Abstract
Background
Recent evidence suggests patients bridged to heart transplant (BTT) have equivalent outcomes as those undergoing conventional heart transplantation (OHT). However, there are limited data on risk factors for early mortality in BTT patients.
Methods
We retrospectively reviewed the United Network for Organ Sharing (UNOS) database of all patients bridged to OHT with a HeartmateII (HMII) from 1/2005–12/2010. The primary outcome was all-cause 90-day mortality. Additional postoperative outcomes were cerebrovascular accident (CVA) and need for renal replacement therapy (RRT). Kaplan-Meier analysis assessed survival. Preoperative variables associated with 90-day mortality on univariate analysis (p<0.2) were included in a multivariable Cox proportional hazards regression.
Results
1,312 patients were bridged to OHT with a HMII. During the study, 171 (13.0%%) patients died, and the unadjusted 90-day survival was 92.3%. Average age was 52±12 years, and the most common indication for OHT was idiopathic cardiomyopathy (N=665, 50.7%). Examining center volume for BTT recipients only, the highest annual average center volume in this cohort was 28 BTT procedures per year. Twenty-nine (2.2%) patients sustained a postoperative CVA and 106 (8.3%) required RRT. Cox regression revealed age, glomerular filtration rate, African-American race, human leukocyte antigen mismatch, serum bilirubin, need for mechanical ventilation, donor age, and prolonged ischemia time were associated with 90-day mortality. There was improved early mortality for patients transplanted at high volume centers (p=0.01).
Conclusions
This is the largest modern study to examine risk factors for early mortality in patients bridged to OHT, and the first to use UNOS data. With increasing use of HMII mechanical assistance to bridge patients to OHT, these findings will aid in identifying patients best suited to benefit from this therapy.
Keywords: Heart Transplantation, UNOS, LVAD, mortality
Introduction
Left ventricular assist devices (LVAD) are increasingly being used to bridge patients to transplant (BTT). There were early concerns that BTT patients would have inferior posttransplant survival compared with conventional OHT.1, 2 However, contemporary devices have smaller profiles and improved reliability.3 In the modern era of continuous flow (CF) devices, several reports suggest that patients bridged to transplant with CF LVADs appear to have equivalent or modestly inferior post-transplant survival compared to conventional OHT.4–12 Yet, actual clinical risk factors for early mortality are not well characterized. Despite growing enthusiasm to place CF LVADs as a bridge to transplantation, given the complexity of device explant, it is important to identify patients in this particular cohort at high risk for early mortality. Therefore, we used United Network for Organ Sharing (UNOS) data to examine risk factors for early post-transplant mortality among patients bridged with CF devices.
Material and Methods
Data Source
The UNOS Standard Transplant Analysis and Research database was used, which represents an open cohort of prospectively collected donor specific and follow-up data from October 1987 to December 2010. The dataset includes all United States patients undergoing thoracic organ transplantation, with follow-up to March 2011. This study was submitted to the institutional review board and granted approval because there were no patient or center identifiers used.
Study Design
This study was a retrospective cohort design, including adult (>17 years) patients undergoing primary OHT as BTT with the Heartmate II (HM2) (Thoratec Corp., Pleasanton, CA) from 1/2005–12/2010. Although several CF LVAD devices are available, the HM2 is the most commonly used CF device in the United States and the only device with FDA approval for a BTT indication. Therefore, we elected to study this device exclusively. Patients without a VAD (n=10,019), older generation pulsatile flow LVADs (n=845), biventricular VAD (n=519), heart-lung transplantation (n=6), simultaneous kidney or liver transplantation (n=68), or prior OHT (n=34) were excluded.
Variables Examined and Outcome Measures
The dataset used contains >550 preoperative, intraoperative, and postoperative variables. Variables with greater than 15% missing data in this cohort were not examined. Variables examined in univariate analysis included: primary diagnosis; demographics (age, gender, race, education level, insurance type, body mass index), co-morbidities (hypertension, diabetes mellitus, chronic obstructive pulmonary disease, prior cardiac surgery, glomerular filtration rate) and markers of acuity (treatment in the intensive care unit, need for intra-aortic balloon pump, IMPACT risk index); hemodynamic measurements (cardiac output, mean pulmonary capillary wedge pressure, pulmonary systolic pressure); donor variables (age, gender, race, tobacco use, serum creatinine); and transplant variables (ischemic time, HLA-mismatch, CMV-mismatch). Average annual center volume (heart transplants performed in patients bridged with HM2 LVAD) was determined as well.
Overall risk was assessed according to the IMPACT risk index. The IMPACT score is a 50-point composite recipient risk index derived and cross-validated using UNOS data, and is highly predictive of 1-year mortality for adult patients receiving first time OHT.11 The risk index utilizes twelve recipient specific preoperative variables (age, gender, race, diagnosis, creatinine clearance, pre-operative dialysis, serum bilirubin, pre-operative infection, need for mechanical ventilation, intra-aortic balloon pump, temporary circulatory support, and ventricular assist device) to assign relative points out of a possible maximum of 50. Because older generation pulsatile VAD devices and continuous flow devices besides the HM2 may contribute up to 8 points, we assigned all patients their appropriate risk index, with the maximum possible points modified to 42. A histogram and test for skewness were used to assure the pattern of scores followed a relatively normal distribution. Quartiles of risk strata were then assigned according to the distribution of risk scores within the cohort.
