Abstract
Ascorbic acid is frequently administered intravenously by alternative health practitioners and, occasionally, by mainstream physicians. Intravenous administration can greatly increase the amount of ascorbic acid that reaches the circulation, potentially increasing the risk of oxalate crystallization in the urinary space. To investigate this possibility, we developed gas chromatography mass spectrometry methodology and sampling and storage procedures for oxalic acid analysis without interference from ascorbic acid and measured urinary oxalic acid excretion in people administered intravenous ascorbic acid in doses ranging from 0.2 to 1.5 g/kg body weight. In vitro oxidation of ascorbic acid to oxalic acid did not occur when urine samples were brought immediately to pH less than 2 and stored at –30°C within 6 hours. Even very high ascorbic acid concentrations did not interfere with the analysis when oxalic acid extraction was carried out at pH 1. As measured during and over the 6 hours after ascorbic acid infusions, urinary oxalic acid excretion increased with increasing doses, reaching approximately 80 mg at a dose of approximately 100 g. We conclude that, when studied using correct procedures for sample handling, storage, and analysis, less than 0.5% of a very large intravenous dose of ascorbic acid is recovered as urinary oxalic acid in people with normal renal function.
1. Introduction
Oxalic acid is a major end product of ascorbic acid oxidation, and it has the potential to crystallize as calcium oxalate in the urinary space. An oral dose of 500 mg ascorbic acid modestly increases urinary oxalic acid excretion and could theoretically increase the risk of stone formation in susceptible people [1-4]. The rate limitation of intestinal ascorbic acid transport makes it unlikely that oral doses greater than 500 mg/d will increase oxalic acid excretion and stone risk proportionately [5,6], but intravenous administration bypasses this barrier. In an early study, 44% of the radioactivity in an intravenous dose of 14C-ascorbic acid was recovered in the urine as oxalic acid [7]. In the setting of renal dysfunction, acute oxalate nephropathy has been reported after intravenous ascorbic acid administration [8-10]. Increased urinary oxalic acid excretion has also been reported in connection with parenteral nutrition solutions that included 200 to 500 mg/d of ascorbic acid [11,12]. Much larger doses than this have been administered therapeutically in recent years [13-16], but without any information about the associated rate of oxalic acid excretion.
Barriers to the study of oxalic acid formation during ascorbic acid treatment are interference with the analysis by high ascorbic acid concentrations and in vitro conversion of excreted ascorbic acid to oxalic acid in the collection vessel or during storage or analysis [2,17-20]. In this study, we overcame these barriers by immediately acidifying urine specimens and promptly storing them at very low temperature and by developing a gas chromatography mass spectrometry (GC-MS) technique that avoids alkaline extraction [21], which oxidizes ascorbic acid to oxalic acid [2]. This method was then used to analyze the pattern of urinary oxalic acid excretion in patients participating in a clinical trial of high-dose intravenous ascorbic acid as cancer therapy.
2. Methods
2.1. Study participants and clinical trial design
Urinary oxalic acid excretion was measured in 11 men and 5 women (age, 58 ± 17 years; weight, 69 ± 18 kg; mean ± SD) participating in a phase I clinical trial of intravenous ascorbic acid in advanced cancer [22]. All participants in the trial had good functional status, were biochemically screened for glucose-6-phosphate dehydrogenase deficiency—which is associated with hemolytic episodes after high-dose ascorbic acid infusions [17]—had undergone an abdominal x-ray examination to screen for radio-opacities suggestive of silent oxalate urolithiasis, and had a serum creatinine concentration not exceeding 175 μmol/L. Complete details about the patients, their clinical responses, and their plasma ascorbic acid kinetics are described elsewhere [22].
2.2. Ascorbic acid administration and urine collection
Ascorbic acid 500 mg/mL for injection was manufactured and provided as a gift by Bioniche Pharma (Belleville, Ontario), with the assistance of Alveda Pharma (Pointe-Claire, Quebec), in glass vials in 0.025% disodium EDTA with the pH adjusted to neutral with sodium bicarbonate. The vitamin was appropriately diluted to adjust the osmolarity and was administered 3 times per week in doses ranging from 0.1 to 1.5 g/kg. For doses greater than 15 g, the diluent was sterile water to achieve a theoretical osmolarity between 500 and 900 mOsm/L. The infusate was brought immediately to the bedside covered by an opaque bag and was administered by calibrated infusion pump. Doses up to 90 g were infused at a constant rate over 90 minutes; doses greater than 90 g were infused over 120 minutes. The ascorbic acid concentration in the residual infusate solution was routinely measured after every infusion.
