Synopsis
Limb contractures are a common impairment in neuromuscular diseases (NMD). They contribute to increased disability due to decreased motor performance, mobility limitations, reduced functional range of motion, loss of function for activities of daily living (ADL), and increased pain. The pathogenesis of contractures is multifactorial. Myopathic conditions are associated with more severe limb contractures in comparison to neuropathic disorders. Although the evidence supporting the efficacy of multiple interventions to improve ROM in NMD in a sustained manner is lacking, there are generally accepted principles with regard to splinting, bracing, stretching, and surgery that help minimize the impact or disability from the contractures.
Keywords: Contractures, range of motion, fibrosis, static positioning, splinting, bracing, stretching, therapy, surgery
INTRODUCTION
Limb contractures are a common impairment in neuromuscular diseases (NMD). They contribute to increased disability due to decreased motor performance, mobility limitations, reduced functional range of motion, loss of function for activities of daily living (ADL), and increased pain. The pathogenesis of contractures is multifactorial. Known contributing extrinsic factors include decreased ability to actively move a limb through its full range of motion, static positioning for prolonged periods of time, and agonist antagonist muscle imbalance.1 Intrinsic factors include fibrotic changes to the muscle resulting in reduced extensibility.2–9 Contracture prophylaxis is important to maintain function, range of motion (ROM), and skin integrity.10–14 Lower limb contractures are much more prevalent than upper limb contractures. Myopathic conditions are associated with more severe limb contractures in comparison to neuropathic disorders. The rate of neuromuscular disease progression is also related to the frequency and severity of contractures with more rapidly progressive conditions resulting in earlier and more severe contracture formation.6 Bracing, stretching programs, and surgery have all been utilized in the prophylaxis and treatment of limb contractures.
PATHOGENESIS
A limb contracture is the lack of full passive ROM due to joint, muscle, or soft tissue limitations. Contractures in neuromuscular diseases develop due to intrinsic myotendinous structural changes and extrinsic factors.
Static Positioning
Weakness and inability to achieve active joint mobilization throughout the full normal range is the single most frequent factor contributing to the occurrence of fixed contractures. For example, less than antigravity knee extension strength places an individual at risk for knee flexion contractures, particularly if the patient no longer ambulates and spends the majority of their time seated with the knee joint positioned in flexion. The position in which a joint is statically positioned influences the number of sarcomeres present in any given muscle. A shortened muscle length may result in up to a 40% loss of sarcomeres.1 A statically positioned limb developing fibrotic changes within the muscle will develop contracture formation in the position of immobilization. Contractures rapidly develop in many NMD after transitioning to a wheelchair.9 The static nature of wheelchair mobility in comparison to the dynamic movement associated with gait contributes to the development of limb contractures. Compensatory strategies used to biomechanically stabilize joints to accommodate for muscle paresis result in reduced active ROM. For example, individuals with NMD resulting in proximal hip and knee extension weakness will exhibit lumbar lordosis, diminished stance phase knee flexion, and equinus posturing at the ankle during stance and gait. The equinus posturing at the ankle is a compensation to keep the weight line and ground reaction force line anterior to the knee. The increased lumbar lordosis moves the weight line and ground reaction force line posterior to the hip. These compensations stabilize the lower limbs by creating a knee extension moment and a hip extension moment. However, these same compensations result in reduced active ROM of the same joints. This is likely why ankle plantar flexion contractures develop in DMD before the onset of wheelchair reliance.10
Imbalance of Agonist and Antagonist Muscles
Asymmetries of strength are an important determinant of contracture formation. The imbalance between flexor and extensor muscle groups has not been shown to be a major factor leading to contracture formation, but contractures are frequently observed when major muscle imbalance is present. This is likely due to reduced active ROM since the movement is dominated by the stronger muscle group. For example, in several NMDs there is more pathologic involvement of the ankle dorsiflexors and evertors than the ankle plantarflexors and invertors. This imbalance in combination with intrinsic muscle changes leads to the frequently observed equinocavovarus foot deformities,10 which become exacerbated if the patient loses ambulation and is no longer weight bearing.
Fibrosis and Fatty Tissue Infiltration
Intrinsic muscle tissue alterations in dystrophic myopathies contribute to contracture formation. The most significant histologic changes are those of muscle fiber loss, abnormal residual dystrophic muscle fibers, segmental necrosis of muscle fibers, and increased amounts of adipose tissue, connective tissue, and fibrosis. Replacement of functioning muscle fibers with collagen and fatty tissue in concert with chronically shortened resting muscle length results in contracture formation. The collagen fibers undergo rearrangement and proliferation causing muscle fibrosis and resistance to passive stretch. Neurogenic atrophy typically results in a diminished degree of fibrosis which lowers the risk of severe contracture formation. 2–7
CONTRACTURES IN SPECIFIC NMD
Duchenne and Becker Muscular Dystrophies
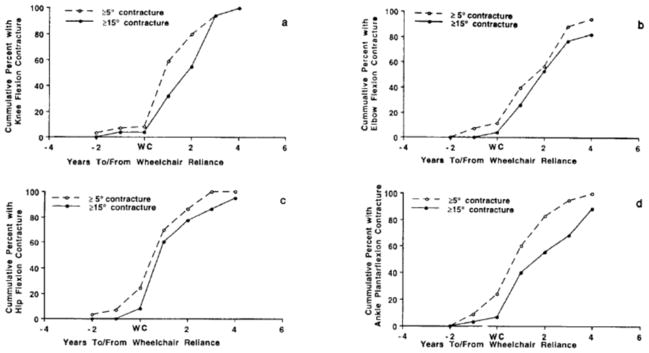
The most common contractures observed in dystrophinopathies in the order of frequency are ankle plantar flexion, knee flexion, hip flexion, hip abduction, elbow flexion, and wrist flexion contractures.9, 15 Proximal lower extremity contractures are rare while DMD subjects are ambulatory, but develop soon after they transition to a sitting position in a wheelchair for most of the day. The occurrence of elbow flexion contractures also appears to be directly related to prolonged static positioning of the limb flexed since these contractures develop soon after fulltime wheelchair reliance (Figure 1). The relationship between wheelchair reliance and hip and knee flexion contractures has been noted by multiple authors.5, 9, 16, 19 Given the tremendous replacement of muscle by fibrotic tissue in individuals with DMD, it is not surprising that a muscle with less than antigravity strength statically positioned in a wheelchair would develop a contracture. Although 20% of DMD subjects in the study by McDonald et al.9 developed ankle plantar flexion contractures of ≥5° before wheelchair reliance, there was a rapid acceleration in severity of these contractures after transition to wheelchair reliance (Figure 1). Ankle plantar flexion contractures were not likely a significant cause of wheelchair reliance since <10% of subjects had plantar flexion contractures of ≥15° before their transition to the wheelchair. The natural history data for DMD was described by McDonald and colleagues.9 The etiology and frequency of contractures in Becker muscular dystrophy (BMD) is similar to DMD when comparing individuals of similar function. As a result, contractures are rare in ambulatory boys with BMD with exception to ankle plantar flexion. As they transition to a wheelchair, the prevalence of contractures increases.15
Figure 1.
