Abstract
Several genome-wide association studies (GWAS) have demonstrated that common genetic variants contribute to obesity. However, studies of this complex trait have focused on ancestrally European populations, despite the high prevalence of obesity in some minority groups. As part of the ‘Population Architecture using Genomics and Epidemiology (PAGE)’ Consortium, we investigated the association between thirteen GWAS-identified SNPs and BMI and obesity in 69,775 subjects, including 6,149 American Indians, 15,415 African-Americans, 2,438 East Asians, 7,346 Hispanics, 604 Pacific Islanders, and 37,823 European Americans. For the BMI-increasing allele of each SNP, we calculated beta coefficients using linear regression (for BMI) and risk estimates using logistic regression (for obesity defined as BMI ≥ 30) followed by fixed-effects meta-analysis to combine results across PAGE sites. Analyses stratified by racial/ethnic group assumed an additive genetic model and adjusted for age, sex, and current smoking. We defined “replicating SNPs” (in European Americans) and “generalizing SNPs” (in other racial/ethnic groups) as those associated with an allele frequency-specific increase in BMI. By this definition, we replicated 9/13 SNP associations (5 out of 8 loci) in European Americans. We also generalized 8/13 SNP associations (5/8 loci) in East Asians, 7/13 (5/8 loci) in African Americans, 6/13 (4/8 loci) in Hispanics, 5/8 in Pacific Islanders (5/8 loci), and 5/9 (4/8 loci) in American Indians. Linkage disequilibrium patterns suggest that tagSNPs selected for European Americans may not adequately tag causal variants in other ancestry groups. Accordingly, fine-mapping in large samples is needed to comprehensively explore these loci in diverse populations.
Background
Obesity is a global health problem, with over 400 million obese adults worldwide[1]. In the US alone, there are over 60 million obese men and women, and obesity is increasingly prevalent among children[2, 3]. Risk factors for obesity in the US include increased age, female sex, and certain minority ancestry groups[2]. Many serious health conditions in the developed world are associated with obesity, including stroke, coronary heart disease, type 2 diabetes mellitus, hypertension, certain cancers, and cardiovascular diseases. Thus, the serious public health implications of unhealthy levels of body fat necessitate the need for deeper understanding of the etiology of obesity.
Several measures of body composition are commonly used in epidemiologic studies, including percentage body fat, waist-to-hip ratio, and body mass index (BMI, calculated as weight (kg) ÷ height (m)2). Although the term “obesity” is often used generally to describe a state of excess adipose tissue, the World Health Organization (WHO) defines obesity as having a BMI of equal or greater than 30 kg/m2 [1]. Variation in body fat and body composition may have a substantial genetic component, with numerous family studies demonstrating that much of the variation in BMI-related measures is heritable [4]. Indeed, our understanding of the role of genetic susceptibility to obesity and the consequences of obesity has increased enormously in recent years as new genotyping technologies have become accessible. First evidence came from linkage and candidate gene studies identifying variants associated with obesity[5]. More recently, genome-wide association studies (GWAS) and replication studies have identified multiple genetic variants across a range of loci that were otherwise unsuspected to be associated with BMI [3, 6–18]. These scans were primarily performed in populations of European ancestry, and while multiple variants of interest have been identified, none explain a substantial amount of population variation in BMI [19].
Investigation of the clinical and public health implications of these genetic discoveries requires not only confirmation in white populations, but importantly generalization of these associations to other ancestries such as African-Americans, Asians, American Indians, and other groups that were not adequately represented in the early GWAS. The purpose of this study is to examine 69,775 participants from diverse racial and ethnic backgrounds as part of the NHGRI-supported ‘Population Architecture using Genomics and Epidemiology (PAGE)’ Consortium to investigate the magnitude and consistency of associations between single-nucleotide polymorphisms (SNPs) previously-identified in genome-wide scans for loci associated with BMI and obesity.
Methods
Study Populations
PAGE involves several studies, described briefly below and in greater detail in the Supplementary Methods and at the PAGE website (https://www.pagestudy.org). All studies collected self-identified racial/ethnic group via questionnaire. All studies were approved by Institutional Review Boards at their respective sites, and all participants provided informed consent.
Causal Variants across the Life Course (CALiCo) is a consortium of six demographically diverse population based studies and a central laboratory, and includes approximately 58,000 men and women ranging in age from adolescence to older adulthood. Five CALiCo studies were involved in the present analysis, and contributed data on a total of 30,291 subjects aged 18 and older: Atherosclerosis Risk in Communities Study (ARIC) (N = 15,525) [20], Coronary Artery Risk in Young Adults (CARDIA) (N = 3,508) [21], Cardiovascular Health Study (CHS) (N = 5,273) [22], Strong Heart Family Study (SHFS) (N = 3,202) [23], and Strong Heart Cohort Study (SHS) (N = 2,846) [24]. In addition to the studies involved in the CALiCo consortium, PAGE includes three other large studies. The Women’s Health Initiative (WHI) is a prospective cohort study investigating post-menopausal women’s health in the U.S [25]. Out of the 161,808 women enrolled in WHI, 19,666 were selected and included in the present study. The Multiethnic Cohort (MEC) is a population-based prospective cohort study of over 215,000 men and women in Hawaii and California aged 45–75 at baseline (1993–1996) and primarily of five ancestries [26]. Participants eligible for the present study were controls in nested case-control studies of breast, colorectal, or prostate cancer or for biomarker studies (N=7,216). Finally, this analysis included data from the Epidemiologic Architecture for Genes Linked to Environment (EAGLE) study. EAGLE accesses the genetic component of three National Health and Nutrition Examination Surveys (NHANES): NHANES III (phase 2 collected between 1991 and 1994), NHANES 1999–2000, and NHANES 2001–2002[27–29]. Overall, 12,539 NHANES participants aged 18 and older were included in these analyses.
At all PAGE sites, we did not select underweight (BMI<18.5 kg/m2) and extremely overweight (BMI>70 kg/m2) individuals with the assumption that these extremes could be attributable to data coding errors, an underlying illness or possibly to a familial syndrome and hence, a rare mutation. After applying the above selection criteria, a total of 69,775 participants were selected from the PAGE consortium for analysis.
Anthropometric measurements
In all PAGE sites except MEC, BMI was calculated from height and weight measured at time of study enrollment in a clinic setting. Measurements collected 1 or 3 years after enrollment were substituted for 140 WHI participants missing enrollment height and/or weight. In MEC, self-reported height and weight were used to calculate baseline BMI. Pilot analyses within MEC have established the validity of self-reported height and weight in that cohort. In a small validation study among white and Japanese American women in the MEC, BMI was under-estimated based on self-reported compared to measured weight, but the difference was small (< 1 BMI unit; data not shown) and comparable to the findings from national surveys [30].
SNP Selection and Genotyping
Thirteen SNPs in 8 genes previously associated with BMI were selected for genotyping based on prior GWAS findings of positive association with BMI or obesity. SNPs were selected from GWAS studies published online as of December 31, 2008. While Supplementary Table 1 lists multiple studies previously investigating these SNPs, 5 papers were used in the selection process. These were Willer et al., 2009[8], Thorleifsson et al., 2009[12], Loos et al. 2008[6], Hinney et al. 2007[10], and Scuteri et al. 2007[11]. We were unable to select all putative obesity-related SNPs from all 5 papers, so we implemented a hierarchical selection strategy. First, we selected all nine SNPs associated with BMI in Willer et al., which was the largest study under consideration (N>70–80,000 subjects). Second, we assumed that SNPs associated with obesity in multiple GWAS were likely targets for our replication study, and this selected one additional SNP that was associated with BMI in three prior studies (rs8050136/FTO). We also noted that SNPs at the FTO locus were associated with BMI in each of the 5 prior GWAS. Because multiple hits in FTO could indicate shared linkage disequilibrium (LD) with an unmeasured causal SNP, we selected the FTO SNP with the strongest effect in each GWAS, if it had not already been selected (rs3751812 from Thorleifsson et al., and rs9930506 from Scuteri et al.). Finally, we noted that SNPs at the MC4R locus were associated with BMI in several prior GWAS, and elected to include an additional SNP at that locus. Having already selected the top MC4R hit from Loos et al. and Willer et al. (rs17782313), as a second SNP we selected the top MC4R hit from Thorleifsson et al. (rs12970134) for grand total of 13 genome-wide significant SNPs. Because LD patterns often vary between racial/ethnic groups, we elected to genotype more than one SNP in FTO and MC4R to maximize our chance of generalizing associations to non-European subjects at these most promising loci. All 13 SNPs were genotyped in all racial/ethnic groups, with the exception of the Pacific Islander group and American Indian groups, for which 8 and 9 SNPs were genotyped, respectively.
