Abstract
The blood-brain barrier (BBB) is formed primarily to protect the brain microenvironment from the influx of plasma components, which may disturb neuronal functions. The BBB is a functional unit that consists mainly of specialized endothelial cells (ECs) lining the cerebral blood vessels, astrocytes and pericytes. The BBB is a dynamic structure that is altered in neurologic diseases, such as stroke. ECs and astrocytes secrete extracellular matrix (ECM) proteins to generate and maintain the basement membranes (BMs). ECM receptors, such as integrins and dystroglycan are also expressed at the brain microvasculature and mediate the connections between cellular and matrix components in physiology and disease. ECM proteins and receptors elicit diverse molecular signals that allow cell adaptation to environmental changes, and regulate growth and cell motility. The composition of the ECM is altered upon BBB disruption and directly affects the progression of neurologic disease. The purpose of this review is to discuss the dynamic changes of ECM composition and integrin receptor expression that control BBB functions in physiology and pathology.
Keywords: integrins, fibrinogen, laminin, basal lamina, ischemia
INTRODUCTION
The brain microenvironment is tightly controlled to ensure proper nervous system functions. The primary layer of protection comes from the blood-brain barrier (BBB) at the capillary endothelium, and the blood-cerebrospinal fluid (CSF) barrier at the choroid epithelium and the arachnoid membrane. These barriers separate the blood from the central nervous system (CNS) microenvironment (Redzic, 2011). In vertebrates, the BBB consists primarily of endothelial cells (ECs) with specialized tight junctions (TJs) lining the blood vessels, astrocytic endfeet surrounding the blood vessels, and pericytes embedded in the basement membranes (BMs) between the ECs and the astrocytes (Figure 1) (Banerjee and Bhat, 2007). The BBB has multiple functions. As a physical barrier, it restricts paracellular transport of cells, proteins and water-soluble agents. As a transport barrier, it regulates nutrient supply and waste removal, through specific transport systems, such as glucose transporter 1 (GLUT-1), and ATP-binding cassette (ABC) transporters, such as P-glycoprotein. As a metabolic barrier, it contains enzymes that metabolize ATP and neuroactive compounds, allowing separation of the central and peripheral pools of neurotransmitters (Abbott et al., 2006). However, the BBB is neither an absolute barrier nor is it static (Carvey et al., 2009; Neuwelt et al., 2011). Rather, this dynamic structure is highly regulated by interactions between its cellular and extracellular matrix (ECM) components along with integrin receptors. In this review, we discuss the contribution of ECM proteins and integrin receptors in the regulation of BBB functions in physiology and pathology.
Figure 1.
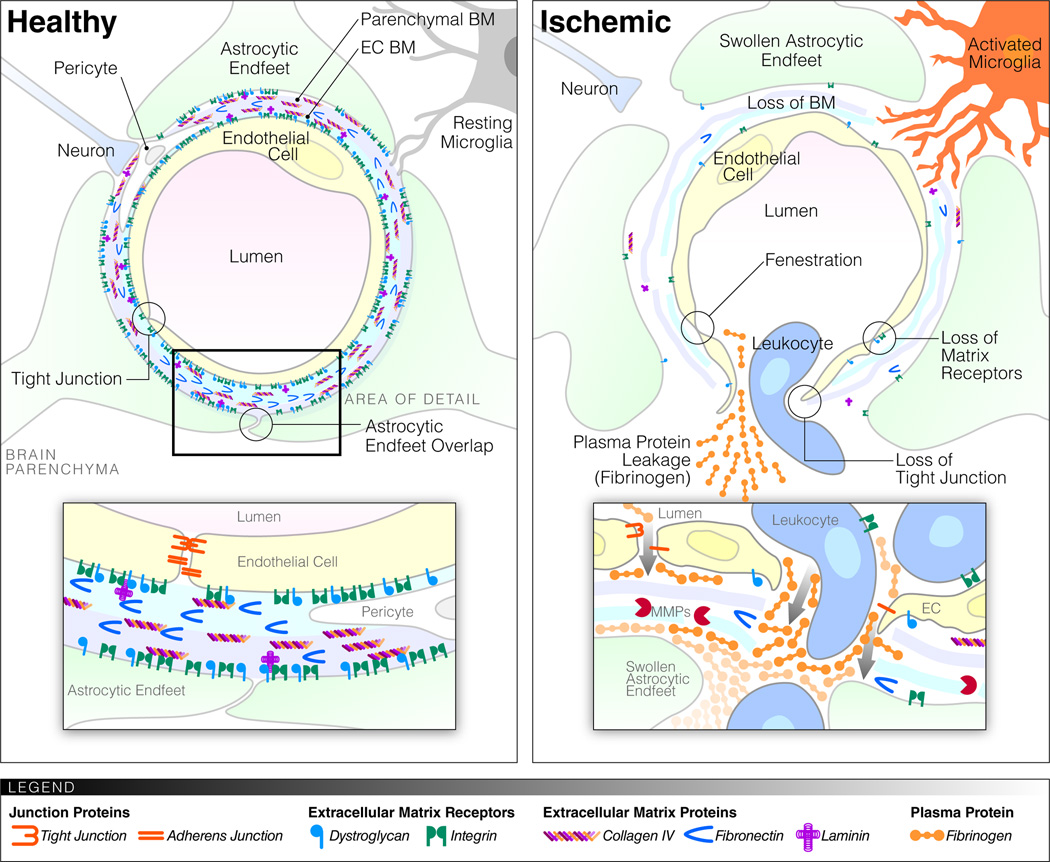
Schematic representation of the BBB before and after ischemia. Healthy microvessels in the brain consist of specialized ECs with TJs, their surrounding endothelial BM (blue) and astrocytic (parenchymal) BM (purple) composed of ECM proteins, and an astrocytic endfeet coverage. Cellular and matrix components of the BBB are connected through a variety of matrix receptors. Pericytes are found within the endothelial BM and occasionally the astrocytic endfeet coverage is interrupted to allow contact of microglia and neurons with the BM. After stroke/ischemia the specialized endothelial characteristics disappear, TJs are lost, fenestrations appear. The BMs become thinner and there is a marked reduction in matrix proteins and receptors. The astrocytic endfeet swell up and the contact with the vessels is lost. There is leakage of fluid, proteins and cells from the vessel lumen. Microglia become activated and might extend processes toward the blood vessels, while pericytes move away. White blood cells transmigrate via an integrin-dependent process across the endothelial BM and via an MMP-dependent process across the parenchymal BM.
BBB DURING DEVELOPMENT AND PATHOLOGY
During embryonic vascularisation of the brain, the BBB forms in parallel with the blood vessels. ECs migrate to and proliferate on a fibronectin-rich ECM, and a laminin-containing BM forms shortly thereafter (Risau and Lemmon, 1988). In brain capillary endothelial cells (BCECs), fibronectin signaling during angiogenesis switches to laminin signaling in adult stages (Milner and Campbell, 2002). Notably, the regulation of exchange between blood and CSF and the composition of CSF are different in the fetus and the adult, as evidenced by higher protein concentrations in CSF and the different plasma-to-CSF ratios in the fetus (Johansson et al., 2008). Whether this is due to ongoing differentiation of ECs into their specialized phenotype or to alternative transport mechanisms and changes in CSF volume during development has been a matter of debate for over 20 years (Engelhardt, 2003; Johansson et al., 2008; Saunders et al., 2008). However, TJs form and the transport of low-molecular-weight markers from the blood into the CNS is restricted very early on (Ek et al., 2006; Saunders et al., 2008). Regardless of the exact time point, formation and maintenance of the BBB as it exists in the adult entail the interaction of several cell-types and ECM components.
The BBB is disrupted in many pathological conditions, such as stroke, Multiple Sclerosis (MS), HIV encephalitis, age-related dementia, and Alzheimer’s Disease (AD) (Abbott et al., 2006). With the exception of hemorrhagic stroke where blood vessels rupture, disruption of the BBB refers to a reduction of the barrier tightness and an increase in leakiness, while the vessels remain largely intact. As a result of these changes in the barrier tightness, several blood proteins deposit in the brain parenchyma. These deposits do not occur in the healthy brain and are often observed very early on in disease, sometimes before the onset of clinical symptoms (Kermode et al., 1990; Huber et al., 2001; Paul et al., 2007). This suggests that BBB disruption may participate in disease onset and progression.