Cumulative percentages of DMD subjects with ≥5° contractures (dashed line) and ≥15° contractures (solid line) versus years to and from wheelchair reliance: a) Knee Flexion; b) Elbow Flexion; c) Hip Flexion; d) Ankle Plantarflexion (From McDonald CM, Abresch RT, Carter GT, Fowler WM Jr, Johnson ER, Kilmer DD, Sigford BJ. Profiles of neuromuscular diseases. Duchenne muscular dystrophy. Am J Phys Med Rehabil. 1995 Sep–Oct;74 (5 Suppl):S70–92. PubMed PMID: 7576424; with permission)
Emery Dreifuss Muscular Dystrophies
Emery Dreifuss muscular dystrophy (EMD) is a group of muscular dystrophies with early and extreme contracture formation disproportional to the degree of muscle weakness and immobility. EMD deserves special attention due to the notorious presence of limb contractures despite the presence of functional strength. EMD results from a group of genes that encode for nuclear proteins. The exact mechanisms of such profound contracture formation in EMD are not fully understood. The condition usually presents in adolescence or early adult life, and many clinical features may be seen in early childhood. An associated cardiomyopathy usually presents with arrhythmia and may lead to sudden death in early adult life. A hallmark of EMD type 1 is the early presence of contractures of the elbow flexors with limitation of full extension. Patients often have striking wasting of the upper arms accentuated by sparing of the deltoids and forearm muscles. The early presence of contractures of the elbow flexors is contrasted by focal wasting of the biceps brachii muscles. Heel cord tightness may be present early in the disorder concomitant with ankle dorsiflexion weakness and toe walking. Unlike DMD, the toe walking in EMD usually is secondary to ankle dorsiflexion weakness and contracture formation, and it is not a compensatory strategy to stabilize the knee due to proximal limb weakness. Tightness of the cervical and lumbar spinal extensor muscles or rigid spine results in limitation of neck and trunk flexion.18
Slowly Progressive Muscular Dystrophies
The slowly progressive muscular dystrophies are a heterogeneous group of muscular dystrophies with a slower progression with a life expectancy into later adulthood. Limb Girdle muscular dystrophies (LGMD), Facioscapulohumeral muscular dystrophy (FSH), Myotonic muscular dystrophy type 1 and 2 (DM1 and DM2) can all be associated with contracture formation. The severity of contractures coincides with the degree of muscle weakness. Severe contractures are infrequent in ambulators but are more prevalent in fulltime wheelchair users.19–22 Contractures in congenital myotonic dystrophy are common affecting greater than 70% of individuals and most commonly affect the ankle but rarely the knees or hips. Congenital myotonic muscular dystrophy patients may be born with clubfoot deformities. Scoliosis is also commonly observed.23
Congenital Muscular Dystrophies and Congenital Myopathies
Congenital muscular dystrophies (CMD) and congenital myopathies represent groups of congenital or infant onset myopathies. These disorders present with hypotonia and early presence of contractures. Subjects with congenital muscular dystrophies or myopathies often exhibit early contractures including ankle plantarflexion, knee flexion, hip flexion, wrist flexion, and long finger flexion. These myopathies have been reported to be fairly slowly progressive or relatively static; however, the contractures become more severe over time with prolonged static positioning and lack of active range of motion.18 Ullrich congenital muscular dystrophy patients have a primary defect in collagen VI in addition to a dystrophic myopathy. These patients have the unique combination of distal ligamentous laxity with hypermobile joints and proximal contractures.
Arthrogryposis
Arthrogryposis is a symptom complex characterized by congenital rigidity of the joints and is not a specific diagnostic entity. By definition, arthrogryposis involves multiple joints with distal joints more often affected than proximal joints. The feet, ankles, hands, and wrists are most commonly affected. A variety of central nervous system disorders such as chromosomal syndromes, developmental disorders, and congenital malformations of the central nervous system may result in arthrogryposis. Alternatively, focal and segmental vascular insufficiency during embryonic development may lead to a focal loss of anterior horn cells and hypomyoplasia or amyoplasia. Arthrogryposis due to amyoplasia leads to embryonic strength imbalance around joints which results in congenital contractures.
