Abstract
The development and homeostasis of adaptive and innate lymphocytes is dependent on the stromal cytokine IL-7. The initial priming of immune responses to pathogenic challenges is executed by innate lymphoid cells (ILCs) with programmed capacity to rapidly secrete effector cytokines. How ILCs are controlled by IL-7 in distinct anatomical locale has evolved into a more complex problem as IL-7 receptor is not only expressed on ILCs, but also on surrounding neighbors, including vascular endothelium and mesenchymal cells that compete for limiting IL-7. For the generation of γδ T and B cells IL-7 is required for the production of antigen receptors, and it is likely that IL-7 performs critical function in facilitating ILC effector programming in addition to its regulatory actions on cell survival and proliferation. Most of our current understanding of the highly calibrated regulatory circuits of IL-7 function and IL-7 receptor signaling has derived from studies of adaptive, conventional lymphocytes. Here we highlight recent advances in mapping the gene circuits and cellular interactions that regulate temporospatial activities of IL-7 in diverse macro and micro niches that have direct relevance to deciphering the sphere of impact of IL-7 on ILC differentiation.
Keywords: Innate lymphoid cells, IL-7 reporter mice, T cell development, gammadelta T cells, gut associated lymphoid tissues, regulation of IL-7R expression
1. Introduction
Homeostasis is the necessary poised state for adaptations to constantly changing environments in biologic systems. The immune system's basal set point is established by a vast array of regulators and reinforcing molecular circuits often tailored with exquisite cell type-specificity. Disturbances of the set point can lead to hyperstasis or disease states and a suboptimal functioning network of sensors tuned to environmental variations. The gene encoding for the cytokine IL-7 was identified 24 years ago [1], and since then, intensive research has established IL-7 as the prototypic regulator of lymphocyte homeostasis. IL-7 controls the birth (as a precursor maintenance factor and a permissive factor of TCRy and Ig gene rearrangement and expression, [2,3]), education (as a CD8+ thymocyte selection tuner [4]), persistence (as a tonic stimulator outside the thymus [5]), maturation (as a system memory promoter [6,7]) and death (as a pro survival factor [8]) of T cells. Several insightful reviews have been published recently on IL-7 function in lymphocyte development and maintenance [9-11]. In this review, we focus on the emerging details on the developmentally regulated spatial distribution of IL-7 in vivo that controls all IL-7 receptor (IL-7R) expressing cell subsets with tissue specific and systemic functions in immunity, with a particular emphasis on innate lymphoid cells (ILCs).
ILCs can be broadly classified into two subtypes based on their origin: the primary lymphoid tissue-derived γδ T, αβ NKT, innate CD8+ αβ T, mucosa associated invariant T cells (MAITs), NK and B-1 cells [12,13], and the gut-associated lymphoid tissue (GALT)-derived Lymphoid Tissue initiator (LTi)-related subsets programmed to produce the Th17 signature cytokines, IL-17 and IL-22 (ILC17 and ILC22), and Th2 cytokines IL-5 and IL-13 (ILC5/13) [14]. As exemplified by innate γδ T effector subsets that mirror the repertoire of adaptive CD4+ T effector subsets (Th1, Th2 and Th17) [15], ILCs are the first responders to environmental insults and as such their effector lineages are programmed in tissues they originate, eliminating the time consuming acquisition of “learned” effector capacity upon pathogen encounter. IL-7 is one of the earliest cytokines consumed by developing ILCs in all anatomical sites where they originate and it has the capacity to program lineage specific gene expression, including the gene circuits that specify the effector identity.
2. In vivo sources of IL-7
Without IL-7 the lymphoid system cannot be built. In rodents, IL-7 is the only cytokine absolutely critical for the generation of innate (γδ T, αβ NKT) and adaptive (αβ T and B) lymphocytes in the primary lymphoid tissues (thymus and bone marrow, BM). In the gut, ILCs capable of IL-17 and IL-22 production express IL-7R and are dependent on IL-7 for their generation [16,17]. In the secondary lymphoid tissues, IL-7 is required for the maintenance of naïve lymphocytes (αβ T and B cells) along with tonic signals transmitted by clonal antigen receptors. While IL-7 has a similar functional profile in humans, B cells are not absolutely beholden to IL-7 for development and survival. For innate lymphocytes (NKT, γδ T and NK cells) and memory T cells that have an activated phenotype and express IL-2Rβ, a component of the high affinity IL-15R, IL-15 is the companion factor of IL-7 that can replace the need for antigen receptor-mediated signals [18-21].
IL-7 is predominantly produced by stromal and vascular endothelial cells, with very low levels of Il7 transcripts detectable in adult animals, consistent with the concept that under basal states there are limited amounts of IL-7 available for lymphocytes in vivo [22]. Stroma-derived IL-7 production can be induced by severe alterations in the environment, such as hypocellularity induced by radiation damage [23] and overt inflammation [24], but in a homeostatic animal the amount of IL-7 produced is thought to be constant. Analyses of five different IL-7 genetic reporter mice have been published (reviewed in [25]). These mice have provided broadly similar results regarding IL-7 expression, with higher levels of IL-7 in sites of lymphopoiesis, including the BM and thymus, than in peripheral lymphoid tissues. Using transgenic mice containing a BAC substrate with Cre knocked into the first exon of the IL-7 locus, we have tracked in detail cells that have expressed IL-7 at some point during their life and/or continue to express it [26]. Analysis of IL-7promoter-Cre Rosa26eYFP reporter mice showed that IL-7 is expressed by specialized lymphoid stromal subsets in BM, lymph node (LN), liver, Peyer's patches (PP), isolated lymphoid follicles, a subset of intestinal epithelium, thymic epithelial cells, specialized vascular endothelium and dermal fibroblasts.
Classically, cytokines and chemokines were assumed to operate with free diffusion characteristics, leading to a protein gradient within the localized microenvironment to which cells respond. However, IL-7 binds strongly to heparin sulfate proteoglycans (HSPGs) in the extracellular matrix (EM) in vivo and does not form a linear gradient from cellular factories. HSPGs on both stromal cells and lymphocytes have key roles in regulating IL-7/IL-7R interactions [27], with the amount of bio-available IL-7 controlled by a combination factors: IL-7 production rate and half-life, production and secretion of soluble IL-7R (sIL-7R), IL-7 binding kinetics to HSPGs, rates of EM deposition by stromal cells and EM degradation by MMPs, interaction properties between IL-7R+ cells and the EM, and IL-7R levels on non-haematopoietic cells in the local microenvironment. Different lymphoid microenvironments, including the thymus, BM and secondary lymphoid tissues, have variable EM composition and HSPG expression patterns, resulting in a finely tuned and complex regulation of IL-7 bio-availability and attendant localized competition among IL-7R+ cells. Unbound IL-7 is found at very low levels in blood serum, thus posing a challenge to its detection in tissues in experimental or clinical settings. Changes to IL-7 levels in serum have been shown to be a predictive biomarker of cardiovascular diseases. This correlation may relate to changes to the EM in vessels associated with cardiovascular disease or localized IL-7 production from inflamed tertiary lymphoid tissue formation associated with atherosclerotic plaques [28]. In contrast, higher relative amounts of sIL-7R to membrane IL-7R are causally linked to T cell mediated autoimmune diseases such as Multiple Sclerosis [29]. Similarly, lower levels of bio-available IL-7 are found in HIV infection, potentially caused by increased levels of sIL-7R detectable in serum samples. The increased sIL-7R levels did not, however, correlate with either viral load or the size of CD4+ T cell population [30]. sIL-7R predominates in lung tissues, perhaps indicating a differential role for sIL-7R in a local microenvironment-specific manner [31]. These data collectively suggest that IL-7 has a diverse functional profile in different localized microenvironments, with roles in both the maintenance and regulation of tissue specific lymphocyte populations and in the development and function of tissues (see below), but the specific dynamics of IL-7 activities in localized microenvironments have not been precisely measured.
3. IL-7R signaling
The IL-7R is composed of the common γ (γc) chain and a unique α chain. IL-7 exhibits a decisive preference for the glycosylated form of IL-7R, and docks onto it using a distinctive two-step binding mode [32]. The α chain of the IL-7R is also utilized by Thymic Stromal Lymphopoietin (TSLP) while the γ chain is the shared component of the cytokine receptors for IL-2, IL-4, IL-9, IL-15 and IL-21, all with essential functions in T effector subset differentiation and maintenance. γc cytokines activate the JAK-STAT pathway and IL-7R and other cytokine receptor signaling has also been shown to engage PI3 Kinase (PI3K) and mTOR pathways [33,34]. The unique activities of each γc cytokine can in part be accounted for by the combinatorial utilization of distinct JAKs, STATs and PI3K stimulation in a cell type-specific manner [34,35]. IL-7Rα recruits JAK1 while phosphorylated γc associates with JAK3. JAKs in close proximity cross-phosphorylate each other, leading to signal amplification. In addition, the IL-7/IL-7R complex partitions into lipid rafts and associates with active JAKs and STATs as well as cytoskeletal proteins [36], suggesting that membrane fluidity may be another parameter distinguishing γc associated receptors and their signaling capacity. TSLP binds to the IL7Rα in complex with the TSLP receptor (encoded by Crlf2) and can partly compensate for the absence of IL-7R signaling in vivo, despite the fact that it does not strongly activate the JAK3-STAT5 pathway, instead recruiting JAK2 for its signaling [37,38].
