Abstract
Beyond their well-established role as triggers for LTP and LTD of fast synaptic transmission mediated by AMPA receptors, an expanding body of evidence indicates that NMDA receptors (NMDARs) themselves are also dynamically regulated and subject to activity-dependent long-term plasticity. NMDARs can significantly contribute to information transfer at synapses particularly during periods of repetitive activity. It is also increasingly recognized that NMDARs participate in dendritic synaptic integration and are critical for generating persistent activity of neural assemblies. Here we review recent advances on the mechanisms and functional consequences of NMDAR plasticity. Given the unique biophysical properties of NMDARs, synaptic plasticity of NMDAR-mediated transmission emerges as a particularly powerful mechanism for the fine tuning of information encoding and storage throughout the brain.
Keywords: LTP, LTD, metaplasticity, glutamate, metabotropic receptor, GluN2A, GluN2B, subunit switch, calcium signaling, receptor trafficking, receptor lateral mobility, bursting, burst firing, integrative functions
INTRODUCTION
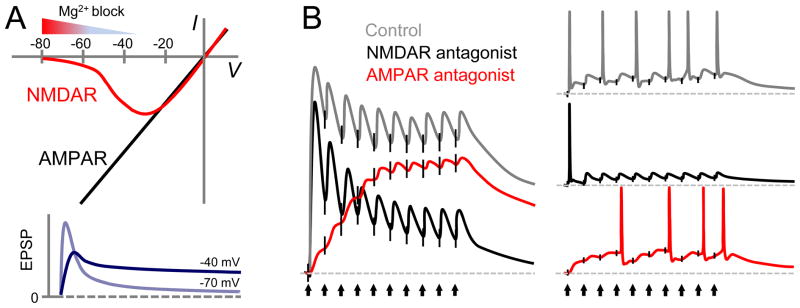
A central task in contemporary neuroscience is to identify the cellular and molecular mechanisms underlying cognitive brain functions, and how alterations of these mechanisms can lead to neuropsychiatric disease states. Several decades of intense research indicate that activity-dependent changes in excitatory synaptic efficacy such as long-term potentiation (LTP) and long-term depression (LTD) likely are cellular correlates to learning and memory [1–4]. In the vertebrate central nervous system the predominant mode of excitatory transmission is mediated by the neurotransmitter glutamate and the ionotropic glutamate receptors [5]. While α-amino-3-hydroxy-5-methyl-4-isoxazolepropionic acid receptors (AMPARs) support fast excitatory transmission at most synapses, N-methyl-D-aspartate receptors (NMDARs) have classically been thought of as coincidence detectors (i.e. coincidence of glutamate release and postsynaptic depolarization) for the induction of long-term plasticity expressed as changes in AMPAR-mediated transmission [6]. According to this view, changes in NMDAR function or expression can effectively modify the induction threshold for AMPAR-mediated plasticity, a phenomenon commonly known as metaplasticity [7]. While classical NMDAR-dependent LTP/LTD of AMPAR-mediated transmission and NMDAR-dependent metaplasticity are important, when considered alone, these dynamic actions of NMDARs constrain their functional capacity to that of simply regulating the induction of AMPAR-mediated plasticity. However, NMDARs can play a much broader functional role in basal synaptic transmission [8], particularly under certain physiological conditions (such as during bursting activity) [9], as well as contribute to the integrative properties of neurons [10]. The integrative functions of synaptic NMDARs stem from the unique nonlinear amplification properties endowed by the Mg2+ block of NMDARs at resting potential, their high permeability to Ca2+, and the slow kinetics of NMDAR-EPSPs, which allow for temporal summation (Fig. 1) [5,11–13].
Figure 1. Unique biophysical properties of NMDARs and the resultant functional impact on temporal summation and firing output.
A) Top; current-voltage relationship comparison between AMPARs and NMDARs. NMDARs exhibit a characteristic region of negative slope from approximately −70 to −35 mV, a property that enables positive feedback, and allows for signal amplification. Bottom; Dual (AMPAR and NMDAR) component EPSPs elicited at −70 and −40 mV membrane potential, highlighting the enhancement of the slow NMDAR component at −40 mV, whereas the fast AMPAR component is diminished. B) Left; Representation of temporal summation of dual (gray) and pharmacologically isolated AMPAR (black) and NMDAR (red) EPSPs elicited by a train of subthreshold synaptic stimulation (vertical black arrows). The relative difference between the AMPAR and NMDAR-mediated responses likely reflects both voltage-dependent Mg2+ block and slow decay kinetics. Right; Suprathreshold repetitive stimulation illustrating the contribution of NMDARs (red) to spike output relative to AMPARs (black). The temporal summation afforded by NMDARs leads to more reliable spike output throughout the train. Modified with permission from Augustinaite & Heggelund, J Physiol 582: 297–315, 2007.
Extensive work, mainly using heterologous expression systems or cultured neurons, has been dedicated to elucidating the molecular mechanisms underlying modulation of NMDAR function and trafficking (for reviews see [14–18]). These studies have shown that a vast array of kinases, phosphatases, receptor-coupled signaling molecules, and intracellular signals can regulate NMDARs under various experimental conditions. However, what modes of regulation are engaged in more intact preparations under more physiological conditions remain a matter of active research. While originally thought to be less dynamic than their AMPAR counterparts [17], increasing evidence demonstrates that NMDARs themselves can be regulated in an activity-dependent manner, and both LTP and LTD of NMDAR-mediated transmission have been reported in several brain areas [15,19] (Table 1). In this review we will focus on recent work in which activity-dependent, long-term plasticity of NMDAR-mediated transmission has been identified and characterized mainly using acute brain slices. We will discuss the properties and molecular mechanisms underlying NMDAR-LTP/LTD throughout the brain (Fig. 2), and where appropriate, attempt to place these forms of plasticity in a physiological context.
Table 1.
Activity-dependent NMDAR-LTP/LTD in the brain*
| Brain area (Synapse type) | Induction protocol | Requirements/Properties | References | |
|---|---|---|---|---|
| LTP | Hippocampus (Sch-CA1) | 20–25 stimuli, 100 Hz | NMDAR activation | [20] |
| (100 stimuli, 100 Hz)x2 | NMDAR activation | [21] | ||
| 10 stimuli, 50 Hz, 0.1 mM (Mg2+)e; 10–20 stimuli, 50 Hz, x2 stim strength; 2.0 mM (Mg2+)e | [23] | |||
| TBS (10 trains at 5Hz, each train consisting of 4 stimuli, 100Hz); 0.05–0.2 mM (Mg2+)e | [24–25] | |||
| 2 trains at 10 intervals, each train consisting of 100 stimuli at 100 Hz. | mGluR5 activation | [41] | ||
| 6 trains at 20 s intervals, each train consisting of 7 stimuli at 200 Hz; depolarization to −45 mV | [100] | |||
| 5 trains at 10 intervals, each train consisting of 20 stimuli at 100 Hz, repeated four times during a period of 10 min; or single train of 20 stimuli at 50 Hz; 0.1 mM (Mg2+)e | [26] | |||
| 2–5 trains at 20 s intervals, each train consisting of 30 stimuli at30 Hz | [27] | |||
| 3 trains at 20 s intervals, each train consisting of 100 stimuli at 100Hz; 0.3 mM (Mg2+)e | [22] | |||
| 4 trains at 30 s intervals, each train consisting of 100 stimuli at 100 Hz; | PKC and Src | [45] | ||
| Pairing-protocol, 2 Hz repetitive stimulation for 90 s delivered at 0 mV | Subunit switch | [53] | ||
| Pairing-protocol, 1 Hz repetitive stimulation for 90 s delivered at 0 mV | NMDAR activation, postsynaptic Ca2+ rise, PKC and Src | [46] | ||
