Abstract
Background
Histone deacetylases (HDACs) influence chromatin organization, representing a key epigenetic regulatory mechanism in cells. Trichostatin A (TSA), a potent HDAC inhibitor, has anti-tumor and anti-inflammatory effects. Allergic contact dermatitis (ACD) is a T-cell-mediated inflammatory reaction in skin and is regulated by epidermal Langerhans cells (LCs).
Objective
The aim of this study was to investigate if TSA treatment prevents 2,4-dinitrofluorobenzene (DNFB)-induced ACD in mice and regulates epidermal LCs and other immune cells during ACD development.
Methods
ACD was induced by sensitizing and challenging with DNFB topically. Mice were treated intraperitoneally with TSA or vehicle DMSO as a control every other day before and during induction of ACD. The ear swelling response was measured and skin biopsies from sensitized skin areas were obtained for histology. Epidermal cells, thymus, spleens and skin draining lymph nodes were collected for immune staining.
Results
TSA treatment ameliorated skin lesion severity of DNFB-induced ACD. The percentages of epidermal LCs and splenic DCs as well as LC maturation were significantly reduced in TSA-treated mice. However, TSA treatment did not significantly affect the homeostasis of conventional CD4+ and CD8+ T cells, Foxp3+CD4+ regulatory T cells, iNKT cells, and γδ T cells in thymus, spleen and draining lymph nodes (dLNs). Furthermore, there were no significant differences in IL-4 and IFN-γ-producing T cells and iNKT cells between TSA- and DMSO-treated mice.
Conclusion
Our findings suggest that TSA may ameliorate ACD through the regulation of epidermal LCs and HDACs could serve as potential therapeutic targets for ACD and other LCs-related skin diseases.
Keywords: Trichostatin A, Histone deacetylases, Allergic contact dermatitis, Langerhans cells, NKT cells, Regulatory T cells
1. Introduction
Allergic contact dermatitis (ACD) is one of the leading causes for occupational diseases and it has great socio-economic impact [1]. Knowledge of the pathophysiology of ACD is derived mainly from animal models in which the skin inflammation is induced by painting haptens on the skin. ACD is a T-cell-mediated skin inflammation caused by skin repeated exposure to the haptens, such as 2,4-dinitrofluorobenzene (DNFB). The hapten first penetrates the epidermis and is taken up by skin dendritic cells (DCs), including epidermis-resident DCs, Langerhans cells (LCs) and dermal DCs, which process the allergen internally into a complete antigen. Skin DCs then migrate to the drain lymph nodes (dLNs) and present haptenated peptide to naïve antigen-specific T lymphocytes. As a result, antigen-specific CD4+ and CD8+ T cells are primed [2, 3]. Upon challenging the skin with the same hapten, the haptenated protein is presented by LCs and/or other DCs to recruited hapten-specific T cells to the skin. An inflammatory response is elicited to the skin and the subsequent production of inflammatory cytokines and chemokines [4]. Both DCs and T cells play critical roles in the development of ACD. LCs are members of the heterogenous family of professional antigen presenting cells (APCs), and specifically express Langerin (CD207), a C type lectin [5]. LCs form a contiguous network to detect invading pathogens or antigens in skin, and have classically been thought to play a pivotal role in the initiation and control of skin immunity and allergy [6]. However, more recent studies suggest LCs may have immunoregulatory functions as well [7–9].
Post-translational modification of histones is an epigenetic regulatory mechanism crucial for the regulation of gene expression. It is widely accepted that densely packed DNA structure is related to histone acetylation status. Histone acetylation status is associated with transcription regulation and controlled by histone acetyltransferases (HATs) and histone deacetylases (HDACs) [10, 11]. HDACs are enzymes that remove acetyl groups from specific lysine residues on histone proteins to regulate chromatin architecture and gene expression [12]. In general, histone acetylation relaxes chromatin structure and promotes gene transcription by allowing transcription factors and regulatory proteins access to DNA [10]. HDAC inhibitors (HDACi) can block HDAC activity and are shown to have anti-tumor and anti-inflammatory effects in a variety of tumors, autoimmune and inflammatory diseases [13–15]. However, the molecular mechanisms of its effects on these diseases are not very clear. Trichostatin A (TSA) is a well-known potent HDAC inhibitor [16], which has been considered a potential therapeutic agent against inflammatory diseases and autoimmune diseases [17, 18]. We recently reported that TSA reduced the number of LCs in vitro. Moreover, histone deacetylase activity is required for normal LC maturation and phagocytosis [19]. In the present study, we investigated the effects of TSA on 2,4-dinitrofluorobenzene (DNFB)-induced ACD and on the regulation on epidermal LCs and other immune cells during the induction of ACD in TSA treated mice.
2. Materials and methods
2.1. Mice
C57BL/6 mice were obtained from the Jackson Laboratory. All experiments were performed with 6 to 8-wk-old mice. Mice were housed in a specific pathogen-free barrier unit. Handling of mice and experimental procedures were approved by the Institutional Animal Care and Use Committee (IACUC) of the Henry Ford Health System.
2.2. Hapten sensitization and elicitation of ACD
Mice were sensitized by applying 25μl 0.5% DNFB (Sigma-Aldrich, St. Louis, MO, USA) (acetone:olive oil=4:1) topically on shaved abdominal skin on day 0. On day 5, sensitized mice were challenged topically with 10μl 0.2% DNFB on the left ear. An identical volume of acetone/olive oil was painted on the right ear. Ear thicknesses were measured in a blinded fashion by comparing challenged (left) and unchallenged (right) ears using a thickness gauge (Digimatic caliper, Mitutoyo, Japan) before and at 24, 48 and 72h after the challenge, and ear thickness increases were calculated by subtracting pre-challenge (0 h) from post-challenge measurements (24h, 48h, 72h).
2.3. TSA treatment
TSA (Sigma-Aldrich, St. Louis, MO, USA) was dissolved in 1% dimethyl sulfoxide (DMSO) and mice were injected intraperitoneally with TSA (1mg/kg) or DMSO only every other day beginning 2 days before applying 0.5% DNFB. Mice were sacrificed at 72h after sensitization with TSA treatment (for three doses) or sacrificed at 72h after challenge with TSA treatment (for five doses).
