Abstract
Mental set switching is a key facet of executive control measured behaviorally through reaction time or accuracy (i.e., ‘switch costs’) when shifting among task types. One of several experimentally-dissociable influences on switch costs is ‘task set inertia’, conceptualized as the residual interference conferred when a previous stimulus-response tendency interferes with subsequent stimulus processing on a new task. Task set inertia is thought to represent the passive decay of the previous stimulus-response set from working memory, and its effects decrease with increased interstimulus interval. Closely spaced trials confer high task set inertia, while sparsely spaced trials confer low task set inertia. This functional magnetic resonance imaging (fMRI) study characterized, for the first time, two opposing brain systems engaged to resolve task set inertia: 1) a frontoparietal ‘cortical control’ network for overcoming high task set inertia interference and 2) a subcortical-motor network more active during trials with low task set inertia. These networks were distinct from brain regions showing general switching effects (i.e., switch > non-switch) and from other previously-characterized interference effects. Moreover, there were ongoing maturational effects throughout adolescence for the brain regions engaged to overcome high task set inertia not seen for generalized switching effects. These novel findings represent a new avenue of exploration of cognitive set switching neural function.
Keywords: fMRI, set switching, set shifting, task set inertia, development
INTRODUCTION
A key facet of executive control is the capacity to rapidly, flexibly, and effectively switch mental sets among different types of information in a quickly-changing environment, commonly referred to as ‘set-switching’ (also termed ‘set-shifting’, ‘task-switching’, ‘attention-switching’, ‘task-shifting’, or ‘attention-shifting’) (Corbetta et al., 1993; Huston et al., 1937; Jersild, 1927; Smith et al., 2001; Wager et al., 2004). Despite the numerous and often conflated terms used to describe this cognitive process, it is generally assumed that the act of switching is subserved by a set of executive control parameters necessary to complete the task, termed a ‘task set’ (Logan and Gordon, 2001; Rogers and Monsell, 1995). Behaviorally, the presence of executive control needed to effect a mental set switch can be quantified in terms of ‘switch costs.’ Switch costs are typically defined as loss of accuracy or response speed when one task is interrupted in order to switch to another. Several behavioral studies have shown that switch costs involve multiple, experimentally dissociable influences (Allport et al., 1994; Meiran, 1996; Rogers and Monsell, 1995). Logan and Gordon (2001) theorized that switch costs measure the time it takes to implement new executive control parameters in a ‘task set’. Others have proposed a proactive component of control that partially reconfigures cognitive resources when impending set switches are prompted by an external cue, termed task-set reconfiguration (Meiran, 1996, 2000; Rogers and Monsell, 1995). Although such cue-guided mental preparation greatly reduces switch costs, it does not eliminate them (Cepeda et al., 2001; Meiran, 1996). The presence of residual behavioral costs implies that there is at least one other important cognitive factor at play in mediating switches of mental set. Another component has been conceptualized as ‘task set inertia’ (Allport et al., 1994; Meiran, 1996). Task set inertia is believed to represent the degree of residual interference conferred when a previous stimulus-response tendency interferes with the next stimulus (Rogers and Monsell, 1995). Unlike task-set reconfiguration, task set inertia is thought to be passive and to decrease with time as the previous task set decays from working memory (Allport et al., 1994). Initially proposed to last several minutes (Allport et al., 1994), subsequent studies have shown that task set inertia resolves immediately following a switch during ongoing performance (Rogers and Monsell, 1995). Experimentally, the influence of task set inertia on switch costs can be seen by altering the interval between trials, with shorter intervals corresponding to greater degree of residual interference to overcome and producing greater behavioral switch costs.
The behavioral independence of task set inertia from other influences on switch costs (Rogers and Monsell, 1995) suggests that overcoming this residual interference could require distinct neural resources, perhaps similar to those engaged to overcome either stimulus-response compatibility interference or Stroop interference (Nee et al., 2007). The neural correlates of mental set switching have been fairly well described in two separate meta-analyses of 31 (Wager et al., 2004) and 18 (Buchsbaum et al., 2005) fMRI and PET studies. These meta-analyses have linked set switching to numerous cortical regions, including the caudal anterior cingulate cortex, premotor cortex, bilateral inferior parietal sulci, and right dorsolateral prefrontal cortex, as well as bilateral insula, and bilateral thalamus. Although many of these regions have been individually linked to other executive abilities such as working memory and response inhibition (Wager et al., 2004; Wager and Smith, 2003), there is evidence that these brain regions taken together are reliably and specifically engaged more during switch trials than during non-switch trials across several types of set switching tasks (Wager and Smith, 2003). Such an overlap between switching and other cognitive processes should not be wholly unexpected as switching is thought to rely on the integrity of several cognitive systems, including attention, inhibition, and working memory (Miyake et al., 2000). Several studies have also suggested that the basal ganglia play a role in set switching (Casey et al., 2004; Casey et al., 2002; Luna et al., 2001; Sohn et al., 2000). However, because basal ganglia engagement has not been consistently supported by set switching neuroimaging studies, it has been proposed that frontostriatal, or perhaps other prefrontal-subcortical, engagement in set switching is required only when the experimental paradigm requires the inhibition of certain competing cognitive or motor responses (Cools, 1980; Mink, 1996). Alternatively, the purpose of subcortical brain activation in this context might instead be to help maintain or switch between competing behavioral sets, as has been previously proposed (Alexander and Crutcher, 1990). Within the mental set switching literature, however, there is no consensus as to a definitive role for the basal ganglia in the underlying cognitive processes.
In sum, although previous behavioral and functional neuroimaging studies have firmly established the existence of residual task-set inertia switch costs, their neural correlates and functional interpretation remain to be determined. No previous investigators have sought to characterize the neural correlates of task-set inertia as distinct from set switching in general, despite the likelihood of its unique and important contribution to cognitive control. At least one study has indirectly examined possible task set inertia effects. Although it was not the primary aim of the study, Loose et al., (2006) contrasted fMRI-measured brain activity to switch trials preceded by a short (850 msec) versus a longer interval (1600 msec). They reported greater bilateral dorsolateral prefrontal activation to shorter intervals, interpreted as greater top-down control (Loose et al., 2006). However, this study was limited by a small sample size, an inability to fully dissociate brain activity to different types of events due to its block design, and not holding task set reconfiguration demands constant to ensure the neural results were specific to overcoming task set inertia. The vast majority of other published neuroimaging studies have examined set switching by contrasting switch and non-switch trials on average, which does little to differentiate the separate contributions of different cognitive processes and brain function to switch costs.
Several studies have shown mental set switching task performance reaches near-adult levels around age 12, with non-significant switch cost decreases between adolescence and adulthood thereafter (Anderson, 2002; Casey et al., 2004; Davidson et al., 2006; Rubia et al., 2006; Waber et al., 2007). A recent behavioral study (Crone et al., 2006a) found switch costs decreased both with increasing interstimulus interval length and age, indicating that children and early adolescents have more difficulty than adults performing mental set shifts in the presence of high task set inertia. Two previous fMRI developmental set switching studies found numerous brain regions exhibiting positive and negative age-related activity changes (Casey et al., 2004; Rubia et al., 2006), suggesting a progressive development in task-specific frontoparietal and striatal regions related to an increased capacity of cognitive control functions. This indicates that although near-adult levels of set switching behavioral performance are achieved by adolescence, there remain important maturational changes in set switching related neural activity until adulthood. No study has yet examined task set inertia development specifically, so it is unclear how similar developmental changes may be instantiated in TSI-related brain activity levels. As with set switching, it is likely that relatively mature networks engaged to overcome task set inertia are already in place by puberty, but that these networks are differentially activated throughout adolescent development as cortical connections and long-distance white matter pathways are refined (Spencer-Smith and Anderson, 2009; Thatcher, 1992, 1997). Such developmental changes might be observed as changes in amplitude of regional brain activity or changes in the overall distributed profile of activation. Current neurodevelopmental theories propose that maturation of some executive functions is accompanied by changes from diffuse activation patterns to more localized patterns (Casey et al., 2005), characterized by greater activation of key task-specialized brain regions and decreased activity in superfluous regions (e.g., subcortical regions). In parallel, it has also been suggested that the functional network organization of these regions develops from being defined by local, anatomical proximity during childhood to being defined by distributed functional roles in adulthood (Fair et al., 2008; Fair et al., 2009; Fair et al., 2007; Kelly et al., 2009; Power et al., 2010; Supekar et al., 2009). Thus, development of cognitive control can be described as going from distributed networks of brain regions characterized by localized connections to more functionally central networks of brain regions characterized by long-distance connections. While evidence from these developmental studies suggests that the ability to overcome task set inertia will exhibit a similar developmental trajectory, it is still not yet know whether this is the case. For example, it is not know whether adolescents engage a similar, more frontally localized network as adults to overcome task set inertia, or if adolescents recruit a more distributed network containing both cortical and subcortical regions. Answering this question will have important implications for understanding the relationships between predominantly cortical versus subcortical systems of neurocognitive development.