| Pairing-protocol, 200 stimuli at 2Hz delivered at 0 mV with a 2.5-min depolarization to 0 mV prior to stimulation; 0.25 mM (Mg2+)e | Postsynaptic Ca2+ rise, NMDAR and mGluR5 co-activation, PKC, SNARE complex, actin stabilization, incorporation of GluN2A-containing receptors | [36] | ||
| Hippocampus (Mossy fiber-CA3) | Single train of 25 stimuli at 25 Hz | Postsynaptic Ca2+ rise, mGluR5 and NMDAR co-activation, PKC, SNARE complex | [38] | |
| Single train of 60 stimuli at 50 Hz | Postsynaptic Ca2+ rise, A2AR mGluR5, NMDAR activation, Src | [39] | ||
| Dentate Gyrus (PP-DGC) | 4 trains at 5 s intervals, each train consisting of 20 stimuli at 10 or 50 Hz | Postsynaptic Ca2+ rise | [28] | |
| 8 trains at 2 s intervals, each train consisting of 8 stimuli at 200 Hz | Postsynaptic Ca2+ rise, mGluR5 and NMDAR activation, PKC | [29–30] [42,47] |
||
| Amygdala (Glutamatergic inputs to basolateral amygdala) | 2 trains at 20 s intervals, each train consisting of 100 stimuli at 100 Hz. | NMDAR activation | [101] | |
| Visual cortex (L5-L5 pyramidal neurons) | Pairing 200 ms pre- and postsynaptic current injections (current clamp mode) 30 times at 0.1–0.2 Hz; 0.5 mM (Mg2+)e; pharmacological blockade of CB1 receptors. | Requires previous AMPAR-LTP (at least in visual cortical cultures). | [35] | |
| Midbrain Dopamine Neurons (Glutamatergic inputs onto SNc and VTA neurons) | 10 trains at 20 s intervals, each train consisting of 70 stimuli at 50 Hz and paired with a burst of five postsynaptic unclamped APs at 20 Hz, pre ->post, 0.1 mM (Mg2+)e | Postsynaptic Ca2+ rise, Ca2+ release from internal stores, NMDAR activation, IP3 signaling, PKA, | [40] | |
| LTD | Hippocampus (Sch-CA1) | Low-frequency stimulation (1Hz, 5 min) paired with 500 ms depolarization steps and cell firing (2–6 action potentials) | NMDAR activation | [50] |
| 2–5 trains at 20 s intervals, each train consisting of 5 stimuli at 5 Hz | mGluR activation | [27] | ||
| Low-frequency stimulation (2 Hz, 10 min); 0.1 mM (Mg2+)e | [26] | |||
| Low-frequency stimulation (1 Hz, 5–7 min); 6 trains at 120 s intervals, each train consisting of 100 stimuli at 10 Hz. | Postsynaptic Ca2+ rise, NMDAR activation | [34] | ||
| Single train of 900 stimuli at 5 Hz delivered at −40 mV | Postsynatpic Ca2+ rise, protein phosphatase 1, actin depolymerization | [51–52] | ||
| Pairing-protocol, 600 stimuli at 1 Hz delivered at −40 mV. | [53] | |||
| Paired-pulse low-frequency stimulation [PP-LFS, 200 paired-pulses (50-ms interval) at 1 Hz] delivered at −40 mV | mAChR activation, postsynaptic Ca2+ rise from IP3-sensitive intracellular stores, hippocalcin, dynamin activity | [54] | ||
| Single train of 900 stimuli at 5 Hz delivered at −40 mV | Postsynaptic Ca2+ rise, NMDAR and mGluR5 co-activation, actin destabilization, incorporation of GluN2B-containing receptors | [36] | ||
| 4 trains at 10 s intervals, each train consisting of 13 glutamate pulses (single dendritic spine 2 photon laser uncaging) at 3.3 Hz | [60] | |||
| Hippocampus (CA3-CA3) | Low-frequency stimulation (1 Hz, 10 min) paired with depolarization to ~ −50mV | NMDAR activation, dynamin activity | [55–56] | |
| Dentate Gyrus (PP-DGC) | Low-frequency stimulation (1Hz, 15 min) | Postsynaptic Ca2+ rise, mGluR5 activation | [42] | |
| 4 trains at 5 s intervals, each train consisting of 20 stimuli at 10 Hz; hyperpolarization to −100 mV | Postsynaptic Ca2+ rise | [28] | ||
| Midbrain Dopamine Neurons (Glutamatergic inputs onto SNc and VTA neurons) | 10 trains at 20 s intervals, each train consisting of 70 stimuli at 50 Hz and paired with a burst of five postsynaptic unclamped APs at 20 Hz, post->pre, 0.1 mM (Mg2+)e, | [40] | ||
| Nucleus Accumbens (Glutamatergic inputs onto NAc neurons) | 1–3 trains at 10 s intervals, each train consisting of 100 stimuli at 100 Hz; pairing-protocol 120 stimuli at 2 Hz delivered at ~ − 40 mV. | Postsynaptic Ca2+ rise, GluN2A-containing NMDARs | [102–103] |
The table includes examples of NMDAR-LTP/LTD in acute brain slices from rodents (rat and mouse), except for one example using rabbit [42].
Abbreviations: TBS, theta-burst stimulation; (Mg2+)e, extracellular magnesium concentration PP, perforant path; DGC, dentate granule cell; STDP, spike timing-dependent plasticity; VGCC, voltage-gated calcium channel; mGluR, metabotropic glutamate receptor; mAChR, muscarinic acetylcholine receptor; NAc, Nucleus Accumbens; Sch, Schaffer Collateral; SNc, Substantia Nigra par compacta; VTA, Ventral tegmental area.
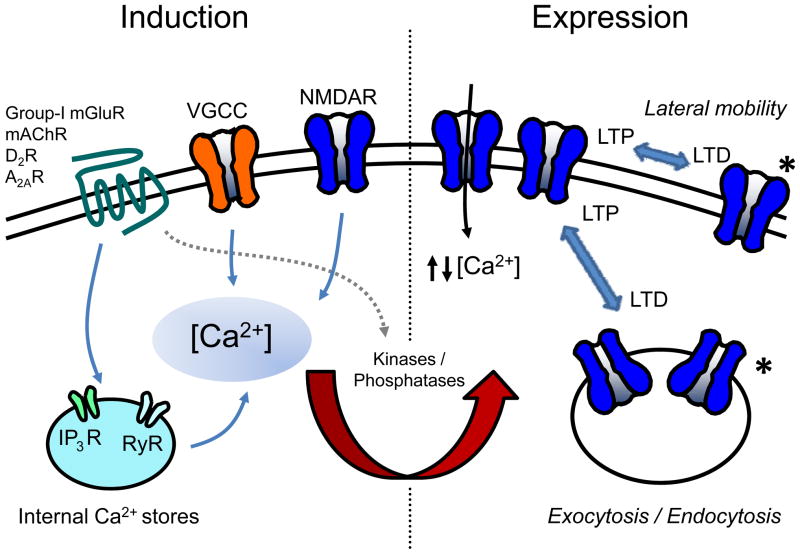
Figure 2. Common pathways of NMDAR plasticity.
Left: Induction of NMDAR plasticity has been shown to be triggered by postsynaptic Ca2+ rise that can be achieved through NMDARs and voltage gated calcium channels (VGCCs). Metabotropic receptors, such as group I mGluR, mAChR, D2R, A2AR, can also contribute by releasing Ca2+ from internal stores (either via inositol triphosphate receptors, IP3Rs or rynanodine receptors, RyRs). Postsynaptic calcium signals activate enzymatic activity required for plasticity, including PKC, PKA, Src, PP1/PP2A. Metabotropic receptors could also directly activate the enzymatic activity (gray dash arrow). Right: Diverse modes of expression have been identified for NMDAR plasticity that involve NMDAR exo/endocytosis as well as lateral mobility between synaptic and extrasynaptic pools. Expression of NMDAR plasticity can also involve changes in the magnitude of fractional Ca2+ current through NMDARs. Modes of expression can also be associated with a shift in NMDAR subunit composition (*).
LONG-TERM PLASTICITY OF NMDAR-MEDIATED TRANSMISSION
Long-term potentiation
The question of whether NMDAR-mediated transmission could undergo both LTP and LTD has been addressed by numerous studies spanning nearly three decades of research. Early studies using hippocampal brain slices showed that similar induction protocols that trigger AMPAR-LTP also induce NMDAR-LTP at the Schaffer collateral to CA1 pyramidal cell synapse (Sch-CA1) [20–27], and at the perforant path to dentate granule cell synapse (PP-DGC) [28–30]. However, the presence of NMDAR-LTP was not confirmed by others [31–34]. While this discrepancy could be due to different experimental conditions, it was suggested that a stronger induction protocol is required to elicit NMDAR-LTP vs AMPAR-LTP, at least at the Sch-CA1 synapse [22,25]. Following these initial descriptions of NMDAR-LTP in CA1 and dentate gyrus, the topic of both NMDAR-mediated transmission and NMDAR plasticity were immediately eclipsed by a massive number of studies on LTP and LTD of AMPAR-mediated transmission. Perhaps this shift in focus was based on the prevailing view in the field that AMPAR-LTP can occur independently of changes in NMDAR-mediated transmission, at least in the CA1 area [31–34]. It was also suggested that NMDARs could be relatively less dynamic at the synapse. Contrary to this notion, a growing body of evidence has demonstrated that NMDAR-mediated transmission can be dynamically regulated independently as well as concomitantly with AMPAR-mediated transmission.
