Abstract
Using 12-year longitudinal data from deceased participants of the Berlin Aging Study (N = 414; 70–103 years at first occasion; M = 87 years) askew examined if and how old and very old individuals exhibit terminal decline in reported life satisfaction at the end of life. Relative to distance from birth (i.e., chronological age), distance to death was associated with steeper average decline per year. Distance to death accounted for more variance in interindividual differences in life satisfaction change than did age. By applying change-point growth models to mortality-related change, we identified a time point about four years prior to death at which decline showed a two-fold increase in steepness relative to the pre-terminal phase. For the oldest old (85+ years at baseline), a three-fold increase was observed. Established mortality predictors such as sex, comorbidities, risk for dementia, and intellectual functioning accounted for only small portions of interindividual differences in mortality-related change in life satisfaction. We conclude that late-life changes in subjective well-being are related to mechanisms predicting death and suggest routes for further inquiry.
Keywords: Longitudinal, selective mortality, terminal decline, successful aging, differential aging, Berlin Aging Study, psychosocial factors, life satisfaction, well-being
The lifespan and gerontological literatures propose that trajectories of psychological change at the end of life reflect a combination of age-related, mortality-related, and pathology-related processes (Baltes, 1997; Berg, 1996; Birren & Cunningham, 1985). The few longitudinal studies that have examined such proposals have focused primarily on questions about the existence of terminal decline in cognitive abilities (i.e., pronounced late-life deterioration as a function of distance-to-death; for reviews, see Bäckman & MacDonald, 2006; Bosworth & Siegler, 2002; Small & Bäckman, 1999). The present study uses longitudinal data to compare models of age-related and death-related change in subjective well-being. This is of interest because whereas, in cross-sectional research, age typically has not been associated with individuals’ reported levels of well-being (e.g., Diener, Lucas, & Scollon, 2006), mortality has associated with reduced well-being (e.g., Danner, Snowdon, & Friesen, 2001; Levy, Slade, & Kunkel, 2002; Maier & Smith, 1999). Specifically, using 12-year longitudinal data from deceased participants of the Berlin Aging Study (BASE: Baltes & Mayer, 1999; N = 414; 70–103 years at T1; M = 87 years) we (a) contrast the age-related and mortality-related trajectories of life satisfaction, a key component of subjective well-being; (b) examine whether decline on life satisfaction is more pronounced with approaching death as would be indicative of terminal decline, and (c) explore factors that may underlie interindividual differences in the observed intraindividual changes in life satisfaction.
Starting with seminal work in the 1960s and 1970s (Kleemeier, 1962; Palmore & Cleveland, 1976; Riegel & Riegel, 1972; Siegler, 1975), a substantial body of evidence has accumulated showing that low levels of, or pronounced decline on, cognitive abilities including perceptual-motor speed, memory, executive functioning, and crystallized abilities are related to imminent death (Anstey, Mack, & von Sanden, 2006; Bosworth & Schaie, 1999; Ghisletta, McArdle, & Lindenberger, 2006; Johansson et al., 2004; Rabbitt et al., 2002; Sliwinski, Hofer, Hall, Buschke, & Lipton, 2003; White & Cunningham, 1988). These findings are robust after accounting for various physical and medical conditions and appear to be relatively independent of cause of death (e.g., cerebrovascular, cardiac, cancer: Anstey et al., 2006; Small, Fratiglioni, von Strauss, & Bäckman, 2003). A diverse set of factors has repeatedly been proposed to potentially account for the noted relationships between cognitive decline and death ranging from APOE genotype to specific disease mediation including neurological problems, (pre)-clinical dementia and depression, decreased global biological vitality as well as reduced vitamin consumption, and fewer cognitively stimulating activities (Hassing et al., 2002; Hofer et al., 2002; Laukka, MacDonald, & Bäckman, 2006; Sliwinksi, Hofer, & Hall, 2003). Although evidence suggests that (somewhat independent of age and health status) proximity to death may have pervasive effects across a range of measures of cognitive functioning, it is not yet known how many other domains of psychological functioning exhibit associations with distance-to-death.
There are several reasons why subjective well-being may be a candidate for terminal decline. Some researchers argue, for example, that mortality-related decline is particularly pronounced on measures that are relatively age-insensitive because mortality-related effects are less obscured by normative age-graded decline on these measures (White & Cunningham 1988). A large body of research suggests relative age-graded stability of well-being across adulthood and old age despite increased risks for social losses and declines in physical health (well-being paradox in old age; Diener et al., 2006; Filipp, 1996; Kunzmann, Little, & Smith, 2000; Larsen, 1978; Mroczek & Kolarz, 1998). It is conceivable, however, that approaching death may represent an additional burden making it increasingly difficult to maintain a sense of well-being. Another position highlights that well-being may not only serve as a consequence of, but also a source for successful aging (Baltes & Baltes, 1990; Lyubomirsky, King, & Diener, 2005; Rowe & Kahn, 1997; Ryff & Singer, 1998). Specifically, despite a general self-enhancement bias, perceptions and evaluations of well-being show reliable interindividual and intraindividual variance allowing subtle differences to be powerful enough to uniquely predict other outcomes of successful aging including changes in perceptual speed, morbidity, and mortality (Danner et al., 2001; Gerstorf, Lövdén, Röcke, Smith, & Lindenberger, in press; Levy et al., 2002; Lyyra, Törmäkangas, Read, Rantanen, & Berg, 2006; Maier & Smith, 1999). For example, Mroczek and Spiro (2005) reported that men from the Normative Aging Study who died within one year after assessment showed steeper decline over time in life satisfaction than those who did not die. These notions suggest that mortality-related decline on measures of well-being might be more pronounced than normative age gradients and thereby provide an important qualification to the stability-despite-loss paradox of well-being.
Kleemeier (1962) proposed that terminal decline phenomena involve two phases of change: A pre-terminal phase of relative stability or gradual decline reflecting rather normative age-graded processes, followed by a terminal phase of pronounced decline likely indexing normative influences as well as an accumulating pathological burden. Recently, “change-point” growth models have been developed for modeling multiple phases of change, and provide an opportunity to empirically address such notions (Cudeck & Klebe, 2002; Hall, Lipton, Sliwinski, & Stewart, 2000; Wilson, Beckett, Bienias, Evans, & Bennett, 2003). For example, Sliwinski and colleagues (2006) used such a model to identify a “change point” at around eight years prior to death at which decline in episodic memory steepened dramatically by a factor of two relative to the pre-terminal phase. Examining whether different phases in mortality-related well-being trajectories can be identified would constitute an important and unique contribution to the extant literature on terminal decline phenomena.
If indicators of well-being show marked mortality-related decline, one open question is whether the effect is merely indicative of an underlying third variable. Besides typical mortality predictors such as age, sex, and socioeconomic status (SES), one factor potentially underlying mortality-related decline may be medical conditions, including cerebrovascular or cardiac diseases, and physical frailty that are known to increase mortality risks in old age (Guralnik, 1991). A second potential factor may be pathological burden encompassing preclinical and clinical stages of dementia (Sliwinski et al., 2003). Specifically, recent evidence from the cognitive aging literature suggests that findings of cognitive terminal decline among the very old may largely be attributable to participants in early phases of dementia (Laukka et al., 2006; for discussion of the interplay between well-being indicators and dementia, see Nussbaum, 1997). Finally, mortality-related decline in aspects of well-being may essentially reflect the effects of low levels of cognitive functioning or cognitive decline and thereby parallel cognitive terminal decline as established in the extant literature (Bäckman & MacDonald, 2006). In the current study we explore whether mortality-related changes in life satisfaction remain evident when statistically controlling for influences known to be associated with impending death including age, sex, SES, comorbidities, risk for dementia, and intellectual functioning.
The present study extends and qualifies insights gained from a long history of theory and research about terminal decline by asking three questions. First, we ask whether interindividual differences in intraindividual change in life satisfaction late in life is best described by chronological age or distance-to-death. To do so, we apply growth curve (e.g., multilevel) modeling to 12-year longitudinal data collected from 414 individuals who died subsequent to their participation in BASE (individuals for whom age and distance-to-death information was available or all time points). We evaluate the relative efficiency of age and distance-to-death time models for representing observed changes in life satisfaction.
Second, we explore whether there is evidence for terminal decline in life satisfaction in that the rate of change is greater closer versus farther from death. Specifically, we test whether a model with two phases of linear decline (one pre-terminal phase and one terminal phase) provides a better representation of the data than a model with a single phase of linear decline over the entire period studied. Given the scarcity of knowledge about the exact location of a transition to a terminal phase of decline, we use empirical means and test a series of models with varying transition points to isolate a general time frame during which the rate of decline increases in steepness.
Third, we examine associations between the observed interindividual differences in life satisfaction changes and well-established mortality predictors such as life period (Third versus Fourth age), sex, and SES as well as comorbidities, risk for dementia, and cognitive functioning. We selected perceptual speed as a measure of cognitive functioning because it represents a powerful and psychometrically sound indicator of (mortality-related) cognitive decline in old age (Bosworth & Schaie, 1999; Ghisletta et al., 2006). In follow-up analyses, we also investigate the role of cognitive terminal decline as a time-varying covariate of change in life satisfaction as well as the seminal Riegel and Riegel (1972) hypothesis of diminished decline among very old individuals relative to old individuals (for a summary of conflicting evidence, see Bäckman & MacDonald, 2006).
Method
We use six-wave longitudinal data from deceased participants of the interdisciplinary BASE collected over 12 years. Detailed descriptions of the variables assessed and procedures used, as well as information about the longitudinal samples and design, are published in Baltes and Mayer (1999), Gerstorf, Herlitz, and Smith (2006), and Smith et al. (2002). A brief overview is given below.
Participants and Procedure
The total cross-sectional BASE sample at T1 (N = 516; mean age = 84.92 years, SD = 8.66, range: 70 – 103) was stratified by age and sex with 43 men and 43 women in each of six different age brackets (70–74, 75–79, 80–84, 85–89, 90–94, and 95+ years; born between 1887 and 1922). To obtain this sample, a total of 1,908 individuals, drawn from the Berlin city registry, were approached for participation. Of those, 516 participants completed a 14-session intensive assessment protocol. This baseline sample was found to be positively selected on a number of variables (e.g., younger age, lower one-year mortality, better physical health and cognitive functioning, and higher well-being), but the amount of selection bias was relatively small and did not exceed .05 SD units for any of the variables examined (Lindenberger et al., 1999). In addition, the sample was not restricted in heterogeneity and did not exhibit major differences in patterns of covariation among variables (Baltes & Smith, 1997). Since baseline, extensive effort has been invested into maintaining contact with the participants in order to avoid voluntary dropout. As expected, given the mean age of the sample, the primary reason for attrition was mortality. On average, only 10% of participants have voluntarily dropped out at each measurement occasion, primarily because of poor health.