Oxalic acid excretion was measured on either 1 or 2 occasions in 16 patients: there were 5 excretion profiles at 0.2 g/kg ascorbic acid and 6 profiles at 0.6, 0.9, and 1.5 g/kg ascorbic acid, for a total of 23 profiles. Patients emptied their bladders to provide a urine sample just before the start of the infusion, then voided again and began a fresh collection when the infusion finished. They then provided urine samples at least every 2 hours for the next 6 hours. After the sample volume was recorded, one 0.2-mL aliquot was prepared for ascorbic acid analysis by mixing it with 0.8 mL of ice-cold 90% methanol–10% 1 mmol/L EDTA and frozen at –80°C; a second aliquot was prepared for oxalic acid analysis by adding 0.5 mL of 6 mol/L hydrochloric acid for every 4.5 mL of urine. These samples were kept on ice for no more than 2 hours before being frozen at –30°C.
2.3. General laboratory procedures
All water was type I or ultrapure water (resistivity, 18.2 MΩ/cm). l-Ascorbic acid for laboratory use was from Sigma Chemical (St Louis, MO), oxalic acid was from BDH (Toronto, Ontario, Canada), and 13C2-oxalate was from CDN Isotopes (Pointe-Claire, Quebec, Canada). Hydrochloric acid, sodium chloride, diethyl ether, and sodium sulfate were from Fisher Scientific (Montreal, Quebec, Canada). Acetonitrile was from Pierce Chemical (Rockford, IL), whereas N-methyl-N-(tert-butyldimethylsilyl)-trifluoroacetamide was from Regis Technologies (Morton Grove, IL).
2.4. Ascorbic acid
Plasma and urinary ascorbic acid concentrations were measured on-site by high-performance liquid chromatography separation with electrochemical detection, using an ESA (Chelmsford, IL) Coulochem III detector equipped with a 5011A analytical cell [23]. Urinary ascorbic acid was measured as the sum of ascorbic acid and dehydroascorbic acid after reducing any dehydroascorbic acid present in the sample to ascorbic acid with dimercaprol, as previously described [23]. Plasma samples were treated the same way as urine samples, except that the reduction step was omitted because dehydroascorbic acid was undetectable (b5% of total ascorbic acid) in plasma samples. Using this analytical methodology, the chromatographic response was highly linear up to a concentration of 10 mmol/L. For concentrations in this range and higher, the sample was diluted 10-fold in EDTA/ methanol before injection on the column.
2.5. Oxalic acid
Oxalic acid was measured as previously described [21], with the following differences: analyte extraction was only into acid, omitting a previous initial alkaline extraction; tert-butyldimethylsilyl (TBDMS) rather than trimethylsilyl derivatives were made; the GC column and separation conditions were different; and electron impact ionization rather than chemical ionization was used. To each 200 μL acidified urine sample was added 120 nmol of the internal standard described below. After the pH was reduced to approximately 1 with 3 drops of 3 mol/L hydrochloric acid, the samples were diluted by adding 200 μL water and saturated with 0.5 to 1.0 g solid sodium chloride. Oxalic acid was extracted by 2-fold addition of 2 mL diethyl ether followed by vigorous shaking. The 2 ether extracts were combined and dried over anhydrous sodium sulfate, and brought to complete dryness in sample vials under a flow of nitrogen gas at 35°C. The oxalic acid in dried residues was converted to TBDMS derivatives by adding 50 μL acetonitrile and 50 μL N-methyl-N-(tert-butyldimethylsilyl)-trifluoroacetamide followed by incubation for 30 minutes at 60°C. The derivatized samples were stable at –30°C for several days.
Calibration standards were prepared in water by combining 120 nmol of the 13C2-oxalate internal standard with 0, 15.9, 31.7, 63.4, and 127 nmol unlabeled oxalic acid. The analysis was carried out on a Hewlett-Packard 5988A GC quadrupole MS. Samples of 1 μL were introduced by splitless injection using an HP 7673 autoinjector onto a fused silica HP-5 (Agilent Technologies, Mississauga, Ontario, Canada) capillary column (15 m × 0.32 mm, 0.25-μm film thickness). The oven temperature was programmed at 10°C/min after a 2-minute hold at 80°C to 300°C. Helium carrier gas was used at a column head pressure of 70 kPa. The injector port and transfer line temperatures were 250°C and 270°C, respectively. Electron ionization energy was 70 eV at an emission current of 300 μA with a source temperature of 200°C. Quantitation was performed by selected ion monitoring (SIM) of m/z 261.1 and 263.1 of the [M-C4H9] positive fragment ions of the bis-TBDMS derivatives of unlabeled oxalate and [13C2]-oxalate, respectively. The retention time was 6.5 to 6.8 minutes. Analyses of the calibration samples yielded intensity ratios that were used to construct a standard curve.
Ascorbate was also observed as the tris-TBDMS derivative, eluting at 16.7 minutes with a major M-57 fragment at m/z 461, and as the tetrakis-TBDMS derivative, eluting at 18.2 minutes with corresponding fragment at m/z 575. Data acquisition and processing were done on an HP-1000A computer using RTE-A primary system software (Mississauga, Ontario, Canada).