We also had access to genotype data for an additional 7 SNPs associated with BMI or obesity in prior GWAS, but not reaching genome-wide significance in a large sample of European American subjects at commonly accepted p-value of 5 × 10−8. Because these SNPs (rs7566605/INSIG2, rs748192/3p26.1, rs10498767/RCAN2, rs1106683/7q32.3, rs6602024/PFKP, rs1333026/13q21.32, and rs6013029/CTNNBL1) may represent false positive findings, analysis results are located in Supplementary Tables 5 and 6, but are not discussed in this manuscript.
DNA extraction and genotyping methods are detailed in the Supplementary Methods. Briefly, each PAGE site employed different genotyping platforms, with similar quality control criteria. CALiCo sites used TaqMan, the Illumina 370CNV BeadChip, and the Affymetrix Genome-Wide Human SNP Array 6.0. Thus, a portion of SNP genotype data was obtained from prior GWAS of CALiCo subjects [31]. EAGLE used Sequenom’s iPLEX® Gold coupled with MassARRAY MALDI-TOF MS detection and Illumina’s BeadXpress with a custom GoldenGate genotyping assay. MEC used Applied Biosystems OpenArray and TaqMan. WHI used Illumina BeadXpress with the Veracode GoldenGate genotyping assay. All sites used appropriate internal and external controls, and excluded genotypes deviating from Hardy-Weinberg expectations or with low concordance (typically, <95% – 99%). In addition to site-specific quality control, all PAGE study sites genotyped 360 DNA samples from the International HapMap Project and submitted these data to the PAGE Coordinating Center for concordance checks [32].
Statistical analysis
We investigated continuous BMI (kg/m2) and binary obesity (defined as BMI ≥ 30). Because the distribution of BMI was non-normal (skewed towards higher BMI), all analyses used natural-log transformed BMI. The association between each SNP and natural log-transformed BMI (lnBMI) was estimated using linear regression with robust standard errors (SEs)[33]. For the association between SNPs and obesity we used logistic regression with robust SEs[34]. SNP genotype was coded assuming an additive genetic model (i.e., 0, 1, or 2 copies of the BMI-increasing allele). All analyses were stratified by self-identified racial/ethnic group, and adjusted for the effects of age (continuous) and sex. We also adjusted for current smoking (yes/no) as smokers are less likely to be overweight[2]. All models included sex*smoking and sex*age interaction terms to account for possible effect modification by sex.
Analyses were performed for each PAGE study separately and results (effect sizes and robust SEs) were combined with fixed-effects meta-analysis using METAL[35]. Family data from the SHFS was analyzed using mixed models (variance component models) to account for relatedness. These mixed models are not amenable to analysis of binary variables, thus subjects from SHFS were excluded from the obesity analyses. Approximately 13% of the overall WHI study cohort was selected to contribute to PAGE. This selection was non-random, and was enriched for subjects with certain incident health conditions (diabetes, cardiovascular disease, and stroke), non-European American race/ethnicity, and BMI>40. Therefore, analyses of WHI data incorporated inverse probability weighting to account for this sampling strategy.
Fixed-effects meta-analysis was used to calculate effect sizes (β for lnBMI and log OR for obesity) and 95% confidence intervals for each SNP by racial/ethnic group. Meta-analyses for which the combined N was fewer than 150 subjects were not performed. We evaluated I2 as a measure of heterogeneity[36], to describe the presence or absence of excess variation between the PAGE cohorts. As a sensitivity analysis, we repeated the meta-analyses after excluding results from subjects with self-reported height and weight (i.e., MEC subjects) and compared these effect sizes and p-values with those from the metaanalyses with all subjects included. A second sensitivity analysis explored using different BMI cutoffs for exclusion from the analyses. In the subset of subjects from WHI and ARIC, we repeated analyses excluding subjects with BMI ≥50 and ≥40.
To investigate possible correlations between multiple SNPs genotyped at a single locus, we calculated the pairwise r2 for 2 SNPs in MC4R and 5 SNPs in FTO. We also compared racial/ethnic-specific linkage disequilibrium (LD) patterns for selected FTO SNPs in HaploView 4.2 (Broad Institute, Cambridge, MA) using data from the International HapMap Project (Version 3, Release R2), specifically the HapMap African Ancestry in Southwestern USA (ASW) analysis panel and the Centre d’Etude du Polymorphisme Humain Utah Residents with Northern and Western European Ancestry (CEU) analysis panel.
Finally, to address the issue of population stratification, we repeated the site-specific analyses for WHI, ARIC, and MEC including the top principal components (PCs) derived from ancestry informative markers (AIMs) in each model, and compared the results to models unadjusted for AIMs. Each PAGE site independently calculated PCs for all racial/ethnic groups combined using the EIGENSTRAT method[37]. The number of PCs required to account for population stratification varied by PAGE site, thus WHI analyses were adjusted for the top 3 PCs, MEC analyses were adjusted for the top 4 PCs, and ARIC analyses were adjusted for the top 10 PCs. The other PAGE sites did not have AIMs data, and were not included in this sub-analysis.
We used regression coefficients (betas) and 95% confidence intervals from each meta-analysis of lnBMI to calculate the racial/ethnic group-specific difference in BMI associated with one copy of the BMIincreasing allele, using the weighted average of BMIs from each PAGE site to estimate the mean BMI in each racial/ethnic group. The formula used was: mean BMI with 1 copy of risk allele = exp [ln(population mean BMI) + beta]. We subtracted the overall population mean from the formula output, to obtain the per-allele difference in BMI associated with each SNP. In this way, we are able to discuss differences in mean BMI without running analyses on the untransformed (and thus skewed) raw BMI variable. To ease interpretation, we selected a set of arbitrary thresholds to aid in distinguishing null results from small, yet relevant effects, using different thresholds based on minor allele frequencies (MAF). Using MAF-based thresholds helps to account for the fact that allele frequencies vary between racial/ethnic groups, and that the strength of association tends to increase with decreases in allele frequency (i.e., rarer alleles may be associated with stronger effects). For interpretation, we evaluated results in terms of absolute BMI units (kg/m2). For SNPs with MAF >0.25, we used a threshold of 0.1 BMI units (kg/m2). For SNPs with MAF 10 – 25%, we used a threshold of 0.15 BMI units (kg/m2), and for SNPs with MAF <10%, we used a threshold of 0.2 BMI units (kg/m2). As allele frequencies often varied by race/ethnic group, different BMI thresholds were used for each racial/ethnic group when required. We labeled meta-analysis results as “replicating” (for European Americans) or “generalizing” (for other racial/ethnic groups) if the effect size conferred a >0.10, >0.15, or >0.20 unit (kg/m2) difference in BMI (for MAFs >0.25, 0.10–0.25, and <0.10, respectively). Effect sizes conferring smaller differences in BMI, or effect sizes in the opposite direction as prior GWAS, were labeled as “not replicating” or “not generalizing”. Statistical significance was not considered when assigning these labels.
For each racial/ethnic group, we estimated the statistical power to detect the GWAS-reported effect sizes for each SNP (Supplementary Table 1) using Quanto (http://hydra.usc.edu/gx/), assuming the same effect size as reported in a prior GWAS, an additive genetic model and a two-sided test of association at p = 0.05. Power calculations were based on allele frequencies specific to each racial/ethnic group, as listed in Table 2. When necessary, the published effect sizes were re-calculated to obtain the appropriate beta associated with BMI. For example, if an effect size was reported in terms of effect on standard deviations of BMI, the published mean and SD BMI in the GWAS population were used to estimate what the corresponding effect size would be in terms of difference in BMI (kg/m2). The published and recalculated betas are provided in Supplementary Table 1. We calculated a weighted average BMI across all PAGE sites, and calculated the weighted average standard deviation for mean BMI by taking the square root of the weighted average of the variance of BMI from each PAGE site.
