Abstract
Aims
Pannexins (Panx) form ATP-release channels and have been proposed to play an important role in the regulation of vascular tone. However, distribution of Panx across the arterial vasculature is not documented.
Methods
We tested antibodies against Panx1, Panx2 and Panx3 on HEK cells (which do not endogenously express Panx proteins) transfected with plasmids encoding each pannexin isoform and Panx1−/− mice. Each of the Panx antibodies was found to be specific and was tested on isolated arteries using immunocytochemistry.
Results
We demonstrate that Panx1 is the primary isoform detected in the arterial network. In large arteries, Panx1 was primarily in endothelial cells, whereas in small arteries and arterioles Panx1 localizes primarily to the smooth muscle cells. In coronary arteries Panx1 was the predominant isoform expressed, except in arteries less than 100 μm Panx3 became detectable. Only Panx3 was expressed in the juxtaglomerular apparatus and cortical arterioles. The pulmonary artery and alveoli had expression of all three Panx isoforms. No Panx isoforms were detected at the myoendothelial junctions.
Conclusion
We conclude that the specific localized expression of Panx channels throughout the vasculature points towards an important role for these channels in regulating the release of ATP throughout the arterial network.
Keywords: pannexins, vasculature, arteries, endothelium, smooth muscle
Introduction
Pannexins (Panx) are a class of glycoproteins that oligomerize to form channels at the plasma membrane [1–3]. Pannexin proteins are found in three different isoforms (Panx1, Panx2 and Panx3) in cultured cells and in vivo. To date, Panx1 has been characterized extensively and has been shown to be ubiquitously expressed. Conversely, Panx2 expression has been found primarily in the central nervous system [4–6] and Panx3 is mainly expressed in the skin, osteoblast and chondrocytes [7–9]. While Panx proteins have been shown to possess a similar membrane topology to the vertebrate gap junction proteins, the connexins, there are key differences in their respective functions within the cell. One of the most important differences is that connexins allow for a direct communication between two cells by forming gap junction channels through the docking of two connexin hemichannels, or connexons, whereas the formation of gap junctions by the docking of two Panx channels has never been demonstrated in vivo [10]. Another key difference is the ability of Panx channels to open and release ATP into the extracellular space under physiological extracellular calcium concentration, whereas hexameric connexin hemichannels present at the plasma membrane have been shown to be closed under these conditions and open when extracellular calcium concentration is reduced [11–13]. Therefore, since their first description in 2000, Panx channels have been suggested to act as paracrine release channels that are strongly implicated in the release of purine nucleotides from cells [3, 14–17].
The role of extracellular purines, including ATP, in the systemic circulation has been shown to be important for several vascular functions including the regulation of vascular tone [18, 19], reactive hyperemia during contraction of skeletal muscle [20, 21] and hypoxia-induced vasodilation [22–24]. Although there are well-described reports of ATP release occurring from both circulating erythrocytes and sympathetic nerves innervating vascular smooth muscle cells [16, 23, 25–31], there is accumulating evidence indicating that endothelial and smooth muscle cells of the vascular wall can also release ATP [24, 32–37]. The conduit for ATP release from these cells continues to be investigated, but several reports suggest that Panx channels may be an important candidate. Indeed, we have recently demonstrated that both smooth muscle and endothelial cells in small arteries express Panx1 and our results showed that vascular smooth muscle cells can release ATP through Panx1 channels [36]. However, the distribution of Panx isoforms across the vasculature is not yet known. This is an important omission considering the potential role these channels may have in the vasculature, where ATP and its breakdown products have been documented to have tremendous physiological importance for decades [21, 30, 38–47]. Importantly, Panx channels in endothelial cells could play essential roles in ATP signaling in the blood vessel lumen which could potentially include vasodilation and monocyte recruitment. Alternatively, expression of Panx channels in smooth muscle cells may be involved in purinergic signaling such as the regulation of vasoconstriction or vascular smooth muscle cell proliferation [48, 49]. Together, the expression of these channels in vascular cells could play important roles in regulating a number of physiological processes.
As blood vessels across the arterial tree experience a variety of different environments, identification of how ATP is released from the endothelial and smooth muscle cells of different arterial beds is essential for understanding how purinergic signaling is regulated in the control of vascular tone in both blood pressure regulation and maintenance of proper organ physiology. Therefore, as Panx proteins have been strongly implicated in ATP release from cells, we sought to characterize the expression of the different Panx isoforms across the vasculature to help provide a more detailed understanding of the potential ATP release mechanisms in these cells.
Methods
HEK293T Cells
Human embryonic kidney cells 293T (HEK293T) were used under passage 20 and grown in DMEM supplemented with 10% FBS, 1% penicillin/streptomycin, 1% L-glutamine and 1% non-essential amino acids. Cells were transfected with mouse Panx1 (pcDNA3.1), mouse Panx2 (pEGFP-N1 [50]) or mouse Panx3 (pEGFP-N1 [50]) plasmids at 750 ng/mL using 8 μg Lipofectamine 2000 on 6×105 cells for 15 hours. Cells were washed with PBS and were either fixed with 4% paraformaldehyde before being processed for immunocytochemistry (as previously described [51]) or protein was extracted for Western blot analysis.
Western blot
Western blots were performed as described previously [51]. For all experiments, 50 μg of protein was loaded into each well.
qRT-PCR
qRT-PCR was performed as described previously [52] with primers for mouse and human Panx1 (mouse forward 5′-TAAGCTGCTTCTCCCCGAGT-3′, mouse reverse 5′-TGGCAAACAGCAGTAGGATG-3′; human forward 5′-TGCAGAGCGAGTCTGGAAAC-3′, human reverse 5′CAGCCTTAATTGCACGGTTG-3′) mouse and human Panx2 (mouse forward 5′AAGCATACCCGCCACTTCTC-3′, mouse reverse 5′-GGGGTACGGGATTTCCTTCT-3′; human forward 5′-GTCACCCTGGTCTTCACCAA-3′, human reverse 5′-GCAGGAACTTGTGCTCAAACA-3′) or mouse and human Panx3 (mouse forward 5′-CCCATTCTCAGCAGCATCAT-3′, mouse reverse 5′-ACTCCTGGGCGAAAGCTAGA-3′; human forward 5′-CTCAAAGGACTGCGTCTGGA-3′, human reverse 5′-CTGCCGGATGCTGAAGTTAC-3′). Transcripts were quantified by comparing to the housekeeping gene β2 microglobulin (B2M) [53].