We present behavioral and functional MRI results from a relatively large sample of 134 healthy adolescents and adults performing a novel attribute set switching task (SST). The primary study goal was to identify the neural correlates of task set inertia and to distinguish them from general set shifting brain function. We constructed a novel fMRI paradigm that systematically manipulated the interval between the end of one trial and the cue heralding the next to experimentally increase or decrease task set inertia. Importantly, the fMRI task design controlled for task set reconfiguration by fixing the cue-to-target interval. It, additionally, kept task-response rules constant and balanced the probability of each task, each response, and stimulus characteristics to ensure that neural effects were specific to task-set inertia. We hypothesized that parametric analysis of response-to-cue interval (RCI) would reveal a subset of brain regions observed in previous fMRI comparisons between switch and non-switch trials studies specifically engaged to resolve residual interference between competing mental sets. Based on previous neuroimaging work (Loose et al., 2006), as well as behavioral work linking task-set inertia to passive decay of previous task sets from working memory (Allport et al., 1994), we anticipated that these brain regions would include regions in the frontal cortices. Additionally, given inconsistent previous set switching findings for activity in subcortical regions we considered whether striatal engagement in set shifting may be related more to overcoming task set inertia. In order to ensure that we had the statistical power to detect any unique effects of overcoming task set inertia, we recruited a sample size much larger than previous set shifting functional neuroimaging studies. Our secondary goal was a cross-sectional analysis of age effects on task set inertia behavioral and neural effects. We aimed to characterize the developmental trajectory of task set inertia, which has not been previously examined. Our analyses again separately examined generalized switching effects and task set inertia effects. Because switching is thought to be one of the last executive functions to fully mature (Anderson, 2002), we hypothesized that late-maturing frontal lobe cortical regions would exhibit increased activity with age, with the most noticeable increases related to overcoming task set inertia. We also hypothesized that older participants would require less reliance on subcortical and lower level sensory cortical involvement to overcome residual set interference on switch trials, presumably because maturing prefrontal networks would not require basal ganglia and other related systems to inhibit competing response tendencies.
In summary, this study aimed to identify the neural correlates of task set inertia and distinguish them from those related to switching, and to characterize the developmental trajectory of task set inertia during adolescence. This was accomplished through: 1) comparison of the neural correlates of task set inertia and general main effects of shifting versus non-shifting task performance and 2) multivariate analyses of the effects of age on both switching and task set inertia.
EXPERIMENTAL PROCEDURES
2.1 Participants
A total of 134 right-handed participants (72 females) were recruited for several studies at the Olin Neuropsychiatric Research Center through advertisements or word of mouth. Participants ranged in age from 12 to 31.3 years (mean (SD) = 19.5 (3.9) years) and were screened to ensure that they were otherwise healthy and had no past head injury, neurologic conditions, learning disability, or other neurodevelopmental conditions. The absence of current or lifetime psychiatric and substance abuse disorders was determined using the screening module of the Structured Clinical Interview for Diagnosis (SCID-IV; (First et al., 1996) or the Schedule for Affective Disorders and Schizophrenia for School-Age Children – Present and Lifetime Version (K-SADS-PL; (Kaufman et al., 1997), depending on the age of the participant. All participants underwent written informed consent using procedures approved by the Hartford Hospital IRB.
2.2 FMRI Stimulus Delivery/Response Recording
The fMRI set switching task was implemented using E-Prime software (Psychology Software Tools, Inc.). Custom behavioral monitoring software was employed to record and analyze the behavioral data online to ensure that all participants performed the task within the specified parameters. Visual stimuli were presented using a projection system (5000 ANSI lumens) and displayed on a high-resolution screen located just behind the participant’s head. The participant viewed the screen using a mirror attached to the head coil. Corrective lenses were provided as needed. An MR-compatible fiber optic response device (Lightwave Medical, Inc., Vancouver, B.C.) was used to acquire behavioral responses.
2.3 Set Shifting Paradigm
The set switching paradigm was designed as a rapid, event-related fMRI task, where each of two unique runs consisted of 81 trials, 41 switch trials (counting the first trial as a switch) and 40 non-switch trials. Each trial was 4 seconds long, preceded by a 1.2 second cue (Cepeda et al., 2001). The cue informed participants whether they would be required to count the number of different colors, sizes, or shapes of the stimulus, and this cue remained on the screen throughout the trial to remove any working memory demands. The cue duration was picked based on previous evidence that 1.2 seconds was sufficient to equalize benefits to behavioral switch costs due to proactive task set reconfiguration in adolescents and adults (Cepeda et al., 2001). This ensured that any residual switch costs would be due to task set inertia. The response type was held constant on all trials (i.e., participants always pressed button 1, 2, or 3 if there were 1, 2, or 3 different dimensions counted), and the task itself (counting) remained the same on all trials. However, participants had to switch among attribute sets to count the relevant stimulus attributes. The stimuli were 3x3 grids of different colors (red, yellow, or blue), sizes (small, medium, or large), and shapes (triangles, circles, or squares) that ranged from very simple (all large, yellow squares; i.e., differing only on one dimension) to complex (differing on three dimensions). Task set inertia effects depended on a systematic variation in response-to-cue intervals (RCI), which ranged from 0.5 to 9.5 seconds, with an average of 2.4 seconds. Figure 1 depicts a sequence of several example stimuli. The task was cross-balanced across all factors, such that the frequency of each color, size, and shape (including combinations and mappings to task) was equiprobable, ensuring that there were no frequency or familiarity confounds. Additionally, no stimulus was repeated. This task allowed us to isolate neural activation on switch trials specifically related to the need to overcome interference from previous mental sets, while holding task set configuration demands, such as novelty, task set mapping, and response mapping constant. Each run lasted 8 minutes and 41 seconds.
Figure 1.
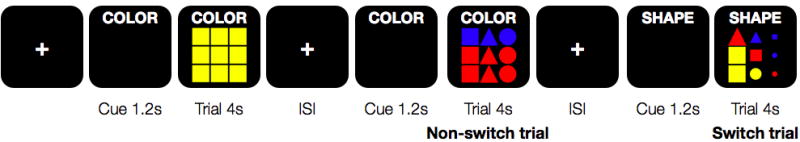
Partial schematic of the paradigm design. Participants were given a verbal cue 1.2 s prior to stimulus onset as to which stimulus attribute to count (color, shape, or size), and this cue remained on the screen throughout the entire trial. Participants then had 4 s to respond with the appropriate button press (1,2,3). ISI stands in for the variable interstimulus interval.
In summary, as the primary goal of our task was to measure the neural and behavioral correlates of task set inertia, we tried to control and balance every other aspect of the task, such that we could be confident that the effects we measured were the result of task set inertia and not contaminated by other factors. There is a growing set of terminology used to describe set switching tasks, but as our task was designed such that the over-arching task was the same across all trials and participants were only required to switch focus among three different stimulus attributes, we consider our task to fall under the guise of ‘set switching’ rather than ‘task switching’, so as not to confuse the experiment presented here with those experiments switching between two different tasks (i.e., dual-task paradigms) (e.g., Dreher and Grafman, 2003).
2.4 Imaging Parameters
MR images were acquired on a 3T Siemens Allegra (Siemens Medical Solutions, Erlangen, Germany) located at the Olin Neuropsychiatry Research Center at the Institute of Living/Hartford Hospital in Hartford, CT. The functional image volumes were collected in axial orientation to the anterior commissure-posterior commissure line using a single-shot-gradient-echo echo-planar sequence (TR/TE = 1,500/28 msec; flip angle = 65°; FOV = 24cm; matrix = 64; 3.4x3.4 mm in plane resolution; slice thickness = 5mm; 30 slices) with whole brain coverage. The two runs each consisted of 347 time points, with an initial 9 second rest session to allow for T1 effects to stabilize. These initial six images were not included in subsequent analyses.
High resolution T1- and T2-weighted images were also acquired on all participants to ensure that all were free from obvious vascular injury that might otherwise influence both neuropsychological test performance results and interpretation of functional imaging results.