Spinal Muscular Atrophy
Spinal muscular atrophy (SMA) is a term used to describe a varied group of inherited disorders characterized by weakness and muscle wasting secondary to degeneration of both anterior horn cells of the spinal cord and brain stem motor nuclei without pyramidal tract involvement. The most common spinal muscular atrophy syndrome is predominantly proximal, autosomal recessive, and linked to chromosome 5q. Contractures are problematic in SMA patients who have lost ambulation or never obtained ambulation. One study found reductions in ROM by > 20° among 22% to 50% of SMA II subjects depending on the joint. Hip, knee, and wrist contractures were most common.21 Lower extremity contractures have been found to be rare in ambulatory SMA patients.24 Patients with SMA perceive their elbow flexion contractures to hinder one or more daily functions, and the contractures were reported to be associated with greater discomfort.25 Contractures are very common in SMA II. In the lower limbs, the knees are most affected followed by the hips and ankles.26 The shoulders are the most severely affected in the upper limbs followed by the elbows and wrists.27
Amyotrophic Lateral Sclerosis
Amyotrophic lateral sclerosis (ALS) is a rapidly progressive motor neuron disorder that results in profound appendicular, bulbar, and respiratory muscle weakness, but only mild joint contractures. In one study, only 26% of ALS subjects had ankle plantar flexion contractures, 13% had shoulder contractures, and only 20% had contractures of any joint measuring ≥20° by goniometry.6 The low prevalence and mild severity of contractures in ALS is likely due to the neurogenic nature of the muscle wasting with less severe fibrosis and fatty tissue infiltration.
Charcot Marie Tooth
Multiple subtypes of Charcot Marie Tooth (CMT) or Hereditary Motor Sensory Neuropathy (HMSN) exist with genetic heterogeneity among both the primarily demyelinating forms as well as the primarily axonal forms. In a study of 53 subjects, reduction in ROM by 20° or more was seen in 9% at the ankle, 8% at the knee, 2% at the elbow, 14% at the hip, and 19% at the wrist. Focal wasting of intrinsic foot and hand musculature is common, and the most common lower limb contracture is an equinocavovarus deformity.28 Cavus foot deformities associated with hindfoot varus and a variety of complex foot deformities due to muscle imbalance is common in Charcot Marie Tooth with disease progression.
MANAGEMENT OF CONTRACTURES
Although the evidence supporting interventions to improve ROM in NMD is lacking,29–31 there are generally accepted principles that may minimize the impact or disability from the contractures in NMD. Contractures in NMD conditions should be managed with the following concepts in mind:
Prevention of contractures requires early diagnosis and initiation of physical medicine approaches such as passive ROM and splinting before contractures are present or while contractures are mild.
Contractures are inevitable in some NMD conditions.
Advanced contractures become fixed and show little response to conservative interventsions such stretching or splinting programs and may require surgical intervention.
A major rationale for controlling lower limb contractures is to minimize their adverse effects on independent ambulation; however, the major cause of wheelchair reliance in NMD is generally weakness and not contracture formation.
Static positioning of both upper and lower limbs is an important cause of contracture formation.
Mild upper limb contractures may not negatively impact function.
Rehabilitation Management of Lower Limb Contractures
Four principal physical therapy modalities must be regularly carried out to prevent or delay the development of lower limb contractures for those at risk for musculoskeletal deformity. These include: (1) regularly prescribed periods of daily standing and/or walking; (2) passive stretching of muscles and joints; (3) positioning of the limbs to promote extension and oppose flexion; and (4) splinting which is a useful measure for the prevention or delay of contractures.32
A minimum of 2 to 3 hours of daily standing and/or walking is necessary in addition to passive stretching for the control of contracture formation in myopathies. Passive stretching to maintain or improve range of motion is an enormously important component of the program to prevent contractures. Such passive range of motion has been documented to be efficacious in slowing the development of contractures in DMD.32–38
A program of passive stretching should be started as early as possible in the course of neuromuscular disease and become part of a regular morning and evening routine. Proper technique is essential for passive stretching to be effective. With each stretch, the position should be held for a count of 15, and each exercise should be repeated 10 to 15 times during a session.39 Stretching should be performed slowly and gently. An overly strenuous stretch may cause discomfort and reduce cooperation. Written instructional materials should be provided to the patient and family as a supplement to verbal instructions and demonstrations by the physical therapist. The specific anatomic focus of stretching exercises prescribed for lower limb contractures will vary with the type of neuromuscular disease. Lower limb positioning may be a useful adjunct for preventing contracture formation. The limb should be placed in a resting position that opposes or minimizes flexion. The prone lying position is an effective method to stretch the hip flexors.
Splinting is another adjunctive measure used to slow the development of contractures in neuromuscular diseases. Ankle-foot orthotics (AFOs) or nighttime resting splints have been used to maintain a 90° angle of the foot relative to the tibia. Some authors indicate that such splinting is effective for reducing heel cord contractures in DMD; however, other investigators do not believe that AFO’s change the natural history of heel cord contracture formation.39–40 Foot deformities are common in peripheral neuropathies such as CMT and distal myopathies such as myotonic dystrophy type 1 (DM1). Treatment of foot deformities depends on the patient’s age, flexibility of the foot, bony deformity, and muscle imbalance. A nighttime or full-time AFO in a neutral ankle position custom molded to the foot deformity may decrease the tendency toward further development of the deformity. A supple foot can be managed non-operatively by a solid ankle AFO in the neutral position.
Few randomized controlled trials (RCT) have evaluated the efficacy of bracing. One such study found no benefit in nocturnal bracing in CMT, but the duration of treatment was only 6 weeks; however, the same group did report benefit from serial night casting.41–42 Burns et al. found no benefit from the use of an ankle foot orthoses in CMT in regards to functional outcomes and pain.43
Any splinting program must be used in conjunction with passive stretching and standing or ambulation. Splinting for prevention of equinus deformity generally has been used at night. However, AFOs may be worn while in the wheelchair throughout the day. Long leg knee-ankle-foot orthoses (KAFO) which immobilize the knee in extension may be worn for ambulation, for standing, or as a night resting brace. Most patients with tight hamstrings generally do not tolerate long leg bracing. Stretching, positioning, and splinting have limited roles in the management of hip and knee contractures in patients who primarily use a wheelchair.
