Abstract
Objectives
To evaluate the association between baseline (BL) replication capacity (RC) [RCBL] and immunologic/virologic parameters (at BL and after 48 weeks on therapy) in HIV-1 infected subjects initiating antiretroviral therapy.
Methods
RCBL was determined using a modified Monogram PhenoSense HIV drug susceptibility assay on plasma HIV-1 from 321 treatment-naïve subjects from ACTG384. Univariate and multivariable analyses were performed to determine the association of RCBL with BL and on-therapy virologic and immunologic outcomes.
Results
Higher RCBL was associated with lower baseline CD4 (CD4BL) (r=−0.23, p<0.0001), higher baseline HIV-1 (RNABL) (r=0.25, p<0.0001), higher CD4BL activation percent (r=0.23, p<0.0001) and lower CD4BL memory count (r=−0.21, p=0.0002).
In a multivariable model, week 48 CD4 increase (ΔCD448) was associated with lower CD4BL memory count and higher CD4BL naive percent (p=0.004, p=0.015, respectively). The interaction between CD4BL and RCBL was significant (p=0.018), with a positive association between RCBL and ΔCD448 in subjects with higher CD4BL, and a negative association at lower absCD4BL.
Conclusions
At baseline, higher RC was significantly associated with higher HIV-1 RNA, higher CD4 cell activation, lower CD4 cell count, and lower CD4 memory cell count. These factors may interact, directly or indirectly, to modify the extent to which CD4 recovery occurs in patients starting antiretroviral therapy at different baseline CD4 counts.
Keywords: HIV, replication capacity, viral fitness, pathogenesis, immune reconstitution, activation, memory
Introduction
Immunologic recovery following the initiation of a potent antiretroviral regimen remains a central goal of HIV-1 therapy. Greater CD4 cell recovery during antiretroviral therapy (ART) has been associated with younger age, female sex, higher BL viral load, and virologic suppression to fewer than 50 copies/ml (1–7). Virologic suppression, however, does not guarantee immunologic recovery. Other factors, such as lower BL CD4 naïve percent, higher BL CD4 and CD8 activation and persistent CD4 or CD8 activation on therapy, have been associated with lower CD4 cell recovery in some individuals despite achieving virologic suppression (1, 8–12).
Another factor which may influence immunologic recovery is viral fitness. Although viral fitness is dependent on many factors in vivo, including immunologic pressure and antiretroviral therapy, RC, as measured in vitro by recombinant virus assays, represents a component of viral fitness. RC is defined as the intrinsic ability of a virus to replicate in the absence of drugs under standardized laboratory conditions, in comparison to a reference wild-type strain (13). RC has been shown to be a predictor of clinical progression to AIDS that is independent of HIV RNA and CD4 cell count (14). Furthermore, higher RC values were significantly associated with a greater rate of decline of CD4 cell count during follow-up (14).
The association between RC and virologic or immunologic response to ART has been tested in several HIV-1 cohorts with varying results. An association between lower pre-therapy RC and greater CD4 cell recovery on-therapy has been suggested in two analyses of patients with acute or early HIV infection who initiated antiretroviral therapy (15, 16). A third analysis of chronically infected patients who achieved virologic suppression on their first ART regimen, however, did not show a relationship between RCBL and CD4 cell recovery after 1 year of therapy (17). In treatment-experienced patients initiating a new regimen, higher RCBL was associated with lower on-therapy CD4 cell gains in patients who achieved virologic suppression and higher on-therapy viral load in non-suppressed patients (18, 19). Thus, while several studies have shown an association between RC measurements and response to treatment, to date no adequately powered and controlled study has demonstrated a definitive role for RC measurements in the management of HIV-1 infection.
The ACTG 384 study provides a unique opportunity to evaluate RC in a large, prospective, randomized, controlled clinical trial, in which many predictors of virologic and immunologic outcomes have been defined (1, 20, 21). RC measurements at a pre-therapy BL were analyzed in the context of other BL covariates and as related to week 48 on-therapy immunologic and virologic response.
Methods
We determined RCBL on plasma HIV-1 from 321 treatment-naïve subjects who participated in the immunology substudy of ACTG 384 (980 subjects randomized to start d4T/ddI or AZT/3TC with NFV, EFV or both NFV/EFV (20, 21). For this analysis, subjects were required to have BL and week 48 CD4 cell counts, flow cytometry, plasma HIV-1 RNA by PCR, and stored plasma samples. In addition, subjects had to have remained on their initial ART regimen assignment through week 48.
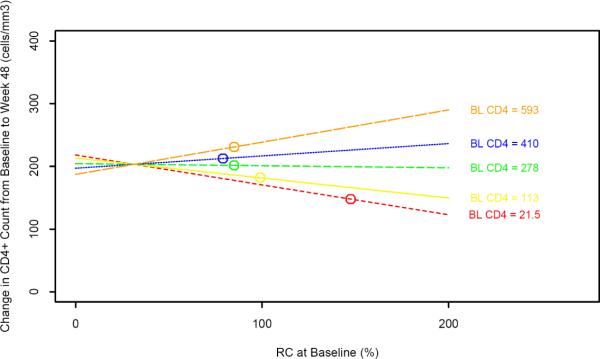
Subjects were grouped into pre-defined BL CD4 cell count strata 1 through 5 (<= 50, 51–200, 201–350, 351–500, and > 500 cells/mm3)(1). For subjects with fewer than 350 CD4 cells/mm3 at BL, we randomly identified 48 subjects with virologic suppression (HIV-1 RNA < 50 copies/ml at week 48) from each of 3 strata. In order to enrich the lower CD4 sample for the evaluation of immunologic endpoints, up to 7 additional subjects per stratum were identified who had virologic suppression and immunologic failure (HIV-1 RNA <50 c/mL and <100 CD4 cell/mm3 rise in CD4 cell count) at week 48. Stored samples were available on a total of 148 subjects in the lower 3 strata. All subjects with greater than 350 CD4 cells/mm3 who had stored samples available were assayed for RC, regardless of virologic or immunologic response; this was a total of 173 subjects. A total of 321 subjects were included in the RC substudy (Figure 1).
Figure 1. Subject Selection.
Subject selection for the ACTG 384 RC substudy. See Methods.
The institutional review board of participating sites approved the ACTG 384 trial, all subjects signed informed consent forms which included the use of stored samples in future ACTG-approved research, and subsequent analyses were approved by the ACTG. All procedures followed were in accordance with the ethical standards of the Helsinki Declaration of 1975, as revised in 2000.