Animals
All mice were male, 10–14 weeks of age, on a C57Bl/6 genetic background and were cared for under the provisions of the University of Virginia Animal Care and Use Committee. The Panx1−/− mice were kind gifts of Genentech Corporation and were cared for under provisions of the University of Western Ontario Animal Care and Use Committee. The Panx1−/− mouse tissue samples were harvested at the University of Western Ontario and sent to the University of Virginia for analysis. All experiments were performed on a minimum of n=3 mice.
Embedding and immunocytochemistry
Prior to tissue harvesting, fixation was performed by perfusing room temperature 4% paraformaldehyde (PFA) made in PBS through the heart. The specific tissues were immediately removed from the animal and placed in 4% PFA for 30 minutes before being placed in 70% ethanol for paraffin embedding. Paraffin sections (4–5 μm in thickness) were de-paraffinized and processed for immunocytochemistry as previously described [36].
Imaging
All images were obtained with an Olympus Fluoview 1000. For each vessel bed, a minimum of 5 sections from each mouse, with a minimum of 3 mice, were evaluated. Figures are representative of composite z-stacks. For z-stacks, a minimum of 5 images at a depth of 0.5 μm/image were compiled. Although each of the different pannexin isoforms was scanned at different settings, the individual pannexin isoforms were scanned at the same settings across tissue beds.
Antibodies
Each of the pannexin antibodies were made in rabbit and initially described in [50]. The Panx1 antibodies were made against amino acids 247–265 (SIKSGVLKNDSTIPDRFQC) in the extracellular loop (anti-Panx1 EL [50]) or amino acids 395–409 (QRVEFKDLDLSSEAA) in the carboxyl tail (anti-Panx1 CT [50]) The Panx2 antibody was made against amino acids 494–508 (ASEKKHTRHFSLDVH) and the Panx3 antibody was made against amino acids 379–392 (KPKHLTQHTYDEHA). AlexaFluor 594 donkey anti-rabbit secondary antibodies were used to observe all primary antibodies. Nuclei were stained with DAPI (Invitrogen). At least five observations were made per mouse, per experiment.
Electron Microscopy
All tissues were processed and stained for immunogold-transmission electron microscopy as previously described [54]. Quantification of gold beads in endothelium (EC), at the myoendothelial junction (MEJ), or vascular smooth muscle cells (VSMC) was performed as previously described [54] on five TEM images per artery that were sectioned 10 μm apart. Using Metamorph analysis software, areas of EC, MEJ and VSMC were determined in μm2 and the number of gold beads in each area was counted. Measurements are representative of the average number of gold beads per μm2 ± SE. For each vessel bed, a minimum of 4 observation from each mouse, with a minimum of 3 mice, were evaluated.
En face imaging of MEJs
The thoracodorsal artery (TDA) was maximally dilated by whole-mouse perfusion with Krebs-HEPES buffer (in mM: 118.4 NaCl, 4.7 KCl, 1.2 MgSO4, 4 NaHCO3, 1.2 KH2PO4, 2 CaCl2, 10 N-2-hydroxyethylpiperazine-N′-2-ethanesu lfonicacid (HEPES) and 6 D-glucose, pH 7.4 with NaOH) containing sodium nitroprusside followed by fixation with 4% PFA. The arteries were removed, cut longitudinally and conventional immunocytochemistry was performed (as described above). The arteries were mounted in DAPI and coverslip sealed to provide a flat surface for confocal imaging. Autofluorescence of the internal elastic lamina between the endothelium and smooth muscle was readily apparent with “holes” between the two layers of cells which has been extensively documented (e.g., [55–57]). In our study, the detection of punctate fluorescence in >25% of the “holes” was considered indicative of protein localization to MEJs.
Results
Initially HEK293T cells were tested for endogenous Panx expression by quantitative RT-PCR for detection of mRNA (Figure 1A) or by Western blot for detection of protein (Figure 1B). There was no detectable Panx3 mRNA, with minimal amounts of Panx1 and Panx2 mRNA as compared to the housekeeping gene B2M [53]; however, no endogenous protein was detected for any of the Panx isoforms in these cells. We therefore transfected the HEK293T cells with plasmids encoding murine Panx1, Panx2 or Panx3. Western blot analysis of HEK293T cells transfected with the Panx plasmids revealed specific bands for each Panx isoform when probed with the respective Panx antibody (Figure 1B). Using immunocytochemistry, we found expression of each transfected Panx isoform at the plasma membrane (Figure 1C–E), as well as intracellular staining for Panx2 which has been shown in a number of cell types, again demonstrating the specificity of the Panx antibodies.
Figure 1. Specificity of pannexin antibodies in cultured cells.
HEK293T cells were tested for endogenous pannexin expression by mRNA via RT-PCR (A) and by protein via Western blot (B). In A, mRNA expression is normalized to the housekeeping gene β2 microglobulin (B2M). The HEK cells were transfected with Panx1, Panx2 or Panx3 plasmids, stained using anti-Panx1 CT antibody, anti-Panx1 EL antibody, anti-Panx2 antibody or anti-Panx3 antibody and detected with anti-rabbit IRDye 800CW (LiCOR) for Western blots (B) or anti-rabbit Alexa 594 for immunofluorescence (C–E). In addition, for immunofluorescence the antibody corresponding to the transfected pannexin isoform was incubated with its respective blocking peptide for negative controls. In C–E, blue are DAPI stained nuclei and red is the expression of each pannexin; scale bar is 10 μm in unstained images and 5 μm in stained images.
Next we tested the specificity of the Panx1 antibodies on thoracodorsal arteries (TDA) as we previously reported that this is the only isoform expressed in the TDA wall [36]. Using TDA harvested from C57Bl/6 mice, we found consistent staining in both endothelial cells and vascular smooth muscle cells using both the anti-Panx1 CT and anti-Panx1 EL antibodies (Figure 2A). We could not detect any Panx1 in TDAs isolated from the Panx1−/− mouse (Figure 2B), again demonstrating the specificity of the Panx1 antibodies.
Figure 2. Specificity of Panx1 antibodies in arteries.
The anti-Panx1 antibodies were tested on mouse TDA from C57Bl/6 (A) and Panx1−/− mice (B). Blue are DAPI stained nuclei, green is autofluorescence of internal elastic lamina and red is pannexin staining. Scale bar in each image is 20 μm and asterisks indicate the lumen of the artery.