3.1. Regulators of IL-7R signaling
Suppressors of cytokine signaling (SOCS) proteins are a family of E3 ubiquitin ligases that inhibit cytokine induced intracellular signals by direct interaction with components of various cytokine receptors, aborting kinase activity and targeting receptor associated kinases such as JAKs and the receptors themselves for degradation [39]. For IL-7, SOCS1 is the major negative regulator of signaling expressed in most phases of developing T and B lymphocytes, tuning sensitivity to γc cytokines. Ectopic over expression of SOCS1 in developing thymocytes phenocopies mice lacking γc or Jak3 [40], and physiological levels of SOCS1, while permissive for IL-7R signaling in T cells, are dynamically regulated. T cell development in the absence of SOCS1 is characterized by hyper IL-7 and IL-15 signaling, primarily impacting the generation of CD8+ cytotoxic T cells with an activated phenotype [41].
While SOCS proteins are induced by a variety of cytokines and thought to function in a negative auto-feedback loop, SOCS1 expression in developing lymphocytes may be less critically dependent on cytokines. IL-7 has not been reported to induce SOCS1 in developing thymocytes. Rather, the transcription factor (TF) Zbtb17 (Miz1), expressed in most lymphoid cells throughout development (Immgen.org), has been shown to regulate IL-7R signaling through direct modulation of Socs1 expression [42,43]. Canonical WNT signaling components might also be a significant regulator, as enforced expression of a stabilized β-catenin transactivator that results in TCF1 activation in T cells led to increased IL-7R expression and signaling, and a coincident downregulation of SOCS1. Conversely, in thymocytes lacking β-catenin, SOCS1 was upregulated, leading to impaired thymocyte development [44].
That the amount and/or duration of IL-7R signaling is tightly calibrated is further illustrated by the observation that additional E3 ubiquitin ligases and a deubiquitinase (DUB) directly and indirectly control IL-7R expression levels: T cells in mice lacking c-Cbl, a negative regulator of TCR signaling and AKT amongst other activities, are endowed with higher IL-7R expression and are hyper responsive to IL-7 in vitro [45]. Innate-like invariant Vα14+ NKT cells (iNKT) lacking the CYLD DUB are prone to apoptosis correlating with decreased IL-7Rα expression as a result of hyperactive NκKb signaling [46]. The reduced IL-7R signaling leads to decreased expression of ICOS, which is necessary for NKT cell homeostasis. These findings not only reveal a Byzantine network of proteins that modulate the IL-7R signaling pathway depending on activation states of the cells, but also attest to the calibration of normal IL-7R signaling to a tight range of active regulators.
3.2. Factors controlling IL-7R expression in lymphocytes
One central characteristic of IL-7R expression is its dynamic regulation by cytokines and by the overall metabolic and differentiation state of the cells. For example, TNFα can upregulate IL-7Rα expression [47], whereas most other trophic cytokines of the γc and gp130 family repress its expression [48]. At basal states, IL-7R expression is relatively high, as exemplified by B and T cell progenitors (e.g., IL-7R+ clonogenic lymphoid progenitors and IL-7R+C-kit+CD25+CD44+CD4−CD8−CD3− proT cells) and resting naïve lymphocytes in secondary lymphoid tissues. Downregulation of IL-7R expression is a necessary step in early B cell differentiation from progenitors [3,49]. Excessive IL-7 elicited signals are detrimental to the expression of B cell lineage TFs EBF and Pax5 as well as Forkhead box O1 (FOXO1) that induces Rag1/2 expression [50,51]. Given that EBF, E2A and FOXO1 coordinately impose B cell lineage specification [52], modulation of IL-7R signaling can be considered as a permissive, but not necessarily deterministic [7,49,53], parameter for cell lineage commitment. The apparent paradox that IL-7R signaling is necessary for regulated B cell receptor (BCR) gene rearrangement and expression [3,54], but also dampens the expression of FOXO1 that turns on Rag1/2 can be resolved by invoking developmentally compartmentalized IL-7R signaling output. In T and B progenitors, the IL-7R-JAK3-STAT5 circuit dominates and primes the chromatin for transcription and rearrangement in conjunction with cofactors, such as E proteins that are also direct targets of IL-7R signaling [55]. Concurrently, the FOXO1-Rag1/2 circuit is dampened by the PI3K-AKT (or possibly MAPK) pathway activated by IL-7R in B cells until the proper chromatin state is established [50,51]. With the decline of IL-7R expression and signaling that accompanies maturation, lineage specific factors are induced that selectively propagate cell type-specific chromatin states permissive for gene rearrangement and transcription as well as TFs such as FOXO1 that promotes the expression of enzymes that act on the modified chromatin to generate lineage-specific proteins.
A dynamic modulation of IL-7R signaling necessary for normal differentiation is also evident in the thymus [53,56]. Generation of innate γδ T cells from proT cells is linked to IL-7R expression with IL-7Rhi proT cells as the preferred source of γδ T cells relative to IL-7Rlo proT cells. Conversely, IL-7Rlo proT cells are biased to differentiate into αβ T cells. This bias can be uncoupled from the possible enhancement of TCRγ gene rearrangement/expression in IL-7Rhi proT cells as the provision of a rearranged functional TCRγ transgene to proT cells did not alter the observed bias [57]. While the γδ T cell lineage specific TF SOX13 [58] has been suggested to be a positive regulator of IL-7R expression [59], developing immature γδ T cell subsets distinguished based on Vγ chain usage exhibit a range of Sox13 expression, with the subset containing the highest amount of Sox13 transcripts expressing the lowest amount of IL-7R on the cell surface (Immgen.org; unpublished). Hence, the link between IL-7R signaling and γδ T cell differentiation remains unclear, exacerbated by more recent findings that different γδ cell subsets may originate asynchronously from multiple developmental intermediates. That distinct IL-7R expression pattern of γδ cell subsets is molecularly linked to the timing of γδ subset generation and serves as a marker of origin rather than function per se is currently being investigated. In this setting γδ subsets arising from the earliest T cell progenitors would inherit gene expression programs distinct from those arising from more mature T lineage-committed precursors, including distinct IL-7 sensitivity and effector function programming.
In contrast to developing innate γδ cell subsets that mostly maintain IL-7R expression, adaptive αβ T cell lineage committed precursor thymocytes (C-kitloCD25+CD44− CD4−CD8−CD3− preT) rapidly lose IL-7R expression, only to regain it after the final steps of maturation prior to thymic egress. For developing αβ T cells, the shutdown of IL-7R expression coincides with the induction of TCR expression, making the cells switch from cytokines for survival to becoming highly focused on preTCR and peptide-MHC complexes for terminal maturation towards the CD4+ or CD8+ T cell lineages. Another consequence of the selective shut down of IL-7R expression is that the vast majority of thymocytes (αβTCRloCD4+CD8+) do not consume IL-7, thus permitting limiting local depots of IL-7 to sustain precursor cells, γδ thymocytes (IL-7Rmid-high) and selected αβ CD4+ and CD8+ thymocytes and promote functional maturation [48]. This exclusivity of consumption to highly segregated subsets imparts distinct, tightly regulated differentiation programs to those cells, such as induction of early cell lineage factors (e.g., E proteins) in the progenitors, facilitation of antigen receptor rearrangements and expression for developing cells, and survival of thymocytes that passed developmental checkpoints. A similar dynamic of IL-7R expression occurs in secondary lymphoid tissues such that antigen activated lymphocytes variably lose IL-7R expression, only to resurface on antigen-experienced cells of the memory compartment. It has been suggested that this downregulation is necessary for the democratization of lymphocytes to ensure that the cells that best compete for limiting IL-7 do not dominate [60].
During development, Il7r transcription in thymocytes requires GABPα [61], which is a member of the ETS family of TFs, and c-Myb [62], whereas in immature B cells, the ETS TF PU.1 is an additional cofactor for Il7r expression [63]. In positively selected CD4+ thymocytes and peripheral CD4+ T cells, RUNX1 activates Il7r transcription [64], with ETS-1 acting as a coactivator in both naïve and central memory CD4+ and CD8+ T cells [65]. Growth factor independence-1 (Gfi-1), on the other hand, is the best-characterized repressor of Il7r transcription in T cells [48]. In CD8+ effector T cells Gfi-1 is expressed highly and antagonizes GABPa binding to, and modification of, the Il7r genomic locus [66]. In peripheral T cells Gfi is turned on by low level ERK-dependent antigen receptor signaling [67] and by IL-7 itself (in CD8+ T cells, [48]) thereby establishing an auto inhibitory feedback loop. Conversely, TGFβ downmodulates Gfi1 expression [68]. In thymocytes, the absence [69] or over-expression of Gfi1 does not lead to altered expression of IL-7R per cell [70], but rather results in increased apoptosis, particularly of early progenitors
Upon egress from the thymus, the homeostasis of naïve αβ T cells requires tonic cytokine and TCR signals as the cells cruise the secondary lymphoid organs, directed by chemokines and endothelial migratory cues. Trophic signals and cell trafficking behaviors are coordinately regulated by the FOX family of TFs. FOXO TFs in particular have emerged as the central nuclear effectors programming the adaptation of diverse cell types to external variations impacting metabolism and stress [71]. Among lymphocytes, Il7r is a direct target of FOXO1 in T, but not B, cells and the absence of Foxo1 results in decreased Il7r expression and the subsequent loss of naïve T cells [60,72]. Concurrently, FOXO1 controls the expression of the primary migratory molecules S1P1, CCR7 and CD62L by directly modulating the TF KLF2 that globally dictates naïve T cell migratory patterns [73]. Cell-extrinsic stimuli of the PI3K-AKT pathway, including TCR ligands, costimulatory B7 molecules that bind to CD28, and pro-survival cytokines such as IL-7, can downregulate molecules of T cell homeostasis by inactivating FOXO1 [50,51]. In naïve CD8+ T cells, FOXP1 is a counter regulator of FOXO1 and therefore an inhibitor of IL-7Rα expression, most likely by direct competition with FOXO1 for docking onto Il7r locus [74] In precursor thymocytes FOXO1 expression is low to negligible (Immgen.org), suggesting that this pathway is not a regulatory loop for IL-7R expression during T cell development. As discussed previously, FOXO1 is a B cell lineage specification factor operating in conjunction with E2A and EBF proteins to induce Rag1/2 expression downstream of IL-7R signaling [50-52]. Whether an auto feedback circular loop consisting of IL-7R and homeostasis regulating FOX family TFs is a general phenomenon in extrathymically-derived ILCs remains to be established.
