Abstract
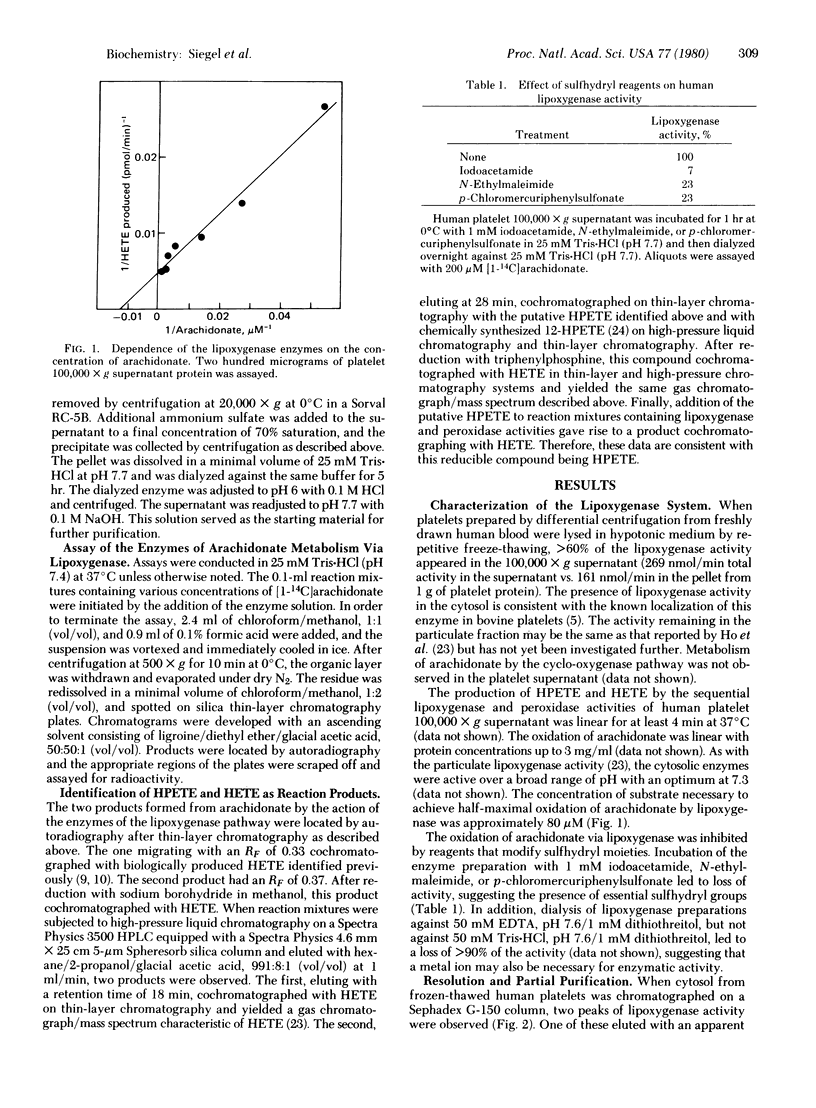
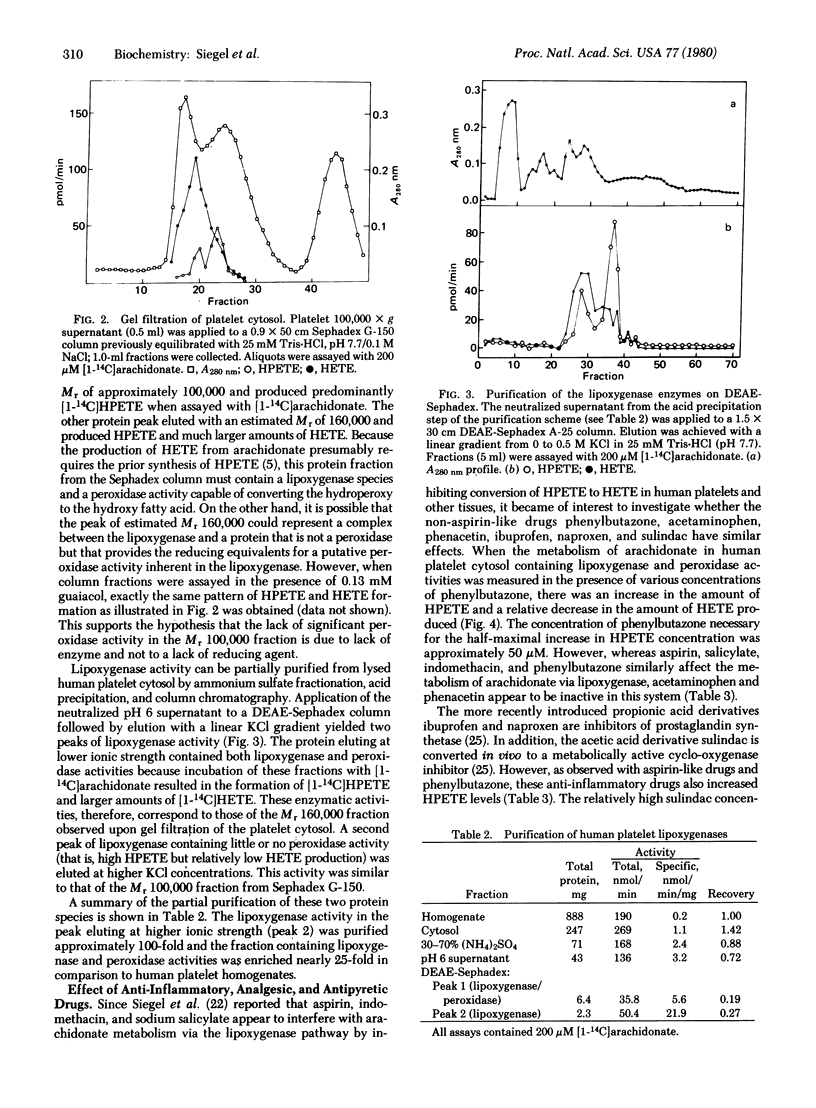
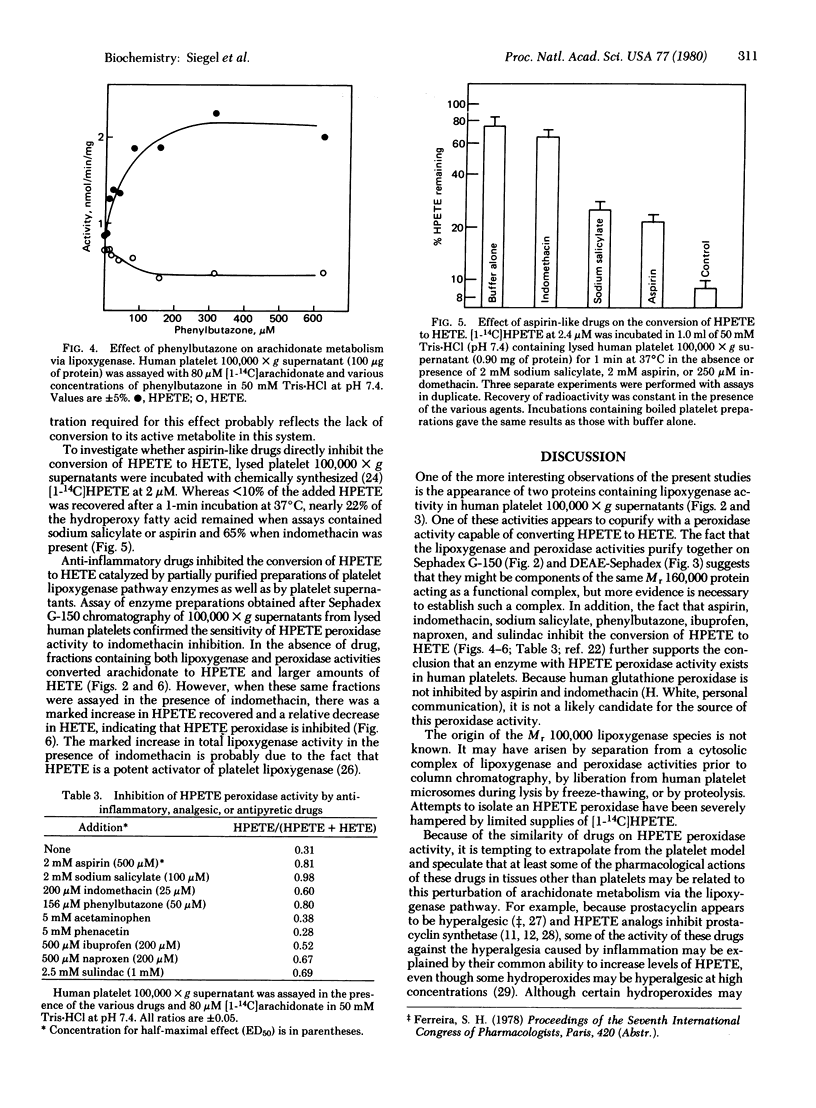
The enzymes of arachidonate metabolism via the lipoxygenase pathway in human platelet cytosol have been characterized and partially purified. The lipoxygenase activity has a pH optimum of 7.3 and reaches half-maximal activity at an arachidonate concentration of 80 microM. The oxidation of arachidonate by these enzymes is inhibited by reagents that modify sulfhydryl groups. Two separable lipoxygenase activities can be detected by chromatography of platelet cytosol on Sephadex G-150 and of partially purified preparations on DEAE-Sephadex. One of these has an apparent Mr of 100,000. A second enzyme species behaves as a Mr 160,000 entity containing, in addition to lipoxygenase, a peroxidase activity that catalyzes the conversion of 12L-hydroperoxy-5,8,10,14-icosatetraenoic acid (HPETE) to 12L-hydroxy-5,8,10,14-icosatetraenoic acid (HETE). Aspirin, indomethacin, sodium salicylate, phenylbutazone, ibuprofen, naproxen, and sulindac, but not acetaminophen or phenacetin, give rise to increased levels of HPETE in the lipoxygenase pathway. This increase in HPETE levels is the result of the ability of these drugs to inhibit directly the enzymatic conversion of HPETE to HETE.
Full text
PDF

Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Adcock J. J., Garland L. G., Moncada S., Salmon J. A. Enhancement of anaphylactic mediator release from guinea-pig perfused lungs by fatty acid hydroperoxides. Prostaglandins. 1978 Aug;16(2):163–177. doi: 10.1016/0090-6980(78)90019-9. [DOI] [PubMed] [Google Scholar]
- Adcock J. J., Garland L. G., Moncada S., Salmon J. A. The mechanism of enhancement by fatty acid hydroperoxides of anaphylactic mediator release. Prostaglandins. 1978 Aug;16(2):179–187. doi: 10.1016/0090-6980(78)90020-5. [DOI] [PubMed] [Google Scholar]
- Bryant R. W., Bailey J. M. Isolation of a new lipoxygenase metabolite of arachidonic acid, 8. 11, 12-trihydroxy-5,9,14-eicosatrienoic acid from human platelets. Prostaglandins. 1979 Jan;17(1):9–18. doi: 10.1016/0090-6980(79)90071-6. [DOI] [PubMed] [Google Scholar]
