Abstract
Purpose
A contact lens (CL) can act as a vector for microorganisms to adhere to and transfer to the ocular surface. Commensal microorganisms that uneventfully cohabitate on lid margins and conjunctivae and potential pathogens that are found transiently on the ocular surface can inoculate CLs in vivo. In the presence of reduced tissue resistance, these resident microorganisms or transient pathogens can invade and colonize the cornea or conjunctiva to produce inflammation or infection.
Methods
The literature was reviewed and used to summarize the findings over the last 30 years on the identification, enumeration, and classification of microorganisms adherent to CLs and their accessories during the course of normal wear and to hypothesize the role that these microorganisms play in CL infection and inflammation.
Results
Lens handling greatly increases the incidence of lens contamination, and the ocular surface has a tremendous ability to destroy organisms. However, even when removed aseptically from the eye, more than half of lenses are found to harbor microorganisms, almost exclusively bacteria. Coagulase-negative Staphylococci are most commonly cultured from worn lenses; however, approximately 10% of lenses harbor Gram-negative and highly pathogenic species, even in asymptomatic subjects. In storage cases, the incidence of positive microbial bioburden is also typically greater than 50%. All types of care solutions can become contaminated, including up to 30% of preserved products.
Conclusions
The process of CL-related microbial keratitis and inflammation is thought to be preceded by the presence or transfer or both of microorganisms from the lens to the ocular surface. Thus, this detailed understanding of lens-related bioburden is important in the understanding of factors associated with infectious and inflammatory complications. Promising mechanisms to prevent bacterial colonization on lenses and lens cases are forthcoming, which may decrease the incidence of microbially driven CL complications.
Keywords: Contact lens, Microbial contamination, Antimicrobial lens surfaces
In developed countries, contact lens (CL) wear, specifically extended wear (EW) with hydrogel lenses, overrides all other risk factors for the development of microbial keratitis in otherwise healthy eyes.1 Thus, the presence of the CL influences development of infection as the lens biomaterial acts as a vector for adherence of microorganisms with subsequent transfer to the ocular surface. Although the presence of microorganisms is necessary, it is not necessarily sufficient for the development of infection. Other factors associated with infection include disruption to the ocular surface, often caused by CL wear. Nevertheless, the presence of bacteria, protozoa, and fungi on CLs clearly predispose a patient to the development of infection. In fact, in studies where microbial keratitis was clinically proven, the isolation of organisms from surfaces of the CLs worn during the event was a more sensitive method to detect the causative microorganisms compared with corneal scrapings.2– 4
Bacterial colonization of CLs has also been implicated in CL-induced inflammation. Specifically, CL acute red eye (CLARE), CL peripheral ulcer (CLPU), and infiltrative keratitis have all been associated with adherence of bacteria to hydrogel CLs.5–11 In particular, CLARE has been associated with Haemophilus influenzae,11 Acinetobacter sp,5 Pseudomonas aeruginosa,6,10 Aeromonas hydrophila,10 Serratia liquefaciens,10 Serratia marcescens,6 Pseudomonas putida,6 and others. Infiltrative keratitis and CLPU have been associated with Staphylococcus aureus,7 Streptococcus pneumoniae,9 Abiotrophia defectiva,8 Acinetobacter sp,5 and others. In animal models, Wu et al.12 have shown that rabbits fit with EW CLs and inoculated with S. aureus develop corneal infiltrative events, and Willcox et al.13 have shown that various microorganisms isolated from previous inflammatory or infectious conditions create inflammation in the mouse cornea. However the pathway between bacterial contamination of CLs and corneal inflammatory events is not as straightforward as the link between CL bioburden and microbial keratitis. There are many other speculative causes of inflammation (lens deposits or defects, hypoxia, cytotoxicity of care solutions, changes in pH and oxygen and CO2 concentration, and corneal surface disruption), which may be present alone or in combination with lens bioburden. Even so, most research has been applied to microbial colonization of CLs as it seems to be the most consistent and repeatable finding and has biologic plausibility. Holden et al.6 have hypothesized that endotoxin released from Gram-negative (Gram−) bacteria is a primary cause of the cellular response and infiltration seen when CLs are highly contaminated with Gram− bacteria, and the primary cell types detected are neutrophils.
Before unraveling the link among corneal infection, inflammation, and CL bioburden, first, one must identify, enumerate, and classify microorganisms adherent to CLs and their accessories during the course of normal wear. This review summarizes the findings over the last 30 years and hypothesizes the role that these microorganisms play in CL-related infection and inflammation. Finally, mechanisms to prevent bacterial colonization on lenses and lens cases and the impact this may have on the incidence of CL complications will be explored (Table 1).
TABLE 1.
Commonly Used Terms, Definitions and Abbreviationsa
| Term | Abbreviation | Definition as used in this review |
|---|---|---|
| Antimicrobial | An antimicrobial substance is one that kills or inhibits the growth of microorganisms such as bacteria, fungi, or protozoans and destroys viruses | |
| Bioburden | The number of microorganisms with which an object is contaminated | |
| Biofilm | An aggregate of microorganisms in which cells are stuck to each other or to a surface or to both. These adherent cells are frequently embedded within a self-produced matrix of extracellular polymeric substance. The cells of a microorganism growing in a biofilm are physiologically distinct from planktonic cells of the same organism, which by contrast, are single cells that may float or swim in a liquid medium | |
| Coagulase-negative Staphylococci | CNS | Coagulase is an enzyme produced by Staphylococcus aureus, which converts fibrinogen to fibrin. In the laboratory, it is used to distinguish between different types of Staphylococcus isolates. Coagulase negativity excludes S. aureus |
| Colony forming unit | CFU | A measure of viable bacterial or fungal numbers. Unlike direct microscopic counts where all cells, dead and living, are counted, CFU measures viable cells |
| Commensal (microorganisms) | A class of relationship between two organisms where one organism benefits but the other is unaffected | |
| Daily wear | DW | Contact lenses wearing modality where lenses are removed nightly |
| Extended wear | EW | Contact lenses wearing modality where lenses are worn continuously, typically at least 6 nights and up to 29 nights |
| Gram-negative bacteria | Gram− | Those bacteria that do not retain crystal violet dye in the Gram staining protocol based on the structure of their cell walls. The pathogenic capability of Gram-negative bacteria is often associated with certain components of their cell walls, in particular the lipopolysaccharide (also known as LPS or endotoxin) layer |
| Gram-positive bacteria | Gram+ | Those bacteria that are stained dark blue or violet by Gram staining. Gram+ organisms are able to retain the crystal violet stain because of the high amount of thick peptidoglycan in the cell wall |
| Normal microbiota | Organisms on the human host that are expected to be present and that under normal circumstances do not cause disease, but instead may participate in maintaining health | |
| Pathogenic organisms | Organisms that have the capability of causing infectious diseases |
Adapted from Wikipedia.99
Commensal Microorganisms of the Ocular Surface
Because of its constant exposure to the external environment, the ocular surface is subject to intense and frequent microbial contamination. In fact, the conjunctiva and the lid margins harbor many microorganisms, yet, in the absence of CL wear, the cornea is considered to be sterile.14 Most environmentally introduced microorganisms are removed by the ocular defense mechanisms with only a low density of microbiota remaining consisting of a reduced number of species.
Normal microbiota of the eyelids and conjunctivae consist principally of aerobic bacteria. In 1935, Khorazo et al.15 found that Staphylococci and diphtheroids (Corynebacterium sp) are the most frequent bacterial inhabitants of the normal conjunctiva. They also showed that the incidence of diphtheroid organisms increased markedly with advancing age, the increase being most pronounced from 30 to 50 years. The normal aerobic bacteria typically include coagulase-negative Staphylococci (CNS; primarily being Staphylococcus epidermidis), Corynebacterium sp, Micrococcus sp, and Bacillus sp.16 –23 Under appropriate culture conditions, anaerobic bacteria can be identified, particularly Propionibacterium sp, and some Candida sp. The normal ocular microbiota undergo minimal change during the first two decades of life, where Streptococci and Pneumococci are more prevalent and S. epidermidis, S. aureus, and Corynebacterium sp remain the predominant bacteria on the conjunctiva and the eyelids.24 With increasing age, however, there is a trend toward the presence of more Gram− bacteria. There is not a great deal of variation in the commensal organisms with geographic regions. Although rates differ among individuals and populations, S. epidermidis, S. aureus, and Corynebacterium sp are the predominant conjunctival microbiota regardless of geographic location.24 Although bacteria and yeast are part of the commensal organisms of the ocular surface, viruses and amoeba are not.
Varied factors enable a particular strain to remain as a nonpathogenic inhabitant of the ocular surface including microbial metabolic end products, tear film components, and bacteriocins. Bacteriocins are proteinaceous toxins secreted by a specific organism, which are harmful to other competitive bacterial species. Of the conjunctival bacterial microflora, Staphylococci, Corynebacteria, and Propionibacteria are all known bacteriocin producers, thus their ability to synthesize and secrete toxins that are detrimental to the growth of other closely related bacteria could afford these species the necessary competitive advantage to survive in the ocular microenvironment.24 Bacterial metabolic end products such as hyaluronidase and hydrogen peroxide also possess antibacterial activity that can alter the surrounding bacterial composition. Finally, tear film components such as lipids, lysozyme, lactoferrin, β-lysin, complement proteins, and immunoglobulins may be selectively toxic to the noncommensal microbiota. For example, lysozyme, which comprises 20% to 40% of the total protein content of tears, is active against many Gram+ bacteria and selectively accelerates the lysis of Gram− bacteria in the presence of complement.24 Staphylococci, Corynebacteria, and Propionibacteria are resistant to lysozyme because of their cell wall composition, thus it is easier for them to remain inhabitants of the ocular surface.24,25 In addition, lactoferrin binds iron and interferes with bacterial iron uptake, which decreases the virulence of several bacteria including Pseudomonas sp.24 Thus, the combination of toxic components, which are part of our ocular defense mechanism or are produced by microorganisms selectively, enhances the ability of some strains to remain as commensal ocular microbiota, whereas others are routinely removed from the ocular environment.
Although some species are considered nonpathogenic commensal organisms of the ocular surface, the classification as pathogenic or nonpathogenic strains remains difficult. Although normal ocular surface flora usually uneventfully cohabitate with the ocular surface, they can also overwhelm the host defense mechanisms and cause external ocular infection and inflammation in the presence of reduced tissue resistance. Resistance is affected by age, trauma, surgery, systemic disease, immunosuppression, or CL use.26 Microorganisms normally resident on the eyelids may invade and colonize the cornea or conjunctiva to produce inflammation or infection.
