Abstract
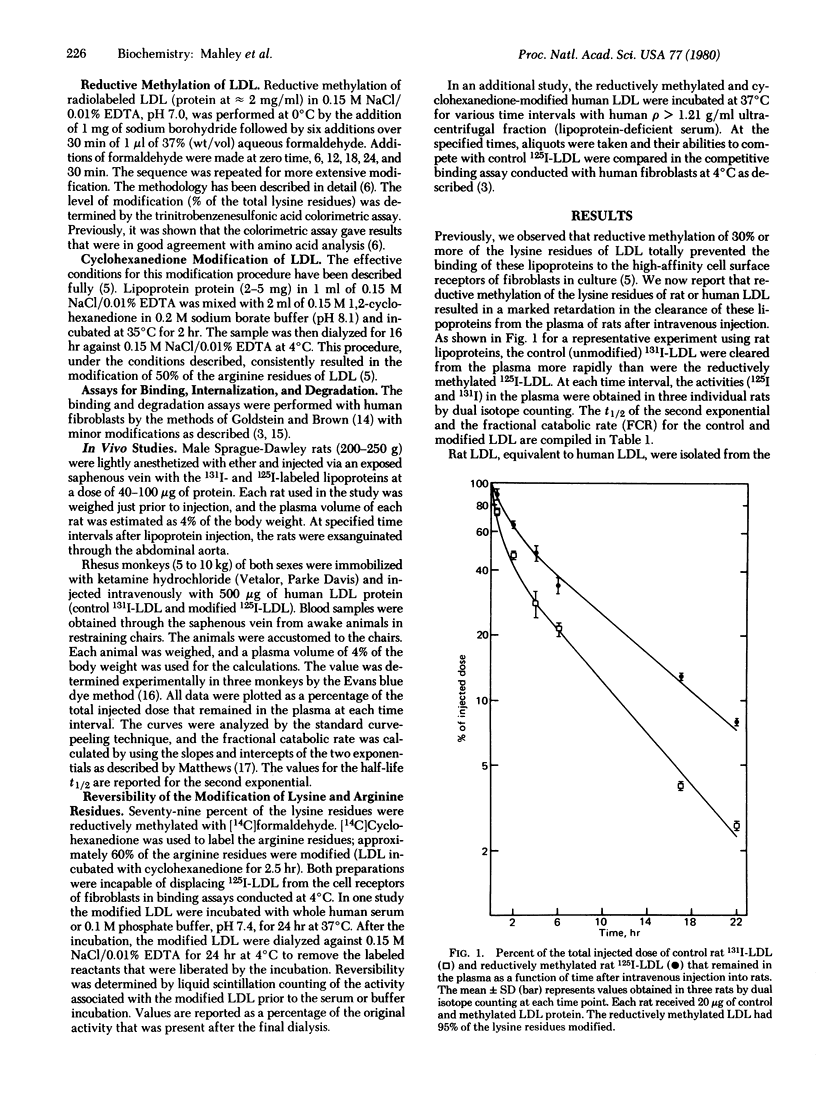
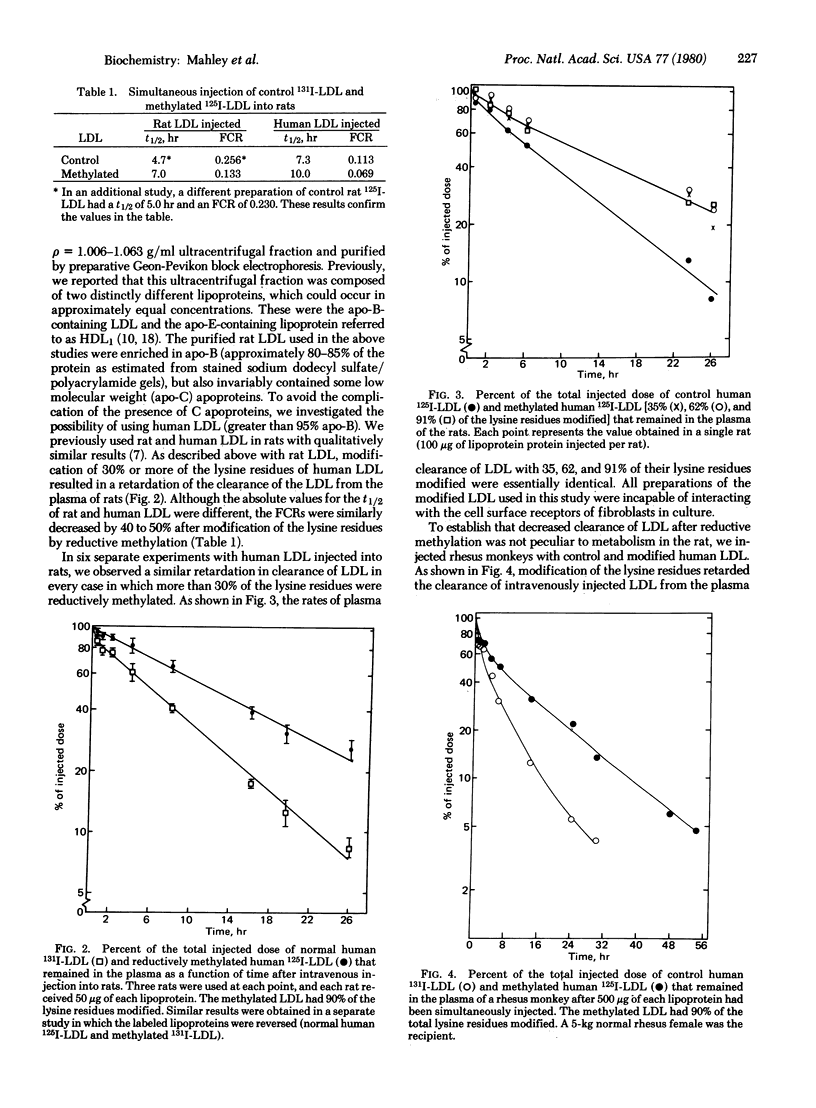
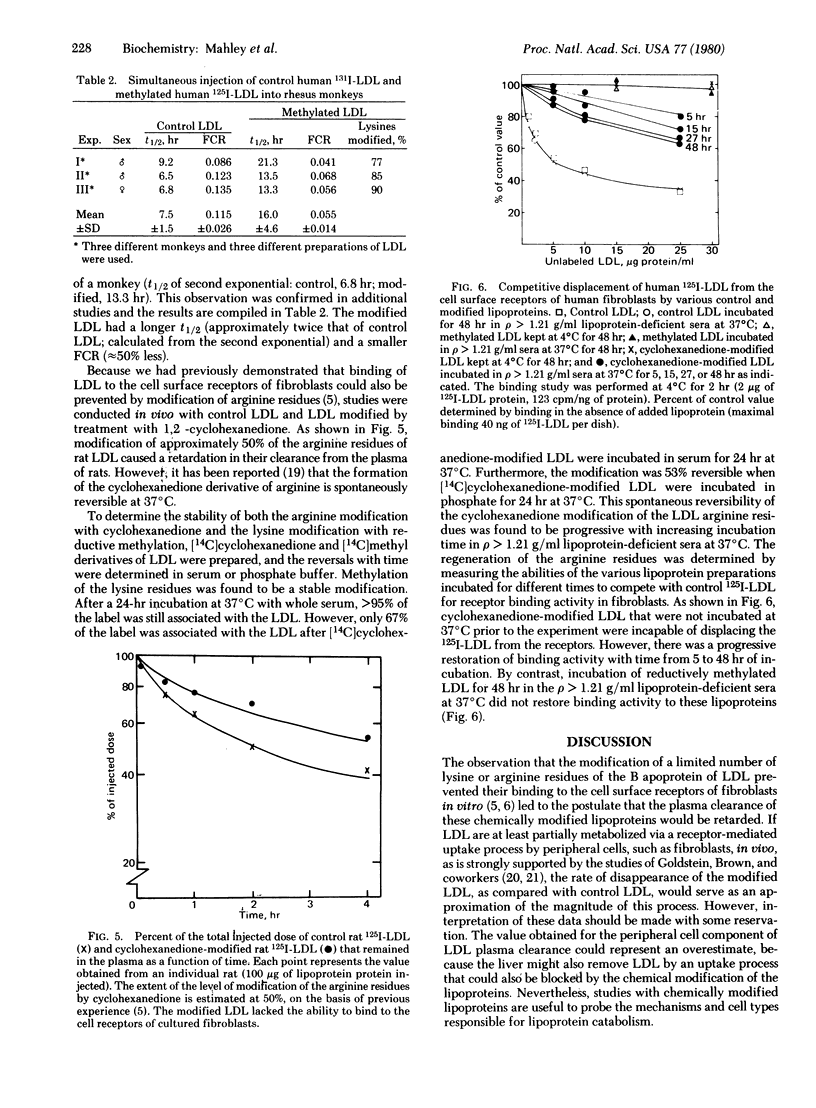
Reductive methylation of at least 30% of the lysine residues or 1,2-cyclohexanedione modification of 45% of the arginine residues prevented low density lipoproteins (LDL) from binding to cell surface receptors of fibroblasts in vitro, without significantly altering other physical or chemical properties of the LDL. When rat or human LDL with more than 30% of the lysine residues methylated were injected intravenously into rats, the clearance of these lipoproteins from the plasma was slowed considerably. The half-life of the reductively methylated LDL was approximately twice that obtained for control (unmodified) LDL, and the value for the fractional catabolic rate was approximately half that of the control. Furthermore, when human LDL modified by reductive methylation were injected into rhesus monkeys, the rate of clearance was similarly retarded, and the value for the fractional catabolic rate was reduced by approximately 50% as compared with the value for control LDL. A dual isotope labeling technique (125I and 131I) was used to compare the disappearance of the control and modified LDL in the same animal. It was demonstrated that not only modification of lysine residues but also modification of the arginine residues with 1,2-cyclohexanedione retarded the plasma clearance of the rat LDL. However, the cyclohexanedione modification was spontaneously reversible at 37°C, whereas reductive methylation of the lysine residues was stable. It is concluded that the selective chemical modification of lysine or arginine residues of LDL interferes with the normal uptake of these lipoproteins in vivo as well as by fibroblasts in vitro. These data provide an estimation of the level of receptor-mediated clearance of LDL from the plasma, a value that may be as high as 50% in rats and monkeys.
