Abstract
The complex of the 39-amino inhibitor (potato) of bovine carboxypeptidase A (carboxypeptidase; peptidyl-L-amino-acid hydrolase, EC 3.4.12.2) was crystallized in space group P32. There are two protein-inhibitor complexes in the asymmetric unit. These crystals exhibited pseudo-P3221 symmetry due to twinning about the a3 axis. Heavy atom difference Patterson maps and rotation functions indicated, however, that the noncrystallographic twofold axis that relates these two complexes is nearly coincident with the a3 axis. Consequently, to a good approximation at low resolution, the space group of the complex is P3221 and the effects of twinning may be ignored. The structure was solved by using multiple isomorphous replacement and molecular replacement techniques. At 5.5-A resolution, the multiple isomorphous replacement map was readily interpretable in terms of the known native carboxypeptidase A structure plus extra density around the active site. The position of this extra density is consistent with the binding mode for extended substrate proposed from earlier model building studies with the native enzyme (Lipscomb, W.N., Hartsuck, J.A., Reeke, G.N., Quiocho, F.A. Bethge, P.H., Ludwig, M.L., Steitz, T.A., Muirhead, H. & Coppola, J.C. (1968) Brookhaven Symp. Biol. 21, 24-90).
Full text
PDF
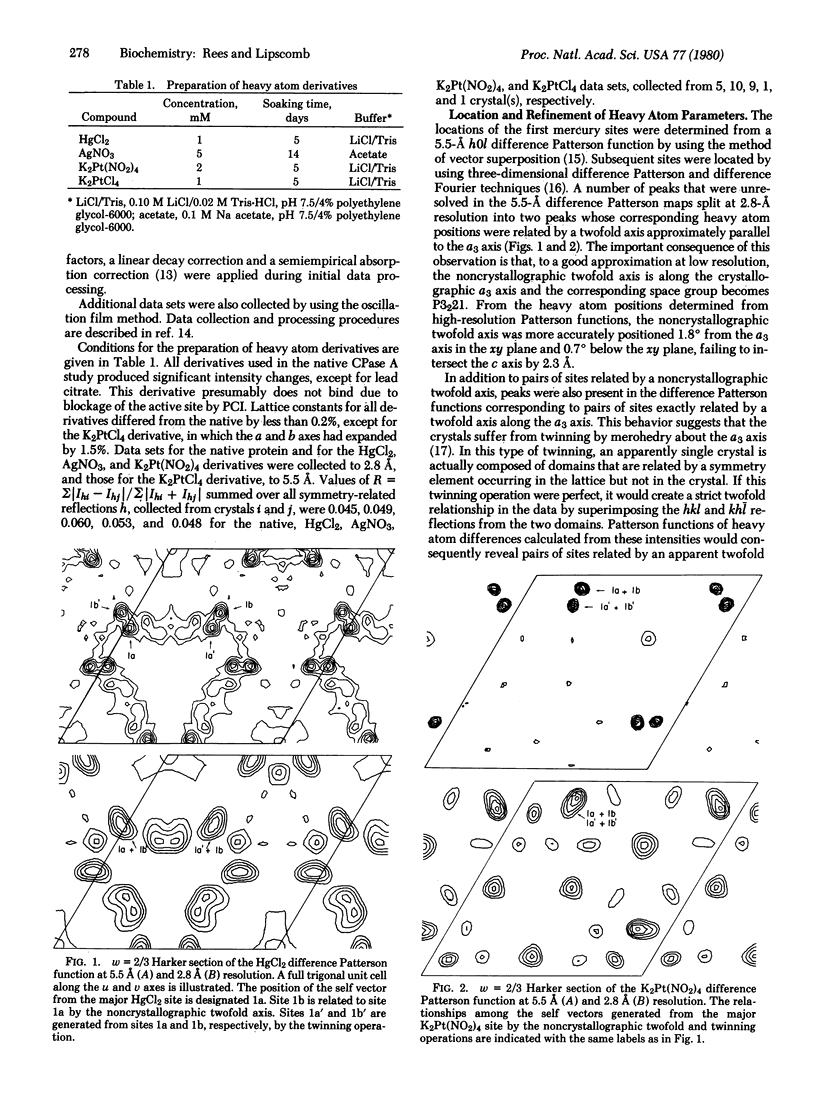
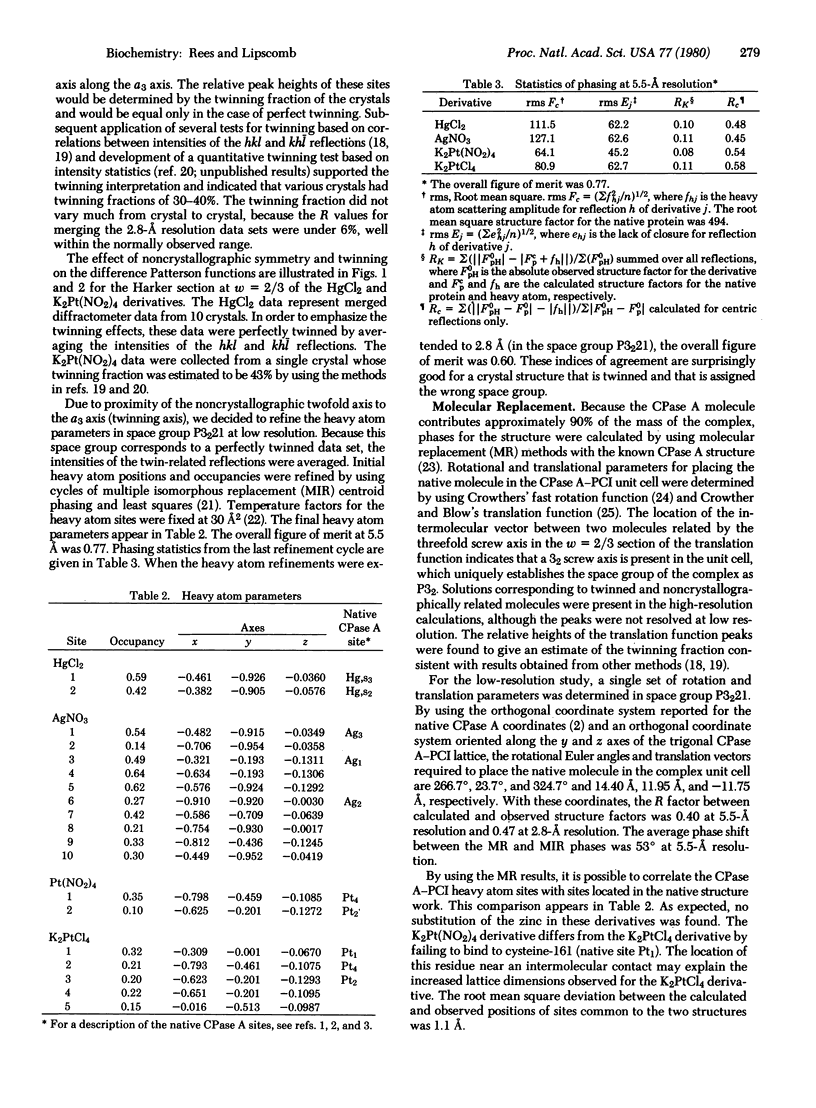

Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Abramowitz N., Schechter I., Berger A. On the size of the active site in proteases. II. Carboxypeptidase-A. Biochem Biophys Res Commun. 1967 Dec 29;29(6):862–867. doi: 10.1016/0006-291x(67)90299-9. [DOI] [PubMed] [Google Scholar]
- Hass G. M., Ako H., Grahn D. T., Neurath H. Carboxypeptidase inhibitor from potatoes. The effects of chemical modifications on inhibitory activity. Biochemistry. 1976 Jan 13;15(1):93–100. doi: 10.1021/bi00646a015. [DOI] [PubMed] [Google Scholar]
- Hass G. M., Nau H., Biemann K., Grahn D. T., Ericsson L. H., Neurath H. The amino acid sequence of a carboxypeptidase inhibitor from potatoes. Biochemistry. 1975 Mar 25;14(6):1334–1342. doi: 10.1021/bi00677a036. [DOI] [PubMed] [Google Scholar]