Aliquots of acidified urine were routinely centrifuged before analysis until it was discovered, in the patient cohort that received 1.5 g/kg ascorbic acid, that despite the addition of acid, the pH of some samples ranged between pH 2 and 4; this was most likely the result of the excretion of large amounts of neutral sodium ascorbate. Because oxalic acid can form a poorly soluble calcium salt at pH greater than 2.0 [24,25], these and several other samples were reanalyzed without centrifugation. In most of the samples with pH greater than 2.0, samples that were not centrifuged had higher oxalate concentrations than after centrifugation. Centrifugation had no effect in samples with pH not exceeding 2.0.
3. Results
3.1. Method validation
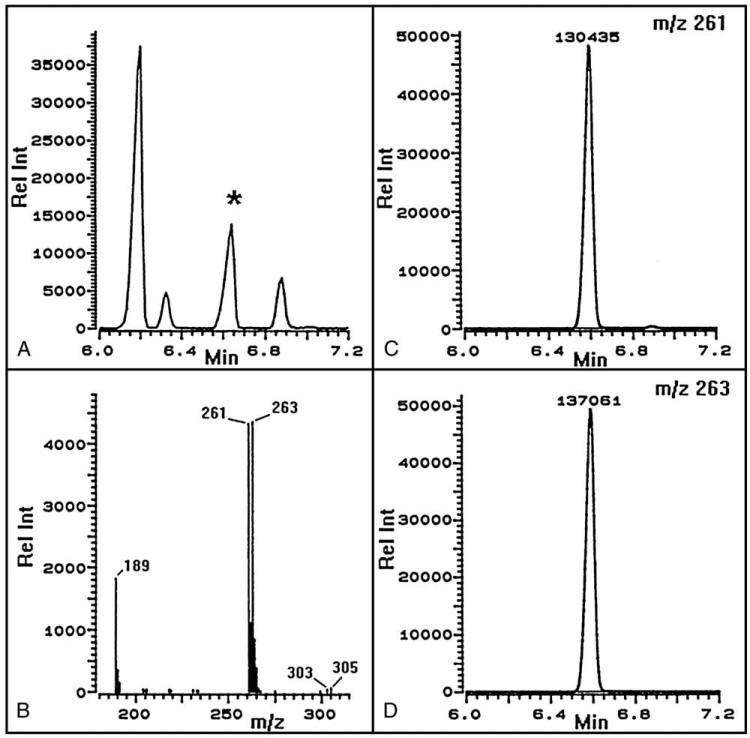
As shown in Fig. 1, the SIM chromatograms for m/z 261 and 263 were well characterized, and a conventional calibration curve relating amounts of [12C2]- and [13C2]-oxalic acid to the measured intensity ratios of m/z 261 to m/z 263 was easily constructed. Recovery of physiologic amounts of oxalic acid added to 6 normal urine samples was 100% ± 6.9% (mean ± SD). Acid-only extraction, as used here, was compared with the conventional method involving base and acid extraction. Addition of ascorbic acid to normal urine samples in concentrations of 0.5 g/L (2.8 mmol/L) and 20 g/L (114 mmol/L) had no effect on the oxalic acid concentration when the new method was used, but increased it by 4- to 14-fold and 12- to 25-fold, respectively, when the method involving base extraction was used.
Fig. 1.
Gas chromatograms and spectrum of the TBDMS derivative of a typical oxalic acid standard containing equimolar 12C and 13C2. The total ion chromatogram (A) is shown with the oxalic acid peak (*) at 6.6 minutes with the corresponding mass spectrum (B) showing the major M-57 fragments (m/z 261 and 263) used for quantification. Selected ion monitoring of m/z 261 and 263 and peak intensities are shown in C and D.
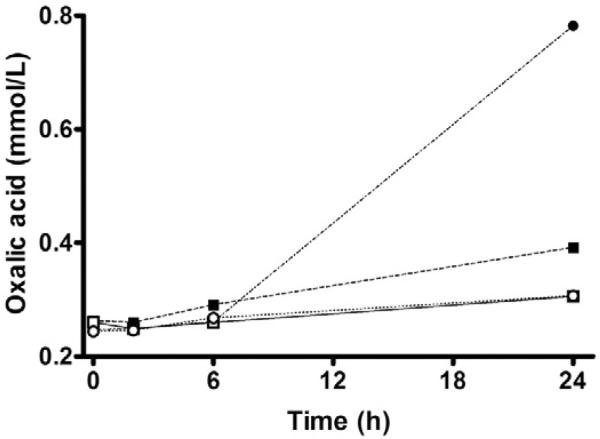
The effect of high ascorbic acid concentrations on urinary oxalic acid was then examined in a time course study in which ascorbic acid was added to freshly voided normal urine samples at concentrations of 5.7 and 455 mmol/L. Sample aliquots were immediately brought either to pH 1.7 with hydrochloric acid or pH 6.3 with sodium bicarbonate, then either analyzed immediately or left on a benchtop at room temperature for 2, 6, and 24 hours before analysis. Neither 5.7 nor 455 mmol/L ascorbic acid affected the measured oxalic acid concentration at zero time or after 6 hours at room temperature (Fig. 2). After 24 hours at pH 6.3, the oxalic acid concentration increased by 50% (in 5.7 mmol/L ascorbic acid) and by 216% (in 455 mmol/L ascorbic acid). Even at pH less than 2, the oxalic acid concentration had increased by 18% (in 5.7 mmol/L ascorbic acid) and by 26% (in 455 mmol/L ascorbic acid) after 24 hours at room temperature. However, when stored in acid at –30°C, oxalic acid concentrations were stable for at least 4 months.