Information about mortality status and date of death for deceased participants has been obtained every six months from the City Registry since the study commencement. The update used in the present analyses was gathered in June 2005, roughly 15 years after the initiation of the study, when, of the total T1 sample (N = 516), only n = 83 were still alive. Mortality status information was missing for n = 19 participants who had moved out of the Berlin area. The sample considered in the current analyses were the n = 414 BASE decedents. Their average age at death was 91.69 years (SD = 7.13; range: 73 – 106 years). Death occurred an average of 4.84 years (SD = 3.27; range: 90 days – 15 years) after initial assessment (T1) and 2.10 years (SD = 2.06; range: 5 days – 13 years) after the last assessment in which decedents took part. As is true for any longitudinal inquiry into old age, the number of observed individuals decreased as the study evolved. Specifically, the following numbers of individuals were observed at each measurement occasion: at baseline in 1990–1993 (T1; N = 414), in 1993–1994 (T2; n = 267), 1995–1996 (T3; n = 159), 1997–1998 (T4; n = 88), 2000 (T5; n = 31), and 2004–2005 (T6; n = 2). T2 took place 1.95 years (SD = 0.67), T3 3.77 years (SD = 0.62), T4 5.51 years (SD = 0.74), T5 9.00 years (SD = 0.84), and T6 12.37 years (SD = 1.59) after T1, respectively.
To examine longitudinal selectivity for the measures under consideration, we used an effect-size metric indicating the degree to which individuals who survived and participated longitudinally differed from the parent sample at T1 (for details, see Lindenberger, Singer, & Baltes, 2002). For example, for the 31 deceased participants who provided data for four or more measurement occasions, the total selectivity amounted to .16 SD units (where SD refers to that of the parent BASE sample) for life satisfaction, − .80 SD for chronological age, .37 SD for SES, .00 SD for comorbidities, − .28 SD for dementia, and .63 SD for perceptual speed. This suggests that higher levels of well-being at T1, younger age, higher SES, and preserved cognitive functioning were associated with subsequently lower mortality and higher participation rates among survivors. Effects of sample selectivity were primarily due to mortality (e.g., life satisfaction: 71% of the total effect of .16 SD units) rather than drop-out for other reasons. The statistical procedures implemented in this study handle this type of non-random attrition (for details, see Statistical Procedures and Discussion), but we acknowledge that participants who provided the most change (i.e., longitudinal) information represent a positively selected subset of the initial sample.
Trained research assistants and medical personnel carried out all testing in individual face-to-face sessions. With the exception of sessions that involved geriatric medicine, testing took place at the participant’s place of residence (i.e., private household or institution). Sessions required an average of 90 minutes and, when necessary, were split into shorter units of assessment. Over time, the same versions of the questionnaires and tests were administered.
Measures
Well-being
As a key aspect of well-being, life satisfaction was measured on six occasions using a unit-weighted composite of four items adapted from the Philadelphia Geriatric Center Morale Scale (PGCMS; Lawton, 1975; for analyses of the factor structure, see Liang & Bollen, 1983) and translated into German. This scale has a specific aging focus and primarily assesses cognitive-evaluative rather than emotional aspects of well-being. For practical reasons, with this older population, items were administered in an interview-type format. Each item (e.g., “Zur Zeit, bin ich zufrieden mit mein Leben,” i.e., “Right now, I am satisfied with my life”) was read aloud by the research assistants and participants were asked to indicate how well items described them using a 5-point Likert-scale with 1 labeled as ‘does not apply to me at all’ and 5 labeled as ‘applies very well to me.’ As in previous publications from BASE, we report measures standardized to a T metric (mean = 50; SD = 10 at T1), with the total T1 sample (N = 516) serving as the reference. This transformation ensured a common metric across variables, while maintaining the psychometric properties of the scores and the longitudinal changes in means and variances. Detailed descriptions of the PGCMS Life Satisfaction subscale as used in BASE and its measurement properties can be found in Smith, Fleeson, Geiselmann, Settersten, and Kunzmann (1999).
Background variables
To examine how various important mortality predictors were related to interindividual differences in life satisfaction change, we included age, sex, SES, comorbidities, dementia, and perceptual speed in some of our models. Age was either used as a continuous variable (grand-mean centered at 87 years) or as a dichotomized age group variable (0 = old individuals, 70–84 years at T1; n = 165; 1 = oldest-old individuals, 85+ years at T1; n = 249). Sex was coded as 0 = men (n = 216) and 1 = women (n = 198). Socioeconomic status was measured using a unit-weighted composite of three measures: (a) equivalent income, defined as the net household income weighted by the number of people sharing the household; (b) occupational prestige, based on a standard rating scale for Germany; and (c) number of years of education (for details, see Mayer, Maas, & Wagner, 1999). The measure for comorbidities was the number of physician-observed medical diagnoses of moderate to severe chronic illnesses including cardiovascular (e.g., coronary heart disease, stroke) and metabolic diseases (e.g., diabetes mellitus), according to the International Classification of Diseases-9. The diagnoses were determined via clinical examination and supported by additional blood and saliva laboratory assessments (for details, see Steinhagen-Thiessen & Borchelt, 1999). To examine potential biases due to dementia, clinical diagnosis of dementia was assessed using standard clinical interview and assessment procedure (for details, see Helmchen et al., 1999). In follow-up analyses, we also examined the effects of preclinical dementia over time by utilizing cohort-specific cutoffs on the Short Mini-Mental State Examination (SMMSE: Klein et al., 1985; range: 0–18 points; 70–84 years: < 12 points; 85+ years: < 11 points; for details, see Gerstorf et al., 2006), separately at each wave of BASE (0 = non-demented, 1 = demented). Independent clinical diagnoses of dementia in BASE at T1 and T3 indicated sufficient specificity (72–98%) and sensitivity of the cut-offs (63–88%) both among the old and the oldest old. Finally, we included perceptual speed, as measured by a unit-weighted composite of the total number of correct responses in the Digit Letter and Identical Pictures tests (for details, see Lindenberger & Baltes, 1997). The Digit Letter test closely resembles the Digit Symbol Substitution test of the WAIS (Wechsler, 1982). Throughout the duration of the test (3 min.), a template with nine digit-letter pairings was presented to the participants. Participants were shown series of digits (six per page) and were required to name the corresponding letter pair, as fast as possible. In the Identical Pictures task, a target figure and five response alternatives were shown on the computer screen (Macintosh SE/30 equipped with a touch-sensitive screen) and participants had to touch the correct response figure (which was identical to the target) as quickly as possible. Up to 32 items were presented and testing terminated automatically after 80s.
Data Preparation and Analysis
To address the research questions of the present study about intraindivdual change in life satisfaction, we examined three sets of models: (1) age-related vs. mortality-related representations of change, (2) single vs. multiple phases of change, and (3) possible correlates of individual differences in change.
Age-related vs. mortality-related representations of change
Our first analytic task was to determine which time dimension, chronological age or distance-to-death, provided for a better representation of the longitudinal changes in life satisfaction. More specifically, we fit two growth curve models (i.e., multilevel models of change) to model interindividual differences in change over time (McArdle & Nesselroade, 2003; Singer & Willett, 2003) for the life satisfaction measures. In the first model, we used age as the time variable, effectively modeling interindividual differences in how each individual’s life satisfaction changed from age 70 to age 100+ years. In the second model, we used distance-to-death as the time variable, modeling how life satisfaction changed in relation to impending mortality (i.e., up to 15 years prior to death). Comparing the relative fit of these models, we determined which time variable provided a better representation of the data.
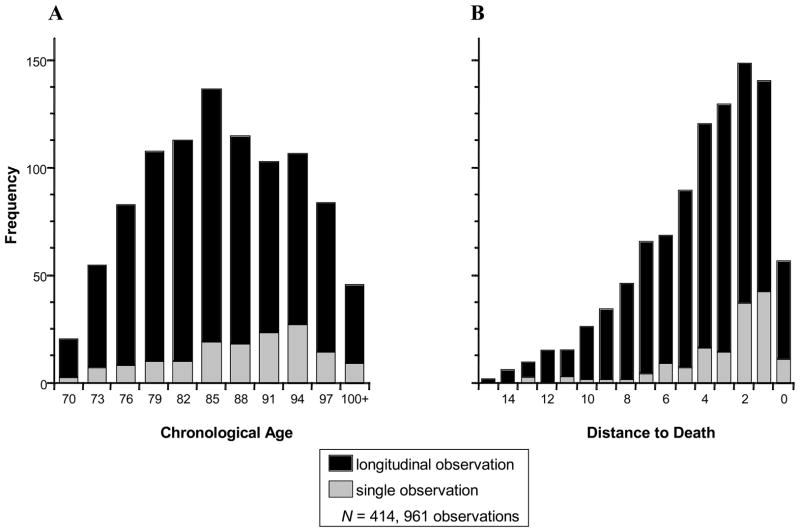
Of the 414 deceased BASE participants, n = 283 (68%) contributed two or more data points thereby providing longitudinal change information. Descriptive statistics for life satisfaction over age and distance-to-death are provided in Tables 1 and 2. Figure 1 illustrates the frequencies of observations for the age models in three-year bins (Panel A) and for the distance-to-death models in yearly bins (Panel B). As can be obtained from both panels of the figure, the large majority of the 961 observations available were longitudinal in nature. Each participant contributed different longitudinal time segments (M observation period = 1.99 years, SD = 2.28; range: 0–12 years) to an age gradient spanning more than 30 years and a distance-to-death gradient of up to 15 years. In the age models, the information for the observations entered into the models was spread relatively equally across the three age decades (70s: n = 188, 80s: n = 406; 90+s: n = 367). In the distance-to-death models, about 50% of the observations were taken in the last three years prior to death (nobservations = 682) suggesting that parameter estimates of these growth models are more stable relatively close to death rather than further away from death. The intercorrelation of age and distance-to-death was of moderate size (r = .45, p < .001), which indicates that there is only partial overlap between the two time dimensions.
Table 1.
