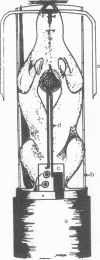
Abstract
High-resolution 31P nuclear magnetic resonance spectra at 73.83 MHz are reported for rat heart in vivo. In live rats, it was possible to observe the cardiac content of ATP, phosphocreatine, and Pi. Only a small amount of whole-blood 2,3-diphosphoglycerate was observed in the spectra, precluding the possibility that blood phosphate compounds were masking the spectra of cardiac phosphate compounds. The 31P nuclear magnetic resonance spectra of in vivo and perfused rat hearts were similar and support the utilization of the perfused rat heart as a model system for studying high-energy phosphate metabolism of the heart in vivo. The dynamic flux of high-energy phosphate compounds was investigated by subjecting the rat to respiratory arrest. In this experiment, the heart followed the classic metabolic pattern known to occur during cardiac arrest; phosphocreatine and then ATP decreased in concentration while Pi increased in concentration. The 31P nuclear magnetic resonance analysis of rat heart in vivo is demonstrated to be a practical and feasible method for studying cardiac high-energy phosphate metabolism.
Full text
PDF
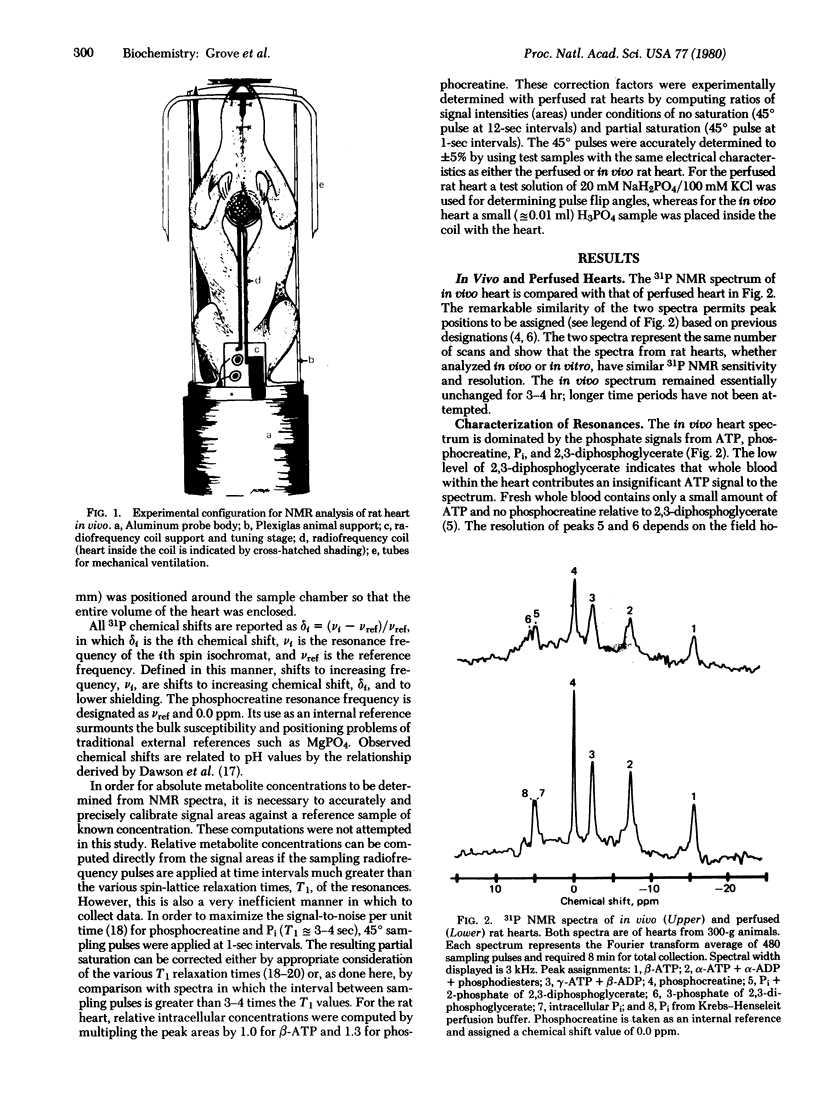
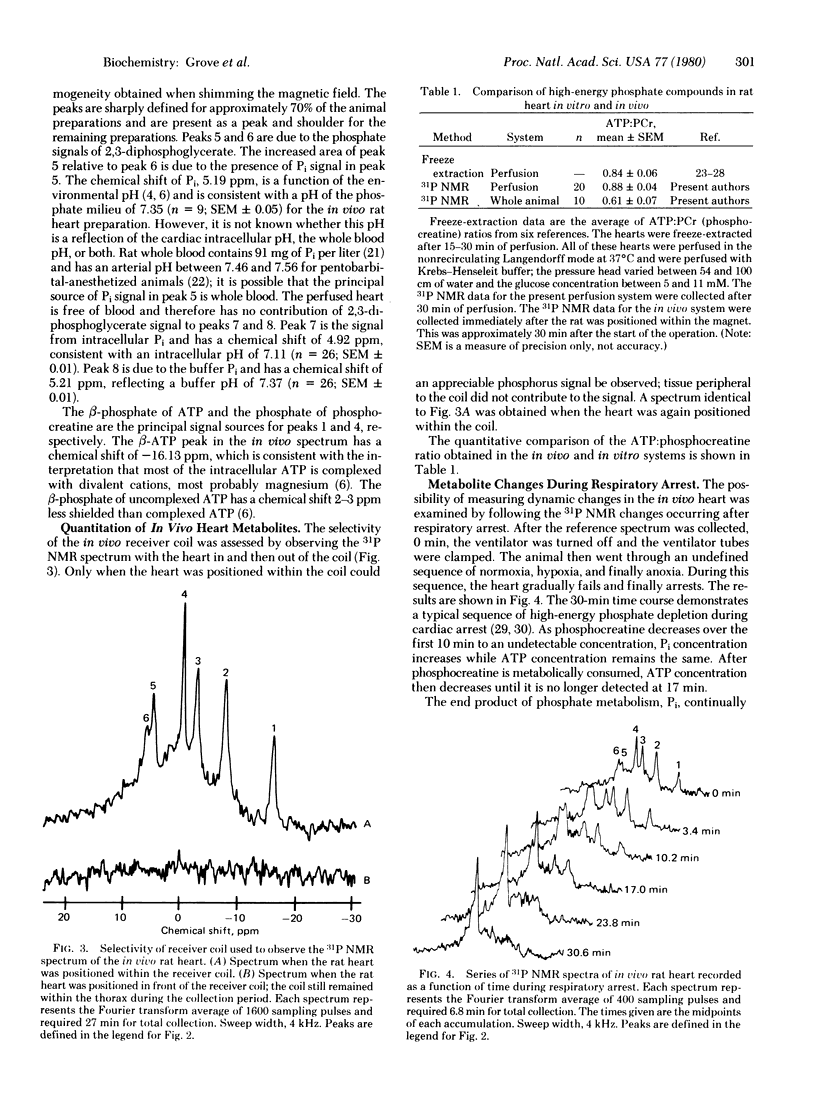

Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Aronson C. E., Serlick E. R. Effects of prolonged perfusion time on the isolated perfused rat heart. Toxicol Appl Pharmacol. 1976 Dec;38(3):479–488. doi: 10.1016/0041-008x(76)90179-4. [DOI] [PubMed] [Google Scholar]
- BENSON E. S., EVANS G. T., HALLAWAYBE, PHIBBS C., FREIER E. F. Myocardial creatine phosphate and nucleotides in aoxic cardiac arrest and recovery. Am J Physiol. 1961 Oct;201:678–693. doi: 10.1152/ajplegacy.1961.201.4.687. [DOI] [PubMed] [Google Scholar]
- Burt C. T., Glonek T., Bárány M. Analysis of phosphate metabolites, the intracellular pH, and the state of adenosine triphosphate in intact muscle by phosphorus nuclear magnetic resonance. J Biol Chem. 1976 May 10;251(9):2584–2591. [PubMed] [Google Scholar]
- Chance B., Nakase Y., Bond M., Leigh J. S., Jr, McDonald G. Detection of 31P nuclear magnetic resonance signals in brain by in vivo and freeze-trapped assays. Proc Natl Acad Sci U S A. 1978 Oct;75(10):4925–4929. doi: 10.1073/pnas.75.10.4925. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dawson M. J., Gadian D. G., Wilkie D. R. Contraction and recovery of living muscles studies by 31P nuclear magnetic resonance. J Physiol. 1977 Jun;267(3):703–735. doi: 10.1113/jphysiol.1977.sp011835. [DOI] [PMC free article] [PubMed] [Google Scholar]