RC was assessed using a modification of the PhenoSense HIV drug susceptibility assay, as previously described (13, 22, 23). Briefly, plasma-derived reverse transcriptase (RT) and protease (PR) sequences [constructs containing gag (3' end from p7), all of PR (aa 1–99), and RT (aa 1–305)] were inserted into a modified retroviral vector (RTV) containing a luciferase indicator gene in place of the HIV-1 envelope gene. Plasmids containing the RTV and a plasmid containing amphotropic murine leukemia virus (aMLV) were co-transfected in HEK-293 cells to generate pseudotyped virus particles containing the patient-derived PR/RT sequences with the aMLV envelope. Fresh HEK-293 cells were infected with the pseudotyped viruses in the absence of drugs, then RC was determined by comparing luciferase activity in infected cells with that of a wild-type reference virus (NL4-3) following a single round of replication. RC was expressed as a percentage relative to the reference strain (NL4-3). An RC of 100% indicates that the RC of the pseudotyped virus population closely approximates the median value of a wild-type (i.e., drug-sensitive) virus population.
Plasma HIV-1 RNA, CD4 and CD8 cell counts and advanced immunology flow cytometry were performed as previously reported (1). Plasma HIV-1 RNA levels were measured using the Roche Ultrasensitive Amplicor v1.0 PCR assay, with a lower limit of detection of 50 copies/mL at a central laboratory. Three-color flow cytometry was performed on fresh cells according to the ACTG advanced flow protocol at BL and week 48. Naïve T cells were defined as triple positive for CD45RA, CD62L and either CD4 or CD8; memory T cells were defined as positive for CD45RO, negative for CD45RA and positive for CD4 or CD8. Activated T cells were defined as triple positive for CD38, HLA-DR and either CD4 or CD8 (1).
BL covariates that have been shown to significantly affect disease progression, CD4 cell decline and/or on-therapy virologic or immunologic outcome were analyzed by univariate and multivariable analysis for association with RCBL. Spearman correlations, logistic regressions and Cox Proportional Hazard models, Wilcoxon Rank Sum and Fisher's Exact tests, as appropriate, were performed to determine the effect of RCBL on virologic and immunologic outcome measures. Multiple linear regression was performed to determine the effect of RCBL on ΔCD448 restricted to subjects with HIV-1 RNA < 50 copies/mL at week 48, controlling for CD4BL, RNABL, BL CD8 count, BL CD4 memory count and BL CD4 naïve percent (all covariates were continuous). All other analyses were based on all 321 subjects. Statistical tests were two-sided exploratory analyses and 0.05 was used for the nominal level of significance (without adjustments for multiple testing).
Results
Patients
BL characteristics and virologic and immunologic outcomes at week 48 are shown in Table 1. The subgroup of individuals with 350 or fewer CD4 cells/mm3 at BL (CD4BL) had significantly higher BL HIV-1 RNA (RNABL) levels than the subgroup with >350 cells/mm3 (p<0.0001).
Table 1.
BL Demographics and Immunologic and Virologic outcomes at Week 48
| CD4 <= 350 | CD4 > 350 | Total | ||
|---|---|---|---|---|
| Sample Sizes | 148 | 173 | 321 | |
| Gender | Male | 128 (86%) | 143 (83%) | 271 (84%) |
| Race/Ethnicitya | White | 68 (46%) | 91 (53%) | 159 (50%) |
| Black | 50 (34%) | 61 (35%) | 111 (35%) | |
| Hispanic | 28 (19%) | 17 (10%) | 45 (14%) | |
| Asian | 2 (1%) | 4 (2%) | 6 (2%) | |
| Intravenous Drug Useb | 9 (6%) | 10 (6%) | 19 (6%) | |
| Age (years) | Median (min-max) | 37 (21–65) | 35 (17–60) | 36 (17–65) |
| CD4 count (cells/mm3) | median (Q1 - Q3) | 118.5 (37, 241) | 479 (413, 593) | 369 (135, 501) |
| Immunologic success (ΔCD448 ≥ 100 cells/mm3) | N (%) | 115 (78%) | 118 (68%) | 233/321 (73%) |
| HIV-1 RNA(log10 copies/mL) | Median (Q1 - Q3) | 5.26 (4.75, 5.66) | 4.44 (4.02, 4.92) | 4.83 (4.28, 5.40) |
| Proportion with RNA < 50 c/ml at week 48 | N (%) | 148 (100%) | 140 (81%) | 288/321 (90%) |
Intravenous drug use – ever (current or past)
Self-reported
Replication Capacity
The distribution of RCBL for each of the five BL CD4 cell strata is illustrated by a box-and-whiskers plot in Figure 2. Median RCBL values for strata 1 through 5 were 148, 99, 85, 90, and 85%, respectively. RCBL was significantly higher in stratum 1 (CD4BL <= 50) than in the other 4 CD4 strata (p<0.0001).
Figure 2. RCBL versus CD4BL Cell Count.
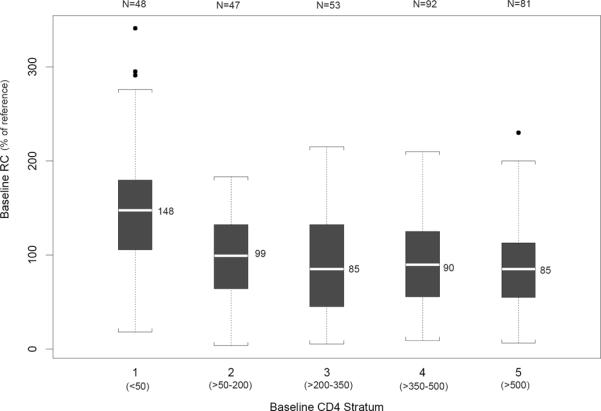
This box-and-whiskers plot illustrates the median RC value for each CD4 stratum (horizontal white line and adjacent value). The Q1 to Q3 (25th to 75th percentile) is depicted by the shaded box, the whiskers are drawn to the nearest value not beyond a standard span (1.5 × the interquartile range, defined as Q3 − Q1) from Q1 and Q3, and points beyond the whiskers (outliers) are drawn individually. RCBL was significantly greater in stratum 1 (CD4 <= 50) compared with the other 4 CD4 strata (p<0.0001).