Keywords: lipoprotein catabolism, protein modification, cell receptors, protein turnover
Full text
PDF

Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bersot T. P., Mahley R. W., Brown M. S., Goldstein J. L. Interaction of swine lipoproteins with the low density lipoprotein receptor in human fibroblasts. J Biol Chem. 1976 Apr 25;251(8):2395–2398. [PubMed] [Google Scholar]
- Bilheimer D. W., Eisenberg S., Levy R. I. The metabolism of very low density lipoprotein proteins. I. Preliminary in vitro and in vivo observations. Biochim Biophys Acta. 1972 Feb 21;260(2):212–221. doi: 10.1016/0005-2760(72)90034-3. [DOI] [PubMed] [Google Scholar]
- Bilheimer D. W., Goldstein J. L., Grundy S. M., Brown M. S. Reduction in cholesterol and low density lipoprotein synthesis after portacaval shunt surgery in a patient with homozygous familial hypercholesterolemia. J Clin Invest. 1975 Dec;56(6):1420–1430. doi: 10.1172/JCI108223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown M. S., Goldstein J. L. Familial hypercholesterolemia: model for genetic receptor disease. Harvey Lect. 1979;73:163–201. [PubMed] [Google Scholar]
- GREGERSEN M. I., RAWSON R. A. Blood volume. Physiol Rev. 1959 Apr;39(2):307–342. doi: 10.1152/physrev.1959.39.2.307. [DOI] [PubMed] [Google Scholar]
- Goldstein J. L., Brown M. S. Binding and degradation of low density lipoproteins by cultured human fibroblasts. Comparison of cells from a normal subject and from a patient with homozygous familial hypercholesterolemia. J Biol Chem. 1974 Aug 25;249(16):5153–5162. [PubMed] [Google Scholar]
- Goldstein J. L., Brown M. S. The low-density lipoprotein pathway and its relation to atherosclerosis. Annu Rev Biochem. 1977;46:897–930. doi: 10.1146/annurev.bi.46.070177.004341. [DOI] [PubMed] [Google Scholar]