Ocular Surface Flora With Lens Wear
To understand how potentially pathogenic organisms gain access to and colonize hydrophilic CLs, a thorough knowledge of ocular surface microorganisms and their changes resulting from lens wear are necessary. Even eye closure in the absence of CL wear has been shown to significantly alter the normal microbiota. Ramachandran et al.27 has shown that the incidence of clinically important Gram+ bacteria increases after 8 hr of eye closure, whereas there are no increases in Gram− or fungal organisms. The effect of eye closure or lens wear on the commensal microorganisms is important, because these organisms produce antimicrobial factors that play a role in defense of the ocular tissues from infection. If changes to the normal flora were prominent with CL wear, suppression of factors that normally inactivate or clear foreign microbes from the eye may facilitate an infectious or inflammatory process.18 Many groups have documented a decrease in microbial colonization of conjunctival surfaces after the introduction of CL wear. In 1979, McBride found an early decrease in the percentage of positive cul-de-sac cultures with as little as 2 days of soft CL wear and speculated that the lenses may actually have an antibacterial effect on the ocular surface. In 1981, Hovding19 found a decrease in positive conjunctival cultures in a group of patients wearing hydrogel lenses after 2 weeks of wear versus a nonwearing control group and speculated this effect was because of a slow release of the chemical disinfectants from the lens onto the ocular surface. It has been postulated that an increase in pathogenic organisms may arise secondary to a decreased presence of commensal organisms. In 1992, Fleiszig and Efron28 have shown that after 2 months of rigid gas permeable (RGP) EW, the incidence of culture-negative eyes increased in conjunction with increased incidence of conjunctival colonization with potentially pathogenic microorganisms. They attributed the increased incidence of potential pathogens to compromises in the mechanisms that normally clear foreign microorganisms, including the reduction of normal ocular flora.28 In 1995, Stapleton et al.22 found that during soft lens EW, increased conjunctival colonization with potential pathogens occurred simultaneously with reductions in colonization of normal flora in established wearers. At least four studies document the effect of decreased conjunctival flora in the first few months of lens wear.19,28 –30 The decrease in conjunctival flora may be a short-term response to lens wear. However, in a cross-sectional study of long-term aphakic soft lens extended wearers and preoperative cataract patients, the established CL wearers showed significantly fewer pathogens recovered from the lids and conjunctivae.31 In addition, in Stapleton et al.’s study,22 the trend of decreasing colonization with time was noted only in established (extended) lens wearers after 12 months of wear. Therefore, decreased conjunctival flora reported in some studies does not likely represent a short-term response to lens wear. Finally, a fewer positive conjunctival cultures has been reported in current (or nonwearers) compared with former wearers in a cross-sectional study.18 This suggests that CL wear permanently altered the microbial flora, or the group of former, presumed failed, CL wearers were predisposed to discomfort and complications from high levels of microbiota on their ocular surface.
In contrast to decreased conjunctival colonization with CL wear, other studies have found evidence of opposing trends, that is, increased16,20 –22 or unchanged17,18,32 microbial loads on the conjunctiva. Specifically, an increase in numbers but not types of organisms were found on lid margins21,22 or conjunctiva16,22 with soft CL daily wear (DW). These three studies have shown that DW of soft lenses is associated with increased colonization by commensal and nonpathogenic bacterial species on the lid margin, conjunctiva, or both.16,21,22 Moreover, the bacterial populations that colonize the lids and conjunctiva during long-term asymptomatic soft lens wear have been hypothesized to change based on the wear schedule. Stapleton et al.22 have shown that more potential pathogens are recoverable from the conjunctiva after EW compared with DW. In addition, during silicone hydrogel (SH) EW, the frequency of positive conjunctival cultures for commensal organisms increased. Iskeleli et al.20 found that the incidence of positive conjunctival cultures rose from 34% before lens wear to 90% after 30 days of continuous wear with lotrafilcon A lenses.
The lid margin consistently shows greater colonization than the conjunctiva during lens wear when both are assessed simultaneously,21–23,33,34 thus it is often assumed that the lid margin is the source of potential pathogens during lens wear. In that regard, Willcox et al.23 found a correlation between the isolation of bacteria from the CL and the lower lid margin, and Szczotka-Flynn et al. has shown that the presence of substantial lid and conjunctival bioburden are associated with a 2.5-fold and more than a 4-fold greater risk of substantial lens bioburden, respectively.34 Thus, the lid margins are likely the major route of contamination and conjunctival contamination precedes or occurs simultaneously with CL contamination.
CL Bioburden
There is a wide variation in the reporting of lens bioburden across studies. This variation stems from sampling techniques, geographical location, differences in lens care systems, modes of wear, materials, subjects sampled, and number of sampling occasions. Table 2 summarizes the results of studies that have assessed bacterial bioburden on CLs during asymptomatic wear. In clinically normal individuals wearing hydrophilic CLs, approximately 40% of lenses remain sterile in vivo, regardless of lens type or mode of wear. The remainder of lenses is colonized with commensal or pathogenic organisms. Table 3 lists the frequencies of isolation of specific species from soft CLs by study. As can be seen in Table 3, most of organisms cultured are CNS in most studies. Approximately half of all patient worn soft lenses are predominantly colonized by normal lid or skin flora including CNS, Propionibacterium, Corynebacterium, Micrococcus, and Bacillus sp and S. epidermidis is the most frequently isolated staphylococcal species.19,29,35 Leitch et al.36 identified and enumerated all the Staphylococci found on traditional hydrophilic lenses on repeated occasions over 1 year in 97 patients. Approximately half (48.8%) of all lenses harbored some Staphylococci in agreement with other groups. However, in contrast to previous studies, they reported that the capitis/warneri group was the most frequently isolated staphylococcal group from both DW and EW soft CLs and the most numerous group isolated from DW lenses, followed by the epidermidis group.36
TABLE 2.
Summary of Studies Assessing Bacterial Bioburden on Contact Lenses During in vivo Asymptomatic Wear
| Study | Sample size and type | Lens type | Disinfection systems used | Length of wear | Frequency of contamination by organisms (%)
|
Median CFU/lens (range)
|
|||
|---|---|---|---|---|---|---|---|---|---|
| Normal flora | Gram− or potentially pathogenic | Sterile | Gram+ | Gram− | |||||
| Removed aseptically | |||||||||
| LOW Dk HYDROGEL MATERIALS | |||||||||
| DAILY WEAR | |||||||||
| Hovding19 | 113 adapted wearers (lenses were not removed aseptically but only back surface was assessed) | HEMA | Chemical or thermal | 2 wk–16 yr | Only back surface assessed: 56% (CNS), 5% (Corynebacteria)a | Only back surface assessed 2.3%a | Only back surface assessed 35%a | NR | NR |
| Fleiszig and Efron18 | 72 adapted DW patients | Various soft and RGP | Chemical, peroxide, and thermal | 1 wk–14 yr | 40% (front surface) 35% (back surface) | 8% (potentially pathogenic) | 51% (front surface) 57% (back surface) | NR | NR |
| Mowry-McKee et al.40 | 20 soft lens wearers | Polymacon | None | 5 hr | NR | NR | 58% | All species; mean: 28; geometric mean: 21.4; range: 6– 300 | All species; mean: 28; geometric mean: 21.4; range: 6–300 |
| Elander et al.17 | 12 CL wearers and nonwearers | Disposable and nondisposable hydrophilic lenses | ReNu Multipurpose (Bausch & Lomb) | 1 wk | NR | NR | 58% | ||
| Hart et al.35 | 82 lenses from 49 patients | Polymacon, etafilcon A, and lidofilcon A | various | 1–48 mo | 32.4% | 1.8% | 69.2% | 0–45 (all species) | 0–45 (all species) |
| Gopinathan et al.53 | 50 neophytes | Etafilcon A and polymacon | ReNu Multipurpose in DW phase | 2 wk DW | 27%ab | 20%ab | 66%ab | All types: 1 (range 1–700) | All types: 1 (range 1–700) |
| Willcox et al.23 | 26 experienced DW users | Etafilcon A and polymacon | ReNu Multipurpose | 20–48 months | 63.6%a | 16.5%a | NR | NR | NR |
| Kozer-Bilgin et al.100 | 46 medical personnel and 35 nonmedical personnel (all CL wearers) | RGP 55%–89%; various soft 11%–45%. Note lenses were not removed, they were cultured in vivoa | Various | 1–108 mo | 0%–21.7% | 6.3%–8.7% | 71.7%–91.4% | NR | NR |
| Iskeleli et al.101 | 75 lenses of 38 soft lens wearers | Polymacon (18 patients) and oculfilcon D (20 patients) | Bausch & Lomb Multipupose solution | 1 mo | 54.2%–87.5% | 20%–75% | 8.6%–15% | ||
| Yung et al.87 | 101 soft contact lens wearers | Various soft lenses | Various | 0.25–10 yr | See sterile lens column | 9% | 91% (no growth or normal flora only) | NR | NR |
| Santos et al.49 | 31 neophytes | Etafilcon A | ReNu MultiPlus | 15-d cycles for 6 mo | NR | NR | NR | All species; 9.3 × 105 ± 3.49 × 105 | All species; 9.3 × 105 ± 3.49 × 105 |
| Weighted averages of low Dk hydrogel materials, daily wear (%) | 41.85 | 12.49 | 44.64 | ||||||
| EXTENDED WEAR | |||||||||
| Hart and Shih39 | 25 soft lens wearers | Unknown FDA group 2 soft lenses | NR | 1–10 d | 0 | 4% | 96% | NR | NR |
| Elander et al.17 | 18 CL wearers and nonwearers | Disposable and nondisposable hydrophilic lenses | NR | 1 wk | NR | NR | 39% | ||
| Hart et al.35 | 26 lenses | Polymacon, etafilcon A, and lidofilcon A | Various | 0.01–48 mo | 5.6% | 3.7% | 69.2% | 0–45 (all species) | 0–45 (all species) |
| Stapleton et al.33 | 11 adapted DW patients | Etafilcon A | None | 8 hr DW, 3 hr EW, 8 hr EW, 1 hr after waking | 63% | 3% | 25% | 36 (0–430) (after 8 hr EW) | NA |