Several groups have noted that NMDAR-LTP can develop over a longer timescale relative to AMPAR-LTP. At unitary connections between layer 5 pyramidal neurons in visual cortical slices, early LTP of the AMPAR-mediated component is followed by a delayed NMDAR-LTP, which seems to restore the AMPAR-to-NMDAR ratio [35]. Delayed NMDAR-LTP has been observed at other synapses as well [25–26,36]. The induction mechanism of this delayed NMDAR-LTP, the reason for delayed potentiation, and whether it requires previous AMPAR-LTP, all remain to be elucidated. Remarkably, in vivo induction of LTP in rat DG increased the surface expression of NMDARs in a delayed and protein synthesis-dependent manner [37], although it is uncertain whether such increase is associated with a measurable increase in synaptic NMDAR transmission (i.e., NMDAR-LTP).
Recent studies have shown that NMDAR-plasticity can occur independently of AMPAR-plasticity. For example, brief bursts of synaptic activity elicit NMDAR-LTP at mossy fiber to CA3 pyramidal cell synapses (MF-CA3) [38–39] and glutamatergic synapses onto dopaminergic neurons in the midbrain (e.g. substantia nigra and ventral tegmental area, or VTA) [40]. NMDAR-LTP at these synapses share several properties, including a postsynaptic mechanism of expression, and the requirement of NMDAR and mGluR5 co-activation for induction. The requirement of mGluR5 activation in NMDAR-LTP has also been reported at other hippocampal synapses [36,41–42], and is consistent with previous observations in cultured neurons and expression systems [14–15,43]. Notably, several studies indicate a direct physical interaction between the NMDAR and mGluRs complexes via PDZ domain-containing proteins in the postsynaptic density [44]. In addition to mGluR5, type 2 adenosine receptors are also required for the induction of NMDAR-LTP at the MF-CA3 synapse [39]. Another commonality between NMDAR-LTP in CA3 pyramidal and dopaminergic midbrain neurons is that induction requires a rise in postsynaptic [Ca2+], which can occur as a result of Ca2+ influx via NMDARs, as well as Ca2+ release from internal stores. The precise Ca2+ sensors involved in detecting these postsynaptic Ca2+ signals that lead to plasticity remain unclear. A point of divergence between these forms of NMDAR-LTP is the requirement of differential kinase activity, where PKC [38] and Src kinases [39] are required for plasticity in CA3 neurons, in agreement with NMDAR-LTP at other synapses [30,45–46], while PKA is required in dopaminergic neurons of the midbrain [40].
Diverse modes of expression have been identified for NMDAR-LTP. At MF-CA3 synapses, NMDAR-LTP appears to be expressed by exocytosis of NMDARs [38] as intracellular loading of a SNAP-25 interfering peptide blocks plasticity. An increase in NMDAR surface expression has also been implicated in NMDAR-LTP at Sch-CA1 synapses [45]. In the dentate gyrus, NMDAR-LTP can be expressed by recruitment of NMDARs from extrasynaptic to synaptic sites [47], in accordance with previous studies in neuronal cultures showing that NMDARs can move laterally between synaptic and extrasynaptic pools [48–49]. Beyond these few examples, the mechanisms of expression of NMDAR-LTP remain largely unexplored.
Long-term depression
Long-term depression of NMDAR-mediated transmission has also been reported at several synapses (Table 1). Similar to the case where AMPA and NMDAR plasticity can be co-induced, induction protocols used to trigger AMPAR-LTD (e.g. low frequency stimulation) can also induce NMDAR-LTD at hippocampal CA1 synapses [26,34,36,50–54], associative/commissural CA3 synapses [55–56], and PP-DGC synapses [42]. “Chemical” NMDAR-LTD at Sch-CA1 synapses can be induced by transient (10 min) perfusion of the group I mGluR agonist DHPG [57], or muscarinic cholinergic receptor (mAChRs) agonists [54]. Intriguingly, brief activation of mAChRs by acetylcholine (e.g., puff application or endogenous release by theta-burst stimulation of the alveus) can cause a long-lasting increase in NMDAR-meditated transmission [58], raising the possibility that the duration, localization or magnitude of mAChR activation could determine the direction of NMDAR plasticity. NMDAR-LTD in midbrain dopaminergic neurons is elicited by a pairing (pre and postsynaptic) bursting protocol; however, little is known about the mechanisms of induction or expression of this form of plasticity [40].
Typically, NMDAR-LTD requires an intracellular [Ca2+] rise for induction and, at least in the dentate gyrus, the direction of NMDAR plasticity (e.g., LTP or LTD) appears to depend on the free Ca2+ concentration triggered by the induction protocol [42]. The precise mechanism by which Ca2+ elicits NMDAR LTD is unclear. However, hippocalcin has recently been identified as a Ca2+ sensor mediating cholinergic induction of NMDAR-LTD at Sch-CA1 synapses [54]. It remains to be determined how this protein can mediate Ca2+ sensing for both AMPAR-LTD [59] and NMDAR-LTD at the same synapse. These results may suggest that exquisite sensitivity to Ca2+ microdomains by spatially delimited signaling machinery may allow for AMPAR or NMDAR plasticity. Furthermore, it seems that the induction protocol can dictate the necessity for downstream phosphatase activity, where induction that requires NMDAR activation also requires PP1/PP2A activity [52], whereas induction via mAChRs does not [54].
Different forms of NMDAR-LTD can exhibit diverse mechanisms of expression. At Sch-CA1 synapses, there is evidence that NMDAR-LTD can be mediated by Ca2+-dependent actin depolymerization, which promotes destabilization of the underlying cytoskeletal framework and lateral diffusion of NMDARs away from synaptic sites [36,52]. A similar mechanism can also underlie DHPG-induced NMDAR-LTD at these synapses [57]. However, when NMDARs are antagonized during induction, paired-pulse low frequency stimulation of Schaffer collaterals induces a form of NMDAR-LTD that requires mAChR activation and is expressed by a dynamin-dependent internalization of NMDARs [54]. At recurrent synapses between CA3 pyramidal cell pairs in organotypic slice cultures, NMDAR-LTD is also expressed by dynamin-dependent endocytosis [55–56].
Recent work has provided evidence for reversibility of NMDAR plasticity. NMDAR-LTP in midbrain dopaminergic neurons can be reversed by a burst of presynaptic stimulation [40], but the mechanism of this de-potentiation has yet to be elucidated. Likewise, de-depression of NMDAR-LTD at Sch-CA1 synapses has been reported, and appears to rely on NMDAR activation and mitogen activated protein kinase activity [51]. While the vast majority of reports focus solely on either LTP or LTD of NMDAR-mediated transmission, reports of bidirectional NMDAR plasticity are sparse (see Table 1).
Beyond the magnitude of NMDAR-mediated synaptic responses
While most forms of NMDAR plasticity discussed so far are expressed as a modification in the amplitude of NMDAR-mediated synaptic responses, several studies indicate that changes in NMDAR function can accompany, or occur irrespective to alterations in response amplitude. In hippocampal CA1 pyramidal neurons, low frequency trains of glutamate uncaging lead to a long-term depression of NMDAR-mediated transmission and, an even stronger depression of Ca2+ signals (i.e. depression of the NMDAR fractional Ca2+ current) at individual dendritic spines in an all-or-none manner [60]. While direct evidence of synaptic activity-dependent changes in NMDAR-mediated Ca2+ signaling without changes in response amplitude is scarce, there is experimental evidence suggesting how this regulation could occur. For instance, in CA1 pyramidal neurons, PKA activity upregulates NMDAR Ca2+ permeability without affecting NMDAR-EPSP amplitude [61]. In agreement with this observation, activation of metabotropic receptors signaling via PKA, such as type 2 dopamine receptors and type 2 adenosine receptors in dorsal striatum [62], and GABAB receptors in prefrontal cortex [63], modulate NMDAR-mediated calcium signals in dendritic spines. Dynamic NMDAR-mediated Ca2+ influx has the potential to influence the induction of NMDAR-dependent AMPAR-LTP/LTD, as well as NMDAR Ca2+-coupled conductances (e.g., BK-type Ca2+-activated K+ channels), the latter of which will impact neuronal excitability [64].