- FEINSTEIN M. B. Effects of experimental congestive heart failure, ouabain, and asphyxia on the high-energy phosphate and creatine content of the guinea pig heart. Circ Res. 1962 Mar;10:333–346. doi: 10.1161/01.res.10.3.333. [DOI] [PubMed] [Google Scholar]
- Frankel H. M., Yousef M. K., Bayer R., Dill D. B. Blood composition in normothermic and hyperthermic kangaroo rats Dipodomys merriami and laboratory rats Rattus norvegicus. Comp Biochem Physiol A Comp Physiol. 1972 Dec 1;43(4):733–738. doi: 10.1016/0300-9629(72)90142-9. [DOI] [PubMed] [Google Scholar]
- GOTTSCHALK C. W., LASSITER W. E., MYLLE M. Localization of urine acidification in the mammalian kidney. Am J Physiol. 1960 Mar;198:581–585. doi: 10.1152/ajplegacy.1960.198.3.581. [DOI] [PubMed] [Google Scholar]
- Gadian D. G., Hoult D. I., Radda G. K., Seeley P. J., Chance B., Barlow C. Phosphorus nuclear magnetic resonance studies on normoxic and ischemic cardiac tissue. Proc Natl Acad Sci U S A. 1976 Dec;73(12):4446–4448. doi: 10.1073/pnas.73.12.4446. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garlick P. B., Radda G. K., Seeley P. J. Phosphorus NMR studies on perfused heart. Biochem Biophys Res Commun. 1977 Feb 7;74(3):1256–1262. doi: 10.1016/0006-291x(77)91653-9. [DOI] [PubMed] [Google Scholar]
- Henderson T. O., Costello A. J., Omachi A. Phosphate metabolism in intact human erythrocytes: determination by phosphorus-31 nuclear magnetic resonance spectroscopy. Proc Natl Acad Sci U S A. 1974 Jun;71(6):2487–2490. doi: 10.1073/pnas.71.6.2487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hollis D. P., Nunnally R. L., Jacobus W. E., Taylor G. J., 4th Detection of regional ischemia in perfused beating hearts by phosphorus nuclear magnetic resonance. Biochem Biophys Res Commun. 1977 Apr 25;75(4):1086–1091. doi: 10.1016/0006-291x(77)91493-0. [DOI] [PubMed] [Google Scholar]
- Hoult D. I., Busby S. J., Gadian D. G., Radda G. K., Richards R. E., Seeley P. J. Observation of tissue metabolites using 31P nuclear magnetic resonance. Nature. 1974 Nov 22;252(5481):285–287. doi: 10.1038/252285a0. [DOI] [PubMed] [Google Scholar]
- Mathur P. P., Case R. B. Phosphate loss during reversible myocardial ischemia. J Mol Cell Cardiol. 1973 Aug;5(4):375–393. doi: 10.1016/0022-2828(73)90029-1. [DOI] [PubMed] [Google Scholar]
- Moon R. B., Richards J. H. Determination of intracellular pH by 31P magnetic resonance. J Biol Chem. 1973 Oct 25;248(20):7276–7278. [PubMed] [Google Scholar]
