Abstract
BACKGROUND & AIMS
Obesity-related insulin resistance contributes to cardiovascular disease. Cannabinoid receptor-1 (CB1) blockade improves insulin sensitivity in obese animals and people, suggesting endocannabinoid involvement. We explored the role of hepatic CB1 in insulin resistance and inhibition of insulin signaling pathways.
METHODS
Wild-type mice and mice with disruption of CB1 (CB1−/− mice) or with hepatocyte-specific deletion or transgenic overexpression of CB1 were maintained on regular chow or a high-fat diet (HFD) to induce obesity and insulin resistance. Hyperinsulinemic-euglycemic clamp analysis was used to analyze the role of the liver and hepatic CB1 in HFD-induced insulin resistance. The cellular mechanisms of insulin resistance were analyzed in mouse and human isolated hepatocytes using small interfering or short hairpin RNAs and lentiviral knockdown of gene expression.
RESULTS
The HFD induced hepatic insulin resistance in wild-type mice, but not in CB1−/− mice or mice with hepatocyte-specific deletion of CB1. CB1−/− mice that overexpressed CB1 specifically in hepatocytes became hyperinsulinemic as a result of reduced insulin clearance due to down-regulation of the insulin-degrading enzyme. However, they had increased hepatic glucose production due to increased glycogenolysis, indicating hepatic insulin resistance; this was further increased by the HFD. In mice with hepatocytes that express CB1, the HFD or CB1 activation induced the endoplasmic reticulum stress response via activation of the Bip-PERK-eIF2α protein translation pathway. In hepatocytes isolated from human or mouse liver, CB1 activation caused endoplasmic reticulum stress-dependent suppression of insulin-induced phosphorylation of akt-2 via phosphorylation of IRS1 at serine-307 and by inducing the expression of the serine and threonine phosphatase Phlpp1. Expression of CB1 was up-regulated in samples from patients with nonalcoholic fatty liver disease.
CONCLUSIONS
Endocannabinoids contribute to diet-induced insulin resistance in mice via hepatic CB1-mediated inhibition of insulin signaling and clearance.
Keywords: NASH, Signal Transduction, Mouse Model, Liver Disease
Insulin resistance is a major public health concern because it can lead to type 2 diabetes, dyslipidemias, and arterio-sclerotic heart disease. The liver plays a central role in obesity-related insulin resistance,1 reflected in impaired insulin suppression of hepatic glucose production by down-regulating gluconeogenesis2 and inhibiting glycogenolysis.3,4 Insulin also promotes de novo hepatic lipogenesis through activation of the transcription factor SREBP1c,5 which is not reduced in insulin-resistant states, but rather increased due to compensatory hyperinsulinemia.6
Endocannabinoids and cannabinoid receptor-1 (CB1) contribute to obesity and its metabolic consequences, as indicated by the effectiveness of CB1 antagonists in reducing body weight and improving insulin resistance and dyslipidemia in subjects with the metabolic syndrome.7–10 Furthermore, CB1-deficient (CB1−/−) mice are resistant to high-fat diet (HFD)-induced obesity, fatty liver, insulin resistance, and dyslipidemia despite a caloric intake similar to that in controls.11,12 Whereas hepatocyte-specific CB1 knockout (hCB1−/−) mice do become obese on HFD, they remain insulin sensitive, suggesting the involvement of hepatic CB1 in whole body insulin resistance.13 Here we explored the involvement of hepatic CB1 in HFD-induced hepatic insulin resistance using a euglycemic hyperinsulinemic clamp. Parallel experiments in hCB1−/− mice and mice with hepatocyte-specific transgenic overexpression of CB1 on a global CB1 knockout background (htgCB1−/− mice) indicated that hepatic CB1 activation is both necessary and sufficient to account for diet-induced hepatic insulin resistance, independent of body weight. The results further indicate that CB1-induced insulin resistance is secondary to endoplasmic reticulum (ER) stress, triggered by activation of the Bip/PERK/eIF2α protein translation pathway, with CB1 inducing serine-307 phosphorylation of IRS1 and activation of the serine/threonine phosphatase Phlpp1, which reverses insulin-induced akt-2 phosphorylation. Finally, we show that the hyperinsulinemia that accompanies CB1-induced insulin resistance is due to decreased insulin clearance, secondary to down-regulation of the insulin-degrading enzyme (IDE) in the liver.
Materials and Methods
Mice
Male C57Bl/6J mice and genetically modified strains backcrossed 10 times to a C57BL/6J background were bred from heterozygote pairs to allow for littermate controls. All experiments were approved by the institutional animal care and use committee. CB1−/− and hCB1−/− mice were generated as described.13,14 A DNA construct containing the mouse albumin promoter and the CB1 receptor coding sequence was injected into fertilized CB1−/− oocytes to generate mice with transgenic expression of CB1 in hepatocytes on a global CB1−/− background (htgCB1−/− mice). Mice aged 8 –12 weeks were placed on standard chow (STD; NIH-31 rodent diet) or an HFD (TD97070; Harlan Teklad, Frederick, MD) containing 33.5% fat (60% of calories) 26.5% carbohydrate, and 27.4% protein for 14 –16 weeks.
Intraperitoneal Glucose Tolerance and Insulin Sensitivity Tests
Mice fasted overnight were injected intraperitoneally with 2 g/kg glucose. Tail blood was collected at indicated time points and glucose levels were determined using a glucometer (Elite; Bayer, Pittsburgh, PA). One week later, mice fasted for 6 hours before the test received an intraperitoneal injection of insulin (0.75 U/kg; Eli Lilly, Indianapolis, IN) and blood glucose levels were determined.
Hyperinsulinemic Euglycemic Clamp
Clamps and glucose tracer analyses in conscious, cannulated mice were performed as described.15
Plasma Hormone Levels
Total plasma insulin was analyzed by the Milliplex Map Mouse Endocrine Panel, human insulin levels were assessed via the Ultrasensitive Human Insulin Assay, and C-peptide in plasma was measured with the Mouse C-Peptide Multiplex Assay (Millipore, Billerica, MA).
Culture of Primary Hepatocytes and Gene Knockdown
Primary mouse hepatocytes were prepared by collagenase perfusion as described.16 After overnight attachment, cells were transfected with Phlpp1 small interfering RNA (siRNA; Qiagen, Valencia, CA) using Lipofectamine 2000 (Invitrogen, Carlsbad, CA) or infected with GFP– eIF2α–short hairpin RNA (shRNA) lentivirus at a multiplicity of infection of 50 for 48 hours. Cells were serum starved for 4 hours and subjected to various treatments. The degree of knockdown relative to mock-transfected cells (60%–70%) was verified by real-time polymerase chain reaction (PCR) of target messenger RNA (mRNA). Primary human hepatocytes were isolated by collagenase digestion of human donor livers not used for transplant.17
Reverse-Transcription PCR
CB1R mRNA expression was assessed by reverse-transcription PCR and normalized with β-actin as described previously.18 Primer sequences are provided in Supplementary Table 1.
Quantitative Real-Time PCR
We used a model PTC-200 Peltier Thermal Cycler (MJ Research, Waltham, MA) and iTaq SYBR Green Supermix with ROX (Bio-Rad, Hercules, CA). Predesigned mouse GAPDH, phosphoenolpyruvate carboxykinase (PEPCK), G6pc, and Glut4 primers were purchased from Qiagen (Valencia, CA). Gene expression values were calculated based on the ΔΔCt method.