2.5 Behavioral Data Analyses
The reaction time data for correct trials were analyzed using a multivariate, repeated-measures ANOVA in SPSS (IBM Corporation, Somers, NY). Trial type (shift, non-shift) and response-to-cue interval (500msec, 1000msec, 1500msec, 2000msec, 3000+msec) were used as within-subjects factors, and participant age, binned into twelve age groups (i.e., 15 yrs, 16 yrs, 17 yrs, …) was entered as a between-subjects factor. To bin the participants’ ages, the youngest (12–14 year olds) and oldest (over 25) were collapsed to preserve stability and ensure that each age bin in the ANOVA had approximately eleven subjects. For similar stability reasons, the reaction time values for the longer RCI intervals were averaged together (2000–2500 msec and 3000–9500 msec) to ensure that each RCI interval contained data from approximately the same number of shift and non-shift trials. Evidence for significant multivariate effects was examined for each within-subject factor (Trial type and RCI interval), as well as for all possible within- and between-subjects interactions.
2.6 Image Processing
Functional images were reconstructed offline. Each run was corrected for slice-timing errors and separately realigned using INRIalign (Freire and Mangin, 2001; Freire et al., 2002) as implemented in SPM5 (Wellcome Department of Cognitive Neurology, London, UK). A mean functional image volume was constructed for each participant for each run from the realigned image volumes and used to determine the parameters for spatial normalization into standardized Montreal Neurological Institute (MNI) space. These normalization parameters were then applied to the corresponding functional image volumes, and the normalized images were smoothed with a 9 mm FWHM Gaussian kernel. All participants’ data were individually inspected to ensure that no subject had translational or rotational head motion greater than the acquired voxel size.
2.7 FMRI Statistics
The regressors from each participant’s fMRI model were derived by extracting stimulus onset timing for all trials and modeled using a synthetic hemodynamic response function. The six motion-correction parameter estimates (x, y, and z displacements and pitch, roll, and yaw rotations) were included as covariates of no interest to statistically control signal change related to head motion. A high-pass filter (cutoff period = 128 s) was incorporated into the model to remove low-frequency signals. For each condition of interest, SPM5 wrote an image in which each voxel represented the estimated amplitude of hemodynamic signal change evaluated by multiple regression. All contrast images written by SPM5 represented brain activity relative to the ‘implicit baseline’ of unmodeled variance.
The functional imaging data of each participant were modeled individually in SPM5 and included regressors for switch trials and non-switch trials. Task set inertia was included as a parametric interaction term that represented the response-to-cue interval preceding a given trial. To preserve approximately equal numbers of trial types at a given task set inertia level, the response-to-cue intervals greater than 1500 msec were binned, such that every trial was assigned to one of six task set inertia levels (0.5, 1.0, 1.5, 2.5, 4.0, 6.85), corresponding to the average inter-stimulus interval duration in seconds. Additionally, as indicated above, the six rigid body motion parameters corresponding to a participant’s head motion were included as covariates of no interest. Three key contrasts corresponding to 1) the comparison of switch trials to non-switch trials, 2) the parametric effects of task set inertia across both trial types, and 3) the comparison of the parametric interaction effects of task set inertia and trial type (switch versus non-switch) were built for each participant. In this modeling approach, parametric terms estimate brain activity in relation to a trial-by-trial interaction term – in this case the response-to-cue interval (RCI) -- that represented the degree of residual task set interference induced per trial. In this way, it is possible to empirically measure how RCI modulates brain response to subsequent stimuli.
To summarize, our model contained regressors encoding switch trials and non-switch trials, as well as an additional parametric term representing response-to-cue interval duration (i.e., level of task-set inertia). This modeling approach allowed us to directly compare switch trials to non-switch trials (i.e., main effects of switching) and the switch trial × RCI interaction to the non-switch trial × RCI interaction (i.e., the interaction of switching and TSI) at the individual subject level. Thus, our choice of parametric analysis yielded the equivalent results as the more commonly used statistical interaction. As such, the main effects of switching were estimated using a one-sample t-test of each participant’s contrast directly comparing the regressors corresponding to switch and non-switch trials. The neural correlates of task set inertia were also measured via a one-sample t-test of the contrast estimating the parametric effects of task set inertia across both trial types. Finally, to determine the parametric effect of task set inertia on a given trial type (i.e., switch versus non-switch), a third one-sample t-test was performed on every participant’s contrast directly comparing the task set inertia by switch trial parametric interaction term to that for non-switch trials. This approach using one-sample t-tests allowed for greater sensitivity than a more typical approach of using group-level two-sample t-tests by preserving the overall number of degrees-of-freedom.
2.8 Region-of-interest Analyses
Two sets of region-of-interest (ROI) analyses were conducted to assess the developmental effects on the neural correlates of: 1) switching and 2) high and low task-set inertia. Spherical regions of interest, with a radius of 8mm, were defined at the peak stereotactic coordinates for the main effects of switching (Figure 2A) and the parametric main effects of task-set inertia (Figure 2B), respectively. For the main effects of switching, ROIs were defined for a carefully selected subset of regions in the frontal cortex, inferior parietal cortex, striatum, and thalamus (N = 12 regions). For the parametric main effects of task set inertia, ROIs were defined for all regions of peak neural activity (Table 2; N = 7 for high TSI, N = 9 for low TSI). Two whole-brain regressions for: 1) age with switching and 2) age with task set inertia were calculated in SPM5, and MarsBar (Brett et al., 2002) was used to extract the mean activation for each respective set of ROIs from each respective age regression map. In all cases, ROIs were deemed to exhibit significant age-related changes in activity only when considering 2-tailed significance and Bonferroni correction for comparing multiple regions simultaneously.
Figure 2.
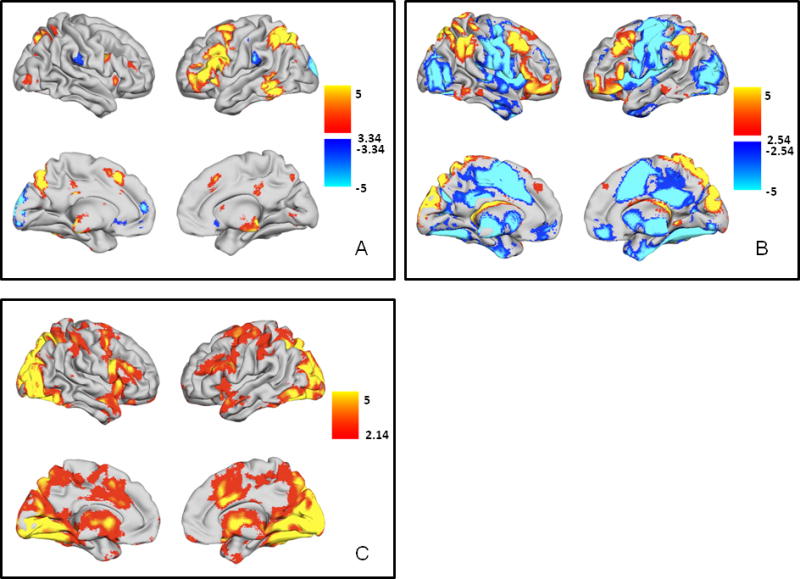
A. Main effects of switching (switch trials > non-switch trials). Regions with greater activity during switch trials are shown in YELLOW/ORANGE, and regions with greater activity during non-switch trials are shown in BLUE/GREEN. B. Parametric effects of task-set inertia. Regions preferentially active during trials with high task-set inertia (i.e., short RCI) are shown in YELLOW/ORANGE. Regions with greater activity during trials with low task-set inertia (i.e., long RCI) are depicted in BLUE/GREEN. C. Regions with greater activity during non-switch trials with low task-set inertia. Regions with greater activity during non-switch trials with low task-set inertia are shown in YELLOW/ORANGE. All t-maps are thresholded at p < 0.05, corrected for multiple comparisons (FDR). Color bars are expressed in terms of t-scores, and images are displayed in normal convention (left = left).
Table 2. Peak stereotactic coordinates (MNI) for the parametric main effects of task-set inertia.