2.4. Histological analysis
Ear samples were taken from sacrificed mice at 72h after challenge, fixed in 10% formalin, embedded in paraffin, sectioned at 3μm, and stained with hematoxylin and eosin (H &E).
2.5. Preparation of single-cell suspensions of epidermis and antigen-uptake assay
Whole body skin was collected from sacrificed mice at 72h after sensitization and 72h after challenge, respectively. The skin were floated on PBS/dispase (2.5mg/ml, Gibco® Grand Island, NY, USA) for 1h in 37°C incubator. Epidermis separated from dermis was digested with DNase (1mg/ml, Worthington Biochemical, Lankewood, NJ, USA) for 50 minutes in 37°C Shaking Bath. Single-cell suspensions were stained with anti-Langerin(clone 929F3.01), anti-MHC- II(M5/114.15.2) and anti-CD45.2 (104) antibodies. For the antigen uptake assay, epidermis cells were incubated at 37°C or 4°C (as control) with FITC-dextran for 45 minutes and then stained with anti-mouse MHC-II and CD45.2 antibodies. For LCs maturation assay, epidermal single-cell suspensions were cultured in RPMI 1640 medium for 60 hours, then stained with anti-Langerin, MHC- II, CD80, CD86 Abs.
2.6. Immune staining for conventional T cells, NKT and Treg cells
Single-cell suspensions from skin dLNs, spleen and thymus from sacrificed mice at 72h after challenge with ACD were resuspended with staining buffer (1×PBS, 2% FBS) and then were stained with anti-CD4 (RM4–5), anti-CD8 (53–6.7), anti-TCRγδ (GL3), anti-TCRβ (H57–597) and α-GalCer/CD1d tetramer (Tetramer Facility, National Institutes of Health). For intracellular staining with anti- Foxp3(FJK-16s), cells were first stained with anti-CD4, then permeabilized and fixed with fixation/permeabilization solution on ice for 40 minutes. Foxp3 was added for extra 30 minutes staining on ice according to the manufacturer’s instructions (eBioscience, San Diego, CA, USA). The data were acquired on a FACS AriaII (BD Bioscience, San Jose, CA, USA) and analyzed using Flowjo software (Tree Star, Inc, Ashland, OR, USA).
2.7. T cell stimulation and intracellular cytokine staining
Single-cell suspensions (2 × 106/ml) were cultured in 24 well plate with RPMI 1640 medium supplemented with 10% FBS, 1% glutamine, 1% penicillin/streptomycin, 1% HEPES, 5.5 μM 2- mercaptoethanol, and stimulated for 5 h (3h for NKT cells intracellular staining) at 37°C with 50ng/ml of PMA, 1μmol/ml of Ionomycin in the presence of 0.67 μl/ml Golgi stop. After incubation, the surface markers were first stained with related antibodies. After fixation/Permeabilization for 20 min at 4°C, intracellular cytokines were stained with anti-IFNγ (XMG1.2), anti-IL-4 (11B11) and anti-IL-17 (eBio17B7). The data were acquired on a FACS AriaII (BD Bioscience, San Jose, CA, USA) and analyzed using Flowjo software (Tree Star, Inc, Ashland, OR, USA). Antibodies were purchased from BD Biosciences or eBioscience.
2.8. Statistics
Data are presented as mean ± SD. Statistics analysis was performed with Microsoft Excel 2007 (Microsoft Corporation, NY, USA) and GraphPad Prism software (GraphPad, San Diego, CA, USA) using a two-tailed Student t test with level of significance: *p<0.05.
3. Results
3.1. TSA ameliorated DNFB-induced allergic contact dermatitis
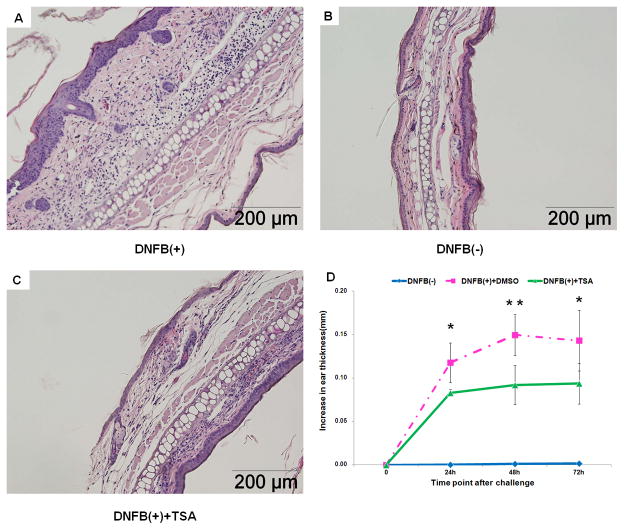
TSA has demonstrated potent anti-inflammatory effects in experimental animal models of inflammatory diseases. We first investigated whether TSA can inhibit DNFB-induced ACD. The mice were treated with TSA (1mg/kg) or DMSO alone as the control every other day for 5 times, starting 2 days before DNFB sensitized and ending 1 day after DNFB challenged. Application of DNFB to ears induced severe inflammation, such as, edema and epidermal hyperplasia, which were associated with the infiltration of large numbers of inflammatory cells into skin lesions (Fig. 1A). As expected, the application of acetone/olive oil only (vehicle) did not induce any obvious inflammation (Fig. 1C). However, TSA administration inhibited DNFB-induced inflammatory cell infiltration (Fig. 1B) and significantly reduced ear thickness at 24h, 48h and 72h after DNFB challenge (Fig. 1D). Hence, TSA treatment ameliorates DNFB-induced ACD.
Fig. 1. TSA treatment ameliorated DNFB-induced allergic contact dermatitis.
Histopathology (H&E staining) of ear lesions 72h after challenge in TSA treated or DMSO treated mice. A, DNFB(+): DNFB-induced ACD treated with DMSO (1%). B, DNFB(+)+TSA: DNFB-induced ACD treated with TSA (1mg/kg). C, DNFB(-): Vehicle alone controls: acetone:olive applied on the right ear. D, Specific ear swelling is shown in different time point during DNFB-induced ACD. They are representative of three experiments with 5 mice per group. Mean±SD values of five mice are shown. *p<0.05, ** p<0.01.