Baseline Associations Between RC and Demographic and Immunologic/Virologic Factors
Higher RCBL was associated with lower CD4BL (r = −0.23, p<0.0001; adjusting for RNABL r = −0.12, p=0.033), higher RNABL (r = 0.25, p<0.0001; adjusting for CD4BL r = 0.16, p=0.005), higher BL CD4 activation percent (r = 0.23, p<0.0001) and lower BL CD4 memory count (r = −0.21, p = 0.0002). RCBL was not significantly associated with BL naïve/memory CD4 ratio, BL CD8 activation percent, gender, intravenous drug use (ever versus never), or race/ethnicity.
Association Between RCBL and Immunologic/Virologic Outcomes at Week 48
In unadjusted analyses, RCBL was not significantly associated with ΔCD448, immunologic success (defined as a greater than 100 cells/mm3 rise in CD4 cell count from BL to week 48), time-to-first virologic failure or time to the primary endpoint of the parent ACTG 384 study (defined as time from first treatment date to second regimen failure for three-drug arms, or time to first regimen failure for four-drug arms)(20,21).
In order to assess the contribution of baseline RC to CD4 recovery on treatment, a multiple regression model was constructed with ΔCD448 as the outcome variable. Univariate analyses (Spearman correlation) in the entire RC substudy cohort (N = 321) demonstrated that ΔCD448 was significantly associated with BL CD4 naïve percent (r = 0.21, p = 0.0002), BL naïve:memory CD4 ratio (r = 0.20, p = 0.0003), RNABL (r = 0.18, p = 0.001), BL memory CD4 count (r = −0.15, p = 0.008), BL CD8 cell count (r = −0.13, p = 0.02) and age (r = −0.13, p = 0.02). Additional significant baseline variables were considered from the multivariable model of Gandhi, et al, from the parent ACTG 384 Immunology Study (1). The final model included RCBL, CD4BL, CD8BL, RNABL, BL CD4 memory count and BL CD4 naïve percent. CD4 naive/memory ratio was not included because of the inclusion of CD4 memory and CD4 naïve variables, and age and gender were not included because they did not remain significant in our multivariable model. This analysis was restricted to subjects with HIV-1 RNA suppression to fewer than 50 copies/ml at week 48 (N = 288).
The multivariable model demonstrated that BL memory CD4 cell count was negatively associated with ΔCD448 (coefficient −0.35, p = 0.004) and BL naïve CD4 percent was positively associated with ΔCD448 (coefficient 1.47, p = 0.015). CD8BL and RNABL failed to show a significant association with ΔCD448 after adjusting for other covariates. In order to put these results in the context of other studies limited to subjects with early HIV infection who, in general, had higher CD4BL counts, a subgroup analysis was performed for subjects with CD4BL <= 350 or > 350 cells/mm3. An interaction between CD4BL and RCBL was suggested, which was then formally tested by including the interaction term (CD4BL * RCBL) in the multiple regression model (Table 2). This interaction was significant (coefficient 0.002, p = 0.018), precluding interpretation of a direct association between RCBL and ΔCD448.
Table 2.
Baseline factors associated with ΔCD448 (restricted to subjects with virologic suppression at week 48)
| BL Factor | Direction | Coefficient (95% C.I.) | P-value |
|---|---|---|---|
| Intercept | -- | 132.79 (−26.34, 291.92) | -- |
| RCBL (%) | neg | −0.51 (−1.01, −0.02) | 0.043+ |
| CD4BL (cells/mm3) | neg | −0.05 (−0.28, 0.18) | 0.065 |
| CD8BL (cells/mm3) | neg | −0.03 (−0.07, 0.01) | 0.144 |
| RNABL (log10 copies/ml) | pos | 24.38 (−1.91, 50.67) | 0.070 |
| BL CD4 Memory Count (cells/mm3) | neg | −0.35 (−0.58, −0.12) | 0.004++ |
| BL CD4 Naïve % | pos | 1.47 (0.30, 2.63) | 0.015+ |
| Interaction: CD4BL *RCBL | -- | 0.002 (0.0003, 0.003) | 0.018+ |
P < 0.05
P < 0.05
The interaction between CD4BL and RCBL in relation to ΔCD448 is illustrated in Figure 3A. Each dot represents one subject. A pattern of ΔCD448 according to RCBL and CD4 stratum is suggested by the univariate least squares fitted lines. It was noted that the vast majority of subjects with very high RCBL (above 200% of reference) were in stratum 1 (CD4BL <= 50) and that these high RC subjects had a ΔCD448 at or below the median for the entire group. In contrast, the highest CD4 stratum (CD4BL > 500) included a group of subjects with low RCBL and a net loss in CD4 cell count from BL to week 48.
Figure 3A. ΔCD448 and RCBL by BL CD4 Stratum (restricted to subjects with virologic suppression at week 48).
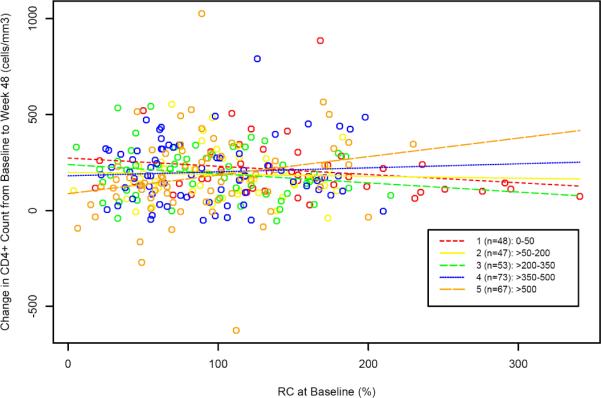
Individual subjects are depicted by colored circles corresponding to the five BL CD4 strata. Each corresponding colored line represents the univariate least squares fit (ΔCD448 on RCBL) generated for each stratum. The lines are drawn beyond the individual points to illustrate the relationships among the lines.