Regions associated with high task-set inertia are listed first, followed by those associated with low task-set inertia. Coordinates are given only for those regions that exhibited significant activity after whole-brain correction for multiple comparisons via False Discovery Rate (FDR) at p < 0.05.
| Region | X | Y | Z | T |
|---|---|---|---|---|
| High Task-Set Inertia | ||||
| Frontal Lobes | ||||
| L middle frontal gyrus (BA 8) | −46 | 22 | 44 | 7.42 |
| R middle frontal gyrus (BA 9) | 46 | 24 | 42 | 7.50 |
| L middle frontal gyrus (BA 10) | −40 | 58 | −4 | 6.64 |
| R middle frontal gyrus (BA 10) | 40 | 60 | −6 | 6.78 |
| Medial middle frontal gyrus (BA 6) | −4 | 40 | 40 | 3.45 |
| L inferior frontal gyrus | −58 | 22 | 8 | 5.70 |
| R inferior frontal gyrus | 62 | 24 | 8 | 4.81 |
| Parietal Lobes | ||||
| L inferior parietal lobe (BA 40) | −52 | −50 | 44 | 7.99 |
| R inferior parietal lobe (BA 40) | 58 | −42 | 40 | 6.41 |
| Precuneus | 4 | −88 | 38 | 13.82 |
| Low Task-Set Inertia | ||||
| Frontal Lobes | ||||
| L middle frontal gyrus (BA 10) | −32 | 42 | 28 | 5.22 |
| R middle frontal gyrus (BA 10) | 32 | 46 | 26 | 5.84 |
| L precentral gyrus | −38 | −20 | 56 | 14.71 |
| R precentral gyrus (BA 6) | 36 | −6 | 56 | 6.62 |
| L postcentral gyrus | −50 | −22 | 18 | 9.74 |
| R postcentral gyrus (BA 6) | 68 | −6 | 28 | 5.46 |
| Supplementary motor area (BA 6) | −2 | −2 | 56 | 12.91 |
| L insula (BA 13) | −44 | −26 | 20 | 9.92 |
| R insula | 52 | 4 | 4 | 7.06 |
| Parietal Lobes | ||||
| Posterior cingulate | 0 | −40 | 28 | 7.48 |
| Occipital Lobes | ||||
| L middle occipital gyrus | −26 | −80 | 22 | 7.56 |
| R superior occipital gyrus | 28 | −80 | 22 | 7.43 |
| L fusiform gyrus | −28 | −70 | −10 | 10.93 |
| R fusiform gyrus | 30 | −64 | −10 | 10.29 |
| Subcortical Regions | ||||
| L putamen | −24 | −4 | −2 | 7.18 |
| R putamen | 16 | 6 | −2 | 7.82 |
| R thalamus | 10 | −16 | −2 | 6.86 |
RESULTS
3.1 Main effects of switching and task set inertia
3.1.1 Behavioral Data
All participants were able to perform the set switching task with high accuracy and consistency. The average response accuracy for non-switch trials was 91.2% and 88.5% for switch trials, and there was < 1% missed trials overall. No participant had less than 75% accuracy or missed more than 10% of responses, regardless of trial type.
Significant multivariate effects on reaction time were observed for Trial type (switch versus non-switch trials), confirming the presence of an overall switch cost of 102 msec (F1,122 = 204.056; p < 8.217 × 10−28), and RCI interval, indicating that altering the response-to-cue interval had an effect on performance (F4,119 = 10.96; p < 1.337 × 10−7). Additionally, a significant Trial × RCI effect was observed, further indicating that switch costs differed with RCI (F4,119 = 20.59; p < 6.489 × 10−13). Therefore, our task robustly produced both generalized switch costs as well as task set inertia effects as designed. In support of the behavioral data summarized here, average reaction time data for each age group for each trial type at each response-to-cue interval bin is presented in Supplemental Table 1 for those interested readers.
3.1.2 Functional Imaging Data
As seen in numerous previous studies (Wager et al., 2004), the neural correlates of the contrast of switch trials versus non-switch trials (Figure 2A, Table 1) were primarily localized to the frontal and parietal cortices, predominantly left middle frontal gyrus (BA 6/46), medial middle frontal gyrus (BA 6), bilateral inferior frontal gyri (BA 44), medial posterior cingulate, bilateral precuneus, left inferior parietal lobe, and left cuneus. Additional switching-related activity was observed in bilateral putamen, bilateral thalamus, bilateral insula, and left middle/inferior temporal gyrus. Only a small region in the left posterior cuneus was preferentially active during non-switch trials.
Table 1. Peak stereotactic coordinates for the main effects of switching (switch trials > non-switch trials).
Coordinates (MNI) of those regions with greater activity during switch trials are listed in the upper portion of the table, while those with greater activity during non-switch trials are listed in the lower portion. Coordinates are given only for those regions that exhibited significant activity after whole-brain correction for multiple comparisons via False Discovery Rate (FDR) at p < 0.05.
| Region | X | Y | Z | T |
|---|---|---|---|---|
| Switch greater than Non-switch | ||||
| Frontal Lobes | ||||
| L middle frontal gyrus (BA 6) | −26 | 10 | 62 | 6.46 |
| L middle frontal gyrus | −42 | 44 | −8 | 4.53 |
| L middle frontal gyrus (BA 46) | −50 | 34 | 16 | 7.01 |
| R middle frontal gyrus (BA 46) | 44 | 38 | 16 | 4.04 |
| L inferior frontal gyrus (BA 6) | −46 | 22 | 28 | 7.57 |
| R inferior frontal gyrus | 44 | 8 | 28 | 5.41 |
| Supplementary motor area (BA 6) | −6 | 20 | 50 | 6.01 |
| L insula | −34 | 20 | −4 | 5.42 |
| R insula | 36 | 22 | −2 | 4.47 |
| Parietal Lobes | ||||
| L inferior parietal lobe | −38 | −56 | 64 | 5.72 |
| L cuneus | −6 | −74 | 40 | 7.58 |
| Posterior cingulate | 0 | −34 | 40 | 4.55 |
| L precuneus | −32 | −70 | 54 | 8.89 |
| R precuneus | 32 | −72 | 52 | 5.53 |
| Temporal Lobes | ||||
| L middle temporal gyrus (BA 37) | −58 | −52 | −12 | 6.59 |
| Occipital Lobes | ||||
| L inferior occipital gyrus (BA 18) | −32 | −98 | −10 | 3.14 |
| R middle occipital gyrus | 24 | −96 | −10 | 2.85 |
| L fusiform gyrus | −22 | −78 | −8 | 3.18 |
| R fusiform gyrus | 32 | −76 | −10 | 3.71 |
| Subcortical Regions | ||||
| L putamen | −16 | 6 | 14 | 3.64 |
| R putamen | 14 | 10 | 8 | 3.59 |
| L thalamus | −10 | −14 | 0 | 4.40 |
| R thalamus | 8 | −14 | −2 | 4.88 |
| Non-switch greater than Switch | ||||
| Frontal Lobes | ||||
| L middle frontal gyrus | −12 | 56 | 14 | 5.35 |
| R postcentral gyrus | 70 | −20 | 24 | 3.80 |
| Parietal Lobes | ||||
| L cuneus | −8 | −102 | 18 | 9.05 |
| L inferior parietal lobe (BA 40) | −54 | −32 | −24 | 4.20 |
The neural correlates of task set inertia (Figure 2B, Table 2) revealed a profile of activation that was distinct compared to generalized switch-related brain function (Figure 2A, Table 1). The profile revealed two opposing sets of regions – a ‘cortical control network’ with greater hemodynamic response during trials with high task set inertia (short RCI) and a ‘subcortical-motor network’ with greater hemodynamic activity during trials with low task set inertia (long RCI). The phrases, ‘cortical control network’ and ‘subcortical-motor network’, are used here and in the Discussion merely as a convenient way to identify the two opposing sets of brain regions observed in Figure 2B. The ‘cortical control network’ consisted of bilateral middle frontal gyri, inferior frontal gyri, inferior parietal lobes, and precuneus. The ‘subcortical-motor network’ included bilateral pre-/post-central gyri, superior frontal gyri, middle occipital gyri, putamen, insula, thalamus, and lingual gyri along with medial middle frontal gyrus and precuneus. At first glance, the main effects of switching and parametric effects of short RCI may appear to share many of the same frontal and parietal regions. Closer inspection of the respective activation maps revealed that these two phenomena activated different parts of these frontal and parietal regions with virtually no overlap in the peak activations, save a small region in the left ventrolateral prefrontal cortex. Additionally, whereas bilateral striatal engagement was observed during switch trials (Figure 2A), only trials with low task set inertia appear to more greatly engage the striatum.
3.1.3 Effect of task set inertia on switch and non-switch trials
We also directly contrasted the degree of task set inertia-related hemodynamic activity between switch and non-switch trials to determine how task set inertia effects would be altered by demands to switch cognitive set. As described above, behaviorally, there was strong evidence for response-cue-interval affecting both overall reaction time as well as switch costs. Analysis of the functional imaging data revealed that the collection of prefrontal and parietal cortical regions (i.e., the ‘cortical control network’) active during trials with high task set inertia (Figure 2B, Table 2) was active regardless of trial type. However, some of the brain regions that were more active during trials with low task set inertia (i.e., the ‘subcortical-motor network’) were also more active during non-switch trials (Figure 2C, Table 3). This subset of regions included the supplementary motor area, anterior cingulate, bilateral thalamus, bilateral basal ganglia, bilateral middle occipital gyri, and brainstem. In contrast, no task set inertia-related brain regions were more active during switch trials.