- Collier H. O. A pharmacological analysis of aspirin. Adv Pharmacol Chemother. 1969;7:333–405. [PubMed] [Google Scholar]
- Ferreira S. H., Moncada S., Vane J. R. Indomethacin and aspirin abolish prostaglandin release from the spleen. Nat New Biol. 1971 Jun 23;231(25):237–239. doi: 10.1038/newbio231237a0. [DOI] [PubMed] [Google Scholar]
- Ferreira S. H. Prostaglandins, aspirin-like drugs and analgesia. Nat New Biol. 1972 Dec 13;240(102):200–203. doi: 10.1038/newbio240200a0. [DOI] [PubMed] [Google Scholar]
- Flower R. J., Vane J. R. Inhibition of prostaglandin synthetase in brain explains the anti-pyretic activity of paracetamol (4-acetamidophenol). Nature. 1972 Dec 15;240(5381):410–411. doi: 10.1038/240410a0. [DOI] [PubMed] [Google Scholar]
- Glenn E. M., Bowman B. J., Rohloff N. A. Anti-inflammatory and PG inhibitory effects of phenacetin and acetaminophen. Agents Actions. 1977 Dec;7(5-6):513–516. doi: 10.1007/BF02111123. [DOI] [PubMed] [Google Scholar]
- Goetzl E. J., Woods J. M., Gorman R. R. Stimulation of human eosinophil and neutrophil polymorphonuclear leukocyte chemotaxis and random migration by 12-L-hydroxy-5,8,10,14-eicosatetraenoic acid. J Clin Invest. 1977 Jan;59(1):179–183. doi: 10.1172/JCI108617. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ham E. A., Egan R. W., Soderman D. D., Gale P. H., Kuehl F. A., Jr Peroxidase-dependent deactivation of prostacyclin synthetase. J Biol Chem. 1979 Apr 10;254(7):2191–2194. [PubMed] [Google Scholar]
- Hamberg M. Inhibition of prostaglandin synthesis in man. Biochem Biophys Res Commun. 1972 Nov 1;49(3):720–726. doi: 10.1016/0006-291x(72)90470-6. [DOI] [PubMed] [Google Scholar]
- Hamberg M., Samuelsson B. Prostaglandin endoperoxides. Novel transformations of arachidonic acid in human platelets. Proc Natl Acad Sci U S A. 1974 Sep;71(9):3400–3404. doi: 10.1073/pnas.71.9.3400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hamberg M., Svensson J., Samuelsson B. Prostaglandin endoperoxides. A new concept concerning the mode of action and release of prostaglandins. Proc Natl Acad Sci U S A. 1974 Oct;71(10):3824–3828. doi: 10.1073/pnas.71.10.3824. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hemler M. E., Graff G., Lands W. E. Accelerative autoactivation of prostaglandin biosynthesis by PGG2. Biochem Biophys Res Commun. 1978 Dec 29;85(4):1325–1331. doi: 10.1016/0006-291x(78)91148-8. [DOI] [PubMed] [Google Scholar]
- Higgs G. A., Harvey E. A., Ferreira S. H., Vane J. R. The effects of antiinflammatory drugs on the production of prostaglandins in vivo. Adv Prostaglandin Thromboxane Res. 1976;1:105–110. [PubMed] [Google Scholar]
- Ho P. P., Walters C. P., Sullivan H. R. A particulate arachidonate lipoxygenase in human blood platelets. Biochem Biophys Res Commun. 1976 May 23;76(2):398–405. doi: 10.1016/0006-291x(77)90738-0. [DOI] [PubMed] [Google Scholar]
- Jones R. L., Kerry P. J., Poyser N. L., Walker I. C., Wilson N. H. The identification of trihydroxyeicosatrienoic acids as products from the incubation of arachidonic acid with washed blood platelets. Prostaglandins. 1978 Oct;16(4):583–589. doi: 10.1016/0090-6980(78)90188-0. [DOI] [PubMed] [Google Scholar]
- Komoriya K., Ohmori H., Azuma A., Kurozumi S., Hashimoto Y., Nicolaou K. C., Barnette W. E., Magolda R. L. Prostaglandin I2 as a potentiator of acute inflammation in rats. Prostaglandins. 1978 Apr;15(4):557–564. doi: 10.1016/0090-6980(78)90052-7. [DOI] [PubMed] [Google Scholar]
- Lapetina E. G., Chandrabose K. A., Cuatrecasas P. Ionophore A-23187- and thrombin-induced platelet aggregation: independence from cycloxygenase products. Proc Natl Acad Sci U S A. 1978 Feb;75(2):818–822. doi: 10.1073/pnas.75.2.818. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lapetina E. G., Schmitges C. J., Chandrabose K., Cuatrecases P. Cyclic adenosine 3',5'-monophosphate and prostacyclin inhibit membrane phospholipase activity in platelets. Biochem Biophys Res Commun. 1977 Jun 6;76(3):828–835. doi: 10.1016/0006-291x(77)91575-3. [DOI] [PubMed] [Google Scholar]