4. Innate lymphoid cells expressing IL-7R in the gut
Recently, unconventional IL-7R+ lymphocytes have gained prominence, including ILCs that are prevalent in the gut-associated lymphoid tissues (GALTs). While their functional relevance is just beginning to emerge, recent findings that they are the source of IL-17 and IL-22 inflammatory/regulatory cytokines and thus impact inflammatory set points and control intestinal homeostasis have elevated their immunological stature [14]. In common with other lymphocytes, IL-7R expression is the key feature of GALT ILCs and their developmental programming and acquisition of effector capacity are regulated by genetic circuits that are critical for thymic ILCs, such as Notch-HES1 and TCF1 ([75,76] and unpublished). The founding subset of intestinal ILCs are the Lymphoid Tissue initiator cells (LTis), first identified in the fetal intestine, and essential for PP and LN organogenesis via the production of Lymphotoxins (LT, see below)[77].
4.1. GALT effector ILCs
Two LTi related ILC subsets are ILC22 (also called NK22, NKp46+ in mice, NKp44+CD56+ in humans) and ILC17 (Thy1+, Ly6a (Sca-1)+, IL-23R+). These cells are required for maintaining homeostasis in the gut during inflammatory responses through the rapid production of IL-22, a key mediator of innate responses in gut epithelial cells against bacterial pathogens, and IL-17, which drives pro-inflammatory responses through the localized induction of inflammatory cytokines and neutrophil recruitment [78-80]. All of these subsets are dependent on IL-7 for their differentiation and survival [16,17]. The effector cytokine producers LTi, ILC17 and ILC22 cells are also commonly marked by the expression of RORγt and Id2. RORγt is the central molecule responsible for production of the signature cytokine IL-17 and mice lacking RORγt lack all effector ILCs in the GALTs [81] and peripheral IL-17 producing iNKT (NKT17) and γδ T cells (Tγδ17) [76,82-84]. Id2 is an inhibitor of the HLH E protein TFs that are also expressed by non-GALT associated ILCs, including IL-7R+ NK progenitors in the BM, NK cells, iNKT cells and innate CD8+ T cells [12,13,85-87]. Although IL-7 has been implicated as a permissive factor for GALT ILC effector cytokine production [88], the molecular link between RORγt/Id2 expression and IL-7 has not been established. In fact, the co-expression of IL-7R and the signature ILC defining TFs is somewhat counterintuitive. Two well characterized TFs, STAT3 and IRF4, induce RORγδt transcription [89,90], but neither is primarily regulated by IL-7. In conventional CD4+ T cells, IL-2 activated STAT5, the primary STAT of IL-7R signaling, is a negative regulator of Rorc [91]. Further, in developing thymocytes, IL-7R signaling is not permissive for Rorc expression [56]. IL-7R signaling is also necessary for E protein induction in lymphoid precursors [55] and it seems unlikely that a direct link exists between IL-7 and Id2. Clearly, regulation of Rorc expression in ILCs is distinct from that of conventional adaptive T cells [17], also evidenced by the abundant expression of IL-7R by RORγt+ Tγδ17 cells upon thymic maturation (unpublished), and a role for IL-7 in modulating effector cytokine production in ILCs, if any, will require further studies. Th2-like ILC subsets, which are mostly RORγt-independent, but Id2-dependent, are found in the BM and secondary lymphoid tissues in mice [92,93] and represent innate sources of IL-5 and IL-13 that are necessary for immunity against worms. These ILC5/13 subsets are IL-25 (IL-17e) and IL-33-dependent for expansion, capable of γc chain signaling [94], and express the IL-7R. A similar functional subset is also found in fat cells of the mouse peritoneal cavity [95] and in humans (Crth2+CD161+CD7+CD3− innate lymphocytes), where they are localized in blood, lung, gut and inflamed nasal tracts [96]. The impact of IL-7R signaling for the members of the ILC5/13 subset has not been studied in detail. IL-7R is also necessary for intestinal enterocytes mediating repair of chemically damaged intestinal epithelial layers. In this inflammatory setting, autocrine TSLP acting on IECs is necessary to induce secretory leukocyte peptidase inhibitor, a tissue repair factor that antagonizes proteases [97,98]. Thus, IL-7R signaling elicited by IL-7 and TSLP in ILCs and stromal cells, respectively, are critical for regulating inflammatory responses in the gut.
4.2. IL-7R signaling-dependent lymphoid organogenesis and maintenance
Cytokines and chemokines not only have key roles in directing lymphocyte trafficking to tissues, but they are essential for organogenesis and maintenance of tissue architecture. For the latter processes, while morphogens dominate in embryogenesis, the IL-7/IL-7R axis along with Lymphotoxins (LTs) and other TNF family of cytokines are the central factors in adult hematopoietic tissue development and organization. IL-7R signaling in LTi (LTα1β2CD45+CD4+CD3−c-Kit+RANK+CCR7+CXCR5+) cells modulates their turnover in developing anlagen [99], as well as inducing CD30L [100] and LTs [77,101]. In humans, LTi cells differ phenotypically, as they do not express CD4, but do respond to IL-7 and are localized to developing LNs [102]. LTi cells persist in adult lymphoid tissues including Tonsils, LNs, intestinal crypotpatches, isolated lymphoid follicles and spleen.
Lymphoid tissues develop as a result of stochastic interactions between IL-7Rhi LTi cells and mesenchymal organizer cells (LTo). In vitro, IL-7 drives the rapid proliferation of LTi cells and is required for their maintenance. In vivo, however, LTi cells in the mid-gut are G0/G1 growth arrested and do not depend on IL-7R signaling for their survival [103](Fig 1.). Rather, IL-7R signaling likely modulates LTi cell responses to chemotactic gradients (IL-7 is not in itself directly chemoattractive [104]), and perhaps most importantly, induces LTp [77,101]. In mice over-expressing IL-7 and TSLP, LTi cell numbers are enhanced and additional ectopic lymphoid tissues develop [37,98,105], confirming that supra-physiological amounts of IL-7 and TSLP can drive LTi expansion in vivo. However, in normal mice, this process is not dependent on either IL-7 or TSLP, since no major impairment in PP formation is observed in IL-7 or TSLP deficient mice [98,106] (Fig. 1). It has been previously speculated that an unknown cytokine signals through IL-7R in a JAK3 dependent pathway and has an essential role in PP formation [107], potentially adding further complexity to IL-7R signaling in organogenesis in the GALT.
Fig. 1.
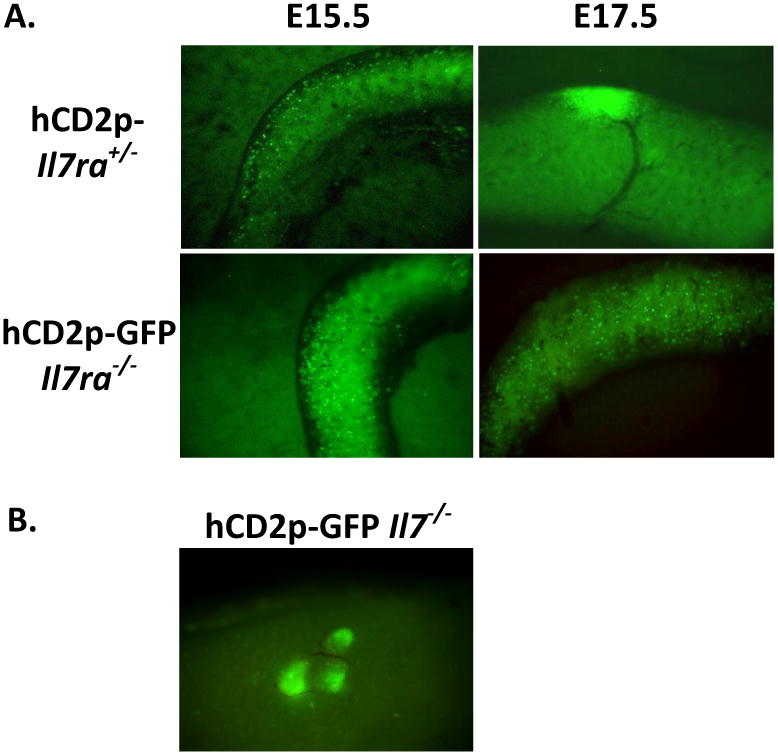
To determine the role of IL-7/IL-7R interactions in the development of Peyer's patches (PP) the human CD2 promoter–GFP reporter (GFPhi LTi cells and T cells) transgene expressing Il7ra−/− and Il7−/− mice were generated and their fetal intestines examined by fluorescent stereo-microscopy (Zeiss M2Bio) for GFP reporter expressing cells to track ILC and T cells. (A) No PP were observed in E17.5 Il7ra−/− embryos (right panels, PP shown as a concentrated mound of GFP+ cells in Il7r+/− intestine) or in adult mice (data not shown). Normal numbers of LTi cells were however observed in the mid-gut at E15.5 (left panels). (B) In contrast to Il7ra−/− mice, development of PP occurred normally in Il7−/− mice. Adult PP in Il7−/− mice are shown.