Table 2.
Meta-analysis of linear regression of putative obesity-related SNPs and per-allele difference in mean BMI (kg/m2), stratified by racial/ethnic group
| Gene | SNP | Location | CA | European Americans; mean BMI = 27.48 | Hispanics; mean BMI = 28.86 | African Americans; mean BMI = 29.80 | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Effect Size (95% CI)* | p-value | N | AF | power | Effect Size (95% CI)* | p-value | N | AF | power | Effect Size (95% CI)* | p-value | N | AF | power | ||||
| NEGR1 | rs2815752 | 1p31.1 | T | 0.14 (0.03 – 0.22) | 9.16E-03 | 28261 | 0.63 | 0.55 | 0.00 (−0.32 – 0.32) | 0.99 | 2891 | 0.71 | 0.09 | 0.03 (−0.12 – 0.18) | 0.7 | 10576 | 0.54 | 0.2 |
| TMEM18 | rs6548238 | 2p25.3 | C | 0.28 (0.17 – 0.39) | 8.59E-08 | 37061 | 0.83 | 0.99 | 0.06 (−0.23 – 0.35) | 0.7 | 6398 | 0.87 | 0.4 | 0.39 (0.18 – 0.60) | 2.41E-04 | 14492 | 0.88 | 0.59 |
| GNPDA2 | rs10938397 | 4p12 | G | 0.08 (0.00 – 0.19) | 0.04 | 31346 | 0.43 | 0.99 | 0.17 (−0.03 – 0.38) | 0.11 | 6369 | 0.38 | 0.44 | 0.27 (0.09 – 0.42) | 1.49E-03 | 14383 | 0.24 | 0.56 |
| MTCH2 | rs10838738 | 11p11.2 | G | 0.14 (0.06 – 0.22) | 1.01E-03 | 34679 | 0.35 | 0.36 | 0.23 (0.03 – 0.41) | 0.03 | 6406 | 0.37 | 0.1 | −0.18 (−0.41 – 0.06) | 0.15 | 13653 | 0.1 | 0.08 |
| SH2B1 | rs7498665 | 16p11.2 | G | 0.06 (−0.03 – 0.17) | 0.22 | 31383 | 0.38 | 0.91 | 0.00 (−0.20 – 0.20) a | 0.98 | 6391 | 0.45 | 0.31 | 0.15 (−0.03 – 0.30) | 0.09 | 13642 | 0.27 | 0.39 |
| FTO | rs1121980 | 16q12.12 | A | 0.42 (0.28 – 0.53) | 7.60E-12 | 21645 | 0.43 | 0.99 | −0.09 (−1.49 – 1.42) | 0.91 | 426 | 0.37 | 0.31 | 0.12 (−0.15 – 0.36) | 0.38 | 4005 | 0.47 | 0.98 |
| FTO | rs3751812 | 16q12.12 | T | 0.39 (0.28 – 0.50) | 4.21E-14 | 25776 | 0.52 | 0.99 | 0.17 (−0.34 – 0.67) | 0.53 | 1911 | 0.275 | 0.41 | 0.12 (−0.33 – 0.60) | 0.6 | 4549 | 0.118 | 0.4 |
| FTO | rs8050136 | 16q12.12 | A | 0.39 (0.28 – 0.47) | 2.78E-14 | 26544 | 0.41 | 0.99 | 0.38 (0.09 – 0.67) | 9.91E-03 | 3674 | 0.29 | 0.68 | 0.18 (−0.01 – 0.33) | 0.06 | 9435 | 0.43 | 0.96 |
| FTO | rs9930506 | 16q12.12 | G | 0.39 (0.28 – 0.53) | 3.27E-11 | 21998 | 0.44 | 0.99 | 0.29 (−0.20 – 0.82) | 0.24 | 1912 | 0.32 | 0.84 | 0.00 (−0.27 – 0.27) | 0.97 | 7065 | 0.21 | 0.99 |
| FTO | rs9939609 | 16q12.12 | A | 0.36 (0.28 – 0.47) | 4.61E-15 | 28286 | 0.4 | 0.99 | −0.37 (−1.68 – 1.00) | 0.58 | 449 | 0.33 | 0.13 | 0.03 (−0.15 – 0.24) | 0.7 | 6492 | 0.47 | 0.82 |
| MC4R | rs12970134 | 18q22 | A | 0.22 (0.08 – 0.33) | 1.31E-03 | 21987 | 0.26 | 0.92 | −0.74 (−2.06 – 0.67) | 0.3 | 448 | 0.17 | 0.07 | 0.18 (−0.21 – 0.54) | 0.38 | 4046 | 0.13 | 0.15 |
| MC4R | rs17782313 | 18q22 | C | 0.06 (0.00 – 0.14) | 0.08 | 35398 | 0.22 | 0.98 | 0.12 (−0.17 – 0.38) | 0.41 | 6388 | 0.13 | 0.26 | 0.18 (0.01 – 0.33) | 0.04 | 13698 | 0.29 | 0.63 |
| KCTD15 | rs11084753 | 19q13.11 | G | 0.03 (−0.05 – 0.11) | 0.61 | 29411 | 0.67 | 0.24 | 0.20 (−0.09 – 0.52) | 0.18 | 2891 | 0.65 | 0.07 | 0.03 (−0.12 – 0.18) | 0.72 | 10795 | 0.64 | 0.1 |
| Gene | SNP | Location | CA | East Asians; mean BMI = 24.99 | Pacific Islanders; mean BMI = 28.60 | American Indians; mean BMI = 31.86 | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Effect Size (95% CI)* | p-value | N | AF | power | Effect Size (95% CI)* | p-value | N | AF | power | Effect Size (95% CI)* | p-value | N | AF | power | ||||
| NEGR1 | rs2815752 | 1p31.1 | T | −0.17 (−0.57 – 0.25) | 0.43 | 2041 | 0.91 | 0.08 | −0.11 (−1.07 – 0.90) | 0.84 | 392 | 0.81 | 0.05 | −0.13 (−0.41 – 0.16) | 0.39 | 6153 | 0.76 | 0.11 |
| TMEM18 | rs6548238 | 2p25.3 | C | −0.02 (−0.37 – 0.35) | 0.94 | 2041 | 0.9 | 0.3 | 0.40 (−0.98 – 1.89) | 0.57 | 392 | 0.92 | 0.07 | 0.06 (−0.22 – 0.35) | 0.61 | 6186 | 0.78 | 0.43 |
| GNPDA2 | rs10938397 | 4p12 | G | −0.02 (−0.25 – 0.23) | 0.92 | 2037 | 0.28 | 0.35 | 0.58 (−0.23 – 1.38) | 0.16 | 392 | 0.27 | 0.07 | 0.32 (0.03 – 0.58) | 0.03 | 6125 | 0.24 | 0.27 |
| MTCH2 | rs10838738 | 11p11.2 | G | 0.18 (−0.07 – 0.40) | 0.16 | 2041 | 0.34 | 0.09 | 0.69 (−0.14 – 1.56) | 0.11 | 392 | 0.34 | 0.05 | −0.13 (−0.35 – 0.1) | 0.25 | 6147 | 0.46 | 0.09 |
| SH2B1 | rs7498665 | 16p11.2 | G | 0.48 (0.13 – 0.81) | 5.77E-03 | 2038 | 0.13 | 0.15 | 0.81 (−0.03 – 1.65) | 0.06 | 392 | 0.25 | 0.06 | 0.38 (0.13 – 0.64) | 2.00E-03 | 6149 | 0.59 | 0.23 |
| FTO | rs1121980 | 16q12.12 | A | 0.13 (−0.57 – 0.81) | 0.73 | 672 | 0.21 | 0.7 | ||||||||||
| FTO | rs3751812 | 16q12.12 | T | 0.28 (−0.45 – 0.99) | 0.46 | 701 | 0.18 | 0.3 | ||||||||||
| FTO | rs8050136 | 16q12.12 | A | 0.40 (0.13 – 0.66) | 3.14E-03 | 2325 | 0.19 | 0.8 | 0.49 (−0.23 – 1.23) | 0.18 | 574 | 0.24 | 0.14 | 0.32 (−0.01 – 0.68) | 0.06 | 6193 | 0.13 | 0.51 |
| FTO | rs9930506 | 16q12.12 | G | 0.20 (−0.42 – 0.84) | 0.53 | 699 | 0.22 | 0.72 | ||||||||||
| FTO | rs9939609 | 16q12.12 | A | 0.28 (−0.42 – 0.99) | 0.45 | 696 | 0.18 | 0.26 | 0.35 (0 – 0.71) | 0.05 | 6168 | 0.14 | 0.47 | |||||
| MC4R | rs12970134 | 18q22 | A | 0.63 (−0.15 – 1.44) | 0.11 | 696 | 0.16 | 0.12 | ||||||||||
| MC4R | rs17782313 | 18q22 | C | 0.10 (−0.17 – 0.38) | 0.46 | 2040 | 0.22 | 0.34 | −0.68 (−1.8 – 0.46) | 0.24 | 392 | 0.14 | 0.06 | 0.32 (−0.19 – 0.87) | 0.21 | 6163 | 0.07 | 0.13 |
| KCTD15 | rs11084753 | 19q13.11 | G | 0.20 (−0.05 – 0.45) | 0.1 | 2041 | 0.62 | 0.08 | −0.03 (−0.85 – 0.78) | 0.93 | 392 | 0.52 | 0.05 | 0.03 (−0.22 – 0.26) | 0.89 | 6163 | 0.69 | 0.07 |
CA: coded allele;
: Effect size is in terms of difference in mean BMI (kg/m2) associated with one copy of CA; AF: risk allele frequency; CI: confidence interval;
= heterogeneity I2 = 72.5, p-value = 0.03;
= heterogeneity I2 = 87.2, p-value = 0.005
Results
BMI (kg/m2) and demographic characteristics of participants in each PAGE site are detailed in Table 1. Across all sites, participant age ranged from 18–100 years, and BMI ranged from 18.5 to 69.7. Except for the Women’s Health Initiative, all studies recruited men and women. After reviewing the results with and without adjustment for ancestry principal components in a smaller sample of subjects with AIMs data, we found little evidence that population stratification affected our results (Supplementary Tables 2 and 3). Thus, the results unadjusted for PCs are presented as the main results of the study given that not all studies have available AIMs.
