Abstract
Background & Aims
Progression of liver fibrosis in experimental models depends on gut-derived bacterial products, but little is known about mechanisms of disruption of the mucosal barrier or translocation. We used a mouse model of cholestatic liver disease to investigate mechanisms of intestinal barrier disruption following liver injury.
Methods
Liver fibrosis and bacterial translocation were assessed in Toll-like receptor (TLR)2- and tumor necrosis factor receptor (TNFR)I-deficient mice subjected to bile duct ligation. Epithelial and lamina propria cells were isolated and analyzed by immunoblot analyses and flow cytometry. We analyzed bone-marrow chimeras and mice with a conditional gain-of-function allele for the TNFRI receptor. By crossing TNFRIflxneo/flxneo mice with mice that expressed the VillinCre transgene specifically in intestinal epithelial cells, we created mice that express functional TNFRI specifically on intestinal epithelial cells (VillinCreTNFRIflxneo/flxneo mice).
Results
Following bile duct ligation, TLR2-deficient mice had less liver fibrosis and intestinal translocation of bacteria and bacterial products than wild-type mice. Mice with hematopoietic cells that did not express TLR2 also had reduced bacterial translocation, indicating that TLR2 expression by hematopoietic cells regulates intestinal barrier function. The number of TLR2+ monocytes that produce TNF_ increased in the intestinal lamina propria of wild-type mice following bile duct ligation; bacterial translocation was facilitated by TNFRI-mediated signals on intestinal epithelial cells.
Conclusion
Intestinal inflammation and bacterial translocation contribute to liver fibrosis via TLR2 signaling on monocytes in the lamina propria and TNFRI signaling on intestinal epithelial cells in mice. Enteric TNFRI therefore is an important mediator of cholestatic liver fibrosis.
Keywords: microbiome, endotoxin, intestinal inflammation, microbiota composition
Introduction
Liver fibrosis is triggered upon chronic liver damage caused by various etiologies, including viral hepatitis, alcohol abuse, chronic cholestasis, and non-alcoholic steatohepatitis (NASH). Fibrosis can progress to cirrhosis as endstage liver disease, which is the 12th most common cause of death in the United States 1. There is an emerging concept that gut-derived bacterial products resulting from bacterial translocation play a central role in the initiation of acute liver injury and progression to chronic liver disease. In rodents, experimental bacterial overgrowth in the intestine induces bacterial translocation as defined by crossing of viable bacteria or bacterial products across the intestinal epithelial lining, and subsequent liver damage 2, 3. In humans, patients with chronic liver disease show intestinal bacterial overgrowth with bacterial translocation, and liver disease severity correlates with systemic levels of endotoxin (or lipopolysaccharide, LPS) 4. Reducing the intestinal bacterial burden by non-absorbable antibiotics reduces endotoxemia and improves chronic liver disease in preclinical animal models 5, 6 and patients 7. Further evidence for the critical involvement of gut-derived bacterial products in liver fibrogenesis comes from genetically modified animals. Mice that are either unresponsive to bacterial products by a deficiency or mutation in Toll-like receptor (TLR)-4 and 9 6, 8, or lack molecules propagating TLR signaling events triggered by bacterial products 9, 10 are resistant to experimental liver fibrosis. Nevertheless, the exact molecular mechanism of increased intestinal permeability and liver fibrosis are not known. In this study we use an animal model of cholestatic liver fibrosis to demonstrate that TLR2+ monocytes in the intestinal lamina propria mediate intestinal barrier disruption by production of TNFα. TNFRI on enterocytes mediates tight junction disruption by activation of the RhoA/myosin II regulatory light chain (MLC) pathway.
Materials and Methods
Animal models of liver disease
TNFRI−/−11 and transgenic mice expressing GFP under the control of β-actin promoter 6 have been described. TNFRIflxneo/flxneo 12 and VillinCreTNFRIflxneo/flxneo 13 were kindly provided by Drs. Manolis Roulis and George Kollias (Biomedical Sciences Research Center ‘Alexander Fleming’, Vari, Greece), and littermates were used for the experiments. TLR2-deficient mice that were originally purchased from Jackson Laboratory were rederived by cesarean section and housed in a specific pathogen free vivarium. Age-matched male mice on a C57BL/6 genetic background were used for the study except when stated otherwise. All animals received humane care in compliance with institutional guidelines. Mice had access to water and irradiated chow diet (PicoLabo Rodent Diet 20 5053) ad libitum. Ligation of the common bile duct or sham operations as control have been described2. One day, 10 days or 3 weeks after bile duct ligation or sham operation, mice were sacrificed for analysis.
Generation of bone marrow chimeric mice
Recipient mice were lethally irradiated with 1000 rads using a 137Cs source and injected intravenously 2–3 h later with approximately 5x106 bone marrow cells derived from the tibia and femur of donors as described 15. Eight weeks after engrafting, animal experiments were performed. To assess reconstitution, GFP expressing transgenic mice under a β-actin promoter were used as bone marrow donors with wild type mice as recipients. Engraftment was assessed by FACS analysis of peripheral blood and isolated intestinal lamina propria cells. After red blood cell lysis, peripheral blood and lamina propria cells were stained with PE-labeled CD45.2 (eBioscience). Cell suspensions were analyzed for the presence of intracellular GFP on a BD LSR II (BD Biosciences). Immunofluorescence was performed on frozen liver sections using anti-GFP (Abcam) and anti-F4/80 (eBioscience) as primary antibodies, and FITC- or Texas Red-conjugated secondary antibodies (Invitrogen).
Epithelial cell and lamina propria cell isolation
Intestines of mice were rinsed with cold PBS, opened longitudinally, and cut into pieces of 1-cm lengths. Intestinal pieces were placed in ice-cold RPMI1640 medium (Invitrogen) with 5% FCS containing DTT (1mM). After vigorous shaking the supernatant was discarded, and tissue was incubated for 20 min in RPMI1640 with 5% FCS containing EDTA (1 mM) at 37 °C with shaking (250 rpm). This step was repeated for a total of two times. Intestinal epithelial cells were collected from supernatant after the first incubation. For lamina propria cell isolation, tissue was further incubated in RPMI1640 with 5% FCS containing Collagenase type VIII (Sigma; 1mg/ml) for 30 min at 37 °C with shaking (250 rpm). Cell suspension was sieved through a cell strainer (100 μm; BD). Lamina propria cells were collected by centrifugation, stained with CD45.2, CD11b, TLR2, Lys6C, Lys6G, CD11c, F4/80, IL-6, or TNFα (all eBiosciences) and analyzed by FACS. Intracellular cytokine staining was performed using the BD Cytofix/Cytoperm kit according to the manufacturer’s instructions.
Bacterial cultures
To assess for bacterial translocation, mesenteric lymph nodes were harvested in a sterile fashion and cultured on Blood Agar Plates (containing 5% sheep blood) for total bacteria as described 2, 16. Plates were incubated for 48–72 hrs at 37°C in aerobic conditions followed by the counting of colony-forming units (CFUs).