Western Blotting and Immunoprecipitation
Protein was extracted from frozen brain or liver samples or from cultured hepatocytes. Western blotting was performed as described,18 and immunoprecipitation was performed using the Pierce (Rockford, IL) Seize Classic (G) Immunoprecipitation Kit.
Tissue Uptake of 2-Deoxyglucose
CB1−/− and htgCB1−/− mice were injected intravenously with 0.2 nmol/g body weight of 2-[1-14C]deoxyglucose (57.7 mCi/mmol; Perkin Elmer, Waltham, MA) and 0.75 U/kg insulin in 150 μL saline and killed 25 minutes later. Tissue samples were collected, weighed, and digested in NCSII solubilizer (Amersham, Piscataway, NJ), and radioactivity was determined by liquid scintillation spectrometry.
Guanosine 5′-[γ-thio]Triphosphate Binding
Agonist-stimulated [35S]guanosine 5′-[γ-thio]triphosphate (GTP[γS]) binding was measured as described.19
G6-Pase, PEPCK, Glycogen Phosphorylase, and IDE Activity
Details of the enzyme assays are provided in Supplementary Materials and Methods.
Results
Anandamide Causes CB1-Mediated Glucose Intolerance and Whole Body Insulin Resistance
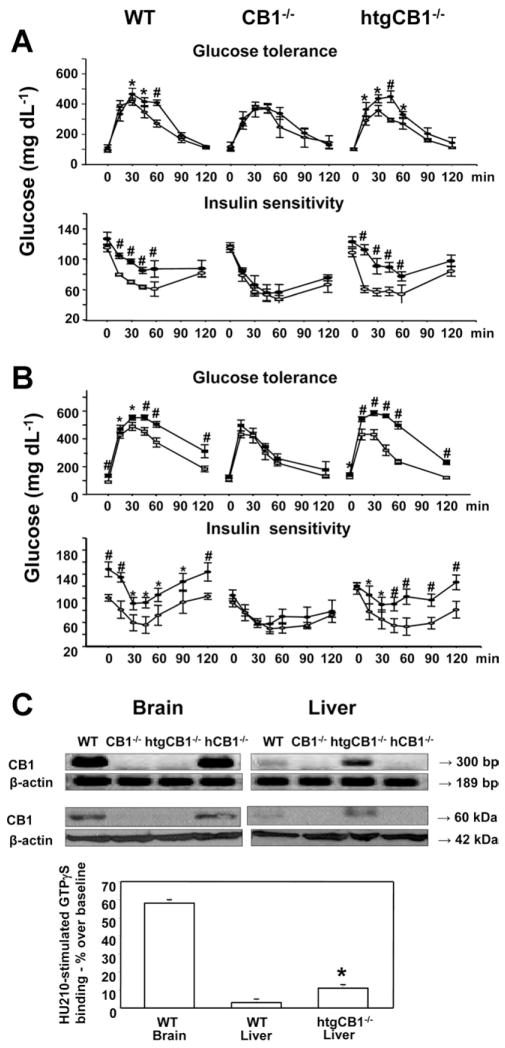
To explore the role of endocannabinoids in glucose homeostasis, we examined the effect of acute in vivo treatment with anandamide on whole body glucose tolerance and insulin sensitivity in mice kept on STD. In wild-type mice, anandamide (10 mg/kg intraperitoneally) caused acute glucose intolerance and insulin resistance, with no such effects observed in CB1−/− mice (Figure 1A). However, the previously described effects of anandamide reappeared in htgCB1−/− mice, in which the overexpression of hepatic CB1 matched the HFD-induced increase in hepatic CB1 in wild-type mice,13 and resulted in increased CB1 agonist efficacy quantified by GTPγS binding (Figure 1C). HtgCB1−/− mice had elevated fasting blood glucose and insulin levels relative to CB1−/− littermates, while the rest of their hormonal/metabolic profile and body composition were similar (Supplementary Figure 1). We next tested the effect of HFD on glucose tolerance and insulin resistance. HtgCB1−/− mice on HFD remained lean and had a moderate increase in liver triglyceride levels (Supplementary Figure 1). Again, wild-type and htgCB1−/− mice, but not CB1−/− mice, developed marked HFD-induced glucose intolerance/insulin resistance (Figure 1B), indicating that activation of hepatic CB1 induces glucose intolerance and insulin resistance and mediates the similar effects of HFD.
Figure 1.
(A) Anandamide induces whole body glucose intolerance and insulin resistance via activation of hepatic CB1. Male wild-type, CB1−/−, and htgCB1−/− mice were subjected to an intraperitoneal glucose tolerance test (GTT) and a week later an insulin sensitivity test (IST). Overnight fasted mice received an intraperitoneal injection of vehicle (open symbols) or 10 mg/kg anandamide (filled symbols). Ten minutes later, the animals received 2 g/kg glucose intraperitoneally, followed by blood glucose measurements at the indicated time points. One week later, each mouse received an intraperitoneal injection of vehicle or 10 mg/kg anandamide. Ten minutes later, 0.75 U/kg insulin was injected and blood glucose monitoring was continued for an additional 2 hours. Note that anandamide caused acute glucose intolerance and insulin resistance in wt and htgCB1−/−, but not in CB1−/−, mice. N = 6 – 8 animals/group. *P < .05 or #P < .005 from corresponding value in vehicle-pretreated mice. (B) GTT and IST were conducted as previously described in wild-type (wt), CB1−/−, and htgCB1−/− mice on STD (open symbols) or HFD (solid symbols) for 14 –16 weeks. N = 6 – 8 animals/group, significance indicated as in A. (C) CB1 receptor expression profile in wt, CB1−/−, htgCB1−/−, and hCB1−/− mice. (Top) CB1 mRNA expression in brain and liver using reverse-transcription PCR. (Middle) Western blotting of CB1 protein using a CB1 receptor N-terminal antibody (Cayman) or a β-actin antibody for loading control. (Bottom) GTPγS binding stimulated by the cannabinoid agonist HU-210 (100 nmol/L) in membranes from wild-type mouse brain and liver and htgCB1−/− liver. Similar results were obtained in 3 independent experiments. Note that CB1 overexpression in ht-gCB1−/− hepatocytes is associated with increased CB1-stimulated GTPγS binding.
Hepatic CB1 Are Necessary for Diet-Induced Hepatic Insulin Resistance
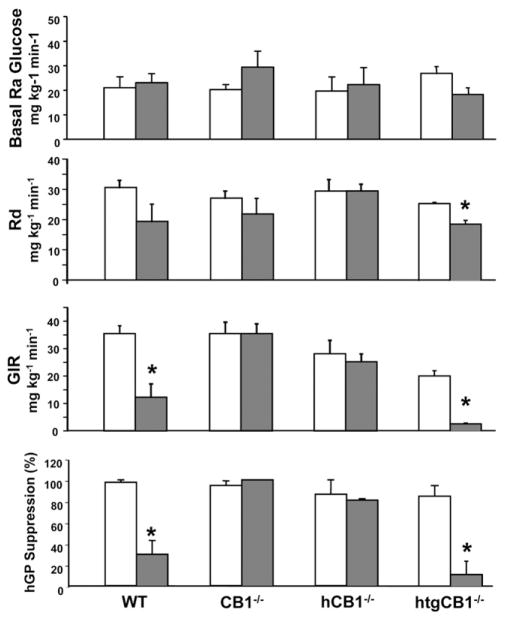
To assess the contribution of the liver to CB1-mediated changes in glucose homeostasis, we conducted euglycemic hyperinsulinemic clamps in wild-type, CB1−/−, hCB1−/−, and htgCB1−/− mice on STD or HFD. Wild-type mice became obese on HFD and displayed hepatic insulin resistance, as indicated by the marked reduction in glucose infusion rates, resulting from the reduced ability of insulin to suppress hepatic glucose production. Whole body glucose uptake tended to decrease in mice receiving HFD, but this did not reach statistical significance (Figure 2). CB1−/− mice receiving HFD remained lean and insulin sensitive, with hepatic glucose production, glucose infusion rate, and whole body glucose uptake that were similar to their controls receiving STD. Interestingly, hCB1−/− mice receiving HFD also remained insulin sensitive despite becoming as obese as wild-type mice,13 whereas ht-gCB1−/− mice receiving HFD were as insulin resistant as wild-type mice receiving HFD despite remaining lean, with marked reduction of glucose infusion rate and a modest decrease in whole body glucose uptake (Figure 2 and Supplementary Figure 1). This indicates that hepatic insulin resistance depends on CB1 activity and is independent of adiposity.
