cv ZPV-292. Although Cu/Zn-SOD II did not respond to Mn toxicity, one might not rule out a direct effect of excess Mn in the chloroplast of mature leaves. Even with oxidative stress caused by paraquat, which is believed to target the chloroplast, increases in cytosolic and plastidic Cu/Zn-SOD have been reported (Perl-Treves and Galun, 1991). Aono et al. (1991) showed that transformed plants expressing bacterial GR in the cytosol were more resistant to paraquat than untransformed plants. More recently, Donahue et al. (1997) described an increment in cytosolic ASPX transcript in pea leaves treated with paraquat, and no responses of any isoform of SOD were detected, but a new Fe-SOD isoform was induced by paraquat that was not associated with tolerance.
Changes in the activity of SODs were reported among several soybean genotypes with different responses to Mn toxicity (Leidi et al., 1987a). The authors found that the susceptible genotype Bragg showed elevated total SOD under conditions of Mn toxicity and low Fe. However, it is difficult to discern a pattern among genotypes because Lee, a genotype regarded as tolerant of excess Mn (Heenan and Carter, 1976), also displayed elevated SOD activity. Leidi et al. (1987b) tested the effect of various Fe and Mn concentrations on soybean. These authors determined the activity of Mn-SOD and Cu/Zn-SOD by assaying the leaf extracts in the presence of 1 mm NaCN. Their results indicated that most of the increased activity in response to the Fe- and Mn-toxicity stress corresponded to Mn-SOD, with very little associated with Cu/Zn-SOD. This function is contrary to the findings of most reports, which indicate that Cu/Zn is the most abundant isozyme in soybean and in most of the species assayed (Bowler et al., 1994). Leidi et al. (1987b) pointed out that these values were unexpectedly low compared with the values observed when SOD was electrophoretically determined. The effect of Mn toxicity, according to the authors, resulted in the elevated activity of Mn-SOD regardless of the Fe level in the growing medium (Leidi et al., 1987b). In our studies we detected a slight increase in Mn-SOD activity, mainly in the susceptible cv ZPV-292, but the most significant response was observed in the cytosolic SOD (Cu/Zn-SOD I) in high-light and excess-Mn treatments.
Several isozymes of ASPX have been identified; some are located in the chloroplast, and the others are thought to occur in the cytosol (Foyer et al., 1994). Of the two isozymes in the chloroplast, one is bound to the thylakoid membranes (Miyake and Asada, 1992) and the other is in the stroma (Chen and Asada, 1989). From our results it is not possible to tell which isozyme of ASPX is responding to the Mn treatment. However, we could speculate that perhaps it is the cytosolic fraction that is being activated under Mn toxicity. This speculation is based on the observed increment of the activity of the cytosolic isozyme of SOD and the consequent production of H2O2. It is more likely that the ASPX activity observed is responding to the accumulation of the substrate (H2O2) in the putative site where it is being produced. After 5 d of Mn treatment, SOD and ASPX increased their activity by 68% and 86%, respectively, and after 10 d this increment was 275% in SOD activity and 235% in ASPX activity, indicating a close coordination in their response to control oxygen reactive species, perhaps within the same cell compartment. Also, Mitler and Zilinskas (1993) reported that the activity of the ASPX detected in native gels co-migrated with the pure cytosolic ASPX enzyme, and that their attempts to detect chloroplastic ASPX were successful only when fresh, intact chloroplasts were ruptured and subjected to native electrophoresis. No detection of chloroplastic ASPX was possible when frozen chloroplasts or leaf extracts were used for native electrophoresis (Mitler and Zilinskas, 1993). When we tested the effect of pCMB by assaying the activity of ASPX in the activity gels, we used conditions similar to those used by Mitler and Zilinskas (1993), and the samples with higher ASPX activity in the spectrophotometric assay also displayed wide bands in the gels, presumably representing the activity of cytosolic ASPX.