3.2. TSA treatment reduced the number and maturation of epidermal Langerhans cells, but did not affect epidermal γδ T cells during the ACD development
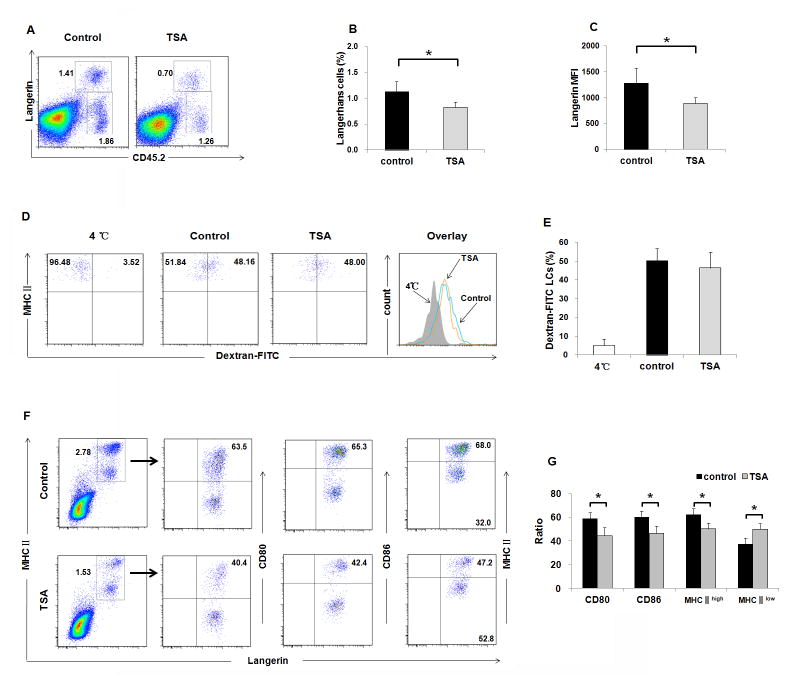
Previous studies indicated that LCs are involved in ACD [20] and epidermal γδ T cells also control cutaneous inflammation [21]. We recently reported that histone deacetylase activity is required for normal skin Langerhans cell maturation and phagocytosis and that TSA reduced the number of LCs but did not affect epidermal γδ T cells in vitro [19]. To test if LCs and γδ T cells were involved in the TSA-mediated amelioration of ACD, we evaluated the number and function of epidermal LCs and γδ T cells after TSA treatment. As shown in Fig. 2A&B, the percentage of epidermal LCs (Langerin+CD45.2 +) was dramatically reduced after TSA treatment (P < 0.05). The percentage of epidermal γδ T cells (Langerin− CD45.2+) was comparable between the TSA-treated group and DMSO-treated control group (Fig. S1A). Consistent with previous finding of TSA treatment in vitro, the expression levels of langerin represented by mean fluorescence intensity (MFI) in LCs were significantly reduced in TSA-treated mice compared to DMSO-treated control mice (Fig. 2C). Due to their physical location, LCs acquire and process antigens, and subsequently migrate to skin dLNs to present both foreign and self-antigens to T lymphocytes, leading to the induction of immunity or tolerance [22]. To evaluate the role of HDACs in antigen uptake function of LCs during the induction of ACD, we used dextran-FITC as an antigen to observe the phagocytic capacity of LCs from mice treated with TSA. As shown in Fig. 2D&E, no significant difference of LCs’ phagocytic capacity was detected comparing TSA-treated mice with DMSO-treated control mice (P > 0.05). When LCs are mature, they highly express some surface proteins necessary for stimulation of T cells, including CD80, CD86, MHC-II. As shown in Fig. 2F&2G, both the percentages of CD80+Langerin+ and CD86+Langerin+ LCs were significant reduced in TSA-treated mice compared to DMSO-treated control mice (P<0.05). Furthermore, the percentage of mature MHC-IIhigh LCs decreased, while immature MHC-IIlow LCs increased in TSA-treated mice (P<0.05). We also examined the phenotype of LCs in the sensitization phase after TSA treatment. As shown in Fig. S2A-F, no significant differences were observed in the number, phagocytic capacity, and maturation of LCs. Thus, in vivo inhibition of HDAC activation reduces the number and maturation of LCs, which could contribute to TSA-mediated ACD protection.
Fig. 2. TSA treatment reduced the number and maturation of epidermal LCs during ACD development.
Epidermal cells were isolated from the skin of ACD mice (TSA-treated mice and DMSO-treated control mice). (A) Representative FACS dot plots for LCs identified as Langerin+ CD45.2+. (B) A significant difference was observed in the frequencies of LCs. P<0.05. (C) The expression levels of langerin represented by mean fluorescence intensity (MFI) in LCs were significantly reduced in TSA-treated mice compared to DMSO-treated control mice, P<0.05. (D&E) LCs from TSA-treated mice were able to phagocyte Dextran-FITC as efficiently as LCs from DMSO-treated mice. Numbers in histogram indicate geometric mean fluorescence of test samples. 4°C indicated that cells were incubated with Dextran-FITC at 4°C, as negative control. (F&G) Epidermal cell suspensions were cultured in RPMI 1640 medium for 60 hours, and the percentages of CD80+Langerin+ and CD86+Langerin+ LCs were significant reduced in TSA-treated mice. The percentage of mature MHC-IIhigh LCs was decreased, while immature MHC-IIlow LCs were increased in TSA-treated mice, P<0.05. Representative results of three independent experiments are shown. Data are presented as mean ± SD (N=5/group).