Figure 2.
HFD induces hepatic insulin resistance via activation of hepatic CB1. Hyperinsulinemic euglycemic clamps were performed in wild-type, CB1−/−, hCB1−/−, and htgCB1−/− mice 5 hours after withdrawal of food, as described in Materials and Methods. The mice had been maintained on HFD (filled columns) or STD (open columns) for 14 –16 weeks before the clamps. Note that HFD results in suppression of glucose infusion rate (GIR) and hepatic glucose production (hGP), indicating decreased insulin-mediated suppression of hepatic glucose production in mice with (wild-type [WT] and htgCB1−/−) but not those without hepatic CB1 (CB1−/− or hCB1−/−). Rd, whole body glucose uptake. Means ± SE from 3– 6 animals/group are shown. *P < .05 relative to corresponding STD values.
Hepatic CB1 Are Sufficient to Induce Hepatic Insulin Resistance Associated With Reduced Insulin Clearance
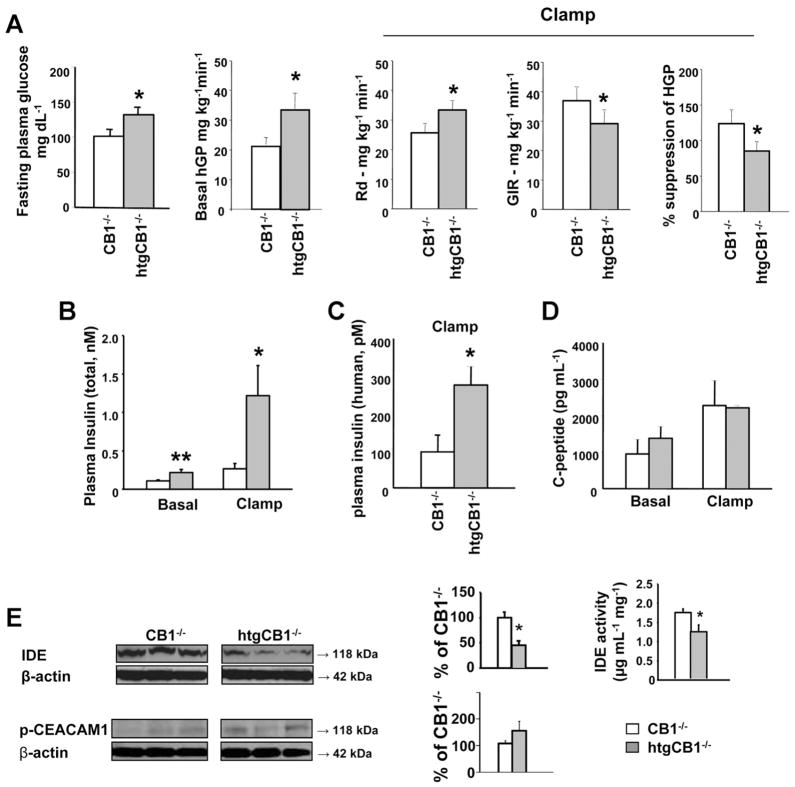
HtgCB1−/− mice on STD had elevated fasting blood glucose relative to their CB1−/− littermates (Figure 3A). When subjected to hyperinsulinemic clamp, basal hepatic glucose production and plasma insulin levels were significantly higher than in CB1−/− littermates (Figure 3A and B and Supplementary Figure 2), indicating hepatic insulin resistance. Unexpectedly, plasma insulin levels remained significantly elevated in htgCB1−/− compared with CB1−/− mice near the end of the clamp, suggesting reduced insulin clearance in the former. This was confirmed by the finding that plasma levels of the infused insulin, determined with an assay selective for human insulin, were similarly ~3 times higher in the htgCB1−/− mice (Figure 3C), whereas plasma levels of C-peptide were similar in the 2 strains, the latter indicating similar insulin secretion rates (Figure 3D). The hepatic levels of IDE were about half of that in CB1−/− littermates, and there was a parallel reduction in IDE catalytic activity in htgCB1−/− mice (Figure 3E), whereas levels of p-CEACAM1, another regulator of insulin degradation,20 were similar in the 2 strains (Figure 3E). This suggests that reduced insulin degradation by IDE contributes to the moderate hyperin-sulinemia of htgCB1−/− mice.
Figure 3.
Selective overexpression of CB1 in the liver is associated with hepatic insulin resistance and reduced insulin clearance. (A) HtgCB1−/− mice on STD (filled columns) are hyperglycemic and show hepatic insulin resistance, compensated by increased whole body glucose uptake, relative to CB1−/− littermates (open columns). (B) Plasma insulin before (basal) and 120 minutes after initiation of euglycemic/hyperinsulinemic clamp. *P < .05, **P < .01 relative to corresponding value in CB1−/− mice, n = 7 (CB1−/−) or 8 (htgCB1−/−). (C) Plasma levels of human insulin, measured in the same “clamp” samples, *P < .05. (D) Plasma levels of C-peptide in same basal and clamp samples. (E) IDE and p-CEACAM1 levels and IDE activity in livers from CB1−/− and htgCB1−/− mice.
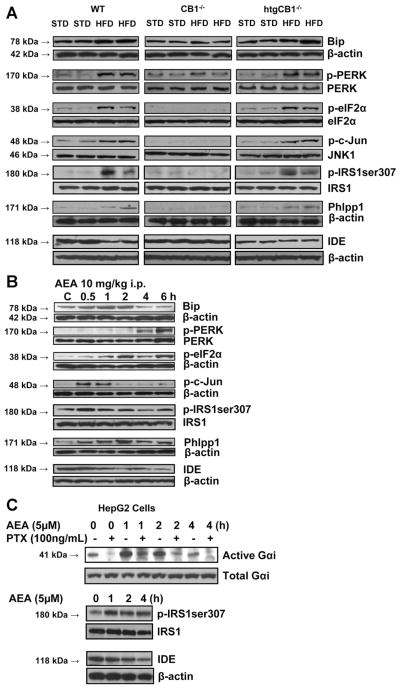
Regulation of IDE by hepatic CB1 was further indicated by the HFD-induced down-regulation of IDE expression in wild-type and htgCB1−/−, but not in CB1−/−, mice (Figure 4A) and by the ability of anandamide to down-regulate IDE expression in vivo in wild-type mice (Figure 4B) or in vitro in HepG2 cells (Figure 4C). CB1-induced down-regulation of IDE expression was associated with increased serine-307 phosphorylation of IRS1 (Figure 4A–C), and it involved pertussis toxin-sensitive activation of Giα (Figure 4C).