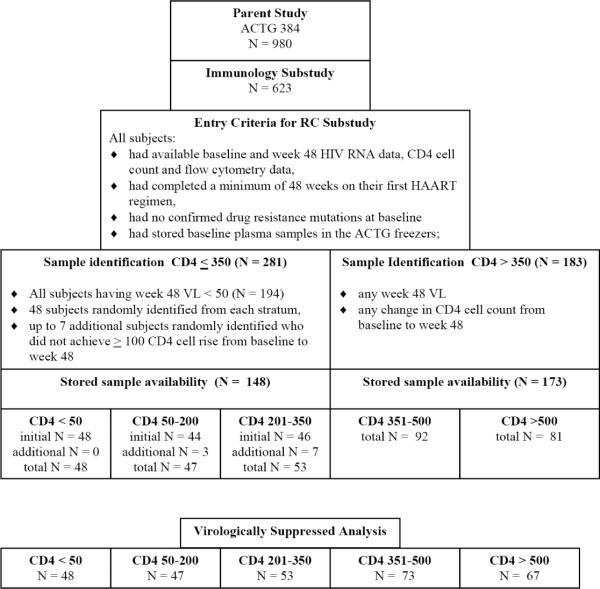
The value for ΔCD448 was associated not only with RCBL and CD4BL as depicted in Figure 3A, but also other BL factors, such as absolute memory CD4 count and the percentage of naïve CD4 cells. In order to focus on the relationship between CD4BL, RCBL and ΔCD448, the multiple regression model described above was used to generate fitted regression lines by controlling for (at their median values) BL CD8 count (779 cells/mm3), RNABL (4.85 log10 copies/ml), BL memory CD4 count (165 cell/mm3) and BL naïve CD4 percent (33%). CD4BL was placed in the model at values corresponding to the median values for the five BL CD4 strata. Figure 3B illustrates the fitted regression lines generated and the median RCBL for each of the five strata. Although there is some variability in these estimates, these lines illustrate the significant interaction term (CD4BL * RCBL) discussed above, with a positive association between RCBL and ΔCD448 in subjects with higher CD4BL and a negative association in subjects with lower CD4BL.
Figure 3B. Fitted Regression Lines for ΔCD448 and RCBL for Various CD4BL Values (restricted to subjects with virologic suppression at week 48).
Fitted regression lines are based on the model described in Table 2 (restricted to subjects with viral suppression at week 48), with controlled BL CD8 count, RNABL, BL CD4 memory count, BL CD4 naive percent at their median values (779 cells/mm3, 4.85 log10 copies/mL, 165 cells/mm3, and 33% respectively), and CD4BL at various values (medians for each of the 5 strata: 21.5, 113, 278, 410, and 593 cells/mm3). Circles represent the model-generated values of ΔCD448 at their median BL RCBL for the 5 strata. When CD4BL = 297, the slope of the fitted regression line is zero. The CD4BL × RCBL interaction was significant (p=0.018), with a positive association between RCBL and ΔCD448 in subjects with higher CD4BL, and a negative association in subjects with lower CD4BL. The lines are drawn beyond the data points used in the model in order to illustrate the relationships among the lines.
Discussion
Variation in CD4 recovery has been noted in previous analyses of treatment-naïve, HIV-1 infected individuals who achieve virologic suppression after initiating ART (1, 2, 8–10, 13, 24, 25). Although overall gains in CD4 cell count do not differ by BL CD4 count strata ((7) and G Robbins, submitted), a small but significant number of subjects do not achieve at least a 100 cell rise in CD4 count. In the analysis of the larger ACTG 384 Immunology substudy, of 608 subjects with virologic suppression to fewer than 50 copies/ml at week 24, only 255 (42%) achieved this definition of “immunologic success”(1). Although 87% of the 148 subjects who maintained virologic suppression through week 144 achieved immunologic success at this time point, 13% did not. These immunologic substudies not only describe changes in lymphocyte subsets on therapy, but attempt to understand why not all patients are able to achieve immunologic success.
The current study was broadly designed to evaluate the relationship between RC and other BL factors, and to evaluate RCBL in relation to immunologic and virologic response to ART. As described above, RCBL was significantly associated with CD4BL, RNABL, BL CD4 activation percent, and BL memory CD4 cell count.
Three previous studies evaluated similar BL associations with RCBL in individuals who were naive or minimally exposed to antiretroviral therapy (14–15,17). The significant negative association between RCBL and CD4BL in our study is in agreement with these previous analyses. In the recently infected cohort of Barbour, et. al., a significant inverse association between RCBL and CD4BL cell count was seen (r = −0.29, P = <0.0001)(15). In contrast, in study populations with more advanced disease, weaker inverse associations between RCBL and CD4BL were seen (r = −0.197, P = 0.03(14) and r = 0.065, P = 0.0031(17)). The current analysis demonstrated a highly significant association (r = −0.23, p < 0.0001), which remained significant after controlling for HIV-1 RNABL (r = −.012, p = 0.033) in a large and uniformly antiretroviral treatment-naïve population across a broad spectrum of CD4BL.
Previous evaluations of RC and plasma HIV-1 RNA levels have shown weak positive associations, at best. One study found no significant association (r = 0.08, p = 0.25), whereas another study found a weak association (r = 0.189, p = 0.03) (14, 15). These study populations differed from the current study in regard to stage of disease, presence of antiretroviral resistance, and/or previous antiretroviral experience. In contrast, our study of a larger number of uniformly treatment-naïve subjects found a highly significant positive association between RCBL and RNABL (r = 0.25, p<0.0001) that remained significant after adjusting for CD4BL (r = 0.16, p = 0.005). Whether higher RC leads directly to an increase in circulating virus or whether indirect effects are exerted through another mechanism remains to be elucidated.
As a substudy of the larger ACTG 384 Immunology Study, this analysis was able to utilize advanced flow analysis results to investigate associations between RCBL and subpopulations of CD4 cells. This analysis demonstrated, for the first time, the highly significant positive association between RCBL and BL CD4 activation percent (r = 0.23, p <0.0001). The level of CD4 activation has been shown to be an independent predictor of CD4 decline and progression to AIDS (26–28). The relationship between immune system activation and lentivirus disease progression is complex and has been demonstrated to be independent of circulating viral load in humans (29–31) and non-human primates (32, 33). The association between RC and CD4 activation demonstrated in this study may suggest one mechanism by which RC contributes to CD4 cell decline and disease progression (14).
High RCBL was also associated with low BL CD4 memory cell count (r = −0.21, p = 0.0002) in this population. Low levels of circulating CD4 memory cells may reflect loss by direct infection by R5 virus (34, 35), loss via activation-induced cell death (36), and/or sequestration into lymph nodes during viremia (37–39). High RC viruses may contribute to CD4 memory cell loss directly through one of these mechanisms, or indirectly by a positive association with high viral load or CD4 cell activation. Thus, viruses with higher RC may contribute to CD4 decline and disease progression through an association with higher viral load, through an increase in the level of CD4 cell activation, and/or through enhanced loss of CD4 memory cells.