Fig. 2.
Effect of pH, ascorbic acid concentration, and time on urinary oxalic acid concentration. Boxes, ascorbic acid concentration of 5.7 mmol/L; circles, ascorbic acid concentration of 455 mmol/L. Open symbols, pH 1.7; closed symbols, pH 6.3.
3.2. Clinical findings
The concentration of ascorbic acid in the infusate remaining at the end of each infusion agreed closely with the calculated dose, thus indicating no detectable ascorbic acid breakdown. Using the methodology developed for this study, we also measured oxalic acid, both in the 500-mg/mL ascorbic acid stock solution provided by the manufacturer and in some of the infusates. The concentration of oxalic acid in ascorbic acid infusates was 26 mg/100 g and remained constant throughout the infusion period. Of interest, the concentration of oxalic acid in a sample of pure dry crystalline United States Pharmacopeia ascorbic acid was 8 mg/100 g.
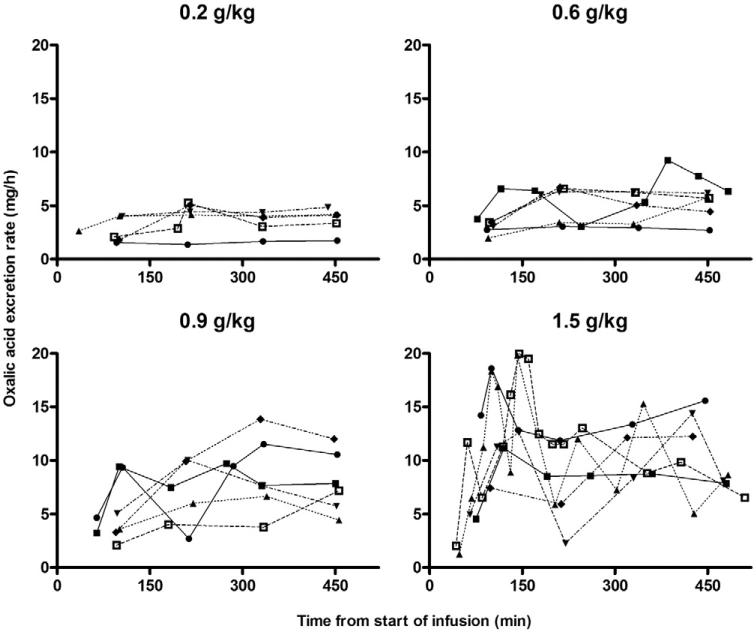
Data related to the study participants and their plasma and urinary ascorbic and oxalic acid excretion are shown in Table 1. As described in detail elsewhere [22], the ascorbic acid infusions were well tolerated despite polyuria and moderate thirst, the anticipated effects of a large intravenous osmotic load. As the infused ascorbic acid was pH neutral, no acid load was delivered. Cumulative urinary oxalic acid excretion increased in a dose-dependent fashion. It was not feasible for patients to return 24-hour urine collections the day after an ascorbic acid infusion, so data were available only up to the time point 6 hours after the end of the infusion. By that time, plasma ascorbic acid concentrations had decreased considerably; and approximately 80% of the administered dose had been excreted in the urine (Table 1). To extrapolate subsequent oxalic acid excretion, we calculated the instantaneous oxalic acid excretion rate associated with each urine specimen as the amount of oxalic acid excreted over the time interval of the collection. As shown in Fig. 3, the instantaneous rate of oxalic acid excretion was relatively constant over the entire observation period for ascorbic acid doses not exceeding 0.9 g/kg, whereas for the 1.5-g/kg dose, a spike in excretion occurred during the infusion, followed by a drop to a lower rate after it. As shown in Table 1, instantaneous oxalic acid excretion rates at the time the infusion ended were related to the ascorbic acid dose administered.
Table 1.