3.3 TSA treatment reduced the frequencies of DCs in spleen, but did not affect the frequencies of migrated epidermal LCs and dermal langerin+ DCs
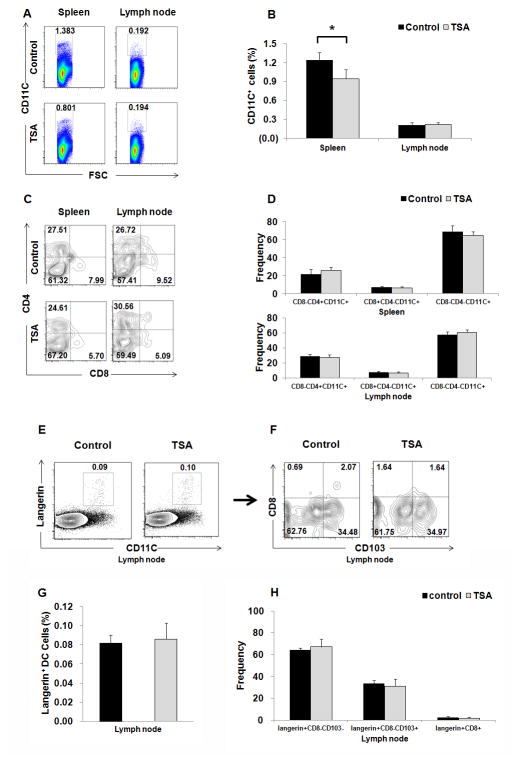
Previous studies indicated that HDACi blocked GM-CSF-induced DC development from bone marrow precursors in vitro [23, 24]. Next, we determined whether TSA administration could affect the frequency of DCs in different lymphoid organs. As depicted in Fig. 3A & 3B, the frequencies of DCs in spleen was significantly reduced in TSA-treated mice compared to DMSO-treated control mice (P < 0.05). However, there were no significant differences on the percentage of CD8+CD4−CD11C+, CD8−CD4−CD11C+, and CD8−CD4+CD11C+ subpopulations of DCs between two groups (Fig. 3C & 3D). The frequencies of DCs in the lymph nodes and their maturation based on CD80 and CD86 expression were comparable between TSA-treated mice and DMSO-treated control mice (Fig. S1C). During the ACD induction, skin dendritic cells first uptake the hapten, and then migrate to dLNs and present haptenated peptide to naïve antigen-specific T lymphocytes. Reduced LCs in epidermis in TSA-treated mice could be caused by enhanced LC migration to dLNs. We next investigated skin migrated DCs in dLNs. There are at least two different migrated skin DCs in dLNs, Langerin+CD103−CD8−CD11C+ migrated LCs and Langerin+CD103+CD8−CD11C+ migrated dermal DCs, while Langerin+CD8+CD11C+ DCs are LN-resident DCs (Fig. 3E & 3F). We did not find any significant differences in these subsets of DCs in dLNs (Fig. 3G & 3H) between TSA-treated group and DMSO-treated control group.
Fig. 3. TSA treatment reduced the frequencies of DCs in spleen during the ACD development, but did not affect the frequencies of migrated epidermal LC and langerin+ dermal DCs in dLNs.
(A) Representative FACS dot plots for CD11C+ cells in spleen and dLNs from ACD mice treated with TSA or DMSO as controls. (B) Summary of data for the frequencies of CD11C+ cells in spleen and dLNs in the two groups, *p<0.05. (C) Representative FACS dot plots for subpopulations of CD11C+DCs based on CD4 and CD8 expression. (D) Summary of data for the frequencies of CD8+CD4−CD11C+, CD8−CD4−CD11C+, and CD8−CD4+CD11C+ cells in spleen and dLNs. (E) Representative FACS dot plots for skin immigrant DCs in dLNs. The migrated epidermal LCs (CD11C+langerin+CD103−CD8−DCs), migrated dermal langerin+ DCs (CD11C+langerin+CD103+CD8−), and LN-resident langerin+ DCs (CD11C+langerin+CD8+) were analyzed on gated Langerin+CD11c+ DCs in dLN. (F) Summary of data for the frequencies of CD11C+langerin+CD8−CD103−, CD11C+ langerin+ CD8− CD103+, and CD11C+ langerin+ CD8+ cells in dLNs. Representative results of three independent experiments are shown. Data are presented as mean ± SD (N=5/group).
3.4. TSA treatment did not influence conventional T-cell number and function
Given that T cells are the critical cellular immune mediators in ACD [25], to elucidate the cellular mechanisms by which TSA suppressed ACD response, we tested whether T cell numbers and function were changed in the different lymphoid organs at 72h after challenge. As shown in Fig. S3A & B, there was no significant difference in the percentages of CD4+ and CD8+ T cells from thymus, spleen and lymph node between TSA-treated mice and DMSO-treated control mice. The γδ T cells in TSA-treated mice were comparable to that in the different lymphoid organs from DMSO-treated control mice (Fig. S1B). DNFB-induced ACD is controlled by IFN-γ, IL-4 and IL-17 produced by Th1, Th2 and Th17 T cells, respectively [26]. We next investigated whether TSA regulated cytokine production by T cells. No significant difference was found in the frequencies of IFN-γ- and IL-4-producing CD4+ (Fig. S3C & D) or CD8+ (Fig. S3E & F) T cells. Furthermore, the frequencies of IL17+CD4+ cells (Fig. S3G & H) in TSA-treated mice were comparable to that in DMSO-treated control mice. Thus, TSA under current condition did not affect the number and function of conventional T cells.