| Gopinathan et al.53 | 50 neophytes | Etafilcon A and polymacon | None | 1 night, 1 wk, 1 mo and every 3 mo until 18 mo | 39.75%ab | 15.25%ab | 55.75%ab | All types: 2 (range 0–700) | All types: 2 (range 0–700) |
| Willcox et al.23 | 26 neophytes and 18 experienced EW users | Etafilcon A and polymacon | 20–48 mo | 67.1%–73.7%a | 14.9%–17.0%a | NR | NR | NR | |
| Sankaridurg et al.56 | 4,203 lenses of 330 neophytes | FDA groups I and IV hydrogels | NR | 1 night–42 mo | 57%a | 1% | 42% | Median ≤15 (exception Streptococcus pneumoniae); range 1–1,800 | Range only reported as 1–2,575 |
| Keay et al.48 | 63 subjects | Etafilcon A results for asymptomatic patients only)a | NR | 6 nights wear at 1, 3, 6, 9, and 12 mo | 69% | 3% | 28% | 6–10 (range 1–720) | 6 (range 1–300) |
| Ahanotu and Ahearn102 | 220 lenses | HEMA | Unknown | 5–30 d | 75% | 33% | 0% | Range: <100–5.6 × 103 CFU/mL | Range: <100–4.1 × 103 CFU/mL |
| Weighted averages of low Dk hydrogel materials, extended wear (%) | 57.37 (Excluding Sankaridurg et al., 60.91) | 2.87 (Excluding Sankaridurg et al., 20.8) | 40.3 (Excluding Sankaridurg et al., 23.55) | ||||||
| MIXED DAILY AND EXTENDED WEAR | |||||||||
| Hart et al.103 | 49 lenses of 18 soft lens wearers | Disposable ionic high water soft lenses | NR | 1–17 d | 54% | 0% | 46% | Mean 4.46; range 0–50 CFU | |
| Sweeney et al.54 | 20 soft extended lens wearers | Etafilcon A and polymacon | None | 1–13 d | 86%a | 66%a | 8% | <10 | NR |
| Weighted averages of low Dk hydrogel materials, mixed daily and extended wear (%) | 63.3 | 19.1 | 35.0 | ||||||
| SILICONE HYDROGEL LENSES | |||||||||
| DAILY WEAR | |||||||||
| Santos et al.49 | 8 neophytes in each material | Balafilcon A, galyfilcon, lotrafilcon A, lotrafilcon B | ReNu MultiPlus | 30-d cycles for 6 mo | NR | NR | NR | all species 2.3 × 105– 2.3 × 106 ± 1.2 × 105–1.45 × 106 | all species 2.3 × 105–2.3 × 106 ± 1.2 × 105– 1.45 × 106 |
| EXTENDED WEAR | |||||||||
| Keay et al.48 | 64 subjects | lotrafilcon A results for asymptomatic patients only)a | NR | 30 nights at 1, 3, 6, 9, and 12 mo | 69% | 2%–4% | 27% | 9–59 (range 1–612) | 6 (range 1–6) |
| Willcox et al.14 | 27 neophytes to extended wear and to silicone hydrogel lenses | Lotrafilcon A | None | 1, 6, and 24 mo | 42%–81%a | 0%–22%a | 19–37%a | Median 2–28 (1–299) | Median 3–4 (1–6) |
| Szczotka-Flynn et al.34 | 186 subjects | Lotrafilcon A | None | 1 mo and 4 mo of CW | NR | 7.4% | 57% | ||
| Weighted averages of silicone hydrogel lenses, extended wear (%) | 50.66 | 8.17 | 43.30 | ||||||
| PATIENT-HANDLED LENSES (nonaseptic lens removal) | |||||||||
| DAILY WEAR | |||||||||
| Hovding19 | 113 adapted wearers | HEMA, polymetyl methacrylate, cellulose acetate butyrate, and silicone elastomer | Chemical or thermal | 2 wk–16 yr | Only back surface assessed HEMA:56% (CNS) 5% (Corynebacteria)a |
Only back surface assessed HEMA: 2.3%a |
Only back surface assessed HEMA:35%a |
NR | NR |
| Barr et al.50 | 46 soft lens wearers | Various hydrogel materials with ≤55% water content | All types: thermal, chemical, and peroxide | 1–48 mo | NR | NR | NR | All species 70% of lenses: <120; 28% of lenses: 140– 9,060; range: 0 to >60,000 | All species 70% of lenses: < 120; 28% of lenses: 140– 9,060 range: 0 to >60,000 |
| Mowry-McKee et al.104 | 196 lenses of 109 soft lens wearers | Polymacon | Thermal | 4 d–2.5 yr | NR | NR | 5% | All species; mean 2,615; range <3– 150,000 | All species; mean 2,615; range <3–150,000 |
| Mowry-McKee et al.40 | 20 soft lens wearers | Polymacon | None | 5 hr | NR | NR | 0 | All species; mean 653; geometric mean 607; range: 12–5,700 | All species; mean 653; geometric mean 607; range: 12– 5,700 |
| Lipener et al.38 | 30 lenses in 15 soft contact lens wearers | Various hydrophilic soft lenses | Various | 1–24 mo | 0 | 80% | 20% | NR | NR |
| Weighted averages of patient-handled lenses, daily wear (%) | NA | NA | 15.40 | ||||||
| EXTENDED WEAR | |||||||||
| Hart and Shih39 | 4 | Unknown FDA group 2 soft lenses | NR | 1–5 d | 100% | 0% | 0% | All species 2–24 | All species 2–24 |
Estimated from data assuming no mixed cultures, very likely overestimates.
Averaged data from multiple sampling occasions or various subgroups.
TABLE 3.
Frequency of isolation (%) of organisms from soft contact lenses by study
| CNS | Bacillus | Corynebacteria sp | Micrococcus | Propionibacterium sp | Pseudomonas sp | Serratia sp | Staphylococcus aureus | Streptococcus pneumoniae | Streptococcus viridans | Yeast/fungi | Other Gram− |
|---|---|---|---|---|---|---|---|---|---|---|---|
| 038,39 | 033,38,39,69,70,87,100,101 | 038,39,69,70,100 | 014,35,38,39,69,70,87,100,101 | 019,39,69,70 | 014,39 | 014,33,35,38,39,69,70,100,101 | 033,35,39,70,100 | 014,33,35,38,39,69,70,87,100,101 | 014,35,38,39,55,70,87,100,101 | 019,35,38,39,55,69,70,87,102 | 014 |
| 2.469 | 0.5102 | 0.935 | 0.5102 | 1.456 | 0.8–1.623 | 0.156 | 0.1736 | 0.4a55 | 1.2 (back surface)18 | 0.5–1.823 | 1.269 |
| 3.870 | 219 | 2.6–4.323 | 0.8–4.323 | 4–5814 | 1.256 | 0.923 | 1.0–1.523 | 0.5–1.323 | 1.5–3.323 | 1.2 (back surface)18 | 219 |
| 19.6100 | 1.8–3.223 | 4102 | 3.332 | 2.1100 | 1.2 (front surface) and 1.2 (back surface)18 | 1.2a55 | 0.656 | 3.769 | 2.1100 | 2a23 | |
| 36.135 | 4.756 | 519 | 633 | 20.2–29.623 | 6102 | 1.2a55 | 219 | 1.2 (front surface) and 1.2 (back surface)18 | 633 | 5.4a56 | 2.1–5.8100 |
| 39–6714 | 5–714 | 633 | 8.456 | 4133 | 754 | 254,87 | 2.3 (front surface) and 1.2 (back surface)18 | 8–1114 | 2.835 | ||
| 39.4–39.923 | 854 | 6.732 | 954 | 7354 | 41.638 | 10102 | 3102 | 933 | 333, 102 | ||
| 41.456 | 8–1014 | 1219 | 487 | 15101 | 3.156 | ||||||
| 42.8–55101 | 9.156 | 514 | 439 | ||||||||
| 46.732 | 11.4–32.5101 | 554 | 587 | ||||||||
| 4836 | 2254 | 6.356 | 6.732 | ||||||||
| 5619 | 12.5101 | 7.2a55 | |||||||||
| 6333 | 21.269 | 7.770 | |||||||||
| 71102 | 5038 | 9.5 (front surface) and 5 (back surface)18 | |||||||||
| 8654 | 20101 | ||||||||||
| 33.3 (Pseudomonas mirabilis) | |||||||||||
| 25 (Pseudomonas vulgaris)38 | |||||||||||
| 38a54 |
Variations in isolation frequencies depend on whether a single occasion was sampled vs. multiple occasions.
Estimated from multiple sampling occasions in the article.
Although Gram− and other pathogenic organisms are also present, they are found much more sporadically and in a smaller percentage of samples (between 2% and 20%). Nonetheless, even this percentage of contaminated lenses compared with the relatively low prevalence of microbially driven CL-related infection (~2 in 10,000 for soft lens DW37) highlights the potency of the ocular defense mechanisms. These defense mechanisms include blinking (which mechanically removes loosely adherent organisms); immunoproteins and mucin, which kill or inhibit organisms; and normal ocular flora, which inhibit the growth of pathogens by consuming nutrients and secreting antimicrobial toxins.
As seen in Table 2, the incidence of Gram− and highly pathogenic organisms cultured from soft lenses in asymptomatic subjects is approximately 10%, but many studies report the presence of such bioburden as less than 5% (Fig. 1). The exception is a study performed by Lipener et al. in Brazil.38 They found an unusually high percentage (80%) of patients with CLs colonized with pathogenic species. The patients initiated lens removal the night before the visit and brought their lenses to the clinic in lens storage cases. The majority of the organisms cultured were Gram− bacteria, and S. aureus was the only Gram+ organism isolated. The large discrepancy between this study and others is partly because of hand contamination from lens removal.
FIG. 1.
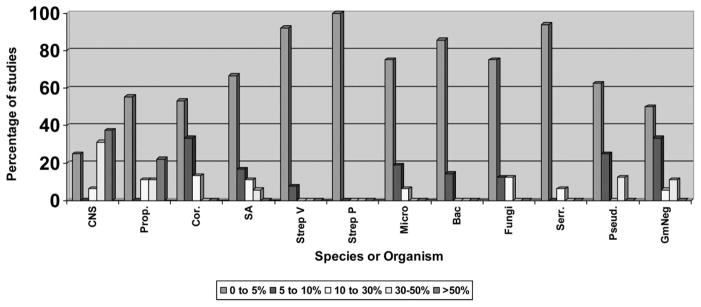
Percentage of studies reporting incidence of lens contamination by species. CNS, coagulase-negative Staphylococci; Prop, Propionibacterium sp; Cor, Corynebacteria sp; SA, Staphylococcus aureus; Strep V, Streptococcus viridans; Strep P, Streptococcus pneumoniae; Micro, Micrococcus; Bac, Bacillus; Fungi, yeast/fungi; Serr, Serratia sp; Pseud, Pseudomonas sp; GmNeg, Other Gram−.
Handling CLs is a major source of CL contamination. Figure 2 shows a daily disposable lens removed and handled with washed hands before culture in an agar sandwich technique. The high levels of colony-forming units (CFU) present are assumed to come from hand contact, because the organisms are almost exclusively CNS normal skin flora. In fact, hand-transported microorganisms usually do not survive and colonize permanently on a lens surface when worn on a normal, healthy eye.39 In 1992, Mowry-McKee et al. performed a study where patients were given two sets of sterile lenses to handle. One set was handled and then worn for 5 hours, and the second set was handled only and then cultured.40 The level of contamination after 5 hours wear was 22 to 65 times lower then the initial contamination level induced by lens handling. In addition, the level of bacterial bioburden before insertion was not predictive of the amount of contamination after wear. For example, in Mowry-McKee et al.’s study,40 the patients presenting with detectable levels of bacteria on lenses after wear did not have greater bacterial bioburden on the corresponding handled-only lenses. This further supports the idea that under normal conditions, the eye has a potent antimicrobial system capable of destroying many organisms introduced during lens handling and insertion and that there are other sources of contamination once the lens is placed on the eye.
FIG. 2.
A daily disposable lens that was removed and handled with washed hands before removal culture in an agar sandwich technique.
Most of the studies exploring bacterial bioburden on CLs have been performed during asymptomatic wear of hydrophilic CLs. There is no doubt that the greater incidence of microbial keratitis in soft lens wearers compared with RGP wearers has driven the preponderance of studies to examine in vivo bacterial adhesion to hydrophilic lenses. Consistent with the notion that the low incidence of microbial keratitis during rigid lens wear is partly because of a low incidence of in vivo bacterial presence on rigid lenses, one study prospectively examined both rigid and hydrophilic lenses and found a significantly lower frequency of CNS and a higher frequency of sterile cultures obtained from hard or RGP lenses.19 No other report has differentiated lens bioburden by rigid or soft lens type.