Effect of intravenous ascorbic acid on urinary oxalic acid excretion
| Ascorbic acid dose (g/kg) | 0.2 | 0.6 | 0.9 | 1.5 |
| No. of patients | 6a | 6 | 6 | 6b |
| Serum creatinine (μmol/L) | 83 ± 21 | 68 ± 20 | 76 ± 29 | 87 ± 22 |
| Absolute dose infused (g) | 14.4 ± 3.9 | 37.6 ± 12.6 | 57.8 ± 12.1 | 100 ± 29.8 |
| Plasma AA (mmol/L) | ||||
| At end of infusion | 4.7 ± 0.5 | 11.3 ± 2.4 | 17 ± 3.6 | 26.2 ± 4.9 |
| 6 h after end of infusion | 0.7 ± 0.3 | 1.7 ± 0.8 | 2.8 ± 1.9 | 5.3 ± 2.6 |
| Total AA excretion (% of dose) | ||||
| By end of infusion | 23 ± 8 | 27 ± 8 | 25 ± 8 | 25 ± 11 |
| By 6 h after end of infusion | 74 ± 14 | 89 ± 4 | 77 ± 15 | 79 ± 23 |
| Total oxalic acid excretion (mg) | ||||
| By end of infusion | 6.5 ± 3.4 | 8.7 ± 2.9 | 10.7 ± 2.8 | 24.1 ± 8.2 |
| By 6 h after end of infusion | 27.2 ± 9.3 | 39.3 ± 9.6 | 56.5 ± 16.0 | 81.3 ± 18.8 |
| As % of dosec | 0.40 ± 0.09 | 0.21 ± 0.04 | 0.19 ± 0.03 | 0.17 ± 0.07 |
| Oxalic acid excretion rate | 4.1 ± 1.6 | 5.2 ± 1.4 | 8.0 ± 2.9 | 9.8 ± 3.4 |
| 6 h after the end of the infusion (mg/h) |
Values are expressed as the mean ± SD. AA indicates ascorbic acid.
n = 5 for oxalic acid measurements.
n = 5 for AA measurements.
Total moles of oxalic acid excreted per 100 mol of ascorbic acid administered.
Fig. 3.
Instantaneous oxalic acid excretion. Oxalic acid excretion rate for each participant, calculated as the amount of oxalic acid excreted over the time interval associated with each urine specimen. The ascorbic acid dose is indicated above each set of curves.
4. Discussion
This article describes a novel method to analyze oxalic acid accurately in the presence of very high concentrations of ascorbic acid and its use to determine the urinary oxalic acid excretion profiles of patients administered very large intravenous doses of ascorbic acid.
Accurate oxalic acid analysis in the presence of ascorbic acid requires a strongly acid environment and sample storage at low temperature to prevent in vitro oxidation of ascorbic acid to oxalic acid. Acid conditions are also necessary during sample preparation for GC-MS analysis. In particular, alkaline extraction oxidizes large amounts of ascorbic acid to oxalic acid. When appropriate precautions are taken, and unlike other methods, GC-MS/SIM permits sensitive and accurate oxalic acid analysis even in the presence of massive amounts of ascorbic acid. Thus, in this study, oxalic acid in amounts of 27 to 81 mg was accurately detected in the presence of 11 to 79 g ascorbic acid.
It appears from this study that intravenous ascorbic acid increases urinary oxalic acid excretion in a dose-dependent fashion. For doses of 0.2 and 0.6 g/kg, less than 40 mg oxalic acid was excreted during and over the 6 hours after the ascorbic acid infusion, at which time oxalic acid excretion was continuing at the rate of 4 to 5 mg/h. At the 1.5-g/kg dose, approximately 80 mg of oxalic acid was excreted 6 hours after the infusion ended (approximately 20 mg of which was already present in the pharmaceutical product before the infusion began); and excretion was continuing at approximately 10 mg/h. By that time, the plasma ascorbic acid concentration had fallen from a peak value of 26.2 to 5.3 mmol/L, and approximately 80% of the ascorbic acid dose had been excreted. Because the circulatory half-life of oxalic acid is approximately 3.6 hours [26], it may be predicted that the oxalic acid excretion rate would fall to normal after a further 4 half-lives or approximately 14 hours. Oxalic acid is absorbed from the diet and synthesized from endogenous sources other than ascorbic acid, but samples were unavailable to measure endogenous oxalic acid excretion rates in the participants in this study. The oxalic acid excretions reported here therefore overestimate the extent to which the infused ascorbic acid was oxidized to oxalic acid. In the absence of ascorbic acid administration, urinary oxalic acid excretion normally ranges from 10 to 60 mg/24 h [2,27], with higher rates occurring during the day [24].
Contrary to the findings in an early human study in which 44% of the radioactivity in an intravenous dose of 14C-ascorbic acid was recovered in the urine as oxalic acid [7], we found that only approximately 0.2% (mol/mol) of large doses of ascorbic acid appeared in the urine as oxalic acid 6 hours postinfusion, and as explained above, this is an overestimation. The situation would be different for people with a reduced glomerular filtration rate, which would increase the circulatory dwell time of ascorbic acid and any oxalic acid resulting from its oxidation. People with end-state renal disease have hyperoxalemia that increases after the administration of even conventional amounts of ascorbic acid [28,29]; the appropriate dose and route of ascorbic acid administration for people with end-stage renal disease are controversial [29-31]. It is important, therefore, to reiterate that only patients whose serum creatinine did not exceed 175 μmol/L participated in this study.