Table 3. Peak stereotactic coordinates (MNI) for the brain regions exhibiting greater activity during non-switch trials with low task-set inertia.
Coordinates are given only for those regions that exhibited significant activity after whole-brain correction for multiple comparisons via False Discovery Rate (FDR) at p < 0.05.
| Region | X | Y | Z | T |
|---|---|---|---|---|
| Non-switch trials with low task-set inertia | ||||
| Frontal Lobes | ||||
| L superior frontal gyrus (BA 6) | −22 | 0 | 58 | 4.90 |
| L inferior frontal gyrus/insula | −40 | 8 | 24 | 5.35 |
| −42 | 6 | 26 | 5.37 | |
| R inferior frontal gyrus/insula | 44 | 8 | 26 | 7.68 |
| R middle frontal gyrus | 34 | 4 | 48 | 5.76 |
| Supplementary motor area (BA 32) | −2 | 8 | 52 | 4.62 |
| Anterior cingulate | 6 | 18 | 26 | 4.84 |
| Parietal Lobes | ||||
| L inferior parietal lobe | −38 | −40 | 44 | 5.52 |
| R inferior parietal lobe | 42 | −36 | 52 | 5.28 |
| L precuneus | −20 | −74 | 50 | 6.59 |
| R precuneus/superior parietal lobe (BA 7) | 24 | −70 | 52 | 7.10 |
| Cingulate gyrus | 2 | −10 | 48 | 3.66 |
| Cuneus | −2 | −78 | 24 | 5.15 |
| Occipital Lobes | ||||
| L middle occipital gyrus | −32 | −90 | 20 | 8.38 |
| R middle occipital gyrus | 36 | −82 | 20 | 9.36 |
| R middle occipital gyrus/fusiform gyrus | 34 | −78 | −16 | 11.12 |
| L lingual gyrus | −20 | −50 | −10 | 6.59 |
| −26 | −80 | −14 | 10.72 | |
| R lingual gyrus (BA 19) | 18 | −48 | −8 | 6.42 |
| Subcortical Regions | ||||
| L caudate | −8 | 4 | 2 | 5.75 |
| R caudate | 4 | 4 | 0 | 5.17 |
| L thalamus | −10 | −16 | 10 | 5.76 |
| R thalamus | 8 | −8 | 4 | 6.10 |
| L hippocampus | −22 | −30 | −4 | 5.21 |
3.2 Developmental Effects
3.2.1 Behavioral Data
The overall linear multivariate effect of age was a non-significant trend (F1,122 = 1.710; p < 0.079), reflecting marginally faster overall performance in older participants regardless of Trial Type or RCI, which is consistent with numerous previous developmental studies. To determine whether age-related gains in response speed were affected differently by response-to-cue interval on switch versus non-switch trials, both the linear and quadratic Trial type × RCI interactions with age were examined. There were no significant linear interaction effects. However, a quadratic effect of Trial type × RCI × Age was observed (F11,122 = 2.033; p < 0.031). Additional investigation revealed that this quadratic effect reflected the fact that the youngest participants (12–14 years) had larger switch costs for trials with the highest task set inertia (shortest RCI), but this effect quickly waned by mid-adolescence. The remaining switch costs were relatively uniform across all age groups and all RCI durations. Thus, there were no significant developmental gains in general switch costs between our adolescent and adult participants that could not be explained by overall developmental speed increases on performance. We did, however, observe a modest but significant developmental effect on performance during trials with high stimulus-response set interference.
3.2.2 Functional Imaging Data
The results of the region-of-interest age effect analyses, did not find any regions exhibiting significant age-related activity for the main effects of switching. In a supplemental whole-brain regression analysis, no brain regions exhibited significant age-related changes in hemodynamic activity.
Results from the region-of-interest age effect analyses yielded significant age-related effects for two regions active during trials with high task set inertia, namely the left middle frontal gyrus (t = 2.88; p < 0.032) and left inferior parietal lobe (t = 2.80; p < 0.042) (Figure 3, Table 4), indicating the presence of developmental gains in these regions engaged to overcome high stimulus-response interference (i.e., high task set inertia) between trials separated by short response-to-cue intervals. No other regions active during trials with high task set inertia exhibited any statistically significant age-related changes in activity. Likewise, no regions active during trials with low task set inertia exhibited any significant age-related changes in neural activity. Supplemental whole-brain regression analysis did not yield any significant age-related changes in hemodynamic activity for those brain regions active during trials with either high or low task set inertia.
Figure 3.
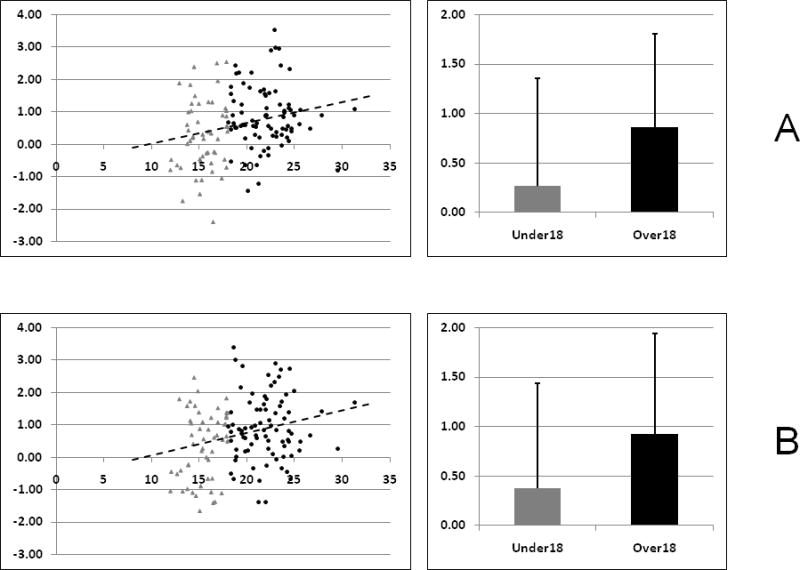
Graphical representations of the region-of-interest analysis results for the two regions showing significant age-related gains in neural activity from the high task-set inertia cortical control network (A. Left middle frontal gyrus, B. Left inferior parietal lobe). For purposes of display, the participants have been divided into those under the age of 18 (shown in GRAY TRIANGLES) and those over the age of 18 (shown in BLACK CIRCLES). The scatter plots on the left depict individual subject beta weights versus age, along with the best linear fit line. The bar plots on the right summarize the mean beta weights for the two age groups (GRAY: under 18; BLACK: over 18).
Table 4. Region-of-interest analysis results describing the age-related gains associated with high task-set inertia.
Coordinates (MNI) given are the location of the center of the 8mm spherical region-of-interest, as defined from the parametric effects of task set inertia. The listed p-values are of 2-tailed significance, corrected for comparing multiple regions of interest simultaneously.
| Region | X | Y | Z | T | P (2-tailed) |
|---|---|---|---|---|---|
| Frontal Lobes | |||||
| L inferior frontal gyrus | −58 | 22 | 8 | 1.12 | 1 |
| R inferior frontal gyrus | 62 | 24 | 8 | 1.3 | 1 |
| L middle frontal gyrus | −46 | 22 | 44 | 2.88 | 0.032 |
| R middle frontal gyrus | 46 | 24 | 42 | 1.72 | 0.54 |
| Parietal Lobes | |||||
| L inferior parietal lobe | −52 | −50 | 44 | 2.8 | 0.042 |
| R inferior parietal lobe | 58 | −42 | 40 | 2.1 | 0.24 |
| Precuneus | 4 | −88 | 38 | −2.23 | 1 |
DISCUSSION
This study was conducted to characterize the neural correlates of task set inertia and to examine the effects of development on brain activity related to resolving stimulus-response set interference. The behavioral and functional imaging main effects of switching are in line with what has been previously summarized in a meta-analysis cited in the introduction (Reimers and Maylor, 2005; Wager et al., 2004). These results served both to confirm that our novel set switching task worked and to add further evidence for frontoparietal engagement in mental set switching. As the primary aim of this study was to be the first to more fully characterize one of several cognitive factors underlying mental set switching, the results describing the neural correlates of the behavioral phenomenon of ‘task set inertia’ are of greater interest.
