Abstract
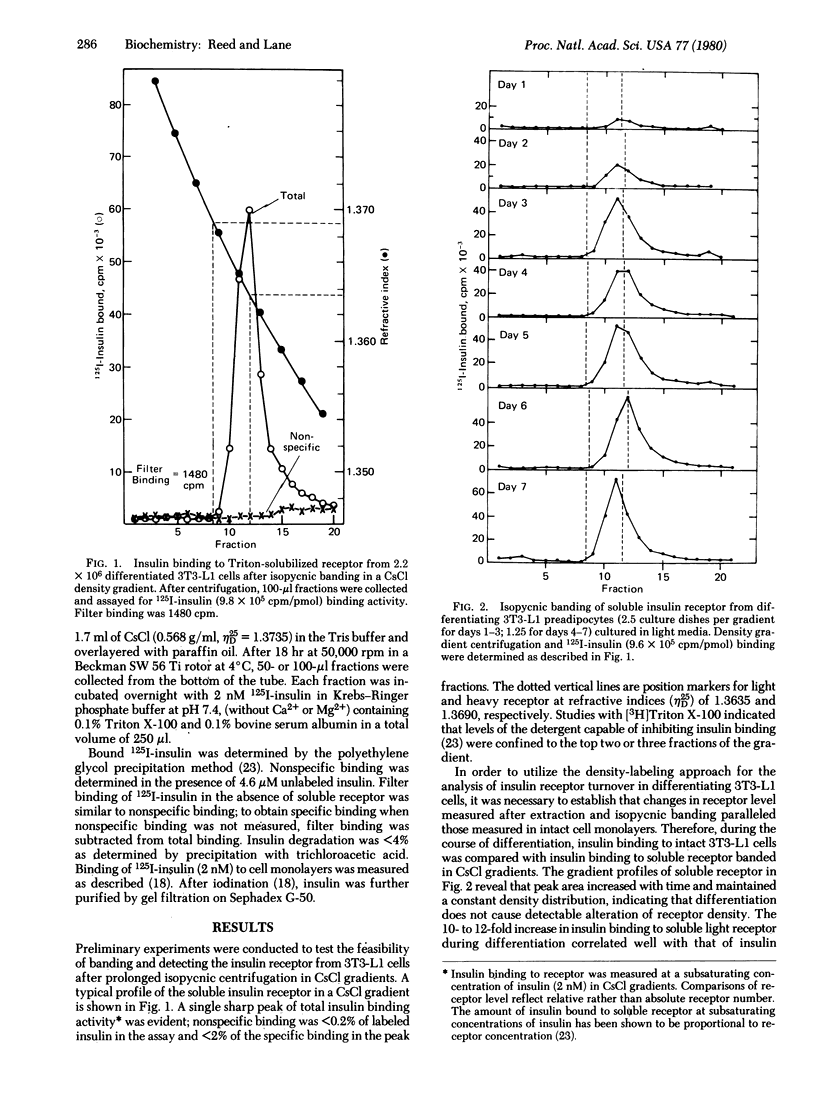
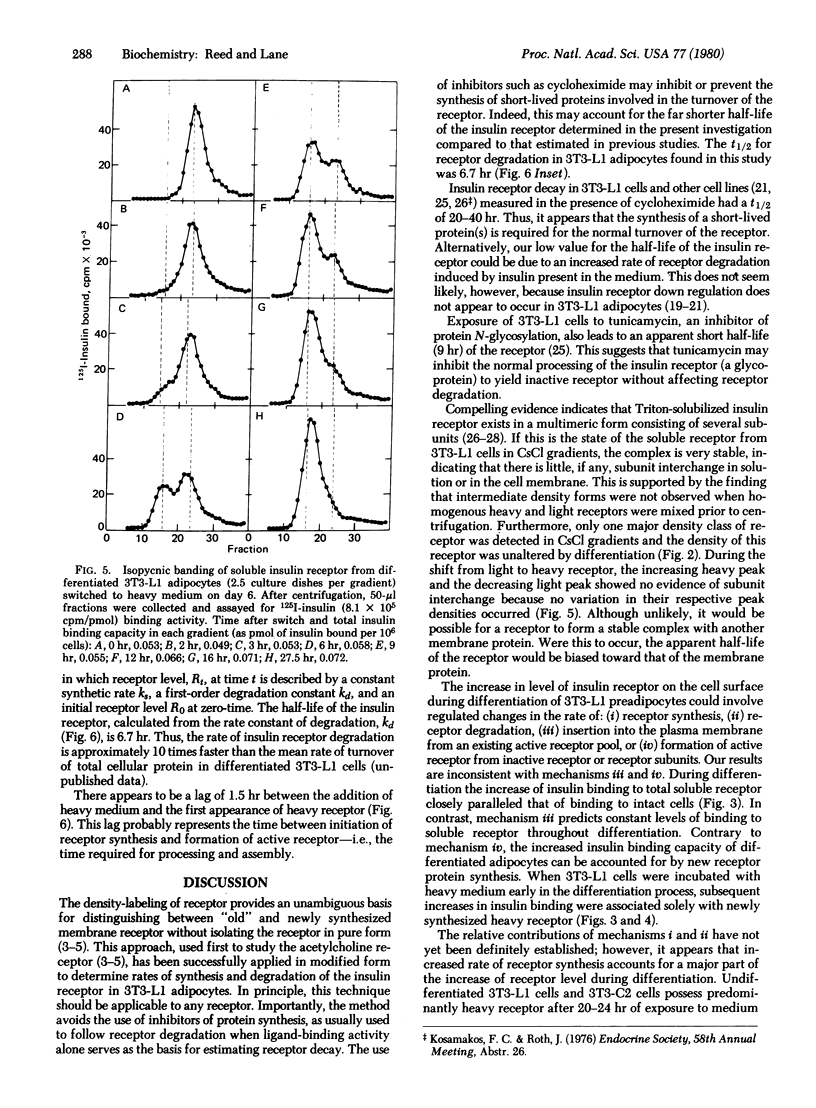
A density-shift method is described for analyzing insulin receptor synthesis and turnover in cultured cells labeled with "heavy" amino acids (2H, 13C, and 15N). Solubilized newly synthesized heavy and old "light" receptors are separated by isopycnic banding on CsCl gradients and then quantitated. Insulin receptor synthesis and turnover were studied by this technique in 3T3-L1 preadipocytes which undergo an increase in insulin binding capacity during differentiation. The results indicate that the increase in insulin binding capacity is a consequence of new receptor synthesis, that the insulin receptor has a relatively short half-life (6.7 hr), and that an increased rate of receptor synthesis contributes to the increase of insulin receptor level during differentiation.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Catt K. J., Harwood J. P., Aguilera G., Dufau M. L. Hormonal regulation of peptide receptors and target cell responses. Nature. 1979 Jul 12;280(5718):109–116. doi: 10.1038/280109a0. [DOI] [PubMed] [Google Scholar]
- Chang T. H., Polakis S. E. Differentiation of 3T3-L1 fibroblasts to adipocytes. Effect of insulin and indomethacin on the levels of insulin receptors. J Biol Chem. 1978 Jul 10;253(13):4693–4696. [PubMed] [Google Scholar]
- Coleman R. A., Reed B. C., Mackall J. C., Student A. K., Lane M. D., Bell R. M. Selective changes in microsomal enzymes of triacylglycerol phosphatidylcholine, and phosphatidylethanolamine biosynthesis during differentiation of 3T3-L1 preadipocytes. J Biol Chem. 1978 Oct 25;253(20):7256–7261. [PubMed] [Google Scholar]
- Cuatrecasas P., Hollenberg M. D. Membrane receptors and hormone action. Adv Protein Chem. 1976;30:251–451. doi: 10.1016/s0065-3233(08)60481-7. [DOI] [PubMed] [Google Scholar]
- Cuatrecasas P. Isolation of the insulin receptor of liver and fat-cell membranes (detergent-solubilized-( 125 I)insulin-polyethylene glycol precipitation-sephadex). Proc Natl Acad Sci U S A. 1972 Feb;69(2):318–322. doi: 10.1073/pnas.69.2.318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cuatrecasas P. Properties of the insulin receptor isolated from liver and fat cell membranes. J Biol Chem. 1972 Apr 10;247(7):1980–1991. [PubMed] [Google Scholar]