IL-7R signaling has an important role in LN development and maturation, but LNs can develop in IL-7R-deficient mice using RANK signaling [101]. RANKL is relatively abundant in LN anlagen, but not in GALTs. Exogenous IL-7 infused into RANKL-deficient mice is sufficient for the early phases of LN genesis, but proper compartmentalization within LNs requires the RANKL-TRAF6 signaling pathway. The selective redundancy in LN development may in part results from differences in both the kinetics and numbers of LTi cells and factors necessary for LTi-LTo interactions present in developing LN vs PP anlagen, with all relevant factors particularly limiting in PP. Compound CXCL13, the chemoattractant for LTi cells, and IL-7R-deficient mice lack nearly all peripheral LNs, correlating with a severe reduction in LTi cell numbers in developing LN anlagen [99]. While the provision of IL-7R+ T and thymic NK cells can restore neonatal LN genesis in IL-7R or γc chain-deficient mice [108], an increased pool of LTi cells in the absence of other lymphocytes can also be curative [98]. Overexpression of TSLP has been shown to drive the expansion in LTi cells in IL-7 deficient background [37,105]. A common quantitative modifier in all these models may be the increased LTβ that has direct stimulatory effects on stromal LTo of LNs.
5. IL-7R signaling in non-haematopoietic cells
Outside of lymphoid cells IL-7R is expressed on blood and lymphatic vascular endothelial cells, dermal fibroblasts and LN and BM stromal cells, albeit at lower levels than found on haematopoietic cells. Functional IL-7R is also found at low levels on a variety of primary human endothelial cell lines [109]. Stimulation of human aortic endothelial cells with IL-7 leads to a rapid increase in E-selectin, ICAM-1, and VCAM-1 in a PI3K-dependent manner, and MCP-1 through a JAK/STAT-dependent pathway [28]. The up-regulation of these factors in turn modulates the migration of monocytes and macrophages to atherosclerotic lesions in vivo. IL-7R is also expressed on breast and lung cancer epithelial cell lines, and stimulation of these cells by IL-7 leads to up regulation of VEGF-D expression. Analysis of tumor biopsies shows a strong correlation between IL-7R expression in the tumor microenvironment, VEGF-D expression and survival probability [110]. While the precise connection between IL-7 and tumorigenesis in vivo has not been established, the VEGF regulated angiogenesis is clearly a potential target of IL-7 that will impact tumor growth and metastasis. Analysis of IL-7p-Cre Rosa26eYFP reporter mice indicates that IL-7 is expressed at some stage(s) of normal mouse breast epithelium differentiation [26]. IL-7 is expressed at physiologically active concentrations in human breast milk, with the levels correlating with thymic function in newborn children and nutritional input from the mothers [111]. Analysis of IL-7 in maternal milk in mice shows that it can cross the intestinal barrier and modulate T cell development in neonatal mice [112]. These results collectively suggest that IL-7R signaling is functionally relevant for non-haematopoietic cells, particularly in the regulation of vascular network.
6. IL-7 production and IL-7R signaling in bone and liver macro- and microenvironment
Our analyses of IL-7R expression patterns on non-lymphoid cells show expression in diverse human BM stromal populations including multi-potent mesenchymal stem cells and BM stromal cells. In the BM, the mesenchymal stroma directs the development of hematopoietic cells. Examination of stromal subpopulations has shown that Nestin+ mesenchymal cells express IL-7 and form niches required for the maintenance of haematopoietic stem cells [113]. Further analysis of the IL-7 expressing BM stroma niches show that these vascular associated microenvironments are required for the first stage (pro-B/pre-BI cells) in B cell development, but subsequent stages of B cell development (pre-BII) occur in IL-7 negative stromal niches expressing Galectin I [114]. Although our understanding of the functional relevance of IL-7R signaling in stromal cells is rudimentary, the effects of IL-7 stimulation in distinct stromal cell lines in vitro have provided some leads. Using a BM stromal line that supports in vitro cobblestone formation arising from co-cultures of hematopoietic stem cells and stroma, it has been shown that IL-7 induces a ten-fold increase in IL-6 expression [115]. The beneficial effects of in vivo IL-7 administration in boosting immunity to chronic viral infection have been shown to be also dependent on IL-6 [116], suggesting that this inflammatory cytokine may yet emerge as a critical effector of IL-7R signaling in diverse biological settings.
In pathological rheumatoid arthritis (RA), a destructive bone joint disease, high levels of IL-7 in serum have been found to correlate strongly with disease progression [117,118]. IL-7 production is similarly increased in postmenopausal bone as a result of estrogen (E2) loss. High levels of IL-7 in the bone microenvironment have been linked to the loss of bone mass in both RA and E2 through the stimulation of osteoclast formation resulting from the downregulation of OPG, a negative regulator of the RANK/RANK-L pathway [119]. Thus, IL-7R signaling may have a key role in regulating bone remodeling.
As alluded to earlier, IL-7 is expressed robustly in fetal liver and is necessary for the development of lymphoid precursors and LTi during late stages of fetal ontogeny. In the adult liver, sinusoidal endothelial cells express IL-7, FLT3 and SCF and thus have the potential to both maintain the homeostasis of liver resident T cell populations and facilitate B cell development. Co-culture of primary liver sinusoidal endothelium with BM precursor cells (lineage marker negative) led to robust B cell differentiation [120]. Although IL-7 is normally a homeostatic cytokine whose bioavailability is primarily controlled by consumption, administration of LPS into mice leads to the induction of IL-7 in both kidney and liver, dependent on the TRIF-IFNα pathway [24]. This acute induction of IL-7 is likely to have a profound influence in regulating resident liver T cells, including innate iNKT and γδ NKT cells [121], early in the immune response.
7. Perspective
Manipulation of IL-7 remains a viable option to restore homeostasis post trauma, to treat immune diseases such as Multiple Sclerosis genetically linked to Il7 [122], and to induce temporary hyperstasis to counter damaging environmental insults and cellular transformation [10]. Advances in the basic mechanistic understanding of the shared IL-7 function in the development and homeostasis of innate and adaptive lymphocytes are permitting rationale forays toward clinical applications of IL-7 modulatory strategies to treat chronic viral infection [116], immunodeficiencies [123] and autoimmune diseases [124], and to improve vaccine efficacy [125]. The pleiotropic effects of IL-7 on the ever-expanding list of IL-7R-expressing cell types, especially outside the adaptive lymphocyte compartment, complicates targeted applications of exogenous IL-7 and modulatory biologicals specific for the IL-7/IL-7R complex. Fine mapping of the gene circuits controlling Il7 transcription in a context-dependent manner is necessary to improve our understanding of the regulation of localized production of IL-7 and its impact on macro and micro niches. Detailed characterization of cell type-specific IL-7R signaling properties, their dynamic integration with other concurrent signals regulating homeostasis locally and system-wide, and the identification of novel downstream gene targets and their function will permit more selective immunotherapies designed to impact specific IL-7-regulated cellular niches of innate and adaptive immunity.
Highlights.
>We discuss newly emerging details on the regulation of IL-7R expression. >IL-7 regulates the differentiation of all innate lymphoid cells in diverse tissues. >IL-7 may be a permissive factor for arming innate effectors. >Complex competition for IL-7 by multiple cell types in a given niche is addressed.