3.5. TSA treatment did not alter Foxp3+ regulatory T cells and iNKT cells
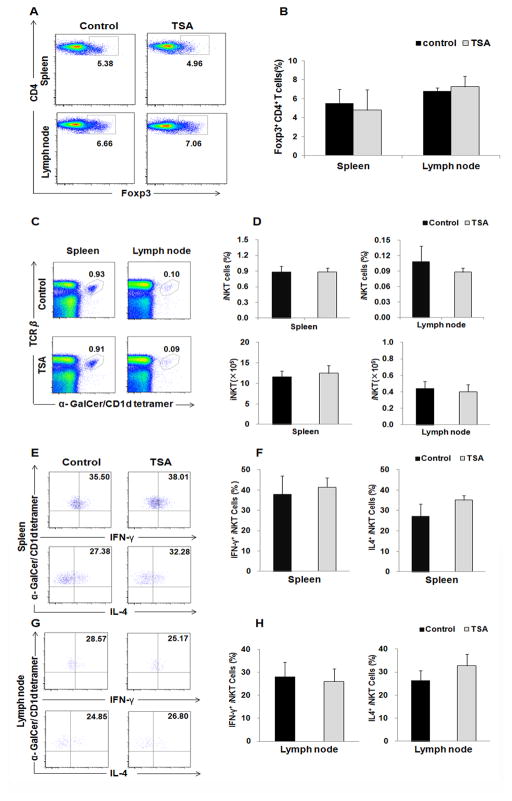
Foxp3+ regulatory T cells (Tregs) suppress ACD development [26]. We next sought to determine whether Tregs participated in TSA suppression of ACD. Analysis of CD4+ T cells from spleen and dLNs showed the equal frequencies of Foxp3+ cells in TSA-treated and DMSO-treated control mice (Fig. 4A & B). Previous studies showed that deletion of iNKT cells prevented ACD while iNKT cell activation enhanced ACD progression, suggesting a role of iNKT cells in ACD pathogenesis [21, 27]. We further assessed the role of TSA treatment in iNKT cells. The percentages of iNKT cells were stained with anti-TCRβ and α-GalCer-loaded CD1d tetramer in the spleen and lymph node (Fig 4C). There was no significant difference in the percentage and absolute number of iNKT cells between the two groups (Fig. 4D). We further examined the function of iNKT cells. No significant difference was identified in IL-4 and IFN-γ-producing iNKT cells in spleens (Fig. 4E & F) and dLNs (Fig. SG & H) between the two groups. Thus, TSA under the current condition did not affect the number of Foxp3+ regulatory T cells and iNKT cells, as well as iNKT cell function.
Fig. 4. TSA treatment did not affect the frequencies of Tregs and iNKT cells, as well as the function of iNKT cells.
(A) Foxp3 expression was detected by intracellular staining. Treg cells were identified as CD4+ Foxp3+ cells. (B) Summary plots showing the frequency of CD4+ Foxp3+ T cells from spleen and lymph node, p>0.05. (C, D) The frequency and absolute number of iNKT cells were shown as α-GalCer-loaded CD1d-tetramer+TCRβ+ lymphocytes gated on B220− cells from spleen and lymph node, p>0.05. (E–H) The production of IL-4 and IFN-γ by iNKT cells in spleen (E&F) and lymph node (G&H) was analyzed with intracellular cytokine staining after PMA/ionomycin stimulation for 3h in vitro. Representative results of three independent experiments are shown. Data are presented as mean ± SD. (N=5, P>0.05).
4. Discussion
Posttranslational modifications of histones in chromatin are emerging as an important mechanism in the regulation of gene expression. Histone acetylation plays key roles in modulating chromatin structure and function. Recent studies indicate that HDACs play a key role in regulating immune cell development and functions and the alterations in HADC expression and function are related to a number of immune-mediated inflammatory diseases, such as rheumatoid arthritis (RA), systemic lupus erythematosus (SLE), and colitis [13, 28, 29]. HDACi could serve as novel drugs for a wide spectrum of inflammatory conditions; however, its anti-inflammatory mechanisms remain elusive. A previous in vitro study from our group demonstrated that histone deacetylase activity is required for skin LCs maturation and phagocytosis and that TSA reduced the number of LCs by enhanced LC apoptosis [30]. In the present study, we found that TSA treatment ameliorated skin lesion severity of DNFB-induced ACD. In consistence with our previous studies in vitro, TSA treatment in vivo significantly reduced the frequency and maturation of epidermal LCs and their expression of langerin. Interestingly, TSA treatment did not significantly affect the homeostasis of conventional CD4+ and CD8+ T cells, Foxp3+CD4+ regulatory T cells, NKT cells, and γδ T cells in different lymphoid organs. Furthermore, there were no significant differences in the IL-4- and IFN-γ-producing T cells andNKT cells between TSA treated mice and controls.
Epidermal LCs have been considered for decades as the prominent DC population, maintaining skin immunity. Previous observations suggested a role for LCs in ACD sensitization. In the last few years, the role of LCs in the development of ACD has been revisited using genetically engineered mutant mice, in which skin LCs were deleted. However, experiments using different LC-deleted models yielded conflicting results as to the role of LCs in ACD. One group reported that inducible ablation of LCs led to diminished ear swelling, suggesting that LCs can activate effector cells to mediate the inflammatory reaction [20, 31]. On the other hand, constitutive or acute depletion of LCs resulted in an enhanced ACD, supporting a regulatory role of LCs in ACD [32, 33]. These inconsistent observations may rely mostly on differences into the genetic constructions for the generation of mutant mice, the haptens and the technical procedures used, and the fact that Langerin is definitely not a specific marker for LCs. Indeed, various DC subsets express Langerin, and notably a particular dermal DC (dDC) subset. To further address this controversy, Zahner recently generated new Langerin-Cre knockin mice, which can delete a gene in both LC and other Langerin+ tissue DC [34]. In agreement with the requirement of TGF-β for LC development, deletion of TGF-βR1 allele mediated by this Langerin-Cre resulted in permanent LC deficiency, whereas dermal Langerin+ DC was unaffected. In the absence of LC, induction of ACD in these Langerin+ DC-specific TGF-βR1–deficient mice elicited decreased ear swelling. This study provided strong evidence against a regulatory function of LC in ACD, suggesting epidermal LCs are required for ACD development. Thus, TSA-mediated epidermal LC reduction may contribute to ameliorated skin lesion severity of DNFB-induced ACD. It would be of interest to test in the future whether TSA also can regulate dDCs, which may work with LCs together involved in TSA-ameliorated ACD.
Three subsets of langerin-expressing DCs are now distinguished in the dLNs of the mouse [8]. LCs that have migrated from the epidermis are characterized by langerin+, CD103−, MHC-II+, CD40+, CD86+, CCR7+, and the absence of CD8 [35]. Migrated dermal langerin+ DCs express CD103 but largely lack EpCAM, and they are found in the CD11C+/CD8− fraction of DCs [36]. A resident langerin+ DCs can be distinguished from migratory langerin+ cells by their expression of CD8 [37]. The percentage of Langerin+CD103−CD8− migrated epidermal LCs and Langerin+CD103+CD8− migrated dermal DCs are comparable between TSA-treated group and DMSO-treated control group, suggesting that the defective epidermal LCs in TSA-treated mice may not be caused by enhanced LC migration to dLNs and could be related to TSA-mediated defective LC survival and maturation. This is further supported by our previous in vitro study [19]. In addition, the frequency of DC in spleen was decreased in TSA-treated group compared to that in DMSO-treated control group, which is consistent with previous in vitro study that TSA treatment reduced the number of bone marrow-derived DCs [24]. The reduced DCs in spleen from TSA-treated mice may also contribute to the suppression of ACD, which is needed to be further investigated.