The primary endpoint was 90-day mortality. Additionally, 1-year mortality was examined. Secondary outcomes examined were stroke, dialysis, and length of stay.
Statistical Analysis
We examined baseline characteristics among the cohort overall. Clinical characteristics were then compared between patients who survived to 90 days versus non-survivors using the student’s t-test (continuous variables) and the chi-square test (categorical variables). 90-day survival was estimated using the Kaplan-Meier method, as this time interval has adequate follow-up in this dataset. Patients were stratified into quartiles according to the IMPACT risk index, and the Mantel-Cox log rank test was used to test for a significant difference in the survival curves.
Multivariable Cox proportional hazards regression was used to estimate risk of death with censoring for death, loss to follow-up, and administrative reasons (end of study period). Two separate multivariable regressions were performed. The first model was constructed using independent covariates (composite IMPACT risk index not included). Covariates with potential for confounding were first tested in a univariate fashion. In addition to variables associated with mortality on exploratory analysis (p<0.2), previously recognized risk factors, and those with biological plausibility were incorporated in a forwards and backwards stepwise fashion into this first multivariable Cox model. The likelihood ratio test and Akaike’s information criterion in a nested model approach identified the covariates that increased the explanatory power of the model. This method for model construction favors more parsimonious models. Because the multivariable analysis was performed with case-wise deletion, all covariates with greater than 15% missing data were not included. Testing of Schoenfeld residuals and visual inspection of complementary log-log plots for each variable confirmed that the assumption of proportional hazards had not been violated.
A second Cox regression was performed using only the composite IMPACT risk index, donor age, and ischemic time. No individual recipient characteristics were included in this regression model. This second regression was performed to determine if the IMPACT risk index was associated with 90-day mortality, after adjusting for donor age and ischemic time.
For statistical analyses, p-values less than 0.05 (two-tailed) were deemed significant. Medians are presented with their interquartile range (IQR), and means are presented with standard deviations. Hazard ratios (HR) are displayed with 95% confidence intervals (CI). Statistical testing was conducted using STATA software (version 12, StataCorp LP, College Station, TX).
Results
Cohort Statistics
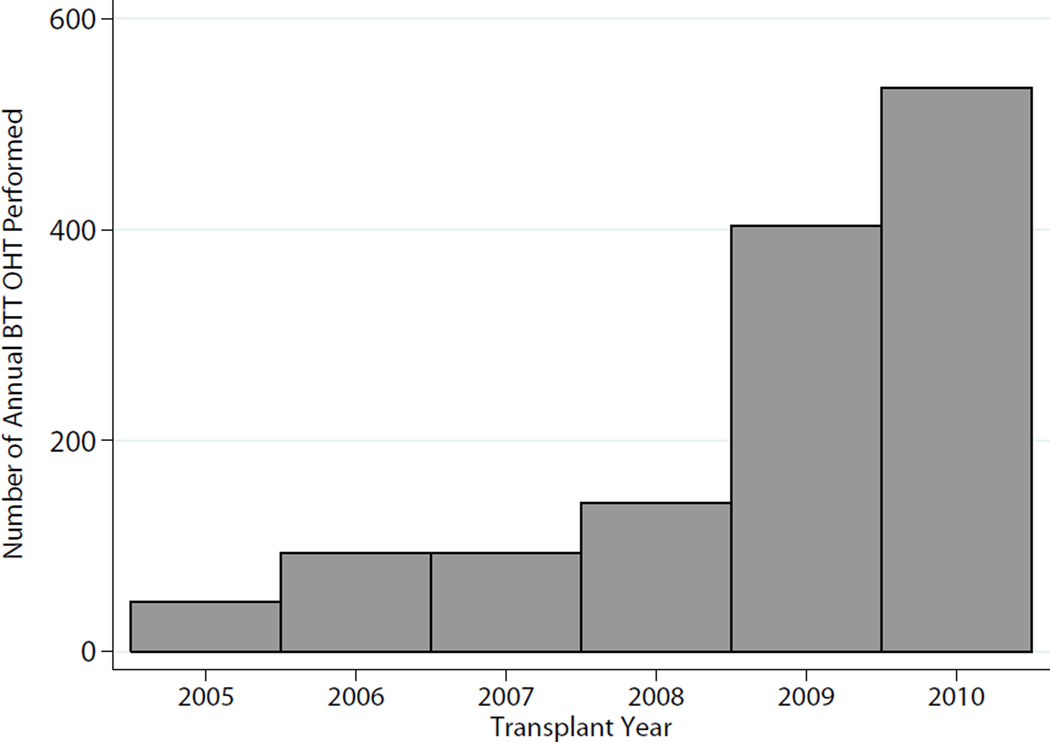
There were 1,312 OHT recipients who were bridged to transplant with a HM2, and these transplants were performed at a total of 95 unique centers. The number of BTT heart transplant recipients each year steadily increased from 47 in 2005 to 534 in 2010 (Figure 1). At the time of listing, 541 (41.2%) patients had already undergone LVAD implant, and the remaining patients (n=771,58.8%) underwent device placement in the interval between listing and transplant. Median waitlist time was 159 days (IQR:68–307), and median overall follow-up time was 11 months (IQR:4–19). During the study, 171 patients died (incidence 10.5 deaths/100 person-years), and the Kaplan-Meier cumulative incidence of 1-year mortality was 14.4% (n=150).