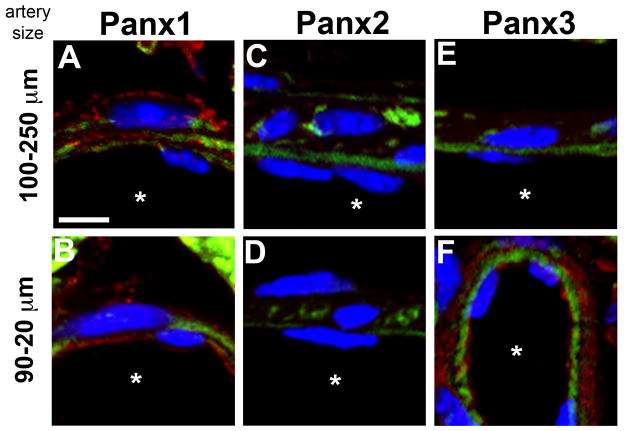
Because we determined the Panx antibodies to be specific and Panx1 to be endogenously expressed in vascular cells of the TDA, we tested for Panx isoform expression through the systemic arterial tree, beginning in the aorta (Figure 3A), then carotid artery (Figure 3B), femoral artery (Figure 3C), renal artery (Figure 3D), TDA (Figure 3E), abdominal arteries (Figure 3F), arterioles in the spinotrapezius muscle (Figure 3G), and finally cremasteric arterioles (Figure 3H). Throughout the arterial tree, Panx1 was the only protein detected and was consistently expressed in endothelium regardless of artery size. However, the expression of Panx1 in smooth muscle cells was poorly detectable in larger conduit arteries (aorta/carotid/femoral). Some expression of Panx1 in smooth muscle cells was detected in renal arteries, whereas in smaller arteries including TDA, abdominal artery, spinotrapezius arterioles and cremasteric arterioles, Panx1 was found throughout the smooth muscle. Similar to the smaller systemic arteries, both small (luminal diameter of 20–90 μm) and large (luminal diameter of 100–250 μm) coronary arteries also expressed Panx1 in endothelium and smooth muscle cells (Figure 4A–B) and no Panx2 expression (Figure 4C–D). Interestingly, in coronary arteries with a luminal diameter less than 100 μm, Panx3 was detected in endothelium and smooth muscle (Figure 4E–F).
Figure 3. Pannexin expression in the murine systemic arterial network.
In A–H, each Panx antibody was tested on arteries of progressively decreasing size starting with the aorta (A), then carotid artery (B), femoral artery (C), renal artery (D), TDA (E), abdominal artery (F), an arteriole from the spinotrapezius muscle (G) and finally a cremasteric arteriole (H). Blue are DAPI stained nuclei, green is autofluorescence of internal elastic lamina and red is pannexin staining. Scale bar in each image is 10 μm and is representative for the row of staining; asterisks indicate the lumen of the artery.
Figure 4. Pannexin expression in the coronary arteries.
Coronary arteries that were between 100–250 μm in diameter (A, C, E) or 20–90 μm in diameter (B, D, F) were assessed for expression of Panx1 (A–B), Panx2 (C–D) or Panx3 (E–F). In each image, blue is DAPI stained nuclei, green is autofluorescence of the internal elastic lamina and red is pannexin staining. Scale bar is 5 μm and representative for all images; asterisks indicate the artery lumen.
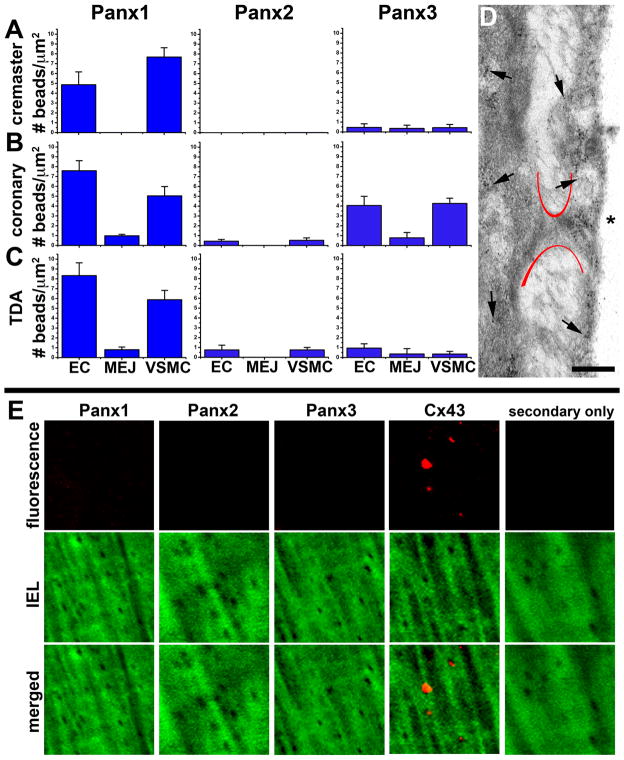
Next we tested whether Panx were expressed at MEJs, where endothelium and smooth muscle make contact in small arteries and arterioles [58, 59]. Using immuno-TEM, we quantified the amount of each Panx isoform expressed in the coronary, cremasteric and TDA and found little detectable Panx expression at myoendothelial junctions in selected arteries (Figure 5A–D). While immuno-TEM can be a valuable method for quantifying the distribution of proteins at distinct subcellular locations within tissues at high resolution, this method has its limitations and results can potentially be confounded by non-uniform antigen distribution across sections. Therefore, we also analyzed Panx isoform expression at the MEJ by performing whole-mount immunocytochemistry on isolated TDA that were fixed in a maximally dilated state to facilitate visualization of MEJs at IEL holes. This method allows for visualization of large areas of antigen distribution. In agreement with our immuno-TEM data, there were no pannexin isoforms detectable; however, Cx43, as previously found to be located at MEJs via quantified immuno-TEM [60]; as used above), was localized to these holes (Figure 5E).
Figure 5. Minimal pannexin expression at myoendothelial junctions.
In A–C, the number of gold beads after staining with Panx1, Panx2 or Panx3 per μm2 in endothelial cells (EC), MEJ and vascular smooth muscle (VSMC) was quantified in cremaster arterioles (20–40 μm; A), coronary arteries (50–100 μm; B), and TDAs (200 μm; C). In D, representative immuno-TEM from coronary arterioles is shown with gold beads representing Panx1. Asterisks indicate the artery lumen and arrows indicate representative location of gold beads. Scale bar is 0.5 μm. In E, holes in the internal elastic lamina from TDAs, corresponding to possible MEJs, were examined for protein expression. Green is autofluorescence of the internal elastic lamina and red is the protein of interest. Only when Cx43 antibody was used punctate fluorescence could be detected in the holes. Each image is 15 μm × 15 μm.