NMDARs are hetero-tetramers typically composed of GluN1 and GluN2 subunits, where the precise subunit composition determines the functional properties of NMDARs [5,65–66]. Notably, the subunit composition can be regulated in response to neural activity [53,67–69], and by sensory experience early in life [70–71]. An acute, activity-driven NMDAR switch in subunit composition was identified at neonatal Sch-CA1 synapses [53], and was later shown to require NMDAR and mGluR5 co-activation, PLC activity, Ca2+ release from IP3R-dependent stores, and PKC activity [67]. It therefore appears that this form of plasticity is mechanistically similar to NMDAR-LTP described at more mature synapses, including MF-CA3, PP-DG and Sch-CA1 synapses (see Table 1). Additional work at mature synapses in the DG and CA1 regions of the hippocampus indicates that expression of NMDAR plasticity can also be accompanied by a shift in subunit composition [36,47], a mechanism that does not appear to be associated with NMDAR-LTP in dopaminergic midbrain neurons [40]. In any case, activity-dependent changes in NMDAR subunit composition will have important implications for NMDAR function, including changes in Ca2+ influx through NMDARs and postsynaptic Ca2+ dynamics, which in turn can regulate the magnitude and sign of NMDAR-dependent forms of synaptic plasticity [70].
FUNCTIONAL SIGNIFICANCE OF NMDAR PLASTICITY
Metaplasticity
An expected consequence of NMDAR plasticity is the potential for a long-term change in the inducibility of NMDAR-dependent forms of plasticity, such as LTP and LTD of AMPAR-mediated transmission. Such a shift in the induction threshold of synaptic plasticity is one form of metaplasticity [72]. This higher order form of synaptic regulation is commonly regarded as a homeostatic mechanism to buffer the extent of synaptic modification against saturation, thereby ensuring the fidelity of synaptically encoded information within a given network [7]. Despite the key role of NMDARs in the induction of conventional LTP/LTD of AMPAR-mediated transmission, there is surprisingly little direct evidence in support of long-term NMDAR plasticity as a mechanism of metaplasticty. This is presumably due to the fact that NMDAR plasticity and the potentially associated metaplasticity are in several cases induced simultaneously, making the analysis of metaplasticty problematic. At hippocampal mossy fiber synapses, where NMDAR-LTP can occur in the absence of AMPAR-LTP [38–39], it has recently been reported that NMDAR-LTP could be a prerequisite for the induction of NMDAR-dependent AMPAR-LTP [73]. This observation is intriguing given previous evidence that the postsynaptic machinery commonly involved in AMPAR-LTP at most synapses is likely missing at the MF-CA3 synapse [74]. As mentioned above, NMDAR plasticity can include a shift in NMDAR subunit composition, and the associated change in receptor function could have an important impact on the magnitude and sign of AMPAR-plasticity [70]. In this context, new research using cultured hippocampal neurons has elegantly shown that prolonged suppression of spontaneous glutamate release up-regulates GluN2B-containing NMDARs and augments Ca2+ influx at single dendritic spines, thus facilitating the induction for AMPAR-LTP [68]. Whether this kind of metaplasticity occurs in more intact preparations awaits confirmation.
Recent studies have shown that in-vivo exposure to ethanol [75] or amphetamine [76] enhances NMDAR-LTP in the rat VTA. This enhancement seems to be due to increased Ca2+ release from internal stores as a result of PKA-dependent sensitization of IP3 receptors. Metaplasticity commonly refers to changes in AMPAR plasticity, but it may include other forms of plasticities as well. Under this more inclusive definition, these studies represent progress towards understanding the mechanisms underlying metaplasticity of NMDAR plasticity.
Bursting activity and integrative functions
NMDAR-mediated currents are minimal at membrane potentials more negative than −70 mV. However, given the region of negative slope conductance from approximately −70 to −35 mV [12–13] (Fig. 1A), current definitely flows through the channel and can be amplified in neurons that are usually depolarized by incoming excitatory activity, a condition commonly found in vivo. Due to the slow decay kinetics of NMDAR-EPSPs temporal summation of these responses can produce a sustained level of excitation, driving neuronal firing upon repetitive synaptic activity (Fig. 1B,C). NMDARs also carry a substantial fraction of the total synaptic charge [13] and may be important for recurrent excitation in cortical networks [77–78]. Thus, in addition to their well-known role as coincidence-detectors in the induction of AMPAR-LTP/LTD [6], NMDARs play an important role in basal synaptic transmission [8–9]. As a result, NMDARs can contribute significantly to the integrative properties of neurons [10], and in generating persistent activity of neural assemblies [79].
Neuronal bursting activity has been described in numerous neuronal subtypes across multiple model organisms [80], and is thought to represent particularly salient information, allowing for selective communication across anatomical brain regions [81–82]. Consistent with this notion, several reports spanning the past decade have indicated that coincident NMDAR activation across multiple synapses can generate non-linear signals in dendrites, such as “NMDA spikes” or “NMDA plateau potentials” (for recent reviews, see [10,83]) (Fig. 3A). This NMDAR-mediated nonlinearity has been shown to be critical in recognizing and subsequently generating bursts of action potentials [84], which likely occur as a result of a strict correlation between the generation of the dendritic NMDA plateau potential, and a prolonged depolarization of the somatic membrane potential. Moreover, NMDARs have been shown to control normal bursting activity as well as the epileptic discharge of granule cells of the DG [85], and play an important role in the transition from tonic firing mode to bursting mode in dopaminergic midbrain neurons [86] (Fig. 3B). As growing evidence indicates that NMDARs in VTA neurons are involved in addictive behaviors [70,75,87–88], it is likely that NMDAR plasticity in the VTA, by regulating bursting activity, may play a pivotal role in the encoding of reward and ultimately, in the development of addictive behaviors. Building on the biophysical properties of NMDARs, it has been shown that synaptic NMDARs contribute to overcoming the electrotonic disadvantage imposed by distal dendritic locations [89], in addition to a role in the spatio-temporal discrimination of synaptic inputs along dendrites [83]. Furthermore, NMDARs have been implicated in the generation of plateau potentials that mediate pathway interactions between different inputs across cortical layers [90]. While significantly more work will be needed to demonstrate directly the impact of NMDAR plasticity on bursting activity and the integrative functions of neurons and neural circuits, the available evidence indicates that synaptic NMDARs, and therefore NMDAR plasticity can be a major factor governing these important neural properties.
Figure 3. Functional consequences of NMDAR plasticity.
A) NMDAR plasticity can modify the threshold for the initiation of an NMDA spike or plateau potential, an important integrative property of cortical neurons. B) NMDARs have been shown to be critical for the transition between single spike and complex spike/bursting output modes in several cell types, enabling the regulation of information transfer. C) Schematic representing the effects of AMPAR (black) vs. NMDAR (red) plasticity in response to repetitive synaptic activation (e.g. bursts like those depicted in Fig. 1B) where AMPAR plasticity generally leads to linear changes in gain while NMDAR plasticity enables nonlinear shifts in output.
CONCLUSIONS AND FUTURE DIRECTIONS
Recent work on NMDAR-mediated transmission has bolstered the notion that synaptic NMDARs, as with their AMPAR counterparts, are dynamically regulated and can undergo activity-dependent plasticity throughout the brain. In some cases, NMDAR plasticity occurs in the absence of AMPAR plasticity. When both components are potentiated or depressed, the signaling cascades involved are not necessarily identical. In addition, some synapses seem to be more susceptible to NMDAR plasticity than others. The unique functional properties of the NMDAR, including high Ca2+ permeability, negative slope conductance (a property that enables signal amplification), and slow NMDAR-EPSP kinetics, makes NMDAR plasticity a particularly powerful mechanism for the fine tuning of information encoding and storage (Fig. 3C). Just considering the role of Ca2+ as a second messenger, NMDAR plasticity would be expected to have far reaching implications beyond amplitude changes of NMDAR-mediated synaptic responses. In addition to triggering AMPAR-LTP/LTD, NMDARs play an important role in other forms of synaptic plasticity, including inhibitory synaptic plasticity [91], thereby expanding the functional impact of activity-dependent NMDAR plasticity not only to excitatory but also inhibitory synapses. Despite extensive information regarding the regulation of NMDAR function and trafficking in cultured neurons and expression systems [14–18,92], much remains to be learned about the molecular basis of activity-dependent NMDAR plasticity in more intact preparations. Thus far, most work has examined NMDAR plasticity in vitro under rather unphysiological experimental conditions. An important future challenge is to determine the precise contribution of NMDAR-LTP/LTD in vivo, and its relationship to experience-driven changes in NMDAR-mediated transmission [71,93].