4.1 Main Effects
As hypothesized, greater task set inertia engaged a collection of bilateral frontal and parietal cortical regions that were distinct from those identified by a general contrast of switch versus non-switch trials. Superficially, the collection of brain regions active during trials with high task set inertia shared many of the same regions as that of brain regions preferentially active during shift trials. However, there was only minimal overlap in the left ventrolateral prefrontal cortex, indicating that overcoming task-set inertia is a unique phenomenon with its own distinct set of neural correlates. As the phenomenon of task set inertia has been linked to passive decay of working memory (Allport et al., 1994), it is unsurprising that overcoming it would result in neural activity in prefrontal and parietal regions. In comparing the collection of brain regions active during trials with high task set inertia to those regions thought to underlie working memory (Rottschy et al., 2012; Wager and Smith, 2003), there is support, based on the qualitative similarities, particularly for the network identified in relation to task sets (Rottschy et al., 2012), for working memory playing a role in overcoming task set inertia. The results further suggested a ‘push-pull’ relationship between a ‘cortical control network’, needed to overcome high task set inertia, and a ‘subcortical-motor network’, more active during trials of low task set inertia. Recall that parametric analyses identify brain regions with relatively greater or lesser activity along a task dimension, in this case, response-to-cue inter-trial intervals. Therefore, as brain activity in the ‘cortical control’ networks increased, activity in the opposing ‘subcortical-motor network’ by definition decreased. The regions identified here as more greatly engaged to overcome high task set inertia include those found by Loose et al. (2006). However, as that prior study did not consider an interstimulus interval longer than 1600 msec, it was not possible to compare the composition of our ‘subcortical-motor network’ with the limited regions identified by Loose et al. (2006) as being active during trials with the ISI of 1600 msec. Overall, the finding that the set of regions necessary for overcoming high task set inertia was distinct from those brain regions that are generally more active during mental set switches suggested that task set inertia may represent a unique neurocognitive influence on switching – likely an additional cognitive flexibility executive function needed to properly complete mental set switching tasks with short response-to-cue intervals.
The imaging results further indicated that the cortical control network identified for overcoming high task set inertia was active regardless of switch versus non-switch trial type. However, for the subcortical-motor network of regions active during trials with low task set inertia, a subset of these regions exhibited greater activity during non-switch trials. This subset of regions shared considerable overlap with the cingulo-opercular set maintenance network identified by Dosenbach et al. (2007, 2008), including anterior cingulate cortex, inferior frontal gyrus/insula, and thalamus. This set maintenance network is thought to be necessary to stay on task, suggesting that the longer the response-to-cue interval between non-switch trials, the more difficult it is for participants to stay on task. The observed subset of brain regions also included the basal ganglia, which contradicted our expectation that striatal control would be necessary to resolve interference during switches. Instead, it appeared that overcoming interference from previous trials relies on both a) a cognitive control composed of frontoparietal regions active during trials with high task set inertia, regardless of whether a set switch has occurred, and b) relatively more engagement of a subcortical-motor network (including basal ganglia) to maintain the active task set during non-switch trials with low task set inertia.
In distinguishing the neural correlates of task set inertia from those of generalized switching, we found that several regions previously associated with set switching, including anterior cingulate cortex and striatum (Wager et al., 2004), were actually engaged more during trials with low task set inertia than with switching in general. These results suggested that anterior cingulate and striatal involvement during switching may have more to do with preserving mental sets and motor programs, respectively, between sparsely spaced trials than in mediating mental set switches. In the context of set switching, activity in the anterior cingulate has been linked to performance monitoring/conflict detection (Rubia et al., 2006), particularly during set switching tasks that involve response and motor remapping (Dove et al., 2000; Rubia et al., 2006; Smith et al., 2006). The lack of observed activity in the anterior cingulate cortex in this present study emphasizes the role paradigmatic differences can play in eliciting specific regional hemodynamic activity. In the more general context of executive functions, the anterior cingulate is thought to be responsible for monitoring response conflict resolution during tasks with high interference (Nee et al., 2007). Stroop tasks are well known to engage the anterior cingulate cortex, thought to contribute to mental effort needed to resolve interference (Nee et al., 2007). In contrast, the interference imparted by task set inertia appeared to be different. Most notably, overcoming task set inertia required engaging a cortical control network as opposed to one dominated by the anterior cingulate cortex. Indeed, the only overlap between the brain regions found in this study and previous Stroop effects was the anterior cingulate. As described above, the profile of cingulate activity observed here did not conform to its role in numerous previous Stroop experiments. However, robust activity in the anterior cingulate, in conjunction with activity in bilateral anterior insula and thalamus, during trials with low task set inertia in this study, suggested that it might play a role in maintaining task set (Dosenbach et al., 2008; Dosenbach et al., 2007). The striatum also has been associated with several attributes during mental set shifting tasks, including mediating response incompatibility (Sylvester et al., 2003), general engagement in executive functions requiring attentional control (Loose et al., 2006), action selection and inhibition of irrelevant information (Yehene et al., 2008), and maintaining and overriding stimulus-response associations (Crone et al., 2006b). In general, the basal ganglia are thought to play a role in modulating operations in the entire frontal lobe, including the maintenance and switching of behavioral sets, as well as the planning and execution of limb and eye movements (Alexander and Crutcher, 1990). Our results from the main effects of switching suggested a role for the striatum in generalized switching that is most likely related to either basic action selection or maintaining stimulus-response associations. Much more robust striatal activity was observed during trials with low task set inertia, indicating that in addition to maintaining stimulus-response sets between like trials, the striatum may play a role in the overall maintenance of behavioral sets between like trials, as previously suggested (Alexander and Crutcher, 1990).
4.2 Developmental Effects
No significant age-related effects were observed for the main effects of switching compared with non-switching in the functional neuroimaging results. The one functional neuroimaging study that previously compared the neural correlates of set switching in adolescents to adults found a number of cortical and subcortical regions that exhibited age-related activity increases and decreases (Rubia et al., 2006). A partial explanation for the discrepancies between the two sets of results may lie in that Rubia et al. (2006) used a modified Meiran-type paradigm, which involved both attribute and motor remapping. It could be that this added task complexity is more prone to showing developmental effects. However, our results indicated that for simpler attribute switching tasks, the cognitive control needed to mediate these mental switches has already matured to near-adult levels by early adolescence.
Our results also suggested that the ability to optimally perform the attribute set switching task specifically in the face of high stimulus response set interference (i.e., overcoming high task-set inertia) appeared to mature somewhat later than more general cognitive flexibility executive functions such as mental set switching. Significant age-related effects were observed in both the behavior and imaging results, respectively, for the parametric effects of task-set inertia. Behaviorally, we observed that the youngest participants had significantly higher switch costs on trials with the highest task-set inertia, which is in line what has previously been shown (Crone et al., 2006a). From the neuroimaging data, significant age effects – specifically age-related increases in neural activity – were observed in the left middle frontal gyrus and left inferior parietal lobe. These neuroimaging results indicated that adolescents do not rely on a more distributed network than adults to overcome high task set inertia, as evidenced by the lack of significant age-related hemodynamic changes in subcortical regions such as the striatum or lower level sensory regions. In contrast, region-of-interest fMRI analyses suggested that adolescents transition from predominant use of the subcortical-motor network to the cortical-control network to overcome high task set inertia by age twelve. The continuing age-related gains in the regions comprising this cortical-control network indicated that while adolescents make use of the same network as adults, they may not be using at as effectively or efficiently as adults. Future functional connectivity analyses may help further elucidate the nature of this developmental trajectory of this cortical-control network, particularly whether certain brain regions are relatively more critical to mediate task set inertia effects with ongoing development. The lack of significant age-related changes in regions in the subcortical-motor network active during trials with low task set inertia indicated that the ability to maintain behavioral and motor sets has matured by early adolescence.