We also examined Panx expression in the arterial network of the kidney (Figure 6). We did not detect any Panx1 or Panx2 in the juxtaglomerular apparatus, (Figure 6A–B), but Panx3 was found throughout the unit in very distinct punctate stains (Figure 6C), which was not detectable when incubated with the Panx3 peptide (Figure 6D). This same pattern of expression was found in arterioles of the cortical kidney with only Panx3 being detectable in the endothelium, with more limited expression in smooth muscle cells (Figure 6E–G), which was again absent when incubated with the Panx3 peptide (Figure 6H).
Figure 6. Pannexin expression in the kidney.
In A–D, the glomerulus of the kidney was stained for Panx1 (A), Panx2 (B) and Panx3 (C), with Panx3 peptide competition shown in D. In E–H, arterioles of the cortical kidney were stained for Panx1 (E), Panx2 (F) and Panx3 (G), with Panx3 peptide competition shown in H. In all images, pannexins are in red and blue represents DAPI stained nuclei. Scale bar in A is 20 μm and representative for all images.
Lastly, as a contrast to the systemic circulation, we examined Panx expression in the pulmonary circulation (Figure 7). The first order intrapulmonary artery had positive staining for Panx1 in the endothelium and smooth muscle, although there was some Panx1 staining in the IEL. It is not clear why staining would be in the IEL but the most likely reason is Z-stack compression. Panx2 was expressed in the smooth muscle cells with some detectable Panx2 in endothelial cells (Figure 7A–B). Peptide competition eliminated the Panx2 staining (Figure 7C). The expression of Panx3 was predominantly in the endothelium, but there remained some detectable expression in the smooth muscle cells (Figure 7D). In the distal lung, Panx1 and Panx3 were the dominant isoforms expressed, with some detectable Panx2 (Figure 7E–H). Of particular note in the alveoli, it appeared that the pannexins could also be localized around the nuclei.
Figure 7. Pannexin expression in the pulmonary artery and lung alveoli.
The pulmonary artery (lumen diameter approximately 400 μm) was stained for each of the pannexin isoforms in A–D. In C, Panx2 peptide competition is shown. The asterisks indicate the luminal side of the arterial wall. In E–H, the distal lung alveoli were also stained with each of the pannexin isoforms, with G showing Panx2 peptide competition. In all images, green is autofluorescence of matrix proteins and red blood cells (E–H), red is the pannexin isoform of interest, and blue represents DAPI stained nuclei. Scale bars in A and E are both 20 μm.
Discussion
Pannexins form plasma membrane channels capable of releasing ATP that provide key physiological functions including regulation of apoptosis and has a potential role in regulation of vasoconstriction by ATP release mechanisms [36, 61]. For this reason, we sought to identify whether the various Panx isoforms were expressed across the arterial vasculature and to describe their expression pattern in several vascular beds. We began by extensively testing each of the Panx antibodies on HEK cells which do not endogenously express Panx proteins, although there was some residual Panx mRNA detectable. After transfection of each Panx isoform in HEK cells, we found reactivity for each Panx isoform with corresponding Panx antibodies by Western blotting and immunocytochemistry.
In the blood vessel wall, endothelial cells and vascular smooth muscle cells are in constant communication both within and often between cell types through multiple signaling mechanisms. One such signaling molecule is ATP, which has been implicated in the control of vasoconstriction and vasodilation [41, 42, 44, 62]. Subsequently, ATP sensitive purinergic receptors in vascular endothelial and smooth muscle cells have been extensively characterized [62–66]. In endothelial cells, ATP binding to P2Y purinergic receptors has been shown to mediate vasodilation, whereas in vascular smooth muscle cells, ATP-sensitive P2X purinergic receptors have been shown to control vasoconstriction [62, 64–66]. While much work has been done characterizing the functional role of extracellular ATP in the vasculature, the conduit for its release from the cells in the arterial wall is a current topic of investigation. As early studies on Panx channels identified the potential for these membrane proteins to release ATP into the extracellular space, their expression in vascular cells may provide critical insight into the regulation of purinergic signaling at the level of purine release from these cells.
Our observation that Panx1 is expressed in endothelial cells across the arterial tree may suggest that this protein is intimately involved in the release of ATP into the blood vessel lumen and thus may be involved in modulating the extent of vasodilation in response to stimuli such as increases in blood flow and hypoxia, which are known to cause ATP release from endothelial cells [22, 24, 35, 67]. At the smooth muscle cell axis, we have previously suggested a pivotal role for ATP release from Panx1 channels in regulating vasoconstriction in response to stimulation of α1D-adrenergic receptors [36]. The observation that Panx isoforms are highly expressed in smooth muscle cells of small arteries and arterioles, versus selected larger conduit arteries, may suggest a role for these channels in the regulation of peripheral resistance by ATP release mechanisms, as small arterioles have the highest resistance and contribute greatest to total peripheral resistance [68]. While we detected very little Panx expression in smooth muscle cells in large arteries, we did find expression of Panx1 in the endothelial cell layer of these arteries. It is possible that Panx1 channels in endothelial cells across the vasculature may also play a role in mediating the inflammatory response, where ATP released into the vascular lumen has been shown to promote monocyte and macrophage recruitment and migration into the vascular intima [69–72]. With the possibility that Panx channels in the vasculature may play dual roles in regulating blood pressure as well as pathological events such as inflammation, it will be important to identify potential binding partners and regulatory mechanisms that control the gating of these channels and thus ATP release from vascular cells.