Acknowledgments
We thank Dr. K. Narayan for editorial inputs. This work was supported by grants NIH RO1 CA100382 (J.K.) and MRC (UK) G0601156 (M.C.).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Namen AE, Lupton S, Hjerrild K, Wignall J, Mochizuki DY, Schmierer A, Mosley B, March CJ, Urdal D, Gillis S. Stimulation of B-cell progenitors by cloned murine interleukin-7. Nature. 1988;333:571–573. doi: 10.1038/333571a0. [DOI] [PubMed] [Google Scholar]
- 2.Ye SK, Agata Y, Lee HC, Kurooka H, Kitamura T, Shimizu A, Honjo T, Ikuta K. The IL-7 receptor controlsthe accessibility of the TCRgamma locus by Stat5 and histone acetylation. Immunity. 2001;15:813–823. doi: 10.1016/s1074-7613(01)00230-8. [DOI] [PubMed] [Google Scholar]
- 3.Chowdhury D, Sen R. Transient IL-7/IL-7R signaling provides a mechanism for feedback inhibition of immunoglobulin heavy chain gene rearrangements. Immunity. 2003;18:229–241. doi: 10.1016/s1074-7613(03)00030-x. [DOI] [PubMed] [Google Scholar]
- 4.Park JH, Adoro S, Guinter T, Erman B, Alag AS, Catalfamo M, Kimura MY, Cui Y, Lucas PJ, Gress RE, et al. Signaling by intrathymic cytokines, not T cell antigen receptors, specifies CD8 lineage choice and promotes the differentiation of cytotoxic-lineage T cells. Nat Immunol. 2010;11:257–264. doi: 10.1038/ni.1840. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Schluns KS, Kieper WC, Jameson SC, Lefrancois L. Interleukin-7 mediates the homeostasis of naive and memory CD8 T cells in vivo. Nat Immunol. 2000;1:426–432. doi: 10.1038/80868. [DOI] [PubMed] [Google Scholar]
- 6.Seddon B, Tomlinson P, Zamoyska R. Interleukin 7 and T cell receptor signals regulate homeostasis of CD4 memory cells. Nat Immunol. 2003;4:680–686. doi: 10.1038/ni946. [DOI] [PubMed] [Google Scholar]
- 7.Hand TW, Morre M, Kaech SM. Expression of IL-7 receptor alpha is necessary but not sufficient for the formation of memory CD8 T cells during viral infection. Proc Natl Acad Sci U S A. 2007;104:11730–11735. doi: 10.1073/pnas.0705007104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Malin S, McManus S, Busslinger M. STAT5 in B cell development and leukemia. Curr Opin Immunol. 2010;22:168–176. doi: 10.1016/j.coi.2010.02.004. [DOI] [PubMed] [Google Scholar]
- 9.Mazzucchelli R, Durum SK. Interleukin-7 receptor expression: intelligent design. Nat Rev Immunol. 2007;7:144–154. doi: 10.1038/nri2023. [DOI] [PubMed] [Google Scholar]
- 10.Mackall CL, Fry TJ, Gress RE. Harnessing the biology of IL-7 for therapeutic application. Nat Rev Immunol. 2011;11:330–342. doi: 10.1038/nri2970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Sprent J, Surh CD. Normal T cell homeostasis: the conversion of naive cells into memory-phenotype cells. Nat Immunol. 2011;12:478–484. doi: 10.1038/ni.2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Yamagata T, Benoist C, Mathis D. A shared gene-expression signature in innate-like lymphocytes. Immunol Rev. 2006;210:52–66. doi: 10.1111/j.0105-2896.2006.00371.x. [DOI] [PubMed] [Google Scholar]
- 13.Berg LJ. Signalling through TEC kinases regulates conventional versus innate CD8(+) T-cell development. Nat Rev Immunol. 2007;7:479–485. doi: 10.1038/nri2091. [DOI] [PubMed] [Google Scholar]
- 14.Spits H, Di Santo JP. The expanding family of innate lymphoid cells: regulators and effectors of immunity and tissue remodeling. Nat Immunol. 2011;12:21–27. doi: 10.1038/ni.1962. [DOI] [PubMed] [Google Scholar]
- 15.O'Brien RL, Born WK. gammadelta T cell subsets: a link between TCR and function? Semin Immunol. 2010;22:193–198. doi: 10.1016/j.smim.2010.03.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Satoh-Takayama N, Lesjean-Pottier S, Vieira P, Sawa S, Eberl G, Vosshenrich CA, Di Santo JP. IL-7 and IL-15 independently program the differentiation of intestinal CD3-NKp46+ cell subsets from Id2-dependent precursors. J Exp Med. 2010;207:273–280. doi: 10.1084/jem.20092029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Vonarbourg C, Mortha A, Bui VL, Hernandez PP, Kiss EA, Hoyler T, Flach M, Bengsch B, Thimme R, Holscher C, et al. Regulated expression of nuclear receptor RORgammat confers distinct functional fates to NK cell receptor-expressing RORgammat(+) innate lymphocytes. Immunity. 2010;33:736–751. doi: 10.1016/j.immuni.2010.10.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Schluns KS, Klonowski KD, Lefrancois L. Transregulation of memory CD8 T-cell proliferation by IL-15Ralpha+ bone marrow-derived cells. Blood. 2004;103:988–994. doi: 10.1182/blood-2003-08-2814. [DOI] [PubMed] [Google Scholar]
- 19.Burkett PR, Koka R, Chien M, Chai S, Boone DL, Ma A. Coordinate expression and trans presentation of interleukin (IL)-15Ralpha and IL-15 supports natural killer cell and memory CD8+ T cell homeostasis. J Exp Med. 2004;200:825–834. doi: 10.1084/jem.20041389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Baccala R, Witherden D, Gonzalez-Quintial R, Dummer W, Surh CD, Havran WL, Theofilopoulos AN. Gamma delta T cell homeostasis is controlled by IL-7 and IL-15 together with subset-specific factors. J Immunol. 2005;174:4606–4612. doi: 10.4049/jimmunol.174.8.4606. [DOI] [PubMed] [Google Scholar]
- 21.French JD, Roark CL, Born WK. {gamma}{delta} T cell homeostasis is established in competition with {alpha}{beta} T cells and NK cells. Proc Natl Acad Sci U S A. 2005(102):14741–14746. doi: 10.1073/pnas.0507520102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Bolotin E, Annett G, Parkman R, Weinberg K. Serum levels of IL-7 in bone marrow transplant recipients: relationship to clinical characteristics and lymphocyte count. Bone Marrow Transplant. 1999;23:783–788. doi: 10.1038/sj.bmt.1701655. [DOI] [PubMed] [Google Scholar]
- 23.Zubkova I, Mostowski H, Zaitseva M. Up-regulation of IL-7, stromal-derived factor-1 alpha, thymus-expressed chemokine, and secondary lymphoid tissue chemokine gene expression in the stromal cells in response to thymocyte depletion: implication for thymus reconstitution. J Immunol. 2005;175:2321–2330. doi: 10.4049/jimmunol.175.4.2321. [DOI] [PubMed] [Google Scholar]
- 24.Sawa Y, Arima Y, Ogura H, Kitabayashi C, Jiang JJ, Fukushima T, Kamimura D, Hirano T, Murakami M. Hepatic interleukin-7 expression regulates T cell responses. Immunity. 2009;30:447–457. doi: 10.1016/j.immuni.2009.01.007. [DOI] [PubMed] [Google Scholar]
- 25.Kim GY, Hong C, Park JH. Seeing is believing: illuminating the source of in vivo interleukin-7. Immune Netw. 2011;11:1–10. doi: 10.4110/in.2011.11.1.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Repass JF, Laurent MN, Carter C, Reizis B, Bedford MT, Cardenas K, Narang P, Coles M, Richie ER. IL7- hCD25 and IL7-Cre BAC transgenic mouse lines: new tools for analysis of IL-7 expressing cells. Genesis. 2009;47:281–287. doi: 10.1002/dvg.20497. [DOI] [PubMed] [Google Scholar]
- 27.Borghesi LA, Yamashita Y, Kincade PW. Heparan sulfate proteoglycans mediate interleukin-7-dependent B lymphopoiesis. Blood. 1999;93:140–148. [PubMed] [Google Scholar]
- 28.Li R, Paul A, Ko KW, Sheldon M, Rich BE, Terashima T, Dieker C, Cormier S, Li L, Nour EA, et al. Interleukin-7 induces recruitment of monocytes/macrophages to endothelium. Eur Heart J. 2011 doi: 10.1093/eurheartj/ehr245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Hoe E, McKay FC, Schibeci SD, Gandhi K, Heard RN, Stewart GJ, Booth DR. Functionally significant differences in expression of disease-associated IL-7 receptor alpha haplotypes in CD4 T cells and dendritic cells. J Immunol. 2010;184:2512–2517. doi: 10.4049/jimmunol.0902900. [DOI] [PubMed] [Google Scholar]
- 30.Crawley AM, Faucher S, Angel JB. Soluble IL-7R alpha (sCD127) inhibits IL-7 activity and is increased in HIV infection. J Immunol. 2010;184:4679–4687. doi: 10.4049/jimmunol.0903758. [DOI] [PubMed] [Google Scholar]
- 31.Rane L, Rahman S, Magalhaes I, Ahmed R, Spangberg M, Kondova I, Verreck F, Andersson J, Brighenti S, Maeurer MJ. Increased (6 exon) interleukin-7 production after M. tuberculosis infection and soluble interleukin-7 receptor expression in lung tissue. Genes Immun. 2011 doi: 10.1038/gene.2011.29. [DOI] [PubMed] [Google Scholar]
- 32.McElroy CA, Dohm JA, Walsh ST. Structural and biophysical studies of the human IL-7/IL-7Ralpha complex. Structure. 2009;17:54–65. doi: 10.1016/j.str.2008.10.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Pallard C, Stegmann AP, van Kleffens T, Smart F, Venkitaraman A, Spits H. Distinct roles of the phosphatidylinositol 3-kinase and STAT5 pathways in IL-7-mediated development of human thymocyte precursors. Immunity. 1999;10:525–535. doi: 10.1016/s1074-7613(00)80052-7. [DOI] [PubMed] [Google Scholar]
- 34.Rathmell JC, Farkash EA, Gao W, Thompson CB. IL-7 enhances the survival and maintains the size of naive T cells. J Immunol. 2001;167:6869–6876. doi: 10.4049/jimmunol.167.12.6869. [DOI] [PubMed] [Google Scholar]