Figure 1.
Annual BTT performed in the United States since 2005
Average age was 52±12 years (yrs), and additional baseline characteristics are shown in Table 1. Donor race distribution was: 67.8% Caucasian (n=889); 16.8% African-American (n=221); 13.2% Hispanic (n=173); and 2.2% Other (n=29). Patients who died within 90 days tended to be older, have worse renal function, higher serum bilirubin, and require mechanical ventilation (Table 1).
Table 1.
Patient characteristics and postoperative outcomes
| Ninety Day Mortality | ||||
|---|---|---|---|---|
| Variable | Cohort Overall (N=1,312) |
Survivorsa (N=1,009) |
Non-Survivors (N=93) |
P-valueb |
| Demographics | ||||
| IMPACT score (±SD) | 4.9 (3.2) | 4.7 ± 3.1 | 6.9 ± 3.5 | <0.01 |
| Age, years (±SD) | 52.0 (12.2) | 51.7 ± 12.2 | 56.5 ± 10.3 | <0.01 |
| Female, n (%) | 238 (18.1%) | 181 (17.9%) | 19 (20.4%) | 0.5 |
| Caucasian, n (%) | 915 (69.7%) | 713 (70.7%) | 60 (64.5%) | 0.7 |
| African-American, n (%) | 280 (21.3%) | 205 (20.3%) | 22 (23.7%) | 0.6 |
| Diagnosis, n (%) | 0.07 | |||
| Idiopathic | 665 (50.7%) | 518 (51.3%) | 36 (38.7%) | |
| Ischemic | 541 (41.2%) | 418 (41.4%) | 46 (49.5%) | |
| Other | 106 (8.1%) | 73 (7.3%) | 11 (11.8%) | |
| Acuity | ||||
| HTN, n (%) | 95/230 (41.3%) | 116/202 (57.4%) | 16/24 (66.7%) | 0.4 |
| Diabetes Mellitus, n (%) | 387 (29.5%) | 703 (69.8%) | 63 (67.7%) | 0.7 |
| Creatinine Clearancec, ml/min (±SD) | 74.1 (26.4) | 75.0 ± 26.2 | 61.5 ± 23.5 | <0.01 |
| Serum bilirubin, mg/ml (±SD) | 1.1 (2.2) | 1.0 ± 1.7 | 2.4 ± 4.9 | <0.01 |
| Preop Mechanical Ventilation, n (%) | 14 (1.1%) | 7 (0.7%) | 5 (5.4%) | <0.01 |
| IABP, n (%) | 22 (1.7%) | 21 (2.1%) | 1 (1.1%) | 0.5 |
| Donor characteristics | ||||
| Age, years | 31.2 (11.2) | 30.9 (11.2) | 34.8 (11.4) | <0.01 |
| Creatinine Clearance, ml/min (±SD) | 82.7 (39.5) | 81.6 (34.7) | 77.7 (30.6) | 0.4 |
| Tobacco use, n (%) | 230 (17.7%) | 174 (17.4%) | 21 (22.8%) | 0.2 |
| Ischemic time, hours (±SD) | 3.3 (1.1) | 3.3 (1.1) | 3.5 (1.0) | 0.07 |
| Postoperative Outcomes | ||||
| Length of stay, days | 15 (IQR: 11–22) | 15 (IQR:11–22) | 12 (IQR: 4–41) | 0.02 |
| Stroke, n (%) | 29 (2.2%) | 19 (1.9%) | 9 (10.0%) | <0.01 |
| New onset dialysis, n (%) | 106 (8.3%) | 52 (5.3%) | 41 (46.6%) | <0.01 |
| Treated rejection in first year, n (%) | 158/669 (23.6%) | -- | -- | -- |
Patients with less than 90 day follow-up time included in Cohort overall column and time-to-event (Kaplan-Meier and Cox regression) analysis, but omitted from 90-day mortality comparisons in this table.
P-value based on comparisons between 90-day non-survivors and patients who survived to 90-days. Student’s t-test used for normally distributed continuous data, and Wilcoxon rank sum used for non-parametric continuous data. Chi-square analysis used for categorical data.
Based on Cockcroft-Gault Calculation
Abbreviations: hrs=hours; HTN=hypertension; IABP=intra-aortic balloon pump; IMPACT=index for mortality prediction after cardiac transplantation; PVR=pulmonary vascular resistance.