Although the main arteries of the systemic arterial vascular tree had only Panx1 expressed, be it in endothelium or smooth muscle, the specialized organs had more variability in terms of the other Panx isoforms expressed. For example, while Panx1 was the main isoform expressed in the coronary circulation, we observed the expression of Panx3 in coronary arteries with an internal diameter less than 100 μm. This is the first evidence for Panx3 expression in vascular cells and may suggest that this isoform plays a special role in cardiac physiology. The coronary arteries play an important role in oxygen delivery to cardiac tissue which has an extremely high metabolic demand and changes in coronary blood flow, and thus changes in oxygen delivery to myocardial tissue, can lead to alterations in myocardial metabolism and ultimately cardiac output. Therefore, changes in coronary blood flow could have profound effects on blood pressure and it is possible that these arteries may require more dynamic ATP release mechanisms to facilitate rapid changes in blood flow to cardiac tissue to match oxygen demand. It will be interesting in the future to determine whether coronary blood flow may be influenced by the expression of the different Panx isoforms in these arteries. Along with Panx1, Panx3 has also been shown to release ATP and expression of this isoform along with Panx1 in critically small arterioles in the coronary circulation may provide additional channels for ATP release. This observation warrants further investigation into the role of Panx channels in the regulation of coronary blood flow, the dynamics of channels composed of Panx3 and whether heteromeric channels can be formed by Panx1 and Panx3 [6, 73].
In the renal vasculature, we observed sole expression of Panx3 in both the juxtaglomerular apparatus as well as in the endothelium of arteries in the cortical kidney, with no detection of Panx1 and Panx2. This was the sole vascular bed in which we did not observe Panx1 expression, which may suggest that the different Panx isoforms may play specific roles in different vascular beds and that the renal vasculature may utilize purinergic signaling cascades distinct from other arterial beds across the arterial tree. This observation may also suggest, as with the coronary circulation, that the different Panx isoforms in the arteries supplying these tissues may play distinct roles in regulating ATP release in different physiological environments that are subject to unique interstitial and intraluminal pressures.
When we examined the pulmonary circulation, we observed expression of Panx1 in both endothelial and smooth muscle cells of the first order intrapulmonary artery while Panx2 was found primarily in the smooth muscle and Panx3 primarily in the endothelium. In the distal lung, we observed expression of Panx1 and Panx3 in the alveoli with Panx2 expressed in a perinuclear fashion.
Lastly, it should be noted that all of the images described in this manuscript were from paraffin embedded tissue blocks. This presents its own unique set of potential issues, including the well-described lack of good signal-to-noise for immunofluorescence and poor antigenicity (as compared to unfixed frozen sections). Although these may be issues, the embedding allowed us to compare directly all the tissue beds and have a consistent amount of antibody applied to each section and consistent setting on the microscope. While these conditions can also be met with frozen tissue sections, the morphology of the tissue is often compromised by sectioning of frozen sections.
In conclusion, the consistent observation from our data was 1. pannexins are expressed throughout the arterial tree, 2. Panx1 is the predominant isoform in the arterial network, 3. Panx1 is predominantly expressed in the endothelium throughout the arterial network and 4. smooth muscle cells in large conduit arteries express less Panx as compared to the small arteries, where Panx1 is found to be abundantly expressed. Further studies on the ways in which these channels are utilized should provide fascinating new data on the regulation of ATP release in the vasculature.
Acknowledgments
We thank the University of Virginia Histology Core for sectioning, Jan Redick and Stacey Guillot at the University of Virginia Advanced Microscopy Core and Genentech Inc for the use of the Panx1−/− mice. This work was supported by National Institutes of Health grant HL088554 (B.E.I.), American Heart Association Scientist Development Grant (B.E.I.), an American Heart Association postdoctoral fellowship (M.B., S.R.J.), a National Research Science Award postdoctoral fellowship from the National Institutes of Health (A.C.S.), a National Institutes of Health Cardiovascular Training Grant (A.W.L.) and the Canadian Institutes of Health Research (D.W.L.).
Footnotes
Declarations
None.
Literature Cited
- 1.Panchin YV. Evolution of gap junction proteins--the pannexin alternative. The Journal of experimental biology. 2005;208:1415–1419. doi: 10.1242/jeb.01547. [DOI] [PubMed] [Google Scholar]
- 2.Baranova A, Ivanov D, Petrash N, Pestova A, Skoblov M, Kelmanson I, Shagin D, Nazarenko S, Geraymovych E, Litvin O, Tiunova A, Born TL, Usman N, Staroverov D, Lukyanov S, Panchin Y. The mammalian pannexin family is homologous to the invertebrate innexin gap junction proteins. Genomics. 2004;83:706–716. doi: 10.1016/j.ygeno.2003.09.025. [DOI] [PubMed] [Google Scholar]
- 3.Bao L, Locovei S, Dahl G. Pannexin membrane channels are mechanosensitive conduits for atp. FEBS Lett. 2004;572:65–68. doi: 10.1016/j.febslet.2004.07.009. [DOI] [PubMed] [Google Scholar]
- 4.Ray A, Zoidl G, Weickert S, Wahle P, Dermietzel R. Site-specific and developmental expression of pannexin1 in the mouse nervous system. Eur J Neurosci. 2005;21:3277–3290. doi: 10.1111/j.1460-9568.2005.04139.x. [DOI] [PubMed] [Google Scholar]
