Abstract
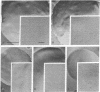
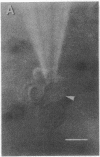
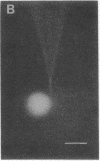
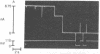
Protein-lipid complexes in apolar solvents reassemble into large bilayer protein-lipid vesicles (PLVs) with diameters of several micrometers. PLVs form spontaneously upon hydration of the protein-lipid complex residue after solvent removal. This procedure has been applied to the following membrane proteins: bovine and squid rhodopsin, reaction centers from Rhodopseudomonas sphaeroides, beef heart cytochrome c oxidase, and acetylcholine receptors from Torpedo californica. PLVs have a large internal aqueous space (e.g., 790 μl/mg of lipid for cattle rhodopsin vesicles). Freeze-fracture replicas of PLVs revealed that both internal and external leaflets contained numerous intramembranous particles with diameters between 80 and 120 Å, depending on the specific protein incorporated in the membrane. The optical spectral properties of rhodopsin and reaction centers in PLVs were similar to those recorded in the respective natural membrane. Furthermore, bovine rhodopsin in PLVs was chemically regenerable with 9-cis-retinal. Actinic illumination induced proton efflux from reaction center vesicles that was abolished by proton ionophores. Therefore, this method is suitable for the incorporation of some membrane proteins in their functional state. PLVs were penetrated with microelectrodes and visualized by the injection of a fluorescent dye. Preliminary electrical recordings were obtained by sealing PLVs to a hole in a septum separating two aqueous compartments. These studies suggest that PLVs assembled by this procedure permit the simultaneous analysis of reconstituted membranes by chemical, optical, and electrical techniques.
Keywords: liposomes, rhodopsin, photosynthetic reaction centers, cytochrome c oxidase, acetylcholine receptors
Full text
PDF
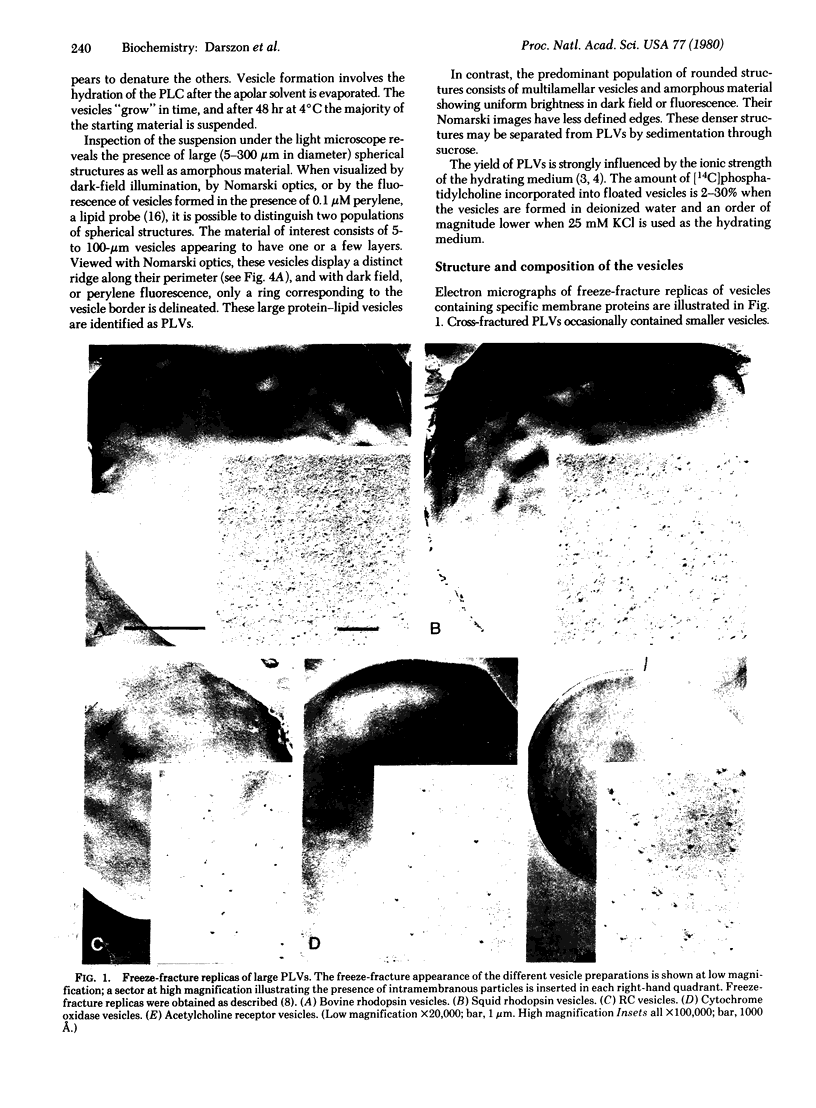
Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- AMES B. N., DUBIN D. T. The role of polyamines in the neutralization of bacteriophage deoxyribonucleic acid. J Biol Chem. 1960 Mar;235:769–775. [PubMed] [Google Scholar]
- Bangham A. D. Properties and uses of lipid vesicles: an overview. Ann N Y Acad Sci. 1978;308:2–7. doi: 10.1111/j.1749-6632.1978.tb22010.x. [DOI] [PubMed] [Google Scholar]