- Goldstein J. L., Ho Y. K., Basu S. K., Brown M. S. Binding site on macrophages that mediates uptake and degradation of acetylated low density lipoprotein, producing massive cholesterol deposition. Proc Natl Acad Sci U S A. 1979 Jan;76(1):333–337. doi: 10.1073/pnas.76.1.333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Innerarity T. L., Mahley R. W. Enhanced binding by cultured human fibroblasts of apo-E-containing lipoproteins as compared with low density lipoproteins. Biochemistry. 1978 Apr 18;17(8):1440–1447. doi: 10.1021/bi00601a013. [DOI] [PubMed] [Google Scholar]
- MATTHEWS C. M. The theory of tracer experiments with 131I-labelled plasma proteins. Phys Med Biol. 1957 Jul;2(1):36–53. doi: 10.1088/0031-9155/2/1/305. [DOI] [PubMed] [Google Scholar]
- Mahley R. W., Innerarity T. L. Interaction of canine and swine lipoproteins with the low density lipoprotein receptor of fibroblasts as correlated with heparin/manganese precipitability. J Biol Chem. 1977 Jun 10;252(11):3980–3986. [PubMed] [Google Scholar]
- Mahley R. W., Innerarity T. L., Pitas R. E., Weisgraber K. H., Brown J. H., Gross E. Inhibition of lipoprotein binding to cell surface receptors of fibroblasts following selective modification of arginyl residues in arginine-rich and B apoproteins. J Biol Chem. 1977 Oct 25;252(20):7279–7287. [PubMed] [Google Scholar]
- Mahley R. W., Weisgraber K. H., Innerarity T. L., Windmueller H. G. Accelerated clearance of low-density and high-density lipoproteins and retarded clearance of E apoprotein-containing lipoproteins from the plasma of rats after modification of lysine residues. Proc Natl Acad Sci U S A. 1979 Apr;76(4):1746–1750. doi: 10.1073/pnas.76.4.1746. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mahley R. W., Weisgraber K. H., Innerarity T., Brewer H. B., Jr, Assmann G. Swine lipoproteins and atherosclerosis. Changes in the plasma lipoproteins and apoproteins induced by cholesterol feeding. Biochemistry. 1975 Jul;14(13):2817–2823. doi: 10.1021/bi00684a005. [DOI] [PubMed] [Google Scholar]