In stroke, blood flow in the brain is disrupted by bleeding (hemorrhagic stroke) or occlusion of the vessel by a blood clot (ischemic stroke). Loss of blood flow results in lack of oxygen and nutrient supply to the brain cells that quickly leads to neurological deficits or death (Ding and Clark, 2006). While blood flow needs to be quickly restored, the reperfusion of ischemic tissue following removal of the thrombus can lead to secondary damage due to oxidative stress induced by the sudden re-exposure to oxygen and inflammatory responses to the damaged tissue. Local changes in oxygen and nutrient supply as well as in blood flow and haemodynamic pressure during the different phases of ischemia and reperfusion result in many interdependent biochemical and cellular events, as reviewed in depth by Sandoval and Witt (2008). For example, ischemia leads to ATP depletion, ionic imbalance, increased acidosis and cell swelling, oxidative stress, release of inflammatory cytokines and chemokines, and upregulation of proteases. Restoration of blood flow increases the pressure on the damaged ECs and their TJs (Sandoval and Witt, 2008). In addition, reperfusion results in a fresh supply of leukocytes, which translocate in the CNS triggering a cascade of cytokine release (Wang et al., 2007). This wide variety of oxidative and inflammatory responses results in a multitude of effects, including loss of microvascular integrity, loss of TJ regulation, multi-phasic changes in permeability, proteolytic degradation of BMs, loss of integrins, loss of cell adhesion, edema, extracellular deposition of plasma proteins, and further inflammation (Figure 1) (Hamann et al., 1995; Wang and Lo, 2003; Wang et al., 2007; Sandoval and Witt, 2008; Baumann et al., 2009; Kwon et al., 2009). Notably, these processes all contribute to BBB disruption, and although the exact cause-and-effect relationships of the different pathological changes are hard to establish, they are likely to be interdependent (Sandoval and Witt, 2008).
CELLULAR PLAYERS OF THE BBB
Endothelial cells
The ECs of the BBB distinguish themselves from ECs elsewhere by the absence of fenestrations, increased mitochondria, reduced pinocytosis, absence of class II major histocompatibility complex, expression of specialized transporters and the higher abundance of specialized TJs (Abbott et al., 2006; Zlokovic, 2008; Carvey et al., 2009). TJs are present between adjacent cells, and the network of parallel, intra-membrane strands of protein acts as a series of barriers, preventing paracellular transport (Figure 1) (Huber et al., 2001). TJs can even restrict the movement of ions, such as Na+ and Cl−, thereby increasing by 50–500-fold the transendothelial electrical resistance (TEER), a measure of endothelial barrier properties. In addition to restricting the paracellular permeability, TJs cause polarization of ECs by dividing them into apical and basal domains. The main proteins forming the TJs at the BBB are claudin-5, -3 and -12, occludin, junctional adhesion molecules (JAM A, B and C) and zona occludens proteins (ZO-1, ZO-2, ZO-3). Besides TJs, there are also adherens junctions (AJs) that are characterized by the intracellular insertion of microfilaments and comprise proteins, such as VE-cadherin (CD144) and PECAM-1 (CD31). The TJs and AJs of the BBB have been extensively reviewed elsewhere (Huber et al., 2001; Wolburg and Lippoldt, 2002; Harhaj and Antonetti, 2004). These properties are not intrinsic to ECs, but are induced by the CNS environment (Stewart and Wiley, 1981). Moreover, in vitro cultures of ECs rapidly lose their barrier properties (Kniesel and Wolburg, 2000), and in vitro models of BBB require co-culture and specific ECM coatings (Tao-Cheng et al., 1987; Daneman et al., 2010). The acquisition of these specialized characteristics by the ECs is mediated by interactions with the other cellular and ECM components that constitute the BBB as discussed below.
Astrocytes
Astrocytes are glial cells that support neurons by regulating the fluid, electrolyte, amino acid and neurotransmitter balances in the neuronal microenvironment. They also have roles in synapse formation and clearance of axonal-derived material (Sofroniew and Vinters, 2010; Zhang and Barres, 2010; Allaman et al., 2011; Nguyen et al., 2011). They are present throughout the CNS and form a network with neighboring astrocytes that communicates with each other through GAP junctions (Rouach and Giaume, 2001). In addition, astrocytes also form connections with neurons, axons and blood vessels, making them ideally positioned to respond to multiple signals in their microenvironment.
Astrocytes and, in particular, perivascular astrocytic endfeet cover microvessels in the CNS and form the glia limitans (Figure 1). Astrocytic coverage of vessel ECs and pericytes is virtually complete (Mathiisen et al., 2010). On the rare occasion where a gap occurs between the endfeet, microglial processes can be observed touching the BM (Lassmann et al., 1991; Mathiisen et al., 2010). Holes in the endfeet coverage of pericytes are more common, allowing BM contact with neuropil components (Mathiisen et al., 2010). Thus, astrocytes separate the vessels and their BMs from the brain parenchyma, while still allowing some direct contacts at specific sites.
Astrocytes are also important for inducing and maintaining EC barrier properties (Janzer and Raff, 1987; Abbott et al., 2006). In freeze-fracture studies, ECs in co-culture with astrocytes for several days have greater TJ numbers, width, length and complexity than ECs in mono-culture where TJs become fragmented and GAP junctions appear (Tao-Cheng et al., 1987). Induction of EC barrier characteristics, such as blocked intercellular passage of horseradish peroxidase, increased glucosamine uptake and visible formation of TJs, can also occur upon stimulation with astrocyte-conditioned medium (Yamagata et al., 1997). With regard to the increase in TJs, co-culture models revealed an upregulation of ZO-1 and occludin and the re-distribution of CD31, claudin-5 and ZO-1 from a diffuse pattern to the cell-borders in ECs in the presence of astrocytes (Siddharthan et al., 2007; Colgan et al., 2008; Al Ahmad et al., 2010). The interaction between astrocytes and ECs is bidirectional, since ECs also enhance growth and differentiation of astrocytes (Mi et al., 2001; Abbott et al., 2006). While astrocytes induce barrier properties in ECs, induction of endothelial barrier properties during embryonic development precedes astrocyte differentiation in several species (Daneman et al., 2010). Thus, while astrocytes most likely regulate the dynamics of the BBB postnatally, astrocytes cannot be the sole or essential regulator of BBB during embryongenesis.
Pericytes
Pericytes are cells likely derived from the vascular smooth muscle cell lineage that are embedded in the BM of ECs at the BBB (Allt and Lawrenson, 2001). Pericytes provide a substantial but incomplete (approximately 30%) coverage of the endothelium (Mathiisen et al., 2010). They extend processes around capillaries and can contain contractile elements, such as alpha-smooth muscle actin (Allt and Lawrenson, 2001; Bandopadhyay et al., 2001; Peppiatt et al., 2006; Mathiisen et al., 2010). Despite a lot of controversy, pericytes appear to contract and modulate cerebral blood flow in capillaries (Allt and Lawrenson, 2001; Peppiatt et al., 2006). Pericytes maintain close contact with ECs through peg-socket contacts, which involve reciprocal interdigitating evaginations from each cell, and GAP junctions and are important regulators of angiogenisis and the formation of the vascular tree (e.g., via Transforming Growth Factor-β (TGF-β) and angiopoietin-1) (Allt and Lawrenson, 2001; Bagley et al., 2005; Lai and Kuo, 2005; Caruso et al., 2009; Fisher, 2009).
The role of pericytes in BBB was studied in mice where pericyte recruitment to the vessels was abolished by mutations that interfere with Platelet-Derived Growth Factor B (PDGF-B) signaling through the PDGF receptor beta (PDGFR-β) (Dohgu et al., 2005; Armulik et al., 2010; Bell et al., 2010; Daneman et al., 2010; Quaegebeur et al., 2010). Daneman et al. (2010) showed that pericytes are necessary for BBB formation during embryonic development. Mice with reduced or complete absence of pericytes (due to hypomorphic or null mutations in Pdgfrb alleles) have increased vessel permeability, which is inversely correlated with the degree of pericyte coverage. Mice lacking pericytes have increased transcytosis, aberrant alignment of TJs, and higher expression of molecules, which increase barrier permeability, such as angiopoietin2 and leukocyte adhesion molecules (LAMs) (Daneman et al., 2010). Interestingly, these mice still express many BBB-specific genes in ECs, pointing towards barrier-forming roles of other cell types, such as neural progenitor cells, at even earlier stages of development (Daneman et al., 2010).