Figure 4.
Both HFD and acute treatment with anandamide inhibit hepatic insulin signaling via CB1 receptor-induced ER stress response, through serine-307 phosphorylation of IRS1 and activation of Phlpp1. (A) HFD increases Bip levels; induces PERK, eIF2α, c-Jun, and IRS1 (serine) phosphorylation; increases Phlpp1; and decreases IDE protein in the liver of wild-type and htgCB1−/−, but not CB1−/−, mice (Western blots). (B) Acute treatment of wild-type mice on STD with anandamide (10 mg/kg intraperitoneally) induces hepatic Bip expression, PERK, eIF2α, c-Jun, and IRS1 (serine) phosphorylation and increases Phlpp1 and decreases IDE expression. (C) In HepG2 cells, anandamide causes pertussis toxin (PTX)-sensitive activation of Giα, serine-307 phosphorylation of IRS1, and inhibition of IDE expression in a time-dependent manner.
CB1-Mediated Hepatic Insulin Resistance Manifests in Increased Glycogenolysis
The activity of the gluconeogenic enzymes glucose-6-phosphatase and PEPCK were similar in liver extracts from htgCB1−/− and CB1−/− mice, whereas glycogen phosphorylase a activity was increased by ~70% in the former (Supplementary Figure 3), which suggests that glycogenolysis is a primary source of the increased hepatic glucose production. Indeed, hepatic glycogen at the end of the clamp was significantly lower in htgCB1−/− than in CB1−/− mice (Supplementary Table 1), most likely due to a compensatory increase in GLUT4 expression and a corresponding increase in glucose uptake in skeletal muscle and kidney, as revealed by [14C]2-deoxyglucose uptake (Supplementary Figure 4). Thus, overexpression of hepatic CB1 leads to increased hepatic glucose production due to increased glycogenolysis, which is partially offset by a compensatory increase in glucose uptake, primarily into skeletal muscle.
CB1-Induced Hepatic Insulin Resistance is ER Stress Dependent
To test whether CB1-mediated insulin resistance may be via increased ER stress, we analyzed the hepatic level of the molecular chaperone Bip and the phosphorylation status of its downstream targets PERK21 and the α subunit of translation initiation factor-2 (eIF2α), key indicators of ER stress.22 The phosphorylation status of c-Jun and serine-307 of IRS1 was also monitored as indicators of the activation of c-Jun NH2-terminal kinase 1 (JNK1), which has been also linked to inhibition of insulin signaling.23,24 In agreement with earlier findings,25 HFD in wild-type mice markedly increased Bip protein levels and the phosphorylation of PERK, eIF2α, c-Jun, and IRS1 (serine-307) in the liver. There was a similar HFD-induced increase in Bip, p-PERK, p-eIF2α, p-c-Jun, and p-serine-307IRS1 in the liver of htgCB1−/−, but not CB1−/−, mice (Figure 4A), whereas markers of 2 alternative pathways of ER stress, ATF6 and the spliced form of XBP1, were unaffected by genotype or HFD (not shown). To test whether activation of hepatic CB1 induces ER stress, we treated wild-type mice with anandamide (10 mg/kg intraperitoneally) and killed them at various times posttreatment. Anandamide caused a rapid increase in Bip, p-c-Jun, and p-IRS1 that peaked at 30 – 60 minutes and a slower, more prolonged increase in p-PERK and p-eIF2α (Figure 4B).
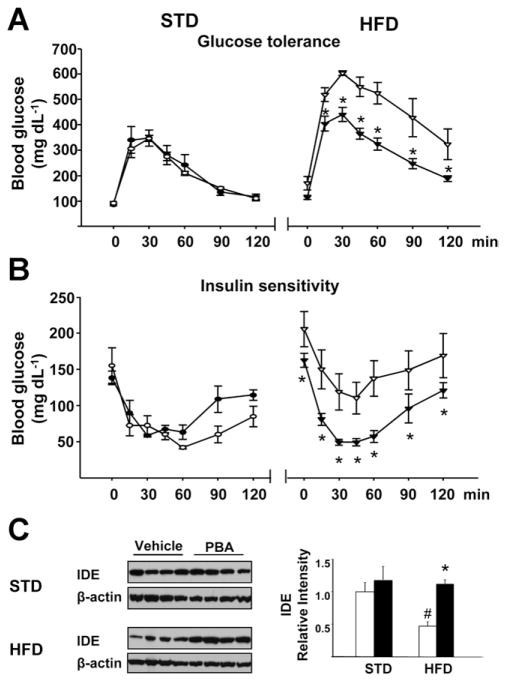
The chemical chaperone 4-phenyl butyric acid (PBA) reverses HFD-induced ER stress and insulin resistance.26 In agreement with those findings, daily oral treatment of HFD-fed mice with 1 g/kg PBA for 20 days reversed the glucose intolerance (Figure 5A) and insulin resistance (Figure 5B) and also reversed the HFD-induced decrease in IDE expression (Figure 5C), thus providing additional evidence for the role of IDE in insulin resistance and its regulation by ER stress.
Figure 5.
In vivo treatment of mice with the chemical chaperone PBA (1 g · kg−1 · day−1 for 20 days, solid symbols) reverses (A) obesity-induced glucose intolerance, (B) insulin resistance, and (C) down-regulation of IDE in the liver. *P < .05 from corresponding value in vehicle-treated mice; #P < .05 relative to corresponding STD values.
CB1 Inhibits Insulin-Induced akt-2 Phosphorylation in Mouse and Human Hepatocytes in an ER Stress-Dependent Manner
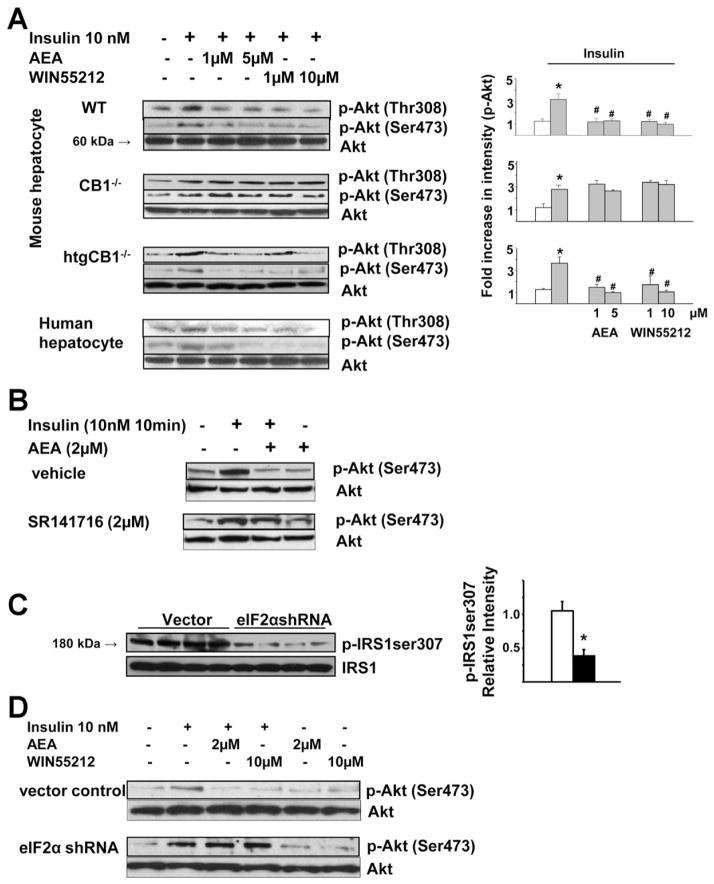
Protein kinase B/akt-2 plays an obligatory role in insulin-induced hypoglycemia by down-regulating gluconeogenic gene expression6 and increasing the activity of glycogen synthase.15 Exposure of human or mouse isolated hepatocytes to 10 nmol/L insulin increased phosphorylation of akt-2 at the thre308 and ser473 sites, which was concentration-dependently inhibited by anandamide or the synthetic cannabinoid WIN55,212-2 in human hepatocytes and in hepatocytes from wild-type or htgCB1−/−, but not CB1−/−, mice (Figure 6A). The role of CB1 was further verified by blocking the effect of anandamide with the CB1 antagonist SR141716 (Figure 6B). The inhibition of akt-2 phosphorylation by anandamide is ER stress dependent. In mouse hepatocytes, shRNA knock-down of eIF2α by 61.6% ± 7.1% resulted in marked suppression of serine-307 phosphorylation of IRS1 (Figure 6C), whereas insulin-induced akt-2 phosphorylation was enhanced and was resistant to inhibition by anandamide or WIN 55,212-2 (Figure 6D).