Table 1.
Demographic Characteristics of PAGE participants, by site
| European Americans | Hispanics | African Americans | ||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| total N | Mean age (SD) | Age range | Mean BMI (SD) | Max BMI | % obese | total N | Mean age (SD) | Age range | Mean BMI (SD) | Max BMI | % obese | total N | Mean age (SD) | Age range | Mean BMI (SD) | Max BMI | % obese | |
|
| ||||||||||||||||||
| ARIC | 11333 | 54.4 (5.7) | 44 – 66 | 27.1 (4.8) | 56.3 | 23 | 4192 | 53.6 (5.8) | 44 – 66 | 29.7 (6.0) | 65.9 | 41 | ||||||
| CARDIA | 1863 | 25.6 (3.3) | 18 – 30 | 23.8 (3.7) | 37.8 | 7 | 1645 | 24.5(3.8) | 18 – 30 | 25.8 (5.4) | 45.9 | 18 | ||||||
| CHS | 4454 | 72.8 (5.6) | 65 – 100 | 26.5 (4.4) | 48.3 | 18 | 819 | 72.9 (5.7) | 65 – 93 | 28.7 (5.4) | 58.8 | 33 | ||||||
| EAGLE | 6085 | 52.6 (19.6) | 18 – 90 | 27.6 (5.7) | 64.5 | 28 | 3513 | 43.5 (17.5) | 18 – 90 | 28.3 (5.5) | 59.7 | 32 | 2941 | 43.5 (17.1) | 18 – 90 | 29.1 (6.8) | 66.4 | 38 |
| MEC | 1193 | 59.5 (8.3) | 45 – 76 | 26.3 (5.0) | 56.8 | 17 | 1821 | 60.0 (7.2) | 45 – 76 | 27.7 (4.6) | 60.9 | 23 | 1858 | 62.0 (8.1) | 45 – 77 | 28.3 (5.1) | 68.7 | 30 |
| WHI | 12895 | 67.1 (6.9) | 50 – 79 | 29.0 (6.6) | 69.6 | 35 | 2012 | 60.5 (6.8) | 50 – 79 | 30.9 (7.2) | 69.9 | 33 | 3960 | 61.4 (7.1) | 50 – 79 | 33.2 (7.7) | 69.2 | 60 |
| Total | 37823 | 7346 | 15415 | |||||||||||||||
|
|
||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| East Asians | Pacific Islanders | American Indians | ||||||||||||||||
| total N | Mean age (SD) | Age range | Mean BMI (SD) | Max BMI | % obese | total N | Mean age (SD) | Age range | Mean BMI (SD) | Max BMI | % obese | total N | Mean age (SD) | Age range | Mean BMI (SD) | Max BMI | % obese | |
|
| ||||||||||||||||||
| MEC | 1740 | 61.0 (8.1) | 45 – 77 | 24.7 (3.3) | 40.7 | 6 | 604 | 56.7 (8.3) | 45 – 76 | 28.6 (6.0) | 60.2 | 35 | ||||||
| WHI | 698 | 64.1 (7.4) | 50 – 79 | 25.7 (4.4) | 56.4 | 12 | 0 | 101 | 60.5 (7.7) | 50 – 79 | 31.0 (6.6) | 49.8 | 50 | |||||
| SHFS | 0 | 0 | 3202 | 42.3 (15.9) | 18 – 93 | 32.7 (7.2) | 69.7 | 60 | ||||||||||
| SHCS | 0 | 0 | 2846 | 56.2 (8.0) | 45 – 75 | 31.0 (6.2) | 69.1 | 52 | ||||||||||
| Total | 2438 | 604 | 6149 | |||||||||||||||
ARIC: Atherosclerosis Risk in Communities Study; CARDIA: Coronary Artery Risk in Young Adults; CHS: Cardiovascular Health Study; EAGLE: Epidemiologic Architecture of Genes Linked to Environment; MEC: Multiethnic Cohort; WHI: Women’s Health Initiative; SHFS: Strong Heart Family Study; SHCS: Strong Heart Cohort Study; SD: standard deviation; BMI: body mass index; Note: minimum BMI was 18.5 for all sites and ancestry groups.
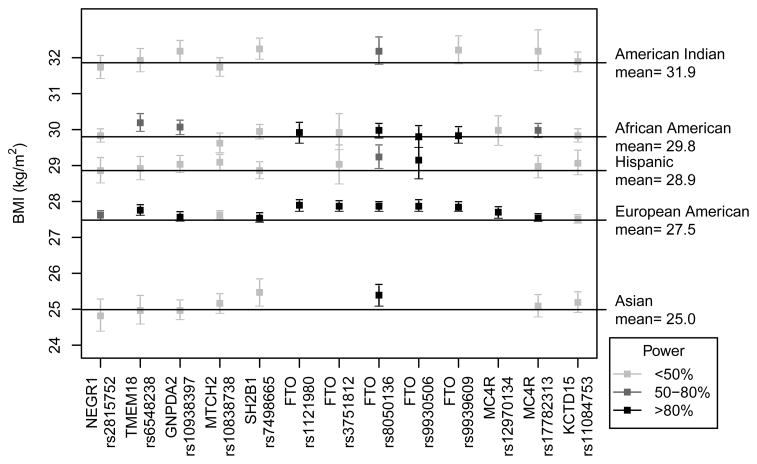
For each SNP, the per-allele difference from mean BMI (kg/m2) for each racial/ancestry group is listed in Table 2, and illustrated in Figure 1. Meta-analyses results for lnBMI and obesity are listed in Supplementary Table 4 and Table 3, respectively. Figure 1 shows that among European Americans, 9/13 SNPs replicated results from prior GWAS studies. Five out of these nine significant SNPs resided in the FTO region, and were likely not independent in our analysis, with pairwise r2 values ranging from 0.82 – 0.99 in European Americans (Supplementary Table 7). An additional SNP, rs10938397/GNPDA2 (MAF = 0.43) was associated with a nominally significant (p = 0.04) 0.08 BMI-unit increase, but did not meet our criteria for “replication” (i.e., >0.10 BMI units, kg/m2). Two SNPs were found to alter mean BMI by less than the generalization threshold, and were not statistically significant despite having adequate power (90 – 99%) (rs7498665/SH2B1 and rs17782313/MC4R). One SNP (rs11084753/KCTD15) was not significantly associated with BMI, but was likely underpowered (power = 24%).