IMPACT Risk Score Generation
After assigning all patients their respective IMPACT risk score index, the average score for the entire cohort was 4.9±3.2 (range: 0–22). When divided into quartiles (Q), the breakdown of scores was: Q1,0 to <3 (n=308,23.5%); Q2, ≥3 to <5 (n=320, 24.4%); Q3, ≥5 to <7 (n=315,24.0%); and Q4, ≥9 (n=369,28.1%). There were 64 (4.9%) patients with a risk score greater than 10.
Risk-Adjusted Cox Regression and Survival Analysis
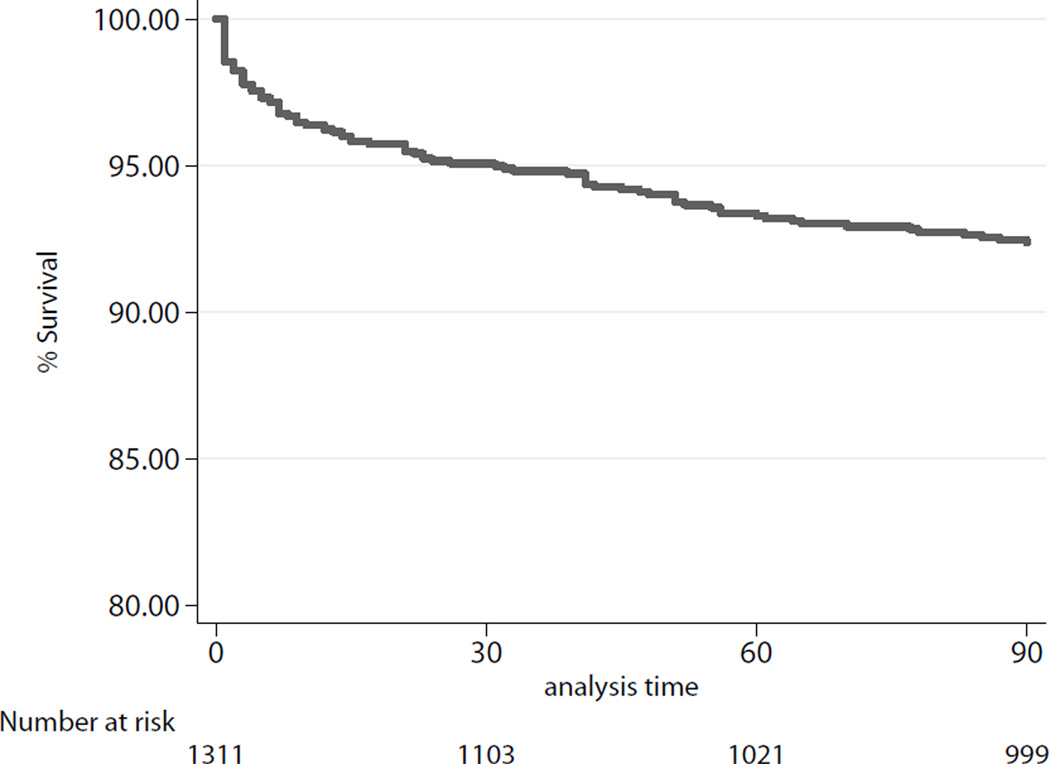
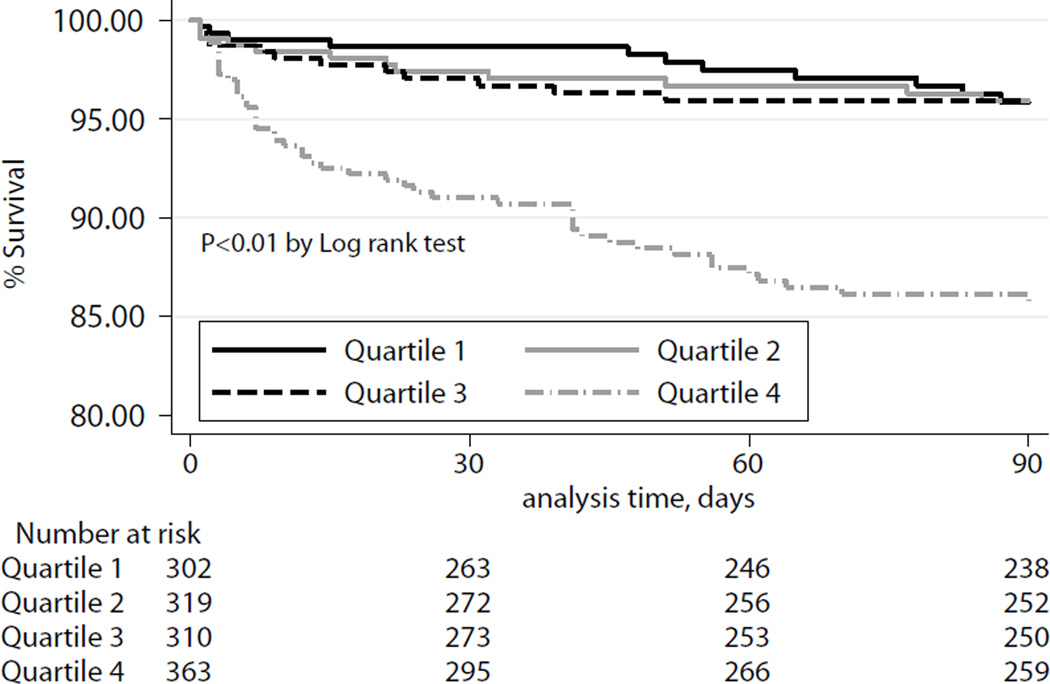
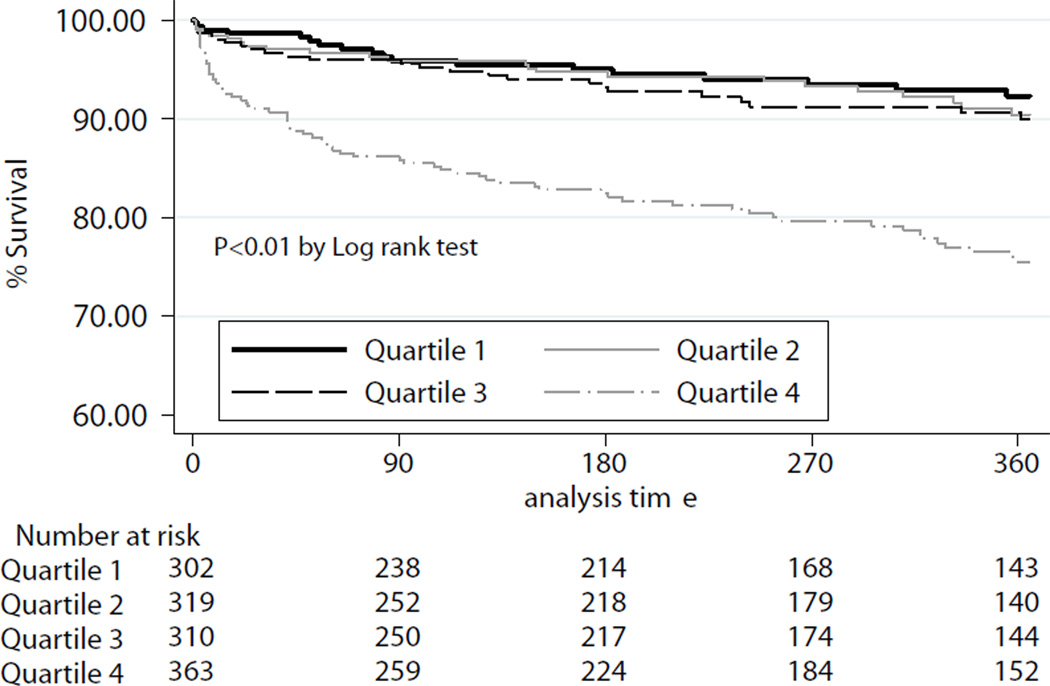
At ninety days, the unadjusted observed survival was 92.3%. Observed 1-year survival was 85.6%, unadjusted for recipient risk. Ninety day survival for the cohort overall is depicted in Figure 2. Ninety-day survival and 1-year survival stratified by quartiles of IMPACT recipient risk index are shown in Figure 3a–b.
Figure 2.
Kaplan-Meier Estimates of 90-day survival, cohort overall.
Figure 3.
a: Kaplan-Meier Estimates of 90-day survival, stratified by quartiles of IMPACT risk index. P-value determined according to Cox-Mantel log rank test.
b: Kaplan-Meier Estimates of 1-year survival, stratified by quartiles of IMPACT risk index. P-value determined according to Cox-Mantel log rank test.