Figure 6.
CB1-mediated inhibition of akt-2 phosphorylation is ER stress dependent. (A) Insulin-induced phosphorylation of akt-2 is inhibited by anandamide or WIN55,212-2 in isolated hepatocytes obtained from wild-type or htgCB1−/− mice or from human livers, but not in hepatocytes from CB1−/− mice (quantified by densitometry, *P < .05 relative to control cells (first lanes, open columns); #P < .05 relative to cells treated with insulin only. (B) Anandamide inhibition of insulin-induced akt-2 phosphorylation is abolished by the CB1 antagonist rimonabant. (C) shRNA knockdown of eIF2α prevents serine-307 phosphorylation of IRS1 in HepG2 cells. (D) shRNA knockdown of eIF2α prevents CB1 agonist-induced inhibition of akt-2 phosphorylation. Each blot in A–D has been replicated 3 times with similar results.
Anandamide Inhibits Insulin Signaling via Activation of Phlpp1
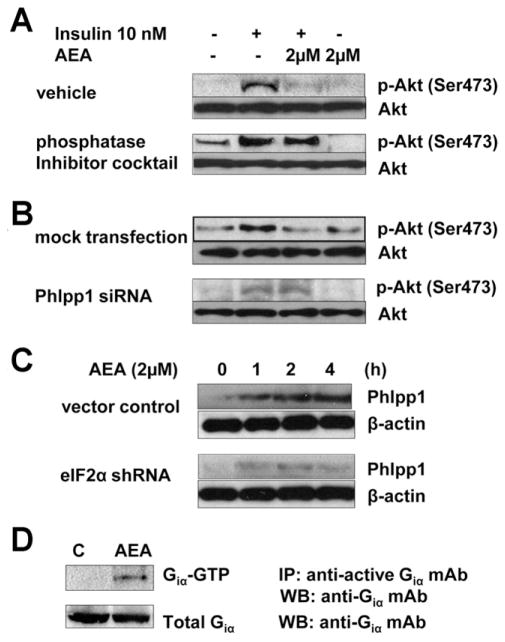
Anandamide inhibition of insulin-induced akt-2 phosphorylation in wild-type mouse hepatocytes was blocked by a phosphatase inhibitor cocktail (Figure 7A), suggesting that anandamide promotes dephosphorylation of akt-2 by a phosphatase. The PH domain leucine-rich repeat protein phosphatase-1 (Phlpp1) has been implicated in the dephosphorylation of akt-2.27,28 In mouse hepatocytes with an siRNA-induced, 68.8% ± 1.3% knockdown of Phlpp1, insulin-induced akt-2 phosphorylation was enhanced and the inhibitory effect of anandamide was blunted (Figure 7B). Hepatic levels of Phlpp1 protein were increased by HFD in a CB1-dependent manner (Figure 4A) and were also increased following in vivo treatment with anandamide (Figure 4B). In wild-type mouse hepatocytes, anandamide increases Phlpp1 expression via the ER stress response, as indicated by its blunted effect in cells with shRNA-mediated knockdown of eIF2α (Figure 7C). CB1 is a Gi/Go-coupled receptor, and the action of anandamide involves activation of Giα (Figure 7D).
Figure 7.
Anandamide inhibits insulin-induced akt-2 phosphorylation in mouse hepatocytes via ER stress-dependent activation of Phlpp1. Anandamide inhibition of insulin-induced akt-2 phosphorylation is abrogated by (A) a phosphatase inhibitory cocktail or by (B) siRNA knockdown of Phlpp1 expression. (C) Anandamide induction of Phlpp1 is inhibited by shRNA-mediated knockdown of eIF2α. (D) Anandamide activates Giα in hepatocytes. Each blot in A–D has been replicated 3 times with similar results.
Up-regulation of Hepatic CB1 in Human Nonalcoholic Fatty Liver Disease
The effects of cannabinoids observed in human hepatocytes predicated the presence of CB1 in human liver. Indeed, CB1 mRNA could be detected by real-time PCR in liver biopsy tissue from 5 patients with no liver pathology, and a 34.2-fold ± 9.7-fold increase (P < .05) in CB1 mRNA relative to these controls was observed in tissue from 26 patients with nonalcoholic fatty liver disease. Increased expression of CB1 protein in fatty versus nonfatty livers could also be documented by immunohistochemistry (Supplementary Figure 5).
Discussion
Increased activity of the endocannabinoid/CB1 system has emerged as a pathogenic factor in visceral obesity.29,30 A common complication of obesity is insulin resistance, and CB1 blockade not only reduces weight and adiposity, but also improves insulin sensitivity both in obese subjects8 and animals,31–33 suggesting a role for endocannabinoids in glycemic control. Mice with selective deletion of CB1 in hepatocytes become obese on HFD but remain glucose tolerant and insulin sensitive, whereas mice that express CB1 only in hepatocytes remain lean on HFD but are insulin resistant, suggesting a weight-independent effect of hepatic CB1 on insulin sensitivity.13 Here we provide in vivo and in vitro evidence that endocannabinoids acting via hepatic CB1 contribute to diet-induced hepatic insulin resistance by inhibiting both insulin signaling and clearance. Hepatic CB1 activate the serine/threonine phosphatase Phlpp1 via the Bip/PERK/eIF2α ER stress pathway, which suppresses insulin signaling by counteracting insulin-induced akt-2 phosphorylation. Hepatic CB1 also induce serine-307 phosphorylation of IRS1, which inhibits insulin signaling.34 Furthermore, activation of hepatic CB1 suppresses insulin clearance via reducing the expression of the IDE.
