Abstract
Objective
Systemic juvenile idiopathic arthritis (SJIA) is an autoinflammatory syndrome where the myelomonocytic lineage is believed to play a pivotal role in pathogenesis. Traditionally, inflammatory macrophages are driven by interferon-γ (IFN-γ), yet recent gene expression studies of peripheral blood mononuclear cells (PBMCs) from active patients fail to demonstrate an IFN-induced signature. The goals of this study were to characterize the status of an IFN-induced signature within affected tissues and to gauge the integrity of IFN-signaling pathways within the peripheral monocytes of patients with SJIA.
Methods
Real-time PCR was used to quantify IFN-induced chemokine gene expression levels from synovial biopsies of 12 active SJIA and 9 active extended oligoarticular JIA patients. Fresh peripheral monocytes were then isolated from 3 inactive SJIA patients on an anti-IL-1β therapeutic, 5 active SJIA patients, and 8 healthy controls (HCs) and incubated with and without IFN-γ to gauge changes in the expression of IFN-induced genes via real-time PCR and to measure phosphorylated-STAT1 levels via FACS.
Results
IFN-induced chemokine gene expression was constrained in active SJIA synovium compared to the known IFN-mediated extended oligoarticular subtype. Expression of IFN-induced genes in unstimulated peripheral monocytes were statistically equal between the 3 groups, except for STAT1, MIG, and PIAS being significantly lower in active SJIA and PIAS1 higher in the inactive SJIA group. There was a trend of basal levels P-STAT1 being higher in SJIA patient monocytes compared to healthy controls, with inactive SJIA monocytes having the highest amount of the two, yet this observation failed to reach significance. Upon stimulation, fold-increase in gene expression was roughly equal between all 3 groups, except for a greater increase in inactive SJIA STAT1 vs controls, and a greater increase of IRF-1 expression in active patients vs inactive ones. Upon stimulation, the fold-increase of P-STAT1 in inactive SJIA peripheral monocytes was the greatest of all groups.
Conclusion
Monocytes from active SJIA patients retain the ability to respond to IFN-γ suggesting that the lack of an IFN-induced signature in active SJIA reflects a limited in vivo exposure to IFN-γ. Moreover, we detected a hyperresponsiveness to IFN-γ in inactive patients on an anti-IL-1β therapeutic.
Systemic juvenile idiopathic arthritis (SJIA) is an autoinflammatory disease characterized by spiking fevers, arthritis, polyserositis, evanescent rash, and lymphadenopathy (1). Evidence suggests that one of the effector cells in the pathogenesis of this disease is of the monocyte/macrophage lineage. Patients with active SJIA not only have an increased proportion of CD14+ monocytes (2), but also increased myelomonocytic precursors within their peripheral blood (3,4). In addition, one of the feared complications of SJIA is the macrophage activation syndrome (MAS) where affected patients experience widespread activation and expansion of T lymphocytes and cells of the monocyte-macrophage lineage, leading to unremitting fever, hyperferritinemia, hypertriglyceridemia, hepatosplenomegaly, a consumptive coagulopathy, and cytopenias. One of its prominent characteristics is an expansion of macrophages actively engaged in the hemophagocytosis of multiple cellular elements within the bone marrow and other lymphoid tissue (5,6). Yet, many of these features can be appreciated in severe SJIA too, in an intermediate phenotype called subclinical or occult MAS, thus suggesting MAS and SJIA may be different extremes of the same clinical spectrum (7,8).
The activated macrophage phenotype can be divided into two general groups, M1 and M2, depending on the stimuli they receive and their subsequent differentiation pathway (9–11). In this model, M1 macrophage differentiation is mainly driven by interferon-γ (IFN-γ) and M2 macrophages result from mostly anti-inflammatory signals, such as IL-4, IL-10, M-CSF, TGF-β, or glucocorticoids. M1 macrophages excel at intracellular pathogen eradication, producing high amounts of IL-12 and TNF-α. Due to their highly phagocytic properties, M2 macrophages are mostly scavengers that are heavily involved in wound healing and tissue remodeling. In contrast to M1 macrophages, M2 macrophages are a prominent source of IL-10.
Currently, the activated macrophage in SJIA and MAS has yet to be characterized into the M1/M2 polar model. SJIA and MAS are both inflammatory conditions, thus it’s likely that the macrophages therein are of the M1 phenotype. However, the data supporting IFN-γ, which is the key facilitator of M1 macrophage differentiation, as a mediator in active SJIA is scant. Gattorno et al. demonstrated elevated serum levels of both IFN-γ and IP-10, regardless of disease activity (12). In contrast, Lasiglie at al. showed that upon overnight stimulation of PBMC populations, no difference in the absolute number of IFN-γ-producing cells between active SJIA patients and healthy controls could be appreciated (13). Congruent with this finding, three independent gene expression studies have failed to detect an IFN-induced genetic signature within the peripheral blood mononuclear cells (PBMCs) of active SJIA patients (3,14,15). In contrast to SJIA, IFN-γ is believed to have a prominent role in the pathogenesis of hemophagocytic lymphohistiocytosis (HLH), both a hereditary and acquired hemophagocytic syndrome clinically similar to MAS, where IFN-γ is found to be highly elevated in the serum (16–18). In fact, blockade of IFN-γ has been suggested as a potential treatment for both inherited and acquired forms of active HLH (19). To this end, the goals of this study were to characterize the status of an IFN-induced signature within affected tissues and to gauge the integrity of IFN-signaling pathways within the peripheral monocytes of patients with SJIA.
IFN-γ is a pleotropic cytokine that canonically signals via the Janus kinase (Jak)-signal transducer and activator of transcription (STAT) pathway, machinery that is common to the many different cytokines (20). Specifically, IFN-γ will lead to the recruitment of Jak1 and Jak2 to the intracellular portion of the IFN-γ receptor, which then becomes phosphorylated on a tyrosine residue. This allows recruitment, docking, and subsequent phosphorylation of STAT1. In its active form, STAT1 will then form a homodimer and translocate into the nucleus in order to bind to a promoter region IFN-γ activation site (GAS) to initiate specific IFN-responsive genes.
Given the inflammatory nature of active SJIA, it seems paradoxical that an IFN-induced genetic signature cannot be detected within the PBMCs of active SJIA. In this study, we explore the role of IFN-γ in the pathogenesis of SJIA. We confirm a restricted IFN-induced genetic signature in not only the peripheral mononuclear cells, but also isolated peripheral monocytes therein. Furthermore, we demonstrate a markedly constrained expression of IFN-responsive genes within peripherally affected SJIA synovial tissue in comparison to extended oligoarticular JIA, a subtype of JIA that is known to express an IFN-induced genetic signature within its synovial cells (21). Upon exploring the ability of SJIA peripheral monocytes to even respond to IFN-γ, we did not find their overall reactivity to be significantly different from healthy control peripheral monocytes, which suggests that the absence of an IFN-induced genetic signature is not due to monocyte hyporesponsiveness to IFN-γ, but likely due to a limited exposure to it. Finally, we also report an increased basal interferon signal and augmented reactivity to IFN-γ within the peripheral monocytes of inactive SJIA patients on an IL-1β blocking agent, a finding that may have an impact on the ability of these patients to appropriately respond to viral illnesses.