Bracing of contractures for prolonged ambulation in NMD conditions with proximal weakness such as DMD was first reported by Spencer and Vignos in 1962.44 Lightweight polypropylene KAFOs with a neutral solid ankle, drop-lock knee joints, and ischial weight-bearing polypropylene upper thigh component has been used in DMD. The ischial seating gives a more upright standing posture with reduction in lordosis. DMD patients who have had excellent stretching programs can be placed immediately into KAFO bracing without surgical interventions. There are some experts who feel the use of KAFO’s prolongs ambulation in DMD by one to three years.45, 46 Although the literature to support this is poor,47 the greater reason why fewer patients are using KAFO bracng in recent years is due to the efficacy of corticosteroids in prolonging ambulation. In addition, the compliance of DMD patients with KAFO bracing is variable.48 The disadvantages of braced ambulation with KAFOs center around the excessive energy cost of such ambulation and safety concerns in the event of falls. DMD subjects prescribed long-leg braces (i.e. KAFOs) need gait training by physical therapy, and they need to be taught fall techniques with locked knees.
Surgical Management of Lower Limb Contractures
While DMD subjects are still ambulating independently, they use the ankle equinus posturing from the gastrocnemius soleus group to create a knee extension moment to stabilize the knee due to weak knee extensors. Several authors have cautioned against isolated tendo achilles lengthening (TAL) while DMD patients are still ambulating independently.53–51 Tendo achilles lengthening without simultaneous bracing above the knee can lead to loss of walking ability unless the quadriceps are grade 4- to 4 or better. Overcorrection (overlengthening) of the heel cord contracture in a patient with DMD may result in immediate loss of the ability to walk.37
Surgery prior to the loss of ambulation in DMD has not been shown to prolong ambulation and some studies have suggested that early surgery may have deleterious effects on ambulation.48 Some surgeons have performed early prophylactic TAL and posterior tibialis lengthening surgery years before anticipated loss of ambulation in patients with relatively mild contractures. In a randomized trial, Manzur and colleagues showed no benefit from early surgical treatment of distal lower extremity contractures in DMD.52 After braced ambulation ceases, surgical heel cord lengthening may have the benefit of better foot positioning on the wheelchair leg rests and occasionally allows improved show wear. Although surgery may improve the ankle ROM and positioning of the foot on the wheelchair footrest in DMD after transitioning to fulltime wheelchair use, it does not usually impact shoe wear, pain, hypersensitivity, or self-perceived cosmesis in a significant manner.53
Distal lower limb surgical interventions in ambulatory patients with slowly progressive NMD, particularly in neuropathies such as CMT, are often utilized. If a fixed deformity is present, surgical intervention may be required to obtain a plantigrade foot. The Coleman block test is commonly used in cavovarus feet to determine whether the contracture involves the forefoot, hindfoot, or both.54–56 The patient stands on a wood block 2.5 to 4 cm thick with the heel and lateral border of foot on the block with the first three metatarsals hanging freely over the block. This negates the effect the forefoot may have on the hindfoot stance. The hindfoot should be bearing the patient’s full weight. The correction of hindfoot varus with the patient standing on the block suggests the hindfoot is flexible and surgical intervention should be directed to correct the forefoot position. If the hindfoot varus does not correct while standing on the block, correction of both the forefoot and hindfoot are likely needed to obtain a more plantigrade foot.57 Rarely is TAL needed to correct a cavovarus foot because the calcaneus is already in calcaneus or dorsiflexion (Figure 2).54–56 TAL may worsen the cavus deformity by tilting the hindfoot into more calcaneus and the forefoot into more equinus to maintain ground contact. A dorsal closing wedge osteotomy of the midfoot at the apex of the cavus foot deformity is often performed in CMT (Figure 3). The osteotomy improves the forefoot position in relation to the hindfoot. This improves weight bearing biomechanics and can reduce pain.54–55
Figure 2.
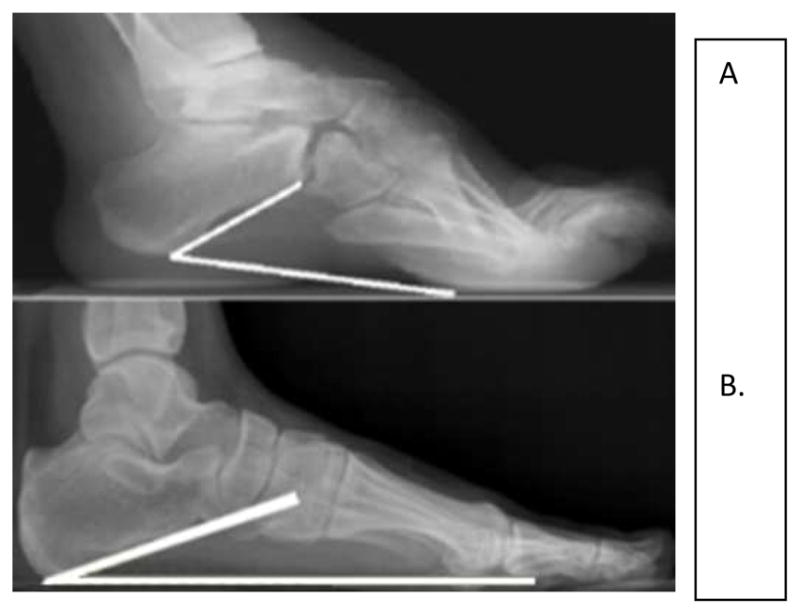
A) The calcaneal inclination angle is increased in the cavus foot since the hindfoot is already calcaneus despite the forefoot equinus. B) Normal calcaneal inclination angle.
Figure 3.