- 35.Osborne LC, Dhanji S, Snow JW, Priatel JJ, Ma MC, Miners MJ, Teh HS, Goldsmith MA, Abraham N. Impaired CD8 T cell memory and CD4 T cell primary responses in IL-7R alpha mutant mice. J Exp Med. 2007;204:619–631. doi: 10.1084/jem.20061871. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Rose T, Pillet AH, Lavergne V, Tamarit B, Lenormand P, Rousselle JC, Namane A, Theze J. Interleukin-7 compartmentalizes its receptor signaling complex to initiate CD4 T lymphocyte response. J Biol Chem. 2010;285:14898–14908. doi: 10.1074/jbc.M110.104232. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 37.Chappaz S, Flueck L, Farr AG, Rolink AG, Finke D. Increased TSLP availability restores T- and B-cell compartments in adult IL-7 deficient mice. Blood. 2007;110:3862–3870. doi: 10.1182/blood-2007-02-074245. [DOI] [PubMed] [Google Scholar]
- 38.Rochman Y, Kashyap M, Robinson GW, Sakamoto K, Gomez-Rodriguez J, Wagner KU, Leonard WJ. Thymic stromal lymphopoietin-mediated STAT5 phosphorylation via kinases JAK1 and JAK2 reveals a key difference from IL-7-induced signaling. Proc Natl Acad Sci U S A. 2010;107:19455–19460. doi: 10.1073/pnas.1008271107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Yoshimura A, Naka T, Kubo M. SOCS proteins, cytokine signalling and immune regulation. Nat Rev Immunol. 2007;7:454–465. doi: 10.1038/nri2093. [DOI] [PubMed] [Google Scholar]
- 40.Fujimoto M, Naka T, Nakagawa R, Kawazoe Y, Morita Y, Tateishi A, Okumura K, Narazaki M, Kishimoto T. Defective thymocyte development and perturbed homeostasis of T cells in STAT-induced STAT inhibitor-1/suppressors of cytokine signaling-1 transgenic mice. J Immunol. 2000;165:1799–1806. doi: 10.4049/jimmunol.165.4.1799. [DOI] [PubMed] [Google Scholar]
- 41.Chong MM, Cornish AL, Darwiche R, Stanley EG, Purton JF, Godfrey DI, Hilton DJ, Starr R, Alexander WS, Kay TW. Suppressor of cytokine signaling-1 is a critical regulator of interleukin-7-dependent CD8+ T cell differentiation. Immunity. 2003;18:475–487. doi: 10.1016/s1074-7613(03)00078-5. [DOI] [PubMed] [Google Scholar]
- 42.Kosan C, Saba I, Godmann M, Herold S, Herkert B, Eilers M, Moroy T. Transcription factor miz-1 is required to regulate interleukin-7 receptor signaling at early commitment stages of B cell differentiation. Immunity. 2010;33:917–928. doi: 10.1016/j.immuni.2010.11.028. [DOI] [PubMed] [Google Scholar]
- 43.Saba I, Kosan C, Vassen L, Moroy T. IL-7R-dependent survival and differentiation of early T-lineage progenitors is regulated by the BTB/POZ domain transcription factor Miz-1. Blood. 2011;117:3370–3381. doi: 10.1182/blood-2010-09-310680. [DOI] [PubMed] [Google Scholar]
- 44.Yu Q, Xu M, Sen JM. Beta-catenin expression enhances IL-7 receptor signaling in thymocytes during positive selection. J Immunol. 2007;179:126–131. doi: 10.4049/jimmunol.179.1.126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Rathinam C, Flavell RA. c-Cbl deficiency leads to diminished lymphocyte development and functions in an age-dependent manner. Proc Natl Acad Sci U S A. 2010;107:8316–8321. doi: 10.1073/pnas.0914496107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Lee AJ, Zhou X, Chang M, Hunzeker J, Bonneau RH, Zhou D, Sun SC. Regulation of natural killer T-cell development by deubiquitinase CYLD. Embo J. 2010;29:1600–1612. doi: 10.1038/emboj.2010.31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Tian B, Nowak DE, Jamaluddin M, Wang S, Brasier AR. Identification of direct genomic targets downstream of the nuclear factor-kappaB transcription factor mediating tumor necrosis factor signaling. J Biol Chem. 2005;280:17435–17448. doi: 10.1074/jbc.M500437200. [DOI] [PubMed] [Google Scholar]
- 48.Park JH, Yu Q, Erman B, Appelbaum JS, Montoya-Durango D, Grimes HL, Singer A. Suppression of IL7Ralpha transcription by IL-7 and other prosurvival cytokines: a novel mechanism for maximizing IL-7-dependent T cell survival. Immunity. 2004;21:289–302. doi: 10.1016/j.immuni.2004.07.016. [DOI] [PubMed] [Google Scholar]
- 49.Purohit SJ, Stephan RP, Kim HG, Herrin BR, Gartland L, Klug CA. Determination of lymphoid cell fate is dependent on the expression status of the IL-7 receptor. Embo J. 2003;22:5511–5521. doi: 10.1093/emboj/cdg522. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Dengler HS, Baracho GV, Omori SA, Bruckner S, Arden KC, Castrillon DH, DePinho RA, Rickert RC. Distinct functions for the transcription factor Foxo1 at various stages of B cell differentiation. Nat Immunol. 2008;9:1388–1398. doi: 10.1038/ni.1667. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Amin RH, Schlissel MS. Foxo1 directly regulates the transcription of recombination-activating genes during B cell development. Nat Immunol. 2008;9:613–622. doi: 10.1038/ni.1612. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Lin YC, Jhunjhunwala S, Benner C, Heinz S, Welinder E, Mansson R, Sigvardsson M, Hagman J, Espinoza CA, Dutkowski J, et al. A global network of transcription factors, involving E2A, EBF1 and Foxo1, that orchestrates B cell fate. Nat Immunol. 2010;11:635–643. doi: 10.1038/ni.1891. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Munitic I, Williams JA, Yang Y, Dong B, Lucas PJ, El Kassar N, Gress RE, Ashwell JD. Dynamic regulation of IL-7 receptor expression is required for normal thymopoiesis. Blood. 2004;104:4165–4172. doi: 10.1182/blood-2004-06-2484. [DOI] [PubMed] [Google Scholar]
- 54.Malin S, McManus S, Cobaleda C, Novatchkova M, Delogu A, Bouillet P, Strasser A, Busslinger M. Role of STAT5 in controlling cell survival and immunoglobulin gene recombination during pro-B cell development. Nat Immunol. 2010;11:171–179. doi: 10.1038/ni.1827. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Singh H, Medina KL, Pongubala JM. Contingent gene regulatory networks and B cell fate specification. Proc Natl Acad Sci U S A. 2005;102:4949–4953. doi: 10.1073/pnas.0500480102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Yu Q, Erman B, Park JH, Feigenbaum L, Singer A. IL-7 receptor signals inhibit expression of transcription factors TCF-1, LEF-1, and RORgammat: impact on thymocyte development. J Exp Med. 2004;200:797–803. doi: 10.1084/jem.20032183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Kang J, Volkmann A, Raulet DH. Evidence that gammadelta versus alphabeta T cell fate determination is initiated independently of T cell receptor signaling. J Exp Med. 2001;193:689–698. doi: 10.1084/jem.193.6.689. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Melichar HJ, Narayan K, Der SD, Hiraoka Y, Gardiol N, Jeannet G, Held W, Chambers CA, Kang J. Regulation of gammadelta versus alphabeta T lymphocyte differentiation by the transcription factor SOX13. Science. 2007;315:230–233. doi: 10.1126/science.1135344. [DOI] [PubMed] [Google Scholar]
- 59.Turchinovich G, Hayday AC. Skint-1 identifies a common molecular mechanism for the development of interferon-gamma-secreting versus interleukin-17-secreting gammadelta T cells. Immunity. 2011;35:59–68. doi: 10.1016/j.immuni.2011.04.018. [DOI] [PubMed] [Google Scholar]
- 60.Kerdiles YM, Beisner DR, Tinoco R, Dejean AS, Castrillon DH, DePinho RA, Hedrick SM. Foxo1 links homing and survival of naive T cells by regulating L-selectin, CCR7 and interleukin 7 receptor. Nat Immunol. 2009;10:176–184. doi: 10.1038/ni.1689. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Xue HH, Bollenbacher J, Rovella V, Tripuraneni R, Du YB, Liu CY, Williams A, McCoy JP, Leonard WJ. GA binding protein regulates interleukin 7 receptor alpha-chain gene expression in T cells. Nat Immunol. 2004;5:1036–1044. doi: 10.1038/ni1117. [DOI] [PubMed] [Google Scholar]