More recently, some authors have attempted to differentiate lens bioburden between low Dk poly(2-hydroxyetthyl methacrylate [pHEMA])-based hydrogels and SH lens types. There is interest in differentiating the bioburden between these lens types, because it may explain why the incidence of inflammatory complications is increased with SH lens continuous wear.41 In this regard, in vitro bacterial adhesion studies have shown that SH lenses have increased adhesion compared with pHEMA lenses, which may explain the higher rate of inflammation observed during SH lens wear.42– 44 Specifically, when compared with etafilcon A, P. aeruginosa shows increased adhesion to balafilcon A, galyfilcon A, and lotrafilcon B lenses,43,44 and S. epidermidis has increased adhesion to galyfilcon A, balafilcon A, and lotrafilcon B lenses.43 However, other in vitro studies found no difference or decreased adhesion on SH lenses compared with etafilcon A.45,46 Alternatively, three in vivo studies that have directly compared the bioburden between pHEMA and SH materials during wear47– 49 have found no difference between the microbial colonization of these materials. Specifically, in 2001, Keay et al.48 indicated that there were no differences in the number and type of bacteria that adhere to a SH material during 30-day continuous wear compared with 7-day EW with a low Dk pHEMA material, when lenses were worn for up to 1 year. Choo et al.47 examined SH- and pHEMA-based lenses after swimming in a chlorinated pool for 30 min and, also, found no difference between levels or frequency of colonization of the two materials. Lastly, Santos et al.49 showed that commensal organisms are more prevalent on balafilcon A compared with etafilcon A lenses, but no differences were seen between lotrafilcon A and B or galyfilcon A compared with etafilcon A lenses.
There is concern that deposition of protein on a lens surface may act as a ligand for bacterial growth or may independently compromise the corneal epithelium, leaving it more susceptible to infection. Thus, the combination of protein deposition supporting microbial growth and potentially compromising the ocular surface would be especially worrisome. In 1988, Barr et al.50 have shown a positive linear association between in vivo protein deposition and bacterial CFU counts on low Dk nondisposable soft lenses. However, in vitro studies are conflicting in this regard. In 1986, Dart et al.,51 using in vitro adherence assays for S. aureus and P. aeruginosa, determined that bacterial adherence onto low Dk soft lenses was not affected by tear film deposits, even on those lenses that were worn for prolonged use with numerous macroscopic nodular deposits present. However, the differences may be due to surface hydrophobicity of the adsorbed tear film components and base substrate. Bruinsma et al.52 reported that the presence of adsorbed tear film decreases the difference in surface hydrophobicity between lens substrates, which alters the relative amount of bacterial adhesion differences seen on unworn lenses. Willcox et al. found that tear film components increase bacterial adhesion in vitro on a SH material (balafilcon A) but not on etafilcon A, with increases in adhesion of P. aeruginosa and Aeromonas hydrophilia ranging from 243% to 1,393%.44
A common misperception is that microbial bioburden increases gradually on lenses over time. Several studies have shown that there is no increase in the colonization of lenses by potential pathogens or normal flora with length of wear or age of lens. In this regard, Willcox et al.14 monitored subjects in lotrafilcon A lenses replaced at monthly intervals over 2 years and sampled lenses at 1 month, 6 months, and 24 months of wear. There was no increase in frequency of contamination or numbers of bacteria during that time period, with the exception of Propionibacterium sp in which the frequency of colonization increased at the 2-year visit. Furthermore, Gopinathan et al.53 followed up 50 patients wearing low Dk disposable lenses for EW over 18 months and also found no difference in lens contamination with time. Alternatively, some studies have assessed the effect of lens age as opposed to patient-years of experience on microbial bioburden of worn CLs. In 1993 Hart et al.35 correlated lens age with the number of CFU cultured per lens in a cross-sectional study of patients wearing nondisposable soft lenses aged between 0.5 and 12 months and found a statistically significant correlation in an EW group of patients (P = 0.028). In 1988, Barr et al.50 did not find a correlation between CFU counts per lens and lens age based on a cross-sectional survey of nondisposable lenses worn between 1 and 48 months. In both the studies, the patients used various lens types, disinfection regimens, and wearing schedules. In a prospective study, Sweeney et al.,54 controlling for lens type, length of wear, and disinfection regimen, assessed 20 subjects wearing low Dk lenses for increasing periods of lens wear between 1 and 13 days and found no predictable increase in bacterial colonization of lenses during those time points. Therefore, it appears that soft CL wearers establish a baseline level of bioburden on their lenses at the first day of lens wear, which remains fairly consistent over time.
The majority of organisms responsible for this persistent level of colonization on CLs are ocular commensal species. Alternatively, colonization of lenses with pathogenic organisms is more commonly transient as opposed to persistent. Boost and Cho55 noted that microbial contaminants on lenses, lens cases, and suction holders of orthokeratology patients were of a transient nature and did not match the organisms colonizing the eye. They found that only 3 of 22 patients experienced repeated isolation of potential pathogens from CLs over 6 visits spanning more than 12 weeks of wear. In addition, Sweeney et al.54 noted that pathogenic colonization of bacteria on low Dk EW CLs was intermittent rather than a gradual accumulation over time. Although they noted that 80% of subjects were colonized at least once with Gram− bacteria (over 14 visits), the colonization was sporadic. These studies concur with data from our laboratory that examined lens bioburden after 1 week and 4 months of continuous wear with lotrafilcon A lenses in 205 patients. Only 9% of subjects with significant lens bioburden at one visit showed persistent bioburden at a subsequent visit. Transient association of pathogenic levels of bacteria on the lens and ocular surface was more common than persistent colonization.34
In general, when contamination is detected, the mean number of CFUs found on CLs removed aseptically from the eye are generally very low, usually below a median of 5 or 10 CFU per lens.35,36,56 However, there are exceptions to this, with ranges as high as 9.3 × 105 CFU/lens. Although usually transient and unpredictable, when Gram− or other pathogenic organisms are present, they are frequently isolated in very high levels (compared with ocular commensal organisms), even during asymptomatic wear. For example, in Sankaridurg et al.’s study,56 where over 4,200 lens cultures were performed on asymptomatic patients during an EW trial in India, 4 pathogenic species were present at a median of 140 CFUs/lens, whereas all commensal organisms colonized at a median of 15 CFU/lens. They postulated that pathogenic organisms increase their colonization of the lens surfaces by mechanisms of adherence through the production of biofilm. The pathogens likely gain access to the eye and lens from other body sites such as the oropharynx and then begin to firmly attach to each other and the lens surface. Pathogenic species may adhere more strongly to lenses and are not removed as easily as normal biota.22 In biofilm formation, a phenotypic and genotypic shift occurs, and the organisms secrete a polysaccharide matrix that envelopes and protects the newly formed multilayer microbial community from host defenses and biocides in CL cleaning agents.57 Biofilm formation does not occur as often in ocular commensal species, which may explain why colonization of lens surfaces by ocular commensal organisms results in lower numbers of CFU per lens and less associated pathogenicity. For example, many of the most common pathogenic species (P. aeruginosa, S. marcescens, and S. aureus) are known biofilm producers, whereas among the commensal flora (CNS, Corynebacteria, and Propionibacteria), only some of the S. epidermidis strains are known biofilm producers.
The relevant findings with regards to lens-related microbial bioburden can be summarized as follows: across all studies during normal CL wear, the incidence of microbial bioburden on traditional hydrophilic or SH CLs is approximately 55% to 85%. Lens handling greatly increases the incidence of lens contamination, and the ocular surface has a tremendous ability to destroy organisms. However, even when removed aseptically from the eye, more than half (~56%– 65%) of lenses are found to harbor microorganisms, almost exclusively bacteria. The bacteria isolated are mostly considered normal microbiota, the greatest percentage being CNS. The presence of ocular commensal organisms on lens surfaces appears to be persistent when assessing groups of patients over time, and the presence of ocular pathogens is typically sporadic and unpredictable. There appears to be an inverse correlation between the frequency of isolation of microbes most commonly isolated from asymptomatic wearers and those believed to be associated with ocular surface pathologic conditions. Lens deposits influence bacterial adherence differentially depending on lens substrate. Silicone hydrogel lenses do not appear to differ from traditional pHEMA lenses in levels or frequency of bacterial colonization in vivo. The process of CL-related microbial keratitis and inflammation is believed to be preceded by the presence or transfer of microorganisms from the lens to the ocular surface or both. Thus, this detailed understanding of lens-related bioburden is important in the understanding of factors associated with infectious and inflammatory complications.
Special Circumstances of CL Bioburden
Orthokeratology
Renewed interest in orthokeratology has lead to global use of this technique and an increased concern over microbial keratitis associated with this procedure.58 Reports of infection in orthokeratology patients in Hong Kong and elsewhere reveal that the most common pathogen is P. aeruginosa.59 – 64 There has been one in vivo study examining the changes of ocular flora of overnight orthokeratology patients and the contamination of orthokeratology lenses and lens accessories. Boost and Cho55 examined 41 newly fitted orthokeratology subjects before and after 12 weeks after initiation of orthokeratology treatment in Hong Kong. Before lens wear, three subjects (7.3%) yielded conjunctival samples positive for potential pathogens (S. aureus), and during treatment, 29% of subjects had potential pathogens isolated from the conjunctiva on at least one of six occasions after orthokeratology treatment began. Overall, 54% of subjects yielded no growth or normal flora only, and 39% yielded potential pathogens on lenses made of hexafocon A (Boston XO; Baush & Lomb, Rochester, NY) throughout the treatment. The pathogens isolated from lenses included P. aeruginosa, other Pseudomonas sp, E. coli, Acinetobacter, yeast, S. aureus, and others. The authors concluded that ocular flora was not altered by orthokeratology treatment in this subset of infection-free patients throughout the study. The frequency of lens colonization is similar to other soft and RGP CL studies; however, this may be underestimated when compared with clinical practice, because positive reinforcement was used to warn subjects of contamination noted at any visit.
Swimming
Water exposure is implicated in many CL infections, most importantly Acanthamoeba keratitis.65– 67 Similarly, short-term swimming in a chlorinated pool significantly increases the frequency of CL colonization with the same bacterial organisms found in the pool water.47 For chlorinated pools, the organisms present in both the water and on the lenses included predominantly S. epidermidis, with small numbers of S. aureus and Streptococcus salivarius.
Shipboard Naval Environment
A shipboard naval environment increases colonization of Enterobacter and Pseudomonas sp on EW CLs as reported by Theng et al.68 CLs worn for 1 week of EW were found to be colonized with S. epidermidis and S. aureus, as in most other studies of normal wear. However, Enterobacter was present at a frequency of approximately 16% and Pseudomonas sp at a frequency of approximately 9%, which is much higher than frequencies cultured during normal wear outside the naval environment (see Table 3). Because of the higher than normal incidence of potential pathogens found on EW lenses and because seamen face prolonged periods with limited access to eye care, EW use of soft contact is not indicated.
Bandage Lenses
Bandage lenses used after photorefractive keratectomy (PRK), laser subepithelial keratectomy (LASEK), or corneal disease are often combined with topical antibiotics and are, thus, infrequently colonized with bacteria.32,69,70 In 1982, Hovding32 explored bandage lens contamination with and without use of topical antibiotics. Although not statistically significant and perhaps confounded by hand contamination, a greater incidence of sterile cultures was found in the group using topical antibiotic therapy (66.7% compared with 46.7% in patients not using antibiotics). In more recent refractive surgery studies, approximately 90% of bandage CLs used are sterile when combined with a regimen of topical medications such as ofloxacin, tromethamine ketorolac, and fluorometholone69 or tobramycin and diclofenac.70 However, when bandage lenses in combination with antibiotic prophylaxis are colonized, nonocular potential pathogens often prevail. One study, which used vifilcon A lenses after LASEK, found 6.2% of lenses colonized with Klebsiella pneumoniae, S. aureus, or Streptococcus viridans.69 Another study using lotrafilcon A lenses after PRK found 7.7% of lenses positive for Stenotrophomonas maltophilia, Acinetobacter sp, or Aeromonas hydrophila. Nonetheless, the organisms found were typically sensitive to the prophylactic antibiotics administered during lens use, thus the risk of infectious keratitis after PRK and LASEK secondary to bandage lens is believed to be low.