This analysis also evaluated the association between RCBL and immunologic/virologic outcomes on therapy. In initial univariate analyses, RCBL was not significantly associated with ΔCD448, immunologic success (ΔCD448 ≥ 100 cells/mm3), or time-to-first virologic failure. A subsequent model utilized ΔCD448 as the outcome variable and independent variables that were highly associated with ΔCD448 (RNABL, BL CD8 cell count, BL CD4 memory count and BL CD4 naïve CD4 percent) as well as CD4BL.
BL memory CD4 cell count was a significant negative predictor and BL naïve CD4 percent a significant positive predictor of ΔCD448. Accounting for these factors overwhelmed the effects of RNABL and BL CD8 cell count, such that ΔCD448 was no longer significantly associated with either of these factors. This relationship between baseline naïve and memory cell number, percent or ratio and CD4 recovery on ART is in agreement with other studies (1, 8, 9).
A significant interaction between RCBL and CD4BL was demonstrated, as illustrated by the fitted regression lines in Figure 3B. The explanation for why a positive relationship between RCBL and ΔCD448 exists in subjects with higher BL CD4BL and a negative relationship exists in subjects with lower BL CD4BL is uncertain. However, as noted in Figure 3A, individual subject data points from the highest and lowest BL CD4 cell strata stand out and provide a basis for generating hypotheses as to the potential role of RCBL in CD4 recovery.
From the model, in patients with higher CD4BL, lower RCBL was associated with a smaller ΔCD448. Figure 2 illustrates that a cluster of subjects with CD4BL > 500 had RCBL values in the lower half of the entire group, and demonstrated a net fall in CD4 cell count from BL to week 48. This suggests that low RC viruses in this population are of low pathogenicity and that suppression of virus replication reverses what is at most a low level of CD4 cell destruction or redistribution. The occurrence of poor CD4 recovery in patients initiating ART at high CD4 counts has been noted by others (2, 40, 41), and the demonstrated association with low RC may in part explain this observation.
Conversely, high RC viruses in this high CD4BL population are associated with a larger ΔCD448. This suggests that high RC viruses are more pathogenic, contributing to greater CD4 cell destruction or redistribution, potentially by effects on the level of CD4 activation or CD4 memory cell numbers. It would follow, then, that abrogation of replication of these high RC viruses by effective antiretroviral therapy would lead to greater CD4 count gains in these subjects, when compared to those subjects with lower RC viruses.
In the lowest BL CD4 stratum (CD4BL <=50 cells/mm3), higher RCBL was associated with a smaller ΔCD448. This suggests that high RC viruses may have exerted an effect on the body prior to the institution of ART, which prevented an optimal CD4 cell recovery. This would be the case if high RC viruses destroyed T cell precursors in the bone marrow or thymus, destroyed CD4 memory cells in lymphoid tissues, prevented their redistribution after viral suppression, or interfered with an effective immune response to the virus. The association of high RC viruses with high levels of CD4 activation and low CD4 memory cell counts at baseline may contribute to these mechanisms. In contrast to the situation in high CD4BL/high RCBL subjects, the poor CD4 recovery seen in low CD4BL/high RCBL subjects suggests that the damage from high RC viruses has already occurred and is relatively irreversible.
The potential role of RC as a contributing factor in HIV-1 disease progression and CD4 cell decline has been suggested by a previous study. In the Hemophilia Growth and Development study by Daar, et. al., RCBL was shown to be a predictor of clinical progression to AIDS, independent of HIV RNABL and CD4BL (p = 0.04) (14). Furthermore, higher RCBL values were significantly associated with a greater rate of decline of CD4 cell count during follow-up, controlling for time on study, antiretroviral use over time, CD4BL and RNABL (P < 0.0001) (14).
In summary, this analysis has demonstrated significant pre-therapy associations between higher RC viruses and either higher HIV-1 RNA level, higher levels of CD4 activation, lower CD4 count, or lower CD4 memory cell numbers. These factors may interact, directly or indirectly, to modify the extent to which CD4 recovery occurs in patients at different baseline CD4 counts. The contribution of other factors, such as viral coreceptor tropism (42), will be evaluated in ongoing analyses.
Acknowlegments
We thank the study participants, the pharmaceutical sponsors and the ACTG 384 protocol team and study sites for their contributions to this study. We are indebted to the members of the ACTG 384 Immunology Substudy team, Charles Hicks, Jason Barbour, Eric Daar and Eoin Coakley for helpful discussions. We also thank Jessica Hass and Monogram Biosciences for operational support.
Supported in part by the AIDS Clinical Trials Group funded by the National Institute of Allergy and Infectious Diseases, Grant # AI38858, AI68636, AI68634.