Following risk adjustment with Cox multivariable analysis, transplant year was not associated with 90-day mortality. Age greater than 60, African-American race, lower glomerular filtration rate, higher serum bilirubin, preoperative recipient mechanical ventilation, donor age, and prolonged allograft ischemic time were all associated with an increased hazard of death (Table 2). Regarding center volume, higher average annual BTT heart transplant procedure volume was associated with lower risk of death.
Table 2.
Results of Cox Regression Analysis
| Variable | Hazard Ratio | 95% CI | P-value |
|---|---|---|---|
| Age >60 years | 1.70 | 1.01–2.85 | 0.05 |
| GFR <60, ml/min | 1.87 | 1.15–3.06 | 0.01 |
| Infection requiring intravenous antibiotics | 1.14 | 0.62–2.08 | 0.7 |
| Serum bilirubin > 4 mg/ml | 2.55 | 1.08–6.30 | 0.02 |
| Mechanical ventilation pre-op | 4.91 | 1.67–14.43 | 0.004 |
| HLA mismatch | 0.49 | 0.30–0.81 | 0.005 |
| Donor age | 1.02 | 1.01–1.04 | 0.03 |
| Ischemic time > 4 hours | 1.90 | 1.16–3.12 | 0.01 |
| Average annual center volume | 0.92 | 0.87–0.98 | 0.01 |
| Race | |||
| Caucasian | --- | Reference | --- |
| African-American | 2.24 | 1.23–4.07 | 0.008 |
| Hispanic | 2.60 | 1.13–5.97 | 0.02 |
| Indication | |||
| Idiopathic CM | --- | Reference | ---- |
| Ischemic | 1.48 | 0.85–2.57 | 0.2 |
CM=cardiomyopathy; HLA=human leukocyte antigen
Regression included transplant year as continuous variable to adjust for time trend.