To assess bacterial translocation from gastrointestinal segments, we used an intestinal loop model as we have previously described 2, 15, 17. E. coli serotype O74:K.:H39 isolated from a patient with peritonitis (ATCC#23535) was transfected with a GFP-expressing plasmid, which conferred chloramphenicol resistance 18. After anesthesia, a midline laparotomy incision was made. A 4cm long segment of the gastrointestinal tract (proximal, mid or distal small intestine, cecum or colon) was created with two vascular hemoclips without disrupting the mesenteric vascular arcades. The length of intestine between the two clips was injected with approximately 106 CFUs. After 4 hrs, mice were sacrificed and mesenteric lymph nodes were cultured on nutrient broth agar plates (containing chloramphenicol 20μg/ml). GFP-expressing CFUs were counted after 48–72 hrs using fluorescence microscopy. The number of translocated CFUs was adjusted for the total number of bacteria injected and expressed as CFUs per 1x106 injected bacteria.
Protein expression analysis
Formalin-fixed liver samples were embedded in paraffin and stained with Sirius red to assess fibrosis. The Sirius red positive area was measured at a final magnification of 40x. The entire median lobe of the liver was imaged using a polarizing filter and analyzed using NIH Image J as described 14. Results are expressed relative to wild type control mice. Frozen intestinal sections were stained using anti-occludin, anti-claudin-4 and anti-ZO-1 antibodies and a FITC-conjugated secondary antibody (all Invitrogen) as described 2. Nuclei are stained with Hoechst (blue). Control sections were stained with an isotype antibody instead of the primary antibody and showed no staining. Western blot analysis was performed as described 16 using anti-phospho MLC (Cell Signaling), anti-RhoA (Santa Cruz), anti-tubulin (Santa Cruz) or anti-β-actin (Sigma) antibodies. Western blots were quantitated using NIH Image J. Alanine Aminotransferase (ALT) levels were measured as described 16. TNFα was measured in the plasma using ELISA (eBioscience) with a detection limit ≤ 3.9pg/ml.
Statistical analysis
Mann-Whitney rank sum test was used for statistical analysis. All data are presented as mean ± SEM. p<0.05 was selected as the level of significance.
Other materials and methods are described in the Supplementary Materials and Methods section.
Results
TLR2 deficient mice are protected from cholestatic liver fibrosis
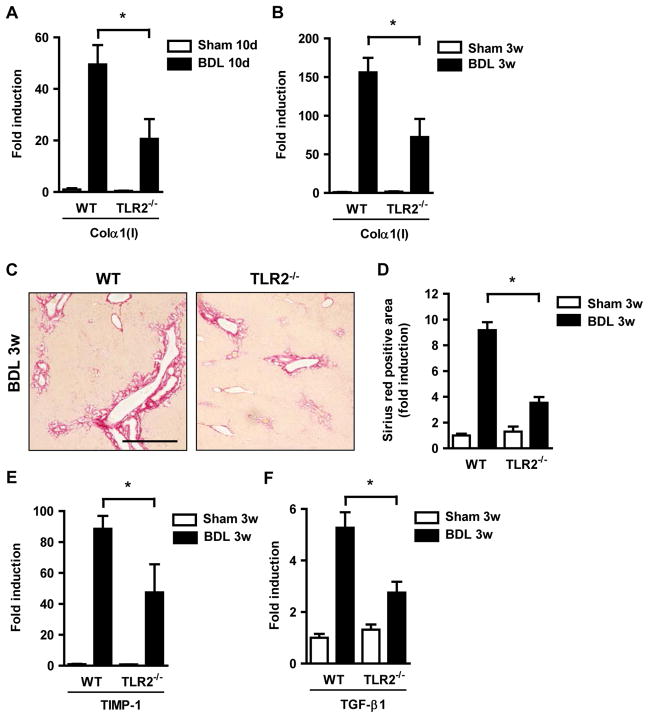
TLR2 is the receptor for products from Gram-positive bacteria such as peptidoglycan. Conflicting results about the importance of TLR2 in experimental liver injury and fibrosis have been published. While one study reported increased survival and protection from liver injury of TLR2 deficient mice following bile duct ligation for up to 30 days 19, another report showed no difference in bile duct ligation-induced liver fibrosis 6. As the phenotype in experimental disease models changes with the intestinal microflora 20 and to eliminate potential opportunistic pathogens present in these mice, TLR2 deficient mice were rederived and housed in a specific pathogen free environment. Mice were subjected to bile duct ligation as a common model for cholestatic liver fibrosis. Following bile duct ligation for 10 days or 3 weeks, TLR2−/− mice showed reduced fibrogenesis as evidenced by decreased hepatic gene expression of collagen α1(I) as compared with wild type C57BL/6 control mice (Fig. 1A and B). TLR2 deficient mice had a reduction in fibrosis as measured by Sirius red staining (Fig. 1C and D). This was associated with a decreased expression of fibrosis related genes, such as Tissue Inhibitor of Matrix Metalloproteinases (TIMP)-1 and Transforming Growth Factor-β1 (TGF-β1) as potent profibrogenic cytokines (Fig. 1E and F). Liver injury as measured by systemic ALT levels was not significantly different in wild type and TLR2−/− mice one day or 3 weeks following bile duct ligation (Supplementary Fig. 1) indicating that TLR2 does not mediate cholestatic hepatotoxicity, but fibrosis progression. Reduced hepatic fibrosis in TLR2−/− mice was confirmed in a second model of toxic liver injury and fibrosis using chronic injections of carbon tetrachloride (CCl4) (Supplementary Fig. 2).
Figure 1. Diminished hepatic fibrosis in TLR2−/− mice.
Wild type and TLR2−/− mice underwent sham operation (n=4) or bile duct ligation (BDL; n=8–13) for indicated time periods. (A and B) Collagen α1(I) mRNA expression was assessed in the liver using qPCR. (C and D) Hepatic fibrosis was assessed by Sirius red staining and quantified using morphometric analysis. Scale bar = 50μm. (E and F) Hepatic TIMP-1 and TGF-β1 mRNA levels were determined by qPCR. *p < 0.05.