- 62.Fahl SP, Crittenden RB, Allman D, Bender TP. c-Myb is required for pro-B cell differentiation. J Immunol. 2009;183:5582–5592. doi: 10.4049/jimmunol.0901187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.DeKoter RP, Schweitzer BL, Kamath MB, Jones D, Tagoh H, Bonifer C, Hildeman DA, Huang KJ. Regulation of the interleukin-7 receptor alpha promoter by the Ets transcription factors PU.1 and GA-binding protein in developing B cells. J Biol Chem. 2007;282:14194–14204. doi: 10.1074/jbc.M700377200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Egawa T, Tillman RE, Naoe Y, Taniuchi I, Littman DR. The role of the Runx transcription factors in thymocyte differentiation and in homeostasis of naive T cells. J Exp Med. 2007;204:1945–1957. doi: 10.1084/jem.20070133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Grenningloh R, Tai TS, Frahm N, Hongo TC, Chicoine AT, Brander C, Kaufmann DE, Ho IC. Ets-1 maintains IL-7 receptor expression in peripheral T cells. J Immunol. 2011;186:969–976. doi: 10.4049/jimmunol.1002099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Chandele A, Joshi NS, Zhu J, Paul WE, Leonard WJ, Kaech SM. Formation of IL-7Ralphahigh and IL- 7Ralphalow CD8 T cells during infection is regulated by the opposing functions of GABPalpha and Gfi-1. J Immunol. 2008;180:5309–5319. doi: 10.4049/jimmunol.180.8.5309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Guo F, Hildeman D, Tripathi P, Velu CS, Grimes HL, Zheng Y. Coordination of IL-7 receptor and T-cell receptor signaling by cell-division cycle 42 in T-cell homeostasis. Proc Natl Acad Sci U S A. 2010;107:18505–18510. doi: 10.1073/pnas.1010249107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Zhu J, Davidson TS, Wei G, Jankovic D, Cui K, Schones DE, Guo L, Zhao K, Shevach EM, Paul WE. Down-regulation of Gfi-1 expression by TGF-beta is important for differentiation of Th17 and CD103+ inducible regulatory T cells. J Exp Med. 2009;206:329–341. doi: 10.1084/jem.20081666. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Yucel R, Karsunky H, Klein-Hitpass L, Moroy T. The transcriptional repressor Gfi1 affects development of early, uncommitted c-Kit+ T cell progenitors and CD4/CD8 lineage decision in the thymus. J Exp Med. 2003;197:831–844. doi: 10.1084/jem.20021417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Doan LL, Kitay MK, Yu Q, Singer A, Herblot S, Hoang T, Bear SE, Morse HC, 3rd, Tsichlis PN, Grimes HL. Growth factor independence-1B expression leads to defects in T cell activation, IL-7 receptor alpha expression, and T cell lineage commitment. J Immunol. 2003;170:2356–2366. doi: 10.4049/jimmunol.170.5.2356. [DOI] [PubMed] [Google Scholar]
- 71.Gross DN, van den Heuvel AP, Birnbaum MJ. The role of FoxO in the regulation of metabolism. Oncogene. 2008;27:2320–2336. doi: 10.1038/onc.2008.25. [DOI] [PubMed] [Google Scholar]
- 72.Ouyang W, Beckett O, Flavell RA, Li MO. An essential role of the Forkhead-box transcription factor Foxo1 in control of T cell homeostasis and tolerance. Immunity. 2009;30:358–371. doi: 10.1016/j.immuni.2009.02.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Carlson CM, Endrizzi BT, Wu J, Ding X, Weinreich MA, Walsh ER, Wani MA, Lingrel JB, Hogquist KA, Jameson SC. Kruppel-like factor 2 regulates thymocyte and T-cell migration. Nature. 2006;442:299–302. doi: 10.1038/nature04882. [DOI] [PubMed] [Google Scholar]
- 74.Feng X, Wang H, Takata H, Day TJ, Willen J, Hu H. Transcription factor Foxp1 exerts essential cell-intrinsic regulation of the quiescence of naive T cells. Nat Immunol. 2011;12:544–550. doi: 10.1038/ni.2034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Possot C, Schmutz S, Chea S, Boucontet L, Louise A, Cumano A, Golub R. Notch signaling is necessary for adult, but not fetal, development of RORgammat(+) innate lymphoid cells. Nat Immunol. 2011;12:949–958. doi: 10.1038/ni.2105. [DOI] [PubMed] [Google Scholar]
- 76.Shibata K, Yamada H, Sato T, Dejima T, Nakamura M, Ikawa T, Hara H, Yamasaki S, Kageyama R, Iwakura Y, et al. Notch-Hes1 pathway is required for the development of IL-17-producing gammadelta T cells. Blood. 2011;118:586–593. doi: 10.1182/blood-2011-02-334995. [DOI] [PubMed] [Google Scholar]
- 77.Yoshida H, Honda K, Shinkura R, Adachi S, Nishikawa S, Maki K, Ikuta K, Nishikawa SI. IL-7 receptor alpha+ CD3(-) cells in the embryonic intestine induces the organizing center of Peyer's patches. Int Immunol. 1999;11:643–655. doi: 10.1093/intimm/11.5.643. [DOI] [PubMed] [Google Scholar]
- 78.Zheng Y, Valdez PA, Danilenko DM, Hu Y, Sa SM, Gong Q, Abbas AR, Modrusan Z, Ghilardi N, de Sauvage FJ, et al. Interleukin-22 mediates early host defense against attaching and effacing bacterial pathogens. Nat Med. 2008;14:282–289. doi: 10.1038/nm1720. [DOI] [PubMed] [Google Scholar]
- 79.Satoh-Takayama N, Vosshenrich CA, Lesjean-Pottier S, Sawa S, Lochner M, Rattis F, Mention JJ, Thiam K, Cerf-Bensussan N, Mandelboim O, et al. Microbial flora drives interleukin 22 production in intestinal NKp46+ cells that provide innate mucosal immune defense. Immunity. 2008;29:958–970. doi: 10.1016/j.immuni.2008.11.001. [DOI] [PubMed] [Google Scholar]
- 80.Zenewicz LA, Yancopoulos GD, Valenzuela DM, Murphy AJ, Stevens S, Flavell RA. Innate and adaptive interleukin-22 protects mice from inflammatory bowel disease. Immunity. 2008;29:947–957. doi: 10.1016/j.immuni.2008.11.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Sawa S, Cherrier M, Lochner M, Satoh-Takayama N, Fehling HJ, Langa F, Di Santo JP, Eberl G. Lineage relationship analysis of RORgammat+ innate lymphoid cells. Science. 2010;330:665–669. doi: 10.1126/science.1194597. [DOI] [PubMed] [Google Scholar]
- 82.Lochner M, Peduto L, Cherrier M, Sawa S, Langa F, Varona R, Riethmacher D, Si-Tahar M, Di Santo JP, Eberl G. In vivo equilibrium of proinflammatory IL-17+ and regulatory IL-10+ Foxp3+ RORgamma t+ T cells. J Exp Med. 2008;205:1381–1393. doi: 10.1084/jem.20080034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Rachitskaya AV, Hansen AM, Horai R, Li Z, Villasmil R, Luger D, Nussenblatt RB, Caspi RR. Cutting edge: NKT cells constitutively express IL-23 receptor and RORgammat and rapidly produce IL-17 upon receptor ligation in an IL-6-independent fashion. J Immunol. 2008;180:5167–5171. doi: 10.4049/jimmunol.180.8.5167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Coquet JM, Chakravarti S, Kyparissoudis K, McNab FW, Pitt LA, McKenzie BS, Berzins SP, Smyth MJ, Godfrey DI. Diverse cytokine production by NKT cell subsets and identification of an IL-17-producing CD4-NK1.1- NKT cell population. Proc Natl Acad Sci U S A. 2008;105:11287–11292. doi: 10.1073/pnas.0801631105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Monticelli LA, Yang Y, Knell J, D'Cruz LM, Cannarile MA, Engel I, Kronenberg M, Goldrath AW. Transcriptional regulator Id2 controls survival of hepatic NKT cells. Proc Natl Acad Sci U S A. 2009;106:19461–19466. doi: 10.1073/pnas.0908249106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Yokota Y, Mansouri A, Mori S, Sugawara S, Adachi S, Nishikawa S, Gruss P. Development of peripheral lymphoid organs and natural killer cells depends on the helix-loop-helix inhibitor Id2. Nature. 1999;397:702–706. doi: 10.1038/17812. [DOI] [PubMed] [Google Scholar]
- 87.Carotta S, Pang SH, Nutt SL, Belz GT. Identification of the earliest NK-cell precursor in the mouse BM. Blood. 2011;117:5449–5452. doi: 10.1182/blood-2010-11-318956. [DOI] [PubMed] [Google Scholar]
- 88.Cella M, Otero K, Colonna M. Expansion of human NK-22 cells with IL-7, IL-2, and IL-1beta reveals intrinsic functional plasticity. Proc Natl Acad Sci U S A. 2010;107:10961–10966. doi: 10.1073/pnas.1005641107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Zhou L, Ivanov II, Spolski R, Min R, Shenderov K, Egawa T, Levy DE, Leonard WJ, Littman DR. IL-6 programs T(H)-17 cell differentiation by promoting sequential engagement of the IL-21 and IL-23 pathways. Nat Immunol. 2007;8:967–974. doi: 10.1038/ni1488. [DOI] [PubMed] [Google Scholar]
- 90.Brustle A, Heink S, Huber M, Rosenplanter C, Stadelmann C, Yu P, Arpaia E, Mak TW, Kamradt T, Lohoff M. The development of inflammatory T(H)-17 cells requires interferon-regulatory factor 4. Nat Immunol. 2007;8:958–966. doi: 10.1038/ni1500. [DOI] [PubMed] [Google Scholar]
- 91.Laurence A, Tato CM, Davidson TS, Kanno Y, Chen Z, Yao Z, Blank RB, Meylan F, Siegel R, Hennighausen L, et al. Interleukin-2 signaling via STAT5 constrains T helper 17 cell generation. Immunity. 2007;26:371–381. doi: 10.1016/j.immuni.2007.02.009. [DOI] [PubMed] [Google Scholar]
- 92.Neill DR, Wong SH, Bellosi A, Flynn RJ, Daly M, Langford TK, Bucks C, Kane CM, Fallon PG, Pannell R, et al. Nuocytes represent a new innate effector leukocyte that mediates type-2 immunity. Nature. 2010;464:1367–1370. doi: 10.1038/nature08900. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Saenz SA, Siracusa MC, Perrigoue JG, Spencer SP, Urban JF, Jr, Tocker JE, Budelsky AL, Kleinschek MA, Kastelein RA, Kambayashi T, et al. IL25 elicits a multipotent progenitor cell population that promotes T(H)2 cytokine responses. Nature. 2010;464:1362–1366. doi: 10.1038/nature08901. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Hurst SD, Muchamuel T, Gorman DM, Gilbert JM, Clifford T, Kwan S, Menon S, Seymour B, Jackson C, Kung TT, et al. New IL-17 family members promote Th1 or Th2 responses in the lung: in vivo function of the novel cytokine IL-25. J Immunol. 2002;169:443–453. doi: 10.4049/jimmunol.169.1.443. [DOI] [PubMed] [Google Scholar]
- 95.Moro K, Yamada T, Tanabe M, Takeuchi T, Ikawa T, Kawamoto H, Furusawa J, Ohtani M, Fujii H, Koyasu S. Innate production of T(H)2 cytokines by adipose tissue-associated c-Kit(+)Sca-1(+) lymphoid cells. Nature. 2010;463:540–544. doi: 10.1038/nature08636. [DOI] [PubMed] [Google Scholar]