PATIENTS AND METHODS
Subjects
For real-time RT-PCR studies of IFN-γ-stimulated peripheral monocytes: 8 age-matched, healthy controls, 5 patients with active SJIA (but who did not fulfill MAS diagnostic criteria (22)), and 3 patients with inactive SJIA on an anti-IL-1β therapeutic were used. SJIA diagnoses were determined using International League of Associations for Rheumatology (ILAR) criteria (23). For STAT1-phosphorylation assays: same patients as above. Please refer to Table 1 for a complete summary of the patients used for both PCR and STAT1-phosphorylation assays. For use in real-time RT-PCR of affected peripheral tissue: previously obtained biopsies from 12 SJIA and 9 extended oligoarticular JIA patients were procured from the Cincinnati Pediatric Rheumatology Tissue Repository based on the presence of a significant inflammatory infiltration of the tissue as described previously (24). Extended oligoarticular arthritis patients were chosen as a comparison group based on observations that IFN-γ-producing Th1 lymphocytes are in abundance within the synovial compartment of these patients. Furthermore, the increased production of IFN-γ within the synovium is associated with a prominent IFN-induced gene signature (21). For all patients in this study, except for those who contributed synovial biopsy tissue where clinical data was lacking, active SJIA was determined by having at least having one of the following findings once other potential causes, such as minor infections, had been clinically excluded; increase erythrocyte sedimentation rate (ESR) and/or C-reactive protein (CRP), evidence of arthritis, SJIA-consistent rash, fever, serositis, or lymphadenopathy. Written consent was collected for all participants and this study was approved by Cincinnati Children’s Hospital Medical Center’s Institutional Review Board.
Table 1.
Demographical and clinical characteristic of the patients with SJIA who contributed samples*
| Patient | Assay | Age (yr), Duration (mo)# |
Status | Active Arthritis |
Systemic Features† |
Cytopenias | ESR / CRP (mg/dL) |
Ferritin (ng/mL) |
Current Medications |
|---|---|---|---|---|---|---|---|---|---|
| 1 | PCR | 10, 51 | Active | + | − | No | 42 / 4.7 | ND | Etanercept, Methotrexate |
| 2 | PCR, FACS |
14, 120 | Active | + | − | No | 44 / 1.6 | 40 | Anakinra |
| 3 | PCR | 6, 36 | Active | + | + | No | 69 / 29.7 | 3,644 | None |
| 4 | PCR, FACS |
11, 75 | Active | + | − | No | 82 / 5.1 | 140 | Prednisone |
| 5 | PCR, FACS |
10, 1 | Active | + | + | No | 53 / 13.7 | 1,126 | None |
| 6 | PCR, FACS |
5, 36 | Inactive | − | − | No | 11 / < 0.50 | ND | Canakinumab |
| 7 | PCR, FACS |
8, 73 | Inactive | − | − | No | 7 / < 0.50 | ND | Anakinra |
| 8 | PCR, FACS |
16, 69 | Inactive | − | − | No | 3 / < 0.50 | ND | Anakinra |
| 9 | FACS | 3, 21 | Active | + | + | No | ND / ND | > 26,000 | Prednisone, Anakinra, CsA |
Age at time of sample collection, duration = time since diagnosis.
Systemic Features = presence of rash and/or fever and/or lymphadenopathy and/or serositis.
SJIA = systemic juvenile idiopathic arthritis; PCR = real-time polymerase chain reaction; FACS = fluoresence-activated cell sorting; NA = not applicable; ND = not done; CsA = cyclosporine A
Real-time RT-PCR studies using peripheral blood monocytes
PBMCs were isolated from peripheral blood collected in acid citrate dextrose tubes (BD, Franklin Lakes, NJ) using Ficoll (GE Healthcare, Piscataway, NJ) gradient method within 1 hour of venipuncture. The whole PBMC populations were then divided into two subpopulations, CD14+ monocytes (positive selection) and CD14- non-monocytes (negative selection), via AutoMACS Pro ™ automated magnetic cell sorting system using anti-human CD14 MicroBeads (Miltenyi Biotec, Auburn, CA). Based on fluorescence-activated cell sorting studies done in our lab, we were able to achieve a greater than 94% CD14+ cell purity in the CD14+ fraction and less than 5% CD14+ cells in the CD14- fraction was achieved. Approximately 1–3×106 CD14+ cells (or 3×106 CD14- cells), plus 3 mL of RPMI 1640 + 10% fetal calf serum (Invitrogen, Carlsbad, CA), were placed into two wells of a 6-well plate (TPP, Switzerland) and then either stimulated with 100 units/ml of human recombinant IFN-γ (R & D Systems, Minneapolis, MN) or left unstimulated for 3 hours at 37° Celsius. Based on preliminary dose-response studies, we determined 100 units/ml of IFN-γ offered a substantial response, while using the least amount of cytokine possible, for both gene expression and STAT1 phosphorylation assays. After stimulation, cells were lysed with Trizol (Invitrogen) and RNA was isolated per manufacturers instructions. Amount and purity of RNA was then analyzed using NanoDrop ND-1000 spectrophotometer with NanoDrop 1000 3.7.1 software (Thermo Fisher Scientific, Wilmington, DE). 1000 ng of RNA was used to synthesize cDNA using iScript cDNA Synthesis Kit (Bio-Rad, Hercules, CA) via Bio-Rad iCycler v4.006. Real-time PCR reactions were performed in 20 µl volumes using an iCycler (Bio-Rad) with gene specific primers and iQ SYBR Green Supermix (Bio-Rad). mRNA copy numbers were normalized against the copy number of the housekeeping gene, GAPDH, of the same sample. Control calculations in our lab determined that GAPDH gene expression did not change in healthy control or SJIA peripheral monocytes upon stimulation with IFN-γ. The following primer pairs were used: GAPDH 5’-CAGCCCCAGCGTCAAAGG and 5’-GCTCTCCAGAACATCATCC, IP-10 5’-AGTGGCATTCAAGGAGTACC and 5’-ATCCTTGGAAGCACTGCATC, I-TAC 5’-GCTATAGCCTTGGCTGTGATAT and 5’-CAGGGCCTATGCAAAGACA, MIG 5’-GAGAAAGGGTCGCTGTTCCT and 5’-TTTGGCTGACCT GTTTCTCC, STAT1 5’-CTTTGGTTGAATCCCCAGGC and 5’-TGCTCCCAGTCTTGCTTTTCTAAC, IRF-1 5’-ATGAGACCCTGGCTAGAG and 5’-AAGCATCCGGTACACTCG, IL-6 5’-TTCACCAGGCAAGTCTCC and 5’- ATACTCGACGGCATCTCAG, IL-1β 5’- TCCCCAGCCCTTTTGTTGA and 5’- TTAGAACCAAATGTGGCCGTG, TNF-α 5’- AACTACAGACCCCCCCTGAAAAC and 5’- AAGAGGCTGAGGAACAAGCACC, PIAS1 5’-AAGCACGGACGCAAACACGAAC and 5’- GTGAGTTGTGGAATGGTAGATGGAG, SOCS3 5’-CACTCTTCAGCATCTCTGTCGGAAG and 5’-CATAGGAGTCCAGGTGGCCGTT, and SOCS1 5’-TCCCCTTCCAGATTTGACCG and 5’-AAGAGGTAGGAGGTGCGAGTTCAG. The specificities of the primers were determined by measuring the PCR-product length on gel electrophoresis and via the examination of the melting point of the PCR product. Relative gene expression was calculated via Comparative CT method (25).