Descriptive Statistics for Life Satisfaction as a Function of Age at Assessment.
| Age | n | Life satisfaction
|
|
|---|---|---|---|
| M | SD | ||
| 70 | 4 | 49.61 | 11.70 |
| 71 | 7 | 54.00 | 10.18 |
| 72 | 9 | 54.99 | 10.93 |
| 73 | 10 | 50.93 | 8.68 |
| 74 | 18 | 53.59 | 8.33 |
| 75 | 26 | 52.74 | 9.00 |
| 76 | 27 | 50.25 | 7.58 |
| 77 | 19 | 53.00 | 7.76 |
| 78 | 36 | 48.18 | 8.16 |
| 79 | 32 | 48.15 | 9.31 |
| 80 | 44 | 50.64 | 7.16 |
| 81 | 31 | 47.85 | 11.73 |
| 82 | 40 | 51.20 | 8.75 |
| 83 | 36 | 49.22 | 9.53 |
| 84 | 36 | 46.46 | 12.21 |
| 85 | 50 | 49.27 | 10.53 |
| 86 | 44 | 48.79 | 10.97 |
| 87 | 42 | 47.55 | 9.17 |
| 88 | 43 | 47.10 | 11.62 |
| 89 | 40 | 48.08 | 8.83 |
| 90 | 31 | 45.87 | 11.62 |
| 91 | 37 | 44.62 | 8.98 |
| 92 | 34 | 47.79 | 9.56 |
| 93 | 31 | 47.45 | 9.63 |
| 94 | 27 | 41.40 | 12.34 |
| 95 | 44 | 47.54 | 9.72 |
| 96 | 35 | 47.89 | 10.86 |
| 97 | 40 | 48.68 | 9.83 |
| 98 | 20 | 47.18 | 10.03 |
| 99 | 23 | 48.56 | 10.72 |
| 100 | 17 | 42.06 | 13.64 |
| 101 | 16 | 40.09 | 12.62 |
| 102 | 7 | 48.76 | 8.39 |
| 103 | 3 | 47.96 | 15.26 |
| 104 | 1 | 42.44 | – |
| 105 | 1 | 35.00 | – |
Note. T-scores standardized to cross-sectional BASE sample at T1 (N = 516, M = 50, SD = 10).
Table 2.
Descriptive Statistics for Life Satisfaction as a Function of Distance-to-Death.
| Distance-to-death | n | Life satisfaction
|
|
|---|---|---|---|
| M | SD | ||
| 15 | 1 | 51.16 | – |
| 14 | 5 | 54.39 | 9.47 |
| 13 | 9 | 55.03 | 5.99 |
| 12 | 14 | 50.30 | 6.06 |
| 11 | 14 | 53.39 | 6.97 |
| 10 | 25 | 49.22 | 11.31 |
| 9 | 33 | 51.90 | 7.70 |
| 8 | 45 | 50.57 | 8.88 |
| 7 | 65 | 48.98 | 8.75 |
| 6 | 68 | 49.02 | 10.96 |
| 5 | 89 | 48.51 | 9.42 |
| 4 | 120 | 48.05 | 11.19 |
| 3 | 129 | 48.59 | 9.43 |
| 2 | 148 | 46.67 | 10.60 |
| 1 | 140 | 46.77 | 10.64 |
| 0 | 56 | 44.34 | 11.67 |
Note. Distance-to-death in years. T-scores standardized to cross-sectional BASE sample at T1 (N = 516, M = 50, SD = 10).
Figure 1.
Frequency of observations in the Berlin Aging Study in relation to chronological age (Panel A) and distance-to-death (Panel B). The large majority of the 961 observations available were in fact longitudinal in nature. In the age models, observations were spread relatively equally across the three age decades (70s: n = 188, 80s: n = 406; 90+s: n = 367). In the distance-to-death models, about 50% of the observations were taken in the last three years prior to death (nobservations = 682).
Single vs. multiple phases of change
Once the time variable was selected, we examined the data for evidence of multiple phases of decline. In particular, we tested models wherein individual trajectories were parameterized by either a single slope parameter, that would characterize individual change over the entire study period, or by two slope parameters that might capture a systematic shift in the prevailing rate of change – either at a particular age or distance-to-death. Following procedures similar to those used in studies of cognitive terminal change (Sliwinski et al., 2006; Wilson et al., 2003), we compared the relative fit of a standard growth model (single slope parameter) with a change-point model (two slope parameters).
More specifically, the first model was parameterized as
| (1) |
where the life satisfaction measure score for person i at time t, lsit, is a function of an individual-specific intercept parameter, Li, an individual-specific slope parameter, S1i, that captures change over the selected time dimension (age or distance-to-death), and residual error, eit. Interindividual difference in level, Li, and slope, S1i, are assumed to be normally distributed around group means, correlated with each other, and uncorrelated with the residual errors, eit.
The second model introduced multi-phase complexity via a second slope parameter, S2i, and a “change point”, k, that identifies when in time individuals move from one phase of change to another (e.g., the point in time when the terminal phase begins). The model was parameterized as
| (2) |
for all t. As before, interindividual differences in the second slope parameter, S2i, are assumed to be normally distributed around a group mean, correlated with the other interindividual parameters, Li and S1i, and uncorrelated with the residual errors, eit.
Of interest was (a) whether or not there was evidence for multiple phases of decline (i.e., Does the change-point model provide a significantly better overall fit to the data than the singe-phase model?) and (b) if so, at what age or distance-to-death does the change point, k, occur?
Exploring interindividual differences in change
In the final set of models, we examined how a number of time-invariant interindividual difference measures (age, sex, SES, comorbidities, dementia, and perceptual speed) were related to interindividual differences in intraindividual change in life satisfaction. More specifically, time-invariant predictors were included as predictors of interindividual differences in the level and slope parameters describing intraindividual change of life satisfaction. Details of the final model are presented below. In an additional step, we also explored whether change in life satisfaction relates to change in an indicator of preclinical dementia. To do so, we included a time-varying indicator of preclinical dementia into our models and examined the intercorrelations between change in life satisfaction and change in preclinical dementia.
All models were fit to the data using SAS (Proc Mixed; Littell, Miliken, Stoup, & Wolfinger, 1996). Incomplete data were treated as missing at random (Little & Rubin, 1987). Results are presented below in step-wise fashion with later analyses having been informed by earlier ones.
Results
Comparing Age-Related and Mortality-Related Changes in Life Satisfaction
To examine age-related and mortality-related changes in life satisfaction, we proceeded in two steps. As a preliminary check, we estimated the relative amount of between-person and within-person variance by considering models that allowed random effects only for the intercept (see Mroczek & Spiro, 2003). The intraclass correlation, as revealed by these models, was .59 for life satisfaction suggesting that 59% of the total variation in this measure was between-person variance, and the remainder (41%) was within-person variation. This suggests that between-person differences accounted for the majority of variability, but there also was substantial variability within persons over time.
With an indication that there was indeed intraindividual variation to model, we proceeded to evaluate whether using age or distance-to-death as a change dimension would be more efficient in describing this within-person change over time. As outlined above, we fitted both age and distance-to-death models for life satisfaction to the longitudinal data. Mean estimates (top half) and variance estimates (bottom half) as well as relative model fit indices of these growth curve models are summarized in Table 3. Two criteria were used to identify which time dimension provided a “better” description of the data, relative overall model fit and relative proportion of variance explained. In terms of relative model fit, non-nested model comparisons indicated that the distance-to-death model for life satisfaction (Akaike Information Criterion, AIC = 6,854; −2 Log Likelihood, −2LL = 6,842) fitted the data better than did the counterpart age models (AIC = 6,880; −2LL = 6,868). We also examined the explained proportion of within-person variance (i.e., pseudo R2) captured by each time metric – alternatively conceptualized as the proportional reduction of prediction error (Snijders & Bosker, 1999). Specifically, for each model we calculated the change in pseudo-R2 that resulted from when either age or distance-to-death was added to the within-person (Level 1) portion of the model.1 In the age model, the time variable contributed an additional 0.077 of explained variance (i.e., Δpseudo-R2) to that portion of variance explained by an intercept-only model. In contrast, when using distance-to-death as the time dimension, the change in pseudo-R2 was 0.168. It was also noted that the inclusion of both time metrics did not further increase the explained variance. In sum, from both relative overall model fit and proportion of explained variance perspectives, it appears that distance-to-death, as a time dimension on which to track longitudinal change, provides a better fitting and more efficient description of the life satisfaction aspects of this data than does chronological age.
Table 3.
Growth Models over Chronological Age and Distance-to-Death for Life Satisfaction.
| Effect (se) | Life satisfaction
|
|
|---|---|---|
| Age | Distance-to-death | |
| Fixed effects estimates | ||
| Intercept | 49.01 (0.46) *** | 45.34 (0.62) *** |
| Slope | −0.33 (0.05) *** | −0.75 (0.10) *** |
| Random effects estimates | ||
| Variance of intercept | 57.63 (6.84) *** | 89.48 (11.19) *** |
| Variance of slope | 0.05 (0.07) | 0.37 (0.24) a |
| Covariance intercept, slope | 0.58 (0.37) | 3.87 (1.46) ** |
| Residual variance | 39.44 (2.49) *** | 35.53 (2.64) *** |
| AIC | 6,880 | 6,854 |
| − 2LL | 6,868 | 6,842 |
Note. N = 414 and 961 observations. T-scores standardized to cross-sectional BASE sample at T1 (N = 516, M = 50, SD = 10). Standard errors in parentheses. AIC = Akaike Information Criterion; −2LL = −2 Log Likelihood, relative model fit statistics.
p = .068,
p < .05,
p < .01,
p < .001.