- Devreotes P. N., Gardner J. M., Fambrough D. M. Kinetics of biosynthesis of acetylcholine receptor and subsequent incorporation into plasma membrane of cultured chick skeletal muscle. Cell. 1977 Mar;10(3):365–373. doi: 10.1016/0092-8674(77)90023-x. [DOI] [PubMed] [Google Scholar]
- Eckel R. H., Fujimoto W. Y., Brunzell J. D. Development of lipoprotein lipase in cultured 3T3-L1 cells. Biochem Biophys Res Commun. 1977 Sep 9;78(1):288–293. doi: 10.1016/0006-291x(77)91252-9. [DOI] [PubMed] [Google Scholar]
- Gardner J. M., Fambrough D. M. Acetylcholine receptor degradation measured by density labeling: effects of cholinergic ligands and evidence against recycling. Cell. 1979 Mar;16(3):661–674. doi: 10.1016/0092-8674(79)90039-4. [DOI] [PubMed] [Google Scholar]
- Ginsberg B. H., Kahn C. R., Roth J., De Meyts P. Insulin-induced dissociation of its receptor into subunits: possible molecular concomitant of negative cooperativity. Biochem Biophys Res Commun. 1976 Dec 20;73(4):1068–1074. doi: 10.1016/0006-291x(76)90232-1. [DOI] [PubMed] [Google Scholar]
- Green H., Kehinde O. An established preadipose cell line and its differentiation in culture. II. Factors affecting the adipose conversion. Cell. 1975 May;5(1):19–27. doi: 10.1016/0092-8674(75)90087-2. [DOI] [PubMed] [Google Scholar]
- Green H., Kehinde O. Spontaneous heritable changes leading to increased adipose conversion in 3T3 cells. Cell. 1976 Jan;7(1):105–113. doi: 10.1016/0092-8674(76)90260-9. [DOI] [PubMed] [Google Scholar]
- Hoffmann S. S., Kolodny G. M. Insulin receptors in 3T3 fibroblasts. Relationship to growth phase, transformation and differentiation into new cell types. Exp Cell Res. 1977 Jul;107(2):293–299. doi: 10.1016/0014-4827(77)90352-4. [DOI] [PubMed] [Google Scholar]
- Karlsson F. A., Grunfeld C., Kahn C. R., Roth J. Regulation of insulin receptors and insulin responsiveness in 3T3-L1 fatty fibroblasts. Endocrinology. 1979 May;104(5):1383–1392. doi: 10.1210/endo-104-5-1383. [DOI] [PubMed] [Google Scholar]
- Krupp M. N., Livingston J. N. Insulin binding to solubilized material from fat cell membranes: evidence for two binding species. Proc Natl Acad Sci U S A. 1978 Jun;75(6):2593–2597. doi: 10.1073/pnas.75.6.2593. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuri-Harcuch W., Green H. Increasing activity of enzymes on pathway of triacylglycerol synthesis during adipose conversion of 3T3 cells. J Biol Chem. 1977 Mar 25;252(6):2158–2160. [PubMed] [Google Scholar]