Lens Case Bioburden
The frequency of storage case contamination is higher than associated lens contamination. Table 4 lists the relevant studies performed on lens case contamination. On average, well more than half of all lens cases assessed were contaminated, mostly with bacteria, and many studies document contamination rates as high as 81%. In addition, in those studies that specifically cultured for the presence of Acanthamoeba sp, the frequency of isolation was approximately 8%. Assessing the lens storage case for contamination may provide insight into organisms that may transfer to the lens surface and ultimately to the ocular surface.
TABLE 4.
Lens Case Bioburden
| Study | Sample size and type | Lens type | Disinfection systems used | Frequency of sterile cases (%) | Frequency of author defined case contamination (%) | Frequency of contamination (%)
|
||
|---|---|---|---|---|---|---|---|---|
| Bacteria | Fungi | Protozoa | ||||||
| Case wells | ||||||||
| Gray et al.72 | 101 asymptomatic daily wear patients | 86 (85%) soft lens; 15 (15%) rigid | Peroxide (75%); chemical (23%) | 19 | 81 | 77 | 24 | 20 (Acanthamoeba sp 8) |
| Pens et al.88 | 81 contact lens wearers | NR | NR | NR | 80.1 | 71 | NA | Acanthamoeba spp 8.9 |
| Fleiszig and Efron18 | 41 contact lens wearers | Rigid and soft | Various | 29 | 71 | 71 | NR | NA |
| Solution in cases | ||||||||
| Midelfart et al.105 | 21 asymptomatic medical students | 20 (95%) soft lens, 1 (5%) RGP | Chemical or peroxide | 76.2 | 24 | 24 | NR | 0 |
| Callender et al.16 | 58 normal patients | hydrophilic | Various chemical | 28.1 | 71.9 | 71.9 | 0 | NR |
| Combined analysis of wells and residual solution | ||||||||
| Donzis et al.82 | 100 asymptomatic lens wearers | 62% soft and 38% rigid | chemical, peroxide, and heat | 54 | 46 | 46 | 6.5 | 0 |
| Larkin et al.73 | 102 asymptomatic lens wearers | 67 (65.7%) soft; 35 (34.2%) rigid | 62 (60.8%) chemical; 19 (18.6%) heat; 20 (19.6% peroxide) | 39.2 negligible counts | 42.2 | 42.2 (>106 viable counts/mL) 18.6 (between 10–106 viable counts/mL) | NA | 8.8 (Acanthamoeba sp 6.9) |
| May et al.74 | 40 experienced lens wearers | Rigid | Chlorhexidine based wetting, soaking, and disinfecting solutions | NR | 55 | 55 | NA | NA |
| Wilson et al.76 | 76.2 | 76.2 | Occasional | NA | ||||
| Clark et al.71 | 178 asymptomatic wearers | 132 (74%) soft; 46 (26%) rigid | 75.3% chemical; 21.9% peroxide; 0.6% heat | 46 | 54 | 50 | 0.75 | 3.9 |
| Velasco106 | 126 asymptomatic wearers | Soft | Polyaminopropylbiguanide preserved solution | 19 | 81.0 | 81.0 | 0 | 0 |
NR, not reported; NA, not assessed.
Unlike lens contamination, which is almost exclusively bacterial, when contamination is detected within solution found in lens cases or on the internal wells, the microorganisms involved are usually mixed contaminants of bacteria, fungi, and protozoa.71–73 Gray et al. reported on 101 consecutive CL patients in an optometry practice in New Zealand and found that in the 24 cases that harbored fungi and the 20 cases that harbored protozoa, almost all the cases showed bacterial co-contamination. Moreover, in the 78 cases that grew bacteria, most (72%) demonstrated mixed bacterial contaminants.72
Biofilm is a common culprit in the failure of care solutions to be effective against storage case contamination. It is well known that biofilm established on lenses or within the lens case becomes much more resistant to the biocide properties of lens care products.57,74,75 Although the incidence of mature, coalesced, biofilm formation on lens cases has not been published, it is mentioned to have occurred frequently in some studies.72,76 Cell surface attachment to the lens case is the early stage of biofilm production, and even this stage of biofilm development is known to contribute to the resistance of care system disinfectants.74 Once glycocalyx formation occurs, the persistence of the cells increases in hostile environments and mature biofilms then provide a seed of inoculum for continued growth.
The influence of care system on case contamination has been noted by several authors.18,72,73,76 In at least two studies, the use of peroxide care systems was associated with greater case contamination.18,72 One study72 noted that all microbial contaminants contained the enzyme catalase, which breaks down hydrogen peroxide to water and oxygen. The authors speculated that repeated use of peroxide care systems may have selected a naturally resistant population of microbes adapted to survive in such an environment. However, in other studies,73,76 the use of peroxide in soft lens wearers was found to decrease bacterial contamination.
Reviewing the literature on lens case contamination and the data in Table 4 lead to the following conclusions: the incidence of positive microbial bioburden within storage cases ranges from 24% to 81% and most (72.7%) studies report an incidence of greater than 50%. Biofilms are considered the major culprit resulting in transfer of resistant organisms from the lens case to the lens surface. Lens care solutions have varying efficacies against biofilm and the ability of organisms to transfer from the case to the lens. In that regard, in 2008, Vermeltfoort et al.77 published a biofilm model to determine efficacy of multipurpose lens care solutions against biofilms grown in lens cases. They then used their in vitro model to determine the efficacy of solutions on bacterial transmission from the biofilm laden case to CLs stored within the case. Compared with biguanide-preserved multipurpose care solutions, they found that a polyquaternium-preserved care solution was the most effective in reducing transfer of bacterial cells from a biofilm laden case to a SH lens soaked for 8 hours within the case.77
Contamination of Care Solutions
The care system bottles themselves can easily become contaminated and become a source of microbes that may contaminate the lens storage cases, adhere to the lens, cause an inflammatory reaction, or infect the cornea. In fact, microbial keratitis has been linked with contaminated care products.78,79 All types of care solutions, including hydrogen peroxide,80,81 have been shown to become contaminated, even in experienced and compliant users,80,82 including unopened factory sealed bottles.83,84 Although CNS is usually detected, potential pathogens such as S. marcescens and P. aeruginosa are commonly isolated,78 which is a concern, because these organisms can directly infect the cornea under compromised conditions. In addition, these microorganisms can serve as a food source for other pathogenic organisms such as Acanthamoeba sp. In fact, Acanthamoeba organisms are almost always found in lens cases or care solutions that also harbor bacteria or fungal organisms.85
Table 5 outlines the incidence rates of contamination in lens care solutions assessed in this review including products such as nonpreserved saline preparations, preserved rewetting drops, saline and multipurpose solutions, and hydrogen peroxide-based care systems. As one would expect, unpreserved saline products are the most likely to become heavily contaminated. Homemade saline is contaminated in 100% of samples, commercially prepared and bottled nonpreserved saline in 25% to 82% of samples, and aerosol saline in up to 40% of samples assessed. In addition, Sweeney et al.86 have shown that unpreserved saline becomes heavily contaminated within 1 week of patient use, predominantly with Gram-negative organisms. The incidence of contamination in preserved products is less; however, some contamination is usually detected, with most studies reporting contamination rates up to 30%. As opposed to nonpreserved saline, preserved saline typically becomes contaminated with Gram+ species. By using a similar approach to their previous study, Sweeney et al.83 found that nozzles of preserved saline were contaminated at a rate of 55% and the contents at a rate of 26%. More than half (53%) of the bottles harbored CNS; Bacillus sp and Corynebacteria sp were also prevalent (22% and 19%, respectively). Pseudomonas sp were cultured only 4% of the time, fungi had a very small presence, and Acanthamoeba sp was never detected.83
TABLE 5.
Care System Bioburden
| Type of care system | Rates of contamination (%) and respective study |
|---|---|
| Homemade saline | 10082 |
| Unpreserved saline | 2582 |
| 7280 | |
| 8278 | |
| Aerosol saline | 082 |
| 4076 | |
| Preserved saline: new, factory sealed | 082 |
| 2583 | |
| Used preserved saline | 1382 |
| 1480 | |
| 2978 | |
| 45–9083 | |
| Soft lens cleaner | 1582 |
| 678 | |
| Soft disinfecting solution | 0a78 |
| 3a82 | |
| 8.181 | |
| 1176 | |
| 1780 | |
| Hydrogen peroxide soft disinfecting solution | 3.681 |
| 876 | |
| 1780 | |
| Eyedrops (artificial tears or contact lens rewetting drops) | 082 |
| 978 | |
| RGP cleaning solution | 1082 |
| RGP wetting and soaking solution | 2182 |
| 4178 |
May include peroxide.
Many of the studies documenting contaminated solutions have assayed the contents of the bottle by squeezing the contents through the bottle tip.81,82 In such instances, the internal solution became contaminated through suction of organisms through the tip, or the bottle’s tip was contaminated. However, when culturing the solution tip separately from the bottle contents, it usually becomes apparent that the microbes are usually confined to the orifice rims or plastic caps on dispenser tips. In 1990, Wilson et al.76 sampled asymptomatic CL wearers and found 11% of soft lens care system bottles were bacterially contaminated, and more than half of aerosol saline canisters were contaminated if drawn through the bottle tip, but the solution withdrawn through the sides of the containers with syringes was always sterile. Sweeney et al.83 also noted that nozzles of preserved saline were twice as likely to be contaminated compared with the solution contents.
In contaminated solution samples with asymptomatic patients, the level of bioburden is typically low (<300 CFU/mL)81,83; however, it can exceed 105 CFU/mL.78,81 Under optimal viewing conditions, contamination with more than 106 CFU/mL is required to note turbidity in the solution.81 Thus, one cannot reliably educate a patient about potential solution contamination by means of turbidity, because high levels of contamination, as noted in several studies, would not be visible to the naked eye.
The length of time since a bottle was opened and used may influence the degree of contamination. Although used bottles have been shown to harbor organisms as soon as 5 days after opening, risk of contamination increases the longer a bottle has been opened.82 Therefore, concern arises for those patients who use lenses and care products on an occasional basis and over an extended period of time, where the risk of high levels of microbial bioburden can rise to a dangerous level.
Lens Wear and Care Habits and Role of Compliance
Good compliance and reinforcement of proper hygiene and care practices are associated with decreased lens case contamination.76 In orthokeratology wearers, good compliance has been significantly associated with no or low levels of lens, lens case, and suction holder contamination.55 Conversely, poor compliance such as avoiding the rinsing step before insertion is associated with soft lens contamination with pathogenic organisms.87 Insufficient care with hand washing has been associated with increased contamination of lens storage cases with Acanthamoeba sp.88 In addition, occasional lens wearers have been found to have increased pathogenic organisms on the surfaces of their soft lenses, but interestingly no differences were found in lens case contamination of occasional users.87 The most likely scenario involves organisms transferred to the lens from the hands during removal which are not destroyed by the disinfecting solution and allowed to proliferate over time in a static lens case.