The role of pericytes in the adult was investigated using several adult-viable pericyte-deficient mouse mutants with a reduction in pericyte coverage (Armulik et al., 2010; Bell et al., 2010). In the adult, loss of pericytes results in reduced barrier properties due to altered gene expression in ECs and loss of astrocytic endfeet polarization (Armulik et al., 2010). Mutations that lead to an age-dependent progressive pericyte loss showed BBB breakdown associated with brain accumulation of blood proteins, such as IgG and fibrinogen (Bell et al., 2010). In addition, age-dependent reduction in TJs and BM proteins in pericyte-deficient mice precede neurodegenerative changes and impairment of learning and memory (Bell et al., 2010). These results are supported by in vitro findings that pericytes induce barrier functions in ECs, resulting in reduced permeability, reduced intracellular space between ECs, increased TEER, increased junctional integrity and secretion of BM components (Dohgu et al., 2005; Dore-Duffy et al., 2006; Brachvogel et al., 2007; Daneman et al., 2010).
Direct comparisons of the effects of pericytes and astrocytes on induction of EC barrier properties are sparse. Co-culture models showed that while the effect of pericytes on TJ protein localization to the cell borders was a lot less than that of astrocytes (Al Ahmad et al., 2010), TEER and permeability were influenced more by pericytes than astrocytes (Nakagawa et al., 2007). Barrier properties were maximal in the presence of both cell types. The evidence presented above indicates that while astrocytes and pericytes work synergistically to regulate BBB functions, their relative contributions may vary during different developmental stages or disease activity.
Cellular Changes in Stroke
BBB permeability increases after ischemia/reperfusion in stroke patients (Kastrup et al., 2008; Israeli et al., 2010) and in animal models of cerebral ischemia (Baumann et al., 2009). EC cultures exposed to hypoxic conditions in vitro also show reduced TEER and increased paracellular permeability (Mark and Davis, 2002; Yamagata et al., 2004; Koto et al., 2007). These defects in the BBB are associated with loss of endothelial TJs (Sandoval and Witt, 2008). In in vitro experiments, hypoxia/reoxygenation, to mimic ischemia/reperfusion in vivo, caused a reduction and disruption of staining for TJ proteins claudin-5, occludin, ZO-1 and ZO-2 at the plasma membrane of confluent ECs (Mark and Davis, 2002; Koto et al., 2007). This appears to be due to, at least for some proteins, a redistribution of the TJ proteins away from cell-cell contacts rather than a reduction in TJ protein or RNA levels, and entails a transient effect with restoration of TJs upon reoxygenation (Mark and Davis, 2002; Koto et al., 2007).
Astrocytes may be less sensitive to ischemia than ECs because of increased glycolytic capacity and high endogenous anti-oxidant content and might initially protect EC TEER in vitro during hypoxia (Kondo et al., 1996; Haseloff et al., 2005). However, astrocytes are clearly affected by hypoxia over time. Transmission electron microscopy (TEM) of middle cerebral artery occlusion (MCAO) in rats showed that astrocytic endfeet swell due to osmosis and obstruct the vessel lumen or lose contact with the vessels (Figure 1) (Melgar et al., 2005; Kwon et al., 2009). The percentage of microvascular surface that was covered by astrocytic endfeet reduced gradually from 94% in sham-operated animals to 74% after 4 hours and 16% after 16 hours, to almost non-existent after 48 hours (Kwon et al., 2009). In accordance, pericytes are found to migrate away from the vessels in models of ischemia and BBB disruption, breaking through the BMs that ensheets them (Dore-Duffy et al., 2000; Melgar et al., 2005; Nishioku et al., 2009). In contrast, microglia appear to increase their proximity to the vasculature by rapid process extension in a model of local opening of the BBB by laser ablation (Nimmerjahn et al., 2005). This indicates that, in addition to direct changes in BBB permeability by TJ disruption, contact with BBB supportive cells is also lost, which is likely to enhance BBB disruption.
MATRIX RECEPTORS OF THE BBB
Two main receptors/adhesion proteins are involved in the cell-cell and cell-matrix interactions of the BBB: dystroglycan and integrins. Dystroglycan consists of a highly glycosylated extracellular alpha subunit and a transmembrane beta subunit (Moore and Winder, 2010). Integrins are a family of transmembrane glycoprotein heterodimers of alpha and beta chains, which bind several ECM ligands and activate a wide range of signaling pathways (Hynes, 1992; Hynes, 2002). Table 1 lists the expression patterns for dystroglycan and integrins in cells of the BBB. Dystroglycan is expressed in perivascular astrocytes, neurons, and ECs (Zaccaria et al., 2001; Engelhardt and Sorokin, 2009; Moore and Winder, 2010), and integrins are present on all cell types involved in BBB formation. Notably, the detection of specific integrins or their subunits on cells of the BBB varies between studies due to differences in detection methods, developmental or physiological state of the tissue, location within the brain, and the presence of an ECM ligand (McGeer et al., 1990; Paulus et al., 1993; Pinkstaff et al., 1999; Sixt et al., 2001; Milner and Campbell, 2002; Wang and Milner, 2006).
Table 1.
Expression of ECM receptors and secretion of ECM ligands by cells of the BBB.
| Cells BBB | Matrix receptors | Secreted ECM ligands |
|---|---|---|
| Endothelial cells | • Integrins: α1β1, α6β1, α6β4, α3β1, αvβ1, αvβ3, α5β1, α4β1 [1–5] • dystroglycan [5] stroke: - Expression P-selectin and E-selectin [6, 7] - Upregulation αvβ3 [7, 8] - Downregulation of α1β1, α6β4, [4] |
Fibronectin [9–12] Collagen IV [9, 11, 12] Laminin, Laminin α5 and Laminin α4 [11–13] SPARC [14] nidogen-1 [12] Agrin [15] |
| Astrocytes | • Integrins: α6β4, α1β1, αvβ8, αvβ6, αvβ5, α3β1, α5β1, α6β1 [1, 5, 16, 17] • dystroglycan [5] stroke: - Loss of α6β4 and β1 integrin [4, 16] - decrease dystrogycan [18] |
Laminin [13, 19] Fibronectin [9, 10] Nidogen-1 [20] Agrin [21] SPARC [9, 14] Collagen IV [9, 10] disease: neurocan, CSPGs [22] |
| Pericytes | Integrins: α4β1, αM [23, 24] | Collagen IV [25] Glycosaminoglycans [25] Laminin [12, 25, 26] Nidogen [12, 26] Fibronectin [10, 12] Perlecan [12] |
| Microglia | integrins: αvβ3, αvβ5, αvβ8, αMβ2 (MAC-1; CD11b/CD18), αLβ 2 (LFA-1; CD11a/CD18), αXβ2 (CD11c/CD18) [27–29] |
SPARC [14] |
Paulus et al., Am J Pathol 143, 154–63 (1993).
Wang, Milner, J Neurochem 96, 148–59 (2006).
Milner, Campbell, Mol Cell Neurosci 20, 616–26 (2002).
Tagaya et al., J Cereb Blood Flow Metab 21, 835–46 (2001).
Engelhardt, Sorokin, Semin Immunopathol 31, 497–511 (2009).
Haring et al., Stroke 27, 1386-91; discussion 91–2 (1996).
Okada et al., Am J Pathol 149, 37–44 (1996).
Abumiya et al., J Cereb Blood Flow Metab 19, 1038–50 (1999).
Webersinke et al., Biochem Biophys Res Commun 189, 877–84 (1992).
Kose et al., Drug Metab Pharmacokinet 22, 255–66 (2007).
Tilling et al., Cell Tissue Res 310, 19–29 (2002).
Stratman et al., Blood 114, 5091–101 (2009).
Sixt et al., J Cell Biol 153, 933–46 (2001).
Vincent et al., Dev Dyn 237, 1449–62 (2008).
Stone, Nikolics, J Neurosci 15, 6767–78 (1995).