Translocation of bacteria and their products is abolished in TLR2−/− mice following bile duct ligation
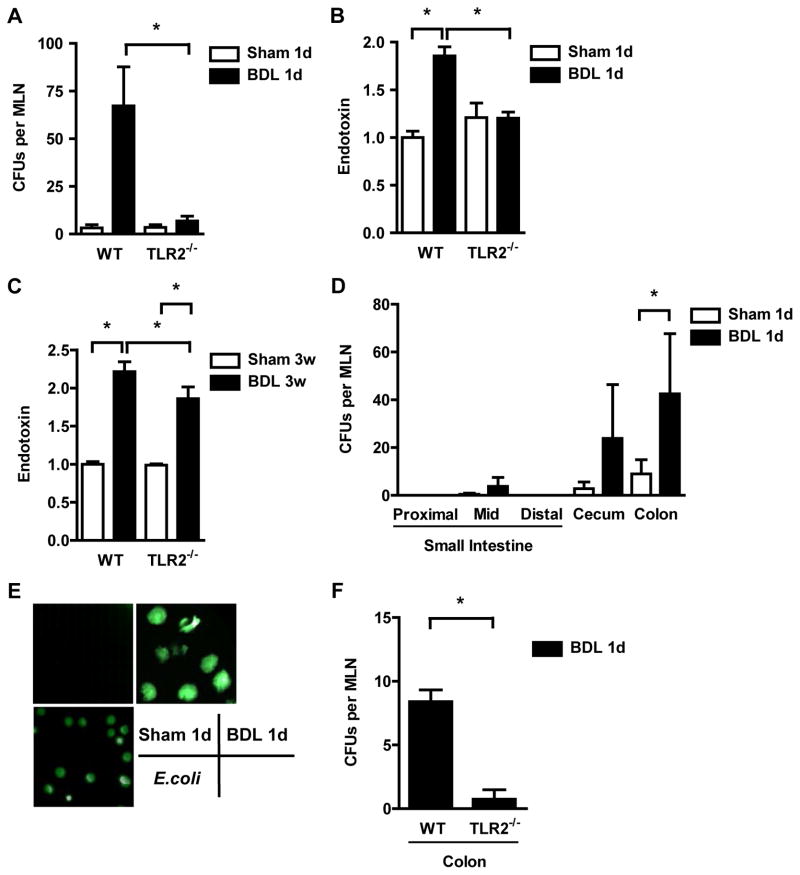
Liver fibrosis is thought to be promoted by translocation of bacteria or their products from the gut to the portal and systemic circulation 6, 8. We therefore investigated whether TLR2 is involved in regulating intestinal barrier function and bacterial translocation following the onset of liver injury and fibrosis. As a measure of bacterial translocation across the mucosal barrier, viable bacteria were quantified in mesenteric lymph nodes as the first organ encountered in the translocation route from the gastrointestinal tract to the portal vein. TLR2−/− mice showed significantly less positive mesenteric lymph node cultures as compared to wild type mice following bile duct ligation for one day (Fig. 2A). Significantly lower systemic endotoxin levels were found in TLR2−/− mice following bile duct ligation for one day or 3 weeks as compared to wild type mice (Fig. 2B and C). To determine the gastrointestinal site of bacterial translocation in vivo, a ligated intestinal loop model was used as previously described by us 2, 15. A 4-cm loop of the proximal, mid or distal small intestine, cecum or colon was ligated (without disrupting the mesenteric vascular arcades and blood supply) in anesthetized mice which have undergone sham operations or bile duct ligation one day prior, and viable GFP-expressing E. coli were injected. At 4 hrs, mice were harvested, mesenteric lymph nodes cultured and translocated bacteria counted using fluorescent microscopy. The large intestine and in particular the colon showed a significant increase in translocated E. coli (Fig. 2D and E). This is consistent with our study demonstrating that the colon shows the largest increase in intestinal permeability following one day of bile duct ligation 2. Translocation of GFP expressing E. coli from colonic loops to mesenteric lymph nodes was markedly attenuated in TLR2 deficient mice as compared to wild type mice suggesting that TLR2 is involved in regulation of the intestinal barrier function (Fig. 2F).
Figure 2. TLR2−/− mice show less bacterial translocation following ligation of the common bile duct.
(A) Aerobic bacteria were cultured and quantified in mesenteric lymph nodes (MLN; n=10–12); CFU = colony forming unit. (B and C) Plasma endotoxin was measured (n=4 for sham, n=8–21 for bile duct ligation (BDL)). (D–E) GFP-expressing E. coli were injected into intestinal loops of BALB/c mice which underwent sham or BDL operations one day prior. MLN were cultured 4 hrs after injection (D; n=4 for small intestine, n=17 for cecum, n=28 for colon). Positive cultures showed GFP expression using fluorescence microscopy. An image with GFP expressing E. coli as positive control is shown (E). (F) Translocation of GFP-E. coli from colonic loops to MLN was assessed in wild type (n=4) and TLR2−/− mice (n=5) one day following BDL. *p<0.05.
TLR2-mediated signaling in hematopoietic cells is required for bacterial translocation following cholestatic liver injury
To investigate whether innate immune signaling in hematopoietic or non-hematopoietic cells mediate bacterial translocation following liver injury, we generated bone marrow chimeric mice in which donor and/or recipients were either wild type or TLR2 deficient. To confirm successful bone marrow transplantation, control animals were transplanted with bone marrow cells from mice expressing GFP under a β-actin promoter and studied 8 weeks after transplantation. Mice were found to be fully chimerized in peripheral blood, colonic lamina propria and liver, as assessed one day following bile duct ligation (Supplementary Fig. 3A and B).
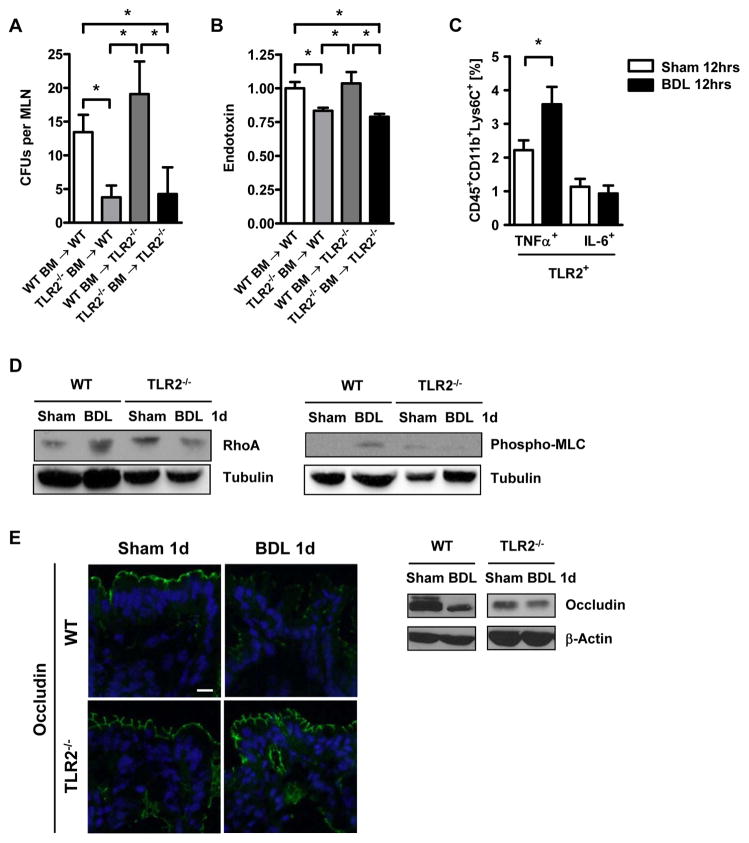
To determine the impact of TLR2-mediated signaling in hematopoietic or non-hematopoietic cells on bacterial translocation, mesenteric lymph nodes from chimeric mice were cultured one day after bile duct ligation. Chimeric mice lacking TLR2 on hematopoietic cells were protected from translocation of bacteria to the mesenteric lymph nodes. In contrast, transplant recipients expressing TLR2 on hematopoietic cells showed bacteria translocated to the mesenteric lymph nodes, regardless of whether the bone marrow recipient was wild type or TLR2 deficient (Fig. 3A). Similarly, plasma endotoxin levels were lowest in transplant recipients lacking TLR2 on hematopoietic cells (Fig. 3B). These findings indicate that TLR2 expression on hematopoietic cells mediates bacterial translocation following cholestatic liver injury.