In parallel with the imaging results discussed above, the behavioral data also indicated that response-to-cue interval (i.e., level of task-set inertia) affects task performance, both in terms of overall reaction time, regardless of trial type, as well as switch costs. Two previous studies examining the behavioral effects of different response-to-cue intervals on switch costs failed to find a similar RCI × Trial Type or RCI × Switch Cost interaction (Badre and Wagner, 2006; Cepeda et al., 2001), however neither of these studies considered response-to-cue intervals longer than 1200 msec. By including RCIs up to 9.5 s, we were able to extend these previous results by demonstrating that switch costs exist at relatively long RCIs. As the paradigm was designed to remove any task-set reconfiguration effects by fixing the cue-to-target interval at 1200 msec (Cepeda et al., 2001), the existence of switch costs at these very long response-to-cue intervals suggested one of three possibilities: 1) task set inertia may take much longer to resolve than previously thought (Rogers and Monsell, 1995), 2) an additional cognitive factor germane to switching may still be at play, or 3) some other phenomenon about how the brain deals with the lack of stimulation for relatively long periods may be engaging to help maintain behavioral set and consequently influences reaction time on subsequent trials. Additional studies specifically designed to examine the neural correlates of mental set switching, as well as other executive functions are warranted, with paradigms designed to include long interstimulus intervals. Despite the significant behavioral effects observed for the whole study population, no significant age-related behavioral effects were observed for the main effects of switching or task set inertia. This was consistent with numerous previous studies examining the maturational trajectory of set switching task performance in children and adolescents (Anderson, 2002; Casey et al., 2004; Davidson et al., 2006; Rubia et al., 2006; Waber et al., 2007) and further confirmed that mental set switching performance matures to near-adult levels by age twelve. While the observed trend for improved performance with increasing age on trials with high task set inertia was in line with the sole previous study examining the effect of variable interstimulus intervals on switching in children and adolescents (Crone et al., 2006a), it also suggested that near-adult levels of performance are not achieved until mid-adolescence on trials with the highest task set inertia interference effects. In conjunction with the neuroimaging results, this further indicated that the ability to overcome task set inertia matures later than that for general switching.
CONCLUSIONS
In summary, the brain regions engaged to overcome stimulus-response interference on trials with high task set inertia are largely distinct from the brain regions repeatedly observed to have greater activation during switch compared to non-switch trials. These results effectively revealed the existence of two previously uncharacterized neural systems that are partly responsible for accomplishing a mental set switch. The study also found that the neural correlates necessary for successfully navigating a set switching task with high and low task set inertia trials appears to be in place by early adolescence, but there is continued maturation of the cortical control network throughout adolescence that allows the systems to be utilized more effectively and efficiently. Future studies should further examine developmental trajectory of the cortical control network engaged for overcoming high task-set inertia, both in adolescents, as we presented here, as well as in younger children. Of particular interest would be whether these networks develop abnormally in youth with developmental disorders that can be characterized by problems flexibly switching among different concepts or behaviors or insistence on preserving sameness, such as autism, obsessive compulsive disorder, and depression. Impairment in this exact system could partially underlie the clinical symptoms, representing neural liability towards various psychiatric disorders. To better understand the age-related effects on task set inertia-related brain function, future work should also consider potential developmental changes in the connectivity among the neural regions active during these two phenomena, particularly for the cortical control network active during trials with high task-set inertia. Finally, it should be noted that these results are specific to attribute and rule switching tasks and may not be applicable to all types of mental set switching (i.e., task switching, motor response remapping). Future behavioral and neuroimaging research should determine to degree to which task set inertia effects are independent from, or complicated by, these other common task demands.
Supplementary Material
Acknowledgments
This research was supported by the National Institutes of Health under grant R01-MH081969 (PI: MCS).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Contributor Information
Suzanne T. Witt, Email: stwitt@harthosp.org.
Michael C. Stevens, Email: msteven@harthosp.org.
References
- Alexander GE, Crutcher MD. Functional architecture of basal ganglia circuits: neural substrates of parallel processing. Trends Neurosci. 1990;13:266–271. doi: 10.1016/0166-2236(90)90107-l. [DOI] [PubMed] [Google Scholar]
- Allport DA, Styles EA, Hsieh S. Shifting intentional set: Exploring the dynamic control of tasks. In: Umilta C, editor. Attention and Performance XV. The MIT Press; Cambridge, MA: 1994. pp. 421–452. [Google Scholar]
- Anderson P. Assessment and development of executive function (EF) during childhood. Child Neuropsychol. 2002;8:71–82. doi: 10.1076/chin.8.2.71.8724. [DOI] [PubMed] [Google Scholar]
- Badre D, Wagner AD. Computational and neurobiological mechanisms underlying cognitive flexibility. Proc Natl Acad Sci U S A. 2006;103:7186–7191. doi: 10.1073/pnas.0509550103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brett M, Anton JL, Valabregue R, Poline JB. Region of interest analysis using an SPM toolbox. Neuroimage. 2002:16. [Google Scholar]
- Buchsbaum BR, Greer S, Chang WL, Berman KF. Meta-analysis of neuroimaging studies of the Wisconsin card-sorting task and component processes. Hum Brain Mapp. 2005;25:35–45. doi: 10.1002/hbm.20128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Casey BJ, Davidson MC, Hara Y, Thomas KM, Martinez A, Galvan A, Halperin JM, Rodriguez-Aranda CE, Tottenham N. Early development of subcortical regions involved in non-cued attention switching. Dev Sci. 2004;7:534–542. doi: 10.1111/j.1467-7687.2004.00377.x. [DOI] [PubMed] [Google Scholar]
- Casey BJ, Galvan A, Hare TA. Changes in cerebral functional organization during cognitive development. Curr Opin Neurobiol. 2005;15:239–244. doi: 10.1016/j.conb.2005.03.012. [DOI] [PubMed] [Google Scholar]
- Casey BJ, Thomas KM, Davidson MC, Kunz K, Franzen PL. Dissociating striatal and hippocampal function developmentally with a stimulus-response compatibility task. J Neurosci. 2002;22:8647–8652. doi: 10.1523/JNEUROSCI.22-19-08647.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cepeda NJ, Kramer AF, Gonzalez de Sather JC. Changes in executive control across the life span: examination of task-switching performance. Dev Psychol. 2001;37:715–730. [PubMed] [Google Scholar]
- Cools AR. Role of the neostriatal dopaminergic activity in sequencing and selecting behavioural strategies: facilitation of processes involved in selecting the best strategy in a stressful situation. Behav Brain Res. 1980;1:361–378. doi: 10.1016/0166-4328(80)90035-2. [DOI] [PubMed] [Google Scholar]
- Corbetta M, Miezin FM, Shulman GL, Petersen SE. A PET study of visuospatial attention. J Neurosci. 1993;13:1202–1226. doi: 10.1523/JNEUROSCI.13-03-01202.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crone EA, Bunge SA, van der Molen MW, Ridderinkhof KR. Switching between tasks and responses: a developmental study. Dev Sci. 2006a;9:278–287. doi: 10.1111/j.1467-7687.2006.00490.x. [DOI] [PubMed] [Google Scholar]
- Crone EA, Wendelken C, Donohue SE, Bunge SA. Neural evidence for dissociable components of task-switching. Cereb Cortex. 2006b;16:475–486. doi: 10.1093/cercor/bhi127. [DOI] [PubMed] [Google Scholar]
- Davidson MC, Amso D, Anderson LC, Diamond A. Development of cognitive control and executive functions from 4 to 13 years: evidence from manipulations of memory, inhibition, and task switching. Neuropsychologia. 2006;44:2037–2078. doi: 10.1016/j.neuropsychologia.2006.02.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dosenbach NU, Fair DA, Cohen AL, Schlaggar BL, Petersen SE. A dual-networks architecture of top-down control. Trends Cogn Sci. 2008;12:99–105. doi: 10.1016/j.tics.2008.01.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dosenbach NU, Fair DA, Miezin FM, Cohen AL, Wenger KK, Dosenbach RA, Fox MD, Snyder AZ, Vincent JL, Raichle ME, Schlaggar BL, Petersen SE. Distinct brain networks for adaptive and stable task control in humans. Proc Natl Acad Sci U S A. 2007;104:11073–11078. doi: 10.1073/pnas.0704320104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dove A, Pollmann S, Schubert T, Wiggins CJ, von Cramon DY. Prefrontal cortex activation in task switching: an event-related fMRI study. Brain Res Cogn Brain Res. 2000;9:103–109. doi: 10.1016/s0926-6410(99)00029-4. [DOI] [PubMed] [Google Scholar]