Some commonalities have emerged regarding the induction mechanism of NMDAR plasticity across synapses, most notably, the need for NMDAR and mGluR5 activation, as well as the role of postsynaptic Ca2+, protein kinases and phosphatases (Fig. 2). A more divergent picture emerges for expression mechanisms since changes in NMDAR function, number and subunit composition have all been implicated. Changes in NMDAR expression can occur via exo/endocytosis or lateral mobilization in and out of the synapse. It is unclear whether these mechanisms of expression coexist at a given synapse type. Unlike AMPARs, which have been shown to have a vast array of auxiliary subunits modulating trafficking and/or function with significant relevance to AMPAR plasticity [94], the identity and function of auxiliary subunits of NMDARs remains relatively unexplored.
While the vast majority of studies support a postsynaptic locus of NMDARs, the existence and functional role of NMDARs localized to the presynapse has recently received greater appreciation (for a review, see [95]). There is evidence for presynaptic NMDARs acting as coincidence-detectors and playing an essential role for some forms of spike timing-dependent plasticity [96–97]. Whether these receptors themselves can undergo plastic changes remains untested. However, the recent observation that the subunit composition of presynaptic NMDARs can be developmentally regulated, thereby modulating the inducibility of spike-timing dependent plasticity [98], raises the possibility that presynaptic NMDARs could undergo some form of plasticity, which remains to be determined. Although we have focused primarily on the mechanisms and implications of rapid, synapse specific NMDAR plasticity, mounting evidence indicates that NMDARs can participate in homeostatic plasticity [92]. Homeostatic mechanisms of NMDAR plasticity act over relatively longer timescales and can underlie metaplasticity [68]. Further investigation of the link between NMDAR homeostatic plasticity and the molecular mechanisms governing NMDAR trafficking may serve to bridge the gap in our understanding of how these processes integrate activity over multiple timescales to support various cognitive functions. Finally, NMDAR dysregulation has been implicated in a variety of neurological and psychiatric disorders, including ischemia/stroke, epilepsy, schizophrenia, drug addiction, chronic pain, and several neurodegenerative diseases [15,99]. While emerging evidence suggests that activity-dependent regulation of NMDARs could play an important role in addictive behaviors, further studies are warranted to directly test the potential involvement of NMDAR plasticity to other neuropsychiatric conditions.
NMDARs exhibit diverse functional role based on their unique biophysical properties
We review current work describing activity-dependent plasticity of NMDAR plasticity
NMDARs can by dynamically regulated by numerous induction and expression mechanisms
NMDAR plasticity is a powerful means by which information can be encoded and stored
Acknowledgments
We wish to thank all scientists whose results are reviewed in this article. We apologize to all the investigators whose work could not be cited owing to strict space constraints. We thank Dr. Reed Carroll and members of the Castillo Lab, in particular Thomas Younts, Paola Haeger and Sachin Makani, for their constructive comments on the manuscript. Supported by NIH/NIMH (R01 MH081935).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Morris RG, Moser EI, Riedel G, Martin SJ, Sandin J, Day M, O’Carroll C. Elements of a neurobiological theory of the hippocampus: the role of activity-dependent synaptic plasticity in memory. Philos Trans R Soc Lond B Biol Sci. 2003;358:773–786. doi: 10.1098/rstb.2002.1264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Bliss TV, Collingridge GL. A synaptic model of memory: long-term potentiation in the hippocampus. Nature. 1993;361:31–39. doi: 10.1038/361031a0. [DOI] [PubMed] [Google Scholar]
- 3.Kandel ER. The molecular biology of memory storage: a dialogue between genes and synapses. Science. 2001;294:1030–1038. doi: 10.1126/science.1067020. [DOI] [PubMed] [Google Scholar]
- 4.Malenka RC, Bear MF. LTP and LTD: an embarrassment of riches. Neuron. 2004;44:5–21. doi: 10.1016/j.neuron.2004.09.012. [DOI] [PubMed] [Google Scholar]
- 5••.Traynelis SF, Wollmuth LP, McBain CJ, Menniti FS, Vance KM, Ogden KK, Hansen KB, Yuan H, Myers SJ, Dingledine R. Glutamate receptor ion channels: structure, regulation, and function. Pharmacol Rev. 2010;62:405–496. doi: 10.1124/pr.109.002451. This thorough review article summarizes our current knowledge on the structure, physiology and pharmacology of ionotropic glutamate receptors, including their regulation by translational and post-translational mechanisms. A must read to all those interested in excitatory synaptic transmission. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Malenka RC, Nicoll RA. NMDA-receptor-dependent synaptic plasticity: multiple forms and mechanisms. Trends Neurosci. 1993;16:521–527. doi: 10.1016/0166-2236(93)90197-t. [DOI] [PubMed] [Google Scholar]
- 7.Abraham WC. Metaplasticity: tuning synapses and networks for plasticity. Nat Rev Neurosci. 2008;9:387. doi: 10.1038/nrn2356. [DOI] [PubMed] [Google Scholar]
- 8.Daw NW, Stein PS, Fox K. The role of NMDA receptors in information processing. Annu Rev Neurosci. 1993;16:207–222. doi: 10.1146/annurev.ne.16.030193.001231. [DOI] [PubMed] [Google Scholar]
- 9.Herron CE, Lester RA, Coan EJ, Collingridge GL. Frequency-dependent involvement of NMDA receptors in the hippocampus: a novel synaptic mechanism. Nature. 1986;322:265–268. doi: 10.1038/322265a0. [DOI] [PubMed] [Google Scholar]
- 10.Larkum ME, Nevian T. Synaptic clustering by dendritic signalling mechanisms. Curr Opin Neurobiol. 2008;18:321–331. doi: 10.1016/j.conb.2008.08.013. [DOI] [PubMed] [Google Scholar]
- 11.Schiller J, Schiller Y. NMDA receptor-mediated dendritic spikes and coincident signal amplification. Curr Opin Neurobiol. 2001;11:343–348. doi: 10.1016/s0959-4388(00)00217-8. [DOI] [PubMed] [Google Scholar]
- 12.McBain CJ, Mayer ML. N-methyl-D-aspartic acid receptor structure and function. Physiol Rev. 1994;74:723–760. doi: 10.1152/physrev.1994.74.3.723. [DOI] [PubMed] [Google Scholar]
- 13.Hestrin S, Nicoll RA, Perkel DJ, Sah P. Analysis of excitatory synaptic action in pyramidal cells using whole-cell recording from rat hippocampal slices. J Physiol. 1990;422:203–225. doi: 10.1113/jphysiol.1990.sp017980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kotecha SA, MacDonald JF. Signaling molecules and receptor transduction cascades that regulate NMDA receptor-mediated synaptic transmission. Int Rev Neurobiol. 2003;54:51–106. doi: 10.1016/s0074-7742(03)54003-x. [DOI] [PubMed] [Google Scholar]
- 15.Lau CG, Zukin RS. NMDA receptor trafficking in synaptic plasticity and neuropsychiatric disorders. Nat Rev Neurosci. 2007;8:413–426. doi: 10.1038/nrn2153. [DOI] [PubMed] [Google Scholar]
- 16.Salter MW, Kalia LV. Src kinases: a hub for NMDA receptor regulation. Nat Rev Neurosci. 2004;5:317–328. doi: 10.1038/nrn1368. [DOI] [PubMed] [Google Scholar]
- 17.Wenthold RJ, Prybylowski K, Standley S, Sans N, Petralia RS. Trafficking of NMDA receptors. Annu Rev Pharmacol Toxicol. 2003;43:335–358. doi: 10.1146/annurev.pharmtox.43.100901.135803. [DOI] [PubMed] [Google Scholar]