Figure 3. Bacterial translocation is dependent on TLR2 signals from hematopoietic cells.
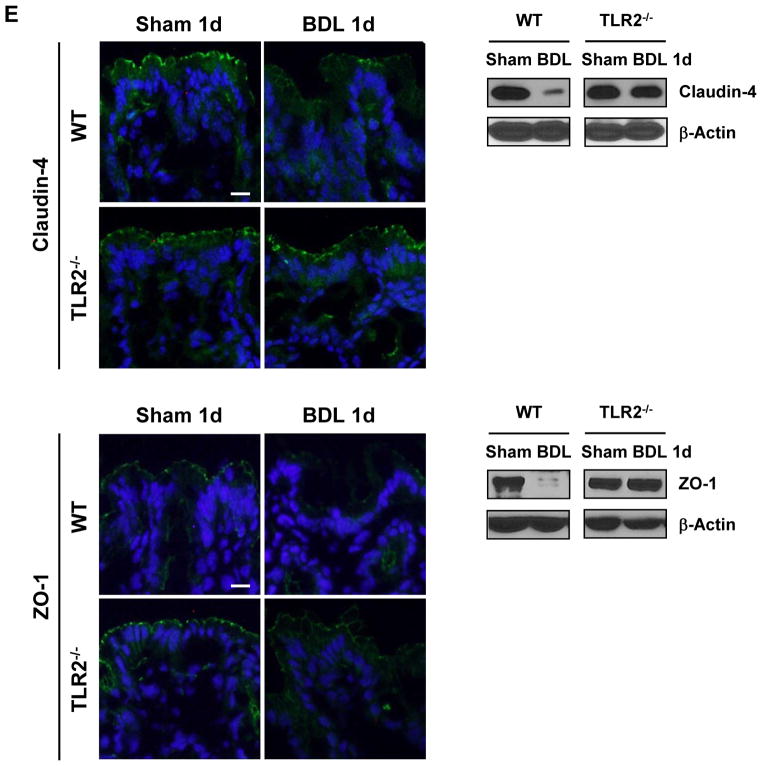
(A and B) Colony forming units (CFUs) were counted of mesenteric lymph nodes cultures (n=8–23), and plasma endotoxin (n=4–15) was measured in wild type and TLR2 bone marrow chimeric mice one day following bile duct ligation (BDL). (C) TLR2+, intracellular TNFα+ (n=4–5 independent experiments), and TLR2+, IL-6+ expression (n=3 independent experiments) of CD45.2+, CD11b+, Lys6C+ cells, was assessed in colonic lamina propria of wild type mice. Lamina propria cells from three mice were pooled in each independent experiment. (D) RhoA, phospho-MLC and tubulin expression was assessed in isolated colon epithelial cells in wild type and TLR2−/− mice. A representative western blot is shown. (E) Occludin, claudin-4 and ZO-1 protein expression in intestinal sections of wild type and TLR2−/− mice were analyzed by immunofluorescence and western blot analysis. Representative fluorescent images of 3-4 independent experiments are shown. Scale bar = 1μm. *p<0.05.
TNFα expressing TLR2+ monocytes in the colonic lamina propria disrupts epithelial tight junctions resulting in bacterial translocation
Several studies demonstrate that intestinal inflammation caused by inflammatory cells in the lamina propria is involved in increasing intestinal permeability leading to translocation of bacteria or their products 21, 22. It is therefore possible that TLR2-mediated signals in cells from the mucosal innate immune system are required for the paracellular leakage pathway in the intestine. We therefore focused on the lamina propria cell infiltrate in the colon as the gastrointestinal site with the largest rate of bacterial translocation and increase in intestinal permeability (Fig. 2D). TNFα is a known downstream target of TLR2 in monocytes and macrophages 23 and it increases the permeability of the tight junction barrier in the intestine 24. We found that the number of infiltrating TLR2+ and TNFα+ monocytes (Fig. 3C), but not TLR2+ and TNFα+ macrophages, dendritic cells or neutrophils (Supplementary Fig. 4A-C), significantly increased 12 hrs after bile duct ligation. The percentage of TLR2+ expressing IL-6 as another proinflammatory cytokine was unchanged in monocytes 12 hrs following bile duct ligation as compared to sham operation (Fig. 3C). In contrast to a local secretion of TNFα, it is also conceivable that systemic TNFα is elevated in response to cholestatic liver injury. However, plasma TNFα levels were below the detection limit, both in sham and bile duct ligated animals 12 hrs following surgery (Supplementary Fig. 4D). TNFα has been reported to activate RhoA or myosin light chain kinase (MLCK) with a subsequent phosphorylation of myosin II regulatory light chain (MLC) 25–27. The degree of MLC activation correlates with local disease activity in patients with inflammatory bowel disease, suggesting that MLC may be regulated by local cytokine signaling rather than systemically 28. Colonic epithelial cells isolated from C57BL/6 mice that have undergone bile duct ligation showed higher protein levels of RhoA and more MLC phosphorylation, a downstream target of RhoA, compared to sham operated mice. This increase in RhoA activation and MLC phosphorylation was blunted in epithelial cells isolated from the colon of TLR2−/− mice following bile duct ligation (Fig. 3D, Supplementary Fig. 5A). However, no increase in the gene expression or phosphorylation of MLCK was observed in isolated colonic enterocytes following various time intervals of bile duct ligation (not shown). Although subsequent molecular events that cause increased intestinal permeability following phosphorylation of MLC are less clear, changes in tight junction protein expression such as occludin are essential effectors of the paracellular leakage pathway 21, 29. Therefore, the integrity of tight junction proteins of colonic epithelial cells was investigated using immunofluorescence and confirmed by western blotting. Following one day of cholestatic liver injury, staining for occludin protein was diminished as compared to sham operated wild type animals as we have reported previously 2. Occludin expression was unchanged in the colon of TLR2 deficient mice after bile duct ligation (Fig. 3E, Supplementary Fig. 5B). Similarly, expression of claudin-4 and zonula occludens (ZO)-1 were decreased one day following bile duct ligation in wild type mice, but not in TLR2−/− mice (Fig. 3E, Supplementary Fig. 5B). We also investigated whether increased cell death of colonic enterocytes might contribute to bacterial translocation, but we did not find a significant increase in apoptosis of the lining epithelial cells between sham and bile duct ligated wild type mice after one day (not shown). Taken together, bacterial translocation occurs following cholestatic liver injury and is facilitated by a disruption of tight junction proteins that involves a TLR2-induced TNFα secretion in lamina propria monocytes activating the RhoA/MLC pathway in enterocytes.
