Abstract
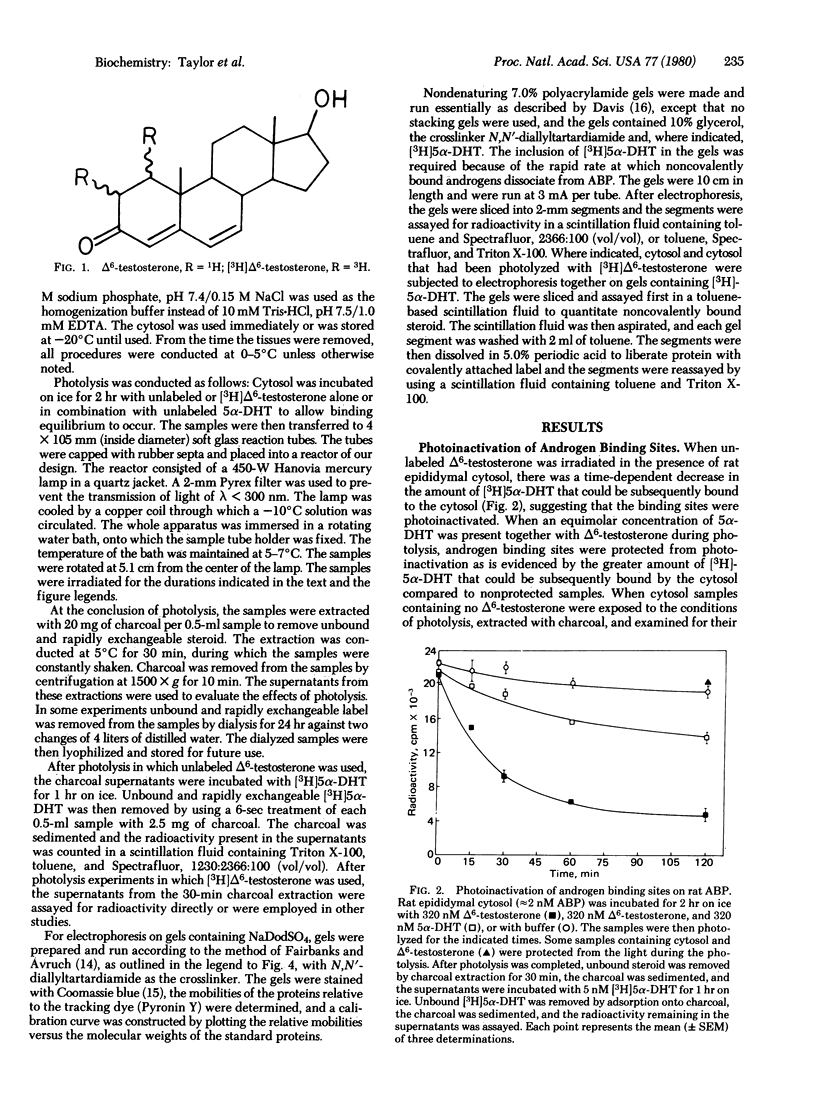
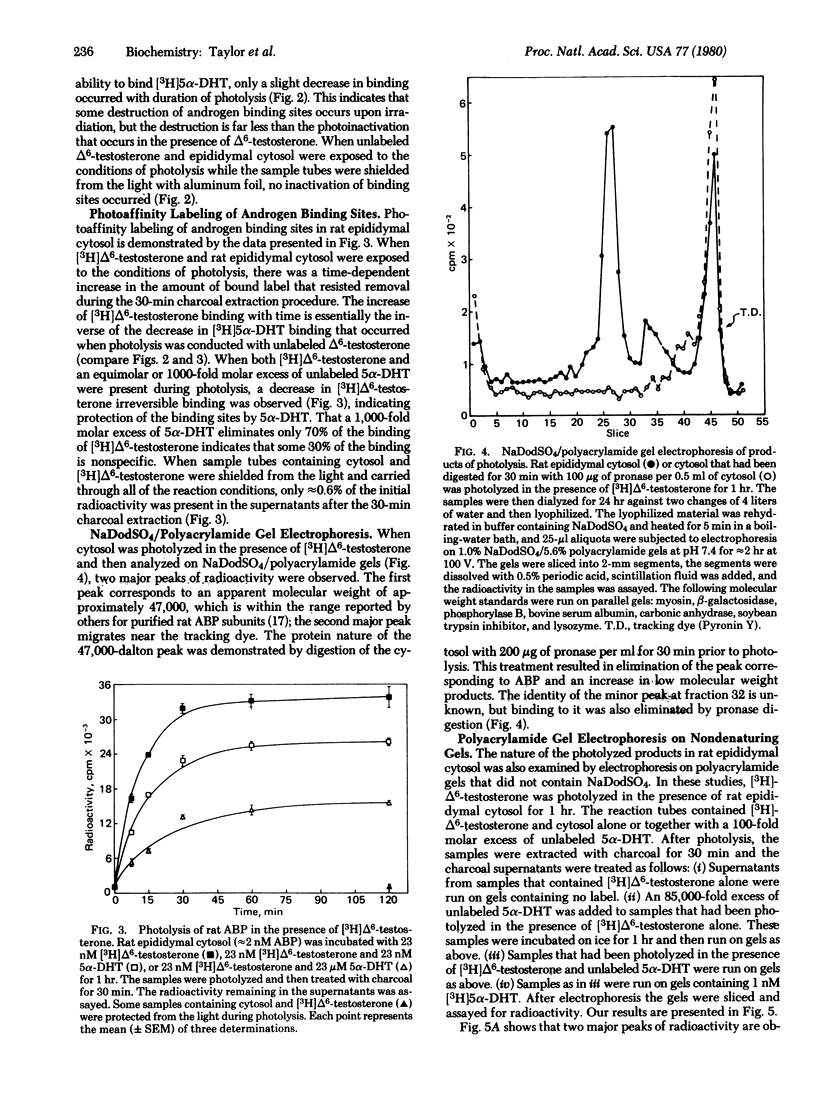
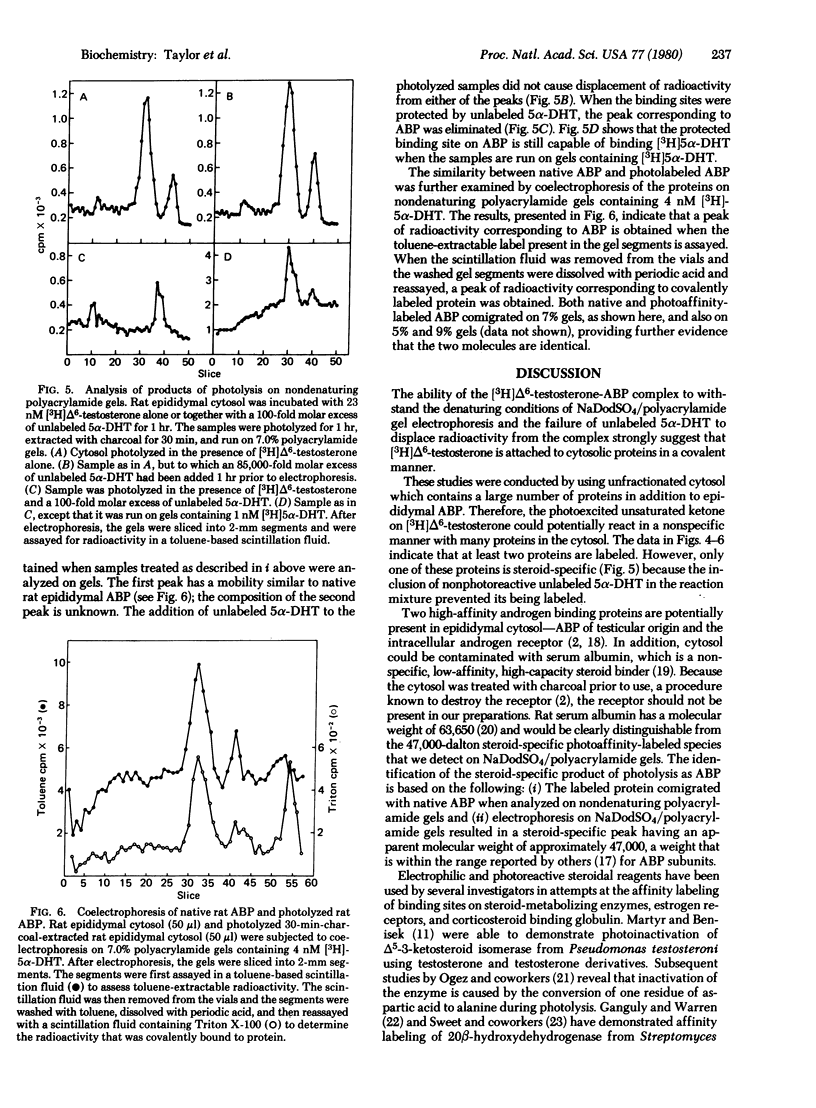
The photoinactivation and photoaffinity labeling of androgen binding sites present in cytosol prepared from intact sexually mature rat epididymides have been demonstrated by using unlabeled and [3H]-labeled 17 beta-hydroxy-4,6-androstadien-3-one. Both photoinactivation and photolabeling are dependent upon exposure to light. These processes are inhibited when photolysis is conducted in the presence of the photoinert compound 17 beta-hydroxy-5 alpha-androstan-3-one, suggesting that steroid-specific sites are involved in the reactions. The labeled steroid-specific product of photolysis is macromolecular with a molecular weight of 47,000 as determined by electrophoresis on polyacrylamide gels containing NaDodSO4, and proteinaceous because digestion of the cytosol with pronase before photolysis eliminates steroid-specific binding. After photolysis, the protein-steroid complex has the ability to withstand dissociation during electrophoresis under denaturing conditions, and unlabeled 17 beta-hydroxy-5 alpha-androstan-3-one fails to displace the label from the complex. Thus, the binding of [3H]17 beta-hydroxy-4,6-androstadien-3-one to the cytosolic protein is covalent. This steroid-specific product is identified as an androgen binding protein of testicular origin by its comigration with native androgen binding protein on nondenaturing polyacrylamide gels and by its molecular weight which is within the range reported for androgen binding protein subunits.
Full text
PDF

Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- DAVIS B. J. DISC ELECTROPHORESIS. II. METHOD AND APPLICATION TO HUMAN SERUM PROTEINS. Ann N Y Acad Sci. 1964 Dec 28;121:404–427. doi: 10.1111/j.1749-6632.1964.tb14213.x. [DOI] [PubMed] [Google Scholar]
- Danzo B. J., Cooper T. G., Orgebin-Crist M. C. Androgen binding protein (ABP) in fluids collected from the rete testis and cauda epididymidis of sexually mature and immature rabbits and observations on morphological changes in the epididymis following ligation of the ductuli efferentes. Biol Reprod. 1977 Aug;17(1):64–77. doi: 10.1095/biolreprod17.1.64. [DOI] [PubMed] [Google Scholar]
- Danzo B. J., Eller B. C. Androgen binding to cytosol prepared from epididymides of sexually mature castrated rabbits: evidence for a cytoplasmic receptor. Steroids. 1975 Apr;25(4):507–524. doi: 10.1016/0039-128x(75)90028-8. [DOI] [PubMed] [Google Scholar]
- Danzo B. J., Eller B. C., Orgebin-Crist M. C. Studies on the site of origin of the androgen binding protein present in epididymal cytosol from mature intact rabbits. Steroids. 1974 Jul;24(1):107–122. doi: 10.1016/0039-128x(74)90049-x. [DOI] [PubMed] [Google Scholar]
- Danzo B. J., Eller B. C. Steroid-binding proteins in rabbit plasma: Separation of testosterone-binding globulin (TeBG) from corticosteroid-binding globulin (CBG), preliminary characterization of TeBG, and changes in TeBG concentration during sexual maturation. Mol Cell Endocrinol. 1975 May;2(5):351–368. doi: 10.1016/0303-7207(75)90022-2. [DOI] [PubMed] [Google Scholar]
- Danzo B. J., Orgebin-Crist M. C., Eller B. C. Changes in 5alpha- dihydrotestosterone binding to epididymal cytosol during sexual maturation in rabbits: correlation with morphological changes in the testis and epididymis. Mol Cell Endocrinol. 1975 Sep;3(3):203–220. doi: 10.1016/0303-7207(75)90045-3. [DOI] [PubMed] [Google Scholar]