HFD-induced hepatic insulin resistance, as revealed by a euglycemic hyperinsulinemic clamp, is dependent on the presence of CB1 in hepatocytes (Figure 2), activation of which suppresses insulin signaling, resulting in increased glucose production. Hepatic CB1 are not only necessary but also sufficient to mediate hepatic insulin resistance; CB1−/− mice with selective hepatic reexpression of CB1 are hyperglycemic and hyperinsulinemic relative to their CB1−/− littermates. These mice are insulin resistant even on normal diet, probably due to the transgenic overexpression of hepatic CB1 to levels similar to those in normal mice on HFD13,35 (Figure 1C), and their insulin resistance is further increased by HFD (Figure 2), likely mediated by the parallel increase in hepatic anandamide (Supplementary Figure 1). The other endocannabinoid, 2-arachidonoyl glycerol, was elevated in the liver of a different experimental model on HFD; however, the magnitude of glucose intolerance was somewhat mitigated in those ApoE−/− mutants compared with wild-type mice on the same HFD.36 The finding of a robust up-regulation of hepatic CB1 expression in people with nonalcoholic fatty liver disease (Supplementary Figure 5), along with evidence for CB1-mediated inhibition of insulin signaling in human hepatocytes (Figure 6A), suggests a similar regulatory function of CB1 in the human liver. Indeed, marijuana smoking was shown more than 30 years ago to induce insulin resistance,37 and cannabis also induces insulin resistance in rodents.38 The key role of hepatic CB1 in insulin resistance is further indicated by the finding that transgenic reexpression of hepatic CB1 in CB1−/− mice rescues the ability of acute in vivo treatment with anandamide to induce insulin resistance (Figure 1A).
The increased hepatic glucose production of htgCB1−/− mice could be attributed to increased glycogenolysis rather than gluconeogenesis, based on ex vivo measurement of glycogen phosphorylase versus glucose-6-phosphatase and PEPCK activities, respectively (Supplementary Figure 3). Nevertheless, a possible contribution of gluconeogenesis cannot be excluded, because regulation may also occur at the level of fructose 2,6-biphosphatase. Indeed, in a recent study in rat and human isolated hepatocytes, direct activation of CB1 receptors by 2-arachidonoylglycerol was found to increase glucose-6-phosphatase and PEPCK gene expression as well as glucose production.39 However, the relative contribution of gluconeogenesis and glycogenolysis to regulated glucose production may be different under in vitro and in vivo conditions. Also, enzyme activities were not measured and the possible contribution of glycogenolysis to the observed increase in glucose production was not explored in the previously described study. Future studies of glucose fluxes could provide a more definitive answer in this regard.
Although diet-induced glucose intolerance is predominantly attributable to hepatic insulin resistance, CB1 can also inhibit glucose uptake into skeletal muscle40,41 and adipose tissue42,43 and inhibit insulin signaling in pancreatic beta cells.44 The relative contributions of these targets to glycemic control by endocannabinoids may be different in rodents and humans and remain to be further explored.
The importance of reduced insulin clearance and IDE as pathogenic factors in diabetes is suggested by reports of an association of type 2 diabetes with loss of function mutations in IDE45 or with polymorphisms in the IDE gene.46,47 Plasma levels of both endogenous and exogenous insulin were higher and hepatic IDE protein and activity levels were lower in htgCB1−/− than in CB1−/− mice (Figure 3E). The HFD-induced decrease in IDE depends on the presence of CB1 in hepatocytes (Figure 4A), and the role of CB1 in regulating IDE expression is further indicated by anandamide down-regulation of IDE in the liver in vivo and in HepG2 cells. Furthermore, obesity-related insulin resistance and reduced IDE expression are both reversed by the chemical chaperone PBA,26 which links ER stress to IDE expression. These findings represent the first direct link among a specific diabetogenic signal, hepatic CB1 activation, and reduced insulin clearance via down-regulation of IDE.
It is well established that obesity is accompanied by activation of the ER stress response in the liver,25,48,49 which inhibits insulin signaling.25 Activation of CB1 can also induce ER stress, as documented recently in human glioma cells.50 Because obesity leads to activation of the endocannabinoid/CB1 system,12,29,35 we hypothesized that the ER stress response in obesity may be mediated by endocannabinoids acting via hepatic CB1. This hypothesis is now supported by both in vivo and in vitro evidence.
Under in vivo conditions, HFD induces the expression of the chaperone Bip and increases the phosphorylation of its downstream targets PERK and eIF2α, a key pathway of ER stress involved in suppressing protein translation,51 whereas ATF6 and XBP1, key components of 2 additional arms of the ER stress response involved in protein folding and degradation,51 appear unaffected. The effects of HFD on the Bip/PERK/eIF2α pathway are CB1 dependent, because they are detectable in the liver of wild-type and htgCB1−/−, but not CB1−/−, mice (Figure 4A). CB1 involvement is further supported by the in vivo action of anandamide to induce Bip expression and PERK and eIF2α phosphorylation (Figure 4B).
In primary cultured mouse hepatocytes, shRNA knock-down of eIF2α abrogated the ability of CB1 agonists to inhibit insulin-induced akt-2 phosphorylation (Figure 6D) or to induce the expression of Phlpp1 (Figure 7C), a serine/threonine phosphatase involved in the dephosphorylation of akt-2.27,28 Furthermore, HFD-induced serine-307 phosphorylation of IRS1, which negatively regulates akt,25 depends on the presence of CB1 in hepatocytes (Figure 4A), and anandamide directly increases IRS1 serine phosphorylation (Figure 4B and C). These findings outline the signaling pathway engaged by hepatic CB1 to inhibit the antiglycemic action of insulin: activation of hepatic CB1 triggers ER stress, which inhibits insulin signaling by suppressing p-akt-2 via serine phosphorylation of IRS1 and at the same time promoting the dephosphorylation of p-akt-2 via activation of Phlpp1.
The possible role of JNK1 in hepatic ER stress and insulin resistance is complex. The protective role of germline deletion of JNK1 against obesity and insulin resistance23,24 could be attributed to loss of JNK1 activity in the brain,52 whereas selective hepatic deletion of JNK1 has the opposite effect, that is, an increase in insulin resistance.53 Thus, activation of JNK by CB1 (Figure 4A and B) is unlikely to contribute to the parallel development of hepatic insulin resistance. This is further suggested by our observation that pretreatment of mice with the JNK inhibitor BI-78D354 failed to prevent anandamide-induced glucose intolerance (not shown).
The ability of a peripherally restricted CB1R antagonist to reverse obesity-related insulin resistance has highlighted the importance of an overactive peripheral endocannabinoid system in the metabolic consequences of obesity33 and is also compatible with the role of hepatic CB1 in this effect, as shown here. The present findings identify hepatic CB1 as a potential novel molecular target for the pharmacotherapy of insulin resistance.
Supplementary Material
Acknowledgments
Jie Liu dedicates this work to the memory of her beloved father, Yulin Liu.
Funding
Supported by intramural funds from the National Institute on Alcohol Abuse and Alcoholism.
Abbreviations used in this paper
- CB1
cannabinoid receptor-1
- ER
endoplasmic reticulum
- GTP[γS]
[35S]guanosine 5′-[γ-thio]triphosphate
- HFD
high-fat diet
- IDE
insulin-degrading enzyme
- JNK
c-Jun NH2-terminal kinase 1
- PBA
4-phenyl butyric acid
- PCR
polymerase chain reaction
- PEPCK
phosphoenolpyruvate carboxykinase
- shRNA
short hairpin RNA
- siRNA
small interfering RNA
- STD
standard chow
Footnotes
Conflicts of interest
The authors disclose no conflicts.
To access the supplementary material accompanying this article, visit the online version of Gastroenterology at www.gastrojournal.org, and at doi: 10.1053/j.gastro.2012.01.032.