- 5.Santiago MF, Veliskova J, Patel NK, Lutz SE, Caille D, Charollais A, Meda P, Scemes E. Targeting pannexin1 improves seizure outcome. PloS one. 2011;6:e25178. doi: 10.1371/journal.pone.0025178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Bruzzone R, Hormuzdi SG, Barbe MT, Herb A, Monyer H. Pannexins, a family of gap junction proteins expressed in brain. Proc Natl Acad Sci U S A. 2003;100:13644–13649. doi: 10.1073/pnas.2233464100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Barbe MT, Monyer H, Bruzzone R. Cell-cell communication beyond connexins: The pannexin channels. Physiology (Bethesda) 2006;21:103–114. doi: 10.1152/physiol.00048.2005. [DOI] [PubMed] [Google Scholar]
- 8.Bond SR, Lau A, Penuela S, Sampaio AV, Underhill TM, Laird DW, Naus CC. Pannexin 3 is a novel target for runx2, expressed by osteoblasts and mature growth plate chondrocytes. Journal of bone and mineral research : the official journal of the American Society for Bone and Mineral Research. 2011;26:2911–2922. doi: 10.1002/jbmr.509. [DOI] [PubMed] [Google Scholar]
- 9.Penuela S, Celetti SJ, Bhalla R, Shao Q, Laird DW. Diverse subcellular distribution profiles of pannexin 1 and pannexin 3. Cell Commun Adhes. 2008;15:133–142. doi: 10.1080/15419060802014115. [DOI] [PubMed] [Google Scholar]
- 10.Dahl G, Locovei S. Pannexin: To gap or not to gap, is that a question? IUBMB life. 2006;58:409–419. doi: 10.1080/15216540600794526. [DOI] [PubMed] [Google Scholar]
- 11.Pfahnl A, Dahl G. Gating of cx46 gap junction hemichannels by calcium and voltage. Pflugers Arch. 1999;437:345–353. doi: 10.1007/s004240050788. [DOI] [PubMed] [Google Scholar]
- 12.Quist AP, Rhee SK, Lin H, Lal R. Physiological role of gap-junctional hemichannels. Extracellular calcium-dependent isosmotic volume regulation. The Journal of cell biology. 2000;148:1063–1074. doi: 10.1083/jcb.148.5.1063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Li H, Liu TF, Lazrak A, Peracchia C, Goldberg GS, Lampe PD, Johnson RG. Properties and regulation of gap junctional hemichannels in the plasma membranes of cultured cells. The Journal of cell biology. 1996;134:1019–1030. doi: 10.1083/jcb.134.4.1019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Qiu F, Dahl G. A permeant regulating its permeation pore: Inhibition of pannexin 1 channels by atp. Am J Physiol Cell Physiol. 2009;296:C250–255. doi: 10.1152/ajpcell.00433.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Ransford GA, Fregien N, Qiu F, Dahl G, Conner GE, Salathe M. Pannexin 1 contributes to atp release in airway epithelia. American journal of respiratory cell and molecular biology. 2009;41:525–534. doi: 10.1165/rcmb.2008-0367OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Sridharan M, Adderley SP, Bowles EA, Egan TM, Stephenson AH, Ellsworth ML, Sprague RS. Pannexin 1 is the conduit for low oxygen tension-induced atp release from human erythrocytes. Am J Physiol Heart Circ Physiol. 2011;299:H1146–1152. doi: 10.1152/ajpheart.00301.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Woehrle T, Yip L, Elkhal A, Sumi Y, Chen Y, Yao Y, Insel PA, Junger WG. Pannexin-1 hemichannel-mediated atp release together with p2x1 and p2x4 receptors regulate t-cell activation at the immune synapse. Blood. 2010;116:3475–3484. doi: 10.1182/blood-2010-04-277707. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Kauffenstein G, Drouin A, Thorin-Trescases N, Bachelard H, Robaye B, D’Orleans-Juste P, Marceau F, Thorin E, Sevigny J. Ntpdase1 (cd39) controls nucleotide-dependent vasoconstriction in mouse. Cardiovascular research. 2010;85:204–213. doi: 10.1093/cvr/cvp265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Kauffenstein G, Furstenau CR, D’Orleans-Juste P, Sevigny J. The ecto-nucleotidase ntpdase1 differentially regulates p2y1 and p2y2 receptor-dependent vasorelaxation. Br J Pharmacol. 2010;159:576–585. doi: 10.1111/j.1476-5381.2009.00566.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Forrester T, Lind AR. Identification of adenosine triphosphate in human plasma and the concentration in the venous effluent of forearm muscles before, during and after sustained contractions. J Physiol. 1969;204:347–364. doi: 10.1113/jphysiol.1969.sp008917. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Mortensen SP, Gonzalez-Alonso J, Bune LT, Saltin B, Pilegaard H, Hellsten Y. Atp-induced vasodilation and purinergic receptors in the human leg: Roles of nitric oxide, prostaglandins, and adenosine. Am J Physiol Regul Integr Comp Physiol. 2009;296:R1140–1148. doi: 10.1152/ajpregu.90822.2008. [DOI] [PubMed] [Google Scholar]
- 22.Bodin P, Burnstock G. Synergistic effect of acute hypoxia on flow-induced release of atp from cultured endothelial cells. Experientia. 1995;51:256–259. doi: 10.1007/BF01931108. [DOI] [PubMed] [Google Scholar]
- 23.Bergfeld GR, Forrester T. Release of atp from human erythrocytes in response to a brief period of hypoxia and hypercapnia. Cardiovascular research. 1992;26:40–47. doi: 10.1093/cvr/26.1.40. [DOI] [PubMed] [Google Scholar]
- 24.Bodin P, Bailey D, Burnstock G. Increased flow-induced atp release from isolated vascular endothelial cells but not smooth muscle cells. Br J Pharmacol. 1991;103:1203–1205. doi: 10.1111/j.1476-5381.1991.tb12324.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Sneddon P, Burnstock G. Atp as a co-transmitter in rat tail artery. Eur J Pharmacol. 1984;106:149–152. doi: 10.1016/0014-2999(84)90688-5. [DOI] [PubMed] [Google Scholar]
- 26.Sprague RS, Ellsworth ML, Stephenson AH, Kleinhenz ME, Lonigro AJ. Deformation-induced atp release from red blood cells requires cftr activity. Am J Physiol. 1998;275:H1726–1732. doi: 10.1152/ajpheart.1998.275.5.H1726. [DOI] [PubMed] [Google Scholar]
- 27.Sneddon P, Burnstock G. Inhibition of excitatory junction potentials in guinea-pig vas deferens by alpha, beta-methylene-atp: Further evidence for atp and noradrenaline as cotransmitters. Eur J Pharmacol. 1984;100:85–90. doi: 10.1016/0014-2999(84)90318-2. [DOI] [PubMed] [Google Scholar]
- 28.Burnstock G. Noradrenaline and atp as cotransmitters in sympathetic nerves. Neurochem Int. 1990;17:357–368. doi: 10.1016/0197-0186(90)90158-p. [DOI] [PubMed] [Google Scholar]