TNFRI deficient mice are protected from liver fibrosis and show decreased bacterial translocation
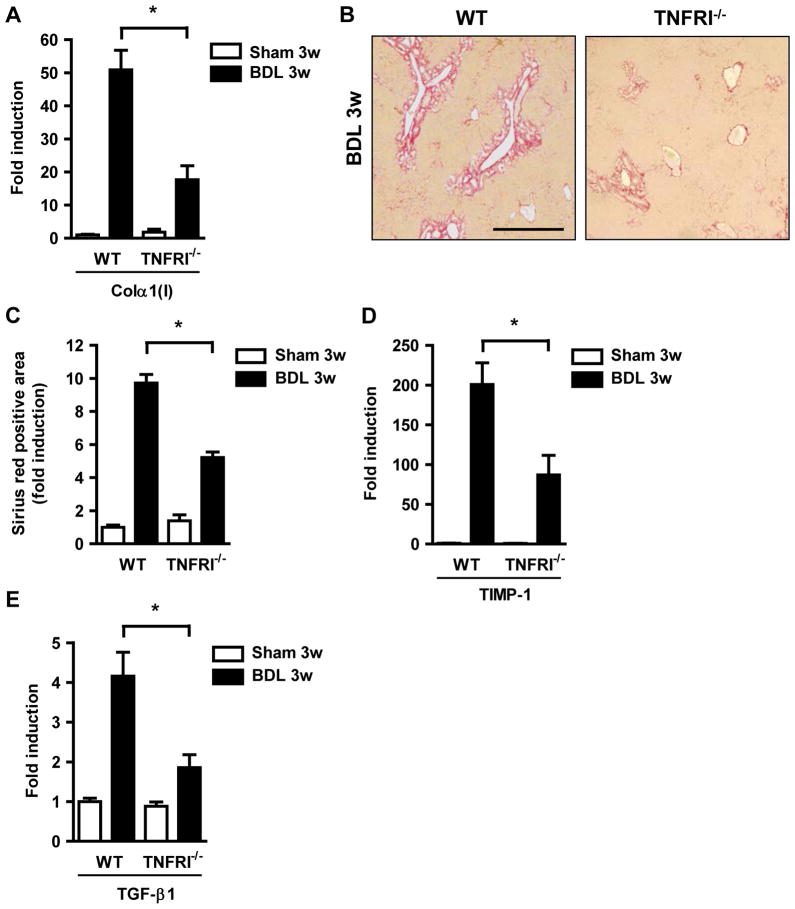
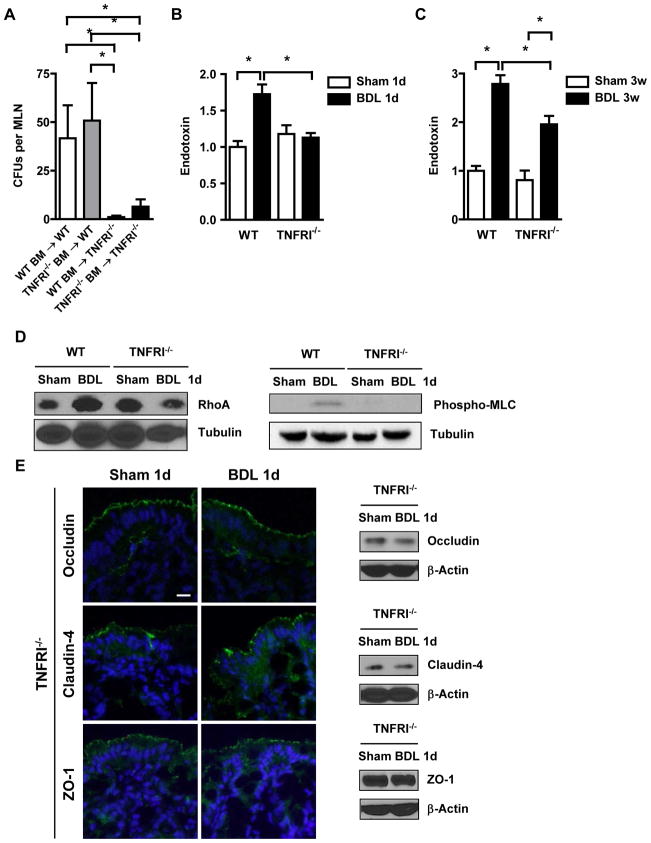
To further define the role of TNFα in mediating tight junction disruption following liver injury, we used TNFRI deficient mice and subjected them to bile duct ligation. TNFRI−/−, but not TNFR2−/−, mice show decreased hepatic fibrogenesis after four days of bile duct ligation 30. Consistent with this report, TNFRI−/− mice were protected from liver fibrosis as shown by decreased collagen α(I) gene expression and deposition of extracellular matrix proteins following bile duct ligation for 3 weeks (Fig. 4A–C). Hepatic gene expression of TIMP-1 and TGFβ-1 was also lower in TNFRI−/− mice following bile duct ligation as compared to C57BL/6 mice (Fig. 4D and E). Liver injury as measured by ALT levels were not statistically significant different in wild type and TNFRI−/− mice one day or 3 weeks following bile duct ligation consistent with previous studies 31, 32 (Supplementary Fig. 1). Moreover, bacterial translocation of viable bacteria to the mesenteric lymph nodes was abrogated in TNFRI−/− mice as compared to C57BL/6 mice after one day of cholestatic liver injury. Inhibition of bacterial translocation did not require TNFRI-mediated signals on hematopoietic cells, but instead was required in non-hematopoietic cells, presumably in intestinal epithelial cells, as demonstrated by bone marrow transplantation (Fig. 5A). Translocation of endotoxin to the systemic circulation was similarly abolished in TNFRI−/− mice following bile duct ligation for one day or 3 weeks (Fig. 5B and C). Consistent with decreased bacterial translocation, RhoA protein levels and MLC phosphorylation were not increased (Fig. 5D). Expression of the tight junction components occludin, claudin-4 and ZO-1 remained intact following bile duct ligation in TNFRI−/− mice (Fig. 5E). Thus, TNFRI on non-hematological cells mediates bacterial translocation following cholestatic liver injury.
Figure 4. TNFRI−/− mice are protected from liver fibrosis after bile duct ligation.
Wild type and TNFRI−/− mice underwent sham operation (n=4) or bile duct ligation (BDL; n=9–13) (A) Hepatic collagen α1(I) mRNA was measured by qPCR. (B and C) Collagen deposition was evaluated by Sirius red staining and quantitated by image analysis. Scale bar = 50μm. (D and E) Hepatic TIMP-1 and TGF-β1 gene expression were assessed by qPCR. *p < 0.05.
Figure 5. TNFRI on non-hematopoietic cells mediates bacterial translocation.
(A) Aerobic bacteria were cultured and quantified in mesenteric lymph nodes (n=7–10); CFU = colony forming unit. (B and C) Endotoxin levels in the plasma were measured (n=4 for sham, n=6–10 for bile duct ligation (BDL)). (D) RhoA, phospho-MLC and tubulin expression was analyzed in isolated epithelial cells from the intestine by western blotting. Images are representative of at least four independent western blots. (E) Immunofluorescent staining or western blotting for occludin, claudin-4 and ZO-1 was performed on colonic sections. Representative images of 3–4 independent experiments are shown. Scale bar = 1μm. *p<0.05.