In a separate Cox regression incorporating the IMPACT risk index, each one point increase in the IMPACT risk score was associated with a 16% increase in the hazard of 90-day mortality, after adjusting for allograft ischemic time and donor age (Table 3).
Table 3.
Adjusted Cox Regression using IMPACT risk index
| Variable | Hazard Ratio | 95% CI | P-value |
|---|---|---|---|
| IMPACT risk index, per 1 point increase | 1.16 | 1.11–1.22 | <0.001 |
| Donor age, years | 1.02 | 1.01–1.03 | 0.03 |
| Ischemic time ≥ 4 hours | 1.67 | 1.06–2.64 | 0.03 |
IMPACT=index for mortality prediction after cardiac transplantation
Postoperative Complications
Commonly encountered postoperative complications are shown at the bottom of Table 1 for the cohort overall. Median length of stay was 15 days (IQR:11–22). When examining patients stratified by 90-day survivors versus non-survivors, patients who died prior to discharge were more likely to require RRT or have sustained a postoperative stroke (Table 1).
Comment
Since 2005, over 1,300 patients are recorded in the UNOS database as having been bridged to OHT with the HM2 device, with a steady increase each year of the study. Extensive health care resources are committed to patients with end stage heart failure requiring LVAD support and ultimately cardiac transplantation. Given the scarcity of donor organs and the complexity of the device explant procedure, it is essential to optimize the odds of a successful operation.
Several clinical risk factors were identified that may aid in identifying BTT patients at risk for poor outcomes following transplantation. We do not intend for these results to be exclusionary and preclude access to OHT for high-risk patients. Rather, these results will facilitate individual transplant programs in establishing a risk profile of BTT patients that they are willing to assume. While we surmise that our findings are generally applicable to HM2 patients, there is the caveat that these observations may not be reproducible in patients with pulsatile flow devices or different models of continuous flow LVADs.
Multivariable Analysis
As center volume has been shown to influence outcomes in previous reports, we also incorporated center volume in our analysis.13, 14 Consistent with these reports, after risk-adjustment higher center volume was independently associated with a lower hazard of 90-day mortality. We speculate that cardiac transplant centers with more experience dealing with LVAD patients are more aware of the myriad complications and complexities that arise in the course of LVAD therapy, thereby explaining this observation. Furthermore, centers with a large number of BTT patients may have greater infrastructure in place, especially with respect to LVAD coordinators and support personnel who are invaluable to these patients’ care, both pre and post-OHT.
The independent effect of preoperative renal dysfunction on outcomes after cardiac surgery has been shown previously for a wide array of cardiac procedures.15, 16 Preoperative renal function is an important predictor of poorer 90-day post-OHT survival in BTT patients, especially given recent evidence that renal function improves during LVAD therapy.17 Another possible use of these data relates to the clinical decision-making regarding simultaneous kidney transplantation. Given the magnitude with which impaired renal function affects 90-day mortality in this patient population, BTT patients with depressed GFR should be given consideration for simultaneous kidney transplantation. However, this study was not designed to delineate a GFR cut-point to recommend simultaneous kidney transplant, and further study is needed to better define this issue.
Past studies have not observed this relationship associated with HLA-mismatch, however different time periods and outcome measures have been used.18 An earlier study by Taylor et al. proposed a differential role for HLA-matching in short and longer-term patient survival, with HLA-A matched grafts having worse outcomes.19 Furthermore, we recently examined panel reactive antibody levels in BTT patients and found that highly sensitized patients experienced equivalent post-OHT outcomes.20 The finding in the current study may reflect an artifact of the study period we chose to include or the main outcome measure of 90-day survival. Future studies are needed to corroborate this finding.
Although the IMPACT risk index was initially devised to predict 1-year mortality, we tested the ability of this risk index to independently predict 90-day mortality in this cohort of patients.11 Each 1-point increase in the risk index was associated with a 16% increase in the hazard of 90-day mortality. It is important to note that the continuous flow HM2 LVAD did not add any points in the original description of this risk index.
Previous Work
In 2009, Patlolla et al. used the UNOS database to compare BTT outcomes with those of conventional OHT, and reported worse survival rates for BTT patients.2 Their study may have been limited by incomplete LVAD data, however. In contrast, Russo and colleagues examined the UNOS database from 2001–2006 to determine if patients bridged to OHT had worse outcomes than patients undergoing conventional OHT.9 They concluded that patients with intracorporeal and paracorporeal devices have equivalent post-transplant survival, although patients with extracorporeal devices had worse early mortality. However, their study did not focus on risk factors for poor outcomes in the specific subset of Heartmate II patients. Nativi et al. reached similar conclusions as Russo and colleagues using the International Society of Heart and Lung Transplantation registry to examine the same issue in 8,557 patients worldwide.7 Importantly, they also observed similar rejection rates between BTT patients and those undergoing conventional OHT, dispelling concerns about sensitization in BTT patients.