References
- 1.Biddinger SB, Hernandez-Ono A, Rask-Madsen C, et al. Hepatic insulin resistance is sufficient to produce dyslipidemia and susceptibility to atherosclerosis. Cell Metab. 2008;7:125–134. doi: 10.1016/j.cmet.2007.11.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Matsumoto M, Han S, Kitamura T, et al. Dual role of transcription factor FoxO1 in controlling hepatic insulin sensitivity and lipid metabolism. J Clin Invest. 2006;116:2464–2472. doi: 10.1172/JCI27047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Ramnanan CJ, Edgerton DS, Rivera N, et al. Molecular characterization of insulin-mediated suppression of hepatic glucose production in vivo. Diabetes. 2010;59:1302–1311. doi: 10.2337/db09-1625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Petersen KF, Laurent D, Rothman DL, et al. Mechanism by which glucose and insulin inhibit net hepatic glycogenolysis in humans. J Clin Invest. 1998;101:1203–1209. doi: 10.1172/JCI579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Brown MS, Goldstein JL. The SREBP pathway: regulation of cholesterol metabolism by proteolysis of a membrane-bound transcription factor. Cell. 1997;89:331–340. doi: 10.1016/s0092-8674(00)80213-5. [DOI] [PubMed] [Google Scholar]
- 6.Brown MS, Goldstein JL. Selective versus total insulin resistance: a pathogenic paradox. Cell Metab. 2008;7:95–96. doi: 10.1016/j.cmet.2007.12.009. [DOI] [PubMed] [Google Scholar]
- 7.Despres JP, Golay A, Sjostrom L. Effects of rimonabant on metabolic risk factors in overweight patients with dyslipidemia. N Engl J Med. 2005;353:2121–2134. doi: 10.1056/NEJMoa044537. [DOI] [PubMed] [Google Scholar]
- 8.Scheen AJ, Finer N, Hollander P, et al. Efficacy and tolerability of rimonabant in overweight or obese patients with type 2 diabetes: a randomised controlled study. Lancet. 2006;368:1660–1672. doi: 10.1016/S0140-6736(06)69571-8. [DOI] [PubMed] [Google Scholar]
- 9.Pi-Sunyer FX, Aronne LJ, Heshmati HM, et al. Effect of rimonabant, a cannabinoid-1 receptor blocker, on weight and cardiometabolic risk factors in overweight or obese patients: RIO-North America: a randomized controlled trial. JAMA. 2006;295:761–775. doi: 10.1001/jama.295.7.761. [DOI] [PubMed] [Google Scholar]
- 10.Nissen SE, Nicholls SJ, Wolski K, et al. Effect of rimonabant on progression of atherosclerosis in patients with abdominal obesity and coronary artery disease: the STRADIVARIUS randomized controlled trial. JAMA. 2008;299:1547–1560. doi: 10.1001/jama.299.13.1547. [DOI] [PubMed] [Google Scholar]
- 11.Ravinet Trillou C, Delgorge C, Menet C, et al. CB1 cannabinoid receptor knockout in mice leads to leanness, resistance to diet-induced obesity and enhanced leptin sensitivity. Int J Obes Relat Metab Disord. 2004;28:640–648. doi: 10.1038/sj.ijo.0802583. [DOI] [PubMed] [Google Scholar]
- 12.Osei-Hyiaman D, DePetrillo M, Pacher P, et al. Endocannabinoid activation at hepatic CB1 receptors stimulates fatty acid synthesis and contributes to diet-induced obesity. J Clin Invest. 2005;115:1298–1305. doi: 10.1172/JCI23057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Osei-Hyiaman D, Liu J, Zhou L, et al. Hepatic CB(1) receptor is required for development of diet-induced steatosis, dyslipidemia, and insulin and leptin resistance in mice. J Clin Invest. 2008;118:3160–3169. doi: 10.1172/JCI34827. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Zimmer A, Zimmer AM, Hohmann AG, et al. Increased mortality, hypoactivity, and hypoalgesia in cannabinoid CB1 receptor knockout mice. Proc Natl Acad Sci U S A. 1999;96:5780–5785. doi: 10.1073/pnas.96.10.5780. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Buettner C, Patel R, Muse ED, et al. Severe impairment in liver insulin signaling fails to alter hepatic insulin action in conscious mice. J Clin Invest. 2005;115:1306–1313. doi: 10.1172/JCI23109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Sun R, Park O, Horiguchi N, et al. STAT1 contributes to dsRNA inhibition of liver regeneration after partial hepatectomy in mice. Hepatology. 2006;44:955–966. doi: 10.1002/hep.21344. [DOI] [PubMed] [Google Scholar]
- 17.Strom SC, Pisarov LA, Dorko K, et al. Use of human hepatocytes to study P450 gene induction. Methods Enzymol. 1996;272:388–401. doi: 10.1016/s0076-6879(96)72044-x. [DOI] [PubMed] [Google Scholar]
- 18.Liu J, Gao B, Mirshahi F, et al. Functional CB1 cannabinoid receptors in human vascular endothelial cells. Biochem J. 2000;346:835–840. [PMC free article] [PubMed] [Google Scholar]
- 19.Wang L, Liu J, Harvey-White J, et al. Endocannabinoid signaling via cannabinoid receptor 1 is involved in ethanol preference and its age-dependent decline in mice. Proc Natl Acad Sci U S A. 2003;100:1393–1398. doi: 10.1073/pnas.0336351100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Poy MN, Yang Y, Rezaei K, et al. CEACAM1 regulates insulin clearance in liver. Nat Genet. 2002;30:270–276. doi: 10.1038/ng840. [DOI] [PubMed] [Google Scholar]
- 21.Harding HP, Zhang Y, Ron D. Protein translation and folding are coupled by an endoplasmic-reticulum-resident kinase. Nature. 1999;397:271–274. doi: 10.1038/16729. [DOI] [PubMed] [Google Scholar]
- 22.Shi Y, Taylor SI, Tan SL, et al. When translation meets metabolism: multiple links to diabetes. Endocr Rev. 2003;24:91–101. doi: 10.1210/er.2002-0018. [DOI] [PubMed] [Google Scholar]
- 23.Hirosumi J, Tuncman G, Chang L, et al. A central role for JNK in obesity and insulin resistance. Nature. 2002;420:333–336. doi: 10.1038/nature01137. [DOI] [PubMed] [Google Scholar]
- 24.Sabio G, Das M, Mora A, et al. A stress signaling pathway in adipose tissue regulates hepatic insulin resistance. Science. 2008;322:1539–1543. doi: 10.1126/science.1160794. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Ozcan U, Cao Q, Yilmaz E, et al. Endoplasmic reticulum stress links obesity, insulin action, and type 2 diabetes. Science. 2004;306:457–461. doi: 10.1126/science.1103160. [DOI] [PubMed] [Google Scholar]
- 26.Ozcan U, Yilmaz E, Ozcan L, et al. Chemical chaperones reduce ER stress and restore glucose homeostasis in a mouse model of type 2 diabetes. Science. 2006;313:1137–1140. doi: 10.1126/science.1128294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Gao T, Furnari F, Newton AC. PHLPP: a phosphatase that directly dephosphorylates Akt, promotes apoptosis, and suppresses tumor growth. Mol Cell. 2005;18:13–24. doi: 10.1016/j.molcel.2005.03.008. [DOI] [PubMed] [Google Scholar]