ACD was reported to be mediated by T cells during skin inflammation. To elucidate the potential role of TSA on T cells during ACD development, we first assessed that the homeostasis of CD4+ and CD8+ T cells from thymus, spleen, and lymph node, and did not find any significant differences in their distributions between TSA-treated mice and DMSO-treated control mice. We further investigated whether TSA regulates cytokine production by T cells, and there was no significant differences observed in IFN-γ- and IL-4- producing CD4+ or CD8+ T cells. Thus, our data suggest that TSA treatment under the current protocol may not alter the homeostasis and function of CD4+ and CD8+ T cells during reduction of ACD.
iNKT cells are restricted to the non-classical MHC-I-like molecule CD1d and preferentially use an invariant TCR consisting predominantly of the Vα14-Jα18/Vβ8 pair in mice [38]. Recent studies indicated iNKT cells are involved in ACD induction [39, 40], and activated NKT cells increase DCs migration and enhance CD8+ T cell responses in the skin [41]. To elucidate if TSA inhibits ACD through blocking iNKT cells, we examined the frequency and function of iNKT cells in the different lymphoid organs in TSA-treated and DMSO-treated control mice. We did not find any significant differences in the number and function of iNKT cells between the two groups. Thus, iNKT cells may not be involved in TSA-mediated prevention of ACD. The γδ T cells have a dual function in the pathophysiology of ACD. γδ T cells are able to inhibit contact sensitivity effector T cells in vivo [42], while they are also involved in initiation and effector phase of DNFB induced ACD collaborated with iNKT cells and assist T cells in the adoptive transfer of ACD [21, 43]. In the present study, we also investigated the frequencies of γδ T cells in the epidermis, skin dLNs, spleen and thymus after TSA treatment, but we did not find any difference between TSA-treated group and DMSO-treated control group. Thus, TSA suppresses ACD response through a γδ T cells- independent mechanism.
Foxp3+CD4+Tregs modulate the immune response to maintain homeostasis and play a pivotal role in the regulation of ACD development. Recently, several groups reported TSA may affect Tregs, but the experiments yielded conflicting results. Early studies indicated that TSA up-regulated Foxp3 expression and increase the generation of Tregs in vivo [44, 45]. More recently, Liu reported that TSA significantly impaired the expression of Foxp3 and reduced the number of Tregs in vivo [46]. In the present study, we found the percentages of Foxp3+CD4+ Tregs was not significantly changed in TSA-treated mice compared to DMSO-treated control mice during ACD induction under our current treatment protocol. TSA has potent effects against cell proliferation and inflammation in a time- and dose-dependent manner [16, 24]. The different outcome after TSA treatment could be related to mouse strains, the doses and duration of TSA treatment. Thus, it is not surprised in our treatment protocol TSA treatment did not affect Treg and other T cells. However, our results challenge that the effects of TSA on ACD are mediated by an increase in the number of regulatory T cells. Further study to determine whether TSA regulates the immunosuppressive function of Treg cells, which may potentially contribute to the reduced ACD, is needed.
In summary, we demonstrate here that TSA treatment results in attenuation of ACD. The reduction of number and maturation of epidermal LCs and the decreased splenic DCs could contribute to the potential mechanisms involved in TSA-mediated ACD protection. Our studies highly suggest the possibility of targeting HDACs as new therapeutic treatment for ACD and LCs-related skin diseases.
Supplementary Material
Acknowledgments
This work was supported by grants from National Institutes of Health Grant R21AR059976, R01Ey017960, and the Henry Ford Health System Research Grant for the Immunology Program (T71016 and T71017). We thank the National Institutes of Health tetramer facility for CD1d-Tetramer, Matthew Weiland and Min Liu for maintaining mouse colonies, Xiaofan Mi for her critical reading, and all members from Mi’s and Zhou’s laboratories for their advice and encouragement.
Footnotes
The authors have no conflict of interest to declare.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Coenraads PJ, Goncalo M. Skin diseases with high public health impact. Contact dermatitis. Eur J Dermatol. 2007;17:564–5. doi: 10.1684/ejd.2007.0299. [DOI] [PubMed] [Google Scholar]
- 2.Banchereau J, Steinman RM. Dendritic cells and the control of immunity. Nature. 1998;392:245–52. doi: 10.1038/32588. [DOI] [PubMed] [Google Scholar]
- 3.Martin SF, Jakob T. From innate to adaptive immune responses in contact hypersensitivity. Curr Opin Allergy Clin Immunol. 2008;8:289–93. doi: 10.1097/ACI.0b013e3283088cf9. [DOI] [PubMed] [Google Scholar]
- 4.Grabbe S, Schwarz T. Immunoregulatory mechanisms involved in elicitation of allergic contact hypersensitivity. Immunol Today. 1998;19:37–44. doi: 10.1016/s0167-5699(97)01186-9. [DOI] [PubMed] [Google Scholar]
- 5.Valladeau J, Ravel O, Dezutter-Dambuyant C, Moore K, Kleijmeer M, Liu Y, et al. Langerin, a novel C-type lectin specific to Langerhans cells, is an endocytic receptor that induces the formation of Birbeck granules. Immunity. 2000;12:71–81. doi: 10.1016/s1074-7613(00)80160-0. [DOI] [PubMed] [Google Scholar]