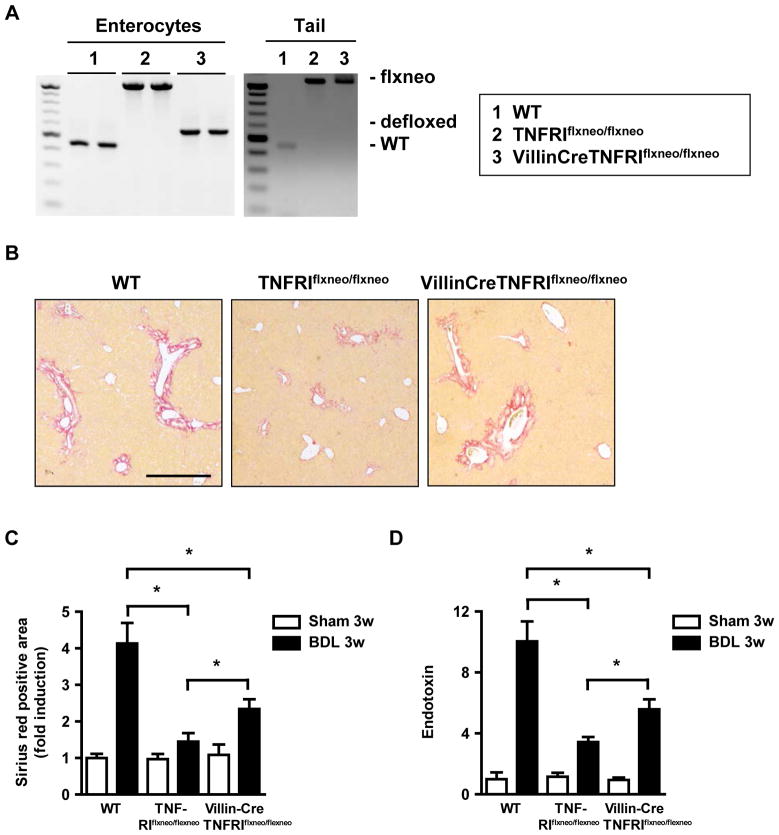
TNFRI deficient mice with reactivation of TNFRI on intestinal epithelial cells lose their protection against bile duct ligation-induced liver fibrosis
To confirm the intestinal epithelial cell-specific function of TNFRI in mediating bacterial translocation in liver fibrosis, we used mutant mice carrying a conditional gain-of-function allele for this receptor with an introduced loxP-flanked neomycin-resistance cassette in intron 5 of the murine p55TnfR gene (TNFRIflxneo/flxneo) 12. A nonfunctional TNFRI allele is engineered to be reactivated specifically in villin expressing intestinal epithelial cells by a Villin Cre-loxP-mediated recombination (VillinCreTNFRIflxneo/flxneo) 12. By crossing TNFRIflxneo/flxneo mice with the intestinal epithelial cell specific VillinCre transgenic mouse, a functional TNFRI is selectively expressed on intestinal epithelial cells, as analyzed by PCR 13. We confirmed the selective reactivation on isolated colonic epithelial cells (Fig. 6A). TNFRIflxneo/flxneo mice carrying a mutation in TNFRI are protected from tight junction disruption in the colon, while wild type mice show diminished expression of tight junction proteins one day following bile duct ligation. VillinCreTNFRIflxneo/flxneo mice that have a functional TNFRI selectively on intestinal epithelial cells show a disruption of tight junction proteins (Supplementary Fig. 6). VillinCreTNFRIflxneo/flxneo mice lose their protection against bile duct ligation-induced liver fibrosis as compared to TNFRIflxneo/flxneo. However, they still develop significantly less liver fibrosis as compared to C57BL/6 wild type mice (Fig. 6B and C). In addition, endotoxin levels were higher in VillinCreTNFRIflxneo/flxneo as compared to TNFRIflxneo/flxneo, but lower as compared to C57BL/6 wild type mice (Fig. 6D). In summary, TNFRI on intestinal epithelial cells contributes to the paracellular leakage pathway resulting in bacterial translocation and liver fibrogenesis.
Figure 6. Reactivation of TNFRI on intestinal epithelial cells contributes to liver fibrosis.
(A) Reactivation of TNFRI on isolated colonic epithelial in VillinCreTNFRIflxneo/flxneo was analyzed by PCR (left panel). Tails from respective mice served as controls (right panel). (B–D) Wild type, TNFRIflxneo/flxneo and VillinCreTNFRIflxneo/flxneo mice underwent sham operation (n=3–4) or bile duct ligation (BDL; n=6 for C57BL/6, n=9–10 for TNFRIflxneo/flxneo and for VillinCreTNFRIflxneo/flxneo mice). (B and C) Collagen deposition was evaluated by Sirius red staining and quantitated by image analysis. Scale bar = 50μm. (D) Plasma endotoxin levels were measured. *p < 0.05.
Discussion
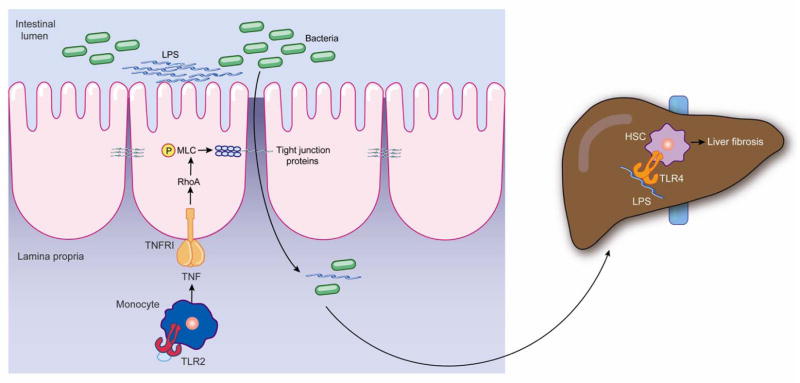
Bacterial translocation is a well characterized phenomenon in patients with liver disease 4. Although experimental studies suggest that liver disease progression, particularly fibrosis progression, is dependent on gut-derived bacterial products 6, 8–10, the molecular mechanisms leading to a leaky gut barrier are unknown. Our study provides important insights into changes of the intestinal barrier function associated with cholestatic liver injury that result in bacterial translocation and liver fibrosis. Following cholestatic liver injury, TLR2 expressing inflammatory monocytes in the lamina propria of the colon produce and secrete TNFα. TNFRI on enterocytes activates the RhoA/MLC pathway ultimately resulting in a disruption of tight junctions. We hypothesize that bacteria and their products 33 translocate across the mucosal barrier, reach the liver via the portal circulation and enhance liver fibrosis (Fig. 7). Taken together, this is the first study functionally linking an intestinal gene to progression of liver fibrosis in vivo.
Figure 7. Proposed model of bacterial translocation in cholestatic liver disease.
Following cholestatic liver injury, TLR2+ monocytes of the intestinal lamina propria are activated and produce TNFα. TNFα binds to TNFRI on enterocytes to activate the RhoA/MLC pathway resulting in disruption of tight junctions. Bacteria and bacterial products such as LPS cross the mucosal barrier to reach the liver via the portal circulation. LPS binds to TLR4 on hepatic stellate cells (HSC) and causes liver fibrosis.