- 96.Mjosberg JM, Trifari S, Crellin NK, Peters CP, van Drunen CM, Piet B, Fokkens WJ, Cupedo T, Spits H. Human IL-25- and IL-33-responsive type 2 innate lymphoid cells are defined by expression of CRTH2 and CD161. Nat Immunol. 2011 doi: 10.1038/ni.2104. [DOI] [PubMed] [Google Scholar]
- 97.Reardon C, Lechmann M, Brustle A, Gareau MG, Shuman N, Philpott D, Ziegler SF, Mak TW. Thymic stromal lymphopoetin-induced expression of the endogenous inhibitory enzyme SLPI mediates recovery from colonic inflammation. Immunity. 2011;35:223–235. doi: 10.1016/j.immuni.2011.05.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Meier D, Bornmann C, Chappaz S, Schmutz S, Otten LA, Ceredig R, Acha-Orbea H, Finke D. Ectopic lymphoid-organ development occurs through interleukin 7-mediated enhanced survival of lymphoid-tissue-inducer cells. Immunity. 2007;26:643–654. doi: 10.1016/j.immuni.2007.04.009. [DOI] [PubMed] [Google Scholar]
- 99.Luther SA, Ansel KM, Cyster JG. Overlapping roles of CXCL13, interleukin 7 receptor alpha, and CCR7 ligands in lymph node development. J Exp Med. 2003;197:1191–1198. doi: 10.1084/jem.20021294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Kim MY, Anderson G, White A, Jenkinson E, Arlt W, Martensson IL, Erlandsson L, Lane PJ. OX40 ligand and CD30 ligand are expressed on adult but not neonatal CD4+CD3- inducer cells: evidence that IL-7 signals regulate CD30 ligand but not OX40 ligand expression. J Immunol. 2005;174:6686–6691. doi: 10.4049/jimmunol.174.11.6686. [DOI] [PubMed] [Google Scholar]
- 101.Yoshida H, Naito A, Inoue J, Satoh M, Santee-Cooper SM, Ware CF, Togawa A, Nishikawa S. Different cytokines induce surface lymphotoxin-alphabeta on IL-7 receptor-alpha cells that differentially engender lymph nodes and Peyer's patches. Immunity. 2002;17:823–833. doi: 10.1016/s1074-7613(02)00479-x. [DOI] [PubMed] [Google Scholar]
- 102.Cupedo T, Crellin NK, Papazian N, Rombouts EJ, Weijer K, Grogan JL, Fibbe WE, Cornelissen JJ, Spits H. Human fetal lymphoid tissue-inducer cells are interleukin 17-producing precursors to RORC+ CD127+ natural killer-like cells. Nat Immunol. 2009;10:66–74. doi: 10.1038/ni.1668. [DOI] [PubMed] [Google Scholar]
- 103.Veiga-Fernandes H, Coles MC, Foster KE, Patel A, Williams A, Natarajan D, Barlow A, Pachnis V, Kioussis D. Tyrosine kinase receptor RET is a key regulator of Peyer's patch organogenesis. Nature. 2007;446:547–551. doi: 10.1038/nature05597. [DOI] [PubMed] [Google Scholar]
- 104.Honda K, Nakano H, Yoshida H, Nishikawa S, Rennert P, Ikuta K, Tamechika M, Yamaguchi K, Fukumoto T, Chiba T, et al. Molecular basis for hematopoietic/mesenchymal interaction during initiation of Peyer's patch organogenesis. J Exp Med. 2001;193:621–630. doi: 10.1084/jem.193.5.621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Chappaz S, Finke D. The IL-7 signaling pathway regulates lymph node development independent of peripheral lymphocytes. J Immunol. 2010;184:3562–3569. doi: 10.4049/jimmunol.0901647. [DOI] [PubMed] [Google Scholar]
- 106.Park LS, Martin U, Garka K, Gliniak B, Di Santo JP, Muller W, Largaespada DA, Copeland NG, Jenkins NA, Farr AG, et al. Cloning of the murine thymic stromal lymphopoietin (TSLP) receptor: Formation of a functional heteromeric complex requires interleukin 7 receptor. J Exp Med. 2000;192:659–670. doi: 10.1084/jem.192.5.659. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Nishikawa S, Honda K, Vieira P, Yoshida H. Organogenesis of peripheral lymphoid organs. Immunol Rev. 2003;195:72–80. doi: 10.1034/j.1600-065x.2003.00063.x. [DOI] [PubMed] [Google Scholar]
- 108.Coles MC, Veiga-Fernandes H, Foster KE, Norton T, Pagakis SN, Seddon B, Kioussis D. Role of T and NK cells and IL7/IL7r interactions during neonatal maturation of lymph nodes. Proc Natl Acad Sci U S A. 2006;103:13457–13462. doi: 10.1073/pnas.0604183103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Dus D, Krawczenko A, Zalecki P, Paprocka M, Wiedlocha A, Goupille C, Kieda C. IL-7 receptor is present on human microvascular endothelial cells. Immunol Lett. 2003;86:163–168. doi: 10.1016/s0165-2478(03)00018-x. [DOI] [PubMed] [Google Scholar]
- 110.Ming J, Zhang Q, Qiu X, Wang E. Interleukin 7/interleukin 7 receptor induce c-Fos/c-Jun-dependent vascular endothelial growth factor-D up-regulation: a mechanism of lymphangiogenesis in lung cancer. Eur J Cancer. 2009;45:866–873. doi: 10.1016/j.ejca.2008.12.006. [DOI] [PubMed] [Google Scholar]
- 111.Ngom PT, Collinson AC, Pido-Lopez J, Henson SM, Prentice AM, Aspinall R. Improved thymic function in exclusively breastfed infants is associated with higher interleukin 7 concentrations in their mothers' breast milk. Am J Clin Nutr. 2004;80:722–728. doi: 10.1093/ajcn/80.3.722. [DOI] [PubMed] [Google Scholar]
- 112.Aspinall R, Prentice AM, Ngom PT. Interleukin 7 from maternal milk crosses the intestinal barrier and modulates T-cell development in offspring. PLoS One. 2011;6:e20812. doi: 10.1371/journal.pone.0020812. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Mendez-Ferrer S, Michurina TV, Ferraro F, Mazloom AR, Macarthur BD, Lira SA, Scadden DT, Ma'ayan A, Enikolopov GN, Frenette PS. Mesenchymal and haematopoietic stem cells form a unique bone marrow niche. Nature. 2010;466:829–834. doi: 10.1038/nature09262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Mourcin F, Breton C, Tellier J, Narang P, Chasson L, Jorquera A, Coles M, Schiff C, Mancini SJ. Galectin-1-expressing stromal cells constitute a specific niche for pre-BII cell development in mouse bone marrow. Blood. 2011;117:6552–6561. doi: 10.1182/blood-2010-12-323113. [DOI] [PubMed] [Google Scholar]
- 115.Iwata M, Graf L, Awaya N, Torok-Storb B. Functional interleukin-7 receptors (IL-7Rs) are expressed by marrow stromal cells: binding of IL-7 increases levels of IL-6 mRNA and secreted protein. Blood. 2002;100:1318–1325. doi: 10.1182/blood-2002-01-0062. [DOI] [PubMed] [Google Scholar]
- 116.Pellegrini M, Calzascia T, Toe JG, Preston SP, Lin AE, Elford AR, Shahinian A, Lang PA, Lang KS, Morre M, et al. IL-7 engages multiple mechanisms to overcome chronic viral infection and limit organ pathology. Cell. 2011;144:601–613. doi: 10.1016/j.cell.2011.01.011. [DOI] [PubMed] [Google Scholar]
- 117.Rihl M, Kellner H, Kellner W, Barthel C, Yu DT, Tak PP, Zeidler H, Baeten D. Identification of interleukin-7 as a candidate disease mediator in spondylarthritis. Arthritis Rheum. 2008;58:3430–3435. doi: 10.1002/art.23998. [DOI] [PubMed] [Google Scholar]
- 118.Hartgring SA, Willis CR, Alcorn D, Nelson LJ, Bijlsma JW, Lafeber FP, van Roon JA. Blockade of the interleukin-7 receptor inhibits collagen-induced arthritis and is associated with reduction of T cell activity and proinflammatory mediators. Arthritis Rheum. 2010;62:2716–2725. doi: 10.1002/art.27578. [DOI] [PubMed] [Google Scholar]
- 119.Weitzmann MN, Roggia C, Toraldo G, Weitzmann L, Pacifici R. Increased production of IL-7 uncouples bone formation from bone resorption during estrogen deficiency. J Clin Invest. 2002;110:1643–1650. doi: 10.1172/JCI15687. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Wittig O, Paez-Cortez J, Cardier JE. Liver sinusoidal endothelial cells promote B lymphopoiesis from primitive hematopoietic cells. Stem Cells Dev. 2010;19:341–350. doi: 10.1089/scd.2009.0300. [DOI] [PubMed] [Google Scholar]
- 121.Felices M, Yin CC, Kosaka Y, Kang J, Berg LJ. Tec kinase Itk in gammadeltaT cells is pivotal for controlling IgE production in vivo. Proc Natl Acad Sci U S A. 2009;106:8308–8313. doi: 10.1073/pnas.0808459106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Zuvich RL, McCauley JL, Oksenberg JR, Sawcer SJ, De Jager PL, Aubin C, Cross AH, Piccio L, Aggarwal NT, Evans D, et al. Genetic variation in the IL7RA/IL7 pathway increases multiple sclerosis susceptibility. Hum Genet. 2010;127:525–535. doi: 10.1007/s00439-010-0789-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Levy Y, Lacabaratz C, Weiss L, Viard JP, Goujard C, Lelievre JD, Boue F, Molina JM, Rouzioux C, Avettand-Fenoel V, et al. Enhanced T cell recovery in HIV-1-infected adults through IL-7 treatment. J Clin Invest. 2009;119:997–1007. doi: 10.1172/JCI38052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Liu X, Leung S, Wang C, Tan Z, Wang J, Guo TB, Fang L, Zhao Y, Wan B, Qin X, et al. Crucial role of interleukin-7 in T helper type 17 survival and expansion in autoimmune disease. Nat Med. 2010;16:191–197. doi: 10.1038/nm.2077. [DOI] [PubMed] [Google Scholar]
- 125.Nanjappa SG, Walent JH, Morre M, Suresh M. Effects of IL-7 on memory CD8 T cell homeostasis are influenced by the timing of therapy in mice. J Clin Invest. 2008;118:1027–1039. doi: 10.1172/JCI32020. [DOI] [PMC free article] [PubMed] [Google Scholar]