Figure 1. Interpretation of association results and statistical power.
Plot of per-allele difference and 95% confidence interval from the population mean BMI for each racial/ethnic group, and statistical power for each analysis. This plot is restricted to analyses that included at least 1000 subjects. All analyses used the BMI-increasing allele named in prior GWAS as the risk allele; thus estimates falling above the mean BMI line are in the same direction as prior GWAS. >Statistical power is indicated by color intensity, with light grey indicating <50% power, medium grey boxes indicating 50–80% power, and black boxes indicating >80% power.
Table 3.
Meta-analysis of logistic re=gression of putative obesity-related SNPs and obesity, stratified by racial/ethnic group
| Gene | SNP | Location | CA | European Americans | Hispanics | African Americans | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Effect Size (95% CI) | p-value | N | AF | Effect Size (95% CI) | p-value | N | AF | Effect Size (95% CI) | p-value | N | AF | ||||
| NEGR1 | rs2815752 | 1p31.1 | T | 1.047 (0.996 – 1.100) | 0.07 | 28261 | 0.63 | 0.997 (0.865 – 1.149) | 0.97 | 2891 | 0.71 | 1.004 (0.942 – 1.070) | 0.91 | 10576 | 0.54 |
| TMEM18 | rs6548238 | 2p25.3 | C | 1.134 (1.075 – 1.196) | 3.50E-06 | 37061 | 0.83 | 0.959 (0.852 – 1.079) | 0.49 | 6398 | 0.87 | 1.159 (1.067 – 1.259) | 4.78E-04 | 14492 | 0.88 |
| GNPDA2 | rs10938397 | 4p12 | G | 1.027 (0.983 – 1.073) | 0.23 | 31346 | 0.43 | 1.024 (0.942 – 1.113) | 0.58 | 6369 | 0.37 | 1.106 (1.040 – 1.176) | 0.00 | 14383 | 0.24 |
| MTCH2 | rs10838738 | 11p11.2 | G | 1.045 (1.001 – 1.091) | 0.04 | 34679 | 0.35 | 1.109 (1.021 – 1.204) | 0.01 | 6406 | 0.37 | 0.947 (0.868 – 1.034) | 0.23 | 14240 | 0.10 |
| SH2B1 | rs7498665 | 16p11.2 | G | 1.020 (0.976 – 1.066) | 0.38 | 31383 | 0.38 | 0.993 (0.916 – 1.077) | 0.87 | 6391 | 0.44 | 1.032 (0.971 – 1.096) | 0.31 | 13642 | 0.27 |
| FTO | rs1121980 | 16q12.12 | A | 1.173 (1.109 – 1.239) a | 1.88E-08 | 21645 | 0.43 | 1.260 (0.835 – 1.901) | 0.27 | 426 | 0.37 | 1.034 (0.939 – 1.137) | 0.50 | 4005 | 0.47 |
| FTO | rs3751812 | 16q12.12 | T | 1.165 (1.107 – 1.225) | 2.91E-09 | 25776 | 0.51 | 1.095 (0.916 – 1.309) | 0.32 | 1911 | 0.28 | 1.056 (0.899 – 1.239) | 0.51 | 4549 | 0.12 |
| FTO | rs8050136 | 16q12.12 | A | 1.169 (1.112 – 1.228) | 7.14E-10 | 26544 | 0.41 | 1.225 (1.085 – 1.384) | 0.001 | 3674 | 0.30 | 1.053 (0.985 – 1.124) | 0.13 | 9435 | 0.43 |
| FTO | rs9930506 | 16q12.12 | G | 1.174 (1.111 – 1.241) b | 1.11E-08 | 21998 | 0.44 | 1.161 (0.977 – 1.379) | 0.09 | 1912 | 0.32 | 1.047 (0.953 – 1.150) | 0.34 | 7065 | 0.21 |
| FTO | rs9939609 | 16q12.12 | A | 1.173 (1.117 – 1.232) | 1.44E-10 | 28286 | 0.41 | 1.051 (0.706 – 1.564) | 0.81 | 449 | 0.33 | 1.024 (0.947 – 1.107) | 0.56 | 6492 | 0.47 |
| MC4R | rs12970134 | 18q22 | A | 1.078 (1.013 – 1.146) | 0.02 | 21987 | 0.26 | 0.527 (0.320 – 0.866) | 0.01 | 448 | 0.17 | 1.049 (0.911 – 1.207) | 0.51 | 4046 | 0.13 |
| MC4R | rs17782313 | 18q22 | C | 1.068 (1.019 – 1.120) | 0.01 | 35398 | 0.23 | 1.049 (0.933 – 1.179) | 0.42 | 6388 | 0.13 | 1.048 (0.987 – 1.112) | 0.12 | 13698 | 0.29 |
| KCTD15 | rs11084753 | 19q13.11 | G | 1.010 (0.961 – 1.062) | 0.69 | 29411 | 0.67 | 1.065 (0.931 – 1.218) | 0.36 | 2891 | 0.65 | 1.002 (0.939 – 1.070) | 0.96 | 10795 | 0.64 |
| Gene | SNP | Location | CA | East Asians | Pacific Islanders | American Indians | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Effect Size (95% CI) | p-value | N | AF | Effect Size (95% CI) | p-value | N | AF | Effect Size (95% CI) | p-value | N | AF | ||||
| NEGR1 | rs2815752 | 1p31.1 | T | 0.688 (0.436 – 1.086) | 0.11 | 2041 | 0.91 | 0.973 (0.638 – 1.484) | 0.90 | 392 | 0.81 | 1.040 (0.910 – 1.188) | 0.57 | 2926 | 0.78 |
| TMEM18 | rs6548238 | 2p25.3 | C | 0.900 (0.586 – 1.381) | 0.63 | 2041 | 0.90 | 1.110 (0.606 – 2.033) | 0.74 | 392 | 0.92 | 0.940 (0.822 – 1.073) | 0.36 | 2950 | 0.75 |
| GNPDA2 | rs10938397 | 4p12 | G | 0.910 (0.683 – 1.214) | 0.52 | 2037 | 0.28 | 1.512 (1.085 – 2.107) | 0.01 | 392 | 0.27 | 0.974 (0.857 – 1.107) | 0.69 | 2933 | 0.24 |
| MTCH2 | rs10838738 | 11p11.2 | G | 1.176 (0.900 – 1.536) | 0.24 | 2041 | 0.34 | 1.161 (0.838 – 1.608) | 0.37 | 392 | 0.34 | 1.019 (0.914 – 1.135) | 0.74 | 2944 | 0.47 |
| SH2B1 | rs7498665 | 16p11.2 | G | 1.846 (1.306 – 2.609) c | 5.16E-04 | 2038 | 0.13 | 1.362 (0.970 – 1.913) | 0.07 | 392 | 0.25 | 0.890 (0.794 – 0.997) | 0.04 | 2946 | 0.58 |
| FTO | rs1121980 | 16q12.12 | A | 1.437 (0.836 – 2.472) | 0.19 | 672 | 0.21 | ||||||||
| FTO | rs3751812 | 16q12.12 | T | 1.607 (0.944 – 2.734) | 0.08 | 701 | 0.18 | ||||||||
| FTO | rs8050136 | 16q12.12 | A | 1.472 (1.109 – 1.954) | 0.01 | 2325 | 0.19 | 1.089 (0.819 – 1.448) | 0.56 | 574 | 0.24 | 1.029 (0.865 – 1.226) | 0.74 | 2945 | 0.12 |
| FTO | rs9930506 | 16q12.12 | G | 1.315 (0.781 – 2.216) | 0.30 | 699 | 0.22 | ||||||||
| FTO | rs9939609 | 16q12.12 | A | 1.541 (0.908 – 2.614) | 0.11 | 696 | 0.18 | 1.019 (0.855 – 1.216) | 0.83 | 2928 | 0.12 | ||||
| MC4R | rs12970134 | 18q22 | A | 1.653 (0.881 – 3.101) | 0.12 | 696 | 0.16 | ||||||||
| MC4R | rs17782313 | 18q22 | C | 1.132 (0.816 – 1.571) | 0.46 | 2040 | 0.22 | 0.848 (0.538 – 1.337) | 0.48 | 392 | 0.14 | 0.816 (0.624 – 1.068) | 0.14 | 2941 | 0.06 |
| KCTD15 | rs11084753 | 19q13.11 | G | 1.205 (0.929 – 1.560) | 0.16 | 2041 | 0.62 | 0.969 (0.713 – 1.318) | 0.84 | 392 | 0.52 | 1.088 (0.968 – 1.222) | 0.16 | 2940 | 0.70 |
CA: coded allele; AF: risk allele frequency; CI: confidence interval;
: heterogeneity I2 = 79.7, p-value = 0.03;
: heterogeneity I2 = 79.6, p-value = 0.03;
: heterogeneity I2 = 82.0, p-value = 0.02
The analyses of obesity in European Americans produced similar results, as listed in Table 3. The 5 correlated FTO SNPs were each associated with 17% increased odds of obesity. A strong association was also observed for rs6548238/TMEM18, for which the C allele was associated with 13% increased odds of obesity. The remaining SNPs were associated with modest increases in odds of obesity, ranging from 0.5% to 8%.