- Danzo B. J., Orgebin-Crist M. C., Toft D. O. Characterization of a cytoplasmic receptor for 5alpha-dihydrotestosterone in the caput epididymidis of intact rabbits. Endocrinology. 1973 Jan;92(1):310–317. doi: 10.1210/endo-92-1-310. [DOI] [PubMed] [Google Scholar]
- Fairbanks G., Avruch J. Four gel systems for electrophoretic fractionation of membrane proteins using ionic detergents. J Supramol Struct. 1972;1(1):66–75. doi: 10.1002/jss.400010110. [DOI] [PubMed] [Google Scholar]
- Fairbanks G., Steck T. L., Wallach D. F. Electrophoretic analysis of the major polypeptides of the human erythrocyte membrane. Biochemistry. 1971 Jun 22;10(13):2606–2617. doi: 10.1021/bi00789a030. [DOI] [PubMed] [Google Scholar]
- French F. S., Ritzén E. M. A high-affinity androgen-binding protein (ABP) in rat testis: evidence for secretion into efferent duct fluid and absorption by epididymis. Endocrinology. 1973 Jul;93(1):88–95. doi: 10.1210/endo-93-1-88. [DOI] [PubMed] [Google Scholar]
- Ganguly M., Warren J. C. Affinity labeling of steroid binding sites. Synthesis of cortisone 21-iodoacetate and study of 20 beta-hydroxysteroid dehydrogenase. J Biol Chem. 1971 Jun 10;246(11):3646–3652. [PubMed] [Google Scholar]
- Hsu A. F., Troen P. An androgen binding protein in the testicular cytosol of human testis. Comparison with human plasma testosterone-estrogen binding globulin. J Clin Invest. 1978 Jun;61(6):1611–1619. doi: 10.1172/JCI109081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kanamarlapudi N. R., Warren J. C. Synthesis and study of the estrogenic activity of 2,4-bis(bromomethyl)estradiol-17 beta 3-methyl ether. J Biol Chem. 1975 Aug 25;250(16):6484–6487. [PubMed] [Google Scholar]