- Martelli N. A., Usandivaras G. Inhibition of aspirin-induced bronchoconstriction by sodium cromoglycate inhalation. Thorax. 1977 Dec;32(6):684–690. doi: 10.1136/thx.32.6.684. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moncada S., Gryglewski R. J., Bunting S., Vane J. R. A lipid peroxide inhibits the enzyme in blood vessel microsomes that generates from prostaglandin endoperoxides the substance (prostaglandin X) which prevents platelet aggregation. Prostaglandins. 1976 Nov;12(5):715–737. doi: 10.1016/0090-6980(76)90048-4. [DOI] [PubMed] [Google Scholar]
- Nugteren D. H. Arachidonate lipoxygenase in blood platelets. Biochim Biophys Acta. 1975 Feb 20;380(2):299–307. doi: 10.1016/0005-2760(75)90016-8. [DOI] [PubMed] [Google Scholar]
- Porter N. A., Wolf R. A., Yarbro E. M., Weenen H. The autoxidation of arachidonic acid: formation of the proposed SRS-A intermediate. Biochem Biophys Res Commun. 1979 Aug 28;89(4):1058–1064. doi: 10.1016/0006-291x(79)92115-6. [DOI] [PubMed] [Google Scholar]
- Salmon J. A., Smith D. R., Flower R. J., Moncada S., Vane J. R. Further studies on the enzymatic conversion of prostaglandin endoperoxide into prostacyclin by porcine aorta microsomes. Biochim Biophys Acta. 1978 Mar 14;523(1):250–262. doi: 10.1016/0005-2744(78)90028-1. [DOI] [PubMed] [Google Scholar]
- Siegel M. I., McConnell R. T., Abrahams S. L., Porter N. A., Cuatrecasas P. Regulation of arachidonate metabolism via lipoxygenase and cyclo-oxygenase by 12-HPETE, the product of human platelet lipoxygenase. Biochem Biophys Res Commun. 1979 Aug 28;89(4):1273–1280. doi: 10.1016/0006-291x(79)92146-6. [DOI] [PubMed] [Google Scholar]
- Siegel M. I., McConnell R. T., Cuatrecasas P. Aspirin-like drugs interfere with arachidonate metabolism by inhibition of the 12-hydroperoxy-5,8,10,14-eicosatetraenoic acid peroxidase activity of the lipoxygenase pathway. Proc Natl Acad Sci U S A. 1979 Aug;76(8):3774–3778. doi: 10.1073/pnas.76.8.3774. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith J. B., Willis A. L. Aspirin selectively inhibits prostaglandin production in human platelets. Nat New Biol. 1971 Jun 23;231(25):235–237. doi: 10.1038/newbio231235a0. [DOI] [PubMed] [Google Scholar]