STAT1-phosphorylation studies via intracellular FACS analysis
CD14+ peripheral blood monocytes from healthy control, inactive SJIA patient, and active SJIA patient groups were collected and isolated as detailed above. Monocytes were then either left unstimulated or stimulated with 100 units/mL of recombinant human IFN-γ (R&D Systems) for 30 min at 37°C. After incubation, 2% Paraformaldehyde was added for 10 min at room temperature to stop the signaling cascade. The cells were then permeabilized in 90% methanol overnight and stained with anti-CD14 (BD Biosciences) and the following phospho-markers: P-STAT1-PE (pY701) and P-STAT6-Alex Fluor® 488 (pY641) (used as a negative control) (both from BD Biosciences). The data was acquired on the FACSCANTO (BD Biosciences) and analyzed using FlowJo (TreeStar).
Statistical analysis
Comparisons between control and SJIA patients groups were performed using unpaired Student’s t tests. For analyzed values, P < 0.05 was considered statistically significant.
RESULTS
Expression of IFN-induced chemokines within SJIA and extended oligoarticular JIA synovium
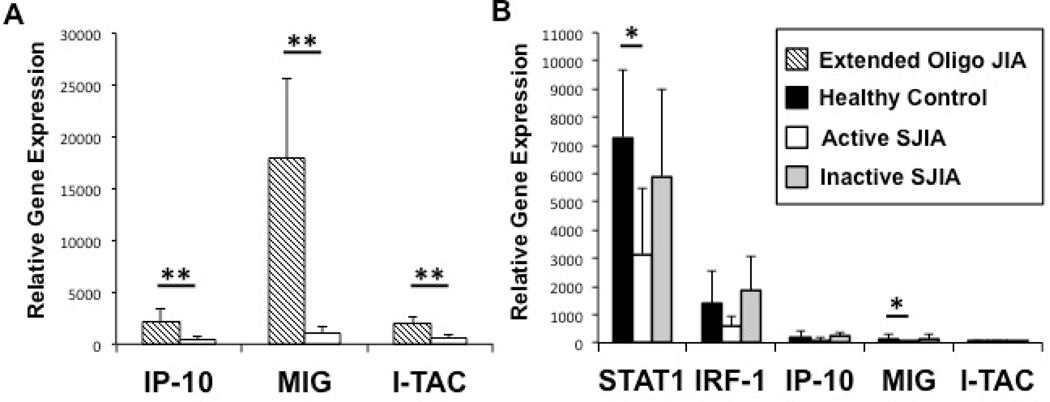
To explore whether or not an IFN-induced gene expression signature exists within peripherally affected tissue of SJIA, we sought to explore this signature within inflamed synovial tissue of active SJIA. Interferon-inducible protein 10 (IP-10, CXCL10) (26), monokine induced by interferon-γ (MIG, CXCL9) (27), and IFN-inducible T cell alpha chemoattractant (I-TAC, CXCL11) (28) are CXC-subfamily chemokines that primarily serve as lymphocyte chemoattractants during inflammation. Each of these proteins experience a highly dramatic fold-increase of expression upon IFN-γ stimulation (29,30), thus serve well as markers to gauge the presence of locally produced IFN within an inflammatory microenvironment. Figure 1A depicts relative synovial tissue gene expression levels of IP-10, MIG, and I-TAC as compared between active SJIA and extended oligoarticular JIA, a disease whose synovium is characterized by an IFN-induced gene signature. Although we did find an IFN-induced chemokine gene signature within SJIA synovial tissue, dramatically lower expression levels of IP-10 (4.5-fold less, p<0.05), MIG (15.8-fold less, p<0.01), and I-TAC (3.6-fold less, p<0.05) were detected in affected SJIA synovium as compared to extended oligoarticular JIA.
Figure 1.
Expression levels of IFN-γ-induced genes, relative to GAPDH, within inflamed synovial tissue (A) and freshly isolated CD14+ peripheral monocytes (B) as determined by real-time PCR. A, Relative expression levels of IFN-γ-induced chemokine genes in inflamed synovial tissue from SJIA and extended oligoarticular JIA patient groups. B, Confirmation of the relative lack of IFN-γ-induced gene expression in unstimulated peripheral monocytes. ( ) Healthy controls; (
) Healthy controls; ( ) Extended oligoarticular JIA (
) Extended oligoarticular JIA ( ) Active SJIA patients; (
) Active SJIA patients; ( ) Inactive SJIA patients. Results shown as mean + SD. JIA = juvenile idiopathic arthritis; SJIA = systemic JIA; IFN-γ = interferon-γ; STAT1 = signal transducer and activator of transcription 1; IRF-1 = interferon regulatory factor 1; IP-10 = interferon gamma-induced protein 10; MIG = monokine induced by interferon-γ; I-TAC = Interferon-inducible T-cell alpha chemoattractant. * = p < 0.05, ** = p < 0.01.
) Inactive SJIA patients. Results shown as mean + SD. JIA = juvenile idiopathic arthritis; SJIA = systemic JIA; IFN-γ = interferon-γ; STAT1 = signal transducer and activator of transcription 1; IRF-1 = interferon regulatory factor 1; IP-10 = interferon gamma-induced protein 10; MIG = monokine induced by interferon-γ; I-TAC = Interferon-inducible T-cell alpha chemoattractant. * = p < 0.05, ** = p < 0.01.
Expression of a restricted interferon-induced genetic signature in peripheral blood CD14+ monocytes
To test for an IFN-induced gene signature within peripheral monocytes, the level of IFN-induced gene expression was compared between patients with active SJIA, inactive SJIA, and healthy controls. In addition to phosphorylated STAT1 directly inducing the expression of I-TAC, MIG, IP-10, and even STAT1 itself, it also causes expression of a secondary transcription factor designated interferon regulatory factor-1 (IRF-1). IRF-1 serves to further propagate the IFN-γ signal by inducing the expression of a second wave of IFN-responsive genes (31). Figure 1B depicts unstimulated levels of STAT1, IRF-1, I-TAC, MIG, and IP-10 gene expression within both control and patient group peripheral blood monocytes. Upon comparing active SJIA patient to health control peripheral monocyte gene expression, while levels of IP-10 (2.3-fold less, p=0.22), I-TAC (1.5-fold less, p=0.47), and IRF-1 (2.4-fold less, p=0.11) were found to be lower in active SJIA patients, only MIG (7.3-fold less, p<0.05) and STAT1 (2.3-fold less, p<0.05) were found to be significantly lower. No statistical difference in the unstimulated expression of these five genes was appreciated between inactive SJIA and healthy control monocytes. Within the active SJIA group, no appreciable differences in gene expressions were seen between those with predominant active arthritis compared to those with mainly systemic features, even though this study was not sufficiently powered to detect such a difference. Although the chemokines studied herein are mainly expressed within the myelomonocytic lineage, to ensure that we were not missing an IFN-induced signature by failing to look at non-monocytes, we studied their expression with the CD14- fraction (a mixture of immature granulocytes, lymphocytes, and NK cells). For the 3 groups tested, IFN-induced chemokine gene expression within the CD14- fraction was found to be approximately 2.5-fold lower than what was measured within the CD14+ peripheral monocytes fraction.