Prevention of Contamination
The widespread contamination of CLs and storage cases sets the stage for the potentially devastating consequences of microbial and inflammatory keratitis. Thus, prevention of lens and storage case microbial contamination is desirable even for compliant patients who use modern care systems. Antimicrobial surfaces have been used for decades on indwelling medical devices; the biggest threat to microbial colonization of a medical device is the formation of resistant biofilm,89 and by preventing the initial colonization of microorganisms, it is believed that biofilms will be likewise inhibited.
In addition to CLs serving as an antibiotic ophthalmic drug delivery system,90,91 CLs and cases can also be formulated with antimicrobial surface treatments. To date, antimicrobial CLs are still in investigational stages, although one type of antimicrobial treated case is commercially available.92 Technologies with potential application to CLs include silver, polyquats, polymeric pyridium compounds, free-radical producing agents, quorum-sensing blockers, and antiinfective agents.93 Silver is a broad spectrum antimicrobial agent against Gram+ and Gram− bacteria including antibiotic resistant strains, protozoa, and certain viruses.94 Silver has been used extensively in other medical devices and dressings, and the large increase in the number of antibiotic resistant bacterial strains has prompted a renewed interest in the use of silver as an antibacterial agent.94 Silver ions interact with the DNA of bacteria preventing cell reproduction, disturb bacterial cell permeability and respiration, and create defects in the bacterial cell wall, resulting in loss of cell contents and cell death.94 Nissen and Furkert95 has shown that nonionic, 55% water, hydrophilic lenses with a silver layer decreased the number of adhered P. aeruginosa organisms by four logs and S. aureus organisms by one log compared with uncoated lenses. In an attempt to decrease case contamination, a silver impregnated lens case has been available in the United Kingdom since 2004 and cleared by the United States Food and Drug Administration in 2005 under the name PRO-GUARD.92 During manufacture of the PRO-GUARD case, ionic silver is added during the injection molding process, thus, it is distributed throughout the entire thickness of the plastic.92 Clinical trials using this case compared with a polypropylene control case show that the silver-impregnated case had between 25% and 41% reduction in bacterial lens case contamination.92
Two other recently published methods of antimicrobial lens technology include quorum-sensing blockers and free-radical producing agents such as selenium. Mathews et al.96 evaluated the safety (in rabbits) and efficacy of a covalent selenium coating on balafilcon A CLs. Selenium in the −2 oxidation state (selenide) assists in reducing oxygen to superoxide, which is toxic to cancer cells, bacteria, and viruses.96 Selenium-coated lenses prevented in vitro colonization by P. aeruginosa compared with uncoated lenses and caused no detectable changes to the corneas of rabbits when worn for up to 60 days.96 Zhu et al.97 evaluated the safety (in humans and guinea pigs) and efficacy of a fimbrolide coating on lotrafilcon A CLs. Fimbrolides (also known as furanones) are inhibitors of bacterial signaling systems and quorum sensing, which were initially isolated from marine algae as they prevented bacterial colonization on the algae’s surface.93 Fimbrolide-coated lenses reduced the adhesion of P. aeruginosa, S. aureus, S. marcescens, and Acanthamoeba sp between 67% and 92% compared with uncoated lenses and caused no significant ocular responses in a 1-month animal model or after 24 hours in human trials.97
The first bit of evidence that antimicrobially treated lenses will reduce the incidence and severity of bacterially driven adverse responses was recently published. Investigators from the Institute for Eye Research in Australia covalently bound etafilcon A lenses with melamine, an antimicrobial cationic peptide, and tested the ability to prevent CLPU and CLARE in animal models.98 In a S. aureus rabbit model of CLPU, the melamine-coated lenses showed significant reductions in scores of symptoms and depth of corneal infiltration, and in a P. aeruginosa guinea pig model of CLARE, there was significant improvement in all ocular response parameters compared with control lenses.
CONCLUSIONS
CLs, lens care solutions, and lens storage cases can transfer high numbers of commensal or potentially pathogenic organisms to the corneal surface. More than half of lenses routinely harbor micro-organisms including potentially pathogenic species; however, the ocular surface tolerates their presence and overcomes potentially devastating consequences under normal conditions. Reduced tissue resistance secondary to trauma, surgery, age, systemic disease, immunosuppression, CL use, and other unknown factors allows microorganisms adherent to CLs to invade and colonize the cornea or conjunctiva to produce inflammation or infection. Thus, the prevention of biofilms and adherent organisms on lens surfaces is desirable to potentially decrease the incidence of microbially driven adverse events. Antimicrobial surfaces have been successfully applied to other biomedical devices, and their application to CLs is a natural step forward. There is early evidence in animal models that antimicrobially treated CLs will decrease the risk of inflammatory complications such as CLARE and CLPU. The influence of antimicrobial surfaces on the reduction of microbial keratitis is not only biologically plausible but quite promising as well.
Acknowledgments
The literature review and preparation of this article was supported by Johnson & Johnson Vision Care, Inc., but under the control of Dr. Szczotka-Flynn.
References
- 1.Dart JK, Stapleton F, Minassian D. Contact lenses and other risk factors in microbial keratitis. Lancet. 1991;338:650– 653. doi: 10.1016/0140-6736(91)91231-i. [DOI] [PubMed] [Google Scholar]
- 2.Das S, Sheoreym H, Taylor HR, et al. Association between cultures of contact lens and corneal scraping in contact lens related microbial keratitis. Arch Ophthalmol. 2007;125:1182–1185. doi: 10.1001/archopht.125.9.1182. [DOI] [PubMed] [Google Scholar]
- 3.Martins EN, Farah ME, Alvarenga LS, et al. Infectious keratitis: Correlation between corneal and contact lens cultures. CLAO J. 2002;28:146–148. [PubMed] [Google Scholar]
- 4.Mela EK, Giannelou IP, Koliopoulos JX, et al. Ulcerative keratitis in contact lens wearers. Eye Contact Lens. 2003;29:207–209. doi: 10.1097/01.icl.0000078102.30635.A7. [DOI] [PubMed] [Google Scholar]
- 5.Corrigan KM, Harmis NY, Willcox MD. Association of Acinetobacter species with contact lens-induced adverse responses. Cornea. 2001;20:463– 466. doi: 10.1097/00003226-200107000-00004. [DOI] [PubMed] [Google Scholar]
- 6.Holden BA, La Hood D, Grant T, et al. Gram-negative bacteria can induce contact lens related acute red eye (CLARE) responses. CLAO J. 1996;22:47–52. [PubMed] [Google Scholar]
- 7.Jalbert I, Willcox MD, Sweeney DF. Isolation of Staphylococcus aureus from a contact lens at the time of a contact lens-induced peripheral ulcer: Case report. Cornea. 2000;19:116–120. doi: 10.1097/00003226-200001000-00023. [DOI] [PubMed] [Google Scholar]
- 8.Keay L, Harmis N, Corrigan K, et al. Infiltrative keratitis associated with extended wear of hydrogel lenses and abiotrophia defectiva. Cornea. 2000;19:864– 869. doi: 10.1097/00003226-200011000-00024. [DOI] [PubMed] [Google Scholar]
- 9.Sankaridurg PR, Sharma S, Willcox M, et al. Colonization of hydrogel lenses with Streptococcus pneumoniae: Risk of development of corneal infiltrates. Cornea. 1999;18:289–295. doi: 10.1097/00003226-199905000-00008. [DOI] [PubMed] [Google Scholar]
- 10.Sankaridurg PR, Vuppala N, Sreedharan A, et al. Gram negative bacteria and contact lens induced acute red eye. Indian J Ophthalmol. 1996;44:29–32. [PubMed] [Google Scholar]
- 11.Sankaridurg PR, Willcox MD, Sharma S, et al. Haemophilus influenzae adherent to contact lenses associated with production of acute ocular inflammation. J Clin Microbiol. 1996;34:2426–2431. doi: 10.1128/jcm.34.10.2426-2431.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Wu PZ, Thakur A, Stapleton F, et al. Staphylococcus aureus causes acute inflammatory episodes in the cornea during contact lens wear. Clin Experiment Ophthalmol. 2000;28:194–196. doi: 10.1046/j.1442-9071.2000.00293.x. [DOI] [PubMed] [Google Scholar]
- 13.Willcox MD, Hume EB. Differences in the pathogenesis of bacteria isolated from contact-lens-induced infiltrative conditions. Aust N Z J Ophthalmol. 1999;27:231–233. doi: 10.1046/j.1440-1606.1999.00189.x. [DOI] [PubMed] [Google Scholar]
- 14.Willcox MD, Harmis NY, Holden BA. Bacterial populations on high-DK silicone hydrogel contact lenses: Effect of length of wear in asymptomatic patients. Clin Exp Optom. 2002;85:172–175. doi: 10.1111/j.1444-0938.2002.tb03031.x. [DOI] [PubMed] [Google Scholar]
- 15.Khorazo D, Thompson R. The bacterial flora of the normal conjunctiva. Am J Ophthalmol. 1935;18:1114–1116. [Google Scholar]
- 16.Callender MG, Tse LS, Charles AM, et al. Bacterial flora of the eye and contact lens. Cases during hydrogel lens wear. Am J Optom Physiol Opt. 1986;63:177–180. doi: 10.1097/00006324-198603000-00002. [DOI] [PubMed] [Google Scholar]
- 17.Elander TR, Goldberg MA, Salinger CL, et al. Microbial changes in the ocular environment with contact lens wear. CLAO J. 1992;18:53–55. [PubMed] [Google Scholar]
- 18.Fleiszig SM, Efron N. Microbial flora in eyes of current and former contact lens wearers. J Clin Microbiol. 1992;30:1156–1161. doi: 10.1128/jcm.30.5.1156-1161.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Hovding G. The conjunctival and contact lens bacterial flora during lens wear. Acta Ophthalmol (Copenh) 1981;59:387– 401. doi: 10.1111/j.1755-3768.1981.tb03004.x. [DOI] [PubMed] [Google Scholar]