GR activity was not altered in plants under Mn-toxicity stress. A small decrease in nonprotein SH groups, an indicator of GSH in beans (Guzy and Heath, 1993), was observed only at 10 d and mainly at high light. This result is quite interesting because glutathione is involved in the regeneration of reduced ascorbic acid, a reaction catalyzed by DAS reductase. If ascorbate is oxidized to monodehydroascorbate and then to DAS, one would expect a depletion of the GSH pool and increased activity of GR, to keep the GSSG-to-GSH ratio in balance. However, ascorbate could also be regenerated by monodehydroascorbate reductase (Foyer, 1993). Measurement of total ascorbate showed that a depletion of AS was not followed by an accumulation of DAS, refuting the possibility that GR or DAS reductase is inhibited under Mn toxicity stress and is the cause of the decreased pool of AS.
Ascorbic acid (for review, see Foyer, 1993) is one of the essential water-soluble antioxidants present in millimolar concentrations in the chloroplast, and is the substrate used by ASPX to control the level of photosynthetically generated H2O2 (Nakano and Asada, 1981) as part of the ascorbate-glutathione cycle. Also, ascorbate is involved in the regeneration of the membrane-bound antioxidants α-tocopherol and zeaxanthin, affording protection against lipid peroxides and singlet oxygen, respectively. The pool of ascorbate in the cell is kept at a fairly constant level, and the loss of ascorbate might reflect the degree of stress imposed (Stegmann et al., 1991). Because ascorbate functions in leaves mainly as a reductant, its redox state is pivotal for its function as an antioxidant (Foyer, 1993). A higher increase in the chloroplast ascorbate content of NaCl-treated leaves was found in a tolerant pea genotype (Hernández et al., 1995). Bean genotypes tolerant of ozone stress had a higher ascorbate content than the susceptible genotypes (Guzi and Heath, 1993). Change in the ascorbate redox state from reduced to oxidized ascorbate was found in tobacco leaves exposed to Fe stress (Kampfenkel et al., 1995) without net changes in the amount of ascorbate. Sirkar and Amin (1974) found a slight increase in total ascorbate in cotton plants suffering Mn toxicity.
We observed that under Mn-toxicity stress both genotypes decreased the total content of ascorbate in leaf tissue, especially in plants grown in high light. The different genotypes did not differ in the ascorbate content of unstressed leaves. However, higher depletion of ascorbate occurred in the Mn-susceptible genotype ZPV-292. The possibility that differences in ascorbate depletion between genotypes is caused by different foliar Mn concentrations is discounted, because the two genotypes accumulated Mn at comparable rates (Fig. 2). In mature leaves of ZPV-292 50% depletion of AS was observed in plants with less than 15% chlorosis. These results indicate that depletion of ascorbate occurred before the onset of visible chlorosis. Although the amount of AS was always lower in Mn-treated leaves, it was not reflected in increments of its oxidized form, DAS. The function of ascorbic acid as an antioxidant is centered on its reduced form, and it would be important to know how plants under Mn-toxicity stress regenerate or restore the pool of ascorbate.
The mechanism of how Mn depletes leaf ascorbate is not clear. The scenario presented by our data indicates that the susceptible genotype is “spending” more of the ascorbate pool to protect against excess Mn in the tissue, but might be left with less protection for normally produced reactive oxygen species. More reactive oxygen species might promote the increase in activities of antioxidant enzymes such as SOD and ASPX, as found in leaves of the susceptible genotype. The mechanism of how excess Mn interacts with ascorbate is not known and requires further investigation. It has been reported that ascorbic acid is the main source of carbon for oxalic acid in several species (Loewus, 1988), and that Mn was bound to oxalate in Mn hyperaccumulator plants (Memon and Yatazawa, 1984). Thus, if susceptible genotypes respond to excess Mn by increasing the synthesis of oxalate at the expense of ascorbic acid, the deleterious effect of Mn toxicity might be indirectly enhanced because of a depletion of this important antioxidant. In nonaqueous fractionation studies (González, 1996) oxalate-like crystals were found to accumulate high levels of Mn in leaves of both genotypes, although no quantitative comparison among genotypes was attempted. Tolerant genotypes might have developed a different strategy to deal with excess Mn in leaf tissue, such as organelle or tissue compartmentation.
Lipid peroxidation was not induced by Mn-toxicity stress in leaf discs of mature leaves. Preliminary experiments with leaf discs of immature leaves showed that necrosis occurred in this type of tissue, matching the symptoms observed in young leaves of intact plants, and perhaps lipid peroxidation occurs in immature leaves. The observation that lipid peroxidation was induced by excess Mn in isolated chloroplasts (Panda et al., 1986) may differ from ours because of the experimental system used.