- 18.Chen BS, Roche KW. Regulation of NMDA receptors by phosphorylation. Neuropharmacology. 2007;53:362–368. doi: 10.1016/j.neuropharm.2007.05.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Rebola N, Srikumar BN, Mulle C. Activity-dependent synaptic plasticity of NMDA receptors. J Physiol. 2010;588:93–99. doi: 10.1113/jphysiol.2009.179382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Bashir ZI, Alford S, Davies SN, Randall AD, Collingridge GL. Long-term potentiation of NMDA receptor-mediated synaptic transmission in the hippocampus. Nature. 1991;349:156–158. doi: 10.1038/349156a0. [DOI] [PubMed] [Google Scholar]
- 21.Berretta N, Berton F, Bianchi R, Brunelli M, Capogna M, Francesconi W. Long-term Potentiation of NMDA Receptor-mediated EPSP in Guinea-pig Hippocampal Slices. Eur J Neurosci. 1991;3:850–854. doi: 10.1111/j.1460-9568.1991.tb00096.x. [DOI] [PubMed] [Google Scholar]
- 22.Aniksztejn L, Ben-Ari Y. Expression of LTP by AMPA and/or NMDA receptors is determined by the extent of NMDA receptors activation during the tetanus. J Neurophysiol. 1995;74:2349–2357. doi: 10.1152/jn.1995.74.6.2349. [DOI] [PubMed] [Google Scholar]
- 23.Asztely F, Wigstrom H, Gustafsson B. The Relative Contribution of NMDA Receptor Channels in the Expression of Long-term Potentiation in the Hippocampal CA1 Region. Eur J Neurosci. 1992;4:681–690. doi: 10.1111/j.1460-9568.1992.tb00177.x. [DOI] [PubMed] [Google Scholar]
- 24.Muller D, Arai A, Lynch G. Factors governing the potentiation of NMDA receptor-mediated responses in hippocampus. Hippocampus. 1992;2:29–38. doi: 10.1002/hipo.450020105. [DOI] [PubMed] [Google Scholar]
- 25.Muller D, Lynch G. Long-term potentiation differentially affects two components of synaptic responses in hippocampus. Proc Natl Acad Sci U S A. 1988;85:9346–9350. doi: 10.1073/pnas.85.23.9346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Xiao MY, Karpefors M, Niu YP, Wigstrom H. The complementary nature of long-term depression and potentiation revealed by dual component excitatory postsynaptic potentials in hippocampal slices from young rats. Neuroscience. 1995;68:625–635. doi: 10.1016/0306-4522(95)00173-g. [DOI] [PubMed] [Google Scholar]
- 27.Yi PL, Chang FC, Tsai JJ, Hung CR, Gean PW. The involvement of metabotropic glutamate receptors in long-term depression of N-methyl-D-aspartate receptor-mediated synaptic potential in the rat hippocampus. Neurosci Lett. 1995;185:207–210. doi: 10.1016/0304-3940(95)11264-w. [DOI] [PubMed] [Google Scholar]
- 28.Xie X, Berger TW, Barrionuevo G. Isolated NMDA receptor-mediated synaptic responses express both LTP and LTD. J Neurophysiol. 1992;67:1009–1013. doi: 10.1152/jn.1992.67.4.1009. [DOI] [PubMed] [Google Scholar]
- 29.O’Connor JJ, Rowan MJ, Anwyl R. Long-lasting enhancement of NMDA receptor-mediated synaptic transmission by metabotropic glutamate receptor activation. Nature. 1994;367:557–559. doi: 10.1038/367557a0. [DOI] [PubMed] [Google Scholar]
- 30.O’Connor JJ, Rowan MJ, Anwyl R. Tetanically induced LTP involves a similar increase in the AMPA and NMDA receptor components of the excitatory postsynaptic current: investigations of the involvement of mGlu receptors. J Neurosci. 1995;15:2013–2020. doi: 10.1523/JNEUROSCI.15-03-02013.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Kauer JA, Malenka RC, Nicoll RA. A persistent postsynaptic modification mediates long-term potentiation in the hippocampus. Neuron. 1988;1:911–917. doi: 10.1016/0896-6273(88)90148-1. [DOI] [PubMed] [Google Scholar]
- 32.Perkel DJ, Nicoll RA. Evidence for all-or-none regulation of neurotransmitter release: implications for long-term potentiation. J Physiol. 1993;471:481–500. doi: 10.1113/jphysiol.1993.sp019911. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Liao D, Hessler NA, Malinow R. Activation of postsynaptically silent synapses during pairing-induced LTP in CA1 region of hippocampal slice. Nature. 1995;375:400–404. doi: 10.1038/375400a0. [DOI] [PubMed] [Google Scholar]
- 34.Selig DK, Hjelmstad GO, Herron C, Nicoll RA, Malenka RC. Independent mechanisms for long-term depression of AMPA and NMDA responses. Neuron. 1995;15:417–426. doi: 10.1016/0896-6273(95)90045-4. [DOI] [PubMed] [Google Scholar]
- 35.Watt AJ, Sjostrom PJ, Hausser M, Nelson SB, Turrigiano GG. A proportional but slower NMDA potentiation follows AMPA potentiation in LTP. Nat Neurosci. 2004;7:518–524. doi: 10.1038/nn1220. [DOI] [PubMed] [Google Scholar]
- 36.Peng Y, Zhao J, Gu QH, Chen RQ, Xu Z, Yan JZ, Wang SH, Liu SY, Chen Z, Lu W. Distinct trafficking and expression mechanisms underlie LTP and LTD of NMDA receptor-mediated synaptic responses. Hippocampus. 2010;20:646–658. doi: 10.1002/hipo.20654. [DOI] [PubMed] [Google Scholar]
- 37.Williams JM, Guevremont D, Mason-Parker SE, Luxmanan C, Tate WP, Abraham WC. Differential trafficking of AMPA and NMDA receptors during long-term potentiation in awake adult animals. J Neurosci. 2007;27:14171–14178. doi: 10.1523/JNEUROSCI.2348-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38•.Kwon HB, Castillo PE. Long-term potentiation selectively expressed by NMDA receptors at hippocampal mossy fiber synapses. Neuron. 2008;57:108–120. doi: 10.1016/j.neuron.2007.11.024. Together with [39], these sudies represent the first description of a specific potentiation of the NMDAR-mediated transmission at hippocampal mossy fiber synapses providing independent evidence for common mechanisms of induction and expression for this form of NMDAR plasticity. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Rebola N, Lujan R, Cunha RA, Mulle C. Adenosine A2A receptors are essential for long-term potentiation of NMDA-EPSCs at hippocampal mossy fiber synapses. Neuron. 2008;57:121–134. doi: 10.1016/j.neuron.2007.11.023. [DOI] [PubMed] [Google Scholar]
- 40••.Harnett MT, Bernier BE, Ahn KC, Morikawa H. Burst-timing-dependent plasticity of NMDA receptor-mediated transmission in midbrain dopamine neurons. Neuron. 2009;62:826–838. doi: 10.1016/j.neuron.2009.05.011. This study utilizes a physiologically relevant burst firing paradigm to elicit NMDAR plasticity on dopaminergic neurons of the midbrain, implicating this form of plasticity in the encoding of reward and addicition. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Jia Z, Lu Y, Henderson J, Taverna F, Romano C, Abramow-Newerly W, Wojtowicz JM, Roder J. Selective abolition of the NMDA component of long-term potentiation in mice lacking mGluR5. Learn Mem. 1998;5:331–343. [PMC free article] [PubMed] [Google Scholar]
- 42.Harney SC, Rowan M, Anwyl R. Long-term depression of NMDA receptor-mediated synaptic transmission is dependent on activation of metabotropic glutamate receptors and is altered to long-term potentiation by low intracellular calcium buffering. J Neurosci. 2006;26:1128–1132. doi: 10.1523/JNEUROSCI.2753-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Kotecha SA, Jackson MF, Al-Mahrouki A, Roder JC, Orser BA, MacDonald JF. Co-stimulation of mGluR5 and N-methyl-D-aspartate receptors is required for potentiation of excitatory synaptic transmission in hippocampal neurons. J Biol Chem. 2003;278:27742–27749. doi: 10.1074/jbc.M301946200. [DOI] [PubMed] [Google Scholar]
- 44.Kim E, Sheng M. PDZ domain proteins of synapses. Nat Rev Neurosci. 2004;5:771–781. doi: 10.1038/nrn1517. [DOI] [PubMed] [Google Scholar]
- 45.Grosshans DR, Clayton DA, Coultrap SJ, Browning MD. LTP leads to rapid surface expression of NMDA but not AMPA receptors in adult rat CA1. Nat Neurosci. 2002;5:27–33. doi: 10.1038/nn779. [DOI] [PubMed] [Google Scholar]
- 46.Li HB, Jackson MF, Yang K, Trepanier C, Salter MW, Orser BA, Macdonald JF. Plasticity of synaptic GluN receptors is required for the Src-dependent induction of long-term potentiation at CA3-CA1 synapses. Hippocampus. 2010 doi: 10.1002/hipo.20818. [DOI] [PubMed] [Google Scholar]