Wagner et al., Stroke 28, 858–65 (1997).
del Zoppo, Milner, Arterioscler Thromb Vasc Biol 26, 1966–75 (2006).
Milner et al., J Cereb Blood Flow Metab 28, 812–23 (2008).
Liesi, Risteli, Exp Neurol 105, 86–92 (1989).
Grimpe et al., Glia 28, 138–49 (1999).
Wolburg et al., Neuroscientist 15, 180–93 (2009).
Silver, Miller, Nat Rev Neurosci 5, 146–56 (2004).
Grazioli et al., Dev Biol 293, 165–77 (2006).
Balabanov et al., Microvasc Res 52, 127–42 (1996).
Allt, Lawrenson, Cells Tissues Organs 169, 1–11 (2001).
Brachvogel et al., Exp Cell Res 313, 2730–43 (2007).
Zhu et al., Development 129, 2891–903 (2002).
Milner, Glia 57, 714–23 (2009).
Akiyama, McGeer, J Neuroimmunol 30, 81–93 (1990).
The matrix receptors perform two major functions: First, they regulate signaling pathways to allow cell adaptations to changes in the microenvironment (Hynes, 2002; Moore and Winder, 2010). Second, they form a physical link between the ECM and the cytoskeleton, thereby anchoring the cells in place and regulating their motility. Integrins signal mainly by a) activating signaling pathways upon binding to ECM ligands, b) activating latent forms of growth factors, or c) via transactivation of growth factor receptors. Integrin activation by ECM ligands affects key cellular processes, such as survival, proliferation, differentiation and migration through signaling pathways, such as focal adhesion kinase/c-Jun N-terminal kinase (FAK/JNK), Ras/ERK (MAP kinase), and the small GTPases such as Rho, Rac and Cdc42 (Giancotti and Ruoslahti, 1999; Miranti and Brugge, 2002). Astrocyte-expressed integrins αvβ6 and αvβ8 (Milner et al., 2001; Cambier et al., 2005) are major activators of latent TGF-β (Annes et al., 2003) through mechanical conformational changes (Munger et al., 1999) and proteolytic mechanisms (Mu et al., 2002), respectively. Integrins activate growth factor receptors, such as PDGF receptor and Epidermal Growth Factor (EGF) receptor, through several mechanisms (Moro et al., 1998; Giancotti and Ruoslahti, 1999; Miranti and Brugge, 2002; Schwartz and Ginsberg, 2002; Yamada and Even-Ram, 2002; ffrench-Constant and Colognato, 2004). Integrin and growth factor receptors can share common downstream signal transduction pathways, such as FAK/JNK and MAP kinase (Schwartz, 1997). As a result integrin activation by ECM ligands might potentiate growth factor receptor activation. In the presence of ECM and growth factors, growth factor receptor transactivation can occur by co-clustering with integrins, as described for example for PDGFRβ in the presence of PDGF with αvβ3 integrin binding to vitronectin, or β1 integrin binding to fibronectin (Miyamoto et al., 1996; Schneller et al., 1997). Moreover, integrins can transactivate growth factor receptors in the absence of growth factor ligands, thus allowing for direct regulation of cellular responses to changes in the extracellular environment. Examples include tyrosine phosphorylation of EGF receptor, PDGFRβ and vascular endothelial growth factor (VEGF) receptor-3 by binding of β1-integrin to ECM ligands such as collagen or fibronectin, even in the absence of EGF, PDGF-B, or VEGF respectively (Sundberg and Rubin, 1996; Moro et al., 1998; Wang et al., 2001). Phosphorylation of EGF receptor by binding of β3-integrins to fibronectin or fibrinogen has also been documented (Moro et al., 1998; Schachtrup et al., 2007). Other mechanisms of cross-talk between integrins and growth factor receptors include modulation of receptor expression and receptor compartmentalization (Miranti and Brugge, 2002).
Cells that regulate BBB permeability express a variety of integrins that regulate diverse biological functions (Table 1). ECs express several laminin-binding receptors, including α6β1, dystroglycan and α3β1, in addition to the collagen IV receptor α1β1 and a vitronectin receptor αvβ3 (Engelhardt and Sorokin, 2009). Astrocytes also express the laminin receptors dystroglycan and α6β4, both of which mediate astrocyte adhesion to laminin in vitro (Milner et al., 2008a), vitronectin receptors αvβ8 and αvβ5 and fibronectin receptor α5β1 (Engelhardt and Sorokin, 2009). As cells express multiple matrix receptors, they interact with a wide range of ECM ligands, as indicated in Table 2 for the CNS. Integrins have multiple functions at the BBB. Fibronectin-stimulated proliferation and survival of BCECs are mediated by fibronectin-binding integrins α5β1 and αvβ3 through the MAP kinase pathway (Wang and Milner, 2006). In contrast, EC growth arrest is observed on laminin through α2β1 integrin activation (Mettouchi et al., 2001). Adhesion and migration of astrocytes are mediated by the vitronectin-binding integrins αvβ5 and αvβ8, respectively (Milner et al., 1999).
Table 2.
Location and function of ECM ligands and their receptors at the BBB and changes in physiology and CNS disease.
| ECM ligand | ECM receptor/anchor | ECM ligand location and function in BBB |
ECM ligand changes in CNS diseases |
|---|---|---|---|
| Laminin isoforms: Laminin 8 (α4β1γ1; 411) Laminin 10 (α5β1γ1; 511) Laminin1 (α1β1γ1; 111) Laminin 2 (α2β1γ1; 211) |
α-dystroglycan Integrins: α6β1, α3β1, α6β4, αvβ3/β1, α5β1, αvβ5, α1β1, α2β1, α7β1 |
Present in endothelial and parenchymal BM [1, 2] BM assembly [3] Ligand for astrocyte endfeet anchoring through dystroglycan Increasing TEER of BCEC [4] Barrier for leukcocyte translocation (laminin α5) [1] |
Stroke model MCAO: BM laminin reduction over time [5] MS: leukocyte degradation of laminin [6] |
| Collagen type IV | Integrins: α1β1, α2β1, α3β1 |
Present in BM of postcapillary venules and capillaries [5] BM stabilization [7] Increasing TEER of BCEC [4] |
Stroke models MCAO, subarachnoid hemorrhage: Reduction collagen IV [5, 8] Mutations in col4a1 linked with hemorrhagic stroke [9] |
| Fibronectin | Integrins: α5β1, α4β1, αvβ3 |
Increasing TEER of BCEC [4] EC proliferation and morphology [10] |
Stroke model MCAO: loss of cellular fibronectin [5] |
| Nidogen (enactin) | Integrins: αvβ3, α3β1 | Stabilization of brain capillary BM; reduced BM in nidogen1 KO [11] |
|
| Agrin (B/z0 isoforms) | α-dystroglycan | EC and parenchymal BM [12] barrier formation [13] |
Stroke model ischemia and reperfusion: loss agrin mRNA and protein [14] Absence in leaky tumor vessels [15] AD: fragmentation at BM and plaque formation [16] |
| Perlecan | α-dystroglycan Integrins: β1 and β3, α2β1 |
EC and parenchymal BM [12] Vessel stabilization [17] |
Stroke model in baboons: loss perlecan [18] |
| SPARC | Expressed in CNS blood vessels and astrocytes in spatiotemporal manner [19] Inhibited collagen IV increased TEER of BCEC in vitro [4] Inhibits VEGF-stimulated EC proliferation [20] |
Stroke model ischemia and reperfusion: loss SPARC [14] |
|
| Fibrinogen | Integrins: αvβ3, αMβ2 (MAC-1) VE-cadherin |
Not present in healthy adult brain | After increased BBB permeability: deposition of fibrinogen and conversion to fibrin in brain [21–23] Functions in animal models of MS [24], AD [25], brain injury [26], and ischemia/hypoxia [27] |
| Osteopontin | Integrins: αvβ1, αvβ3, αvβ5, α4β1, α5β1, α8β1, α9β1 |
Not present in healthy adult brain | Stroke models hypoxia- ischemia and MCAO: upregulation and secretion of osteopontin by microglia, macrophages, neurons and astrocytes [28–30] Protective [30, 31] |
Sixt et al., J Cell Biol 153, 933–46 (2001).
van Horssen et al., J Neuropathol Exp Neurol 64, 722–9 (2005).