The fact that plants under Mn-toxicity stress experienced more chlorosis under high irradiance suggests that Mn toxicity includes a photooxidative component. We have presented evidence that the antioxidant system is highly stimulated under Mn-toxicity stress, mainly in the susceptible genotype ZPV-292. This observation does not rule out the possibility that tolerant genotypes might have a better antioxidant system that protects them from damage by Mn, but certainly SOD or ASPX do not seem to be responsible for the tolerance manifested by the genotype CALIMA, because they had higher activity in the susceptible genotype. Recently, Foy et al. (1995) found that the Mn-tolerant soybean genotype Lee was more tolerant to ozone stress than the Mn-susceptible genotype Bragg. The tolerance of soybean genotypes to ozone stress was associated with the endogenous presence of kaempferol glycosides, particularly K3 through K6 and K9. Flavonoids can absorb short wavelengths and protect the plants against the damaging effect of UV light. Thus, because light is a determining element in both the onset of chlorosis and the increment of antioxidant enzyme activities in Mn-treated plants, the relationship between light-absorbing substances and tolerance to Mn toxicity deserves further study.
Our hypothesis to explain chlorosis in mature leaves is related to the redox state and the content of ascorbic acid in plants under Mn-toxicity stress. We have seen that mature leaves of excess Mn-stressed plants usually do not develop severe chlorosis under reduced irradiance, but chlorophyll is lost shortly after low-light-adapted leaves are exposed to high light. If the presence of Mn decreases the content of ascorbic acid, then leaf tissue is left unprotected against reactive oxygen species normally produced in the chloroplast and at faster rates under high-light conditions. These oxygen species might be responsible for the chlorosis observed in mature leaves. Because the content of ascorbic acid in the chloroplast depends on a constant supply from the cytosol (Foyer, 1993), where ascorbic acid is synthesized (Loewus and Loewus, 1987), events happening outside of the chloroplast leave this organelle with lower concentrations of antioxidant, a situation that promotes chlorosis when the tissue is exposed to higher irradiance. This chain of events would not require excess Mn in chloroplasts of mature leaves.
Abbreviations:
- AS
reduced ascorbate
- ASPX
ascorbate peroxidase
- DAS
dehydroascorbate
- DTNB
5,5′-dithiobis(2-nitrobenzoic acid)
- GPX
guaiacol peroxidase
- GR
glutathione reductase
- GSSG
oxidized glutathione
- MDA
malondialdehyde
- pCMB
p-chloromercuribenzoate
- SOD
superoxide dismutase
Footnotes
This research was partially supported by the Centro Internacional de Agricultura Tropical.
LITERATURE CITED
- Allen RD. Dissection of oxidative stress tolerance using transgenic plants. Plant Physiol. 1995;107:1049–1054. doi: 10.1104/pp.107.4.1049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alscher RG, Hess JL. Antioxidants in Higher Plants. Boca Raton, FL: CRC Press; 1993. [Google Scholar]
- Amako K, Chen G, Azada K. Separate assays of ascorbate peroxidase and guaiacol peroxidase and for the chloroplastic and cytosolic isozymes of ascorbate peroxidase in plants. Plant Cell Physiol. 1994;35:497–504. [Google Scholar]
- Aono M, Kubo A, Saji H, Natori T, Tanaka K, Kondo N. Resistance to active oxygen toxicity of transgenic Nicotiana tabacum that express the gene for glutathione reductase from Escherichia coli. Plant Cell Physiol. 1991;32:691–697. [Google Scholar]