- 20.Iskeleli G, Bahar H, Eroglu E, et al. Microbial changes in conjunctival flora with 30-day continuous-wear silicone hydrogel contact lenses. Eye Contact Lens. 2005;31:124–126. doi: 10.1097/01.icl.0000141923.63458.df. [DOI] [PubMed] [Google Scholar]
- 21.Larkin DF, Leeming JP. Quantitative alterations of the commensal eye bacteria in contact lens wear. Eye. 1991;5(Pt 1):70–74. doi: 10.1038/eye.1991.14. [DOI] [PubMed] [Google Scholar]
- 22.Stapleton F, Willcox MD, Fleming CM, et al. Changes to the ocular biota with time in extended- and daily-wear disposable contact lens use. Infect Immun. 1995;63:4501– 4505. doi: 10.1128/iai.63.11.4501-4505.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Willcox MD, Power KN, Stapleton F, et al. Potential sources of bacteria that are isolated from contact lenses during wear. Optom Vis Sci. 1997;74:1030–1038. doi: 10.1097/00006324-199712000-00025. [DOI] [PubMed] [Google Scholar]
- 24.Osato M. Normal ocular flora. In: Holland G, Pepose J, Wilhelmus K, editors. Ocular Infection & Immunity. St. Louis, Mosby-Year Book, Inc; 1996. [Google Scholar]
- 25.Weidenmaier C, Kristian SA, Peschel A. Bacterial resistance to antimicrobial host defenses—An emerging target for novel antiinfective strategies? Curr Drug Targets. 2003;4:643– 649. doi: 10.2174/1389450033490731. [DOI] [PubMed] [Google Scholar]
- 26.Ly CN, Pham JN, Badenoch R, et al. Bacteria commonly isolated from keratitis specimens retain antibiotic susceptibility to fluoroquinolones and gentamicin plus cephalothin. Clin Experiment Ophthalmol. 2006;34:44–50. doi: 10.1111/j.1442-9071.2006.01143.x. [DOI] [PubMed] [Google Scholar]
- 27.Ramachandran L, Sharma S, Sankaridurg R, et al. Examination of the conjunctival microbiota after 8 hours of eye closure. CLAO J. 1995;21:195–199. [PubMed] [Google Scholar]
- 28.Fleiszig SM, Efron N. conjunctival flora in extended wear of rigid gas permeable contact lenses. Optom Vis Sci. 1992;69:354–357. doi: 10.1097/00006324-199205000-00004. [DOI] [PubMed] [Google Scholar]
- 29.McBride ME. Evaluation of microbial flora of the eye during wear of soft contact lenses. Appl Environ Microbiol. 1979;37:233–236. doi: 10.1128/aem.37.2.233-236.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Rauschl R, Rodgers JJ. The effect of hydrophilic contact lens wear on the bacterial flora of the human conjunctiva. Int Contact Lens Clin. 1978;5:56– 62. [Google Scholar]
- 31.Smolin G, Okumoto M, Nozik RA. The microbial flora in extended-wear soft contact-lens wearers. Am J Ophthalmol. 1979;88(3 Pt 2):543–547. doi: 10.1016/0002-9394(79)90512-9. [DOI] [PubMed] [Google Scholar]
- 32.Hovding G. Conjunctival and contact lens bacterial flora during continuous ‘bandage’ lens wear. Acta Ophthalmol (Copenh) 1982;60:439– 448. doi: 10.1111/j.1755-3768.1982.tb03036.x. [DOI] [PubMed] [Google Scholar]
- 33.Stapleton F, Willcox MD, Sansey N, et al. Ocular microbiota and polymorphonuclear leucocyte recruitment during overnight contact lens wear. Aust N Z J Ophthalmol. 1997;25(suppl 1):S33–S35. doi: 10.1111/j.1442-9071.1997.tb01751.x. [DOI] [PubMed] [Google Scholar]
- 34.Szczotka-Flynn LB, Bajaksouzian S, Jacobs MR, et al. Risk factors for contact lens bacterial contamination during continuous wear. Optom Vis Sci. 2009;86:1216–1226. doi: 10.1097/OPX.0b013e3181bbca18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Hart DE, Reindel W, Proskin HM, et al. Microbial contamination of hydrophilic contact lenses: Quantitation and identification of microorganisms associated with contact lenses while on the eye. Optom Vis Sci. 1993;70:185–191. doi: 10.1097/00006324-199303000-00002. [DOI] [PubMed] [Google Scholar]
- 36.Leitch EC, Harmis NY, Corrigan KM, et al. Identification and enumeration of staphylococci from the eye during soft contact lens wear. Optom Vis Sci. 1998;75:258–265. doi: 10.1097/00006324-199804000-00022. [DOI] [PubMed] [Google Scholar]
- 37.Stapleton F, Keay L, Edwards K, et al. The incidence of contact lens-related microbial keratitis in Australia. Ophthalmology. 2008;115:1655–1662. doi: 10.1016/j.ophtha.2008.04.002. [DOI] [PubMed] [Google Scholar]
- 38.Lipener C, Nagoya FR, Zamboni FJ, et al. Bacterial contamination in soft contact lens wearers. CLAO J. 1995;21:122–124. [PubMed] [Google Scholar]
- 39.Hart DE, Shih KL. Surface interactions on hydrogel extended wear contact lenses: Microflora and microfauna. Am J Optom Physiol Opt. 1987;64:739–748. doi: 10.1097/00006324-198710000-00005. [DOI] [PubMed] [Google Scholar]
- 40.Mowrey-McKee MF, Sampson HJ, Proskin HM. Microbial contamination of hydrophilic contact lenses. Part II: Quantitation of microbes after patient handling and after aseptic removal from the eye. CLAO J. 1992;18:240–244. [PubMed] [Google Scholar]
- 41.Szczotka-Flynn L, Diaz M. Risk of corneal inflammatory events with silicone hydrogel and low DK hydrogel extended contact lens wear: A meta-analysis. Optom Vis Sci. 2007;84:247–256. doi: 10.1097/OPX.0b013e3180421c47. [DOI] [PubMed] [Google Scholar]
- 42.Henriques MC, Sousa M, Lira M, et al. Adhesion of Pseudomonas aeruginosa and Staphylococcus epidermidis to silicone-hydrogel contact lenses. Optom Vis Sci. 2005;82:446– 450. doi: 10.1097/01.opx.0000168585.53845.64. [DOI] [PubMed] [Google Scholar]
- 43.Kodjikian L, Casoli-Bergeron E, Malet F, et al. Bacterial adhesion to conventional hydrogel and new silicone-hydrogel contact lens materials. Graefes Arch Clin Exp Ophthalmol. 2008;246:267–273. doi: 10.1007/s00417-007-0703-5. [DOI] [PubMed] [Google Scholar]
- 44.Willcox MD, Harmis N, Williams CT, et al. Bacterial interactions with contact lenses: Effects of lens material, lens wear and microbial physiology. Biomaterials. 2001;22:3235–3247. doi: 10.1016/s0142-9612(01)00161-2. [DOI] [PubMed] [Google Scholar]
- 45.Borazjani RN, Levy B, Ahearn DG. Relative primary adhesion of Pseudomonas aeruginosa, Serratia marcescens and Staphylococcus aureus to hema-type contact lenses and an extended wear silicone hydrogel contact lens of high oxygen permeability. Cont Lens Anterior Eye. 2004;27:3– 8. doi: 10.1016/j.clae.2003.08.001. [DOI] [PubMed] [Google Scholar]
- 46.Santos L, Rodrigues D, Lira M, et al. Bacterial adhesion to worn silicone hydrogel contact lenses. Optom Vis Sci. 2008;85:520–525. doi: 10.1097/OPX.0b013e31817c92f3. [DOI] [PubMed] [Google Scholar]
- 47.Choo J, Vuu K, Burnham BK, et al. Bacterial populations on silicone hydrogel and hydrogel contact lenses after swimming in a chlorinated pool. Optom Vis Sci. 2005;82:134–137. doi: 10.1097/01.opx.0000153168.54495.da. [DOI] [PubMed] [Google Scholar]
- 48.Keay L, Willcox MD, Sweeney DF, et al. Bacterial populations on 30-night extended wear silicone hydrogel lenses. CLAO J. 2001;27:30–34. [PubMed] [Google Scholar]
- 49.Santos L, Rodrigues D, Lira M, et al. The influence of surface treatment on hydrophobicity, protein adsorption and microbial colonisation of silicone hydrogel contact lenses. Cont Lens Anterior Eye. 2007;30:183–188. doi: 10.1016/j.clae.2006.12.007. [DOI] [PubMed] [Google Scholar]
- 50.Barr JT, Lapple WJ, Snyder AC, et al. Evaluation of contact lenses by microbial enumeration and protein determination. Am J Optom Physiol Opt. 1988;65:476– 480. doi: 10.1097/00006324-198806000-00007. [DOI] [PubMed] [Google Scholar]
- 51.Dart JK, Badenoch R. Bacterial adherence to contact lenses. CLAO J. 1986;12:220–224. [PubMed] [Google Scholar]
- 52.Bruinsma GM, van der Mei HC, Busscher HJ. Bacterial adhesion to surface hydrophilic and hydrophobic contact lenses. Biomaterials. 2001;22:3217–3224. doi: 10.1016/s0142-9612(01)00159-4. [DOI] [PubMed] [Google Scholar]
- 53.Gopinathan U, Stapleton F, Sharma S, et al. Microbial contamination of hydrogel contact lenses. J Appl Microbiol. 1997;82:653– 658. doi: 10.1111/j.1365-2672.1997.tb03598.x. [DOI] [PubMed] [Google Scholar]
- 54.Sweeney DF, Stapleton F, Leitch C, et al. Microbial colonization of soft contact lenses over time. Optom Vis Sci. 2001;78:100–105. doi: 10.1097/00006324-200102000-00010. [DOI] [PubMed] [Google Scholar]
- 55.Boost MV, Cho P. Microbial flora of tears of orthokeratology patients, and microbial contamination of contact lenses and contact lens accessories. Optom Vis Sci. 2005;82:451– 458. doi: 10.1097/01.opx.0000168587.72893.ec. [DOI] [PubMed] [Google Scholar]
- 56.Sankaridurg PR, Sharma S, Willcox M, et al. Bacterial colonization of disposable soft contact lenses is greater during corneal infiltrative events than during asymptomatic extended lens wear. J Clin Microbiol. 2000;38:4420– 4424. doi: 10.1128/jcm.38.12.4420-4424.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Szczotka-Flynn LB, Imamura Y, Chandra J, et al. Increased resistance of contact lens-related bacterial biofilms to antimicrobial activity of soft contact lens care solutions. Cornea. 2009;28:918–926. doi: 10.1097/ICO.0b013e3181a81835. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Schein OD. Microbial keratitis associated with overnight orthokeratology: What we need to know. Cornea. 2005;24:767–769. doi: 10.1097/01.ico.0000186535.57765.85. [DOI] [PubMed] [Google Scholar]
- 59.Chen KH, Kuang TM, Hsu WM. Serratia marcescens corneal ulcer as a complication of orthokeratology. Am J Ophthalmol. 2001;132:257–258. doi: 10.1016/s0002-9394(01)00931-x. [DOI] [PubMed] [Google Scholar]
- 60.Hutchinson K, Apel A. Infectious keratitis in orthokeratology. Clin Experiment Ophthalmol. 2002;30:49–51. doi: 10.1046/j.1442-9071.2002.00483.x. [DOI] [PubMed] [Google Scholar]
- 61.Lau LI, Wu CC, Lee SM, et al. Pseudomonas corneal ulcer related to overnight orthokeratology. Cornea. 2003;22:262–264. doi: 10.1097/00003226-200304000-00017. [DOI] [PubMed] [Google Scholar]