Miner et al., Development 131, 2247–56 (2004).
Tilling et al., J Neurochem 71, 1151–7 (1998).
Hamann et al., Stroke 26, 2120–6 (1995).
Oki et al., J Neurol Sci 222, 7–11 (2004).
Poschl et al., Development 131, 1619–28 (2004).
Scholler et al., Brain Res 1142, 237–46 (2007).
Gould et al., N Engl J Med 354, 1489–96 (2006).
Wang, Milner, J Neurochem 96, 148–59 (2006).
Dong et al., Lab Invest 82, 1617–30 (2002).
Agrawal et al., J Exp Med 203, 1007–19 (2006).
Barber, Lieth, Dev Dyn 208, 62–74 (1997).
Baumann et al., Brain Res 1269, 185–97 (2009).
Rascher et al., Acta Neuropathol 104, 85–91 (2002).
Berzin et al., Neurobiol Aging 21, 349–55 (2000).
Costell et al., J Cell Biol 147, 1109–22 (1999).
Fukuda et al., Stroke 35, 998–1004 (2004).
Vincent et al., Dev Dyn 237, 1449–62 (2008).
Brekken, Sage, Matrix Biol 19, 569–80 (2000).
Claudio et al., Acta Neuropathol 90, 228–38 (1995).
Okada et al., Stroke 25, 1847–53; discussion 53–4 (1994).
Ryu, McLarnon, J Cell Mol Med 13, 2911–25 (2009).
Adams et al., J Exp Med 204, 571–82 (2007).
Cortes-Canteli et al., Neuron 66, 695–709 (2010).
Schachtrup et al., J Neurosci 30, 5843–54 (2010).
Adhami et al., Am J Pathol 169, 566–83 (2006).
Ellison et al., Stroke 29, 1698–706; discussion 707 (1998).
Wang et al., J Neurosci 18, 2075–83 (1998).
Chen et al., Stroke 42, 764–9 (2011).
Meller et al., J Cereb Blood Flow Metab 25, 217–25 (2005).
An additional mechanism that regulates vascular permeability is integrin-mediated regulation of VE-cadherin. Integrins and cadherins transduce signals to the cell cytoskeleton to modulate cellular behavior, such as migration and attachment. While integrins signal upon binding to ECM proteins, cadherins mediate cell-cell adhesion processes (Dejana et al., 2009). Interestingly, signaling through integrin receptors alters the cadherin junctions between ECs. Activation of αvβ3 integrin results in disruption of VE-cadherin localization at AJs in ECs resulting in increased vascular permeability (Alghisi et al., 2009). Activation of αvβ3 integrin by fibronectin disrupts localization of VE-cadherin via Src activation (Wang et al., 2006b). Disruption of VE-cadherin localization is associated with increased tyrosine phosphorylation of catenins and dissociation of γ-catenin from VE-cadherin (Wang et al., 2006b). Tyrosine phosphorylation of VE-cadherin is associated with increased permeability of centrally-derived vascular endothelium (Shen et al., 2011). It is therefore possible that decreases in VE-cadherin-mediated cell-cell interaction of ECs in the CNS as a result of integrin activation may provide a mechanism for the coordination of BBB permeability in response to extracellular signals.
Integrin-deficient mice have elucidated the role of αvβ8 integrin in BBB functions: αv integrin knock-out (KO) mice developed hemorrhage, but ECs and pericytes appeared normal, while vessel attachment to parenchymal neuroepithelial cells, glia, and neuronal precursors was interrupted (McCarty et al., 2002). Follow-up studies with conditional αv KO in ECs, neurons or glia showed that the hemorrhage is due to loss of αv in radial glial cells and astrocytes (McCarty et al., 2005). While αv associates with β1, β3, β5, β6 and β8 subunits, only the β8 integrin KO mice showed similar intracerebral hemorrhage, indicating that αvβ8 was the integrin involved in regulating vascular permeability (Zhu et al., 2002; McCarty, 2009). Targeted deletion of β8 in the brain from vascular ECs or from migrating neurons had no effect, but deletion from neuroepithelium, from which astrocytes derive, resulted in hemorrhage and leaky vasculature during development (Proctor et al., 2005). While αvβ8 is a receptor for vitronectin and thus involved in cell adhesion, strikingly, the phenotype of the αvβ8 KO is mimicked by KO of active TGF-β1 and TGF-β3 (Mu et al., 2008). Interestingly, TGF-β is secreted in a latent form and binding to αv integrin resulted in TGF-β activation through release from the latent binding protein (McCarty, 2009; Nishimura, 2009). Importantly, TGF-β is involved in the regulation of EC differentiation, BM protein secretion and BBB formation (Kose et al., 2007). Astrocytic αvβ8 integrin activation of TGF-β induces EC differentiation (Cambier et al., 2005). Thus, these findings suggest that αvβ8 can affect the BBB through TGF-β -mediated EC differentiation.
Matrix Receptors in Stroke
After a stroke, cell contact with the vessels is often lost, suggesting a change in the receptors that anchor the cells in place. In baboons, levels of several integrins and dystroglycan were reduced after MCAO (Okada et al., 1996; Wagner et al., 1997; Tagaya et al., 2001; Milner et al., 2008b). The number of vessels that stained positive for α1 or β1 integrin showed a reduction of roughly 30% after 2 hours and even 75% 24 hours after MCAO in the ischemic core, indicating an early loss of α1β1 from ECs (Tagaya et al., 2001). In astrocytes, a marked decrease in the laminin-binding proteins α6β4 and dystroglycan was observed in a similar time frame 2–4 hours after MCAO, coincident with astrocyte swelling (Wagner et al., 1997; Tagaya et al., 2001; Milner et al., 2008b). Loss of integrin-ECM binding may not only result in loss of cell anchoring, but could also lead to detachment-mediated cell death (anoikis), through loss of integrin signaling (Sandoval and Witt, 2008). While several integrins are downregulated in stroke, upregulation of integrins is also observed. In ECs after MCAO, αvβ3, which is otherwise only present during angiogenesis, is induced in selected microvessels (Okada et al., 1996; Wang and Milner, 2006). In addition, integrins mediate the secondary inflammation that follows stroke and are particularly important for leukocyte rolling and diapedesis (Ley et al., 2007).
ECM PROTEINS OF THE BBB IN PHYSIOLOGY
In addition to the cellular players, ECM proteins are vital components of the BBB playing both structural and modulatory roles. ECM proteins in the BMs of the microvessels in the CNS between the ECs and the astrocytic endfeet are involved in BBB functions (Figure 1). BMs are extracellular matrices associated with cell surfaces. They form a sheet-like structure and support epithelia and vascular ECs (Yurchenco and Patton, 2009). BMs consist of structural elements (e.g., type IV collagens and elastin), specialized proteins (e.g., laminins, entactin/nidogen, fibronectin, and vitronectin), and proteoglycans (e.g., heparan sulfate proteoglycans (HSPG) perlecan and agrin) (Barber and Lieth, 1997; Lukes et al., 1999; Fukuda et al., 2004; Yurchenco et al., 2004; Agrawal et al., 2006; Baumann et al., 2009; Cardoso et al., 2010). Assembly and organization of BMs in general involve polymerization of laminins and collagens, linkage by nidogens and a critical regulatory role of laminin (Yurchenco et al., 2004; Yurchenco and Patton, 2009).
The BBB, located at the capillaries and postcapillary venules in the CNS, contains two BMs: a vascular wall/endothelial BM and a parenchymal BM of the glia limitans of astrocytes (Sixt et al., 2001; Owens et al., 2008). Under normal conditions, the two BMs are in close contact and appear as one under light microscopy (Sixt et al., 2001). The parenchymal BM is formed by astrocytes and its ECM composition differs somewhat from other BMs, containing mainly laminin isoforms 1 and 2 as discussed in detail below (Sixt et al., 2001; van Horssen et al., 2005; Owens et al., 2008). In contrast, most ECM proteins are ubiquitously expressed at the BM. The parenchymal BM also lines the perivascular space and forms a barrier for leukocyte migration into the brain parenchyma, which results under inflammatory conditions in the perivascular cuff, consisting of infiltrating leukocytes (van Horssen et al., 2005).