- Beauchamp C, Fridovich I. Superoxide dismutase: improved assays and assays applicable to acrylamide gels. Anal Biochem. 1971;44:276–287. doi: 10.1016/0003-2697(71)90370-8. [DOI] [PubMed] [Google Scholar]
- Bowler C, Van Camp W, Van Montagu M, Inze D. Superoxide dismutase in plants. Crit Rev Plant Sci. 1994;13:199–218. [Google Scholar]
- Bowler C, Van Montagu, Inze D. Superoxide dismutase and stress tolerance. Annu Rev Plant Physiol Plant Mol Biol. 1992;43:83–116. [Google Scholar]
- Burke JJ, Oliver MJ. Differential temperature sensitivity of pea superoxide dismutases. Plant Physiol. 1992;100:1595–1598. doi: 10.1104/pp.100.3.1595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burnell JN. The biochemistry of manganese in plants. In: Graham RD, Hannam J, Uren NC, editors. Manganese in Soils and Plants. Dordrecht, The Netherlands: Kluwer Academic Publishers; 1988. pp. 125–133. [Google Scholar]
- Campbell LC, Nable RS. Physiological function of manganese in plants. In: Graham RD, Hannam J, Uren NC, editors. Manganese in Soils and Plants. Dordrecht, The Netherlands: Kluwer Academic Publishers; 1988. pp. 87–95. [Google Scholar]
- Carver BF, Ownby JD. Acid soil tolerance in wheat. Adv Agron. 1995;54:117–173. [Google Scholar]
- Chen GX, Azada K. Ascorbate peroxidase in tea leaves: occurrence of two isozymes and the differences in their enzymatic and molecular properties. Plant Cell Physiol. 1989;30:987–998. [Google Scholar]
- Corpas FJ, Sandalio LM, Palma JM, Leidi EO, Hernández JA, Sevilla F, del Rio LA. Subcellular distribution of superoxide dismutase in leaves of ureide-producing leguminous plants. Physiol Plant. 1991;82:285–291. [Google Scholar]
- Daza MC, Sandalio LM, Quijano-Rico M, del Rio LA. Isoenzyme pattern of superoxide dismutase in coffee leaves from genotypes susceptible and resistant to the rust Hemileia vastatrix. J Plant Physiol. 1993;141:521–526. [Google Scholar]
- del Rio LA, Sandalio LM, Yanez J, Gomez M. Induction of a manganese-containing superoxide dismutase in leaves of Pisum sativum L. by high nutrient levels of zinc and manganese. J Inorg Biochem. 1985;24:25–34. [Google Scholar]
- De Vos CHR, Schat H (1991) Free radicals and heavy metal tolerance. In J Rozeman, JAC Verkleij, eds, Ecological Responses to Environmental Stresses. Kluwer Academic Publishers, Dordrecht, The Netherlands, pp 22–30
- Donahue JL, Okpodu CM, Cramer CL, Grabau EA, Alscher RG. Response of antioxidants to paraquat in pea leaves. Plant Physiol. 1997;113:249–257. doi: 10.1104/pp.113.1.249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Du Z, Bramlage WJ. Modified thiobarbituric acid assay for measuring lipid oxidation in sugar-rich plant tissue extracts. J Agric Food Chem. 1992;40:1566–1570. [Google Scholar]
- Elamin OM, Wilcox GE. Effect of magnesium and manganese nutrition on muskmelon growth and manganese toxicity. J Am Soc Hortic Sci. 1986;111:582–587. [Google Scholar]
- Foy CD, Chaney RL, White MC. The physiology of metal toxicity in plants. Annu Rev Plant Physiol. 1978;29:511–566. [Google Scholar]
- Foy CD, Lee EH, Rowland R, Devine TE, Buzzell RI. Ozone tolerance related to flavonol glycoside genes in soybean. J Plant Nutr. 1995;18:637–647. [Google Scholar]
- Foyer C. Ascorbic acid. In: Alscher RG, Hess JL, editors. Antioxidant in Higher Plants. Boca Raton, FL: CRC Press; 1993. pp. 31–58. [Google Scholar]
- Foyer C, Mullineaux P. Causes of Photooxidative Stress and Amelioration of Defense System in Plants. Boca Raton, FL: CRC Press; 1994. [Google Scholar]
- Foyer CH, Descourvières P, Kunert KJ. Protection against oxygen radicals: an important defence mechanism studied in transgenic plants. Plant Cell Environ. 1994;17:507–523. [Google Scholar]