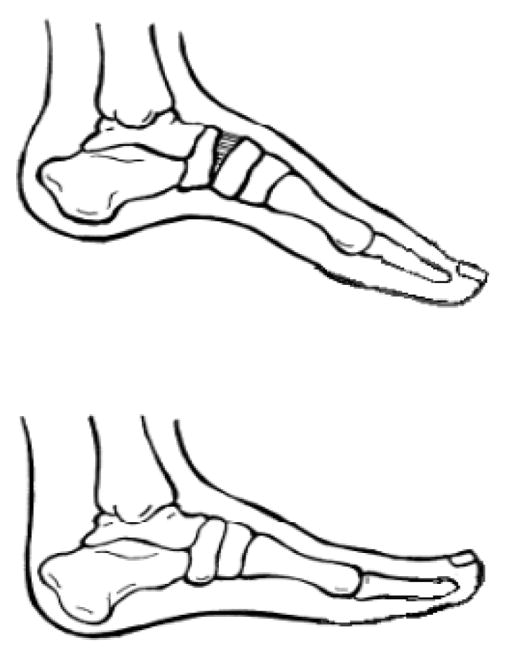
The dorsal osteotomy improves the forefoot equinus without worsening the hindfoot calcaneus.
Rehabilitation Management of Upper Limb Contractures
The primary focus in the literature has been on the management of lower limb contractures because contractures of the arm and hand cause little functional deficit until the late stages of NMD. Upper limb contractures rarely develop when there is functional strength of upper limb musculature with the exception of EMD. Elbow flexion contractures in DMD occur soon after fulltime wheelchair reliance and are likely secondary to the static positioning of the arms in elbow flexion on the arm rests of the wheelchair (figure 1).9 In DMD, deterioration in upper limb function lags approximately two years behind the loss of lower limb function. Other common upper limb contractures include forearm pronation and wrist flexion with ulnar deviation in the later stages of NMD. Although mild elbow flexion contractures of 15° or less are of minimal functional consequence, elbow flexion contractures greater than 30° can interfere with effective gait aid use in ambulatory patients with NMD. Severe elbow flexion contractures greater than 60° decreases distal upper limb function by reducing the reachable workspace and makes dressing difficult.58 Shoulder contractures are less problematic in patients with profound proximal muscle weakness. Combined shoulder adduction contractures and elbow flexion contractures may interfere with independent feeding. Severe shoulder adduction contractures complicate dependent dressing and can lead to hygiene difficulties including skin breakdown in the axilla.
Prophylactic occupational therapy management of wrist and hand ROM is recommended to slow the development of contractures and to maintain fine motor skills for functional tasks such as power wheelchair control. Nighttime resting splints which promote wrist extension, metacarpophalangeal extension, and proximal interphalangeal flexion are recommended to maintain active ROM as late as possible. Daytime positioning should emphasize wrist and finger extension, but any splinting should not compromise sensation or hand function. Passive stretching of the elbow flexors may be combined with passive stretch into forearm supination.
NMD patients with proximal shoulder girdle weakness have been managed with mobile arm supports; however, in the author’s experience, these often are prescribed but rarely accepted and used long term. A raised tray placed on the existing wheelchair lap tray or an elevating hospital bedside table can support the upper limbs in the same plane as the head. This allows the hand to still be brought to the mouth when less than antigravity strength of the elbow flexors is present. Alternatively, some patients choose to eat meals at higher counters or tables.
Surgical Management of Upper Limb Contractures
Upper limb contractures rarely require surgical intervention.58 Release of elbow flexion contractures in Emery Dreifuss muscular dystrophy is not usually performed due to the high rate of contracture recurrence. Surgical intervention may be warranted if the reduced upper limb ROM is impeding care and hygiene. This is especially important if the contracture is leading to skin breakdown or intolerable pain.
SUMMARY
Contractures are exceedingly common impairments in NMD, but weakness more often leads to disability. Less than antigravity strength produces an inability to achieve full active range of motion. Static positioning of limbs and lack of active ROM result in progressive contractures. Aggressive rehabilitation interventions including stretching, positioning, and splinting in addition to orthopedic surgical interventions may help minimize the degree of disability due to contractures in NMD.
Key points.
Known contributing extrinsic factors include decreased ability to actively move a limb through its full range of motion, static positioning for prolonged periods of time, and agonist antagonist muscle imbalance.
Known intrinsic factors contributing to contractures include fibrotic changes to the muscle resulting in reduced extensibility and disruption of muscle fiber architecture; thus myopathic conditions are associated with more severe limb contractures in comparison to neuropathic disorders.
A major rationale for controlling lower limb contractures is to minimize their adverse effects on independent ambulation; however, the major cause of wheelchair reliance in NMD is generally weakness and not contracture formation.
Static positioning of both upper and lower limbs is an important cause of contracture formation.
The primary focus of surgery has been on the management of lower limb contractures to achieve a braceable lower extremity or plantigrade foot or because contractures of the arm and hand cause little functional deficit until the late stages of NMD.
Acknowledgments
This work was supported by Grant# H133B0900001 from the National Institute of Disability and Rehabilitation Research. The authors take full responsibility for the contents of this paper, which do not represent the views of the National Institute of Disability and Rehabilitation Research or the United States Government.
Footnotes
Disclosures. The authors have nothing to disclose.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Contributor Information
Andrew J. Skalsky, University of California San Diego School of Medicine, Director Pediatric Rehabilitation Program, Co-Director MDA Neuromuscular Disease Clinic, Rady Children’s Hospital, San Diego, CA.
Craig M. McDonald, Department of Physical Medicine and Rehabilitation, Professor of Pediatrics, University of California Davis School of Medicine, Sacramento, CA.