- Leary T. R., Grahn D. T., Neurath H., Hass G. M. Structure of potato carboxypeptidase inhibitor: disulfide pairing and exposure of aromatic residues. Biochemistry. 1979 May 29;18(11):2252–2256. doi: 10.1021/bi00578a018. [DOI] [PubMed] [Google Scholar]
- Lipscomb W. N., Hartsuck J. A., Reeke G. N., Jr, Quiocho F. A., Bethge P. H., Ludwig M. L., Steitz T. A., Muirhead H., Coppola J. C. The structure of carboxypeptidase A. VII. The 2.0-angstrom resolution studies of the enzyme and of its complex with glycyltyrosine, and mechanistic deductions. Brookhaven Symp Biol. 1968 Jun;21(1):24–90. [PubMed] [Google Scholar]
- McPherson A., Jr Crystallization of proteins from polyethylene glycol. J Biol Chem. 1976 Oct 25;251(20):6300–6303. [PubMed] [Google Scholar]
- Quiocho F. A., Lipscomb W. N. Carboxypeptidase A: a protein and an enzyme. Adv Protein Chem. 1971;25:1–78. doi: 10.1016/s0065-3233(08)60278-8. [DOI] [PubMed] [Google Scholar]
- Ryan C. A., Hass G. M., Kuhn R. W. Purification and properties of a carboxypeptidase inhibitor from potatoes. J Biol Chem. 1974 Sep 10;249(17):5495–5499. [PubMed] [Google Scholar]
- Tulinsky A., Vandlen R. L., Morimoto C. N., Mani N. V., Wright L. H. Variability in the tertiary structure of alpha-chymotrypsin at 2.8-A resolution. Biochemistry. 1973 Oct 9;12(21):4185–4192. doi: 10.1021/bi00745a023. [DOI] [PubMed] [Google Scholar]
- Warren S. G., Edwards B. F., Evans D. R., Wiley D. C., Lipscomb W. N. Aspartate transcarbamoylase from Escherichia coli: electron density at 5.5 A resolution. Proc Natl Acad Sci U S A. 1973 Apr;70(4):1117–1121. doi: 10.1073/pnas.70.4.1117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wright C. S. The crystal structure of wheat germ agglutinin at 2-2 A resolution. J Mol Biol. 1977 Apr 25;111(4):439–457. doi: 10.1016/s0022-2836(77)80063-6. [DOI] [PubMed] [Google Scholar]
- Wyckoff H. W., Doscher M., Tsernoglou D., Inagami T., Johnson L. N., Hardman K. D., Allewell N. M., Kelly D. M., Richards F. M. Design of a diffractometer and flow cell system for X-ray analysis of crystalline proteins with applications to the crystal chemistry of ribonuclease-S. J Mol Biol. 1967 Aug 14;27(3):563–578. doi: 10.1016/0022-2836(67)90059-9. [DOI] [PubMed] [Google Scholar]