Much smaller doses of ascorbic acid than administered in this study have been reported to increase urinary oxalic acid far more than observed here. Several factors could explain this apparent anomaly. First, despite ample documentation that ascorbic acid in urine samples will lead to an overestimation of oxalic acid when the sample is not appropriately handled, stored, and analyzed [2,17-20], sufficient attention is not always paid to this issue. Thus, in 2 articles that report a considerable percentage increase in oxalic acid excretion when the amount of ascorbic acid included in home parenteral nutrition solutions was increased [11,12], urine samples were acidified only after their delivery to the analytical laboratory, by which time considerable artifactual oxalic acid formation can be assumed to have taken place. It should also be noted that ascorbic acid solutions for clinical infusion may be unstable over time [32-34]. In our study, ascorbic acid was rapidly administered shortly after the infusate was prepared, whereas parenteral nutrition solutions are commonly infused over 12 to 24 hours, during which time considerable ascorbic acid degradation is known to occur [32-34]. It is unclear how much ascorbic acid is converted to oxalic acid in parenteral nutrition mixtures [35,36].
Finally, it is conceivable that intravenous ascorbic acid is less prone to oxidation to oxalic acid than high-dose oral ascorbic acid. Because its intestinal absorption is limited, much of a large oral dose of ascorbic acid could remain for a long time in the alkaline medium of the small and large intestines, favoring its oxidation to oxalic acid with absorption of the resulting sodium oxalate into the bloodstream [20].
The findings in this study should be considered in light of case reports describing renal abnormalities or acute renal failure in patients who ingested or received large doses of ascorbic acid orally or intravenously [8-10,37,38]. Careful study of those cases suggests that, in some cases, they occurred in people with preexisting advanced renal failure and, in others, they occurred in people who were consuming or being administered high-dose ascorbic acid when acute renal failure developed for other reasons and precipitated oxalate nephropathy.
Does the excretion of 80 mg of oxalic acid over the 6 hours after a 100-g intravenous dose of ascorbic acid substantially increase the risk of calcium oxalate stone formation? Oxalate nephrocalcinosis and calcium oxalate stones develop over months to years in primary hyperoxaluria, a disease in which oxalic acid excretion exceeds 100 mg/d and can reach 400 mg/d [20]. The relatively slow natural history of primary hyperoxaluria suggests that a time-limited course of ascorbic acid infusions 3 times per week would not create a severe or immediate risk of oxalate stone accumulation. Nevertheless, oxalate stones occur commonly in the general population; for people already at high risk of oxalate stone, one may assume that even intermittent high-dose ascorbic acid infusions could further increase this risk. The present data are important because they indicate a remarkable lack of severe hyperoxaluria after massive intravenous doses of ascorbic acid in people with normal renal function.
Acknowledgment
This study was funded by a grant from the Lotte and John Hecht Memorial Foundation and from Research Resource Program Grant PRG-80160 from the Canadian Institutes of Health Research. WHM is a Chercheur National of the Fonds de la Recherche en Santé du Québec. ML is supported by the Intramural Research Program, National Institutes of Health/National Institute of Diabetes and Digestive and Kidney Diseases.
References
- 1.Dietary reference intakes: vitamin C, vitamin E, selenium, and beta-carotene and other carotenoids. National Academy Press; Washington, DC: 2000. Panel on Dietary Antioxidants and Related Compounds, Subcommittees on Upper Reference Levels of Nutrients and Interpretation and Uses of Dietary Reference Intakes, Standing Committee on the Scientific Evaluation of Dietary Reference Intakes. [Google Scholar]