References
- 1.Spector SA, Simard CP, Fournier M, Sternlicht E, Edgerton VR. Architectural alterations of rat hind-limb skeletal muscles immobilized at different lengths. Exp Neurol. 1982 Apr;76(1):94–110. doi: 10.1016/0014-4886(82)90104-2. [DOI] [PubMed] [Google Scholar]
- 2.Brooke MH, Fenichel GM, Griggs RC, Mendell JR, Moxley R, Florence J, King WM, Pandya S, Robison J, Schierbecker J, et al. Duchenne muscular dystrophy: patterns of clinical progression and effects of supportive therapy. Neurology. 1989 Apr;39(4):475–81. doi: 10.1212/wnl.39.4.475. [DOI] [PubMed] [Google Scholar]
- 3.Johnson EW, Zeiter Walter J. Lecture: pathokinesiology of Duchenne muscular dystrophy: implications for management. Arch Phys Med Rehabil. 1977 Jan;58(1):4–7. [PubMed] [Google Scholar]
- 4.Sutherland DH, Olshen R, Cooper L, Wyatt M, Leach J, Mubarak S, Schultz P. The pathomechanics of gait in Duchenne muscular dystrophy. Dev Med Child Neurol. 1981 Feb;23(1):3–22. doi: 10.1111/j.1469-8749.1981.tb08442.x. [DOI] [PubMed] [Google Scholar]
- 5.Archibald KC, Vignos PJ., Jr A study of contractures in muscular dystrophy. Arch Phys Med Rehabil. 1959 Apr;40(4):150–7. [PubMed] [Google Scholar]
- 6.Johnson ER, Fowler WM, Jr, Lieberman JS. Contractures in neuromuscular disease. Arch Phys Med Rehabil. 1992;73:807–10. [PubMed] [Google Scholar]
- 7.Hsu JD, Furumasu J. Gait and posture changes in the Duchenne muscular dystrophy child. Clin Orthop Relat Res. 1993 Mar;(288):122–5. Review. [PubMed] [Google Scholar]
- 8.D’Angelo MG, Berti M, Piccinini L, Romei M, Guglieri M, Bonato S, Degrate A, Turconi AC, Bresolin N. Gait pattern in Duchenne muscular dystrophy. Gait Posture. 2009 Jan;29(1):36–41. doi: 10.1016/j.gaitpost.2008.06.002. [DOI] [PubMed] [Google Scholar]
- 9.McDonald CM, Abresch RT, Carter GT, et al. Profiles of neuromuscular diseases. Duchenne muscular dystrophy. Am J Phys Med Rehabil. 1995;74( suppl):S70–92. doi: 10.1097/00002060-199509001-00003. [DOI] [PubMed] [Google Scholar]
- 10.Dubowitz V. Progressive muscular dystrophy: Prevention of deformities. Clin Pediatr (Phila) 1964 Jun;3:323–8. doi: 10.1177/000992286400300520. [DOI] [PubMed] [Google Scholar]
- 11.Dubowitz V. Prevention of deformities. Isr J Med Sci. 1977 Feb;13(2):183–8. [PubMed] [Google Scholar]
- 12.Fowler WM., Jr Rehabilitation management of muscular dystrophy and related disorders: II. Comprehensive care. Arch Phys Med Rehabil. 1982 Jul;63(7):322–8. Review. [PubMed] [Google Scholar]
- 13.Vignos PJ., Jr Physical models of rehabilitation in neuromuscular disease. Muscle Nerve. 1983 Jun;6(5):323–38. doi: 10.1002/mus.880060502. [DOI] [PubMed] [Google Scholar]
- 14.Siegel IM, Weiss LA. Postural substitution in Duchenne’s muscular dystrophy. JAMA. 1982 Feb 5;247(5):584. doi: 10.1001/jama.1982.03320300014014. [DOI] [PubMed] [Google Scholar]
- 15.McDonald CM, Abresch RT, Carter GT, Fowler WM, Jr, Johnson ER, Kilmer DD. Profiles of neuromuscular diseases. Becker’s muscular dystrophy. Am J Phys Med Rehabil. 1995 Sep-Oct;5(Suppl):S93–103. doi: 10.1097/00002060-199509001-00004. [DOI] [PubMed] [Google Scholar]
- 16.Scott OM, Hyde SA, Goddard C, Dubowitz V. Quantitation of muscle function in children: a prospective study in Duchenne muscular dystrophy. Muscle Nerve. 1982 Apr;5(4):291–301. doi: 10.1002/mus.880050405. [DOI] [PubMed] [Google Scholar]
- 17.Vignos PJ., Jr Management of musculoskeletal complications in neuromuscular disease: Limb contractures and the role of stretching, braces and surgery. Phys Med Rehabil: State of the Art Reviews. 1988:2509–536. [Google Scholar]
- 18.Dubowitz V. Muscle disorders in childhood. 2. Philadelphia: WB Saunders; 1995. [Google Scholar]
- 19.Johnson ER, Abresch RT, Carter GT, Kilmer DD, Fowler WM, Jr, Sigford BJ, Wanlass RL. Profiles of neuromuscular diseases. Myotonic dystrophy. Am J Phys Med Rehabil. 1995 Sep-Oct;74(5 Suppl):S104–16. [PubMed] [Google Scholar]
- 20.Kilmer DD, Abresch RT, McCrory MA, Carter GT, Fowler WM, Jr, Johnson ER, McDonald CM. Profiles of neuromuscular diseases. Facioscapulohumeral muscular dystrophy. Am J Phys Med Rehabil. 1995 Sep-Oct;74(5 Suppl):S131–9. doi: 10.1097/00002060-199509001-00007. [DOI] [PubMed] [Google Scholar]
- 21.Carter GT, Abresch RT, Fowler WM, Jr, Johnson ER, Kilmer DD, McDonald CM. Profiles of neuromuscular diseases. Spinal muscular atrophy. Am J Phys Med Rehabil. 1995 Sep-Oct;74(5 Suppl):S150–9. doi: 10.1097/00002060-199509001-00009. [DOI] [PubMed] [Google Scholar]