Acknowledgement Appendix for ACTG 384: sites and grant numbers
University of Cincinnati (A2401) Grant #AI-25897
Howard University (A5301)
University of California, San Diego (A0701) Grant # AI 27670
University of North Carolina at Chapel Hill (A3201) Grant #AI50410, AI25868 and RR00046
University of Miami (A0901) Grant # AI027675
Case Western Reserve University (A2501) Grant #AI25879
Ohio State University (A2301) Grant # AI25924
University of Southern California (A1201) Grant # AI27673
University of Pennsylvania, Philadelphia (A6201) Grant # AI32783
University of Puerto Rico (A5401) Grant # AI034832
University of Texas, Galveston (A6301) Grant # Al32782
University of Minnesota (A1501) Grant # AI27661
Indiana University Hospital (A2601) Grant # AI25859
Washington University (St. Louis) (A2101) Grant # AI25903
Northwestern University, Rush Medical Center and Cook County COREcenter A2705) Grant # AI25915
New York University/NYC HHC at Bellevue (A0401) Grant # AI -27665 and GCRC Grant # M01RR00096
University of Alabama at Birmingham (A5801) Grant # AI32775
University of Colorado Health Sciences Center, Denver (A6101) GCRC Grant # RR00051, ACTG Grant # AI32770
Stanford University (A0501) Grant #ACTG AI27666
Duke University Medical Center (A1601) Grant # AI3915607
Mass General Hospital, Beth Israel Deaconess Medical Center, Boston Medical Center Harvard (Massachusetts General Hospital)(A0101) Grant # AI27659
University of Hawaii (A5201) Grant AI27675
Johns Hopkins University (A0201) NIH grant # RR00052 and AI27668
University of Rochester Medical Center (A1101) Grant #AI27658, GCRC Grant # 5MO1RR00044
Tulane-LSU AIDS Clinical Trials Unit (A1701, A1702) Grant #AI38844, GCRC grant # 5M01RR05096
University of California, Los Angeles (A0601) Grant # AI27660
University of Washington (A1401) Grant # AI 27664
Mt. Sinai (A1801)
San Francisco General Hospital (A0801)
Cornell University (A2201) Grant # AI 46386 (Columbia-Cornell ACTU) and RR00047 (Cornell GCRC)
Dr. Stefano Vella, Dr. Antonio Chiesi, Dr. Romano Arcieri, Dr. Maria Franca Pirillo, Dr. Clementina Maria Galluzzo, Dr. Elena Angela Pia Germinario, Miss Roberta Amici, Mr. Massimo Marzi, Miss Anna Nobile, Miss Rossella Di Nallo, and Mr. Cosimo Polizzi – Istituto Superiore di Sanita (A7901) Grant: HIV Clinical Research Programme, Istituto Superiore di Sanità 1999–2002
Dr. Olga Coronado and Dr. Giovanni Fasulo - Ospedale Maggiore (A7902)
Prof. Giampiero Carosi and Prof. Francesco Castelli – Spedali Civili - Carosi (A7903)
Dr. Massimo Di Pietro and Dr. Francesca Vichi - Ospedale S. M. Annunziata (A7904)
Dr. Gaetana Sterrantino and Dr. Silvia Ambu – Ospedale Careggi (A7905)
Prof. Antonietta Cargnel and Dr. Paola Meraviglia – Ospedale Luigi Sacco – Cargnel (A7907)
Dr. Fosca Niero and Dr. Amedeo Capetti – Ospedale Luigi Sacco - Milazzo (A7908)
Dr. Antonella d'Arminio Monforte, Dr. Salvatore Sollima and Dr. Claudia Balotta – Ospedale Luigi Sacco - Moroni (A7909)
Prof. Salvatore Delia and Dr. Maria Ciardi – Universita di Roma- Delia (A7910)
Dr. Maria Luisa Soranzo and Dr. Antonio Macor – Ospedale Amadeo Di Savoia (A7911)
Dr. Gabriella d'Ettorre and Dr. Gabriele Forcina – Universita di Roma - Vullo (A7912)
Prof. Dante Bassetti and Dr. Antonio Di Biagio – Universita di Genova (A7915)
Prof. Lorenzo Minoli and Dr. Renato Maserati – IRCCS Policlinico S. Matteo – Minoli (A7918)
Prof. Florio Ghinelli and Dr. Laura Sighinolfi – Archispedale S. Anna (A7920)
Dr. Alessandra Riva and Prof. Giorgio Scalise – Azienda Ospedaliera Umberto I (A7921)
Dr. Domenico Santoro and Dr. Enrico Rinaldi – Ospedale Sant'Anna (A7922)
Prof. Francesco Chiodo and Dr. Marco Borderi – Policlinico S. Orsola (A7923)
Dr. Giovanni Guaraldi and Prof. Roberto Esposito – Universita delgi Studi di Modena (A7924)
Prof. Carlo Ferrari and Dr. Giancarlo Pasetti – Azienda Ospedaliera di Parma (A7925)
Prof. Nicola Abrescia and Dr. Annunziata Busto – Azienda Ospedaliera D. Cotugno – Abrescia (A7927)
Prof. Antonio Chirianni and Dr. Miriam Gargiulo – Azienda Opedaliera D. Cotugno- Chirianni (A7928)
Prof. Crescenzo M. Izzo and Costanza Sbreglia – Azienda Ospedaliera D. Cotugno - Izzo (A7929)
Prof. Francesco Alberici and Dr. Daria Sacchini – Azienda U.S.L. of Piacenza- Ospedale Civile (A7930)
Dr. Giacomo Magnani and Dr. Giuliana Zoboli – Arcispedale S. Maria Nuova (A7931)
grant support This project was supported by SBIR Grant Number R44AI050321 from the National Institute of Allergy and Infectious Diseases (NIAID) to Monogram Biosciences. ACTG 384 has been supported in part by Bristol Myers Squibb, GlaxoSmithKline, Agouron/Pfizer, Inc., and the National Institutes of Allergy and Infectious Diseases (NIAID), National Institutes of Health (NIH), grant numbers U01 AI38858, AI68636, AI68634.
Additional grant support: GS AI46381, GKR K01AI062435, RTG 1P30AI060354 and R01 AI066992, VAJ: U01 AI38858 (contract 201VC001).
Footnotes
conflict of interestGS: GlaxoSmithKline, Tobira Therapeutics (honoraria), Monogram Biosciences (travel grant); JS: none; JW, YL, and MB are employees of Monogram Biosciences; GKR: Boehringer Ingelheim Pharmaceuticals, Schering, Gilead (grants), Abbott Pharm, Boehringer Ingelheim (honoraria); VAJ: grant and research support from Agouron Pharmaceuticals, Bristol-Myers Squibb, GlaxoSmithKline, Monogram Biosciences, Inc, and Visible Genetics, Inc (later Bayer, now Siemens Medical Solutions Diagnostics); has served on the speaker's bureaus or received honoraria from Abbott Laboratories, GlaxoSmithKline, Monogram Biosciences, Inc; and has served on medical or clinical advisory boards of Bristol-Myers Squibb, GlaxoSmithKline, Monogram Biosciences, Inc, and Virco Lab, Inc.
EC: none; DMA: none; RTG: unrestricted educational grants from Tibotec, Gilead, Bristol-Myers Squibb, Abbott and Boehringer-Ingelheim, and research grant support from Pfizer and Glaxo-Smith Kline; RBP: grant support and been a consultant for BMS.