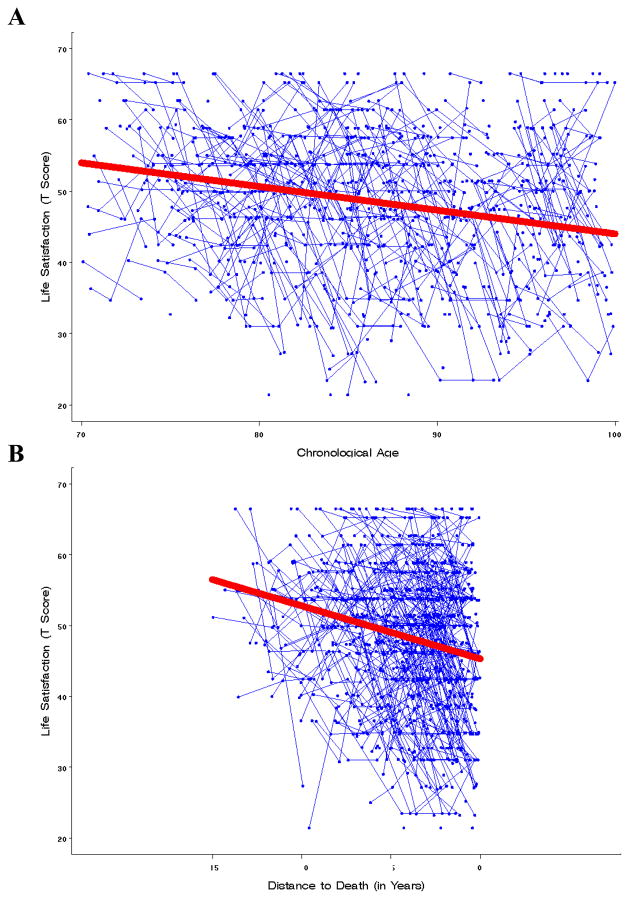
Note also that, on average, the age model shows significant, but relatively minor age-associated decline (− 0.33 T-score units per year). In contrast, decline in the distance-to-death model was more pronounced in that, relative to the age model, decline was steeper by a factor of 2.3 (− 0.75 T-score units per year). For illustration purposes, Figure 2 contrasts the average amount of decline observed over age and distance-to-death, thereby indicating that, on average, life satisfaction remains relatively stable with advancing age, but exhibits considerable decline when viewed in relation to individuals’ proximity to death.
Figure 2.
Average amount of decline observed over chronological age (Panel A) and distance-to-death (Panel B). The age model showed, on average, significant, but relatively minor decline (− 0.33 T-score units per year). Relative to the age model, decline in the distance-to-death model was steeper by a factor of 2.3 (− 0.75 T-score units per year).
To examine whether the noted mortality-related decline differs by age group, we introduced a dichotomized age group variable into our models to compare life satisfaction decline between the old (70–84 years at T1) and the oldest old (85+ years at T1). Our findings indicated that old individuals showed, as compared with the age model, somewhat stronger decline on life satisfaction with approaching death (− 0.54 [SE = 0.12], p < .001). However, the oldest old were found to show an even steeper decline that was approximately doubled relative old individuals (− 1.12 [SE = 0.21], p < .001). In contrast, the age model of life satisfaction did not reveal evidence for more pronounced decline among the oldest old (p > .10). In sum, our analyses suggest more pronounced decline in life satisfaction during the last years of life among very old individuals relative to old individuals contrary to the Riegel and Riegel (1972) hypothesis of diminished terminal decline in late senescence.
Applying Change-Point Models to Mortality-Related Decline in Life Satisfaction
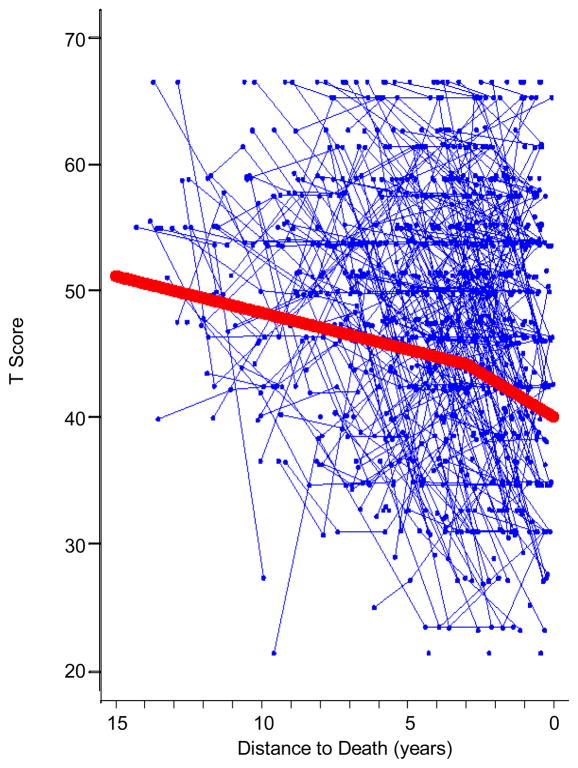
Having determined that the distance-to-death time dimension provides for a better fit to the BASE data, we proceeded to examine if and where multiple phases of change (i.e., pre-terminal and terminal decline phases) might be incorporated. In fitting the change-point model described above, we found that the variance terms, capturing interindividual differences within each phase of change, became non-significant. Thus, we considered various possible models wherein the variances in either phase were fixed to zero, eventually settling on a model with no interindividual differences in the pre-terminal phase (zero variance in S1i), but with interindividual differences in the terminal phase (significant variance in S2i). Constraing the model in this manner then allowed us to estimate and fit a set of change-point models where k, the point of transition between phases, was successively varied from 10 to 2 years prior to death by increments of roughly one-month units (1/10 of years). Our intent was to identify empirically the optimal location of the change point, k, as indicated by the model with the best overall fit (i.e., lowest log-likelihood, see Wilson et al., 2003). The best fitting change-point model, under the non-zero variance constraint, was one wherein the change point was located 4 years prior to death. Figure 3 shows the model-implied means for the optimal change-point model indicating that the rate of decline was twice as steep during the terminal phase (− 1.16 T-Score units per year) as compared to the pre-terminal phase (− 0.59 T-Score units per year; see Table 4).
Figure 3.
Model-implied means for the optimal change-point model (2.9 years prior to death) over distance-to-death in life satisfaction. The rate of decline increased from the pre-terminal phase (− 0.58 T-Score units per year) to the terminal phase (− 1.39 T-Score units per year) by a factor of 2.4.
Table 4.
Two-Phase Growth Model over Distance-to-Death for Life Satisfaction, Change-Point Location at Four Years Prior to Death: The Effects of Covariates.
| Parameter | Estimate | SE |
|---|---|---|
| Fixed effects | ||
| Life satisfaction intercept | 43.34 *** | 1.26 |
| Life satisfaction pre-terminal slope (15 – 4 years) | − 0.59 *** | 0.14 |
| Life satisfaction terminal slope (4 – 0 years) | − 1.16 ** | 0.35 |
| Age | − 0.15 | 0.12 |
| Sex | − 1.29 | 1.69 |
| SES | 0.01 | 0.09 |
| Comorbidities | − 0.18 * | 0.08 |
| Dementia | 1.17 | 2.15 |
| Perceptual Speed | 0.04 | 0.11 |
| Age x life satisfaction terminal slope | − 0.05 | 0.03 |
| Sex x life satisfaction terminal slope | 0.37 | 0.49 |
| SES x life satisfaction terminal slope | − 0.02 | 0.02 |
| Comorbidities x life satisfaction terminal slope | − 0.03 | 0.02 |
| Dementia x life satisfaction terminal slope | 0.13 | 0.68 |
| Perceptual Speed x life satisfaction terminal slope | − 0.01 | 0.03 |
| Random effects | ||
| Life satisfaction Variance of intercept | 111.81 *** | 17.18 |
| Life satisfaction Variance of pre-terminal slope | n. e. | |
| Life satisfaction Variance of terminal slope | 4.53 ** | 1.48 |
| Life satisfaction Covariance intercept, terminal slope | 15.56 *** | 4.66 |
| Life satisfaction Residual variance | 31.34 *** | 2.40 |
| AIC | 6,844 | |
| − 2LL | 6,806 | |
Note. N = 414 and 961 observations. T-scores standardized to cross-sectional BASE sample at T1 (N = 516, M = 50, SD = 10). n. e. = not estimated, SES = socioeconomic status. AIC = Akaike Information Criterion; −2LL = −2 Log Likelihood, relative model fit statistics.
p < .05,
p < .01,
p < .001.
Although optimal, this model did not fit significantly better than models with change points located anywhere in the 2 to 6 year distance-to-death range. Stated differently, the “standard error” of the change-point location only allowed us to isolate the general time frame during which a transition to a terminal phase of decline might occur (i.e., 4 ± 2.5 years prior to death). Regardless of the exact location, however, it is clear that models including a change point and two phases of change fit the data significantly better than a single-phase model. For example, a two-phase model with a change point four years prior to death (−2LL = 6,832) described the structure of intraindividual changes in life satisfaction better than a single-phase model covering the complete 15-year period (−2LL = 6,842; Δ−2LL = 10, Δdf = 1).2 Comparisons among similarly structured age-based models revealed no evidence for a shift to more pronounced decline anchored to chronological age (e.g., age 85 years as change point: Δ−2LL = 1.4).3
Exploring Potential Factors Underlying Mortality-Related Decline in Life Satisfaction
In the final set of analyses we introduced a variety of time-invariant predictor variables into the model and explored their relationships with the modeled interindividual differences in terminal-phase change. The model took the form
| (3) |
Specifically, age, sex, SES, comorbidities, dementia status, and perceptual speed were included as predictors of interindividual differences in level, Li and terminal-phase slope, S2i.
Results of these analyses are presented in Table 4. Inspection of parameter estimates for the effects of these variables revealed that, of the measures included, only the number of comorbidities was associated with reports of life satisfaction (− 0.18 [SE = 0.08], p < .05). None of the covariates included was found to account for significant portions of interindividual differences in terminal-phase decline in life satisfaction.
Follow-up analyses
We also carried out a series of follow-up analyses to substantiate these results. First, rather than using age as a continuous variable, we re-ran the above analysis contrasting old (70 to 85 at first assessment) and very-old (85+) individuals. Findings indicated that, compared with the pre-terminal slope on life satisfaction (− 0.59 [SE = 0.14], p < .001), the terminal slope was only marginally steeper for the old (− 0.68 [SE = 0.42], p > .10) but substantially steeper for the oldest old (− 1.71 [SE = 0.49], p < .05) suggesting a three-fold increase in the rate of decline between the pre-terminal and the terminal phase for participants 85+ years.