TNFα levels produced by monocytes are increased in mesenteric lymph nodes indicative of an immune response to enteric bacteria in rats with cirrhosis. Non-absorbable antibiotics, which reduce the intestinal microflora and bacterial translocation to mesenteric lymph nodes, attenuate the expansion of monocytes in the mesenteric lymph nodes and normalize TNFα in the mesenteric lymph nodes 34. Most interestingly, bacterial translocation is reduced in cirrhotic rats treated with anti-TNFα antibodies 35. These findings support a role for TNFα in the disruption of the intestinal barrier as it might contribute to intestinal leakiness.
We demonstrate that enteric TNFRI mediates bacterial translocation in early stages after cholestasis. We show that MLC as a downstream target of RhoA activation is phosphorylated following cholestatic liver injury. MLC is activated and plays a central role as a common final pathway of barrier disruption in response to TNFα in enterocytes 21. Although bacterial translocation is reduced to baseline in early stages of cholestatic liver disease, endotoxin levels are higher in TNFRI deficient mice following 3 weeks of bile duct ligation as compared to sham operated mice. This indicates that later in disease, other pathways contribute to gut leakiness and subsequent bacterial translocation. This might include TNFR2 or other cytokines and their receptors such as interleukin-1β or interferon-γ that are known to play a role in the regulation of tight junction permeability 21. Similar to bacterial translocation, reactivation of TNFRI specifically on enterocytes increases liver fibrosis as compared to mice deficient in TNFRI, but the degree of fibrosis is still lower compared to wild type mice. TNFα regulates proliferation and extracellular matrix production in hepatic stellate cells by binding to TNFRI 30, which might account for differences in the fibrogenic response.
The question remains how is TNFRI on enterocytes activated? We have no evidence that systemic TNFα possibly coming from the injured liver reaches the TNFRI receptor on enterocytes via the bloodstream to cause tight junction disruption, because TNFα was undetectable in plasma early after bile duct ligation. Our experiments, however, established that TLR2+ monocytes, but not other inflammatory cells, are activated locally in the lamina propria of the colon to secrete TNFα and disrupt the tight junction barrier. We have previously reported a rapid onset of increased intestinal permeability and of intestinal bacterial overgrowth along the entire intestinal tract following bile duct ligation 2. It is therefore conceivable that an abundance of bacterial ligands in the intestinal lumen results also in a higher concentration of TLR2 ligands in the lamina propria to activate these cells and to cause intestinal inflammation. It is less likely that the monocytes are activated in the liver and migrate to the lamina propria to cause intestinal inflammation and initiate a paracelluar leakage pathway. TLR2 is expressed on Kupffer cells (Supplementary Fig. 7) and future studies need to address its contribution to liver fibrogenesis.
Our study demonstrates that intestinal TNFRI functionally contributes to cholestatic liver fibrosis by increasing intestinal permeability and facilitating translocation of bacterial products. Our findings are consistent with the large body of evidence that chronic liver disease and in particular liver fibrogenesis is driven by the gut emphasizing the importance of the gut-liver axis. Treatment targeting intestinal inflammation and bacterial translocation might contribute to the clinical management of patients with chronic liver disease.
Supplementary Material
Acknowledgments
The study was supported in part by NIH grants K08 DK081830, R01 AA020703 (to BS), by the UCSD Digestive Diseases Research Development Center (DK080506) (to BS), and by the AGA Fellowship to Faculty Transition Award (FFTA) (to BS). We thank Dr. Keiko Iwaisako for assistance with intestinal loops, Drs. Jerrold Turner, Alan Hofmann and Reiner Wiest for helpful discussion. We thank the Flow Cytometry Research Core Facility of the VA San Diego Healthcare System, the San Diego Center for AIDS Research (AI 36214) and the VA Research Center for AIDS & HIV Infection.
Abbreviations
- ALT
Alanine Aminotransferase
- BDL
bile duct ligation
- CCl4
carbon tetrachloride
- CFU
colony-forming unit
- HSC
hepatic stellate cells
- KC
Kupffer cells
- LPS
lipopolysaccharide
- MLC
myosin II regulatory light chain
- MLCK
myosin light chain kinase
- TGF-β1
Transforming Growth Factor-β1
- TIMP-1
Tissue Inhibitor of Matrix Metalloproteinases-1
- TLR
Toll-like receptor
- TNFRI
TNF receptor I
- ZO-1
zonula occludens
Footnotes
None of the authors has a financial, personal or professional conflict of interest to disclose.
P.H., M.H. and M.M. were responsible for data acquisition and analysis; D.A.B. and B.S. were responsible for the study concept and design, acquisition of data, analysis and interpretation of data, and writing of the manuscript.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Minino AM, Heron MP, Murphy SL, et al. Deaths: final data for 2004. Natl Vital Stat Rep. 2007;55:1–119. [PubMed] [Google Scholar]
- 2.Fouts DE, Torralba M, Nelson KE, et al. Bacterial translocation and changes in the intestinal microbiome in mouse models of liver disease. J Hepatol. 2012;56:1283–92. doi: 10.1016/j.jhep.2012.01.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Lichtman SN, Sartor RB, Keku J, et al. Hepatic inflammation in rats with experimental small intestinal bacterial overgrowth. Gastroenterology. 1990;98:414–23. doi: 10.1016/0016-5085(90)90833-m. [DOI] [PubMed] [Google Scholar]
- 4.Lin RS, Lee FY, Lee SD, et al. Endotoxemia in patients with chronic liver diseases: relationship to severity of liver diseases, presence of esophageal varices, and hyperdynamic circulation. J Hepatol. 1995;22:165–72. doi: 10.1016/0168-8278(95)80424-2. [DOI] [PubMed] [Google Scholar]
- 5.Enomoto N, Ikejima K, Bradford B, et al. Alcohol causes both tolerance and sensitization of rat Kupffer cells via mechanisms dependent on endotoxin. Gastroenterology. 1998;115:443–51. doi: 10.1016/s0016-5085(98)70211-2. [DOI] [PubMed] [Google Scholar]
- 6.Seki E, De Minicis S, Osterreicher CH, et al. TLR4 enhances TGF-beta signaling and hepatic fibrosis. Nat Med. 2007;13:1324–32. doi: 10.1038/nm1663. [DOI] [PubMed] [Google Scholar]
- 7.Cirera I, Bauer TM, Navasa M, et al. Bacterial translocation of enteric organisms in patients with cirrhosis. J Hepatol. 2001;34:32–7. doi: 10.1016/s0168-8278(00)00013-1. [DOI] [PubMed] [Google Scholar]