References
- 1.Gandhi RT, Spritzler J, Chan E, et al. Effect of baseline- and treatment-related factors on immunologic recovery after initiation of antiretroviral therapy in HIV-1-positive subjects: results from ACTG 384. J Acquir Immune Defic Syndr. 2006;42(4):426–34. doi: 10.1097/01.qai.0000226789.51992.3f. [DOI] [PubMed] [Google Scholar]
- 2.Wolbers M, Battegay M, Hirschel B, et al. CD4+ T-cell count increase in HIV-1-infected patients with suppressed viral load within 1 year after start of antiretroviral therapy. Antivir Ther. 2007;12(6):889–97. [PubMed] [Google Scholar]
- 3.Landay A, da Silva BA, King MS, et al. Evidence of ongoing immune reconstitution in subjects with sustained viral suppression following 6 years of lopinavir-ritonavir treatment. Clin Infect Dis. 2007;44(5):749–54. doi: 10.1086/511681. [DOI] [PubMed] [Google Scholar]
- 4.Kaufmann GR, Perrin L, Pantaleo G, et al. CD4 T-lymphocyte recovery in individuals with advanced HIV-1 infection receiving potent antiretroviral therapy for 4 years: the Swiss HIV Cohort Study. Arch Intern Med. 2003;163(18):2187–95. doi: 10.1001/archinte.163.18.2187. [DOI] [PubMed] [Google Scholar]
- 5.Hunt PW, Deeks SG, Rodriguez B, et al. Continued CD4 cell count increases in HIV-infected adults experiencing 4 years of viral suppression on antiretroviral therapy. AIDS. 2003;17(13):1907–15. doi: 10.1097/00002030-200309050-00009. [DOI] [PubMed] [Google Scholar]
- 6.Kaufmann GR, Furrer H, Ledergerber B, et al. Characteristics, determinants, and clinical relevance of CD4 T cell recovery to <500 cells/microL in HIV type 1-infected individuals receiving potent antiretroviral therapy. Clin Infect Dis. 2005;41(3):361–72. doi: 10.1086/431484. [DOI] [PubMed] [Google Scholar]
- 7.Moore RD, Keruly JC. CD4+ cell count 6 years after commencement of highly active antiretroviral therapy in persons with sustained virologic suppression. Clin Infect Dis. 2007;44(3):441–6. doi: 10.1086/510746. [DOI] [PubMed] [Google Scholar]
- 8.Mildvan D, Bosch RJ, Kim RS, et al. Immunophenotypic markers and antiretroviral therapy (IMART): T cell activation and maturation help predict treatment response. J Infect Dis. 2004;189(10):1811–20. doi: 10.1086/383277. [DOI] [PubMed] [Google Scholar]
- 9.Goicoechea M, Smith DM, Liu L, et al. Determinants of CD4+ T cell recovery during suppressive antiretroviral therapy: association of immune activation, T cell maturation markers, and cellular HIV-1 DNA. J Infect Dis. 2006;194(1):29–37. doi: 10.1086/504718. [DOI] [PubMed] [Google Scholar]
- 10.Fernandez S, Price P, McKinnon EJ, Nolan RC, French MA. Low CD4+ T-cell counts in HIV patients receiving effective antiretroviral therapy are associated with CD4+ T-cell activation and senescence but not with lower effector memory T-cell function. Clin Immunol. 2006;120(2):163–70. doi: 10.1016/j.clim.2006.04.570. [DOI] [PubMed] [Google Scholar]
- 11.Hunt PW, Martin JN, Sinclair E, et al. T cell activation is associated with lower CD4+ T cell gains in human immunodeficiency virus-infected patients with sustained viral suppression during antiretroviral therapy. J Infect Dis. 2003;187(10):1534–43. doi: 10.1086/374786. [DOI] [PubMed] [Google Scholar]
- 12.Anthony KB, Yoder C, Metcalf JA, et al. Incomplete CD4 T cell recovery in HIV-1 infection after 12 months of highly active antiretroviral therapy is associated with ongoing increased CD4 T cell activation and turnover. J Acquir Immune Defic Syndr. 2003;33(2):125–33. doi: 10.1097/00126334-200306010-00002. [DOI] [PubMed] [Google Scholar]
- 13.Deeks SG, Wrin T, Liegler T, et al. Virologic and immunologic consequences of discontinuing combination antiretroviral-drug therapy in HIV-infected patients with detectable viremia. N Engl J Med. 2001;344(7):472–80. doi: 10.1056/NEJM200102153440702. [DOI] [PubMed] [Google Scholar]
- 14.Daar ES, Kesler KL, Wrin T, et al. HIV-1 pol replication capacity predicts disease progression. AIDS. 2005;19(9):871–7. doi: 10.1097/01.aids.0000171400.15619.e1. [DOI] [PubMed] [Google Scholar]
- 15.Barbour JD, Hecht FM, Wrin T, et al. Higher CD4+ T cell counts associated with low viral pol replication capacity among treatment-naive adults in early HIV-1 infection. J Infect Dis. 2004;190(2):251–6. doi: 10.1086/422036. [DOI] [PubMed] [Google Scholar]
- 16.Barbour JD, Hecht FM, Little SJ, et al. Greater CD4 T-cell gains after one year of antiretroviral therapy are associated with lower HIV-1 pol replication capacity. AIDS. 2006;20(16):2123–5. doi: 10.1097/01.aids.0000247583.38943.95. [DOI] [PubMed] [Google Scholar]
- 17.Hicks C, Eron J, Keiser P, et al. HIV-1 replication capacity as an independent predictor of pre-treatment CD4 lymphocyte count. 12th Conference on Retroviruses and Opportunistic Infections; Boston, MA. 2005. Abstract #345. [Google Scholar]
- 18.De Luca A, Weidler J, Di Giambenedetto S, et al. Association of HIV-1 replication capacity with treatment outcomes in patients with virologic treatment failure. J Acquir Immune Defic Syndr. 2007;45(4):411–7. doi: 10.1097/QAI.0b013e318074f008. [DOI] [PubMed] [Google Scholar]
- 19.Haubrich R, Wrin T, Hellmann N, McCutchan JA, Keiser P, Kemper C, Witt M, Leedom J, Forthal D, Richman D, the CCTG Replication Capacity (RC) as a Predictor of Immunologic and Virologic Benefit Despite Virologic Failure of an Antiretroviral Regimen. Antiviral Therapy. 2002;7:S101. [Google Scholar]