- Smith M. J., Ford-Hutchinson A. W., Elliott P. N. Prostaglandins and the anti-inflammatory activities of aspirin and sodium salicylate. J Pharm Pharmacol. 1975 Jul;27(7):473–478. doi: 10.1111/j.2042-7158.1975.tb09487.x. [DOI] [PubMed] [Google Scholar]
- Tainer J. A., Turner S. R., Lynn W. S. New aspects of chemotaxis. Specific target-cell attraction by lipid and lipoprotein fractions of Escherichia coli chemotactic factor. Am J Pathol. 1975 Nov;81(2):401–410. [PMC free article] [PubMed] [Google Scholar]
- Turner S. R., Tainer J. A., Lynn W. S. Biogenesis of chemotactic molecules by the arachidonate lipoxygenase system of platelets. Nature. 1975 Oct 23;257(5528):680–681. doi: 10.1038/257680a0. [DOI] [PubMed] [Google Scholar]
- Vane J. R. Inhibition of prostaglandin synthesis as a mechanism of action for aspirin-like drugs. Nat New Biol. 1971 Jun 23;231(25):232–235. doi: 10.1038/newbio231232a0. [DOI] [PubMed] [Google Scholar]
- Vargaftig B. B. Salicylic acid fails to inhibit generation of thromboxane A2 activity in platelets after in vivo administration to the rat. J Pharm Pharmacol. 1978 Feb;30(2):101–104. doi: 10.1111/j.2042-7158.1978.tb13171.x. [DOI] [PubMed] [Google Scholar]
- Vinegar R., Truax J. F., Selph J. L. Quantitative comparison of the analgesic and anti-inflammatory activities of aspirin, phenacetin and acetaminophen in rodents. Eur J Pharmacol. 1976 May;37(1):23–30. doi: 10.1016/0014-2999(76)90004-2. [DOI] [PubMed] [Google Scholar]
- Vinegar R., Truax J. F., Selph J. L. Quantitative studies of the pathway to acute carrageenan inflammation. Fed Proc. 1976 Nov;35(13):2447–2456. [PubMed] [Google Scholar]
- Vinegar R., Truax J. F., Selph J. L. Some quantitative temporal characteristics of carrageenin-induced pleurisy in the rat. Proc Soc Exp Biol Med. 1973 Jul;143(3):711–714. doi: 10.3181/00379727-143-37397. [DOI] [PubMed] [Google Scholar]
- Zenser T. V., Mattammal M. B., Herman C. A., Joshi S., Davis B. B. Effect of acetaminophen on prostaglandin E2 and prostaglandin F2alpha synthesis in the renal inner medulla of rat. Biochim Biophys Acta. 1978 Sep 6;542(3):486–495. doi: 10.1016/0304-4165(78)90378-1. [DOI] [PubMed] [Google Scholar]