The BM is involved in barrier formation at several levels. First, with its anatomical location between ECs and astrocytes, the BM is ideally positioned to directly regulate barrier function (Barber and Lieth, 1997). Second, the BM provides physical support for the different cells and anchors them into place. Third, BM components are ECM ligands that might regulate cellular processes and signaling between the different cell types through their interactions with integrin and other ECM receptors (Lukes et al., 1999; Sixt et al., 2001; Tilling et al., 2002; Agrawal et al., 2006; Owens et al., 2008; Cardoso et al., 2010). Thus, while ECM proteins of the BM are secreted by the endothelial and parenchymal cells, the relationship between cells and ECM is bidirectional in that, once secreted, the ECM proteins interact with the cells to induce and maintain barrier properties (Table 1 and 2).
Laminin
Laminins are glycoproteins with several isoforms, comprising a combination of alpha, beta and gamma chains (Miner et al., 2004; Hallmann et al., 2005). At the BBB, the four main laminin isoforms are laminin411, 511, 111 and 211. Laminin411, consisting of laminin chains α4β1γ1 and also called laminin 8, and laminin511 (α5β1γ1 or laminin 10) are secreted by the ECs and are present in the endothelial BM, as is the case throughout the body (Sorokin et al., 1994; Sixt et al., 2001; Tilling et al., 2002). In contrast, laminin111 (α1β1γ1, laminin 1) and laminin211 (α2β1γ1, laminin 2) are specific for the brain microvasculature. Laminin 1 and 2 are secreted by astrocytes and located in the parenchymal BM, and their secretion is regulated by pericytes (Sorokin et al., 1994; Sixt et al., 2001; Tilling et al., 2002; Armulik et al., 2010).
Laminin is essential for BM assembly (Miner et al., 2004) and mice KO for the major subunit laminin γ1, which is present in all BM laminin isoforms, lack BMs resulting in embryonic death at E5.5 (Smyth et al., 1999). Other mutations in laminin subunits also lead to disrupted BMs (Miyagoe et al., 1997; Miner et al., 1998; Halfter et al., 2002; Knoll et al., 2007). Laminin may affect barrier functions, since BCECs show increased TEER when grown on laminin (Tilling et al., 1998). Moreover, local formation of inflammatory cuffs does not occur in areas where Laminin α5 is present in the BM, suggesting a possible barrier function for leukocyte infiltration (Sixt et al., 2001; Wang et al., 2006a). More detailed studies are needed to show a causal relationship between laminin and the inhibition of leukocyte migration.
Collagen IV and Fibronectin
Collagen IV and fibronectin are secreted by ECs, astrocytes and pericytes and found in the periphery and in the vasculature of the CNS. They have an important function in BM assembly, as collagen IV stabilizes the BM by retaining laminin, nidogen and perlecan (Poschl et al., 2004). Similar to laminin, both collagen IV and fibronectin affect the barrier properties of ECs as shown by the increased TEER of BCECs in vitro (Tilling et al., 1998). In addition, fibronectin stimulates the proliferation and survival of BCECs in vitro (Wang and Milner, 2006). Null mutations of either fibronectin or collagen IV are embryonic lethal due to mesoderm defects or impaired BM stability respectively; and more modest mutations in the Col4a1 gene are associated with fragile vessels, which predispose to stress-induced hemorrhage and adult-onset stroke in humans and mice (George et al., 1993; Poschl et al., 2004; Gould et al., 2006; Alamowitch et al., 2009).
Nidogen
Nidogen, also called enactin, is a protein component of most BMs that links laminin and collagen IV and binds to many other ECM proteins. Nidogen does not appear to be necessary for BM formation since KOs of nidogen, both in Caenorhabditis elegans and mice, still form BMs that appear normal (Kang and Kramer, 2000; Murshed et al., 2000). However, nidogen plays a role in BM structure, since nidogen KO mice show reduced BMs specifically in brain capillaries (Dong et al., 2002). In addition, antisense treatment of astrocytes in culture that normally express nidogen results in astrocyte detachment (Grimpe et al., 1999). Thus, loss of nidogen might compromise the BBB.
Agrin and Perlecan
Agrin and perlecan are heparan sulphate proteoglycans found in the BM (Iozzo et al., 1994; Burgess et al., 2000; Agrawal et al., 2006; Wolburg et al., 2009). Agrin undergoes differential splicing, and the splice variants have different expression patterns and functions (Smith and Hilgenberg, 2002). B/z-containing isoforms are expressed in neurons and are essential for the formation of neuromuscular junctions, while non-neuronal cells express only the B/z-negative forms found in the BM (Gautam et al., 1996). Interestingly, the distribution of agrin in BMs is restricted to vascular endothelium that performs a barrier function, such as in the CNS, testes and thymus (Barber and Lieth, 1997). Moreover, the time frame of agrin appearance in CNS vessels coincides with that of BBB formation during development, suggesting that agrin has a role in barrier formation (Barber and Lieth, 1997). The exact mechanism by which agrin contributes to the barrier is unknown, but agrin is a major binding protein for α-dystroglycan and, thus, could be involved in anchoring ECs and astrocytes to the BM (Gesemann et al., 1998). Agrin might also be involved in other barrier properties. For example, in the presence of agrin, TJs contain occludin, while leaky vessels of human glioblastoma that lack agrin also lack occludin and claudin-5 (Rascher et al., 2002). Agrin might also influence astrocyte polarization and aquaporin 4 clustering into orthogonal arrays of particles, but their significance to BBB maintenance is unknown (Noell et al., 2009).
Perlecan, also called heparan sulfate proteoglycan 2 (HSPG2), is a component of most BMs throughout the body and also an important constituent of cartilage (Knox and Whitelock, 2006). It might help to stabilize BMs. Perlecan KO mice are embryonic-neonatal lethal and show deterioration of brain vesicles and myocardial BM, possibly due to mechanical stress (Arikawa-Hirasawa et al., 1999; Costell et al., 1999). Mice lacking perlecan in the BM of the ECs have dilations and microvessel bleeds (Hallmann et al., 2005).
Secreted Protein, Acidic and Rich in Cysteine (SPARC)
SPARC (also called BM-40 or osteonectin) is not a structural component of the BM in the way that laminin, collagen IV and fibronectin are. Instead, this matricellular protein mediates cell-matrix interactions (Brekken and Sage, 2000). SPARC is expressed in CNS blood vessels and astrocytes in spatiotemporal manner (Vincent et al., 2008). It has several functions and may affect blood vessels, for example by inhibition of VEGF-stimulated EC proliferation (Brekken and Sage, 2000). SPARC KO mice showed no apparent changes in BBB properties, despite obvious disruptions of vascular BMs in the periphery (Arnold et al., 2010). SPARC actually promotes barrier dysfunction in EC monolayers (Goldblum et al., 1994), inhibits collagen IV-mediated increase in TEER of low TEER BCECs in vitro (Tilling et al., 2002), and has counter-adhesive properties that result in rounding of ECs, inhibition of EC spreading and loss of focal adhesions through a tyrosine-kinase-dependent pathway (Motamed and Sage, 1998).
ECM PROTEINS IN STROKE
After a stroke, several changes occur in the ECM of the brain. While BMs of the BBB are degraded, new ECM proteins are deposited in the parenchyma, either by secretion of ECM proteins, such as the chondroitin sulfate proteoglycan neurocan and osteopontin from activated glia, or by leakage of plasma proteins, such as fibrinogen, into the CNS (Baumann et al., 2009; Schachtrup et al., 2010). The significance and consequences of these changes may vary with the time point after injury. Some of the changes, such as loss of BMs, contribute directly to BBB disruption, and others mediate secondary inflammation and further BBB breakdown. On the other hand, altered ECM composition may also inhibit or initiate remodeling and repair in the parenchyma (Ellison et al., 1999; Gould et al., 2006).