- 47.Harney SC, Jane DE, Anwyl R. Extrasynaptic NR2D-containing NMDARs are recruited to the synapse during LTP of NMDAR-EPSCs. J Neurosci. 2008;28:11685–11694. doi: 10.1523/JNEUROSCI.3035-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Groc L, Heine M, Cognet L, Brickley K, Stephenson FA, Lounis B, Choquet D. Differential activity-dependent regulation of the lateral mobilities of AMPA and NMDA receptors. Nat Neurosci. 2004;7:695–696. doi: 10.1038/nn1270. [DOI] [PubMed] [Google Scholar]
- 49.Tovar KR, Westbrook GL. Mobile NMDA receptors at hippocampal synapses. Neuron. 2002;34:255–264. doi: 10.1016/s0896-6273(02)00658-x. [DOI] [PubMed] [Google Scholar]
- 50.Gean PW, Lin JH. D-2-amino-5-phosphonovaleate blocks induction of long-term depression of the NMDA receptor-mediated synaptic component in rat hippocampus. Neurosci Lett. 1993;158:170–172. doi: 10.1016/0304-3940(93)90256-k. [DOI] [PubMed] [Google Scholar]
- 51.Morishita W, Malenka RC. Mechanisms underlying dedepression of synaptic NMDA receptors in the hippocampus. J Neurophysiol. 2008;99:254–263. doi: 10.1152/jn.01011.2007. [DOI] [PubMed] [Google Scholar]
- 52.Morishita W, Marie H, Malenka RC. Distinct triggering and expression mechanisms underlie LTD of AMPA and NMDA synaptic responses. Nat Neurosci. 2005;8:1043–1050. doi: 10.1038/nn1506. [DOI] [PubMed] [Google Scholar]
- 53•.Bellone C, Nicoll RA. Rapid bidirectional switching of synaptic NMDA receptors. Neuron. 2007;55:779–785. doi: 10.1016/j.neuron.2007.07.035. This study decribes for the first time a rapid shift in NMDAR subunit composition triggered by activity in early postnatal life. [DOI] [PubMed] [Google Scholar]
- 54•.Jo J, Son GH, Winters BL, Kim MJ, Whitcomb DJ, Dickinson BA, Lee YB, Futai K, Amici M, Sheng M, et al. Muscarinic receptors induce LTD of NMDAR EPSCs via a mechanism involving hippocalcin, AP2 and PSD-95. Nat Neurosci. 2010;13:1216–1224. doi: 10.1038/nn.2636. The authors describe a form of mAChR-dependent LTD of NMDAR transmission. They also provide a detailed mechanistic description of this plasticity highlighted by the identification of the calcium sensing protein hippocalcin as a critical mediator of plasticity. [DOI] [PubMed] [Google Scholar]
- 55.Montgomery JM, Selcher JC, Hanson JE, Madison DV. Dynamin-dependent NMDAR endocytosis during LTD and its dependence on synaptic state. BMC Neurosci. 2005;6:48. doi: 10.1186/1471-2202-6-48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Montgomery JM, Madison DV. State-dependent heterogeneity in synaptic depression between pyramidal cell pairs. Neuron. 2002;33:765–777. doi: 10.1016/s0896-6273(02)00606-2. [DOI] [PubMed] [Google Scholar]
- 57.Ireland DR, Abraham WC. Mechanisms of group I mGluR-dependent long-term depression of NMDA receptor-mediated transmission at Schaffer collateral-CA1 synapses. J Neurophysiol. 2009;101:1375–1385. doi: 10.1152/jn.90643.2008. [DOI] [PubMed] [Google Scholar]
- 58.Fernandez de Sevilla D, Buno W. The muscarinic long-term enhancement of NMDA and AMPA receptor-mediated transmission at Schaffer collateral synapses develop through different intracellular mechanisms. J Neurosci. 2010;30:11032–11042. doi: 10.1523/JNEUROSCI.1848-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Palmer CL, Lim W, Hastie PG, Toward M, Korolchuk VI, Burbidge SA, Banting G, Collingridge GL, Isaac JT, Henley JM. Hippocalcin functions as a calcium sensor in hippocampal LTD. Neuron. 2005;47:487–494. doi: 10.1016/j.neuron.2005.06.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60••.Sobczyk A, Svoboda K. Activity-dependent plasticity of the NMDA-receptor fractional Ca2+ current. Neuron. 2007;53:17–24. doi: 10.1016/j.neuron.2006.11.016. This study represents the first demonstration that the calcium permeability of NMDARs can be a mode by which NMDAR plasticity is expressed in addition to a decrease in the amplitude of NMDAR-mediated responses. [DOI] [PubMed] [Google Scholar]
- 61•.Skeberdis VA, Chevaleyre V, Lau CG, Goldberg JH, Pettit DL, Suadicani SO, Lin Y, Bennett MV, Yuste R, Castillo PE, et al. Protein kinase A regulates calcium permeability of NMDA receptors. Nat Neurosci. 2006;9:501–510. doi: 10.1038/nn1664. First demonstration that NMDAR calcium permeability can be regulated by PKA. Subsequent studies [62,63] have convincingly shown that activation of metabotropic receptors signaling via PKA can also regulate calcium influx through NMDARs. [DOI] [PubMed] [Google Scholar]
- 62.Higley MJ, Sabatini BL. Competitive regulation of synaptic Ca2+ influx by D2 dopamine and A2A adenosine receptors. Nat Neurosci. 2010;13:958–966. doi: 10.1038/nn.2592. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Chalifoux JR, Carter AG. GABAB receptors modulate NMDA receptor calcium signals in dendritic spines. Neuron. 2010;66:101–113. doi: 10.1016/j.neuron.2010.03.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Isaacson JS, Murphy GJ. Glutamate-mediated extrasynaptic inhibition. direct coupling of NMDA receptors to Ca(2+)-activated K+ channels. Neuron. 2001;31:1027–1034. doi: 10.1016/s0896-6273(01)00428-7. [DOI] [PubMed] [Google Scholar]
- 65.Cull-Candy SG, Leszkiewicz DN. Role of distinct NMDA receptor subtypes at central synapses. Sci STKE. 2004;2004:re16. doi: 10.1126/stke.2552004re16. [DOI] [PubMed] [Google Scholar]
- 66.Paoletti P, Neyton J. NMDA receptor subunits: function and pharmacology. Curr Opin Pharmacol. 2007;7:39–47. doi: 10.1016/j.coph.2006.08.011. [DOI] [PubMed] [Google Scholar]
- 67•.Matta JA, Ashby MC, Sanz-Clemente A, Roche KW, Isaac JT. mGluR5 and NMDA receptors drive the experience- and activity-dependent NMDA receptor NR2B to NR2A subunit switch. Neuron. 2011;70:339–351. doi: 10.1016/j.neuron.2011.02.045. The authors provide a detailed description of the mechanisms underlying experience- and activity-dependent NR2B to NR2A subunit switch in the neonatal hippocampus. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68••.Lee MC, Yasuda R, Ehlers MD. Metaplasticity at single glutamatergic synapses. Neuron. 2010;66:859–870. doi: 10.1016/j.neuron.2010.05.015. Elegant demonstration in cultured neurons that spontaneous glutamate release adjusts plasticity threshold at individual spines by local regulation of NMDARs. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Barria A, Malinow R. Subunit-specific NMDA receptor trafficking to synapses. Neuron. 2002;35:345–353. doi: 10.1016/s0896-6273(02)00776-6. [DOI] [PubMed] [Google Scholar]
- 70•.Yashiro K, Philpot BD. Regulation of NMDA receptor subunit expression and its implications for LTD, LTP, and metaplasticity. Neuropharmacology. 2008;55:1081–1094. doi: 10.1016/j.neuropharm.2008.07.046. Good summary of the mechanisms controlling the subunit composition of NMDARs, including a thorough discussion on the functional impact of this regulation as a mechanism of metaplasticity. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Quinlan EM, Philpot BD, Huganir RL, Bear MF. Rapid, experience-dependent expression of synaptic NMDA receptors in visual cortex in vivo. Nat Neurosci. 1999;2:352–357. doi: 10.1038/7263. [DOI] [PubMed] [Google Scholar]
- 72.Abraham WC, Bear MF. Metaplasticity: the plasticity of synaptic plasticity. Trends Neurosci. 1996;19:126–130. doi: 10.1016/s0166-2236(96)80018-x. [DOI] [PubMed] [Google Scholar]
- 73•.Rebola N, Carta M, Lanore F, Blanchet C, Mulle C. NMDA receptor-dependent metaplasticity at hippocampal mossy fiber synapses. Nat Neurosci. 2011;14:691–693. doi: 10.1038/nn.2809. Here the authors introduce for the first time the role of NMDAR-LTP as a prerequisite for the induction of postsynaptic AMPAR-LTP at the hippocampal mossy fiber to CA3 pyramidal cell synapse. [DOI] [PubMed] [Google Scholar]