- 6.Romani N, Holzmann S, Tripp CH, Koch F, Stoitzner P. Langerhans cells - dendritic cells of the epidermis. APMIS. 2003;111:725–40. doi: 10.1034/j.1600-0463.2003.11107805.x. [DOI] [PubMed] [Google Scholar]
- 7.Merad M, Ginhoux F, Collin M. Origin, homeostasis and function of Langerhans cells and other langerin-expressing dendritic cells. NatRevImmunol. 2008;8:935–47. doi: 10.1038/nri2455. [DOI] [PubMed] [Google Scholar]
- 8.Romani N, Clausen BE, Stoitzner P. Langerhans cells and more: langerin-expressing dendritic cell subsets in the skin. Immunol Rev. 2010;234:120–41. doi: 10.1111/j.0105-2896.2009.00886.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Kaplan DH. In vivo function of Langerhans cells and dermal dendritic cells. Trends Immunol. 2010;31:446–51. doi: 10.1016/j.it.2010.08.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Haberland M, Montgomery RL, Olson EN. The many roles of histone deacetylases in development and physiology: implications for disease and therapy. Nat Rev Genet. 2009;10:32–42. doi: 10.1038/nrg2485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Wang Z, Zang C, Cui K, Schones DE, Barski A, Peng W, et al. Genome-wide mapping of HATs and HDACs reveals distinct functions in active and inactive genes. Cell. 2009;138:1019–31. doi: 10.1016/j.cell.2009.06.049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Minucci S, Pelicci PG. Histone deacetylase inhibitors and the promise of epigenetic (and more) treatments for cancer. Nat Rev Cancer. 2006;6:38–51. doi: 10.1038/nrc1779. [DOI] [PubMed] [Google Scholar]
- 13.Glauben R, Batra A, Fedke I, Zeitz M, Lehr HA, Leoni F, et al. Histone hyperacetylation is associated with amelioration of experimental colitis in mice. J Immunol. 2006;176:5015–22. doi: 10.4049/jimmunol.176.8.5015. [DOI] [PubMed] [Google Scholar]
- 14.Reilly CM, Regna N, Mishra N. HDAC inhibition in lupus models. Mol Med. 2011;17:417–25. doi: 10.2119/molmed.2011.00055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Horwitz SM. The emerging role of histone deacetylase inhibitors in treating T-cell lymphomas. Curr Hematol Malig Rep. 2011;6:67–72. doi: 10.1007/s11899-010-0067-5. [DOI] [PubMed] [Google Scholar]
- 16.Finnin MS, Donigian JR, Cohen A, Richon VM, Rifkind RA, Marks PA, et al. Structures of a histone deacetylase homologue bound to the TSA and SAHA inhibitors. Nature. 1999;401:188–93. doi: 10.1038/43710. [DOI] [PubMed] [Google Scholar]
- 17.Choi JH, Oh SW, Kang MS, Kwon HJ, Oh GT, Kim DY. Trichostatin A attenuates airway inflammation in mouse asthma model. Clin Exp Allergy. 2005;35:89–96. doi: 10.1111/j.1365-2222.2004.02006.x. [DOI] [PubMed] [Google Scholar]
- 18.Zhou X, Hua X, Ding X, Bian Y, Wang X. Trichostatin differentially regulates Th1 and Th2 responses and alleviates rheumatoid arthritis in mice. J Clin Immunol. 2011;31:395–405. doi: 10.1007/s10875-011-9508-8. [DOI] [PubMed] [Google Scholar]
- 19.Qi R, Liu M, Gao XH, Yu FS, Chen HD, Lim HW, et al. Histone deacetylase activity is required for skin Langerhans cell maturation and phagocytosis. J Dermatol Sci. 2012;65:152–5. doi: 10.1016/j.jdermsci.2011.11.009. [DOI] [PubMed] [Google Scholar]
- 20.Bennett CL, Noordegraaf M, Martina CA, Clausen BE. Langerhans cells are required for efficient presentation of topically applied hapten to T cells. J Immunol. 2007;179:6830–5. doi: 10.4049/jimmunol.179.10.6830. [DOI] [PubMed] [Google Scholar]
- 21.Askenase PW, Majewska-Szczepanik M, Kerfoot S, Szczepanik M. Participation of iNKT cells in the early and late components of Tc1-mediated DNFB contact sensitivity: cooperative role of gammadelta-T cells. Scand J Immunol. 2011;73:465–77. doi: 10.1111/j.1365-3083.2011.02522.x. [DOI] [PubMed] [Google Scholar]
- 22.Kaplan DH, Kissenpfennig A, Clausen BE. Insights into Langerhans cell function from Langerhans cell ablation models. Eur J Immunol. 2008;38:2369–76. doi: 10.1002/eji.200838397. [DOI] [PubMed] [Google Scholar]
- 23.Singh N, Thangaraju M, Prasad PD, Martin PM, Lambert NA, Boettger T, et al. Blockade of dendritic cell development by bacterial fermentation products butyrate and propionate through a transporter (Slc5a8)-dependent inhibition of histone deacetylases. J Biol Chem. 2010;285:27601–8. doi: 10.1074/jbc.M110.102947. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Kim ES, Lee JK. Histone deacetylase inhibitors decrease the antigen presenting activity of murine bone marrow derived dendritic cells. Cell Immunol. 2010;262:52–7. doi: 10.1016/j.cellimm.2009.12.007. [DOI] [PubMed] [Google Scholar]
- 25.Nosbaum A, Vocanson M, Rozieres A, Hennino A, Nicolas JF. Allergic and irritant contact dermatitis. Eur J Dermatol. 2009;19:325–32. doi: 10.1684/ejd.2009.0686. [DOI] [PubMed] [Google Scholar]
- 26.Vocanson M, Hennino A, Rozieres A, Poyet G, Nicolas JF. Effector and regulatory mechanisms in allergic contact dermatitis. Allergy. 2009;64:1699–714. doi: 10.1111/j.1398-9995.2009.02082.x. [DOI] [PubMed] [Google Scholar]
- 27.Campos RA, Szczepanik M, Lisbonne M, Itakura A, Leite-de-Moraes M, Askenase PW. Invariant NKT cells rapidly activated via immunization with diverse contact antigens collaborate in vitro with B-1 cells to initiate contact sensitivity. J Immunol. 2006;177:3686–94. doi: 10.4049/jimmunol.177.6.3686. [DOI] [PubMed] [Google Scholar]