Unstimulated STAT1 phosphorylation within peripheral blood CD14+ monocytes
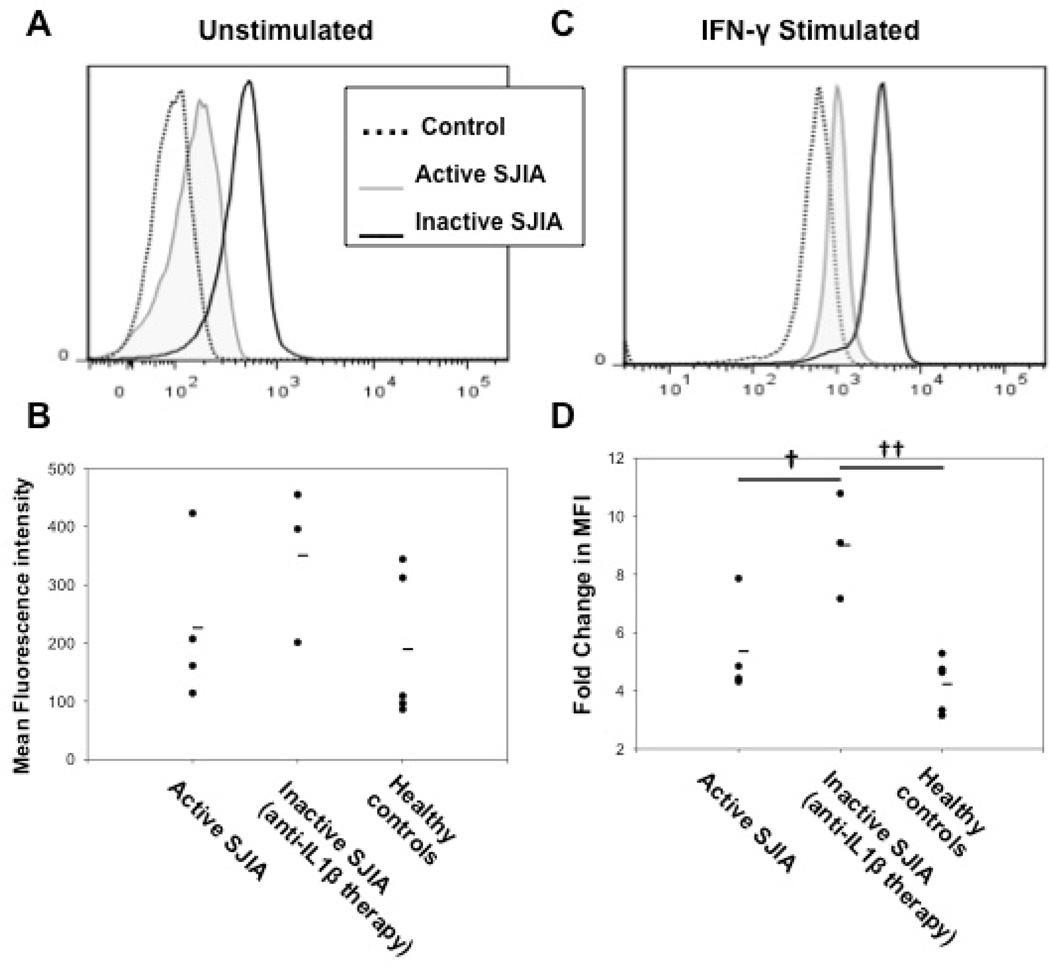
Since most of the effects of IFN-γ are mediated through the phosphorylation of STAT1 (20), to assess whether the isolated cells had been previously exposed to circulating levels of IFN-γ in vivo, we next measured unstimulated levels of phosphorylated STAT1 (P-STAT1) within freshly isolated peripheral blood CD14+ monocytes from active SJIA patients, inactive SJIA patients on IL-1β-blocking therapy, and healthy controls. Figure 2A depicts a representative example of the detection of P-STAT1 via intracellular FACS. Figure 2B summarizes the mean fluorescence intensity of P-STAT1 within each unstimulated group and shows that while both active (226±137 vs 189±127, p=0.69) and inactive (351±133 vs 189±127, p=0.14) SJIA patient groups had higher amounts of P-STAT1 compared to healthy controls, these differences were not statistically significant. Amounts of P-STAT6, used as a negative control, weren’t statistically different between each unstimulated group (data not shown).
Figure 2.
STAT1 phosphorylation within peripheral monocytes upon IFN-γ stimulation. Monocytes were left unstimulated or stimulated with 100 units/mL of IFN-γ for 30 minutes to assess the degree of STAT1 phosphorylation by intracellular flow cytometry. A, Representative example of P-STAT1 experiment on unstimulated peripheral monocytes. B, MFI of P-STAT1 within unstimulated peripheral monocytes. C, Representative example of P-STAT1 experiment on peripheral monocytes stimulated with IFN-γ. D, Fold-change increase in P-STAT1 MFI following stimulation with IFN-γ. Active SJIA patients (gray-line histogram); inactive SJIA patients on anti-IL-1 β therapy (black-line histogram); healthy controls (dotted-line histogram). Lines within dot-plots represent mean values. STAT1 = signal transducer and activator of transcription 1; IFN-γ = interferon-γ, P-STAT1 = phosphorylated STAT1, MFI = mean fluorescence intensity; IL-1β = interleukin-1β; SJIA = systemic juvenile idiopathic arthritis. † = p < 0.05, †† = p < 0.01.
Transcriptional effects of IFN-γ on peripheral blood CD14+ monocytes
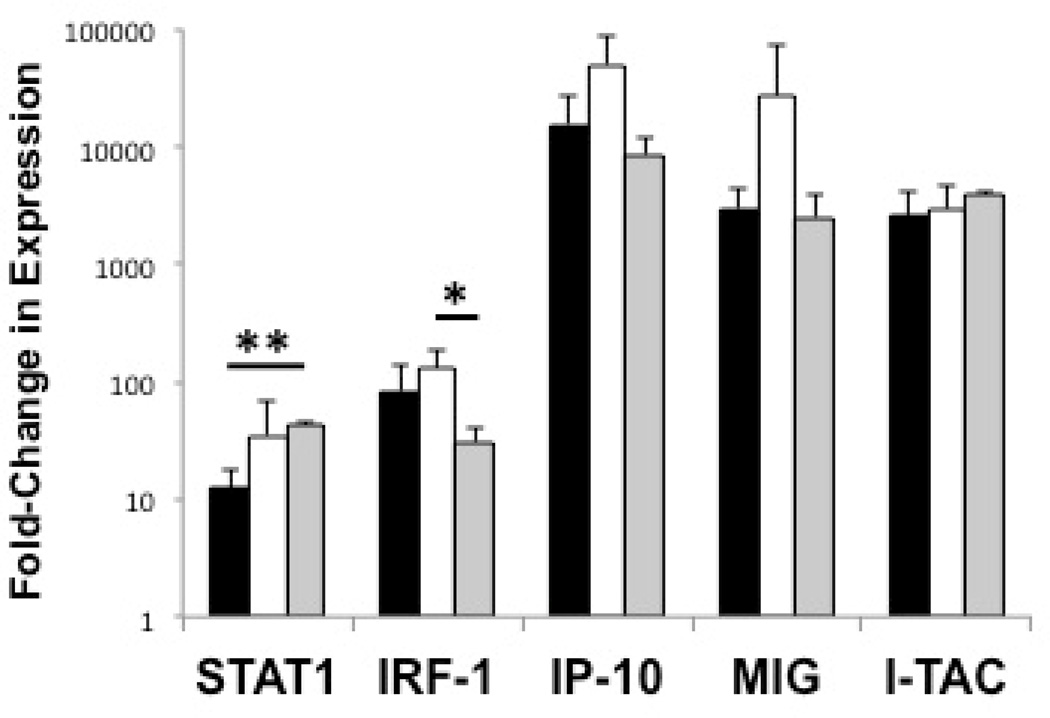
After exploring the unstimulated IFN-induced genetic signature within both peripheral monocytes and CD14- cells, we next studied the effects of IFN-γ stimulation on both of these populations. Seen in Figure 3, upon stimulation of monocytes with IFN-γ, the fold-change in expression levels of STAT1 (34.8±33.7-fold increase vs 12.5±5.8-fold increase, p=0.21), IRF-1 (131±51.2 vs 83.0±58.5, p=0.18), IP-10 (49,994±38,969 vs 15,488±11,293, p=0.12), MIG (27,301±44,486 vs. 2,973±1,486, p=0.29), and I-TAC (2,970±1,572 vs 2,610±1,428, p=0.70) were higher in active SJIA patients compared to healthy controls, although not statistically significant. With the exception of STAT1 induction, fold-change in IRF-1, IP-10, MIG, and I-TAC gene expression was similar between inactive and healthy controls. Surprisingly, STAT1 gene expression induction by IFN-γ was significantly higher in the inactive SJIA patient monocytes compared to healthy controls (42.8±3.5 vs 12.5±5.8-fold increase, p<0.01). This study was not sufficiently powered to detect a difference in IFN-responsiveness between those active SJIA with predominant arthritis versus those with primarily systemic features. Upon stimulation of the CD14- fraction of each group, no significant differences in gene expression induction, which were several magnitudes lower than their CD14+ counterparts, were detected (data not shown).