We further analyzed these 13 SNPs to determine whether these associations generalized to other ancestral groups, and relied upon pairwise r2 (Supplementary Table 7) to determine whether multiple associations at the same locus were likely to be independent. In African Americans, seven SNPs generalized results from prior GWAS. LD in the FTO region was much lower compared to European Americans, with r2 ranging from 0.07 to 0.92 (mean r2 = 0.38). Two out of five FTO SNPs generalized in this group, but no associations were statistically significant despite adequate power. Both MC4R SNPs generalized in African Americans, yielding identical betas. These SNPs were not correlated (mean r2 = 0.12), and may represent independent associations. In the analyses of obesity, we observed nominally significant associations for 2 SNPs: rs6548238/TMEM18 (OR = 1.159, p = 0.0005) and rs10938397/GNPDA2 (OR = 1.106, p = 0.001).
In Hispanics, six SNPs generalized results from prior GWAS. LD in the FTO region was slightly lower compared to European Americans (r2 ranging from 0.63 – 0.91, mean = 0.75). Analyses in Hispanics were largely underpowered, with only one SNP having greater than 80% power (rs9930506/FTO). Three out of five FTO SNPs generalized in this group, although these 3 SNPs are moderately correlated (pairwise r2 ranging from 0.66 – 0.84) and may not represent independent associations. In the analyses of obesity, we observed nominally significant associations for 3 SNPs: rs10838738/MTCH2 (OR = 1.109, p = 0.01), rs8050136/FTO (OR = 1.225, p = 0.001), and a significant inverse association for rs12970134/GNPDA2 (OR = 0.527, p = 0.01).
In East Asians, eight SNPs generalized results from prior GWAS. Most analyses were underpowered, with only one SNP having at least 80% power (rs8050136/FTO). Four out of five FTO SNPs generalized in East Asians, although these SNPs are highly correlated and unlikely to represent independent associations (r2 ranging from 0.75 – 0.99, mean = 0.87). In the analyses of obesity, we observed nominally significant associations for 2 SNPs: rs7498665/SH2B1 (OR = 1.846, p = 0.0005) and rs8050136/FTO (OR = 1.472, p = 0.01).
Of the eight SNPs analyzed in Pacific Islanders, five generalized results from prior GWAS, including 1 FTO SNP, rs8050136. Power was very limited in this group, with no analysis having more than 14% power to detect a significant association (note that results from Pacific Islanders are not displayed in Figure 1 as they included <1000 subjects). In the analyses of obesity, we observed a nominally significant association for 1 SNP: rs10938397/GNPDA2 (OR = 1.512, p = 0.01).
Of the nine SNPs analyzed in American Indians, five SNPs showed a per-allele difference in BMI exceeding the generalization threshold in the same direction as in the initial GWAS. Both FTO SNPs analyzed generalized in American Indians, but these SNPs are highly correlated (r2 = 0.98), and are unlikely to represent independent associations. In the analyses of obesity, we observed a nominally significant inverse association for 1 SNP: rs7498665/SH2B1 (OR = 0.890, p = 0.04). This is opposite the association observed for BMI, in which each copy of the risk allele was associated with a 0.38-unit (kg/m2) increase in BMI (p = 0.002). This discrepancy could be attributable to chance, or variation between the subjects included in the obesity analysis (N = 2946) vs. the analysis of lnBMI (N = 6149).
We found evidence for excess heterogeneity across studies in five SNPs. There was evidence for excess heterogeneity for two summary statistics in European Americans. The heterogeneity p-value was 0.03 for the analyses of two FTO SNPs and obesity: rs9930506 and rs1121980. For these SNPs, the magnitude of effect sizes differed between ARIC and WHI, the only two sites contributing data to the analyses, although the directions of effect were the same. These FTO SNPs are highly correlated in WHI and ARIC European Americans (r2 = 0.86 in both studies), and study-specific results yielded identical betas for each SNP: 0.08 for both SNPs in WHI and 0.21 for both SNPs in ARIC. The heterogeneity pvalue for rs7498665/SH2B1 and BMI was 0.03 in Hispanics, and the beta coefficients from the three studies (EAGLE, WHI, and MEC) contributing to this meta-analysis were not consistent (0.007, −0.005, and −0.02, respectively). Finally, in East Asians, the heterogeneity p-value for rs10838738/MTCH2 and BMI was 0.005, and for rs7498665/SH2B1 and obesity was 0.02. For rs10838738/MTCH2, the beta coefficients from MEC and WHI were in opposite directions (0.01 and −0.02, respectively). The observed differences in beta coefficients may be due to underlying genetic differences in Asian subjects in MEC versus WHI, or because height and weight were directly measured in WHI, and self-reported in MEC. For rs7498665/SH2B1, the beta coefficients from MEC and WHI were in the same direction, but of different magnitudes (0.29 and 1.14, respectively).
The sensitivity analyses in European Americans and African Americans revealed that the meta-analyses performed without subjects with self-reported height and weight produced nearly identical effect sizes and p-values as the analyses including all subjects. However, the sensitivity analysis in East Asian subjects revealed several associations that changed direction after excluding subjects with self-reported height and weight (data not shown). In addition to the underlying heterogeneity described above, this instability could be attributable to sample size changes, because nearly two-thirds (N = 1740) of the East Asian sample had self-reported height and weight. The sensitivity analysis comparing results using different exclusion criteria (i.e., BMI ≥50 or BMI ≥40, rather than BMI ≥70) revealed no systematic differences between results when different BMI exclusion cutoffs were used (Supplementary Table 8). Effect sizes did vary, but there was no universal shift towards stronger or weaker effects when high-BMI subjects were excluded.
Discussion
In this large study including a total of 69,775 participants from six racial/ethnic groups, we found that 9 out of 13 SNPs (5/8 loci) genotyped in European Americans replicated results from prior GWAS. We observed the greatest amount of generalization in East Asians (8/13 SNPs, 5/8 loci), with lesser amounts in African Americans (7/13 SNPs, 5/8 loci, Hispanics (6/13 SNPs, 4/8 loci), Pacific Islanders (5/8 SNPs, 5/8 loci), and American Indians (5/9 SNPs, 4/8 loci).