Loss of basement membranes
The degradation of the BMs after a stroke is very striking and can be observed by TEM or immunohistochemistry for BM proteins (Del Zoppo et al., 2006; Kwon et al., 2009). Notably, the changes in BMs only occur at later time points after injury, while the changes in matrix receptors occur early within a few hours (Kwon et al., 2009). TEM shows the dramatic change from a well-defined, sharply delineated and electron-dense BM to a fuzzy, faint and low-density appearance at 12–48 hours after MCAO (Kwon et al., 2009). Subarachnoid hemorrhage or MCAO, followed by reperfusion in baboons and rats, results in BBB disruption and breakdown of BM collagen IV 24–72 hours after injury (Hamann et al., 1995; Hamann et al., 2002; Scholler et al., 2007). Similarly, MCAO and reperfusion (24 hours) also resulted in a reduction in laminin and cellular fibronectin (Hamann et al., 1995). Other BM proteins, such as agrin, SPARC and perlecan, are also degraded after ischemia (Fukuda et al., 2004; Baumann et al., 2009). Perlecan is degraded soon after MCAO and shows a 50% reduction after only 2 hours (Fukuda et al., 2004).
BM proteins are degraded by proteolysis, mainly by matrix metalloproteinases (MMPs) and plasmin (Liotta et al., 1981; Lukes et al., 1999; Fukuda et al., 2004). These enzyme systems are important for ECM degradation during remodeling, and their activity is highly regulated. In addition to transcriptional regulation, and the presence of inhibitors, plasmin and MMPs are secreted in their inactive zymogen forms and require activation (Lukes et al., 1999; Sachs et al., 2007). However, in pathological conditions, such as stroke, an upregulation of MMPs and their activation systems is evident (Clark et al., 1997; Lukes et al., 1999; Chang et al., 2003; Candelario-Jalil et al., 2009). Microvascular collagen and perlecan can be degraded ex vivo by ischemic brain tissue proteases. Together with laminin, they are degraded by mixtures of MMP-2 or -9 and plasmin, or by the cycteine proteases cathepsin B and L (Fukuda et al., 2004). Perlecan appears to be most sensitive to degradation by cathepsin B and L, which only appear upon ischemia. This might explain why it is degraded before the other BM proteins (Fukuda et al., 2004). Degradation of the BM is related to increased BBB disruption, secondary inflammation, edema, and hemorrhagic transformation, and inhibiting MMPs may be beneficial for treating stroke, as reviewed elsewhere (Morancho et al., 2010).
ECM proteins deposited in stroke
Osteopontin
Osteopontin (OPN) is a secreted glycosylated phosphoprotein that can bind several integrin receptors including αvβ1, αvβ3, αvβ5, α4β1, α5β1, α8β1, α9β1, and CD44 and can act on several cell types (Ellison et al., 1999; Meller et al., 2005). OPN is not normally present in the brain, except during development, or after induction in gliomas or ischemic injury (Ellison et al., 1998; Wang et al., 1998a; Chen et al., 2011). After ischemia, OPN is expressed in neurons, macrophages and astrocytes in the neonate, while in the adult brain OPN is upregulated in microglia and macrophages around the infarct zone (Ellison et al., 1998; Wang et al., 1998a; Chen et al., 2011). Interestingly, expression of the OPN receptor αvβ3 also increases in astrocytes and is associated with astrocyte migration to the OPN-rich infarct zone (Ellison et al., 1998). Glial scar formation by activated astrocytes could limit the expansion of the injured zone and protect the surrounding tissue (Ellison et al., 1999). It is unclear whether astrocyte migration is due to OPN deposition, or whether another mechanism is involved. Nevertheless, OPN appears to be beneficial after an ischemic insult: intracerebroventricular injection of OPN reduces infarct volume and improves functional recovery following neonatal hypoxia/ischemia in rat pups (Chen et al., 2011). Moreover, although lack of endogenous OPN does not affect infarcts in CNS as shown in OPN KO mice, injected OPN has direct protective effects on neurons after ischemia (Meller et al., 2005).
Fibrinogen
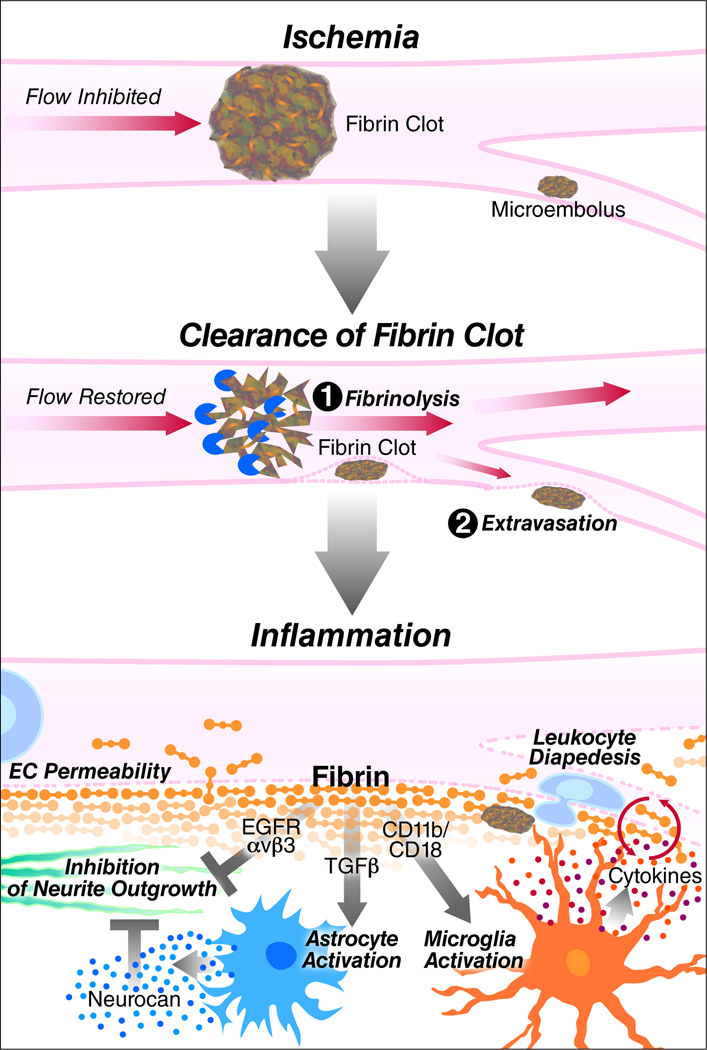
Fibrinogen is a major plasma protein and acute-phase reactant that is secreted by the liver and that has a major role in blood coagulation. In normal conditions, fibrinogen circulates in the blood stream and cannot enter the CNS parenchyma because of the BBB. In stroke, fibrinogen is the major protein component of thrombotic emboli that occlude blood vessels (Figure 2) (Schultz and Arnold, 1990; Okada et al., 1994; Ninomia et al., 2000; Murray et al., 2010). Fibrinogen also leaks in the brain after stroke to form fibrin deposits and may thus participate to secondary damage by increasing inflammation and inhibiting repair processes in the CNS (Figure 2) (Adams et al., 2007b; Ryu et al., 2009). In a stroke model for hypoxia/ischemia, brain damage is decreased in fibrinogen-deficient mice suggesting that fibrinogen participates in ischemic neurodegeneration (Adhami et al., 2006). During thrombus formation, fibrinogen acts as a bridging molecule for activated platelets, causing platelet aggregation through binding to GPIIb/IIIa (integrin αIIbβ3) on the platelet surface (Shattil et al., 1985). Stabilization of the occluding thrombus occurs when fibrinogen is converted to insoluble fibrin by enzymatic removal of fibrinopeptide A and B by thrombin and crosslinking by factor XIII at the end of the coagulation cascade (Yang et al., 2000; Mosesson, 2005). In the occluded area, ischemia and hypoxia result in extra fibrin deposits in the microvasculature, where they negatively influence reperfusion. This sequence has been demonstrated in post-mortem human brains (Heye and Cervos-Navarro, 1996) and in animal stroke models (Okada et al., 1994; Tabrizi et al., 1999; Zhang et al., 1999; Ninomia et al., 2000; Adhami et al., 2006).
Figure 2.
Fibrin during thrombus formation and secondary damage in stroke. In stroke, a fibrin clot or fibrin-stabilized thrombus occludes a vessel, stopping blood flow and causing ischemia. The fibrin clot is cleared enzymatically by fibrinolysis or by extravasation in the CNS. Ischemia–reperfusion is followed by inflammation and disruption of the BBB. Deposition of fibrin into the CNS parenchyma results in microglia activation via CD11b/CD18, leading to increased inflammation; astrocyte activation via TGF-β, resulting in neurocan deposition; and inhibition of neurite outgrowth through αvβ3 and EGF receptor. Fibrinogen-induced proinflammatory and neurodegenerative changes in the CNS may exacerbate damage after stroke.