- 62.Young AL, Leung AT, Cheng LL, et al. Orthokeratology lens-related corneal ulcers in children: A case series. Ophthalmology. 2004;111:590–595. doi: 10.1016/j.ophtha.2003.06.003. [DOI] [PubMed] [Google Scholar]
- 63.Young AL, Leung AT, Cheung EY, et al. Orthokeratology lens-related Pseudomonas aeruginosa infectious keratitis. Cornea. 2003;22:265–266. doi: 10.1097/00003226-200304000-00018. [DOI] [PubMed] [Google Scholar]
- 64.Ying-Cheng L, Chao-Kung L, Ko-Hua C, et al. Daytime orthokeratology associated with infectious keratitis by multiple gram-negative bacilli: Burkholderia cepacia, Pseudomonas putida, and Pseudomonas aeruginosa. Eye Contact Lens. 2006;32:19–20. doi: 10.1097/01.icl.0000167714.53847.8c. [DOI] [PubMed] [Google Scholar]
- 65.Joslin CE, Tu EY, Shoff ME, et al. The association of contact lens solution use and Acanthamoeba keratitis. Am J Ophthalmol. 2007;144:169–180. doi: 10.1016/j.ajo.2007.05.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Lindsay RG, Watters G, Johnson R, et al. Acanthamoeba keratitis and contact lens wear. Clin Exp Optom. 2007;90:351–360. doi: 10.1111/j.1444-0938.2007.00172.x. [DOI] [PubMed] [Google Scholar]
- 67.Nwachuku N, Gerba CP. Health Effects of Acanthamoeba spp. and its potential for waterborne transmission. Rev Environ Contam Toxicol. 2004;180:93–131. doi: 10.1007/0-387-21729-0_2. [DOI] [PubMed] [Google Scholar]
- 68.Theng JT, Kiak LW, Lee BG, et al. Microbiological profile of a shipboard environment and the flora on contact lenses of seamen. CLAO J. 2001;27:47–52. [PubMed] [Google Scholar]
- 69.Dantas PE, Nishiwaki-Dantas MC, Ojeda VH, et al. Microbiological study of disposable soft contact lenses after photorefractive keratectomy. CLAO J. 2000;26:26–29. [PubMed] [Google Scholar]
- 70.Hondur A, Bilgihan K, Cirak MY, et al. Microbiologic study of soft contact lenses after laser subepithelial keratectomy for myopia. Eye Contact Lens. 2008;34:24–27. doi: 10.1097/ICL.0b013e31805881c2. [DOI] [PubMed] [Google Scholar]
- 71.Clark BJ, Harkins LS, Munro FA, et al. Microbial contamination of cases used for storing contact lenses. J Infect. 1994;28:293–304. doi: 10.1016/s0163-4453(94)91893-7. [DOI] [PubMed] [Google Scholar]
- 72.Gray TB, Cursons RT, Sherwan JF, et al. Acanthamoeba, bacterial, and fungal contamination of contact lens storage cases. Br J Ophthalmol. 1995;79:601– 605. doi: 10.1136/bjo.79.6.601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Larkin DF, Kilvington S, Easty DL. Contamination of contact lens storage cases by Acanthamoeba and bacteria. Br J Ophthalmol. 1990;74:133–135. doi: 10.1136/bjo.74.3.133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.May LL, Gabriel MM, Simmons RB, et al. Resistance of adhered bacteria to rigid gas permeable contact lens solutions. CLAO J. 1995;21:242–246. [PubMed] [Google Scholar]
- 75.Imamura Y, Chandra J, Mukherjee K, et al. Fusarium and Candida albicans biofilms on soft contact lenses: Model development, influence of lens type, and susceptibility to lens care solutions. Antimicrob Agents Chemother. 2008;52:171–182. doi: 10.1128/AAC.00387-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Wilson LA, Sawant AD, Simmons RB, et al. Microbial contamination of contact lens storage cases and solutions. Am J Ophthalmol. 1990;110:193–198. doi: 10.1016/s0002-9394(14)76991-0. [DOI] [PubMed] [Google Scholar]
- 77.Vermeltfoort PB, Hooymans JM, Busscher HJ, et al. Bacterial transmission from lens storage cases to contact lenses-effects of lens care solutions and silver impregnation of cases. J Biomed Mater Res B Appl Biomater. 2008;87:237–243. doi: 10.1002/jbm.b.31102. [DOI] [PubMed] [Google Scholar]
- 78.Mayo MS, Schlitzer RL, Ward MA, et al. Association of Pseudomonas and Serratia corneal ulcers with use of contaminated solutions. J Clin Microbiol. 1987;25:1398–1400. doi: 10.1128/jcm.25.8.1398-1400.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Wilson LA, Schlitzer RL, Ahearn DG. Pseudomonas corneal ulcers associated with soft contact-lens wear. Am J Ophthalmol. 1981;92:546–554. doi: 10.1016/0002-9394(81)90649-8. [DOI] [PubMed] [Google Scholar]
- 80.Kanpolat A, Kalayci D, Arman D, et al. Contamination in contact lens care systems. CLAO J. 1992;18:105–107. [PubMed] [Google Scholar]
- 81.Collins M, Coulson J, Shuley V, et al. Contamination of disinfection solution bottles used by contact lens wearers. CLAO J. 1994;20:32–36. [PubMed] [Google Scholar]
- 82.Donzis PB, Mondino BJ, Weissman BA, et al. Microbial contamination of contact lens care systems. Am J Ophthalmol. 1987;104:325–333. doi: 10.1016/0002-9394(87)90219-4. [DOI] [PubMed] [Google Scholar]
- 83.Sweeney DF, Willcox MD, Sansey N, et al. Incidence of contamination of preserved saline solutions during normal use. CLAO J. 1999;25:167–175. [PubMed] [Google Scholar]
- 84.Durban JJ, Villaverde EH, Monteoliva-Sanchez M, et al. Bacterial contamination of hydrophilic contact lens solutions marketed in Spain. Optom Vis Sci. 1996;73:529–532. doi: 10.1097/00006324-199608000-00002. [DOI] [PubMed] [Google Scholar]
- 85.Donzis PB, Mondino BJ, Weissman BA, et al. Microbial analysis of contact lens care systems contaminated with Acanthamoeba. Am J Ophthalmol. 1989;108:53–56. doi: 10.1016/s0002-9394(14)73260-x. [DOI] [PubMed] [Google Scholar]
- 86.Sweeney DF, Taylor P, Holden BA, et al. Contamination of 500 ml bottles of unpreserved saline. Clin Exp Optom. 1992;75:67–75. [Google Scholar]
- 87.Yung MS, Boost M, Cho P, et al. Microbial contamination of contact lenses and lens care accessories of soft contact lens wearers (university students) in Hong Kong. Ophthalmic Physiol Opt. 2007;27:11–21. doi: 10.1111/j.1475-1313.2006.00427.x. [DOI] [PubMed] [Google Scholar]
- 88.Pens CJ, da Costa M, Fadanelli C, et al. Acanthamoeba spp. and bacterial contamination in contact lens storage cases and the relationship to user profiles. Parasitol Res. 2008;103:1241–1245. doi: 10.1007/s00436-008-1120-3. [DOI] [PubMed] [Google Scholar]
- 89.Behlau I, Gilmore MS. Microbial biofilms in ophthalmology and infectious disease. Arch Ophthalmol. 2008;126:1572–1581. doi: 10.1001/archopht.126.11.1572. [DOI] [PubMed] [Google Scholar]
- 90.Danion A, Arsenault I, Vermette P. Antibacterial activity of contact lenses bearing surface-immobilized layers of intact liposomes loaded with levofloxacin. J Pharm Sci. 2007;96:2350–2363. doi: 10.1002/jps.20871. [DOI] [PubMed] [Google Scholar]
- 91.Lesher GA, Gunderson GG. Continuous drug delivery through the use of disposable contact lenses. Optom Vis Sci. 1993;70:1012–1018. doi: 10.1097/00006324-199312000-00004. [DOI] [PubMed] [Google Scholar]
- 92.Amos CF, George MD. Clinical and laboratory testing of a silver-impregnated lens case. Cont Lens Anterior Eye. 2006;29:247–255. doi: 10.1016/j.clae.2006.09.007. [DOI] [PubMed] [Google Scholar]
- 93.Weisbarth RE, Gabriel MM, George M, et al. Creating antimicrobial surfaces and materials for contact lenses and lens cases. Eye Contact Lens. 2007;33(6 Pt 2):426–429. doi: 10.1097/ICL.0b013e318157f488. discussion 434. [DOI] [PubMed] [Google Scholar]
- 94.Monteiro DR, Gorup LF, Takamiya AS, et al. The growing importance of materials that prevent microbial adhesion: antimicrobial effect of medical devices containing silver. Int J Antimicrob Agents. 2009;34:103–110. doi: 10.1016/j.ijantimicag.2009.01.017. [DOI] [PubMed] [Google Scholar]
- 95.Nissen S, Furkert FH. Antimicrobial efficacy of a silver layer on hydrogel lenses [Citation] Ophthalmologe. 2000;97:640– 643. doi: 10.1007/s003470070054. [DOI] [PubMed] [Google Scholar]
- 96.Mathews SM, Spallholz JE, Grimson MJ, et al. Prevention of bacterial colonization of contact lenses with covalently attached selenium and effects on the rabbit cornea. Cornea. 2006;25:806– 814. doi: 10.1097/01.ico.0000224636.57062.90. [DOI] [PubMed] [Google Scholar]
- 97.Zhu H, Kumar A, Ozkan J, et al. Fimbrolide-coated antimicrobial lenses: Their in vitro and in vivo effects. Optom Vis Sci. 2008;85:292–300. doi: 10.1097/OPX.0b013e31816bea0f. [DOI] [PubMed] [Google Scholar]
- 98.Cole N, Hume E, Vijay AK, et al. In vivo performance of melimine as an antimicrobial coating for contact lenses in models of CLARE and CLPU. Invest Ophthalmol Vis Sci. 2010;51:390–395. doi: 10.1167/iovs.09-4068. [DOI] [PubMed] [Google Scholar]
- 99.Wikimedia Foundation. [Accessed December 20, 2009.];Wikipedia, the Free Encyclopedia. Available at: http://en.wikipedia.org/wiki/Main_Page.
- 100.Kozer-Bilgin L, Demir N, Altan-Yaycioglu R. Microbiological evaluation of contact lenses and contact lens disinfection solutions in an asymptomatic population and in medical personnel. CLAO J. 1999;25:228–232. [PubMed] [Google Scholar]
- 101.Iskeleli G, Bahar H, Unal M, et al. Microbiologic evaluation of frequent-replacement soft contact lenses. CLAO J. 2002;28:192–195. doi: 10.1097/01.ICL.0000024118.45191.9B. [DOI] [PubMed] [Google Scholar]
- 102.Ahanotu EN, Ahearn DG. Association of Pseudomonas aeruginosa and Serratia marcescens with extended-wear soft contact lenses in asymptomatic patients. CLAO J. 2002;28:157–159. [PubMed] [Google Scholar]
- 103.Hart DE, Hosmer M, Georgescu M, et al. Bacterial assay of contact lens wearers. Optom Vis Sci. 1996;73:204–207. doi: 10.1097/00006324-199603000-00014. [DOI] [PubMed] [Google Scholar]
- 104.Mowrey-McKee MF, Monnat K, Sampson HJ, et al. Microbial contamination of hydrophilic contact lenses. Part I: Quantitation of microbes on patient worn-and-handled lenses. CLAO J. 1992;18:87–91. [PubMed] [Google Scholar]
- 105.Midelfart J, Midelfart A, Bevanger L. Microbial contamination of contact lens cases among medical students. CLAO J. 1996;22:21–24. [PubMed] [Google Scholar]
- 106.Velasco BJ. Comparative study of the microbial flora on contact lenses, in lens cases, and in maintenance liquids. Int Contact Lens Clin. 1996;23:55–58. [Google Scholar]