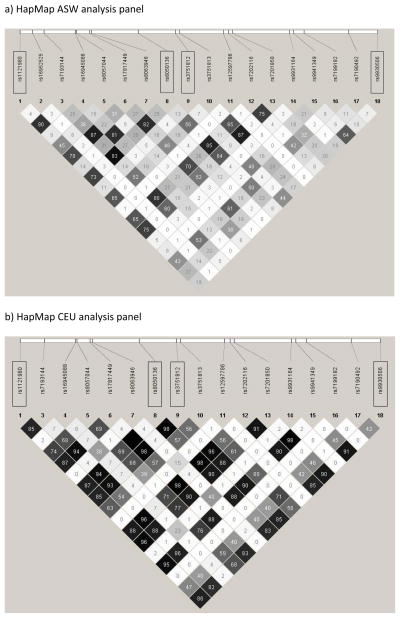
Associations of the five SNPs in the FTO gene with lnBMI and obesity were particularly strong in multiple racial/ethnic groups; and reached genome-wide significance at 5 × 10−8 in European Americans. However, these SNPs are correlated in almost all racial/ethnic groups studied, and therefore likely represent association with a single, unmeasured causal variant. As an illustration of LD in intron 1 of FTO, figure 2 uses HapMap data to illustrate a high degree of correlation among the FTO SNPs in European Americans (note that HapMap data for rs9939609 is not available, but this SNP is located between rs3751813 and rs12597786 on Figure 2). LD in the FTO region is substantially lower in African Americans (r2 from 0.07 – 0.92) versus other racial/ethnic groups, as illustrated in Figure 2 and Supplementary Table 7. LD is likewise lower in other non-European racial/ethnic groups, but there is still evidence for substantial correlation among most FTO SNPs in Hispanics (r2 from 0.63 −0.91), East Asians (r2 from 0.75 – 0.99) and American Indians (r2 of 0.98). A recent fine-mapping analysis illustrated that LD at the FTO locus is much lower in African Americans compared to European Americans, which translates to fewer FTO SNPs associated with BMI in African Americans [38]. This LD pattern (or lack thereof) may explain lack of generalization of several FTO SNPs in PAGE African Americans, despite sufficient power (rs9930506 with 99% power and rs9939609 with 82% power). If rs9930506 and rs9939609 are tagging (i.e., in LD with) a true causal SNP, then lower overall LD in this region could “decouple” the tagging SNPs from the underlying causal variant(s), leading to a true null association between rs9930506 and rs9939609 in African Americans. Likewise, the fine-mapping study cited above found no association between rs9939609 and BMI in African Americans [38]. In addition, Figure 1 shows that although the directions of the associations between the other three FTO SNPs (rs1121980, rs3751812, and rs8050136) and BMI were the same in European Americans and African Americans, the magnitudes of effect were substantially attenuated in African Americans, while the effect sizes observed in other racial/ethnic groups more closely mirrored those in European Americans. Although rs8050136/FTO was marginally associated with lnBMI in African Americans (p-value = 0.06), two SNPs correlated with rs8050136 were not associated with lnBMI in this group despite adequate power (rs1121980, r2 = ~0.75, p-value = 0.38; rs9939609, r2 = ~0.88, p-value = 0.70). This suggests that regions of LD span smaller portions of the FTO locus in African Americans, compared to European Americans. Accordingly, fine-mapping is needed to comprehensively investigate if susceptibility loci observed in European Americans are universally important to obesity in African Americans and other minority racial/ethnic groups. Because LD patterns are more similar between European Americans, Hispanics, East Asians, and American Indians, fine-mapping would likely be more fruitful in limiting the number of potentially functional variants in African Americans than in other racial/ethnic groups.
Figure 2. Linkage disequilibrium (LD) plots for FTO region in ASW and CEU HapMap analysis panels.
LD plots and were constructed in Haploview 4.2 using data from the International HapMap Project (Version 3, Release R2). The meta-analyses presented here involved 5 FTO SNPs, 4 of which are available in HapMap data (boxed). The HapMap CEU (Centre d’Etude du Polymorphisme Humain (Utah residents with ancestry from northern and western Europe)) analysis panel was used to represent European-ancestry individuals, and the HapMap ASW (African Ancestry in Southwest USA) analysis panel was used to represent African-Americans. The number in each square is the correlation coefficient (r2) for each pair of SNPs, with darker shading indicating higher values of r2.
Power limitations will become increasingly challenging for newly discovered GWAS findings given that the “low hanging fruits” with stronger effect sizes, such as FTO, have already been harvested in European-American populations and effect sizes of newly discovered SNPs will become increasingly smaller. In this respect, it is not surprising that many of the associations in minority racial/ethnic groups did not reach statistical significance in our study, despite sizable samples sizes particularly for African Americans (15,415), Hispanics (7,346) and American Indians (6,149). Accordingly, we did not attempt to use a statistical significance threshold as a criterion for generalization into other racial/ethnic groups. Rather, our definition of generalization as > 0.1, >0.15, or >0.20 unit (kg/m2) (depending on MAF) increase in BMI are arbitrary cutoff points for the minimum perceptible difference in BMI.
Our analyses were limited by several factors. As described above, LD patterns vary by race/ethnicity and the SNPs selected for this analysis are almost certainly not the ideal set of tagSNPs for each group. Second, our analyses do not account for known effect modifiers such as diet [2]and physical activity[39], and thus additional studies are needed to investigate the influence of the environment on the associations reported herein. Third, only 13 SNPs in 8 loci met our criteria for inclusion in this analysis as of December 31, 2008. Most of the SNPs under consideration were drawn from Willer et al.[8], and hence, SNP selection was not complete. However, our aim was not to comprehensively genotype all putative obesity-associated SNPs in non-European populations, rather, our goal was to illustrate generalization (or lack thereof) of the most promising obesity-related SNPs to multiple racial/ethnic groups. Including additional SNPs would have strengthened the conclusions of our study. Since the end of 2008, numerous studies have identified additional loci associated with BMI and obesity in European subjects. Notably, a recent GWAS conducted in nearly 250,000 European-descent subjects identified 18 novel loci associated with BMI, and confirmed several known associations, including two investigated in our PAGE analysis (rs10938397/GNPDA2 and rs2815752/NEGR1)[40]. These newly-discovered SNPs should be analyzed in large samples of non-European subjects to improve understanding of genetic risk factors for obesity in minority populations. It is of particular interest to note our a statistically significant association between the reported BMI-increasing allele of rs10938397 and decreased BMI in Hispanics – this finding may be due to chance, or to epistasis, or a gene-environment interaction specific to Hispanic populations.
Finally, in an attempt to maximize the generalizability of these findings, we applied limited exclusion criteria, and included individuals with BMI ranging from 18.5 to 70. We did not exclude individuals with obesity-associated disorders such as diabetes or cardiovascular disease. It is possible that one or more of the SNPs under study are also associated with an obesity-related illness, and thus an underlying condition may have confounded our results. However, diabetes and cardiovascular disease are understood to be consequences of obesity, thus it is unlikely that an obesity-related comorbidity would confound these associations. Further, our exploration of using stricter BMI exclusion criteria (i.e., BMI ≥ 40 kg/m2 and BMI ≥ 50 kg/m2 did not yield any systematic changes in effect size or statistical significance, suggesting that the effects of the SNPs in this analysis are consistent across a wide range of BMI.
The PAGE consortium offers a unique opportunity to investigate associations between candidate SNPs and BMI and obesity in ancestrally diverse cohorts with well-characterized phenotypes. Although PAGE studies recruited participants in a wide age range in many diverse regions of the United States, we observed little evidence of heterogeneity across studies, and only five estimates had a p for heterogeneity <0.05. This not significantly different (p = 0.08) from the number of heterogeneous estimates expected by chance alone. The substantial strength of PAGE is the large samples of ancestrally diverse participants, in which very little is known about genetic risk factors for obesity. However, these sample sizes did not prove to be adequate for SNPs with very small effect sizes. Aside from rs8050136/FTO, very few associations generalized to non-European American racial/ethnic groups and reached statistical significance.
In conclusion, in this large and diverse study we were able to replicate and generalize associations between 13 SNPs and BMI. The fraction of SNPs that generalized to non-European other racial/ethnic groups varied substantially, and appeared to be somewhat dependent on LD patterns. Failure to find statistically significant results underscores the importance of recruiting large samples of diverse participants for genetic studies of BMI and obesity. Race/ethnicity-based differences in LD highlight the need for fine-mapping studies in very large samples, in order to comprehensively explore the behavior of these loci in ancestrally diverse populations. In addition, an investigation of gene-environment interactions may help resolve ancestry-based differences in genetic risk factors for obesity.