- 28.Brognard J, Sierecki E, Gao T, et al. PHLPP and a second isoform, PHLPP2, differentially attenuate the amplitude of Akt signaling by regulating distinct Akt isoforms. Mol Cell. 2007;25:917–931. doi: 10.1016/j.molcel.2007.02.017. [DOI] [PubMed] [Google Scholar]
- 29.Di Marzo V. The endocannabinoid system in obesity and type 2 diabetes. Diabetologia. 2008;51:1356–1367. doi: 10.1007/s00125-008-1048-2. [DOI] [PubMed] [Google Scholar]
- 30.Kunos G, Osei-Hyiaman D, Liu J, et al. Endocannabinoids and the control of energy homeostasis. J Biol Chem. 2008;283:33021–33025. doi: 10.1074/jbc.R800012200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Doyon C, Denis RG, Baraboi ED, et al. Effects of rimonabant (SR141716) on fasting-induced hypothalamic-pituitary-adrenal axis and neuronal activation in lean and obese Zucker rats. Diabetes. 2006;55:3403–3410. doi: 10.2337/db06-0504. [DOI] [PubMed] [Google Scholar]
- 32.Ravinet Trillou C, Arnone M, Delgorge C, et al. Anti-obesity effect of SR141716, a CB1 receptor antagonist, in diet-induced obese mice. Am J Physiol Regul Integr Comp Physiol. 2003;284:R345–R353. doi: 10.1152/ajpregu.00545.2002. [DOI] [PubMed] [Google Scholar]
- 33.Tam J, Vemuri VK, Liu J, et al. Peripheral CB1 cannabinoid receptor blockade improves cardiometabolic risk in mouse models of obesity. J Clin Invest. 2010;120:2953–2966. doi: 10.1172/JCI42551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Hotamisligil GS, Peraldi P, Budavari A, et al. IRS-1-mediated inhibition of insulin receptor tyrosine kinase activity in TNF-alpha- and obesity-induced insulin resistance. Science. 1996;271:665–668. doi: 10.1126/science.271.5249.665. [DOI] [PubMed] [Google Scholar]
- 35.Jourdan T, Djaouti L, Demizieux L, et al. CB1 antagonism exerts specific molecular effects on visceral and subcutaneous fat and reverses liver steatosis in diet-induced obese mice. Diabetes. 2010;59:926–934. doi: 10.2337/db09-1482. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Bartelt A, Orlando P, Mele C, et al. Altered endocannabinoid signalling after a high-fat diet in Apoe (−/−) mice: relevance to adipose tissue inflammation, hepatic steatosis and insulin resistance. Diabetologia. 2011;54:2900–2910. doi: 10.1007/s00125-011-2274-6. [DOI] [PubMed] [Google Scholar]
- 37.Hollister LE, Reaven GM. Delta-9-tetrahydrocannabinol and glucose tolerance. Clin Pharmacol Ther. 1974;16:297–302. doi: 10.1002/cpt1974162297. [DOI] [PubMed] [Google Scholar]
- 38.Bermudez-Siva FJ, Serrano A, Diaz-Molina FJ, et al. Activation of cannabinoid CB1 receptors induces glucose intolerance in rats. Eur J Pharmacol. 2006;531:282–284. doi: 10.1016/j.ejphar.2005.12.016. [DOI] [PubMed] [Google Scholar]
- 39.Chanda D, Kim DK, Li T, et al. Cannabinoid receptor type 1 (CB1R) signaling regulates hepatic gluconeogenesis via induction of ER-bound transcription factor CREBH in primary hepatocytes. J Biol Chem. 2011;286:27971–27979. doi: 10.1074/jbc.M111.224352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Eckardt K, Sell H, Taube A, et al. Cannabinoid type 1 receptors in human skeletal muscle cells participate in the negative crosstalk between fat and muscle. Diabetologia. 2009;52:664–674. doi: 10.1007/s00125-008-1240-4. [DOI] [PubMed] [Google Scholar]
- 41.Song D, Bandsma RH, Xiao C, et al. Acute cannabinoid receptor type 1 (CB1R) modulation influences insulin sensitivity by an effect outside the central nervous system in mice. Diabetologia. 2011;54:1181–1189. doi: 10.1007/s00125-011-2082-z. [DOI] [PubMed] [Google Scholar]
- 42.Bajzer M, Olivieri M, Haas MK, et al. Cannabinoid receptor 1 (CB1) antagonism enhances glucose utilisation and activates brown adipose tissue in diet-induced obese mice. Diabetologia. 2011;54:3121–3131. doi: 10.1007/s00125-011-2302-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Nogueiras R, Veyrat-Durebex C, Suchanek PM, et al. Peripheral, but not central, CB1 antagonism provides food intake-independent metabolic benefits in diet-induced obese rats. Diabetes. 2008;57:2977–2991. doi: 10.2337/db08-0161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Kim W, Doyle ME, Liu Z, et al. Cannabinoids inhibit insulin receptor signaling in pancreatic beta-cells. Diabetes. 2011;60:1198–1209. doi: 10.2337/db10-1550. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Farris W, Mansourian S, Leissring MA, et al. Partial loss-of-function mutations in insulin-degrading enzyme that induce diabetes also impair degradation of amyloid beta-protein. Am J Pathol. 2004;164:1425–1434. doi: 10.1016/s0002-9440(10)63229-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Karamohamed S, Demissie S, Volcjak J, et al. Polymorphisms in the insulin-degrading enzyme gene are associated with type 2 diabetes in men from the NHLBI Framingham Heart Study. Diabetes. 2003;52:1562–1567. doi: 10.2337/diabetes.52.6.1562. [DOI] [PubMed] [Google Scholar]
- 47.Sladek R, Rocheleau G, Rung J, et al. A genome-wide association study identifies novel risk loci for type 2 diabetes. Nature. 2007;445:881–885. doi: 10.1038/nature05616. [DOI] [PubMed] [Google Scholar]
- 48.Gregor MF, Yang L, Fabbrini E, et al. Endoplasmic reticulum stress is reduced in tissues of obese subjects after weight loss. Diabetes. 2009;58:693–700. doi: 10.2337/db08-1220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Wang Y, Vera L, Fischer WH, et al. The CREB coactivator CRTC2 links hepatic ER stress and fasting gluconeogenesis. Nature. 2009;460:534–537. doi: 10.1038/nature08111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Salazar M, Carracedo A, Salanueva IJ, et al. Cannabinoid action induces autophagy-mediated cell death through stimulation of ER stress in human glioma cells. J Clin Invest. 2009;119:1359–1372. doi: 10.1172/JCI37948. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Cunard R, Sharma K. The endoplasmic reticulum stress response and diabetic kidney disease. Am J Physiol Renal Physiol. 2011;300:F1054–F1061. doi: 10.1152/ajprenal.00021.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Sabio G, Cavanagh-Kyros J, Barrett T, et al. Role of the hypothalamic-pituitary-thyroid axis in metabolic regulation by JNK1. Genes Dev. 2010;24:256–264. doi: 10.1101/gad.1878510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Sabio G, Cavanagh-Kyros J, Ko HJ, et al. Prevention of steatosis by hepatic JNK1. Cell Metab. 2009;10:491–498. doi: 10.1016/j.cmet.2009.09.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Stebbins JL, De SK, Machleidt T, et al. Identification of a new JNK inhibitor targeting the JNK-JIP interaction site. Proc Natl Acad Sci U S A. 2008;105:16809–16813. doi: 10.1073/pnas.0805677105. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.