- Mackall J. C., Lane M. D. Role of pyruvate carboxylase in fatty acid synthesis: alterations during preadipocyte differentiation. Biochem Biophys Res Commun. 1977 Dec 7;79(3):720–725. doi: 10.1016/0006-291x(77)91171-8. [DOI] [PubMed] [Google Scholar]
- Mackall J. C., Student A. K., Polakis S. E., Lane M. D. Induction of lipogenesis during differentiation in a "preadipocyte" cell line. J Biol Chem. 1976 Oct 25;251(20):6462–6464. [PubMed] [Google Scholar]
- Maturo J. M., 3rd, Hollenberg M. D. Insulin receptor: interaction with nonreceptor glycoprotein from liver cell membranes. Proc Natl Acad Sci U S A. 1978 Jul;75(7):3070–3074. doi: 10.1073/pnas.75.7.3070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reed B. C., Kaufmann S. H., Mackall J. C., Student A. K., Lane M. D. Alterations in insulin binding accompanying differentiation of 3T3-L1 preadipocytes. Proc Natl Acad Sci U S A. 1977 Nov;74(11):4876–4880. doi: 10.1073/pnas.74.11.4876. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rubin C. S., Hirsch A., Fung C., Rosen O. M. Development of hormone receptors and hormonal responsiveness in vitro. Insulin receptors and insulin sensitivity in the preadipocyte and adipocyte forms of 3T3-L1 cells. J Biol Chem. 1978 Oct 25;253(20):7570–7578. [PubMed] [Google Scholar]
- Rubin C. S., Lai E., Rosen O. M. Acquisition of increased hormone sensitivity during in vitro adipocyte development. J Biol Chem. 1977 May 25;252(10):3554–3557. [PubMed] [Google Scholar]
- Spooner P. M., Chernick S. S., Garrison M. M., Scow R. O. Development of lipoprotein lipase activity and accumulation of triacylglycerol in differentiating 3T3-L1 adipocytes. Effects of prostaglandin F2alpha, 1-methyl-3-isobutylxanthine, prolactin, and insulin. J Biol Chem. 1979 Feb 25;254(4):1305–1311. [PubMed] [Google Scholar]
- Tell G. P., Haour F., Saez J. M. Hormonal regulation of membrane receptors and cell responsiveness: a review. Metabolism. 1978 Oct;27(10):1566–1592. doi: 10.1016/s0026-0495(78)80029-8. [DOI] [PubMed] [Google Scholar]
- Wise L. S., Green H. Studies of lipoprotein lipase during the adipose conversion of 3T3 cells. Cell. 1978 Feb;13(2):233–242. doi: 10.1016/0092-8674(78)90192-7. [DOI] [PubMed] [Google Scholar]