More recently, John et al. concluded that post-transplant survival is not influenced by duration of LVAD support.8 Their study is strengthened by the robust HM2 dataset and prospective data collection methodology in 250 BTT patients who underwent OHT. They also observed a trend toward worse 1-year post-OHT survival in patients with percutaneous driveline infections during LVAD support (p=0.07). We observed a similar trend on univariate analysis, but in our study this finding did not persist on multivariable analysis.
Limitations
Due to the retrospective study design, we are unable to certify that all potential confounders have been identified and examined. The UNOS dataset is composed of many variables available for analysis, however the possibility remains that potentially influential variables are not included in this analysis. Furthermore, large registry databases such as UNOS depend heavily on accurate and honest coding. In this study, we have assumed that all coding errors or missing data in the database are random and are thus unlikely to render any bias. If this assumption is incorrect, our conclusions may be influenced by residual bias. To lessen the effects of missing data, we did not incorporate variables with >15% missing data into the regression analysis. Furthermore, the duration of pre-OHT LVAD support is thought to be important, and this information is not encoded in the database. We advocate inclusion of this information in large national registry datasets in order to further address this important issue. Additionally, mortality during the pre-OHT support period is unknown, and should be incorporated into future investigations.
This study is further limited by the relatively short follow-up period. We elected to begin the study in 2005, as prior to then there were few HM2 BTT patients in the UNOS registry. As the collective experience with the HM2 device accumulates, it will be important to understand how these risk factors may change over time.
Conclusions
This is the largest modern study to examine risk factors for early mortality in patients bridged to OHT with the Heartmate II continuous flow device, and the first to use UNOS data. With increasing use of HM2 mechanical assistance to bridge patients to OHT, these findings will aid in identifying patients best suited to benefit from this therapy.
Acknowledgements
Drs. Arnaoutakis, Weiss, and Beaty are Irene Piccinini Investigators in Cardiac Surgery. Dr. George is the Hugh R. Sharp Cardiac Surgery Research Fellow. This research was supported in part by the National Institutes of Health Grant 1T32CA126607-01A2 (GJA), and also supported by a Health Resources and Services Administration contract 231-00-0115. The content is the responsibility of the authors alone and does not necessarily reflect the views or policies of the Department of Health and Human Services, nor does mention of trade names, commercial products, or organizations imply endorsement by the U.S. government.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Presented at: 58th Annual Meeting of the Southern Thoracic Surgical Association, November 11, 2011, San Antonio, TX.
References
- 1.Taylor DO, Edwards LB, Aurora P, Christie JD, Dobbels F, Kirk R, et al. Registry of the International Society for Heart and Lung Transplantation: twenty-fifth official adult heart transplant report--2008. J Heart Lung Transplant. 2008 Sep;27(9):943–956. doi: 10.1016/j.healun.2008.06.017. [DOI] [PubMed] [Google Scholar]
- 2.Patlolla V, Patten RD, Denofrio D, Konstam MA, Krishnamani R. The effect of ventricular assist devices on post-transplant mortality an analysis of the United network for organ sharing thoracic registry. J Am Coll Cardiol. 2009 Jan 20;53(3):264–271. doi: 10.1016/j.jacc.2008.08.070. [DOI] [PubMed] [Google Scholar]
- 3.Caccamo M, Eckman P, John R. Current state of ventricular assist devices. Curr Heart Fail Rep. 2011 Jun;8(2):91–98. doi: 10.1007/s11897-011-0050-z. [DOI] [PubMed] [Google Scholar]
- 4.Drakos SG, Kfoury AG, Long JW, Stringham JC, Gilbert EM, Moore SA, et al. Effect of mechanical circulatory support on outcomes after heart transplantation. J Heart Lung Transplant. 2006 Jan;25(1):22–28. doi: 10.1016/j.healun.2005.07.014. [DOI] [PubMed] [Google Scholar]
- 5.Pal JD, Piacentino V, Cuevas AD, Depp T, Daneshmand MA, Hernandez AF, et al. Impact of left ventricular assist device bridging on posttransplant outcomes. Ann Thorac Surg. 2009 Nov;88(5):1457–1461. doi: 10.1016/j.athoracsur.2009.07.021. discussion 61. [DOI] [PubMed] [Google Scholar]