Figure 3.
SJIA peripheral blood monocyte responsiveness to IFN-γ stimulation. Monocyte responsiveness expressed as fold-change in expression levels of IFN-γ-induced genes upon stimulation as determined by real-time RT-PCR. Monocytes stimulated with 100 units/mL of IFN-γ for 3 hours. ( ) Healthy controls; (
) Healthy controls; ( ) Active SJIA patients; (
) Active SJIA patients; ( ) Inactive SJIA patients. Results shown as mean + SD. SJIA = systemic JIA; IFN-γ = interferon-γ; STAT1 = signal transducer and activator of transcription 1; IRF-1 = interferon regulatory factor 1; IP-10 = interferon gamma-induced protein 10; MIG = monokine induced by interferon-γ; I-TAC = Interferon-inducible T-cell alpha chemoattractant. * = p < 0.05, ** = p < 0.01.
) Inactive SJIA patients. Results shown as mean + SD. SJIA = systemic JIA; IFN-γ = interferon-γ; STAT1 = signal transducer and activator of transcription 1; IRF-1 = interferon regulatory factor 1; IP-10 = interferon gamma-induced protein 10; MIG = monokine induced by interferon-γ; I-TAC = Interferon-inducible T-cell alpha chemoattractant. * = p < 0.05, ** = p < 0.01.
Effects of IFN-γ stimulation on STAT1-phosphorylation within peripheral blood monocytes
We next examined the effects of stimulation with IFN-γ on STAT1 phosphorylation within both active and inactive SJIA patient and healthy control group peripheral monocytes. A representative example of the fold-increase in phosphorylated STAT1 level upon IFN-γ stimulation can be seen in Figure 2C. Perhaps most interestingly, as seen in the summary Figure 2D, it was the inactive SJIA group on an anti-IL-1β blocking therapy that experienced the greatest fold-change in phosphorylated STAT1 levels upon IFN-γ stimulation compared to both active SJIA patient (9.0±1.8-fold increase vs 5.4±1.7-fold increase, p<0.05) and healthy control (9.0±1.8 vs 4.2±0.9, p<0.01) groups. As a negative control, no appreciable increase in phosphorylated STAT6 was detected in any of the groups upon IFN-γ stimulation (data not shown).
Gene expression of negative regulators of activated STAT1
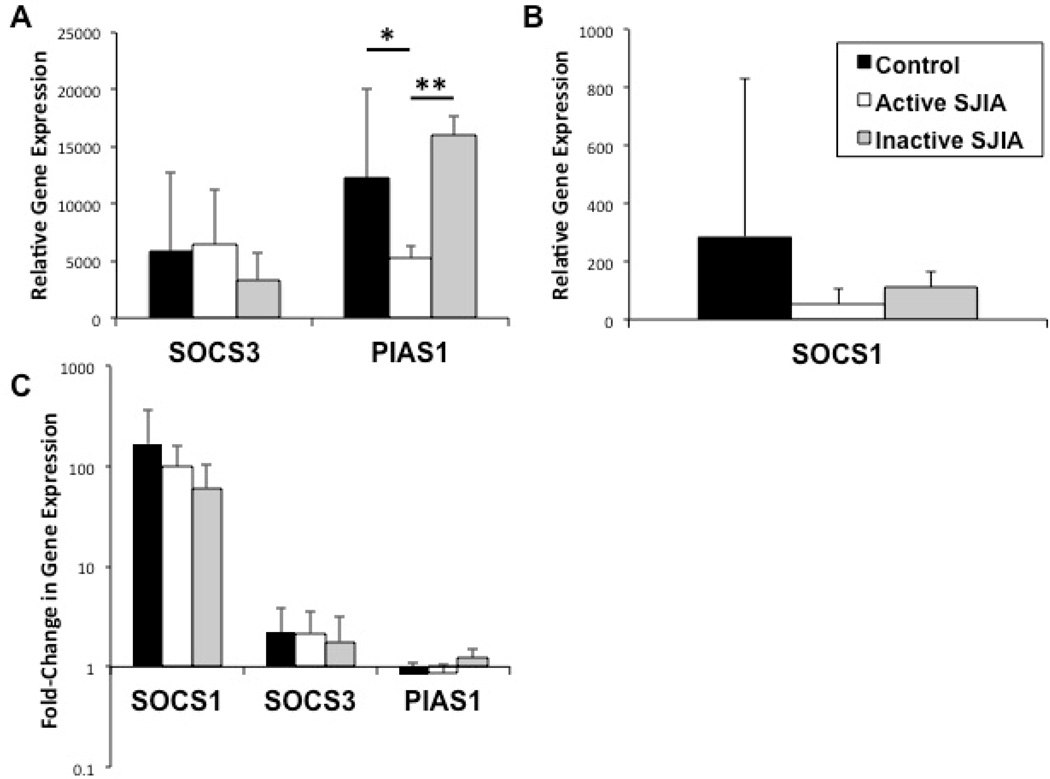
In an attempt to limit the potential deleterious effects of an overly exuberant inflammatory response, our immune system employs safeguards to limit interferon signaling. The proteins suppressor of cytokine signaling 1 (SOCS1) (32), SOCS3 (33), and protein inhibitor of activated STAT1 (PIAS1) (34) serve as important negative regulators of STAT1 signaling. As such, we next studied whether an increased expression of STAT1 negative regulators could be demonstrated in conjunction with the reserved IFN-induced gene signature and, if not, whether these negative regulators could potentially be induced by the presence of IFN-γ. As seen in Figures 4A and 4B, although unstimulated SOCS1 gene expression was lower in both patient groups (active and inactive) compared to healthy controls, this finding was not statistically significant. Unstimulated SOCS3 expression was not found to be significantly different in either SJIA group compared to controls. Unstimulated PIAS1 gene expression was lower in active SJIA patients compared to healthy controls (5,270±1,090 vs 12,260±7,770, p<0.05) and higher in inactive SJA patients compared to active SJIA (15,990±1,660 vs 5,270±1,090, p<0.01). As illustrated in Figure 4C, no differences between groups in PIAS1 or SOCS3 gene induction was detected upon IFN-γ stimulation. Similarly, although not statistically significant, gene induction of SOCS1 was depressed in both active (98.9±63.8 vs 168±196-fold increase, p=0.38) and inactive (61.0±44.4 vs 168±196-fold increase, p=0.18) SJIA patients compared to induction in healthy controls.