- 2.Baxmann AC, De OG, Mendonca C, Heilberg IP. Effect of vitamin C supplements on urinary oxalate and pH in calcium stone-forming patients. Kidney Int. 2003;63:1066–71. doi: 10.1046/j.1523-1755.2003.00815.x. [DOI] [PubMed] [Google Scholar]
- 3.Chai W, Liebman M, Kynast-Gales S, Massey L. Oxalate absorption and endogenous oxalate synthesis from ascorbate in calcium oxalate stone formers and non-stone formers. Am J Kidney Dis. 2004;44:1060–9. doi: 10.1053/j.ajkd.2004.08.028. [DOI] [PubMed] [Google Scholar]
- 4.Traxer O, Huet B, Poindexter J, Pak CY, Pearle MS. Effect of ascorbic acid consumption on urinary stone risk factors. J Urol. 2003;170:397–401. doi: 10.1097/01.ju.0000076001.21606.53. [DOI] [PubMed] [Google Scholar]
- 5.Johnston CS. Biomarkers for establishing a tolerable upper intake level for vitamin C. Nutr Rev. 1999;57:71–7. doi: 10.1111/j.1753-4887.1999.tb06926.x. [DOI] [PubMed] [Google Scholar]
- 6.Padayatty SJ, Sun H, Wang Y, Riordan HD, Hewitt SM, Katz A, et al. Vitamin C pharmacokinetics: implications for oral and intravenous use. Ann Intern Med. 2004;140:533–7. doi: 10.7326/0003-4819-140-7-200404060-00010. [DOI] [PubMed] [Google Scholar]
- 7.Hellman L, Burns JJ. Metabolism of l-ascorbic acid-1-C14 in man. J Biol Chem. 1958;230:923–30. [PubMed] [Google Scholar]
- 8.McAllister CJ, Scowden EB, Dewberry FL, Richman A. Renal failure secondary to massive infusion of vitamin C. JAMA. 1984;252:1684. [PubMed] [Google Scholar]
- 9.Wong K, Thomson C, Bailey RR, McDiarmid S, Gardner J. Acute oxalate nephropathy after a massive intravenous dose of vitamin C. Aust N Z J Med. 1994;24:410–1. doi: 10.1111/j.1445-5994.1994.tb01477.x. [DOI] [PubMed] [Google Scholar]
- 10.Lawton JM, Conway LT, Crosson JT, Smith CL, Abraham PA. Acute oxalate nephropathy after massive ascorbic acid administration. Arch Intern Med. 1985;145:950–1. [PubMed] [Google Scholar]
- 11.Fairholm L, Saqui O, Baun M, Allard J. Influence of multivitamin regimen on urinary oxalate in home parenteral nutrition patients. Nutr Clin Pract. 2003;18:366–9. doi: 10.1177/0115426503018005366. [DOI] [PubMed] [Google Scholar]
- 12.Pena de la Vega L, Lieske JC, Milliner D, Gonyea J, Kelly DG. Urinary oxalate excretion increases in home parenteral nutrition patients on a higher intravenous ascorbic acid dose. JPEN J Parenter Enteral Nutr. 2004;28:435–8. doi: 10.1177/0148607104028006435. [DOI] [PubMed] [Google Scholar]
- 13.Gaby AR. Intravenous nutrient therapy: the “Myer's Cocktail”. Altern Med Rev. 2002;7:389–403. [PubMed] [Google Scholar]
- 14.Nathens AB, Neff MJ, Jurkovich GJ, Klotz P, Farver K, Ruzinski JT, et al. Randomized, prospective trial of antioxidant supplementation in critically ill surgical patients. Ann Surg. 2002;236:814–22. doi: 10.1097/00000658-200212000-00014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Tanaka H, Matsuda T, Miyagantani Y, Yukioka T, Matsuda H, Shimazaki S. Reduction of resuscitation fluid volumes in severely burned patients using ascorbic acid administration: a randomized, prospective study. Arch Surg. 2000;135:326–31. doi: 10.1001/archsurg.135.3.326. [DOI] [PubMed] [Google Scholar]
- 16.Hininger I, Waters R, Osman M, Garrel C, Fernholz K, Roussel AM, et al. Acute prooxidant effects of vitamin C in EDTA chelation therapy and long-term antioxidant benefits of therapy. Free Rad Biol Med. 2005;38:1565–70. doi: 10.1016/j.freeradbiomed.2005.02.016. [DOI] [PubMed] [Google Scholar]
- 17.Levine M, Rumsey SC, Daruwala R, Park JB, Wang Y. Criteria and recommendations for vitamin C intake. JAMA. 1999;281:1415–23. doi: 10.1001/jama.281.15.1415. [DOI] [PubMed] [Google Scholar]
- 18.Chalmers AH, Cowley DM, McWhinney BC. Stability of ascorbate in urine: relevance to analyses for ascorbate and oxalate. Clin Chem. 1985;31:1703–5. [PubMed] [Google Scholar]
- 19.Lemann J, Jr, Hornick LJ, Pleuss JA, Gray RW. Oxalate is overestimated in alkaline urines collected during administration of bicarbonate with no specimen pH adjustment. Clin Chem. 1989;35:2107–10. [PubMed] [Google Scholar]
- 20.Asplin JR. Hyperoxaluric calcium nephrolithiasis. Endocrinol Metab Clin North Am. 2002;31:927–49. doi: 10.1016/s0889-8529(02)00030-0. [DOI] [PubMed] [Google Scholar]