- Gerretsen FC. Manganese in relation to photosynthesis. III. Uptake of oxygen by illuminated crude chloroplasts suspensions. Plant Soil. 1950;2:323–342. [Google Scholar]
- Gillham DJ, Dodge AD. Chloroplast superoxide and hydrogen peroxide scavenging systems from pea leaves: seasonal variations. Plant Sci. 1987;50:105–109. [Google Scholar]
- González A (1996) Identification of the mechanisms of Mn toxicity and tolerance in common bean. PhD thesis. The Pennsylvania State University, University Park
- González A, Lynch J. Effects of manganese toxicity on leaf CO2 assimilation of contrasting common bean genotypes. Physiol Plant. 1997;11:872–880. [Google Scholar]
- Gossett DR, Millhollon EP, Lucas MC. Antioxidant response to NaCl stress in salt-tolerant and salt-sensitive genotypes of cotton. Crop Sci. 1994;34:706–714. [Google Scholar]
- Grace SC, Logan BA. Acclimation of foliar antioxidant system to growth irradiance in three broad-leaved evergreen species. Plant Physiol. 1996;112:1631–1640. doi: 10.1104/pp.112.4.1631. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guikema JA, Sherman LA. Electrophoretic profiles of cyanobacterial membrane polypeptides showing heme-dependent peroxidase activity. Biochim Biophys Acta. 1980;637:189–201. [Google Scholar]
- Guzy M, Heath RL. Response to ozone of varieties of common bean (Phaseolus vulgaris L.) New Phytol. 1993;124:617–625. doi: 10.1111/j.1469-8137.1993.tb03851.x. [DOI] [PubMed] [Google Scholar]
- Heenan DP, Carter OG. Tolerance of soybean genotypes to manganese toxicity. Crop Sci. 1976;16:389–391. [Google Scholar]
- Heenan DP, Carter OG. Influence of temperature on the expression of manganese toxicity by two soybean varieties. Plant Soil. 1977;47:219–227. [Google Scholar]
- Hernández JA, Olmos E, Corpas FJ, Sevilla F, del Rio LA. Salt-induced oxidative stress in chloroplasts of pea plants. Plant Sci. 1995;105:151–167. [Google Scholar]
- Horiguchi T. Mechanism of manganese toxicity and tolerance of plants VII. Effect of light intensity on manganese-induced chlorosis. J Plant Nutr. 1988;11:235–245. [Google Scholar]
- Horst WJ. The physiology of manganese toxicity. In: Graham RD, Hannam J, Uren NC, editors. Manganese in Soils and Plants. Dordrecht, The Netherlands: Kluwer Academic Publishers; 1988. pp. 175–188. [Google Scholar]
- Inskeep WP, Bloom PR. Extinction coefficients of chlorophyll a and b in N,N-dimethylformamide and 80% acetone. Plant Physiol. 1985;77:483–485. doi: 10.1104/pp.77.2.483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kampfenkel K, Van Montagu M, Inze D. Effects of iron excess on Nicotiana plumbaginifolia plants. Implications to oxidative stress. Plant Physiol. 1995;107:725–735. doi: 10.1104/pp.107.3.725. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Law MY, Charles SA, Halliwell B. Glutathione and ascorbic acid in spinach (Spinacia oleracea) chloroplasts: the effect of hydrogen peroxide and of paraquat. Biochem J. 1983;210:899–903. doi: 10.1042/bj2100899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leidi EO, Gomez M, de la Guardia MD. Soybean genetic differences in response to Fe and Mn: activity of metalloenzymes. Plant Soil. 1987a;99:139–146. [Google Scholar]
- Leidi EO, Gomez M, del Rio LA. Evaluation of biochemical indicators of Fe and Mn nutrition for soybean plants. II. Superoxide dismutase, chlorophyll contents and photosystem II activity. J Plant Nutr. 1987b;10:261–271. [Google Scholar]
- Loewus FA. Ascorbic acid and its metabolic products. In: Preiss J, editor. The Biochemistry of Plants: A Comprehensive Treatise. London: Academic Press; 1988. pp. 85–107. [Google Scholar]
- Loewus FA, Loewus MW. Biosynthesis and metabolism of ascorbic acid in plants. Crit Rev Plant Sci. 1987;5:101–119. [Google Scholar]