- 74.Kakegawa W, Tsuzuki K, Yoshida Y, Kameyama K, Ozawa S. Input- and subunit-specific AMPA receptor trafficking underlying long-term potentiation at hippocampal CA3 synapses. Eur J Neurosci. 2004;20:101–110. doi: 10.1111/j.1460-9568.2004.03461.x. [DOI] [PubMed] [Google Scholar]
- 75•.Bernier BE, Whitaker LR, Morikawa H. Previous ethanol experience enhances synaptic plasticity of NMDA receptors in the ventral tegmental area. J Neurosci. 2011;31:5205–5212. doi: 10.1523/JNEUROSCI.5282-10.2011. Together with [76] these studies show that previous in-vivo exposure to ethanol or amphetamine can modulate the induction of subsequent NMDAR plasticity in dopaminergic neurons of the midbrain, providing the first mechanistic evidence for metaplasticity of NMDAR-LTP. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Ahn KC, Bernier BE, Harnett MT, Morikawa H. IP3 receptor sensitization during in vivo amphetamine experience enhances NMDA receptor plasticity in dopamine neurons of the ventral tegmental area. J Neurosci. 2010;30:6689–6699. doi: 10.1523/JNEUROSCI.4453-09.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Wang H, Stradtman GG, 3rd, Wang XJ, Gao WJ. A specialized NMDA receptor function in layer 5 recurrent microcircuitry of the adult rat prefrontal cortex. Proc Natl Acad Sci U S A. 2008;105:16791–16796. doi: 10.1073/pnas.0804318105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Wang XJ. Probabilistic decision making by slow reverberation in cortical circuits. Neuron. 2002;36:955–968. doi: 10.1016/s0896-6273(02)01092-9. [DOI] [PubMed] [Google Scholar]
- 79.Major G, Tank D. Persistent neural activity: prevalence and mechanisms. Curr Opin Neurobiol. 2004;14:675–684. doi: 10.1016/j.conb.2004.10.017. [DOI] [PubMed] [Google Scholar]
- 80.Krahe R, Gabbiani F. Burst firing in sensory systems. Nat Rev Neurosci. 2004;5:13–23. doi: 10.1038/nrn1296. [DOI] [PubMed] [Google Scholar]
- 81.Izhikevich EM, Desai NS, Walcott EC, Hoppensteadt FC. Bursts as a unit of neural information: selective communication via resonance. Trends Neurosci. 2003;26:161–167. doi: 10.1016/S0166-2236(03)00034-1. [DOI] [PubMed] [Google Scholar]
- 82.Lisman JE. Bursts as a unit of neural information: making unreliable synapses reliable. Trends Neurosci. 1997;20:38–43. doi: 10.1016/S0166-2236(96)10070-9. [DOI] [PubMed] [Google Scholar]
- 83.Branco T, Hausser M. The single dendritic branch as a fundamental functional unit in the nervous system. Curr Opin Neurobiol. 2010;20:494–502. doi: 10.1016/j.conb.2010.07.009. [DOI] [PubMed] [Google Scholar]
- 84•.Polsky A, Mel B, Schiller J. Encoding and decoding bursts by NMDA spikes in basal dendrites of layer 5 pyramidal neurons. J Neurosci. 2009;29:11891–11903. doi: 10.1523/JNEUROSCI.5250-08.2009. This paper indicates that the nonlinear amplification properties of synaptic NMDARs can play a definitive role in detecting afferent bursting activity as well as their concomitant role in the subsequent burst firing output of cortical pyramidal neurons. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Lynch M, Sayin U, Golarai G, Sutula T. NMDA receptor-dependent plasticity of granule cell spiking in the dentate gyrus of normal and epileptic rats. J Neurophysiol. 2000;84:2868–2879. doi: 10.1152/jn.2000.84.6.2868. [DOI] [PubMed] [Google Scholar]
- 86.Overton PG, Clark D. Burst firing in midbrain dopaminergic neurons. Brain Res Brain Res Rev. 1997;25:312–334. doi: 10.1016/s0165-0173(97)00039-8. [DOI] [PubMed] [Google Scholar]
- 87.Borgland SL, Taha SA, Sarti F, Fields HL, Bonci A. Orexin A in the VTA is critical for the induction of synaptic plasticity and behavioral sensitization to cocaine. Neuron. 2006;49:589–601. doi: 10.1016/j.neuron.2006.01.016. [DOI] [PubMed] [Google Scholar]
- 88.Zweifel LS, Argilli E, Bonci A, Palmiter RD. Role of NMDA receptors in dopamine neurons for plasticity and addictive behaviors. Neuron. 2008;59:486–496. doi: 10.1016/j.neuron.2008.05.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89•.Branco T, Hausser M. Synaptic integration gradients in single cortical pyramidal cell dendrites. Neuron. 2011;69:885–892. doi: 10.1016/j.neuron.2011.02.006. This study demonstrates the role of NMDARs in overcoming the electrotonic disadvantage of distal dendritic inputs. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Takahashi H, Magee JC. Pathway interactions and synaptic plasticity in the dendritic tuft regions of CA1 pyramidal neurons. Neuron. 2009;62:102–111. doi: 10.1016/j.neuron.2009.03.007. [DOI] [PubMed] [Google Scholar]
- 91.Castillo PE, Chiu CQ, Carroll RC. Long-term plasticity at inhibitory synapses. Curr Opin Neurobiol. 2011;21:328–338. doi: 10.1016/j.conb.2011.01.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Perez-Otano I, Ehlers MD. Homeostatic plasticity and NMDA receptor trafficking. Trends Neurosci. 2005;28:229–238. doi: 10.1016/j.tins.2005.03.004. [DOI] [PubMed] [Google Scholar]
- 93.Carmignoto G, Vicini S. Activity-dependent decrease in NMDA receptor responses during development of the visual cortex. Science. 1992;258:1007–1011. doi: 10.1126/science.1279803. [DOI] [PubMed] [Google Scholar]
- 94.Jackson AC, Nicoll RA. The expanding social network of ionotropic glutamate receptors: TARPs and other transmembrane auxiliary subunits. Neuron. 2011;70:178–199. doi: 10.1016/j.neuron.2011.04.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Corlew R, Brasier DJ, Feldman DE, Philpot BD. Presynaptic NMDA receptors: newly appreciated roles in cortical synaptic function and plasticity. Neuroscientist. 2008;14:609–625. doi: 10.1177/1073858408322675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Duguid I, Sjostrom PJ. Novel presynaptic mechanisms for coincidence detection in synaptic plasticity. Curr Opin Neurobiol. 2006;16:312–322. doi: 10.1016/j.conb.2006.05.008. [DOI] [PubMed] [Google Scholar]
- 97.Rodriguez-Moreno A, Banerjee A, Paulsen O. Presynaptic NMDA Receptors and Spike Timing-Dependent Depression at Cortical Synapses. Front Synaptic Neurosci. 2010;2:18. doi: 10.3389/fnsyn.2010.00018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Larsen RS, Corlew RJ, Henson MA, Roberts AC, Mishina M, Watanabe M, Lipton SA, Nakanishi N, Perez-Otano I, Weinberg RJ, et al. NR3A-containing NMDARs promote neurotransmitter release and spike timing-dependent plasticity. Nat Neurosci. 2011;14:338–344. doi: 10.1038/nn.2750. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Waxman EA, Lynch DR. N-methyl-D-aspartate receptor subtypes: multiple roles in excitotoxicity and neurological disease. Neuroscientist. 2005;11:37–49. doi: 10.1177/1073858404269012. [DOI] [PubMed] [Google Scholar]
- 100.Lozovaya NA, Grebenyuk SE, Tsintsadze T, Feng B, Monaghan DT, Krishtal OA. Extrasynaptic NR2B and NR2D subunits of NMDA receptors shape ‘superslow’ afterburst EPSC in rat hippocampus. J Physiol. 2004;558:451–463. doi: 10.1113/jphysiol.2004.063792. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Gean PW, Chang FC, Huang CC, Lin JH, Way LJ. Long-term enhancement of EPSP and NMDA receptor-mediated synaptic transmission in the amygdala. Brain Res Bull. 1993;31:7–11. doi: 10.1016/0361-9230(93)90003-t. [DOI] [PubMed] [Google Scholar]
- 102.Chergui K. Dopamine induces a GluN2A-dependent form of long-term depression of NMDA synaptic responses in the nucleus accumbens. Neuropharmacology. 2011;60:975–981. doi: 10.1016/j.neuropharm.2011.01.047. [DOI] [PubMed] [Google Scholar]
- 103.Kombian SB, Malenka RC. Simultaneous LTP of non-NMDA- and LTD of NMDA-receptor-mediated responses in the nucleus accumbens. Nature. 1994;368:242–246. doi: 10.1038/368242a0. [DOI] [PubMed] [Google Scholar]