In another set of follow-up analyses, we further explored the uniqueness of the observed terminal decline in life satisfaction in comparison to the previously referenced terminal decline in cognitive functioning. For example, if mortality-related decline in life satisfaction was primarily attributable to pronounced cognitive decline with impending death (e.g., preclinical dementia, perceptual speed), we would expect high intercorrelations among these two distance-to-death slope factors. To address this question, we first included a variable representing cohort-specific cutoffs on the SMMSE at each occasion (0 = non-demented, 1 = demented) as time-varying predictors into our models. Both pre-terminal and terminal slopes on life satisfaction remained significant (− 0.60 [SE = 0.14], p < .001 and − 1.11 [SE = 0.38], p < .001, respectively), and the intercorrelations of the SMMSE with the intercept and terminal slope factors on life satisfaction were not significantly different from zero (15.41 [SE = 14.37], p > .10 and 4.65 [SE = 3.08], p > .10, respectively).4
Second, we examined Correlated (change point) Growth models of life satisfaction and perceptual speed anchored at four years prior to death. Although both variables showed pronounced terminal decline (perceptual speed: − 1.39 [SE = 0.20], p < .001; life satisfaction: − 1.03 [SE = 0.24], p < .001), slope intercorrelations were very low and not statistically significantly different from zero (r = −.06, p > .10). Finally, we also used the bivariate latent difference score models of change (Gerstorf et al., 2006; McArdle & Hamagami, 2001) to explore whether mortality-related decline in perceptual speed may precede and predict decline in life satisfaction. These analyses provided no evidence that perceptual speed could be regarded a leading indicator for terminal decline in life satisfaction. In sum, these follow-up analyses indicated that terminal decline effects in life satisfaction appear to be primarily driven by very-old individuals, but no evidence was found for a primacy of cognitive terminal decline for mortality-related decline in life satisfaction.
Discussion
The major objective of the present study was to explore mortality associations of changes in aspects of subjective well-being in old and very old age. In a first step, we empirically demonstrated that interindividual differences in intraindividual change in life satisfaction among a subsample of deceased participants of the BASE were better described by distance from death rather than distance from birth (chronological age). Indeed, the death-related slope was much steeper than expected on the basis of age-related models. In a second step, we applied “change-point” growth models to mortality-related change in life satisfaction. These analyses identified a point some four years prior to death after which the rate of decline in life satisfaction was steeper by a factor of two relative to the pre-terminal phase. This finding provides empirical evidence that terminal decline phenomena occur in reports of life satisfaction. In a third step, we explored how various mortality predictors were related to the late-life changes in life satisfaction. We found that terminal decline in life satisfaction was most pronounced among the oldest old (85+ years) with a three-fold increase in the rate of terminal decline relative to the rate of pre-terminal decline. In contrast, other mortality predictors including sex, comorbidities, dementia, and intellectual functioning accounted for only small portions of interindividual differences in intraindividual change in life satisfaction.
Comparing Age-Related and Mortality-Related Changes in Life Satisfaction
Our age-related change model suggested relative stability in the average level of life satisfaction. This finding parallels previous cross-sectional and longitudinal reports from various large-scale studies suggesting that well-being remains relatively stable throughout the adult lifespan and into old age (Diener et al., 2006; Filipp, 1996; Kunzmann et al., 2000; Mroczek & Kolarz, 1998; Larsen, 1978). In contrast, distance-to-death models of life satisfaction were better representations of interindividual differences in longitudinal change in the BASE data and indicated pronounced average linear decline (approximately double that found in the age-related model), amounting to three quarters of a standard deviation over a 10-year period.5 These results add to the increasing body of literature reporting mortality associations for level of functioning on numerous psychosocial measures including personality (Friedman et al., 1995; Swan & Carmelli, 1996), feelings of loneliness (Seeman, 2000; Sugisawa, Liang, & Liu, 1994), self-reported health (Idler & Benyamini, 1997), and aspects of well-being (Danner et al., 2001; Levy, 2003; Maier & Smith, 1999). Whereas previous studies examined the long-term predictive effects of level of well-being, our results highlight how change in life satisfaction is structured over distance-to-death (see also Mroczek & Spiro, 2005) – an essential element of terminal decline phenomena.
Our findings provide important qualifications to the literature on well-being across adulthood and into old age (for the effect of major life events on changes in well-being, see Charles, Reynolds, & Gatz, 2001; Lucas, Clark, Georgellis, & Diener, 2003, 2004). At the end of life, it appears that individuals do find it increasingly difficult to maintain the characteristic level of well-being (Baltes & Smith, 2003: Diener et al., 2006). The markedly different trajectories revealed by the age-related and death-related models indicate that approaching death encompasses progressive processes that may be distinct from normative age-graded processes. The nominal differences in the rates of decline (− 0.42 T-score units per year) provide a rough quantification of the effects of processes that precede death on well-being decline that are added to age-related effects. That the increased steepness of decline in life satisfaction prior to death was not linked to distance from birth (i.e., chronological age), but rather to distance from death, further highlights the importance of explicitly modeling terminal decline (cf. Sliwinski et al., 2006). In psychological studies on old age, however, information about how close participants are to their death is typically not available. If a sizeable portion of participants in such studies is already in the terminal phase for some time, then true change is likely be underestimated.
Applying Change-Point Models to Mortality-Related Decline in Life Satisfaction
In the current study, we took advantage of a change-point growth model that allows for modeling of multiple phases of change (e.g., Hall et al., 2000) and data on when individuals died. Although the size of the standard error around the change point did not allow for an exact estimate of the location of the change point, statistically nested model comparisons isolated a general time frame between two and six years prior to death during which a transition from a pre-terminal to a terminal phase of decline in life satisfaction might occur. The rate of pre-terminal decline was already stronger than expected on the basis of the age models, and this rate of decline was steeper by a factor of 2.4 in the terminal phase. Empirical evidence for the existence of multiple phases in mortality-related change have so far been reported from measures of cognitive functioning including perceptual speed and memory (Sliwinski et al., 2006; Wilson et al., 2003). To the best of our knowledge, this study is the first to demonstrate that substantially steeper decline in the last years of life extends to aspects of well-being. The general time window identified overlaps with and is roughly consistent with findings from the cognitive domain. Another similarity to recent evidence from the cognitive aging literature (Ghisletta et al., 2006; Sliwinski et al., 2006) is that life satisfaction decline within both the pre-terminal and terminal phase was constant (i.e., linear as opposed to quadratic) suggesting that the shape of the distance-to-death trajectories appears to represent steady terminal decline rather than precipitous terminal drop. We note, however, that the location of the transition to increased steepness of decline has so far been determined by empirical means only, thereby requiring future studies to examine the generalizability of these findings.
We acknowledge that any application of change-point models, in analogy to other multivariate-structural analyses, is based on some untested statistical assumptions, including ergodicity (Molenaar, Huizenga, & Nesselroade, 2003) and sample homogeneity (Borsboom, Mellenbergh, & van Heerden, 2003). For example, our “average” results (and those of other studies) are population level estimates and do not necessarily represent the changes occurring within a given individual. Similarly, in our analysis the change point between the pre-terminal and the terminal phase was specified as a population parameter with no variance (e.g., “fixed effect”), reflecting the strong assumption that the location of the change point is invariant across individuals. The limited density of longitudinal observations in BASE in relation to distance-to-death did not allow determining change points at the individual level. To do so, one would ideally need at minimum five data points for each person, two defining pre-terminal change, two defining terminal change, and at least one (and likely more) additional observations to help define the location of the change point. In future research, random effect change-point models should be examined to explore, for example, whether mortality-related decline on facets of well-being may be characterized by an acute terminal drop for some (groups of) individuals, but a more protracted decline for others (see also Bäckman & MacDonald, 2006). Taken together, however, the results reported here do provide further support for the terminal decline hypothesis that imminent death may have pervasive effects across domains of functioning and provide a quantitative description of the onset, extent, and temporal course of terminal decline in life satisfaction.
Exploring Potential Factors Underlying Mortality-Related Decline in Life Satisfaction
We explored the role of well-established mortality predictors (Anstey et al., 2006; Berkman, 1988; Johansson & Zarit, 1997) to account for individual differences in mortality-related change in life satisfaction. Although chronological age per se did not account for terminal decline in life satisfaction, the effect was most pronounced among participants older than age 85+, with a three-fold increase in the rate of decline from the pre-terminal to the terminal phase. Their rate of mortality-related decline was approximately doubled relative to old individuals suggesting that the additional burden of approaching death appears to be especially pronounced among the very old. In this regard, our results are consistent with the view that the very old are at the limits of their adaptive capacity (Baltes & Smith, 2003; Smith & Gerstorf, 2004). The system of self-protective processes associated with the maintenance of well-being appears to become increasingly vulnerable. This is also in line with the demise of the Riegel and Riegel (1972) hypothesis in the cognitive aging literature, which stated that the effects of terminal decline may diminish in late senescence due to more random causes of death (see Bäckman & MacDonald, 2006).
One possible scenario for the effects of the other mortality predictors examined would be a temporal sequence of steps involving an initial decline in, for example, intellectual functioning (as represented by perceptual speed as the most powerful indicator of mortality-related cognitive decline), followed by a decline in subjective well-being, and finally death (cf. Maier & Smith, 1999). It was thus somewhat surprising that (with the exception of age group) none of the factors examined was associated with individual variation in mortality-related decline on life satisfaction. Additional follow-up analyses also indicated low intercorrelations of cognitive terminal decline with terminal decline in life satisfaction suggesting that these processes may show only little overlap. However, lack of statistical power may have made it difficult to provide definitive answers regarding cross-daomin links. For example, Hertzog, Lindenberger, Ghisletta, and von Oertzen (2006) demonstrated that even with large samples (N = 500) and six measurement occasions as in the present study, statistical power to detect slope correlations between two variables was moderate to low unless growth curve reliability at study onset was above .90. Although our initial finding of lack of variation in terminal slope could be interpreted as evidence for normative decline in relation to death such that the same slope describes all individuals, we again caution that the low density of observations precludes any definitive statements. In a similar vein, one constraint in our multi-phase models was that the variance component in the pre-terminal decline phase was non-significant, thereby precluding inferences regarding the intercorrelation of decline in the pre-terminal and terminal phase. It would have been instructive to examine the extent to which individual differences in change within each phase may relate across phases.
Leaving issues of statistical power aside, one possible interpretation is that the terminal decline on life satisfaction cannot completely be attributed to factors that broadly represent past, cumulative, and current developmental contexts or conditions that are pathological in nature such as moderate to severe chronic illnesses at baseline assessment and preclinical dementia over time. Another interpretation refers to notions that much of the observed change in advanced old age might be stochastic and reflective of the incomplete biogenetic architecture of ontogeny (Baltes, 1997; Finch & Kirkwood, 2000; Thaler, 2000). Consistent with this line of reasoning, Johansson et al. (1997) reported increased heterogeneity in timing and rates of mortality-related cognitive decline among genetically identical twins aged 80 and older. In this context, we acknowledge that more in-depth studies are needed to specifically pinpoint potential sources of inter- and intraindividual variability in mortality-associated decline in well-being. Although cognitive terminal decline was found to be relatively independent of cause of death (Anstey et al., 2006; Small et al., 2003), it is conceivable that various causes of death (e.g., cerebrovascular, cardiac, and cancer) and the conditions associated with the process of dying may account for differential portions of individual differences in terminal decline of well-being. The present data did not allow an examination of these speculations.