- 20.Shafer RW, Smeaton LM, Robbins GK, et al. Comparison of four-drug regimens and pairs of sequential three-drug regimens as initial therapy for HIV-1 infection. N Engl J Med. 2003;349(24):2304–15. doi: 10.1056/NEJMoa030265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Robbins GK, De Gruttola V, Shafer RW, et al. Comparison of sequential three-drug regimens as initial therapy for HIV-1 infection. N Engl J Med. 2003;349(24):2293–303. doi: 10.1056/NEJMoa030264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Petropoulos CJ, Parkin NT, Limoli KL, et al. A novel phenotypic drug susceptibility assay for human immunodeficiency virus type 1. Antimicrob Agents Chemother. 2000;44(4):920–8. doi: 10.1128/aac.44.4.920-928.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Campbell TB, Schneider K, Wrin T, Petropoulos CJ, Connick E. Relationship between in vitro human immunodeficiency virus type 1 replication rate and virus load in plasma. J Virol. 2003;77(22):12105–12. doi: 10.1128/JVI.77.22.12105-12112.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Moore DM, Hogg RS, Yip B, et al. Discordant immunologic and virologic responses to highly active antiretroviral therapy are associated with increased mortality and poor adherence to therapy. J Acquir Immune Defic Syndr. 2005;40(3):288–93. doi: 10.1097/01.qai.0000182847.38098.d1. [DOI] [PubMed] [Google Scholar]
- 25.Schechter M, Tuboi SH. Discordant immunological and virological responses to antiretroviral therapy. J Antimicrob Chemother. 2006;58(3):506–10. doi: 10.1093/jac/dkl263. [DOI] [PubMed] [Google Scholar]
- 26.Carbone J, Gil J, Benito JM, et al. Increased levels of activated subsets of CD4 T cells add to the prognostic value of low CD4 T cell counts in a cohort of HIV-infected drug users. AIDS. 2000;14(18):2823–9. doi: 10.1097/00002030-200012220-00003. [DOI] [PubMed] [Google Scholar]
- 27.Leng Q, Borkow G, Weisman Z, Stein M, Kalinkovich A, Bentwich Z. Immune activation correlates better than HIV plasma viral load with CD4 T-cell decline during HIV infection. J Acquir Immune Defic Syndr. 2001;27(4):389–97. doi: 10.1097/00126334-200108010-00010. [DOI] [PubMed] [Google Scholar]
- 28.Hazenberg MD, Otto SA, van Benthem BH. Persistent immune activation in HIV-1 infection is associated with progression to AIDS. AIDS. 2003;17(13):1881–8. doi: 10.1097/00002030-200309050-00006. [DOI] [PubMed] [Google Scholar]
- 29.Choudhary SK, Vrisekoop N, Jansen CA, et al. Low immune activation despite high levels of pathogenic human immunodeficiency virus type 1 results in long-term asymptomatic disease. J Virol. 2007;81(16):8838–42. doi: 10.1128/JVI.02663-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Sousa AE, Carneiro J, Meier-Schellersheim M, Grossman Z, Victorino RM. CD4 T cell depletion is linked directly to immune activation in the pathogenesis of HIV-1 and HIV-2 but only indirectly to the viral load. J Immunol. 2002;169(6):3400–6. doi: 10.4049/jimmunol.169.6.3400. [DOI] [PubMed] [Google Scholar]
- 31.Deeks SG, Hoh R, Grant RM, et al. CD4+ T cell kinetics and activation in human immunodeficiency virus-infected patients who remain viremic despite long-term treatment with protease inhibitor-based therapy. J Infect Dis. 2002;185(3):315–23. doi: 10.1086/338467. [DOI] [PubMed] [Google Scholar]
- 32.Silvestri G, Sodora DL, Koup RA, et al. Nonpathogenic SIV infection of sooty mangabeys is characterized by limited bystander immunopathology despite chronic high-level viremia. Immunity. 2003;18(3):441–52. doi: 10.1016/s1074-7613(03)00060-8. [DOI] [PubMed] [Google Scholar]
- 33.Broussard SR, Staprans SI, White R, Whitehead EM, Feinberg MB, Allan JS. Simian immunodeficiency virus replicates to high levels in naturally infected African green monkeys without inducing immunologic or neurologic disease. J Virol. 2001;75(5):2262–75. doi: 10.1128/JVI.75.5.2262-2275.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Mehandru S, Poles MA, Tenner-Racz K, et al. Primary HIV-1 infection is associated with preferential depletion of CD4+ T lymphocytes from effector sites in the gastrointestinal tract. J Exp Med. 2004;200(6):761–70. doi: 10.1084/jem.20041196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Brenchley JM, Schacker TW, Ruff LE, et al. CD4+ T cell depletion during all stages of HIV disease occurs predominantly in the gastrointestinal tract. J Exp Med. 2004;200(6):749–59. doi: 10.1084/jem.20040874. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Hazenberg MD, Hamann D, Schuitemaker H, Miedema F. T cell depletion in HIV-1 infection: how CD4+ T cells go out of stock. Nat Immunol. 2000;1(4):285–9. doi: 10.1038/79724. [DOI] [PubMed] [Google Scholar]
- 37.Pakker NG, Notermans DW, de Boer RJ, et al. Biphasic kinetics of peripheral blood T cells after triple combination therapy in HIV-1 infection: a composite of redistribution and proliferation. Nat Med. 1998;4(2):208–14. doi: 10.1038/nm0298-208. [DOI] [PubMed] [Google Scholar]
- 38.Bucy RP, Hockett RD, Derdeyn CA, et al. Initial increase in blood CD4+ lymphocytes after HIV antiretroviral therapy reflects redistribution from lymphoid tissues. J Clin Invest. 1999;103(10):1391–8. doi: 10.1172/JCI5863. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Lederman MM, Connick E, Landay A, et al. Immunologic responses associated with 12 weeks of combination antiretroviral therapy consisting of zidovudine, lamivudine, and ritonavir: results of AIDS Clinical Trials Group Protocol 315. J Infect Dis. 1998;178(1):70–9. doi: 10.1086/515591. [DOI] [PubMed] [Google Scholar]
- 40.Montaner JLM, Hill A, Cutrell A, Tortell S, Thornborn D. Discordant CD4/RNA responses to HAART are strongly associated with high-baseline CD4 count and low HIVRNA: Analysis of 406 naive patients. Abstr Intersci Conf Antimicrob Agents Chemother. 1999 Sep 26–29; Abstract #1993. [Google Scholar]
- 41.Lederman MM, Valdez H. Immune restoration with antiretroviral therapies: implications for clinical management. JAMA. 2000;284(2):223–8. doi: 10.1001/jama.284.2.223. [DOI] [PubMed] [Google Scholar]
- 42.Daar ES, Kesler KL, Petropoulos CJ, et al. Baseline HIV type 1 coreceptor tropism predicts disease progression. Clin Infect Dis. 2007;45(5):643–9. doi: 10.1086/520650. [DOI] [PubMed] [Google Scholar]