- McCain DC, Markley JL. More manganese accumulates in maple sun leaves than in shade leaves. Plant Physiol. 1989;90:1417–1421. doi: 10.1104/pp.90.4.1417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McCool MM. Effect of light intensity on the manganese content of plants. Contrib Boyce Thompson Inst. 1935;7:427–437. [Google Scholar]
- Memon AR, Yatazawa M. Nature of manganese complexes in manganese accumulator plant Anthopanax sciadophylloides. J Plant Nutr. 1984;7:961–974. [Google Scholar]
- Mitler R, Zilinskas BA. Detection of ascorbate peroxidase activity in native gels by inhibition of the ascorbate-dependent reduction of nitroblue tetrazolium. Anal Biochem. 1993;212:540–546. doi: 10.1006/abio.1993.1366. [DOI] [PubMed] [Google Scholar]
- Miyake C, Asada K. Thylakoid-bound ascorbate peroxidase in spinach chloroplasts and photoreduction of its primary oxidation product monodehydroascorbate radicals in thylakoids. Plant Cell Physiol. 1992;35:541–553. [Google Scholar]
- Nable RO, Houtz RL, Cheniae GM. Early inhibition of photosynthesis during development of Mn toxicity in tobacco. Plant Physiol. 1988;86:1136–1142. doi: 10.1104/pp.86.4.1136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakano Y, Asada K. Hydrogen peroxide is scavenged by ascorbate-specific peroxidase in spinach chloroplasts. Plant Cell Physiol. 1981;22:867–880. [Google Scholar]
- Panda S, Mishra AK, Biswal UC. Manganese-induced modification of membrane lipid peroxidation during aging of isolated wheat chloroplasts. Photobiochem Photobiophys. 1986;13:53–61. [Google Scholar]
- Perl-Treves R, Galun E. The tomato Cu,Zn superoxide dismutase genes are developmentally regulated and respond to light and stress. Plant Mol Biol. 1991;17:745–760. doi: 10.1007/BF00037058. [DOI] [PubMed] [Google Scholar]
- Rufty TW, Miner GS, Raper CD., Jr Temperature effects on growth and manganese tolerance in tobacco. Agron J. 1979;71:638–644. [Google Scholar]
- Sevilla F, Almansa MS, Hellin E, Alcaraz CF (1988) Influence of the citrus species and varieties on the isozyme profile of iron-superoxide dismutase. In R Goren, K Mendel, N Goren, eds, Proceedings of the Sixth International Citrus Congress. Balaban Publishers, Rehovot, Israel, pp 561–570
- Sirkar S, Amin JV. The manganese toxicity of cotton. Plant Physiol. 1974;54:539–543. doi: 10.1104/pp.54.4.539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Slooten L, Capiau K, Van Camp W, Van Montagu M, Sybesma C, Inze D. Factors affecting the enhancement of oxidative stress tolerance in transgenic tobacco overexpressing manganese superoxide dismutase in the chloroplasts. Plant Physiol. 1995;107:737–750. doi: 10.1104/pp.107.3.737. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith IK, Vierheller TLTCA. Assay of glutathione reductase in crude tissues homogenates using 5,5′-dithiobis(2-nitrobenzoic acid) Anal Biochem. 1988;175:408–413. doi: 10.1016/0003-2697(88)90564-7. [DOI] [PubMed] [Google Scholar]
- Stegmann HB, Schuler P, Ruff HJ, Knollmuller M, Loreth W. Ascorbic acid as an indicator of damage to forest: a correlation with air quality. Z Naturforsch. 1991;46C:67–70. [Google Scholar]
- Tanaka K, Suda Y, Kondo N, Sugahara K. O3 tolerance and ascorbate dependent H2O2 decomposing systems in chloroplasts. Plant Cell Physiol. 1985;26:1425–1431. [Google Scholar]
- Vallejos E. Enzyme activity staining. In: Stansley SD, Orton TJ, editors. Isozymes in Plant Genetics and Breeding, Part A. Amsterdam: Elsevier Science Publishers; 1983. pp. 469–516. [Google Scholar]
- Wissemeier AH, Horst WJ. Effect of light intensity on manganese toxicity symptoms and callose formation in cowpea (Vigna unguiculata) Plant Soil. 1992;143:299–309. [Google Scholar]