Abstract
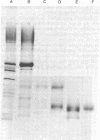
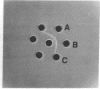
Trypsin digestion of Hansenula wingei 21-cells releases a protein (21-factor-T) that inhibits the agglutination of 21-cells by purified 5-agglutinin obtained from 5-cells by subtilisin digestion [Crandall, M. A. & Brock, T. D. (1968) Bacteriol. Rev. 32, 139-163]. We have purified this inhibitor 415-fold by ion-exchange chromatography, affinity adsorption to 5-cells, and gel permeation chromatography. The material shows a diffuse band, on polyacrylamide gel electrophoresis in the presence of sodium dodecyl sulfate, with an apparent Mr of 27,000. It has a pI of 3.8, is rich in acidic amino acids, contains 5% mannose and a trace of glucosamine, and is stable to reducing agents but is inactivated by heat. Zymolyase (β1→3-glucanase) digestion of 21-cells releases a similar inhibitor that, after purification, has a larger size than 21-factor-T. This 21-factor-Z appears to contain an additional portion that may serve to anchor 21-factor in the cell wall. Haploid cells of the yeasts Pichia amethionina and Saccharomyces kluyveri also show a constitutive sexual agglutination, and little or no crossreactivity is observed in heterologous mixtures. The agglutination factors in all three genera, however, have parallel properties; one cell type of each pair is heat stable and is inactivated by reducing agents (H. wingei 5-cells, P. amethionina α-cells, and S. kluyveri 16-cells), and the other is heat labile and is unaffected by reducing agents H. wingei 21-cells, P. amethionina a-cells, and S. kluyveri 17-cells). Because S. kluyveri 16-cells respond to Saccharomyces cerevisiae α-factor with the typical morphogenetic change of a mating half-reaction, the heat-stable agglutinin appears related to the S. cerevisiae a mating type and the heat-labile factor to the S. cerevisiae α mating type.
Keywords: Saccharomyces, Pichia, 5-agglutinin, receptor-ligand, cognor-cognon
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Barker E. R., Miller M. W. Some properties of Saccharomyces kluyveri. Antonie Van Leeuwenhoek. 1969;35(2):159–171. doi: 10.1007/BF02219126. [DOI] [PubMed] [Google Scholar]
- Crandall M. A., Brock T. D. Molecular basis of mating in the yeast hansenula wingei. Bacteriol Rev. 1968 Sep;32(3):139–163. doi: 10.1128/br.32.3.139-163.1968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cumsky M., Zusman D. R. Myxobacterial hemagglutinin: a development-specific lectin of Myxococcus xanthus. Proc Natl Acad Sci U S A. 1979 Nov;76(11):5505–5509. doi: 10.1073/pnas.76.11.5505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dazzo F. B., Yanke W. E., Brill W. J. Trifolin: a Rhizobium recognition protein from white clover. Biochim Biophys Acta. 1978 Mar 20;539(3):276–286. doi: 10.1016/0304-4165(78)90032-6. [DOI] [PubMed] [Google Scholar]
- Duntze W., Stötzler D., Bücking-Throm E., Kalbitzer S. Purification and partial characterization of -factor, a mating-type specific inhibitor of cell reproduction from Saccharomyces cerevisiae. Eur J Biochem. 1973 Jun;35(2):357–365. doi: 10.1111/j.1432-1033.1973.tb02847.x. [DOI] [PubMed] [Google Scholar]
- Fehrenbacher G., Perry K., Thorner J. Cell-cell recognition in Saccharomyces cerevisiae: regulation of mating-specific adhesion. J Bacteriol. 1978 Jun;134(3):893–901. doi: 10.1128/jb.134.3.893-901.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jones G. H., Ballou C. E. Studies on the structure of yeast mannan. I. Purification and some properties of an alpha-mannosidase from an arthrobacter species. J Biol Chem. 1969 Feb 10;244(3):1043–1051. [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Lipke P. N., Taylor A., Ballou C. E. Morphogenic effects of alpha-factor on Saccharomyces cerevisiae a cells. J Bacteriol. 1976 Jul;127(1):610–618. doi: 10.1128/jb.127.1.610-618.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McCullough J., Herskowitz I. Mating pheromones of Saccharomyces kluyveri: pheromone interactions between Saccharomyces kluyveri and Saccharomyces cerevisiae. J Bacteriol. 1979 Apr;138(1):146–154. doi: 10.1128/jb.138.1.146-154.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakajima T., Ballou C. E. Structure of the linkage region between the polysaccharide and protein parts of Saccharomyces cerevisiae mannan. J Biol Chem. 1974 Dec 10;249(23):7685–7694. [PubMed] [Google Scholar]
- ORNSTEIN L. DISC ELECTROPHORESIS. I. BACKGROUND AND THEORY. Ann N Y Acad Sci. 1964 Dec 28;121:321–349. doi: 10.1111/j.1749-6632.1964.tb14207.x. [DOI] [PubMed] [Google Scholar]
- Richards R. L., Moss J., Alving C. R., Fishman P. H., Brady R. O. Choleragen (cholera toxin): a bacterial lectin. Proc Natl Acad Sci U S A. 1979 Apr;76(4):1673–1676. doi: 10.1073/pnas.76.4.1673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simpson D. L., Rosen S. D., Barondes S. H. Discoidin, a developmentally regulated carbohydrate-binding protein from Dictyostelium discoideum. Purification and characterization. Biochemistry. 1974 Aug 13;13(17):3487–3493. doi: 10.1021/bi00714a011. [DOI] [PubMed] [Google Scholar]
- TAYLOR N. W. INACTIVATION OF SEXUAL AGGLUTINATION IN HANSENULA WINGEI AND SACCHAROMYCES KLUYVERI BY DISULFIDE-CLEAVING AGENTS. J Bacteriol. 1964 Oct;88:929–936. doi: 10.1128/jb.88.4.929-936.1964. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tarentino A. L., Maley F. Purification and properties of an endo-beta-N-acetylglucosaminidase from Streptomyces griseus. J Biol Chem. 1974 Feb 10;249(3):811–817. [PubMed] [Google Scholar]
- Taylor N. W., Orton W. L. Cooperation among the active binding sites in the sex-specific agglutinin from the yeast, Hansenula wingei. Biochemistry. 1971 May 25;10(11):2043–2049. doi: 10.1021/bi00787a012. [DOI] [PubMed] [Google Scholar]
- Yen P. H., Ballou C. E. Partial characterization of the sexual agglutination factor from Hansenula wingei Y-2340 type 5 cells. Biochemistry. 1974 May 21;13(11):2428–2437. doi: 10.1021/bi00708a030. [DOI] [PubMed] [Google Scholar]