Conceptually, it is an open question what underlies the pronounced deterioration of well-being linked to death and interindividual differences therein. To avoid a possible misunderstanding of our findings, it is important to note that we certainly do not contend that changes in well-being constitute a major source of mortality. Instead, well-being ratings might represent evaluations that reflect quite accurate summary perceptions of an individual’s level and change in functioning in a variety of domains (Maier & Smith, 1999). Increasingly negative evaluations themselves may thus not be directly related to mortality, but rather reflect potential causes from other domains of functioning (e.g., health or biological functioning) that we have not captured in this study. From another perspective, however, aspects of well-being might be key factors for preserved functioning in other domains that more directly relate to mortality. For example, several authors have pointed to the physiological effects of positive or negative self-perceptions of one’s life and aging with regard to cardiovascular and immune functioning as well as recovery after heart attack (Danner et al., 2001; Kiecolt-Glaser, McGuire, Robles, & Glaser, 2002; Pressman & Cohen, 2005; Steptoe, Wardle, & Marmot, 2005). Such physiological effects could also influence the brain and thus have an impact on subsequent functioning and long-term survival. A similar line of reasoning refers to the profound motivational and behavioral consequences (e.g., engagement and persistence) of well-being that may in the long-run either restrain or help to exploit an individual’s resources (Furry & Baltes, 1973; Levy et al., 2002) and thereby also relate to mortality.
Conclusions and Outlook
Our findings gathered from 414 decedents of the BASE indicated that an exacerbated rate of decline in the last years of life may not be specific to intellectual and sensory functioning, but also extend to subjective well-being. Whereas the White and Cunningham (1988) hypothesis of steeper mortality-related decline in relatively age-insensitive measures has been largely refuted for cognitive abilities (cf. Bäckman & MacDonald, 2006), our results suggest that it may apply to other areas of psychological functioning, especially self-related dimensions. Proximity to death may not only bring about greater losses than expected in age-sensitive domains such as multiple facets of cognitive functioning, but also substantial decline in domains of functioning, such as well-being, that are usually well preserved into old and advanced old age.
It remains an open question, however, to what extent terminal decline also entails qualitative changes in structure or cascading effects across domains of functioning (Birren & Cunningham, 1985; Siegler, 1975). We also acknowledge that the focus of our study has been on relatively proximal influences on mortality by utilizing data from old and oldest old individuals as assessed at maximum 15 years prior to death. This is to say that the scenario identified may be effective in addition to variables and processes that may affect terminal decline and subsequent death over a much larger time frame to the event of death. For example, findings from the Scottish Mental Survey indicate that childhood IQ is predictive of mortality over a 70-year period (Deary, Whiteman, Starr, Whalley, & Fox, 2004; see also Rabbitt et al., 2002). Future research is thus needed to isolate the unique and conjoint contribution of distant and more proximal factors to terminal decline processes. It would also be instrumental to examine whether pronounced decline in well-being in the last years of life generalizes to younger age groups (e.g., individuals who have died in their 50s or 60s).
Our findings warrant more in-depth analyses in future studies. One open question is whether the terminal decline phenomenon extends to other psychosocial variables. It would be instructive to examine whether more emotion-based measures of well-being, aspects of personality, self-reported health, or feelings of loneliness, all of which have been identified as long-term predictors of mortality, also show distinctively different trajectories of change over age and distance-to-death. Individual differences in intraindividual variation in subjective well-being represent a persistent phenomenon not only across years but across much shorter time frames as well, such as days and months (e.g., Eid & Diener, 1999; Nesselroade & Featherman, 1997; Röcke, 2006). A route worth exploring in future studies would be to extend the findings of the present paper to include an examination of the role of mortality-related over and above age-related changes in longer-term trajectories of short-term variability in well-being. The increase of losses associated with advanced old age may bring about not only a steady but sudden increase in variability in the oldest old, possibly indicating a terminal decline in the psychological system’s ability for equilibrium and regulation (e.g., Eizenman, Nesselroade, Featherman, & Rowe, 1997; Thaler, 2000). Intensive multivariate longitudinal assessments of collections of individuals from early to old age to advanced old age are needed to separate and integrate the operation of age-related, death-related, and pathology-related mechanisms on changes in the organization of psychological functioning during the last phase of life (Jones & Nesselroade, 1990).
Acknowledgments
The Berlin Aging Study (BASE) was financially supported by two German Federal Departments: the Department of Research and Technology (13 TA 011: 1988–1991) and the Department of Family and Senior Citizens (1991–1998). Since 1999, BASE has been funded by the Max Planck Institute for Human Development, Berlin, where the study is located. Members of the Steering Committee are P. B. Baltes and J. Smith (psychology), K. U. Mayer (sociology), E. Steinhagen-Thiessen and M. Borchelt (internal medicine and geriatrics), and H. Helmchen and F. Reischies (psychiatry). Field research was coordinated at various phases by R. Nuthmann, M. Neher, and K. Fröhlich.
Footnotes
| (4) |
Consistent with our finding of increased steepness of decline prior to death, a model specifying linear and quadratic change in life satisfaction over distance-to-death was found to provide slightly, but not significantly, better relative fit to our data (−2LL = 6,840) than a model with linear change only (−2LL = 6,842). However, the two-phase model with a change point four years prior to death still provided better relative model fit than this single-phase model (Δ−2LL = 8).
This also suggests that age-related change was not considerably different between the first and the second 15 years of the 30-year age range, thereby making it unlikely that differences in span between chronological age (30 years) and distance-to-death (15 years) have been a major factor underlying our results. In addition, estimates from the less parsimonious two-phase model revealed that the oldest old showed nominally shallower age-related decline than the old (− 0.25 [SE = 0.09], p < .01 vs. − 0.45 [SE = 0.11], p < .001), although their mortality-related decline was steeper.
In line with this general pattern, follow-up analyses that included other measures available in the BASE including initial cognitive functioning (fluency, episodic memory, and verbal knowledge), social losses (of a partner, family member or friend in the year preceding the interview, n = 260; no loss, n = 256), or balance-gait (for details, see Lindenberger & Baltes, 1997) yielded the same basic pattern of results, i.e., that the covariates did not account for hardly any of the individual differences in level or change of life satisfaction.
Follow-up analyses revealed that the age gradient for BASE participants who had not died by June 2005 (n = 83) was nominally shallower as compared with those who had died (− 0.28 vs. − 0.33 T-score units per year), but this difference was not statistically significant (p > .10). This lack of a significant difference may reflect the fact that some of the not-yet-dead participants were already relatively close to death and may thus have already “entered” their mortality-related change trajectory.
Contributor Information
Denis Gerstorf, Email: gerstorf@virginia.edu.
Nilam Ram, Email: nilam.ram@psu.edu.
Christina Röcke, Email: croecke@brandeis.edu.
Ulman Lindenberger, Email: seklindenberger@mpib-berlin.mpg.de.
Jacqui Smith, Email: smitjacq@isr.umich.edu.