Supplementary Material
References
- 1.Obesity and Overweight: Factsheet No 311. World Health Organization; Geneva: 2011. [Google Scholar]
- 2.Ogden CL, et al. The epidemiology of obesity. Gastroenterology. 2007;132(6):2087–102. doi: 10.1053/j.gastro.2007.03.052. [DOI] [PubMed] [Google Scholar]
- 3.Grant SF, et al. Association analysis of the FTO gene with obesity in children of Caucasian and African ancestry reveals a common tagging SNP. PLoS One. 2008;3(3):e1746. doi: 10.1371/journal.pone.0001746. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Yang W, Kelly T, He J. Genetic epidemiology of obesity. Epidemiol Rev. 2007;29:49–61. doi: 10.1093/epirev/mxm004. [DOI] [PubMed] [Google Scholar]
- 5.Mohlke KL, Boehnke M, Abecasis GR. Metabolic and cardiovascular traits: an abundance of recently identified common genetic variants. Hum Mol Genet. 2008;17(R2):R102–8. doi: 10.1093/hmg/ddn275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Loos RJ, et al. Common variants near MC4R are associated with fat mass, weight and risk of obesity. Nat Genet. 2008;40(6):768–75. doi: 10.1038/ng.140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Cotsapas C, et al. Common body mass index-associated variants confer risk of extreme obesity. Hum Mol Genet. 2009;18(18):3502–7. doi: 10.1093/hmg/ddp292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Willer CJ, et al. Six new loci associated with body mass index highlight a neuronal influence on body weight regulation. Nat Genet. 2009;41(1):25–34. doi: 10.1038/ng.287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Fox CS, et al. Genome-wide association to body mass index and waist circumference: the Framingham Heart Study 100K project. BMC Med Genet. 2007;8(Suppl 1):S18. doi: 10.1186/1471-2350-8-S1-S18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Hinney A, et al. Genome wide association (GWA) study for early onset extreme obesity supports the role of fat mass and obesity associated gene (FTO) variants. PLoS One. 2007;2(12):e1361. doi: 10.1371/journal.pone.0001361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Scuteri A, et al. Genome-wide association scan shows genetic variants in the FTO gene are associated with obesity-related traits. PLoS Genet. 2007;3(7):e115. doi: 10.1371/journal.pgen.0030115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Thorleifsson G, et al. Genome-wide association yields new sequence variants at seven loci that associate with measures of obesity. Nat Genet. 2009;41(1):18–24. doi: 10.1038/ng.274. [DOI] [PubMed] [Google Scholar]
- 13.Meyre D, et al. Genome-wide association study for early-onset and morbid adult obesity identifies three new risk loci in European populations. Nat Genet. 2009;41(2):157–9. doi: 10.1038/ng.301. [DOI] [PubMed] [Google Scholar]
- 14.Liu YJ, et al. Genome-wide association scans identified CTNNBL1 as a novel gene for obesity. Hum Mol Genet. 2008;17(12):1803–13. doi: 10.1093/hmg/ddn072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Herbert A, et al. A common genetic variant is associated with adult and childhood obesity. Science. 2006;312(5771):279–83. doi: 10.1126/science.1124779. [DOI] [PubMed] [Google Scholar]
- 16.Renstrom F, et al. Replication and extension of genome-wide association study results for obesity in 4923 adults from northern Sweden. Hum Mol Genet. 2009;18(8):1489–96. doi: 10.1093/hmg/ddp041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Frayling TM, et al. A common variant in the FTO gene is associated with body mass index and predisposes to childhood and adult obesity. Science. 2007;316(5826):889–94. doi: 10.1126/science.1141634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Bressler J, et al. Risk of type 2 diabetes and obesity is differentially associated with variation in FTO in whites and African-Americans in the ARIC study. PLoS One. 2010;5(5):e10521. doi: 10.1371/journal.pone.0010521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Bogardus C. Missing heritability and GWAS utility. Obesity (Silver Spring) 2009;17(2):209–10. doi: 10.1038/oby.2008.613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.The Atherosclerosis Risk in Communities (ARIC) Study: design and objectives. The ARIC investigators. Am J Epidemiol. 1989;129(4):687–702. [PubMed] [Google Scholar]
- 21.Hughes GH, et al. Recruitment in the Coronary Artery Disease Risk Development in Young Adults (Cardia) Study. Control Clin Trials. 1987;8(4 Suppl):68S–73S. doi: 10.1016/0197-2456(87)90008-0. [DOI] [PubMed] [Google Scholar]
- 22.Fried LP, et al. The Cardiovascular Health Study: design and rationale. Ann Epidemiol. 1991;1(3):263–76. doi: 10.1016/1047-2797(91)90005-w. [DOI] [PubMed] [Google Scholar]
- 23.North KE, et al. Genetic and environmental contributions to cardiovascular disease risk in American Indians: the strong heart family study. Am J Epidemiol. 2003;157(4):303–14. doi: 10.1093/aje/kwf208. [DOI] [PubMed] [Google Scholar]
- 24.Lee ET, et al. The Strong Heart Study. A study of cardiovascular disease in American Indians: design and methods. Am J Epidemiol. 1990;132(6):1141–55. doi: 10.1093/oxfordjournals.aje.a115757. [DOI] [PubMed] [Google Scholar]
- 25.Design of the Women’s Health Initiative clinical trial and observational study. The Women’s Health Initiative Study Group. Control Clin Trials. 1998;19(1):61–109. doi: 10.1016/s0197-2456(97)00078-0. [DOI] [PubMed] [Google Scholar]
- 26.Kolonel LN, et al. A multiethnic cohort in Hawaii and Los Angeles: baseline characteristics. Am J Epidemiol. 2000;151(4):346–57. doi: 10.1093/oxfordjournals.aje.a010213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.National Health and Nutrition Examination Survey (NHANES) DNA Samples: Guidelines for Proposals to Use Samples and Cost Schedule. Centers for Disease Control and Prevention. Fed Regist. 2010;75(108):32191–32195. [Google Scholar]
- 28.National Center for Health Statistics. Plan and Operation of the Third National Health and Nutrition Examination Survey, 1988–94. Vol. 1994. Hyattsville, MD: National Center for Health Statistics; 1994. (Vital and Health Statistics, Series 1: Programs and Collection Procedures, no. 32) (DHHS publication no. (PHS) 94-1308) (GPO no. 017-022-01260-0) [Google Scholar]
- 29.Chang MH, et al. Prevalence in the United States of selected candidate gene variants: Third National Health and Nutrition Examination Survey, 1991–1994. Am J Epidemiol. 2009;169(1):54–66. doi: 10.1093/aje/kwn286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Gorber SC, Tremblay MS. The bias in self-reported obesity from 1976 to 2005: a Canada- US comparison. Obesity (Silver Spring) 2010;18(2):354–61. doi: 10.1038/oby.2009.206. [DOI] [PubMed] [Google Scholar]
- 31.Psaty BM, et al. Cohorts for Heart and Aging Research in Genomic Epidemiology (CHARGE) Consortium: Design of prospective meta-analyses of genome-wide association studies from 5 cohorts. Circ Cardiovasc Genet. 2009;2(1):73–80. doi: 10.1161/CIRCGENETICS.108.829747. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Matise T, et al. The next PAGE in understanding complex traits: study design for analysis of Population Architecture using Genomics and Epidemiology. submitted. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Kleinbaum D, Kupper L, Muller K. Applied Regression Analysis and Other Multivariable Models. 2. Belmont, CA: Duxbury Press; 1988. [Google Scholar]
- 34.Hosmer D, Lemeshow S. Applied Logistic Regression. New York: Wiley; 1989. [Google Scholar]
- 35.Willer CJ, Li Y, Abecasis GR. METAL: fast and efficient meta-analysis of genomewide association scans. Bioinformatics. 2010;26(17):2190–1. doi: 10.1093/bioinformatics/btq340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Higgins JP, et al. Measuring inconsistency in meta-analyses. BMJ. 2003;327(7414):557–60. doi: 10.1136/bmj.327.7414.557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Price AL, et al. Principal components analysis corrects for stratification in genome-wide association studies. Nat Genet. 2006;38(8):904–9. doi: 10.1038/ng1847. [DOI] [PubMed] [Google Scholar]
- 38.Hassanein MT, et al. Fine mapping of the association with obesity at the FTO locus in African-derived populations. Hum Mol Genet. 2010;19(14):2907–16. doi: 10.1093/hmg/ddq178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Rankinen T, et al. FTO genotype is associated with exercise training-induced changes in body composition. Obesity (Silver Spring) 2010;18(2):322–6. doi: 10.1038/oby.2009.205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Speliotes EK, et al. Association analyses of 249,796 individuals reveal 18 new loci associated with body mass index. Nat Genet. 2010;42(11):937–48. doi: 10.1038/ng.686. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.