- 22.McDonald CM, Johnson ER, Abresch RT, Carter GT, Fowler WM, Jr, Kilmer DD. Profiles of neuromuscular diseases. Limb-girdle syndromes. Am J Phys Med Rehabil. 1995 Sep-Oct;74(5 Suppl):S117–30. doi: 10.1097/00002060-199509001-00006. [DOI] [PubMed] [Google Scholar]
- 23.Canavese F, Sussman MD. Orthopaedic manifestations of congenital Myotonic dystrophy during childhood and adolescence. J Pediatr Orthop. 2009 Mar;29(2):208–13. doi: 10.1097/BPO.0b013e3181982bf6. [DOI] [PubMed] [Google Scholar]
- 24.Iannaccone ST, Browne RH, Samaha FJ, Buncher CR. Prospective study of spinal muscular atrophy before age 6 years. DCN/SMA Group. Pediatr Neurol. 1993 May-Jun;9(3):187–93. doi: 10.1016/0887-8994(93)90082-n. [DOI] [PubMed] [Google Scholar]
- 25.Willig TN, Bach JR, Rouffet MJ, Krivickas LS, Maquet C. Correlation of flexion contractures with upper extremity function and pain for spinal muscular atrophy and congenital myopathy patients. Am J Phys Med Rehabil. 1995 Jan-Feb;74(1):33–8. doi: 10.1097/00002060-199501000-00006. [DOI] [PubMed] [Google Scholar]
- 26.Fujak A, Kopschina C, Gras F, Forst R, Forst J. Contractures of the lower extremities in spinal muscular atrophy type II. Descriptive clinical study with retrospective data collection. Ortop Traumatol Rehabil. 2011 Jan-Feb;13(1):27–36. doi: 10.5604/15093492.933792. [DOI] [PubMed] [Google Scholar]
- 27.Fujak A, Kopschina C, Gras F, Forst R, Forst J. Contractures of the upper extremities in spinal muscular atrophy type II. Descriptive clinical study with retrospective data collection. Ortop Traumatol Rehabil. 2010 Sep-Oct;12(5):410–9. [PubMed] [Google Scholar]
- 28.Carter GT, Abresch RT, Fowler WM, Jr, Johnson ER, Kilmer DD, McDonald CM. Profiles of neuromuscular diseases. Hereditary motor and sensory neuropathy, types I and II. Am J Phys Med Rehabil. 1995 Sep-Oct;74(5 Suppl):S140–9. doi: 10.1097/00002060-199509001-00008. [DOI] [PubMed] [Google Scholar]
- 29.Rose KJ, Burns J, Wheeler DM, North KN. Interventions for increasing ankle range of motion in patients with neuromuscular disease. Cochrane Database Syst Rev. 2010 Feb 17;(2) doi: 10.1002/14651858.CD006973.pub2. [DOI] [PubMed] [Google Scholar]
- 30.Sackley C, Disler PB, Turner-Stokes L, Wade DT, Brittle N, Hoppitt T. Rehabilitation interventions for foot drop in neuromuscular disease. Cochrane Database Syst Rev. 2009 Jul 8;(3):CD003908. doi: 10.1002/14651858.CD003908.pub3. Review. [DOI] [PubMed] [Google Scholar]
- 31.Young P, De Jonghe P, Stögbauer F, Butterfass-Bahloul T. Treatment for Charcot-Marie-Tooth disease. Cochrane Database Syst Rev. 2008 Jan 23;(1):CD006052. doi: 10.1002/14651858.CD006052.pub2. Review. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Scott OM, Hyde SA, Goddard C, Dubowitz V. Prevention of deformity in Duchenne muscular dystrophy. A prospective study of passive stretching and splintage. Physiotherapy. 1981 Jun;67(6):177–80. [PubMed] [Google Scholar]
- 33.Abramson AS, Rogoff J. An approach to rehabilitation of children with muscular dystrophy. Proceedings of the First and Second Medical Conferences of the MDAA, Inc; New York, Muscular Dystrophy Association of America. 1953. pp. 122–245. [Google Scholar]
- 34.Allsop KG, Ziter FA. Loss of strength and functional decline in Duchenne’s dystrophy. Arch Neurol. 1981 Jul;38(7):406–11. doi: 10.1001/archneur.1981.00510070040004. [DOI] [PubMed] [Google Scholar]
- 35.Harris SE, Cherry DB. Childhood progressive muscular dystrophy and the role of physical therapy. Phys Ther. 1974 Jan;54(1):4–12. doi: 10.1093/ptj/54.1.4. [DOI] [PubMed] [Google Scholar]
- 36.Seeger BR, Caudrey DJ, Little JD. Progression of equinus deformity in Duchenne muscular dystrophy. Arch Phys Med Rehabil. 1985 May;66(5):286–8. [PubMed] [Google Scholar]
- 37.Williams EA, Read L, Ellis A, Morris P, Galasko CS. The management of equinus deformity in Duchenne muscular dystrophy. J Bone Joint Surg Br. 1984 Aug;66(4):546–50. doi: 10.1302/0301-620X.66B4.6746690. [DOI] [PubMed] [Google Scholar]
- 38.Vignos PJ., Jr . Rehabilitation in the myopathies. In: Vinken PJ, Bruyn GW, editors. Handbook of Clinical Neurology. Chapter 13. Amsterdem: North Holland Publishing; 1980. pp. 457–500. [Google Scholar]
- 39.Vignos PJ, Jr, Archibald KC. Maintenance of ambulation in childhood muscular dystrophy. J Chronic Dis. 1960 Aug;12:273–90. doi: 10.1016/0021-9681(60)90105-3. [DOI] [PubMed] [Google Scholar]