- 6.Williams ML, Trivedi JR, McCants KC, Prabhu SD, Birks EJ, Oliver L, et al. Heart transplant vs left ventricular assist device in heart transplant-eligible patients. Ann Thorac Surg. 2011 May;91(5):1330–1333. doi: 10.1016/j.athoracsur.2011.01.062. discussion 3-4. [DOI] [PubMed] [Google Scholar]
- 7.Nativi JN, Drakos SG, Kucheryavaya AY, Edwards LB, Selzman CH, Taylor DO, et al. Changing outcomes in patients bridged to heart transplantation with continuous-versus pulsatileflow ventricular assist devices: an analysis of the registry of the International Society for Heart and Lung Transplantation. J Heart Lung Transplant. 2011 Aug;30(8):854–861. doi: 10.1016/j.healun.2011.03.019. [DOI] [PubMed] [Google Scholar]
- 8.John R, Pagani FD, Naka Y, Boyle A, Conte JV, Russell SD, et al. Post-cardiac transplant survival after support with a continuous-flow left ventricular assist device: impact of duration of left ventricular assist device support and other variables. J Thorac Cardiovasc Surg. 2010 Jul;140(1):174–181. doi: 10.1016/j.jtcvs.2010.03.037. [DOI] [PubMed] [Google Scholar]
- 9.Russo MJ, Hong KN, Davies RR, Chen JM, Sorabella RA, Ascheim DD, et al. Posttransplant survival is not diminished in heart transplant recipients bridged with implantable left ventricular assist devices. J Thorac Cardiovasc Surg. 2009 Dec;138(6):1425, 1432. doi: 10.1016/j.jtcvs.2009.07.034. e1-3. [DOI] [PubMed] [Google Scholar]
- 10.Smedira NG, Hoercher KJ, Yoon DY, Rajeswaran J, Klingman L, Starling RC, et al. Bridge to transplant experience: factors influencing survival to and after cardiac transplant. J Thorac Cardiovasc Surg. 2010 May;139(5):1295–1305. 305 e1–305 e4. doi: 10.1016/j.jtcvs.2009.12.006. [DOI] [PubMed] [Google Scholar]
- 11.Weiss ES, Allen JG, Arnaoutakis GJ, George TJ, Russell SD, Shah AS, et al. Creation of a quantitative recipient risk index for mortality prediction after cardiac transplantation (IMPACT) Ann Thorac Surg. 2011 Sep;92(3):914–921. doi: 10.1016/j.athoracsur.2011.04.030. discussion 21-2. [DOI] [PubMed] [Google Scholar]
- 12.Urban M, Pirk J, Dorazilova Z, Netuka I. How does successful bridging with ventricular assist device affect cardiac transplantation outcome? Interact Cardiovasc Thorac Surg. 2011 Oct;13(4):405–409. doi: 10.1510/icvts.2011.273722. [DOI] [PubMed] [Google Scholar]
- 13.Russo MJ, Iribarne A, Easterwood R, Ibrahimiye AN, Davies R, Hong KN, et al. Post-heart transplant survival is inferior at low-volume centers across all risk strata. Circulation. 2010 Sep 14;122(11 Suppl):S85–S91. doi: 10.1161/CIRCULATIONAHA.109.926659. [DOI] [PubMed] [Google Scholar]
- 14.Weiss ES, Meguid RA, Patel ND, Russell SD, Shah AS, Baumgartner WA, et al. Increased mortality at low-volume orthotopic heart transplantation centers: should current standards change? Ann Thorac Surg. 2008 Oct;86(4):1250–1259. doi: 10.1016/j.athoracsur.2008.06.071. discussion 9-60. [DOI] [PubMed] [Google Scholar]
- 15.Arnaoutakis GJ, Bihorac A, Martin TD, Hess PJ, Jr, Klodell CT, Ejaz AA, et al. RIFLE criteria for acute kidney injury in aortic arch surgery. J Thorac Cardiovasc Surg. 2007 Dec;134(6):1554–1560. doi: 10.1016/j.jtcvs.2007.08.039. discussion 60-1. [DOI] [PubMed] [Google Scholar]
- 16.Chertow GM, Levy EM, Hammermeister KE, Grover F, Daley J. Independent association between acute renal failure and mortality following cardiac surgery. Am J Med. 1998 Apr;104(4):343–348. doi: 10.1016/s0002-9343(98)00058-8. [DOI] [PubMed] [Google Scholar]
- 17.Russell SD, Rogers JG, Milano CA, Dyke DB, Pagani FD, Aranda JM, et al. Renal and hepatic function improve in advanced heart failure patients during continuous-flow support with the HeartMate II left ventricular assist device. Circulation. 2009 Dec 8;120(23):2352–2357. doi: 10.1161/CIRCULATIONAHA.108.814863. [DOI] [PubMed] [Google Scholar]
- 18.Nwakanma LU, Williams JA, Weiss ES, Russell SD, Baumgartner WA, Conte JV. Influence of pretransplant panel-reactive antibody on outcomes in 8,160 heart transplant recipients in recent era. Ann Thorac Surg. 2007 Nov;84(5):1556–1562. doi: 10.1016/j.athoracsur.2007.05.095. discussion 62-3. [DOI] [PubMed] [Google Scholar]
- 19.Taylor CJ, Smith SI, Sharples LD, Parameshwar J, Cary NR, Keogan M, et al. Human leukocyte antigen compatibility in heart transplantation: evidence for a differential role of HLA matching on short- and medium-term patient survival. Transplantation. 1997 May 15;63(9):1346–1351. doi: 10.1097/00007890-199705150-00024. [DOI] [PubMed] [Google Scholar]
- 20.Arnaoutakis GJ, George TJ, Kilic A, Weiss ES, Russell SD, Conte JV, et al. Effect of sensitization in US heart transplant recipients bridged with a ventricular assist device: Update in a modern cohort. J Thorac Cardiovasc Surg. 2011 Nov;142(5):1236–1245. doi: 10.1016/j.jtcvs.2011.07.019. e1. [DOI] [PMC free article] [PubMed] [Google Scholar]