References
- Akaike H. Information theory and an extension of the maximum likelihood principle. In: Petrov BN, Csaki F, editors. Proceedings of the Second International Symposium on Information Theory. Budapest, Bulgaria: Akademiai Kiado; 1973. pp. 267–281. [Google Scholar]
- Anstey K, Mack HA, von Sanden C. The relationship between cognition and mortality in patients with stroke, coronary heart disease, or cancer. European Psychologist. 2006;11:182–195. [Google Scholar]
- Bäckman L, MacDonald SWS. Death and cognition: Synthesis and outlook. European Psychologist. 2006;11:224–235. [Google Scholar]
- Baltes PB. On the incomplete architecture of human ontogeny: Selection, optimization, and compensation as foundation of developmental theory. American Psychologist. 1997;52:366–380. doi: 10.1037//0003-066x.52.4.366. [DOI] [PubMed] [Google Scholar]
- Baltes PB, Baltes MM, editors. Successful aging: Perspectives from the behavioral sciences. New York, NY: Cambridge University Press; 1990. [Google Scholar]
- Baltes PB, Mayer KU, editors. The Berlin Aging Study: Aging from 70 to 100. New York, NY: Cambridge University Press; 1999. [Google Scholar]
- Baltes PB, Smith J. New frontiers in the future of aging: From successful aging of the young old to the dilemmas of the fourth age. Gerontology. 2003;49:123–135. doi: 10.1159/000067946. [DOI] [PubMed] [Google Scholar]
- Baltes PB, Smith J. A systemic-wholistic view of psychological functioning in very old age: Introduction to a collection of articles from the Berlin Aging Study. Psychology and Aging. 1997;12:395–409. doi: 10.1037//0882-7974.12.3.395. [DOI] [PubMed] [Google Scholar]
- Berg S. Aging, behavior, and terminal decline. In: Birren JE, Schaie KW, editors. Handbook of the psychology of aging. 4. San Diego, CA: Academic Press; 1996. pp. 323–337. [Google Scholar]
- Berkman LF. The changing and heterogeneous nature of aging and longevity: A social and biomedical perspective. In: Maddox GL, Lawton MP, editors. Annual review of gerontology and geriatrics: Varieties of aging. Vol. 8. New York, NY: Springer; 1988. pp. 37–68. [PubMed] [Google Scholar]
- Birren JE, Cunningham W. Research on the psychology of aging: Principles, concepts, and theory. In: Birren JE, Schaie KW, editors. Handbook of the psychology of aging. 2. New York, NY: Van Nostrand Reinhold; 1985. pp. 3–34. [Google Scholar]
- Borsboom D, Mellenbergh GJ, van Heerden J. The theoretical status of latent variables. Psychological Review. 2003;110:203–219. doi: 10.1037/0033-295X.110.2.203. [DOI] [PubMed] [Google Scholar]
- Bosworth HB, Schaie KW. Survival effects in cognitive function, cognitive style, and sociodemographic variables in the Seattle Longitudinal Study. Experimental Aging Research. 1999;25:121–139. doi: 10.1080/036107399244057. [DOI] [PubMed] [Google Scholar]
- Bosworth HB, Siegler IC. Terminal change in cognitive function: An updated review of longitudinal studies. Experimental Aging Research. 2002;28:299–315. doi: 10.1080/03610730290080344. [DOI] [PubMed] [Google Scholar]
- Charles ST, Reynolds CA, Gatz M. Age-related differences and change in positive and negative affect over 23 years. Journal of Personality and Social Psychology. 2001;80:136–151. [PubMed] [Google Scholar]
- Cudeck R, Klebe KJ. Multiphase mixed-effects model for repeated measure data. Psychological Methods. 2002;7:41–63. doi: 10.1037/1082-989x.7.1.41. [DOI] [PubMed] [Google Scholar]
- Danner DD, Snowdon DA, Friesen WV. Positive emotions in early life and longevity: Findings from the Nun Study. Journal of Personality and Social Psychology. 2001;80:804–813. [PubMed] [Google Scholar]
- Deary IJ, Whiteman MC, Starr JM, Whalley LJ, Fox HC. The impact of childhood intelligence on later life: Following up the Scottish Mental Surveys of 1932 and 1947. Journal of Personality and Social Psychology. 2004;86:130–147. doi: 10.1037/0022-3514.86.1.130. [DOI] [PubMed] [Google Scholar]
- Diener E, Lucas RE, Scollon CN. Beyond the hedonic treadmill: Revising the adaptation theory of well-being. American Psychologist. 2006;61:305–314. doi: 10.1037/0003-066X.61.4.305. [DOI] [PubMed] [Google Scholar]
- Eid M, Diener E. Intraindividual variability in affect: Reliability, validity, and personality correlates. Journal of Personality and Social Psychology. 1999;76:662–676. [Google Scholar]
- Eizenman DR, Nesselroade JR, Featherman DL, Rowe JW. Intraindividual variability in perceived control in an older sample: The MacArthur Successful Aging Studies. Psychology and Aging. 1997;12:489–502. doi: 10.1037//0882-7974.12.3.489. [DOI] [PubMed] [Google Scholar]
- Filipp SH. Motivation and emotion. In: Birren JE, Schaie KW, editors. Handbook of the psychology of aging. 4. San Diego, CA: Academic Press; 1996. pp. 218–235. [Google Scholar]
- Finch CE, Kirkwood TBL. Chance, development, and aging. New York, NY: Oxford University Press; 2000. [Google Scholar]
- Friedman HS, Tucker JS, Schwartz JE, Tomlinson-Keasey C, Martin LR, Wingard DL, et al. Psychosocial and behavioral predictors of longevity: The aging and death of the “Termites”. American Psychologist. 1995;50:69–78. doi: 10.1037//0003-066x.50.2.69. [DOI] [PubMed] [Google Scholar]
- Furry CA, Baltes PB. The effect of age differences in ability-extraneous performance variables on the assessment of intelligence in children, adults, and the elderly. Journal of Gerontology. 1973;28:73–80. doi: 10.1093/geronj/28.1.73. [DOI] [PubMed] [Google Scholar]
- Gerstorf D, Herlitz A, Smith J. Stability of sex differences in cognition in advanced old age: The role of education and attrition. Journals of Gerontology Series B: Psychological Sciences. 2006;61B:P245–P249. doi: 10.1093/geronb/61.4.p245. [DOI] [PubMed] [Google Scholar]
- Gerstorf D, Lövdén M, Röcke C, Smith J, Lindenberger U. Well-being affects changes in perceptual speed in advanced old age: Longitudinal evidence for a dynamic link. Developmental Psychology. doi: 10.1037/0012-1649.43.3.705. (in press) [DOI] [PubMed] [Google Scholar]
- Ghisletta P, McArdle JJ, Lindenberger U. Longitudinal cognition-survival relations in old and very old age: 13-year data from the Berlin Aging Study. European Psychologist. 2006;11:204–223. [Google Scholar]
- Guralnik JM. Prospects for the compression of morbidity: The challenge posed by increasing disability in the years prior to death. Journal of Aging and Health. 1991;3:138–154. [Google Scholar]
- Hall CB, Lipton RB, Sliwinski MJ, Stewart WF. A change point model for estimating the onset of cognitive decline in preclinical Alzheimer’s disease. Statistics in Medicine. 2000;19:1555–1566. doi: 10.1002/(sici)1097-0258(20000615/30)19:11/12<1555::aid-sim445>3.0.co;2-3. [DOI] [PubMed] [Google Scholar]
- Hassing LB, Johansson B, Berg S, Nilsson SE, Pedersen NL, Hofer SM, et al. Terminal decline and markers of cerebro- and cardiovascular disease: Findings from a longitudinal study of the oldest old. Journals of Gerontology: Series B Psychological Sciences. 2002;57B:P268–P276. doi: 10.1093/geronb/57.3.p268. [DOI] [PubMed] [Google Scholar]
- Helmchen H, Baltes MM, Geiselmann B, Kanowski S, Linden M, Reischies FM, et al. Psychiatric illnesses in old age. In: Baltes PB, Mayer KU, editors. The Berlin Aging Study: Aging from 70 to 100. New York, NY: Cambridge University; 1999. pp. 167–196. [Google Scholar]
- Hertzog C, Lindenberger U, Ghisletta P, von Oertzen T. On the power of multivariate latent growth curve models to detect correlated change. Psychological Methods. 2006;11:244–252. doi: 10.1037/1082-989X.11.3.244. [DOI] [PubMed] [Google Scholar]
- Hofer SM, Christensen H, Mackinnon AJ, Korten AE, Jorm AF, Henderson AS, et al. Change in cognitive functioning associated with ApoE genotype in a community sample of older adults. Psychology and Aging. 2002;17:194–208. [PubMed] [Google Scholar]
- Idler EL, Benyamini Y. Self-rated health and mortality: A review of twenty-seven community studies. Journal of Health and Social Behavior. 1997;38:21–37. [PubMed] [Google Scholar]
- Johansson B, Hofer SM, Allaire JC, Maldonado-Molina MM, Piccinin AM, Berg S, et al. Change in cognitive capabilities in the oldest old: The effects of proximity to death in genetically related individuals over a 6-year period. Psychology and Aging. 2004;19:145–156. doi: 10.1037/0882-7974.19.1.145. [DOI] [PubMed] [Google Scholar]
- Johansson B, Zarit SH. Early cognitive markers of the incidence of dementia and mortality: A longitudinal population-based study of the oldest old. International Journal of Geriatric Psychiatry. 1997;12:53–59. doi: 10.1002/(sici)1099-1166(199701)12:1<53::aid-gps507>3.0.co;2-m. [DOI] [PubMed] [Google Scholar]
- Jones CJ, Nesselroade JR. Multivariate, replicated, single-subject, repeated measures designs and p-technique factor analysis: A review of intraindividual change studies. Experimental Aging Research. 1990;16:171–183. doi: 10.1080/03610739008253874. [DOI] [PubMed] [Google Scholar]
- Kiecolt-Glaser JK, McGuire L, Robles TF, Glaser R. Emotions, morbidity, and mortality: New perspectives from psychoneuroimmunology. Annual Review of Psychology. 2002;53:83–107. doi: 10.1146/annurev.psych.53.100901.135217. [DOI] [PubMed] [Google Scholar]
- Kleemeier RW. Intellectual changes in the senium. Proceedings of the Social Statistics Section of the American Statistical Association. 1962;1:290–295. [Google Scholar]
- Klein LE, Roca RP, McArthur J, Vogelsang G, Klein GB, Kirby SM, et al. Diagnosing dementia. Univariate and multivariate analyses of the mental status examination. Journal of the American Geriatrics Society. 1985;33:483–488. doi: 10.1111/j.1532-5415.1985.tb05460.x. [DOI] [PubMed] [Google Scholar]
- Kunzmann U, Little TD, Smith J. Is age-related stability of subjective well-being a paradox? Cross-sectional and longitudinal evidence from the Berlin Aging Study. Psychology and Aging. 2000;15:511–526. doi: 10.1037//0882-7974.15.3.511. [DOI] [PubMed] [Google Scholar]
- Larson R. Thirty years of research on the subjective well-being of older Americans. Journal of Gerontology. 1978;33:109–125. doi: 10.1093/geronj/33.1.109. [DOI] [PubMed] [Google Scholar]
- Laukka EJ, MacDonald SWS, Bäckman L. Contrasting cognitive trajectories of impending death and preclinical dementia in the very old. Neurology. 2006;66:833–838. doi: 10.1212/01.wnl.0000203112.12554.f4. [DOI] [PubMed] [Google Scholar]
- Lawton MP. The Philadelphia Geriatric Center Morale Scale: A revision. Journal of Gerontology. 1975;30:85–89. doi: 10.1093/geronj/30.1.85. [DOI] [PubMed] [Google Scholar]