- 21.Mamer OA, Osei-Twum EY, Reimer MLJ, Lepine F, Montgomery JA. Determination of oxalic, glycolic, glyoxylic, glyceric and hydroxypyruvic acids in a single GC/MS analysis useful for distinguishing hyperoxaluria types I and II. In: Matsumoto I, Kuhara T, Mamer OA, Sweetman L, Calderhead RG, editors. Advances in chemical diagnosis and treatment of metabolic disorders. Vol. 2. Kanazawa Medical University Press; Kanazawa: 1994. pp. 85–95. [Google Scholar]
- 22.Hoffer LJ, Levine M, Assouline S, Melnychuk D, Padayatty SJ, Rosadiuk K, et al. Phase I clinical trial of intravenous ascorbic acid in advanced malignancy. Ann Oncol. 2008;19:1969–74. doi: 10.1093/annonc/mdn377. [DOI] [PubMed] [Google Scholar]
- 23.Levine M, Wang Y, Rumsey SC. Analysis of ascorbic acid and dehydroascorbic acid in biological samples. Methods Enzymol. 1999;299:65–76. doi: 10.1016/s0076-6879(99)99009-2. [DOI] [PubMed] [Google Scholar]
- 24.Hodgkinson A. Determination of oxalic acid in biological material. Clin Chem. 1970;16:547–57. [Google Scholar]
- 25.Wilson DM, Liedtke RR. Modified enzyme-based colorimetric assay of urinary and plasma oxalate with improved sensitivity and no ascorbate interference: reference values and sample handling procedures. Clin Chem. 1991;37:1229–35. [PubMed] [Google Scholar]
- 26.Elder TD, Wyngaarden JB. The biosynthesis and turnover of oxalate in normal and hyperoxaluric subjects. J Clin Invest. 1960;39:1337–44. doi: 10.1172/JCI104151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Painter PC, Cope JY, Smith JL. Reference information for the clinical laboratory. In: Burtis CA, Ashwood ER, editors. Tietz fundamentals of clinical chemistry. Saunders; Philadelphia: 2001. pp. 955–1028. [Google Scholar]
- 28.Canavese C, Petrarulo M, Massarenti P, Berutti S, Fenoglio R, Pauletto D, et al. Long-term, low-dose, intravenous vitamin C leads to plasma calcium oxalate supersaturation in hemodialysis patients. Am J Kidney Dis. 2005;45:540–9. doi: 10.1053/j.ajkd.2004.10.025. [DOI] [PubMed] [Google Scholar]
- 29.Handelman GJ. Vitamin C deficiency in dialysis patients—are we perceiving the tip of an iceberg? Nephrol Dial Transplant. 2007;22:328–31. doi: 10.1093/ndt/gfl534. [DOI] [PubMed] [Google Scholar]
- 30.Chan D, Irish A, Dogra G. Efficacy and safety of oral versus intravenous ascorbic acid for anaemia in haemodialysis patients. Nephrology. 2005;10:336–40. doi: 10.1111/j.1440-1797.2005.00424.x. [DOI] [PubMed] [Google Scholar]
- 31.Jacobs C. Intravenous vitamin C can improve anemia in erythropoietin-hyporesponsive hemodialysis patients. Nat Clin Pract Nephrol. 2006;2:552–3. doi: 10.1038/ncpneph0281. [DOI] [PubMed] [Google Scholar]
- 32.Allwood MC, Kearney MC. Compatibility and stability of additives in parenteral nutrition admixtures. Nutrition. 1998;14:697–706. doi: 10.1016/s0899-9007(98)00063-x. [DOI] [PubMed] [Google Scholar]
- 33.Dupertuis YM, Morch A, Fathi M, Sierro C, Genton L, Kyle UG, et al. Physical characteristics of total parenteral nutrition bags significantly affect the stability of vitamins C and B1: a controlled prospective study. JPEN J Parenter Enteral Nutr. 2002;26:310–6. doi: 10.1177/0148607102026005310. [DOI] [PubMed] [Google Scholar]
- 34.Lavoie JC, Chessex P, Rouleau T, Migneault D, Comte B. Light-induced byproducts of vitamin C in multivitamin solutions. Clin Chem. 2004;50:135–40. doi: 10.1373/clinchem.2003.025338. [DOI] [PubMed] [Google Scholar]
- 35.Rockwell GF, Campfield T, Nelson BC, Uden PC. Oxalogenesis in parenteral nutrition solution components. Nutrition. 1998;14:836–9. doi: 10.1016/s0899-9007(98)00104-x. [DOI] [PubMed] [Google Scholar]
- 36.Allwood M. Oxalogenesis in parenteral nutrition mixtures. Nutrition. 1999;15:70. doi: 10.1016/s0899-9007(98)00142-7. [DOI] [PubMed] [Google Scholar]
- 37.Swartz RD, Wesley JR, Somermeyer MG, Lau K. Hyperoxaluria and renal insufficiency due to ascorbic acid administration during total parenteral nutrition. Ann Intern Med. 1984;100:530–1. doi: 10.7326/0003-4819-100-4-530. [DOI] [PubMed] [Google Scholar]
- 38.Nasr SH, Kashtanova Y, Levchuk V, Markowitz GS. Secondary oxalosis due to excess vitamin C intake. Kidney Int. 2006;70:1672. doi: 10.1038/sj.ki.5001724. [DOI] [PubMed] [Google Scholar]