- Crofts A. R., Crowther D., Celis H., De Celis S. A., Tierney G. Proton pumps in bacterial photosynthesis. Biochem Soc Trans. 1977;5(2):491–495. doi: 10.1042/bst0050491. [DOI] [PubMed] [Google Scholar]
- Deamer D. W. Preparation and properties of ether-injection liposomes. Ann N Y Acad Sci. 1978;308:250–258. doi: 10.1111/j.1749-6632.1978.tb22027.x. [DOI] [PubMed] [Google Scholar]
- Enoch H. G., Strittmatter P. Formation and properties of 1000-A-diameter, single-bilayer phospholipid vesicles. Proc Natl Acad Sci U S A. 1979 Jan;76(1):145–149. doi: 10.1073/pnas.76.1.145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- HUBBARD R., ST GEORGE R. C. The rhodopsin system of the squid. J Gen Physiol. 1958 Jan 20;41(3):501–528. doi: 10.1085/jgp.41.3.501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- HUBBARD R., WALD G. Cis-trans isomers of vitamin A and retinene in the rhodopsin system. J Gen Physiol. 1952 Nov;36(2):269–315. doi: 10.1085/jgp.36.2.269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hamilton S. L., McLaughlin M., Karlin A. Formation of disulfide-linked oligomers of acetylcholine receptor in membrane from torpedo electric tissue. Biochemistry. 1979 Jan 9;18(1):155–163. doi: 10.1021/bi00568a024. [DOI] [PubMed] [Google Scholar]
- Hara T., Hara R. Rhodopsin and retinochrome in the squid retina. Nature. 1967 May 6;214(5088):573–575. doi: 10.1038/214573a0. [DOI] [PubMed] [Google Scholar]
- Hopfer U., Lehninger A. L., Thompson T. E. Protonic conductance across phospholipid bilayer membranes induced by uncoupling agents for oxidative phosphorylation. Proc Natl Acad Sci U S A. 1968 Feb;59(2):484–490. doi: 10.1073/pnas.59.2.484. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang C. Studies on phosphatidylcholine vesicles. Formation and physical characteristics. Biochemistry. 1969 Jan;8(1):344–352. doi: 10.1021/bi00829a048. [DOI] [PubMed] [Google Scholar]
- Michels P. A., Konings W. N. The electrochemical proton gradient generated by light in membrane vesicles and chromatophores from Rhodopseudomonas sphaeroides. Eur J Biochem. 1978 Apr;85(1):147–155. doi: 10.1111/j.1432-1033.1978.tb12222.x. [DOI] [PubMed] [Google Scholar]
- Montal M., Darszon A., Trissl H. W. Transmembrane channel formation in rhodopsin-containing bilayer membranes. Nature. 1977 May 19;267(5608):221–225. doi: 10.1038/267221a0. [DOI] [PubMed] [Google Scholar]
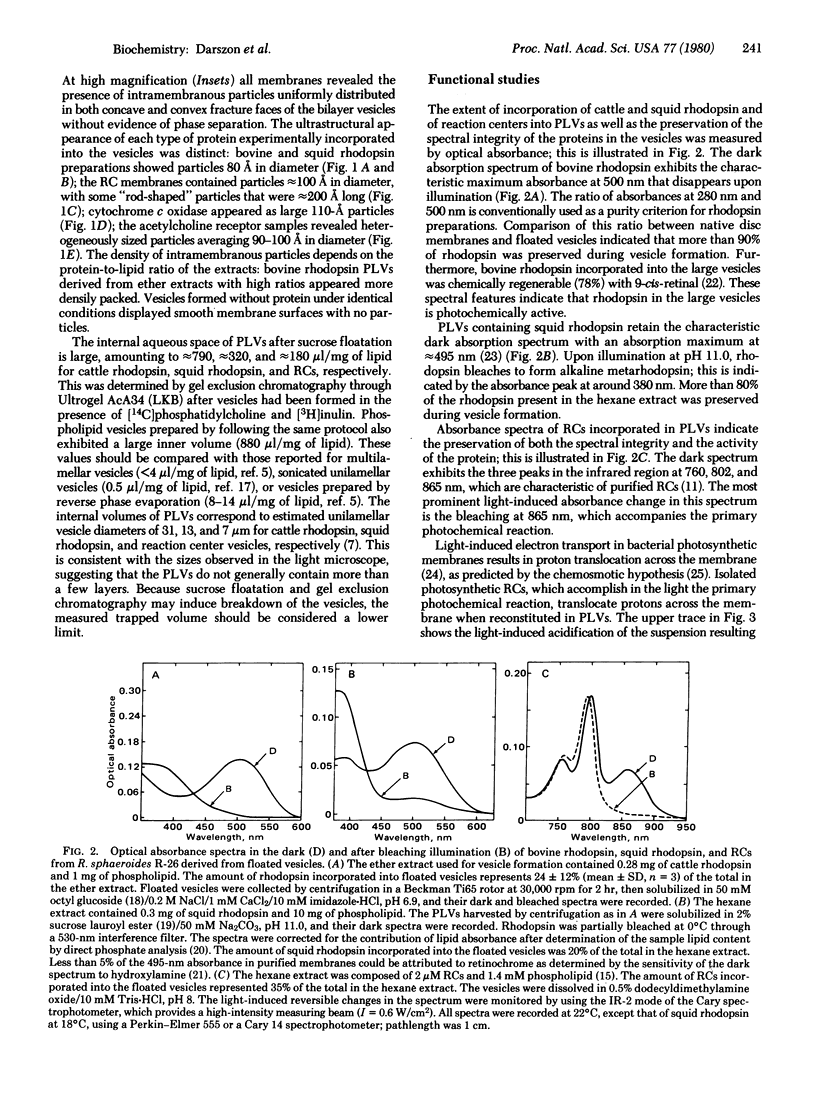
- Montal M. Experimental membranes and mechanisms of bioenergy transductions. Annu Rev Biophys Bioeng. 1976;5:119–175. doi: 10.1146/annurev.bb.05.060176.001003. [DOI] [PubMed] [Google Scholar]