- Navon G., Ogawa S., Shulman R. G., Yamane T. High-resolution 31P nuclear magnetic resonance studies of metabolism in aerobic Escherichia coli cells. Proc Natl Acad Sci U S A. 1977 Mar;74(3):888–891. doi: 10.1073/pnas.74.3.888. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Neely J. R., Denton R. M., England P. J., Randle P. J. The effects of increased heart work on the tricarboxylate cycle and its interactions with glycolysis in the perfused rat heart. Biochem J. 1972 Jun;128(1):147–159. doi: 10.1042/bj1280147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Neely J. R., Liebermeister H., Battersby E. J., Morgan H. E. Effect of pressure development on oxygen consumption by isolated rat heart. Am J Physiol. 1967 Apr;212(4):804–814. doi: 10.1152/ajplegacy.1967.212.4.804. [DOI] [PubMed] [Google Scholar]
- Ogawa S., Shulman R. G., Glynn P., Yamane T., Navon G. On the measurement of pH in Escherichia coli by 31P nuclear magnetic resonance. Biochim Biophys Acta. 1978 Apr 11;502(1):45–50. doi: 10.1016/0005-2728(78)90130-5. [DOI] [PubMed] [Google Scholar]
- Opie L. H., Mansford K. R., Owen P. Effects of increased heart work on glycolysis and adenine nucleotides in the perfused heart of normal and diabetic rats. Biochem J. 1971 Sep;124(3):475–490. doi: 10.1042/bj1240475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Opie L. H., Thomas M., Owen P., Shulman G. Increased coronary venous inorganic phosphate concentrations during experimental myocardial ischemia. Am J Cardiol. 1972 Oct;30(5):503–513. doi: 10.1016/0002-9149(72)90041-0. [DOI] [PubMed] [Google Scholar]
- Salhany J. M., Yamane T., Shulman R. G., Ogawa S. High resolution 31P nuclear magnetic resonance studies of intact yeast cells. Proc Natl Acad Sci U S A. 1975 Dec;72(12):4966–4970. doi: 10.1073/pnas.72.12.4966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scheuer J., Stezoski S. W. Effects of high-energy phosphate depletion and repletion on the dynamics and electrocardiogram of isolated rat hearts. Circ Res. 1968 Oct;23(4):519–530. doi: 10.1161/01.res.23.4.519. [DOI] [PubMed] [Google Scholar]
- Scheuer J., Stezoski S. W. Relationship of ATP to the electrocardiogram in the isolated rat heart. J Lab Clin Med. 1968 Oct;72(4):621–630. [PubMed] [Google Scholar]
- Sehr P. A., Radda G. K. A model kidney transplant studied by phosphorus nuclear magnetic resonance. Biochem Biophys Res Commun. 1977 Jul 11;77(1):195–202. doi: 10.1016/s0006-291x(77)80182-4. [DOI] [PubMed] [Google Scholar]
- Williamson J. R. Glycolytic control mechanisms. II. Kinetics of intermediate changes during the aerobic-anoxic transition in perfused rat heart. J Biol Chem. 1966 Nov 10;241(21):5026–5036. [PubMed] [Google Scholar]