- Dreher JC, Grafman J. Dissociating the roles of the rostral anterior cingulate and the lateral prefrontal cortices in performing two tasks simultaneously or successively. Cereb Cortex. 2003;13:329–339. doi: 10.1093/cercor/13.4.329. [DOI] [PubMed] [Google Scholar]
- Fair DA, Cohen AL, Dosenbach NU, Church JA, Miezin FM, Barch DM, Raichle ME, Petersen SE, Schlaggar BL. The maturing architecture of the brain’s default network. Proc Natl Acad Sci U S A. 2008;105:4028–4032. doi: 10.1073/pnas.0800376105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fair DA, Cohen AL, Power JD, Dosenbach NU, Church JA, Miezin FM, Schlaggar BL, Petersen SE. Functional brain networks develop from a “local to distributed” organization. PLoS Comput Biol. 2009;5:e1000381. doi: 10.1371/journal.pcbi.1000381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fair DA, Dosenbach NU, Church JA, Cohen AL, Brahmbhatt S, Miezin FM, Barch DM, Raichle ME, Petersen SE, Schlaggar BL. Development of distinct control networks through segregation and integration. Proc Natl Acad Sci U S A. 2007;104:13507–13512. doi: 10.1073/pnas.0705843104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- First MB, Spitzer RL, Gibbon M, Williams JB. Structured Clinical Interview for the DSM-IV Axis I Disorders. 1996. [DOI] [PubMed] [Google Scholar]
- Freire L, Mangin JF. Motion correction algorithms may create spurious brain activations in the absence of subject motion. Neuroimage. 2001;14:709–722. doi: 10.1006/nimg.2001.0869. [DOI] [PubMed] [Google Scholar]
- Freire L, Roche A, Mangin JF. What is the best similarity measure for motion correction in fMRI time series? IEEE Trans Med Imaging. 2002;21:470–484. doi: 10.1109/TMI.2002.1009383. [DOI] [PubMed] [Google Scholar]
- Huston PE, Shakow D, Riggs LA. Studies of motor function in schizophrenia. Journal of General Psychology. 1937 doi: 10.1080/00221309.1946.10544529. [DOI] [PubMed] [Google Scholar]
- Jersild AT. Mental set and shift. Archives of psychology. 1927:89. [Google Scholar]
- Kaufman J, Birmaher B, Brent D, Rao U, Flynn C, Moreci P, Williamson D, Ryan N. Schedule for Affective Disorders and Schizophrenia for School-Age Children-Present and Lifetime Version (K-SADS-PL): initial reliability and validity data. J Am Acad Child Adolesc Psychiatry. 1997;36:980–988. doi: 10.1097/00004583-199707000-00021. [DOI] [PubMed] [Google Scholar]
- Kelly AM, Di Martino A, Uddin LQ, Shehzad Z, Gee DG, Reiss PT, Margulies DS, Castellanos FX, Milham MP. Development of anterior cingulate functional connectivity from late childhood to early adulthood. Cereb Cortex. 2009;19:640–657. doi: 10.1093/cercor/bhn117. [DOI] [PubMed] [Google Scholar]
- Logan GD, Gordon RD. Executive control of visual attention in dual-task situations. Psychol Rev. 2001;108:393–434. doi: 10.1037/0033-295x.108.2.393. [DOI] [PubMed] [Google Scholar]
- Loose R, Kaufmann C, Tucha O, Auer DP, Lange KW. Neural networks of response shifting: influence of task speed and stimulus material. Brain Res. 2006;1090:146–155. doi: 10.1016/j.brainres.2006.03.039. [DOI] [PubMed] [Google Scholar]
- Luna B, Thulborn KR, Munoz DP, Merriam EP, Garver KE, Minshew NJ, Keshavan MS, Genovese CR, Eddy WF, Sweeney JA. Maturation of widely distributed brain function subserves cognitive development. Neuroimage. 2001;13:786–793. doi: 10.1006/nimg.2000.0743. [DOI] [PubMed] [Google Scholar]
- Meiran N. Reconfiguration of processing mode prior to task performance. Journal of Experimental Psychology-Learning Memory and Cognition. 1996;22:1423–1442. [Google Scholar]
- Meiran N. Modeling cognitive control in task-switching. Psychol Res. 2000;63:234–249. doi: 10.1007/s004269900004. [DOI] [PubMed] [Google Scholar]
- Mink JW. The basal ganglia: focused selection and inhibition of competing motor programs. Prog Neurobiol. 1996;50:381–425. doi: 10.1016/s0301-0082(96)00042-1. [DOI] [PubMed] [Google Scholar]
- Miyake A, Friedman NP, Emerson MJ, Witzki AH, Howerter A, Wager TD. The unity and diversity of executive functions and their contributions to complex “Frontal Lobe” tasks: a latent variable analysis. Cogn Psychol. 2000;41:49–100. doi: 10.1006/cogp.1999.0734. [DOI] [PubMed] [Google Scholar]
- Nee DE, Wager TD, Jonides J. Interference resolution: insights from a meta-analysis of neuroimaging tasks. Cogn Affect Behav Neurosci. 2007;7:1–17. doi: 10.3758/cabn.7.1.1. [DOI] [PubMed] [Google Scholar]
- Power JD, Fair DA, Schlaggar BL, Petersen SE. The development of human functional brain networks. Neuron. 2010;67:735–748. doi: 10.1016/j.neuron.2010.08.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reimers S, Maylor EA. Task switching across the life span: effects of age on general and specific switch costs. Dev Psychol. 2005;41:661–671. doi: 10.1037/0012-1649.41.4.661. [DOI] [PubMed] [Google Scholar]
- Rogers RD, Monsell S. Costs of a predictable switch between simple cognitive tasks. Journal of Experimental Psychology-General. 1995;124:207–230. [Google Scholar]
- Rottschy C, Langner R, Dogan I, Reetz K, Laird AR, Schulz JB, Fox PT, Eickhoff SB. Modelling neural correlates of working memory: A coordinate-based meta-analysis. Neuroimage. 2012;60:830–846. doi: 10.1016/j.neuroimage.2011.11.050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rubia K, Smith AB, Woolley J, Nosarti C, Heyman I, Taylor E, Brammer M. Progressive increase of frontostriatal brain activation from childhood to adulthood during event-related tasks of cognitive control. Hum Brain Mapp. 2006;27:973–993. doi: 10.1002/hbm.20237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith AB, Taylor E, Brammer M, Toone B, Rubia K. Task-specific hypoactivation in prefrontal and temporoparietal brain regions during motor inhibition and task switching in medication-naive children and adolescents with attention deficit hyperactivity disorder. Am J Psychiatry. 2006;163:1044–1051. doi: 10.1176/ajp.2006.163.6.1044. [DOI] [PubMed] [Google Scholar]
- Smith EE, Geva A, Jonides J, Miller A, Reuter-Lorenz P, Koeppe RA. The neural basis of task-switching in working memory: effects of performance and aging. Proc Natl Acad Sci U S A. 2001;98:2095–2100. doi: 10.1073/pnas.98.4.2095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sohn MH, Ursu S, Anderson JR, Stenger VA, Carter CS. The role of prefrontal cortex and posterior parietal cortex in task switching. Proc Natl Acad Sci U S A. 2000;97:13448–13453. doi: 10.1073/pnas.240460497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spencer-Smith M, Anderson V. Healthy and abnormal development of the prefrontal cortex. Dev Neurorehabil. 2009;12:279–297. doi: 10.3109/17518420903090701. [DOI] [PubMed] [Google Scholar]
- Supekar K, Musen M, Menon V. Development of large-scale functional brain networks in children. PLoS Biol. 2009;7:e1000157. doi: 10.1371/journal.pbio.1000157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sylvester CY, Wager TD, Lacey SC, Hernandez L, Nichols TE, Smith EE, Jonides J. Switching attention and resolving interference: fMRI measures of executive functions. Neuropsychologia. 2003;41:357–370. doi: 10.1016/s0028-3932(02)00167-7. [DOI] [PubMed] [Google Scholar]
- Thatcher RW. Cyclic cortical reorganization during early childhood. Brain Cogn. 1992;20:24–50. doi: 10.1016/0278-2626(92)90060-y. [DOI] [PubMed] [Google Scholar]
- Thatcher RW. Neural coherence and the content of consciousness. Conscious Cogn. 1997;6:42–49. doi: 10.1006/ccog.1997.0291. [DOI] [PubMed] [Google Scholar]
- Waber DP, De Moor C, Forbes PW, Almli CR, Botteron KN, Leonard G, Milovan D, Paus T, Rumsey J. The NIH MRI study of normal brain development: performance of a population based sample of healthy children aged 6 to 18 years on a neuropsychological battery. J Int Neuropsychol Soc. 2007;13:729–746. doi: 10.1017/S1355617707070841. [DOI] [PubMed] [Google Scholar]
- Wager TD, Jonides J, Reading S. Neuroimaging studies of shifting attention: a meta-analysis. Neuroimage. 2004;22:1679–1693. doi: 10.1016/j.neuroimage.2004.03.052. [DOI] [PubMed] [Google Scholar]
- Wager TD, Smith EE. Neuroimaging studies of working memory: a meta-analysis. Cogn Affect Behav Neurosci. 2003;3:255–274. doi: 10.3758/cabn.3.4.255. [DOI] [PubMed] [Google Scholar]
- Yehene E, Meiran N, Soroker N. Basal ganglia play a unique role in task switching within the frontal-subcortical circuits: evidence from patients with focal lesions. J Cogn Neurosci. 2008;20:1079–1093. doi: 10.1162/jocn.2008.20077. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