- 40.Vignos PJ, Jr, Spencer GE, Archibald KC. Management of progressive muscular dystrophy in childhood. JAMA. 1963 Apr 13;184:89–96. doi: 10.1001/jama.1963.03700150043007. [DOI] [PubMed] [Google Scholar]
- 41.Refshauge KM, Raymond J, Nicholson G, van den Dolder PA. Night splinting does not increase ankle range of motion in people with Charcot-Marie-Tooth disease: a randomised, cross-over trial. Aust J Physiother. 2006;52(3):193–9. doi: 10.1016/s0004-9514(06)70028-9. [DOI] [PubMed] [Google Scholar]
- 42.Rose KJ, Raymond J, Refshauge K, North KN, Burns J. Serial night casting increases ankle dorsiflexion range in children and young adults with Charcot-Marie-Tooth disease: a randomised trial. J Physiother. 2010;56(2):113–9. doi: 10.1016/s1836-9553(10)70041-2. [DOI] [PubMed] [Google Scholar]
- 43.Crosbie J, Burns J. Predicting outcomes in the orthotic management of painful, idiopathic pes cavus. Clin J Sport Med. 2007 Sep;17(5):337–42. doi: 10.1097/JSM.0b013e31814c3e9e. [DOI] [PubMed] [Google Scholar]
- 44.Spencer GE, Vignos PJ., Jr Bracing for ambulation in childhood progressive muscular dystrophy. J Bone Joint Surg Am. 1962 Mar;44-A:234–42. [PubMed] [Google Scholar]
- 45.Pardo AC, Do T, Ryder T, Meyer A, Miles L, Wong BL. Combination of steroids and ischial weight-bearing knee ankle foot orthoses in Duchenne’s muscular dystrophy prolongs ambulation past 20 years of age--a case report. Neuromuscul Disord. 2011 Nov;21(11):800–2. doi: 10.1016/j.nmd.2011.06.006. [DOI] [PubMed] [Google Scholar]
- 46.Heckmatt JZ, Dubowitz V, Hyde SA, Florence J, Gabain AC, Thompson N. Prolongation of walking in Duchenne muscular dystrophy with lightweight orthoses: review of 57 cases. Dev Med Child Neurol. 1985 Apr;27(2):149–54. doi: 10.1111/j.1469-8749.1985.tb03763.x. [DOI] [PubMed] [Google Scholar]
- 47.Bakker JP, de Groot IJ, Beckerman H, de Jong BA, Lankhorst GJ. The effects of knee-ankle-foot orthoses in the treatment of Duchenne muscular dystrophy: review of the literature. Clin Rehabil. 2000 Aug;14(4):343–59. doi: 10.1191/0269215500cr319oa. Review. [DOI] [PubMed] [Google Scholar]
- 48.Garralda ME, Muntoni F, Cunniff A, Caneja AD. Knee-ankle-foot orthosis in children with Duchenne muscular dystrophy: user views and adjustment. Eur J Paediatr Neurol. 2006 Jul;10(4):186–91. doi: 10.1016/j.ejpn.2006.07.002. [DOI] [PubMed] [Google Scholar]
- 49.Eyring EJ, Johnson EW, Burnett C. Surgery in muscular dystrophy. JAMA. 1972 Nov 20;222(8):1056–7. [PubMed] [Google Scholar]
- 50.Siegel IM, Miller JE, Ray RD. Subcutaneous lower limb tenotomy in the treatment of pseudohypertrophic muscular dystrophy. Description of technique and presentation of twenty-one cases. J Bone Joint Surg Am. 1968 Oct;50(7):1437–43. [PubMed] [Google Scholar]
- 51.Spencer GE., Jr Orthopaedic care of progressive muscular dystrophy. J Bone Joint Surg Am. 1967 Sep;49(6):1201–4. [PubMed] [Google Scholar]
- 52.Manzur AY, Hyde SA, Rodillo E, Heckmatt JZ, Bentley G, Dubowitz V. A randomized controlled trial of early surgery in Duchenne muscular dystrophy. Neuromuscul Disord. 1992;2(5–6):379–87. doi: 10.1016/s0960-8966(06)80009-x. [DOI] [PubMed] [Google Scholar]
- 53.Leitch KK, Raza N, Biggar D, Stephen D, Wright JG, Alman B. Should foot surgery be performed for children with Duchenne muscular dystrophy? J Pediatr Orthop. 2005 Jan-Feb;25(1):95–7. doi: 10.1097/00004694-200501000-00021. [DOI] [PubMed] [Google Scholar]
- 54.Johnson BM, Child B, Hix J, Mendicino RW, Catanzariti AR. Cavus foot reconstruction in 3 patients with Charcot-Marie-Tooth disease. J Foot Ankle Surg. 2009 Mar-Apr;48(2):116–24. doi: 10.1053/j.jfas.2008.11.013. [DOI] [PubMed] [Google Scholar]
- 55.Tullis BL, Mendicino RW, Catanzariti AR, Henne TJ. The Cole midfoot osteotomy: a retrospective review of 11 procedures in 8 patients. J Foot Ankle Surg. 2004 May-Jun;43(3):160–5. doi: 10.1053/j.jfas.2004.03.009. [DOI] [PubMed] [Google Scholar]
- 56.Krause FG, Wing KJ, Younger AS. Neuromuscular issues in cavovarus foot. Foot Ankle Clin. 2008 Jun;13(2):243–58. vi. doi: 10.1016/j.fcl.2008.02.003. Review. [DOI] [PubMed] [Google Scholar]
- 57.Coleman SS, Chesnut WJ. A simple test for hindfoot flexibility in the cavovarus foot. Clin Orthop Relat Res. 1977 Mar-Apr;(123):60–2. [PubMed] [Google Scholar]
- 58.Do T. Orthopedic management of the muscular dystrophies. Curr Opin Pediatr. 2002 Feb;14(1):50–3. doi: 10.1097/00008480-200202000-00009. Review. [DOI] [PubMed] [Google Scholar]