- 29.Sneddon P, Westfall DP. Pharmacological evidence that adenosine triphosphate and noradrenaline are co-transmitters in the guinea-pig vas deferens. J Physiol. 1984;347:561–580. doi: 10.1113/jphysiol.1984.sp015083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Sprague RS, Hanson MS, Achilleus D, Bowles EA, Stephenson AH, Sridharan M, Adderley S, Procknow J, Ellsworth ML. Rabbit erythrocytes release atp and dilate skeletal muscle arterioles in the presence of reduced oxygen tension. Pharmacol Rep. 2009;61:183–190. doi: 10.1016/s1734-1140(09)70020-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Lew MJ, White TD. Release of endogenous atp during sympathetic nerve stimulation. Br J Pharmacol. 1987;92:349–355. doi: 10.1111/j.1476-5381.1987.tb11330.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Bodin P, Burnstock G. Atp-stimulated release of atp by human endothelial cells. J Cardiovasc Pharmacol. 1996;27:872–875. doi: 10.1097/00005344-199606000-00015. [DOI] [PubMed] [Google Scholar]
- 33.Moser TL, Kenan DJ, Ashley TA, Roy JA, Goodman MD, Misra UK, Cheek DJ, Pizzo SV. Endothelial cell surface f1-f0 atp synthase is active in atp synthesis and is inhibited by angiostatin. Proc Natl Acad Sci U S A. 2001;98:6656–6661. doi: 10.1073/pnas.131067798. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Bodin P, Burnstock G. Evidence that release of adenosine triphosphate from endothelial cells during increased shear stress is vesicular. J Cardiovasc Pharmacol. 2001;38:900–908. doi: 10.1097/00005344-200112000-00012. [DOI] [PubMed] [Google Scholar]
- 35.Yamamoto K, Shimizu N, Obi S, Kumagaya S, Taketani Y, Kamiya A, Ando J. Involvement of cell surface atp synthase in flow-induced atp release by vascular endothelial cells. Am J Physiol Heart Circ Physiol. 2007;293:H1646–1653. doi: 10.1152/ajpheart.01385.2006. [DOI] [PubMed] [Google Scholar]
- 36.Billaud M, Lohman AW, Straub AC, Looft-Wilson R, Johnstone SR, Araj CA, Best AK, Chekeni FB, Ravichandran KS, Penuela S, Laird DW, Isakson BE. Pannexin1 regulates alpha1-adrenergic receptor- mediated vasoconstriction. Circ Res. 2011;109:80–85. doi: 10.1161/CIRCRESAHA.110.237594. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Goedecke S, Roderigo C, Rose CR, Rauch BH, Goedecke A, Schrader J. Thrombin-induced atp release from human umbilical vein endothelial cells. American journal of physiology Cell physiology. 2011 doi: 10.1152/ajpcell.00283.2010. [DOI] [PubMed] [Google Scholar]
- 38.Mustafa SJ, Morrison RR, Teng B, Pelleg A. Adenosine receptors and the heart: Role in regulation of coronary blood flow and cardiac electrophysiology. Handb Exp Pharmacol. 2009:161–188. doi: 10.1007/978-3-540-89615-9_6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Berne RM. Cardiac nucleotides in hypoxia: Possible role in regulation of coronary blood flow. Am J Physiol. 1963;204:317–322. doi: 10.1152/ajplegacy.1963.204.2.317. [DOI] [PubMed] [Google Scholar]
- 40.Burnstock G. Control of vascular tone by purines and pyrimidines. Br J Pharmacol. 2010;161:527–529. doi: 10.1111/j.1476-5381.2010.00937.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Winbury MM, Papierski DH, Hemmer ML, Hambourger WE. Coronary dilator action of the adenine-atp series. J Pharmacol Exp Ther. 1953;109:255–260. [PubMed] [Google Scholar]
- 42.Burnstock G. Dual control of vascular tone and remodelling by atp released from nerves and endothelial cells. Pharmacol Rep. 2008;60:12–20. [PubMed] [Google Scholar]
- 43.Jones RD, Berne RM. Evidence for a metabolic mechanism in autoregulation of blood flow in skeletal muscle. Circ Res. 1965;17:540–554. doi: 10.1161/01.res.17.6.540. [DOI] [PubMed] [Google Scholar]
- 44.Drury AN, Szent-Gyorgyi A. The physiological activity of adenine compounds with especial reference to their action upon the mammalian heart. J Physiol. 1929;68:213–237. doi: 10.1113/jphysiol.1929.sp002608. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Rubio R, Berne RM. Release of adenosine by the normal myocardium in dogs and its relationship to the regulation of coronary resistance. Circ Res. 1969;25:407–415. doi: 10.1161/01.res.25.4.407. [DOI] [PubMed] [Google Scholar]
- 46.Dobson JG, Jr, Rubio R, Berne RM. Role of adenine nucleotides, adenosine, and inorganic phosphate in the regulation of skeletal muscle blood flow. Circ Res. 1971;29:375–384. doi: 10.1161/01.res.29.4.375. [DOI] [PubMed] [Google Scholar]
- 47.Nishiyama A, Rahman M, Inscho EW. Role of interstitial atp and adenosine in the regulation of renal hemodynamics and microvascular function. Hypertension research : official journal of the Japanese Society of Hypertension. 2004;27:791–804. doi: 10.1291/hypres.27.791. [DOI] [PubMed] [Google Scholar]
- 48.Erlinge D. Extracellular atp: A growth factor for vascular smooth muscle cells. General pharmacology. 1998;31:1–8. doi: 10.1016/s0306-3623(97)00420-5. [DOI] [PubMed] [Google Scholar]
- 49.Wang DJ, Huang NN, Heppel LA. Extracellular atp and adp stimulate proliferation of porcine aortic smooth muscle cells. Journal of cellular physiology. 1992;153:221–233. doi: 10.1002/jcp.1041530202. [DOI] [PubMed] [Google Scholar]