- 28.Joosten LA, Leoni F, Meghji S, Mascagni P. Inhibition of HDAC activity by ITF2357 ameliorates joint inflammation and prevents cartilage and bone destruction in experimental arthritis. Mol Med. 2011;17:391–6. doi: 10.2119/molmed.2011.00058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Reilly CM, Thomas M, Gogal R, Jr, Olgun S, Santo A, Sodhi R, et al. The histone deacetylase inhibitor trichostatin A upregulates regulatory T cells and modulates autoimmunity in NZB/W F1 mice. J Autoimmun. 2008;31:123–30. doi: 10.1016/j.jaut.2008.04.020. [DOI] [PubMed] [Google Scholar]
- 30.Qi R, Liu M, Gao XH, Yu FS, Chen HD, Lim HW, et al. Histone deacetylase activity is required for skin Langerhans cell maturation and phagocytosis. J Dermatol Sci. 2012 doi: 10.1016/j.jdermsci.2011.11.009. [DOI] [PubMed] [Google Scholar]
- 31.Bennett CL, van Rijn E, Jung S, Inaba K, Steinman RM, Kapsenberg ML, et al. Inducible ablation of mouse Langerhans cells diminishes but fails to abrogate contact hypersensitivity. J Cell Biol. 2005;169:569–76. doi: 10.1083/jcb.200501071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Kaplan DH, Jenison MC, Saeland S, Shlomchik WD, Shlomchik MJ. Epidermal langerhans cell-deficient mice develop enhanced contact hypersensitivity. Immunity. 2005;23:611–20. doi: 10.1016/j.immuni.2005.10.008. [DOI] [PubMed] [Google Scholar]
- 33.Bobr A, Olvera-Gomez I, Igyarto BZ, Haley KM, Hogquist KA, Kaplan DH. Acute ablation of Langerhans cells enhances skin immune responses. J Immunol. 2010;185:4724–8. doi: 10.4049/jimmunol.1001802. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Zahner SP, Kel JM, Martina CA, Brouwers-Haspels I, van Roon MA, Clausen BE. Conditional deletion of TGF-betaR1 using Langerin-Cre mice results in Langerhans cell deficiency and reduced contact hypersensitivity. J Immunol. 2011;187:5069–76. doi: 10.4049/jimmunol.1101880. [DOI] [PubMed] [Google Scholar]
- 35.Flacher V, Douillard P, Ait-Yahia S, Stoitzner P, Clair-Moninot V, Romani N, et al. Expression of langerin/CD207 reveals dendritic cell heterogeneity between inbred mouse strains. Immunology. 2008;123:339–47. doi: 10.1111/j.1365-2567.2007.02785.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Bursch LS, Wang L, Igyarto B, Kissenpfennig A, Malissen B, Kaplan DH, et al. Identification of a novel population of Langerin+ dendritic cells. J Exp Med. 2007;204:3147–56. doi: 10.1084/jem.20071966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Douillard P, Stoitzner P, Tripp CH, Clair-Moninot V, Ait-Yahia S, McLellan AD, et al. Mouse lymphoid tissue contains distinct subsets of langerin/CD207 dendritic cells, only one of which represents epidermal-derived Langerhans cells. J Invest Dermatol. 2005;125:983–94. doi: 10.1111/j.0022-202X.2005.23951.x. [DOI] [PubMed] [Google Scholar]
- 38.Zhou L, Seo KH, He HZ, Pacholczyk R, Meng DM, Li CG, et al. Tie2cre-induced inactivation of the miRNA-processing enzyme Dicer disrupts invariant NKT cell development. Proc Natl Acad Sci U S A. 2009;106:10266–71. doi: 10.1073/pnas.0811119106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Askenase PW, Itakura A, Leite-de-Moraes MC, Lisbonne M, Roongapinun S, Goldstein DR, et al. TLR-dependent IL-4 production by invariant Valpha14+Jalpha18+ NKT cells to initiate contact sensitivity in vivo. J Immunol. 2005;175:6390–401. doi: 10.4049/jimmunol.175.10.6390. [DOI] [PubMed] [Google Scholar]
- 40.Askenase PW, Szczepanik M, Itakura A, Kiener C, Campos RA. Extravascular T-cell recruitment requires initiation begun by Valpha14+ NKT cells and B-1 B cells. Trends Immunol. 2004;25:441–9. doi: 10.1016/j.it.2004.06.003. [DOI] [PubMed] [Google Scholar]
- 41.Gorbachev AV, Fairchild RL. Activated NKT cells increase dendritic cell migration and enhance CD8+ T cell responses in the skin. Eur J Immunol. 2006;36:2494–503. doi: 10.1002/eji.200636075. [DOI] [PubMed] [Google Scholar]
- 42.Szczepanik M, Anderson LR, Ushio H, Ptak W, Owen MJ, Hayday AC, et al. Gamma delta T cells from tolerized alpha beta T cell receptor (TCR)-deficient mice inhibit contact sensitivity-effector T cells in vivo, and their interferon-gamma production in vitro. J Exp Med. 1996;184:2129–39. doi: 10.1084/jem.184.6.2129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Yokozeki H, Watanabe K, Igawa K, Miyazaki Y, Katayama I, Nishioka K. Gammadelta T cells assist alphabeta T cells in the adoptive transfer of contact hypersensitivity to para-phenylenediamine. Clin Exp Immunol. 2001;125:351–9. doi: 10.1046/j.1365-2249.2001.01570.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Lucas JL, Mirshahpanah P, Haas-Stapleton E, Asadullah K, Zollner TM, Numerof RP. Induction of Foxp3+ regulatory T cells with histone deacetylase inhibitors. Cell Immunol. 2009;257:97–104. doi: 10.1016/j.cellimm.2009.03.004. [DOI] [PubMed] [Google Scholar]
- 45.Tao R, de Zoeten EF, Ozkaynak E, Chen C, Wang L, Porrett PM, et al. Deacetylase inhibition promotes the generation and function of regulatory T cells. Nat Med. 2007;13:1299–307. doi: 10.1038/nm1652. [DOI] [PubMed] [Google Scholar]
- 46.Liu Z, Zhang C, Sun J. Deacetylase inhibitor trichostatin A down-regulates Foxp3 expression and reduces CD4+CD25+ regulatory T cells. Biochem Biophys Res Commun. 2010;400:409–12. doi: 10.1016/j.bbrc.2010.08.090. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.