- Levy BR, Slade MD, Kunkel SR. Journal of Personality and Social Psychology. 2002;83:261–270. doi: 10.1037//0022-3514.83.2.261. [DOI] [PubMed] [Google Scholar]
- Liang J, Bollen KA. The structure of the Philadelphia Geriatric Center Morale Scale: A reinterpretation. Journal of Gerontology. 1983;38:181–189. doi: 10.1093/geronj/38.2.181. [DOI] [PubMed] [Google Scholar]
- Lindenberger U, Baltes PB. Intellectual functioning in old and very old age: Cross-sectional results from the Berlin Aging Study. Psychology and Aging. 1997;12:410–432. doi: 10.1037//0882-7974.12.3.410. [DOI] [PubMed] [Google Scholar]
- Lindenberger U, Gilberg R, Little TD, Nuthmann R, Pötter U, Baltes PB. Sample selectivity and generalizability of the results of the Berlin Aging Study. In: Baltes PB, Mayer KU, editors. The Berlin Aging Study: Aging from 70 to 100. New York, NY: Cambridge University Press; 1999. pp. 56–82. [Google Scholar]
- Lindenberger U, Singer T, Baltes PB. Longitudinal selectivity in aging populations: Separating mortality-associated versus experimental components in the Berlin Aging Study (BASE) Journals of Gerontology Series B: Psychological Sciences. 2002;57B:P474–P482. doi: 10.1093/geronb/57.6.p474. [DOI] [PubMed] [Google Scholar]
- Littell RC, Miliken GA, Stoup WW, Wolfinger RD. SAS system for mixed models. Cary, NC: SAS Institute; 1996. [Google Scholar]
- Lucas RE, Clark AE, Georgellis Y, Diener E. Unemployment alters the set point for life satisfaction. Psychological Science. 2004;15:8–13. doi: 10.1111/j.0963-7214.2004.01501002.x. [DOI] [PubMed] [Google Scholar]
- Lucas RE, Clark AE, Georgellis Y, Diener E. Reexamining adaptation and the set point model of happiness: Reactions to changes in marital status. Journal of Personality and Social Psychology. 2003;84:527–539. doi: 10.1037//0022-3514.84.3.527. [DOI] [PubMed] [Google Scholar]
- Lyubomirsky S, King L, Diener E. The benefits of frequent positive affect: Does happiness lead to success? Psychological Bulletin. 2005;131:803–855. doi: 10.1037/0033-2909.131.6.803. [DOI] [PubMed] [Google Scholar]
- Lyyra TM, Törmäkangas TM, Read S, Rantanen T, Berg S. Satisfaction with present life predicts survival in octogenarians. Journals of Gerontology: Series B Psychological Sciences. 2006;61B:P319–P326. doi: 10.1093/geronb/61.6.p319. [DOI] [PubMed] [Google Scholar]
- Maier H, Smith J. Psychological predictors of mortality in old age. Journals of Gerontology: Series B Psychological Sciences. 1999;54B:P44–P54. doi: 10.1093/geronb/54b.1.p44. [DOI] [PubMed] [Google Scholar]
- Mayer KU, Maas I, Wagner M. Socioeconomic conditions and social inequalities in old age. In: Baltes PB, Mayer KU, editors. The Berlin Aging Study: Aging from 70 to 100. New York, NY: Cambridge University Press; 1999. pp. 227–258. [Google Scholar]
- McArdle JJ, Hamagami F. Latent difference score structural models for linear dynamic analyses with incomplete longitudinal data. In: Collins LM, Sayer AG, editors. New methods for the analysis of change. Washington, DC: American Psychological Association; 2001. pp. 137–176. [Google Scholar]
- McArdle JJ, Nesselroade JR. Growth curve analysis in contemporary psychological research. In: Shinka J, Velicer W, editors. Comprehensive handbook of psychology, Volume two: Research methods in psychology. New York, NY: Wiley; 2003. pp. 447–480. [Google Scholar]
- Molenaar PCM, Huizenga HM, Nesselroade JR. The relationship between the structure of interindividual and intraindividual variability: A theoretical and empirical vindication of developmental systems theory. Understanding human development. In: Staudinger UM, Lindenberger U, editors. Dialogues with lifespan psychology. Dordrecht, NL: Kluwer; 2003. pp. 339–360. [Google Scholar]
- Mroczek DK, Kolarz CM. The effect of age on positive and negative affect: A developmental perspective on happiness. Journal of Personality and Social Psychology. 1998;75:1333–1349. doi: 10.1037//0022-3514.75.5.1333. [DOI] [PubMed] [Google Scholar]
- Mroczek DK, Spiro A., III Change in life satisfaction during adulthood: Findings from the Veterans Affairs Normative Aging Study. Journal of Personality and Social Psychology. 2005;88:189–202. doi: 10.1037/0022-3514.88.1.189. [DOI] [PubMed] [Google Scholar]
- Mroczek DK, Spiro A., III Modeling intraindividual change in personality traits: Findings from the Normative Aging Study. Journals of Gerontology Series B: Psychological Sciences. 2003;58B:P153–P165. doi: 10.1093/geronb/58.3.p153. [DOI] [PubMed] [Google Scholar]
- Nesselroade JR, Featherman DL. Establishing a reference frame against which to chart age-related changes. In: Hardy MA, editor. Studying aging and social change: Conceptual and methodological issues. Thousand Oaks, CA: Sage; 1997. pp. 191–205. [Google Scholar]
- Nussbaum PD. Late-life depression: A neuropsychological perspective. In: Nussbaum PD, editor. Handbook of neuropsychology and aging. New York, NY: Plenum Press; 1997. pp. 260–270. [Google Scholar]
- Palmore E, Cleveland W. Aging, terminal decline, and terminal drop. Journal of Gerontology. 1976;31:76–81. doi: 10.1093/geronj/31.1.76. [DOI] [PubMed] [Google Scholar]
- Pressman SD, Cohen S. Does positive affect influence health? Psychological Bulletin. 2005;131:925–971. doi: 10.1037/0033-2909.131.6.925. [DOI] [PubMed] [Google Scholar]
- Rabbitt P, Watson P, Donlan C, McInnes L, Horan M, Pendleton N, et al. Effects of death within 11 years on cognitive performance in old age. Psychology and Aging. 2002;17:468–481. doi: 10.1037//0882-7974.17.3.468. [DOI] [PubMed] [Google Scholar]
- Riegel KF, Riegel RM. Development, drop, and death. Developmental Psychology. 1972;6:306–419. [Google Scholar]
- Röcke C. Doctoral dissertation. Free University; Berlin: 2006. Intraindividual variability in positive and negative affect: Age-related and individual differences in magnitude and coupling with cognitive performance. http://www.diss.fu-berlin.de/2006/669/indexe.html. [Google Scholar]
- Rowe JW, Kahn RL. Successful aging. Gerontologist. 1997;37:433–440. doi: 10.1093/geront/37.4.433. [DOI] [PubMed] [Google Scholar]
- Ryff CD, Singer B. The contours of positive human health. Psychological Inquiry. 1998;9:1–28. [Google Scholar]
- SAS Institute. SAS/STAT software: Changes and enhancements through Release 6.12. San Diego, CA: Academic Press; 1997. [Google Scholar]
- Seeman TE. Health promoting effects of friends and family on health outcomes in older adults. American Journal of Health Promotion. 2000;14:362–370. doi: 10.4278/0890-1171-14.6.362. [DOI] [PubMed] [Google Scholar]
- Siegler IC. The terminal drop hypothesis: Fact or artifact? Experimental Aging Research. 1975;1:169–185. doi: 10.1080/03610737508257957. [DOI] [PubMed] [Google Scholar]
- Singer JD, Willett JB. Applied longitudinal data analysis: Modeling change and event occurrence. NY, NY: Oxford University Press; 2003. [Google Scholar]
- Sliwinski MJ, Hofer SM, Hall C, Buschke H, Lipton RB. Modeling memory decline in older adults: The importance of preclinical dementia, attrition, and chronological age. Psychology and Aging. 2003;18:658–671. doi: 10.1037/0882-7974.18.4.658. [DOI] [PubMed] [Google Scholar]
- Sliwinski MJ, Stawski RS, Hall RB, Katz M, Verghese J, Lipton RB. On the importance of distinguishing pre-terminal and terminal cognitive decline. European Psychologist. 2006;11:172–181. [Google Scholar]
- Small BJ, Bäckman L. Time to death and cognitive performance. Current Directions in Psychological Science. 1999;8:168–172. [Google Scholar]
- Small BJ, Fratiglioni L, von Strauss E, Bäckman L. Terminal decline and cognitive performance in very old age: Does cause of death matter? Psychology and Aging. 2003;18:193–202. doi: 10.1037/0882-7974.18.2.193. [DOI] [PubMed] [Google Scholar]
- Smith J, Fleeson W, Geiselmann B, Settersten RA, Jr, Kunzmann U. Sources of well-being in very old age. In: Baltes PB, Mayer KU, editors. The Berlin Aging Study: Aging from 70 to 100. New York, NY: Cambridge University Press; 1999. pp. 450–471. [Google Scholar]
- Smith J, Gerstorf D. Aging differently: Potentials and limits. In: Daatland SO, Biggs S, editors. Ageing and diversity: Multiple pathways and cultural migrations. Bristol, UK: Policy Press; 2004. pp. 13–28. [Google Scholar]
- Smith J, Maas I, Mayer KU, Helmchen H, Steinhagen-Thiessen E, Baltes PB. Two-wave longitudinal findings from the Berlin Aging Study: Introduction to a collection of articles. Journals of Gerontology Series B: Psychological Sciences. 2002;57B:P471–P473. doi: 10.1093/geronb/57.6.p471. [DOI] [PubMed] [Google Scholar]
- Snijders TAB, Bosker RJ. Multilevel analysis: An introduction to basic and advanced multilevel modeling. London, UK: Sage; 1999. [Google Scholar]
- Steinhagen-Thiessen E, Borchelt M. Morbidity, medication, and functional limitations in very old age. In: Baltes PB, Mayer KU, editors. The Berlin Aging Study: Aging from 70 to 100. New York, NY: Cambridge University Press; 1999. pp. 131–166. [Google Scholar]
- Steptoe A, Wardle J, Marmot M. Positive affect and health-related neuroendocrine, cardiovascular, and inflammatory processes. Proceedings of the National Academy of Science. 2005;102:6508–6512. doi: 10.1073/pnas.0409174102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sugisawa H, Liang J, Liu X. Social networks, social support, and mortality among older people in Japan. Journals of Gerontology Series B: Social Sciences. 1994;49B:S3–S13. doi: 10.1093/geronj/49.1.s3. [DOI] [PubMed] [Google Scholar]
- Swan GE, Carmelli D. Curiosity and mortality in aging adults: A 5-year follow-up of the Western Collaborative Group Study. Psychology and Aging. 1996;11:449–453. doi: 10.1037//0882-7974.11.3.449. [DOI] [PubMed] [Google Scholar]
- Thaler DS. Design for an aging brain. Neurobiology of Aging. 2002;23:13–15. doi: 10.1016/s0197-4580(01)00262-7. [DOI] [PubMed] [Google Scholar]
- Wechsler D. Manual for the Hamburg Wechsler Intelligence Tests for adults. Bern, Switzerland: Huber; 1982. Handanweisung zum Hamburg-Wechsler-Intelligenztest fuer Erwachsene (HAWIE) [Google Scholar]
- White N, Cunningham WR. Is terminal drop pervasive or specific? Journal of Gerontology: Psychological Sciences. 1988;43:P141–P144. doi: 10.1093/geronj/43.6.p141. [DOI] [PubMed] [Google Scholar]
- Wilson RS, Beckett LA, Bienias JL, Evans DA, Bennett DA. Terminal decline in cognitive function. Neurology. 2003;60:1782–1787. doi: 10.1212/01.wnl.0000068019.60901.c1. [DOI] [PubMed] [Google Scholar]