- 50.Penuela S, Bhalla R, Nag K, Laird DW. Glycosylation regulates pannexin intermixing and cellular localization. Mol Biol Cell. 2009;20:4313–4323. doi: 10.1091/mbc.E09-01-0067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Johnstone SR, Ross J, Rizzo MJ, Straub AC, Lampe PD, Leitinger N, Isakson BE. Oxidized phospholipid species promote in vivo differential cx43 phosphorylation and vascular smooth muscle cell proliferation. Am J Pathol. 2009;175:916–924. doi: 10.2353/ajpath.2009.090160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Lee MY, Garvey SM, Baras AS, Lemmon JA, Gomez MF, Schoppee Bortz PD, Daum G, LeBoeuf RC, Wamhoff BR. Integrative genomics identifies dscr1 (rcan1) as a novel nfat-dependent mediator of phenotypic modulation in vascular smooth muscle cells. Human molecular genetics. 2010;19:468–479. doi: 10.1093/hmg/ddp511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Kadl A, Meher AK, Sharma PR, Lee MY, Doran AC, Johnstone SR, Elliott MR, Gruber F, Han J, Chen W, Kensler T, Ravichandran KS, Isakson BE, Wamhoff BR, Leitinger N. Identification of a novel macrophage phenotype that develops in response to atherogenic phospholipids via nrf2. Circ Res. 2010;107:737–746. doi: 10.1161/CIRCRESAHA.109.215715. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Heberlein KR, Straub AC, Best AK, Greyson MA, Looft-Wilson RC, Sharma PR, Meher A, Leitinger N, Isakson BE. Plasminogen activator inhibitor-1 regulates myoendothelial junction formation. Circ Res. 2010;106:1092–1102. doi: 10.1161/CIRCRESAHA.109.215723. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Haddock RE, Grayson TH, Brackenbury TD, Meaney KR, Neylon CB, Sandow SL, Hill CE. Endothelial coordination of cerebral vasomotion via myoendothelial gap junctions containing connexins 37 and 40. Am J Physiol Heart Circ Physiol. 2006;291:H2047–2056. doi: 10.1152/ajpheart.00484.2006. [DOI] [PubMed] [Google Scholar]
- 56.Sandow SL, Neylon CB, Chen MX, Garland CJ. Spatial separation of endothelial small- and intermediate-conductance calcium-activated potassium channels (k(ca)) and connexins: Possible relationship to vasodilator function? J Anat. 2006;209:689–698. doi: 10.1111/j.1469-7580.2006.00647.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Sandow SL, Haddock RE, Hill CE, Chadha PS, Kerr PM, Welsh DG, Plane F. What’s where and why at a vascular myoendothelial microdomain signalling complex. Clinical and experimental pharmacology & physiology. 2009;36:67–76. doi: 10.1111/j.1440-1681.2008.05076.x. [DOI] [PubMed] [Google Scholar]
- 58.Heberlein KR, Straub AC, Isakson BE. The myoendothelial junction: Breaking through the matrix? Microcirculation. 2009;16:307–322. doi: 10.1080/10739680902744404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Isakson BE, Duling BR. Heterocellular contact at the myoendothelial junction influences gap junction organization. Circ Res. 2005;97:44–51. doi: 10.1161/01.RES.0000173461.36221.2e. [DOI] [PubMed] [Google Scholar]
- 60.Straub AC, Billaud M, Johnstone SR, Best AK, Yemen S, Dwyer ST, Looft-Wilson R, Lysiak JJ, Gaston B, Palmer L, Isakson BE. Compartmentalized connexin 43 s-nitrosylation/denitrosylation regulates heterocellular communication in the vessel wall. Arteriosclerosis, thrombosis, and vascular biology. 2011;31:399–407. doi: 10.1161/ATVBAHA.110.215939. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Chekeni FB, Elliott MR, Sandilos JK, Walk SF, Kinchen JM, Lazarowski ER, Armstrong AJ, Penuela S, Laird DW, Salvesen GS, Isakson BE, Bayliss DA, Ravichandran KS. Pannexin 1 channels mediate ‘find-me’ signal release and membrane permeability during apoptosis. Nature. 2010;467:863–867. doi: 10.1038/nature09413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Hopwood AM, Burnstock G. Atp mediates coronary vasoconstriction via p2x-purinoceptors and coronary vasodilatation via p2y-purinoceptors in the isolated perfused rat heart. Eur J Pharmacol. 1987;136:49–54. doi: 10.1016/0014-2999(87)90777-1. [DOI] [PubMed] [Google Scholar]
- 63.Ralevic V, Mathie RT, Alexander B, Burnstock G. Characterization of p2x- and p2y-purinoceptors in the rabbit hepatic arterial vasculature. Br J Pharmacol. 1991;103:1108–1113. doi: 10.1111/j.1476-5381.1991.tb12308.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Kennedy C, Delbro D, Burnstock G. P2-purinoceptors mediate both vasodilation (via the endothelium) and vasoconstriction of the isolated rat femoral artery. Eur J Pharmacol. 1985;107:161–168. doi: 10.1016/0014-2999(85)90055-x. [DOI] [PubMed] [Google Scholar]
- 65.Buvinic S, Briones R, Huidobro-Toro JP. P2y(1) and p2y(2) receptors are coupled to the no/cgmp pathway to vasodilate the rat arterial mesenteric bed. Br J Pharmacol. 2002;136:847–856. doi: 10.1038/sj.bjp.0704789. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Raqeeb A, Sheng J, Ao N, Braun AP. Purinergic p2y2 receptors mediate rapid ca(2+) mobilization, membrane hyperpolarization and nitric oxide production in human vascular endothelial cells. Cell Calcium. 2011;49:240–248. doi: 10.1016/j.ceca.2011.02.008. [DOI] [PubMed] [Google Scholar]
- 67.Woodward HN, Anwar A, Riddle S, Taraseviciene-Stewart L, Fragoso M, Stenmark KR, Gerasimovskaya EV. Pi3k, rho, and rock play a key role in hypoxia-induced atp release and atp-stimulated angiogenic responses in pulmonary artery vasa vasorum endothelial cells. Am J Physiol Lung Cell Mol Physiol. 2009;297:L954–964. doi: 10.1152/ajplung.00038.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Mohrman DE, Heller LJ. Cardiovascular physiology. 3. 1991. [Google Scholar]
- 69.Kukulski F, Ben Yebdri F, Lecka J, Kauffenstein G, Levesque SA, Martin-Satue M, Sevigny J. Extracellular atp and p2 receptors are required for il-8 to induce neutrophil migration. Cytokine. 2009;46:166–170. doi: 10.1016/j.cyto.2009.02.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Bours MJ, Dagnelie PC, Giuliani AL, Wesselius A, Di Virgilio F. P2 receptors and extracellular atp: A novel homeostatic pathway in inflammation. Front Biosci (Schol Ed) 2011;3:1443–1456. doi: 10.2741/235. [DOI] [PubMed] [Google Scholar]
- 71.Erlinge D, Burnstock G. P2 receptors in cardiovascular regulation and disease. Purinergic Signal. 2008;4:1–20. doi: 10.1007/s11302-007-9078-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Bours MJ, Swennen EL, Di Virgilio F, Cronstein BN, Dagnelie PC. Adenosine 5′-triphosphate and adenosine as endogenous signaling molecules in immunity and inflammation. Pharmacology & therapeutics. 2006;112:358–404. doi: 10.1016/j.pharmthera.2005.04.013. [DOI] [PubMed] [Google Scholar]
- 73.Bruzzone R, Barbe MT, Jakob NJ, Monyer H. Pharmacological properties of homomeric and heteromeric pannexin hemichannels expressed in xenopus oocytes. Journal of neurochemistry. 2005;92:1033–1043. doi: 10.1111/j.1471-4159.2004.02947.x. [DOI] [PubMed] [Google Scholar]