- Katzenellenbogen J. A. Photoaffinity labeling of estrogen receptors. Fed Proc. 1978 Feb;37(2):174–178. [PubMed] [Google Scholar]
- Khan M. S., Rosner W. Investigation of the binding site of human corticosteroid-binding globulin by affinity labeling. Demonstration of a cysteinyl residue in the binding site. J Biol Chem. 1977 Mar 25;252(6):1895–1900. [PubMed] [Google Scholar]
- Martyr R. J., Benisek W. F. Affinity labeling of the active sites of delta 5 -ketosteroid isomerase using photoexcited natural ligands. Biochemistry. 1973 May 22;12(11):2172–2178. doi: 10.1021/bi00735a025. [DOI] [PubMed] [Google Scholar]
- Marver D., Chiu W., Wolff M. E., Edelman I. S. Photoaffinity site-specific covalent labeling of human corticosteroid-binding globulin. Proc Natl Acad Sci U S A. 1976 Dec;73(12):4462–4466. doi: 10.1073/pnas.73.12.4462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ogez J. R., Tivol W. F., Benisek W. F. A novel chemical modification of delta 5-3-ketosteroid isomerase occurring during its 3-oxo-4-estren-17 beta-yl acetate-dependent photoinactivation. J Biol Chem. 1977 Sep 10;252(17):6151–6155. [PubMed] [Google Scholar]
- Steinberger A., Heindel J. J., Lindsey J. N., Elkington J. S., Sanborn B. M., Steinberger E. Isolation and culture of FSH responsive Sertoli cells. Endocr Res Commun. 1975;2(3):261–272. doi: 10.3109/07435807509053853. [DOI] [PubMed] [Google Scholar]
- Sweet F., Strickler R. C., Warren J. C. Affinity labeling of steroid binding sites. Synthesis of 21-bromoacetylaminoprogesterone and study of 20beta-hydroxysteroid dehydrogenase. J Biol Chem. 1978 Mar 10;253(5):1385–1392. [PubMed] [Google Scholar]