- Mahley R. W., Weisgraber K. H., Innerarity T. Canine lipoproteins and atherosclerosis. II. Characterization of the plasma lipoproteins associated with atherogenic and nonatherogenic hyperlipidemia. Circ Res. 1974 Nov;35(5):722–733. doi: 10.1161/01.res.35.5.722. [DOI] [PubMed] [Google Scholar]
- Patthy L., Smith E. L. Reversible modification of arginine residues. Application to sequence studies by restriction of tryptic hydrolysis to lysine residues. J Biol Chem. 1975 Jan 25;250(2):557–564. [PubMed] [Google Scholar]
- Pitas R. E., Innerarity T. L., Arnold K. S., Mahley R. W. Rate and equilibrium constants for binding of apo-E HDLc (a cholesterol-induced lipoprotein) and low density lipoproteins to human fibroblasts: evidence for multiple receptor binding of apo-E HDLc. Proc Natl Acad Sci U S A. 1979 May;76(5):2311–2315. doi: 10.1073/pnas.76.5.2311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simons L. A., Reichl D., Myant N. B., Mancini M. The metabolism of the apoprotein of plasma low density lipoprotein in familial hyperbetalipoproteinaemia in the homozygous form. Atherosclerosis. 1975 Mar-Apr;21(2):283–298. doi: 10.1016/0021-9150(75)90087-8. [DOI] [PubMed] [Google Scholar]
- Weisgraber K. H., Innerarity T. L., Mahley R. W. Role of lysine residues of plasma lipoproteins in high affinity binding to cell surface receptors on human fibroblasts. J Biol Chem. 1978 Dec 25;253(24):9053–9062. [PubMed] [Google Scholar]
- Weisgraber K. H., Mahley R. W., Assmann G. The rat arginine-rich apoprotein and its redistribution following injection of iodinated lipoproteins into normal and hypercholesterolemic rats. Atherosclerosis. 1977 Oct;28(2):121–140. doi: 10.1016/0021-9150(77)90150-2. [DOI] [PubMed] [Google Scholar]