- 8.Gabele E, Muhlbauer M, Dorn C, et al. Role of TLR9 in hepatic stellate cells and experimental liver fibrosis. Biochem Biophys Res Commun. 2008;376:271–6. doi: 10.1016/j.bbrc.2008.08.096. [DOI] [PubMed] [Google Scholar]
- 9.Hritz I, Mandrekar P, Velayudham A, et al. The critical role of toll-like receptor (TLR) 4 in alcoholic liver disease is independent of the common TLR adapter MyD88. Hepatology. 2008;48:1224–31. doi: 10.1002/hep.22470. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Isayama F, Hines IN, Kremer M, et al. LPS signaling enhances hepatic fibrogenesis caused by experimental cholestasis in mice. Am J Physiol Gastrointest Liver Physiol. 2006;290:G1318–28. doi: 10.1152/ajpgi.00405.2005. [DOI] [PubMed] [Google Scholar]
- 11.Inokuchi S, Aoyama T, Miura K, et al. Disruption of TAK1 in hepatocytes causes hepatic injury, inflammation, fibrosis, and carcinogenesis. Proc Natl Acad Sci U S A. 2010;107:844–9. doi: 10.1073/pnas.0909781107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Victoratos P, Lagnel J, Tzima S, et al. FDC-specific functions of p55TNFR and IKK2 in the development of FDC networks and of antibody responses. Immunity. 2006;24:65–77. doi: 10.1016/j.immuni.2005.11.013. [DOI] [PubMed] [Google Scholar]
- 13.Roulis M, Armaka M, Manoloukos M, et al. Intestinal epithelial cells as producers but not targets of chronic TNF suffice to cause murine Crohn-like pathology. Proc Natl Acad Sci U S A. 2011;108:5396–401. doi: 10.1073/pnas.1007811108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Iwaisako K, Haimerl M, Paik YH, et al. Protection from liver fibrosis by a peroxisome proliferator-activated receptor delta agonist. Proc Natl Acad Sci U S A. 2012;109:E1369–76. doi: 10.1073/pnas.1202464109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Brandl K, Plitas G, Mihu CN, et al. Vancomycin-resistant enterococci exploit antibiotic-induced innate immune deficits. Nature. 2008;455:804–7. doi: 10.1038/nature07250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Yan AW, Fouts DE, Brandl J, et al. Enteric Dysbiosis Associated with a Mouse Model of Alcoholic Liver Disease. Hepatology. 2011;53:96–105. doi: 10.1002/hep.24018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Brandl K, Plitas G, Schnabl B, et al. MyD88-mediated signals induce the bactericidal lectin RegIII gamma and protect mice against intestinal Listeria monocytogenes infection. J Exp Med. 2007;204:1891–900. doi: 10.1084/jem.20070563. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Guzman LM, Belin D, Carson MJ, et al. Tight regulation, modulation, and high-level expression by vectors containing the arabinose PBAD promoter. J Bacteriol. 1995;177:4121–30. doi: 10.1128/jb.177.14.4121-4130.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Yin D, Zhang X, Xie N, et al. TLR2 promotes hepatocyte and cholangiocyte apoptosis via a p38-dependent mechanism. Hepatology. 2009;50:623A. [Google Scholar]
- 20.Vijay-Kumar M, Aitken JD, Carvalho FA, et al. Metabolic syndrome and altered gut microbiota in mice lacking Toll-like receptor 5. Science. 2010;328:228–31. doi: 10.1126/science.1179721. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Turner JR. Intestinal mucosal barrier function in health and disease. Nat Rev Immunol. 2009;9:799–809. doi: 10.1038/nri2653. [DOI] [PubMed] [Google Scholar]
- 22.Barreau F, Madre C, Meinzer U, et al. Nod2 regulates the host response towards microflora by modulating T cell function and epithelial permeability in mouse Peyer’s patches. Gut. 2010;59:207–17. doi: 10.1136/gut.2008.171546. [DOI] [PubMed] [Google Scholar]
- 23.Moresco EM, LaVine D, Beutler B. Toll-like receptors. Curr Biol. 2011;21:R488–93. doi: 10.1016/j.cub.2011.05.039. [DOI] [PubMed] [Google Scholar]
- 24.Taylor CT, Dzus AL, Colgan SP. Autocrine regulation of epithelial permeability by hypoxia: role for polarized release of tumor necrosis factor alpha. Gastroenterology. 1998;114:657–68. doi: 10.1016/s0016-5085(98)70579-7. [DOI] [PubMed] [Google Scholar]
- 25.McKenzie JA, Ridley AJ. Roles of Rho/ROCK and MLCK in TNF-alpha-induced changes in endothelial morphology and permeability. J Cell Physiol. 2007;213:221–8. doi: 10.1002/jcp.21114. [DOI] [PubMed] [Google Scholar]
- 26.Clayburgh DR, Barrett TA, Tang Y, et al. Epithelial myosin light chain kinase-dependent barrier dysfunction mediates T cell activation-induced diarrhea in vivo. J Clin Invest. 2005;115:2702–15. doi: 10.1172/JCI24970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Zolotarevsky Y, Hecht G, Koutsouris A, et al. A membrane-permeant peptide that inhibits MLC kinase restores barrier function in in vitro models of intestinal disease. Gastroenterology. 2002;123:163–72. doi: 10.1053/gast.2002.34235. [DOI] [PubMed] [Google Scholar]
- 28.Blair SA, Kane SV, Clayburgh DR, et al. Epithelial myosin light chain kinase expression and activity are upregulated in inflammatory bowel disease. Lab Invest. 2006;86:191–201. doi: 10.1038/labinvest.3700373. [DOI] [PubMed] [Google Scholar]
- 29.Shen L, Black ED, Witkowski ED, et al. Myosin light chain phosphorylation regulates barrier function by remodeling tight junction structure. J Cell Sci. 2006;119:2095–106. doi: 10.1242/jcs.02915. [DOI] [PubMed] [Google Scholar]
- 30.Tarrats N, Moles A, Morales A, et al. Critical role of tumor necrosis factor receptor 1, but not 2, in hepatic stellate cell proliferation, extracellular matrix remodeling, and liver fibrogenesis. Hepatology. 2011;54:319–27. doi: 10.1002/hep.24388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Simeonova PP, Gallucci RM, Hulderman T, et al. The role of tumor necrosis factor-alpha in liver toxicity, inflammation, and fibrosis induced by carbon tetrachloride. Toxicol Appl Pharmacol. 2001;177:112–20. doi: 10.1006/taap.2001.9304. [DOI] [PubMed] [Google Scholar]
- 32.Sudo K, Yamada Y, Moriwaki H, et al. Lack of tumor necrosis factor receptor type 1 inhibits liver fibrosis induced by carbon tetrachloride in mice. Cytokine. 2005;29:236–44. doi: 10.1016/j.cyto.2004.11.001. [DOI] [PubMed] [Google Scholar]
- 33.Kaser A, Ludwiczek O, Waldenberger P, et al. Endotoxin and its binding proteins in chronic liver disease: the effect of transjugular intrahepatic portosystemic shunting. Liver. 2002;22:380–7. doi: 10.1034/j.1600-0676.2002.01666.x. [DOI] [PubMed] [Google Scholar]
- 34.Munoz L, Albillos A, Nieto M, et al. Mesenteric Th1 polarization and monocyte TNF-alpha production: first steps to systemic inflammation in rats with cirrhosis. Hepatology. 2005;42:411–9. doi: 10.1002/hep.20799. [DOI] [PubMed] [Google Scholar]
- 35.Frances R, Chiva M, Sanchez E, et al. Bacterial translocation is downregulated by anti-TNF-alpha monoclonal antibody administration in rats with cirrhosis and ascites. J Hepatol. 2007;46:797–803. doi: 10.1016/j.jhep.2006.11.018. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.