- Nashima K., Mitsudo M., Kito Y. Studies on cephalopod rhodopsin. Fatty acid esters of sucrose as effective detergents. Biochim Biophys Acta. 1978 Sep 26;536(1):78–87. doi: 10.1016/0005-2795(78)90053-3. [DOI] [PubMed] [Google Scholar]
- Papahadjopoulos D., Vail W. J. Incorporation of macromolecules within large unilamellar vesicles (LUV). Ann N Y Acad Sci. 1978;308:259–267. doi: 10.1111/j.1749-6632.1978.tb22028.x. [DOI] [PubMed] [Google Scholar]
- Papermaster D. S., Dreyer W. J. Rhodopsin content in the outer segment membranes of bovine and frog retinal rods. Biochemistry. 1974 May 21;13(11):2438–2444. doi: 10.1021/bi00708a031. [DOI] [PubMed] [Google Scholar]
- Reeves J. P., Dowben R. M. Formation and properties of thin-walled phospholipid vesicles. J Cell Physiol. 1969 Feb;73(1):49–60. doi: 10.1002/jcp.1040730108. [DOI] [PubMed] [Google Scholar]
- Rudy B., Gitler C. Microviscosity of the cell membrane. Biochim Biophys Acta. 1972 Oct 23;288(1):231–236. doi: 10.1016/0005-2736(72)90242-8. [DOI] [PubMed] [Google Scholar]
- Schindler H., Rosenbusch J. P. Matrix protein from Escherichia coli outer membranes forms voltage-controlled channels in lipid bilayers. Proc Natl Acad Sci U S A. 1978 Aug;75(8):3751–3755. doi: 10.1073/pnas.75.8.3751. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schönfeld M., Montal M., Feher G. Functional reconstitution of photosynthetic reaction centers in planar lipid bilayers. Proc Natl Acad Sci U S A. 1979 Dec;76(12):6351–6355. doi: 10.1073/pnas.76.12.6351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stewart W. W. Functional connections between cells as revealed by dye-coupling with a highly fluorescent naphthalimide tracer. Cell. 1978 Jul;14(3):741–759. doi: 10.1016/0092-8674(78)90256-8. [DOI] [PubMed] [Google Scholar]
- Stubbs G. W., Litman B. J. Effect of alterations in the amphipathic microenvironment on the conformational stability of bovine opsin. 1. Mechanism of solubilization of disk membranes by the nonionic detergent, octyl glucoside. Biochemistry. 1978 Jan 24;17(2):215–219. doi: 10.1021/bi00595a003. [DOI] [PubMed] [Google Scholar]
- Szoka F., Jr, Papahadjopoulos D. Procedure for preparation of liposomes with large internal aqueous space and high capture by reverse-phase evaporation. Proc Natl Acad Sci U S A. 1978 Sep;75(9):4194–4198. doi: 10.1073/pnas.75.9.4194. [DOI] [PMC free article] [PubMed] [Google Scholar]