Figure 4.
Gene expression levels of negative-regulators of IFN-γ signaling relative to GAPDH expression within peripheral monocytes (A and B, unstimulated and C, post-stimulation) as determined by real-time RT-PCR. A and B, Unstimulated gene expression of SOCS3, PIAS1, and SOCS1 in peripheral monocytes. C, Fold-change in expression levels of indicated genes after stimulation with 100 units/mL of IFN-γ for 3 hours. ( ) Healthy controls; (
) Healthy controls; ( ) Active SJIA patients; (
) Active SJIA patients; ( ) Inactive SJIA patients. Results shown as mean + SD. IFN-γ = interferon-γ; GAPDH = glyceraldehyde 3-phosphate dehydrogenase; SOCS1/3 = suppressor of cytokine signaling 1/3; PIAS1 = protein inhibitor of activated STAT1; IL-1β = interleukin-1β. * = p < 0.05, ** = p < 0.01.
) Inactive SJIA patients. Results shown as mean + SD. IFN-γ = interferon-γ; GAPDH = glyceraldehyde 3-phosphate dehydrogenase; SOCS1/3 = suppressor of cytokine signaling 1/3; PIAS1 = protein inhibitor of activated STAT1; IL-1β = interleukin-1β. * = p < 0.05, ** = p < 0.01.
DISCUSSION
Although SJIA is an inflammatory disorder where the monocyte-macrophage lineage appears to be expanded and activated, it is unclear if this phenomenon is driven by IFN-γ. In this study we provide evidence suggesting a limited role for IFN-γ in influencing the monocyte-macrophage phenotype in SJIA. We demonstrate that the expression of IFN-induced chemokines in SJIA are markedly limited within both circulating monocytes and within the inflamed synovial tissue compared to extended oligoarticular JIA, a subtype of JIA in which IFN-γ-producing Th1 lymphocytes are in abundance within the synovial compartment (21). Furthermore, we demonstrate that this absence of an IFN-induced signature is not due to monocyte hyporesponsiveness towards IFN-γ, as SJIA peripheral monocytes are just as reactive to IFN-γ when compared to healthy control monocytes. Combined, these findings suggest that the lack of an IFN-induced signature within SJIA monocytes is due to a limited in vivo exposure to IFN-γ. Although neither serum nor synovial IFN-γ levels were directly measured in this study, in agreement with the lack of an IFN-induced PBMC gene expression signature seen in SJIA (3,14,15), serum and synovial IFN-γ levels have been previously found to be lower in SJIA compared to both oligoarticular and polyarticular JIA subtypes (35).
In contrast to active SJIA, there is mounting evidence that implicates IFN-γ as a key pathogenic mediator in hemophagocytic syndromes including MAS that is secondary to SJIA (16–18). Strikingly high levels of IFN-γ have been reported in familial HLH (16,17) and active hemophagocytosis within the primary HLH animal model, the perforin-knockout mouse, requires the presence of IFN-γ (36). Although the data on serum levels of IFN-γ in SJIA/MAS is less abundant, Billiau et al. reported an expansion of IFN-γ-producing lymphocytes within a liver biopsy of a patient with SJIA/MAS (37). Furthermore, neopterin, a molecule produced specifically by IFN-γ-stimulated macrophages (38), was found to be highly elevated in MAS in contrast to only mildly increased in active SJIA (39). In fact, a highly increased serum neopterin has been proposed as a potential diagnostic marker for hemophagocytic syndromes in general (40). Based on these observations, the role of IFN-γ in the pathogenesis of SJIA versus MAS represents a divergence, thus suggesting that these are two distinct pathophysiologic phenomena rather than “the opposite ends of the same spectrum” as it has been proposed in the literature (41).
Based on the M1/M2 macrophage polarization paradigm, IFN-γ is the key driver of M1 differentiation (9–11). SJIA peripheral monocytes do not appear to be exposed to significant amounts of IFN-γ in vivo, thus do not display the typical characteristics of the M1 macrophage. However, their preserved ability to respond to IFN-γ suggests that they could acquire them given the appropriate microenvironment, such as during an intercurrent viral illness.
Lastly and perhaps most interestingly, we demonstrate a paradoxically increased basal interferon signal and increased IFN-γ responsiveness within the inactive SJIA patient group being treated with an anti-IL-1β agent. Supporting this finding is the work done by Quartier et al. that revealed an upregulation of interferon-related gene expression data from SJIA patients treated with anakinra, regardless if they were responders or not (42). These observations suggest the existence of a cross-regulation between IL-1β and IFN-γ-induced signaling. Consistent with this idea, in some experimental systems, IFN-γ has been shown to harbor distinct anti-inflammatory and immunomodulatory properties (43,44). For example, in collagen-induced arthritis (CIA) induced by immunization with collagen type-II plus complete Freund’s adjuvant (containing heat-inactivated mycobacteria), IFN-γ seems to suppress excessive myeloid expansion (45). Similarly, blocking IFN-γ in the experimental autoimmune encephalitis (EAE) model produces a more severe phenotype (46). In light of the apparent pathogenic role IL-1β in SJIA (15), IFN-γ has been reported to decrease the production of IL-1β-induced matrix metalloproteinases (MMPs) in fibroblast-like synoviocytes from human donors with rheumatoid arthritis and to also reduce IL-1β expression within the synovium of the antigen-induced arthritis model (47). In fact, IFN-γ was shown to cause a near global dampening of human macrophage IL-1β-mediated inflammatory cytokine production and tissue destruction; a phenomenon that was believed to be due to a STAT1-dependent downregulation of the IL-1 type I receptor (48). This same group also found that IFN-γ serves as a STAT1-dependent negative regulator of MCP-1/CCL2-mediated human monocytic chemotaxis (49). Whether these observations are relevant to SJIA is not presently clear, but increased monocyte reactivity to IFN-γ in patients being treated with an anti-IL-1β therapeutic deserves further investigations since it may have an impact on the ability of these patients to appropriately respond to viral illnesses. Since MAS is often triggered by viral infections in patients with SJIA, such monocyte hyperresponsiveness to IFN-γ may also contribute to the development of this complication.
In summary, we provide evidence supporting the notion that the role of IFN-γ is limited in active SJIA, a finding that cannot be explained by peripheral monocyte hyporesponsiveness to IFN-γ. Interestingly, based on phosphorylated-STAT1 data, we also show that the peripheral monocytes from inactive SJIA patients on an IL-1β blocking agent display an increased responsiveness to IFN-γ stimulation. Whether this increased reactivity to IFN-γ in patients being treated with an anti-IL-1β agent may impact their ability to respond to infections, particularly viral illnesses, or alters their risk for developing MAS needs to be further investigated.
Acknowledgments
Supported in part by NIH grants P01 AR-048929 and R01 AR-059049.
Dr. Grom has received consulting fees from Novartis Pharmaceuticals Corporation.
REFERENCES
- 1.Schneider R, Laxer R. Systemic-onset juvenile idiopathic rheumatoid arthritis [review] Baillieres Clin Rheumatol. 1998;12:245–271. doi: 10.1016/s0950-3579(98)80018-6. [DOI] [PubMed] [Google Scholar]
- 2.Macaubas C, Nguyen K, Deshpande C, Phillips C, Peck A, Lee T, et al. Distribution of circulating cells in systemic juvenile idiopathic arthritis across disease activity states. Clin Immunol. 2010;134:206–216. doi: 10.1016/j.clim.2009.09.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Fall N, Barnes M, Thornton S, Luyrink L, Olson J, Ilowite NT, et al. Gene expression profiling of peripheral blood from patients with new-onset systemic juvenile idiopathic arthritis reveals molecular heterogeneity that may predict macrophage activation syndrome. Arthritis Rheum. 2007;56:3793–3804. doi: 10.1002/art.22981. [DOI] [PubMed] [Google Scholar]
- 4.Hinze CH, Fall N, Thornton S, Mo JQ, Aronow BJ, Layh-Schmitt G, et al. Immature cell populations and an erythropoiesis gene-expression signature in systemic juvenile idiopathic arthritis: implications for pathogenesis. Arthritis Res Ther. 2010;12:R123. doi: 10.1186/ar3061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Stéphan JL, Zeller J, Hubert P, Herbelin C, Dayer JM, Prieur AM. Macrophage activation syndrome and rheumatic disease in childhood: a report of four new cases. Clin Exp Rheumatol. 1993;11:451–456. [PubMed] [Google Scholar]
- 6.Ravelli A. Macrophage activation syndrome [review] Curr Opin Rheumatol. 2002;14:548–552. doi: 10.1097/00002281-200209000-00012. [DOI] [PubMed] [Google Scholar]
- 7.Bleesing J, Prada A, Siegel DM, Villanueva J, Olson J, Ilowite NT, et al. The diagnostic significance of soluble CD163 and soluble interleukin-2 alpha-chain in macrophage activation syndrome and untreated new-onset systemic juvenile idiopathic arthritis. Arthritis Rheum. 2007;56:956–971. doi: 10.1002/art.22416. [DOI] [PubMed] [Google Scholar]
- 8.Behrens EM, Beukelman T, Paessler M, Cron RQ. Occult macrophage activation syndrome in patients with systemic juvenile idiopathic arthritis. J Rheumatol. 2007;34:1133–1138. [PubMed] [Google Scholar]
- 9.Martinez FO, Sica A, Mantovani A, Locati M. Macrophage activation and polarization [review] Front Biosci. 2007;12:2836–2848. doi: 10.2741/2692. [DOI] [PubMed] [Google Scholar]
- 10.Martinez FO, Gordon S, Locati M, Mantovani A. Transcriptional profiling of the human monocyte-to-macrophage differentiation and polarization: new molecules and patterns of gene expression. J Immunol. 2006;177:7303–7311. doi: 10.4049/jimmunol.177.10.7303. [DOI] [PubMed] [Google Scholar]
- 11.Murray PJ, Wynn TA. Obstacles and opportunities for understanding macrophage polarization [review] J Leukoc Biol. 2011;89:557–563. doi: 10.1189/jlb.0710409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Gattorno M, Piccini A, Lasigliè D, Tassi S, Brisca G, Carta S, et al. The pattern of response to anti-interleukin-1 treatment distinguishes two subsets of patients with systemic-onset juvenile idiopathic arthritis. Arthritis Rheum. 2008;58:1505–1515. doi: 10.1002/art.23437. [DOI] [PubMed] [Google Scholar]
- 13.Lasigliè D, Traggiai E, Federici S, Alessio M, Buoncompagni A, Accogli A, et al. Role of IL-1 beta in the development of human T(H)17 cells: lesson from NLPR3 mutated patients. PLoS One. 2011;6:e20014. doi: 10.1371/journal.pone.0020014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Ogilvie EM, Khan A, Hubank M, Kellam P, Woo P. Specific gene expression profiles in systemic juvenile arthritis. Arthritis Rheum. 2007;56:1954–1965. doi: 10.1002/art.22644. [DOI] [PubMed] [Google Scholar]
- 15.Pascual V, Allantaz F, Arce E, Punaro M, Banchereau J. Role of interleukin-1 (IL-1) in the pathogenesis of systemic onset juvenile idiopathic arthritis and clinical response to IL-1 blockade. J Exp Med. 2005;201:1479–1486. doi: 10.1084/jem.20050473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Xu XJ, Tang YM, Song H, Yang SL, Xu WQ, et al. Diagnostic accuracy of a specific cytokine pattern in hemophagocytic lymphohistiocytosis in children. J Pediatr. 2012 Jan 6; doi: 10.1016/j.jpeds.2011.11.046. [Epub ahead of print]. [DOI] [PubMed] [Google Scholar]
- 17.Henter JI, Elinder G, Söder O, Hansson M, Andersson B, Andersson U. Hypercytokinemia in familial hemophagocytic lymphohistiocytosis. Blood. 1991;78:2918–2922. [PubMed] [Google Scholar]
- 18.Fujiwara F, Hibi S, Imashuku S. Hypercytokinemia in hemophagocytic syndrome. Am J Pediatr Hematol Oncol. 1993;15:92–98. doi: 10.1097/00043426-199302000-00012. [DOI] [PubMed] [Google Scholar]
- 19.de Saint Basile G, Ménasché G, Latour S. Inherited defects causing hemophagocytic lymphohistiocytic syndrome [review] Ann N Y Acad Sci. 2011;1246:64–76. doi: 10.1111/j.1749-6632.2011.06307.x. [DOI] [PubMed] [Google Scholar]
- 20.Schroder K, Hertzog P, Ravasi T, Hume D. Interferon-gamma: an overview of signals, mechanisms and functions [review] J Leukoc Biol. 2004;75:163–189. doi: 10.1189/jlb.0603252. [DOI] [PubMed] [Google Scholar]
- 21.Hunter PJ, Nistala K, Jina N, Eddaoudi A, Thomson W, et al. Biologic predictors of extension of oligoarticular juvenile idiopathic arthritis as determined from synovial fluid cellular composition and gene expression. Arthritis Rheum. 2010;62:896–907. doi: 10.1002/art.27284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Davì S, Consolaro A, Guseinova D, Pistorio A, Ruperto N, Martini A, et al. An international consensus survey of diagnostic criteria for macrophage activation syndrome in systemic juvenile idiopathic arthritis. J Rheumatol. 2011;38:764–768. doi: 10.3899/jrheum.100996. [DOI] [PubMed] [Google Scholar]
- 23.Petty RE, Southwood TR, Manners P, Baum J, Glass DN, Goldenberg J, et al. International League of Associations for Rheumatology classification of juvenile idiopathic arthritis: second revision, Edmonton, 2001. J Rheumatol. 2004;31:390–392. [PubMed] [Google Scholar]
- 24.Murray KJ, Luyrink L, Grom AA, Passo MH, Emery H, Witte D, Glass DN. Immunohistological characteristics of T cell infiltrates in different forms of childhood onset chronic arthritis. J Rheumatol. 1996;23:2116–2124. [PubMed] [Google Scholar]
- 25.Schmittgen T, Livak K. Analyzing real-time PCR data by the comparative C(T) method. Nat Protoc. 2008;3:1101–1108. doi: 10.1038/nprot.2008.73. [DOI] [PubMed] [Google Scholar]
- 26.Luster AD, Weinshank RL, Feinman R, Ravetch JV. Molecular and biochemical characterization of a novel gamma-interferon-inducible protein. J Biol Chem. 1988;263:12036–12043. [PubMed] [Google Scholar]
- 27.Wright TM, Farber JM. 5' regulatory region of a novel cytokine gene mediates selective activation by interferon gamma. J Exp Med. 1991;173:417–422. doi: 10.1084/jem.173.2.417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Cole KE, Strick CA, Paradis TJ, Ogborne KT, Loetscher M, Gladue RP, et al. Interferon-inducible T cell alpha chemoattractant (I-TAC): a novel non-ELR CXC chemokine with potent activity on activated T cells through selective high affinity binding to CXCR3. J Exp Med. 1998;187:2009–2021. doi: 10.1084/jem.187.12.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Farber JM. Mig and IP-10: CXC chemokines that target lymphocytes [review] J Leukoc Biol. 1997;61:246–257. [PubMed] [Google Scholar]
- 30.Hu X, Park-Min KH, Ho HH, Ivashkiv LB. IFN-gamma-primed macrophages exhibit increased CCR2-dependent migration and altered IFN-gamma responses mediated by STAT1. J Immunol. 2005;175:3637–3647. doi: 10.4049/jimmunol.175.6.3637. [DOI] [PubMed] [Google Scholar]
- 31.Saha B, Prasanna JS, Chandrasekar B, Nandi D. Gene modulation and immunoregulatory roles of interferon gamma [review] Cytokine. 2010;50:1–14. doi: 10.1016/j.cyto.2009.11.021. [DOI] [PubMed] [Google Scholar]
- 32.Alexander WS, Starr R, Fenner JE, Scott CL, Handman E, Sprigg NS, et al. SOCS1 is a critical inhibitor of interferon gamma signaling and prevents the potentially fatal neonatal actions of this cytokine. Cell. 1999;98:597–608. doi: 10.1016/s0092-8674(00)80047-1. [DOI] [PubMed] [Google Scholar]
- 33.Song M, Shuai K. The suppressor of cytokine signaling (SOCS) 1 and SOCS3 but not SOCS2 proteins inhibit interferon-mediated antiviral and antiproliferative activities. J Biol Chem. 1998;273:35056–35062. doi: 10.1074/jbc.273.52.35056. [DOI] [PubMed] [Google Scholar]
- 34.Liu B, Liao J, Rao X, Kushner SA, Chung CD, Chang DD, Shuai K. Inhibition of STAT1-mediated gene activation by PIAS1. Proc Natl Acad Sci U S A. 1998;95:10626. doi: 10.1073/pnas.95.18.10626. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.de Jager W, Hoppenreijs EP, Wulffraat NM, Wedderburn LR, Kuis W, Prakken BJ. Blood and synovial fluid cytokine signatures in patients with juvenile idiopathic arthritis: a cross-sectional study. Ann Rheum Dis. 2007;66:589–598. doi: 10.1136/ard.2006.061853. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Jordan MB, Hildeman D, Kappler J, Marrack P. An animal model of hemophagocytic lymphohistiocytosis (HLH): CD8+ T cells and interferon gamma are essential for the disorder. Blood. 2004;104:735–743. doi: 10.1182/blood-2003-10-3413. [DOI] [PubMed] [Google Scholar]
- 37.Billiau AD, Roskams T, Van Damme-Lombaerts R, Matthys P, Wouters C. Macrophage activation syndrome: characteristic findings on liver biopsy illustrating the key role of activated, IFN-gamma-producing lymphocytes and IL-6- and TNF-alpha-producing macrophages. Blood. 2005;105:1648–1651. doi: 10.1182/blood-2004-08-2997. [DOI] [PubMed] [Google Scholar]
- 38.Huber C, Batchelor JR, Fuchs D, Hausen A, Lang A, Niederwieser D. Immune response-associated production of neopterin. Release from macrophages primarily under control of interferon-gamma. J Exp Med. 1984;160:310–316. doi: 10.1084/jem.160.1.310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Shimizu M, Yokoyama T, Yamada K, Kaneda H, Wada H, Wada T, et al. Distinct cytokine profiles of systemic-onset juvenile idiopathic arthritis-associated macrophage activation syndrome with particular emphasis on the role of Interleukin-18 in its pathogenesis. Rheumatology (Oxford) 2010;49:1645–1653. doi: 10.1093/rheumatology/keq133. [DOI] [PubMed] [Google Scholar]
- 40.Ibarra MF, Klein-Gitelman M, Morgan E, Proytcheva M, Sullivan C, Morgan G, et al. Serum neopterin levels as a diagnostic marker of hemophagocytic lymphohistiocytosis syndrome. Clin Vaccine Immunol. 2011;18:609–614. doi: 10.1128/CVI.00306-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Bloom BJ. Macrophage activation is a key component in the pathogenesis of systemic juvenile idiopathic arthritis: comment on the article by Blessing et al. Arthritis Rheum. 2007;56:3877–3878. doi: 10.1002/art.22969. [DOI] [PubMed] [Google Scholar]
- 42.Quartier P, Allantaz F, Cimaz R, Pillet P, Messiaen C, Bardin C, et al. A multicentre, randomised, double-blind, placebo-controlled trial with the interleukin-1 receptor antagonist anakinra in patients with systemic-onset juvenile idiopathic arthritis (ANAJIS trial) Ann Rheum Dis. 2011;70:747–754. doi: 10.1136/ard.2010.134254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Kelchtermans H, Billiau A, Matthys P. How interferon-gamma keeps autoimmune diseases in check [review] Trends Immunol. 2008;29:479–486. doi: 10.1016/j.it.2008.07.002. [DOI] [PubMed] [Google Scholar]
- 44.Hu X, Ivashkiv LB. Cross-regulation of signaling pathways by interferon-gamma: implications for immune responses and autoimmune diseases [review] Immunity. 2009;31:539–550. doi: 10.1016/j.immuni.2009.09.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Matthys P, Vermeire K, Mitera T, Heremans H, Huang S, Schols D, et al. Enhanced autoimmune arthritis in IFN-gamma receptor-deficient mice is conditioned by mycobacteria in Freund's adjuvant and by increased expansion of Mac-1+ myeloid cells. J Immunol. 1999;163:3503–3510. [PubMed] [Google Scholar]
- 46.Billiau A, Heremans H, Vandekerckhove F, Dijkmans R, Sobis H, Meulepas E, Carton H. Enhancement of experimental allergic encephalomyelitis in mice by antibodies against IFN-gamma. J Immunol. 1988;140:1506–1510. [PubMed] [Google Scholar]
- 47.Page CE, Smale S, Carty SM, Amos N, Lauder SN, Goodfellow RM, et al. Interferon-gamma inhibits interleukin-1beta-induced matrix metalloproteinase production by synovial fibroblasts and protects articular cartilage in early arthritis. Arthritis Res Ther. 2010;12:R49. doi: 10.1186/ar2960. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Hu X, Ho HH, Lou O, Hidaka C, Ivashkiv LB. Homeostatic role of interferons conferred by inhibition of IL-1-mediated inflammation and tissue destruction. J Immunol. 2005;175:131–138. doi: 10.4049/jimmunol.175.1.131. [DOI] [PubMed] [Google Scholar]
- 49.Hu Y, Hu X, Boumsell L, Ivashkiv LB. IFN-gamma and STAT1 arrest monocyte migration and modulate RAC/CDC42 pathways. J Immunol. 2008;180:8057–8065. doi: 10.4049/jimmunol.180.12.8057. [DOI] [PMC free article] [PubMed] [Google Scholar]