Fibrin must be cleared from the vessel to re-establish blood flow (delivery of oxygen and glucose), which is necessary to prevent further tissue damage and neuronal death (Ding and Clark, 2006). Two major mechanisms are responsible for fibrin clearance. In fibrinolysis, fibrin is enzymatically degraded to soluble products by plasmin (Sidelmann et al., 2000; Lijnen, 2001). Plasminogen activators (PA), tissue-type PA (tPA) or urokinase (uPA), activate plasminogen to plasmin, a reaction that is enhanced on a fibrin or cell surface (Hoylaerts et al., 1982; Ranby, 1982; Miles and Plow, 1985; Ellis et al., 1991; Gong et al., 2001). Fibrin breakdown is regulated at different levels. For example, plasminogen activator inhibitor-1 (PAI-1) is released from activated platelets and from ECs where it is upregulated in ischemic regions after stroke (Booth et al., 1985; Booth, 1999; Zhang et al., 1999). Fibrinolysis by tPA in stroke has been extensively studied (Wang et al., 1998b; Sheehan and Tsirka, 2005; Adibhatla and Hatcher, 2008; Gravanis and Tsirka, 2008) and forms the basis of thrombolytic therapy with recombinant tPA, currently the only FDA-approved treatment for stroke (1995) (Murray et al., 2010).
A second mechanism of fibrin clearance was recently described. Lam et al. (2010) showed that microemboli injected in mice (fibrin clots, cholesterol emboli or microspheres) that had not been cleared by fibrinolysis and haemodynamic forces within 48 hours can undergo extravasation in the following days. In vivo two-photon microscopy and high-resolution confocal and electron microscopy showed engulfment of intravascular microemboli by endothelial membrane and the extravascular localization of the microembolus several days later (Lam et al., 2010). The cellular and molecular mechanisms that underlie this phenomenon have not yet been elucidated. However, translocation across the EC layer after the engulfment is likely to involve disruption of intercellular TJs, or the formation of a large transcellular channel, as well as ECM remodeling, suggested by the importance of MMP2/9 activity (Lam et al., 2010). This mechanism, together with increased BBB permeability, might contribute to the extravascular fibrin deposits observed in several stroke models (Okada et al., 1994; Ninomia et al., 2000; Baumann et al., 2009).
Disruption of the BBB, extravasation of fibrin clots and hemorrhagic stroke with ruptured blood vessels all result in fibrin(ogen) deposition in the brain. Fibrin(ogen) interacts with different cell types in the brain through a variety of integrin binding sites within the fibrinogen molecule (Adams et al., 2004; Adams et al., 2007b) and, in that way, may aggravate the damage after stroke. Fibrin exerts proinflammatory functions in the CNS primarily as an activator of microglia, the resident immune cells of the brain and spinal cord (Adams et al., 2007a). Fibrin binding to the integrin αMβ2 (also called CD11b/CD18, Mac-1 or complement receptor 3) receptor in microglia occurs through a binding site in its gamma chain (γ390–396), which is exposed in fibrin and immobilized fibrinogen, but not in soluble fibrinogen (Ugarova et al., 2003; Flick et al., 2004; Adams et al., 2007a). A study in our laboratory by Adams et al. (2007a) shows that the binding of fibrin to αMβ2 on microglia results in microglia activation, which is characterized by an increase in cell size and induction of phagocytosis. The activation of microglia by fibrin is inhibited when the interaction of fibrin γ390–396 and Mac-1 is blocked, by M1/70 antibody against CD11b or fibrin γ377–395 peptide or in mice that have a mutation in the Mac-1 binding site of fibrinogen (fibrinogen γ390–396A) (Flick et al., 2004). Inhibition of the interaction of fibrinogen with the microglial αMβ2 receptor suppressed neurologic symptoms and inflammatory demyelination in animal models of MS (Adams et al., 2007a). Importantly, none of these inhibitory mechanisms interferes with the clotting function of fibrinogen, making this interaction an attractive therapeutic target (Adams et al., 2007a; Adams et al., 2007b).
In addition to microglia activation, fibrinogen increases inflammation by promoting leukocyte translocation and EC permeability (Languino et al., 1993; Languino et al., 1995; Petzelbauer et al., 2005; Sahni et al., 2009). Fibrin(ogen) binds to VE-cadherin in ECs via a site in its beta-chain (β15–42), which is only exposed after removal of fibrinopeptide B during fibrin formation (Gorlatov and Medved, 2002). Blocking the interactions of fibrin with VE-cadherin using Bβ15–42 inhibits transmigration of leukocytes across Human Umbilical Vein Endothelial Cells (HUVECs) in vitro (Petzelbauer et al., 2005). In vivo, the infarct size from myocardial reperfusion injury is significantly lower in fibrinogen KO mice than in wild-type mice, confirming a role for fibrinogen in that process (Petzelbauer et al., 2005). Increased translocation of leukocytes across the EC barrier in the presence of fibrin(ogen) fragments is likely due to the physical linkage of ECs and leukocytes by fibrin(ogen) (Petzelbauer et al., 2005). The inhibitory fibrin Bβ15–42 peptide shows therapeutic promise. When administered in reperfusion injury models in the presence of both leukocytes and fibrinogen, it reduces infarct size, myocardial inflammation and scar formation (Petzelbauer et al., 2005). Other receptors for fibrinogen in ECs include ICAM-1 and CD54, which can also facilitate leukocyte adhesion and transendothelial migration (Languino et al., 1993; Languino et al., 1995).
Regeneration is also affected by ECM. In addition to ECM expressed in situ (Kwok and Fawcett, 2011), BBB disruption directly contributes to the inhibition of regeneration via fibrinogen leakage in the CNS. Fibrinogen that enters the CNS upon increased BBB permeability inhibits neurite outgrowth by either directly interacting with neurons (Schachtrup et al., 2007) or by stimulating the secretion of inhibitory proteoglycans from astrocytes (Schachtrup et al., 2010). These mechanisms might also hinder recovery after stroke (Figure 2). Fibrinogen causes inhibition of neurite outgrowth in vitro on two different rodent cell types, cerebellar granule neurons and superior cervical ganglia neurons (Schachtrup et al., 2007). The inhibition by fibrinogen is concentration-dependent and is observed not only at its physiological concentration in the plasma, but also at concentrations 10-fold less at 0.3 mg/ml. Fibrinogen is as potent as myelin in inhibiting neurite outgrowth. Fibrinogen (Aα572–574) binds to the αvβ3 integrin receptor expressed on neurons, which results in phosphorylation of the EGF receptor, mediating inhibition of neurite outgrowth (Schachtrup et al., 2007). In addition to inhibition of neurite outgrowth, induction of astrocyte activation and glial scar formation by fibrinogen may contribute to the inhibition of axon regeneration. In a mouse model of stab wound injury, genetic loss of fibrinogen suppressed astrocyte activation and neurocan deposition (Schachtrup et al., 2010). Neurocan secreted from activated astrocytes inhibits neurite outgrowth (Friedlander et al., 1994; Asher et al., 2000). Decrease of neurite outgrowth in cortical neurons by conditioned medium from fibrinogen-treated astrocytes was rescued by chondroitinase ABC that degrades proteoglycans such as neurocan (Schachtrup et al., 2010). In this case, the effect of fibrinogen is not through direct binding of fibrinogen to integrins, but through regulation of the bioavailability of TGF-β (Schachtrup et al., 2010). Indeed, fibrinogen was identified as a novel carrier of the latent form of TGF-β (Schachtrup et al., 2010). Upon BBB disruption, fibrinogen-bound latent TGF-β interacts with local perivascular astrocytes, leading to active TGF-β formation, which induces reactive astrocytosis by regulating the TGF-β/Smad signaling pathway, resulting in scar formation (Schachtrup et al., 2010). Therefore, fibrin(ogen) influences both the occurrence of stroke as the main component of thrombotic emboli and the development of secondary edema through its interactions with CNS cells as a ligand for integrin receptors or a carrier for growth factors in the CNS.
CONCLUDING REMARKS
The extracellular environment undergoes frequent changes during development, upon adaptation to physiological needs, and during disease pathogenesis. Changes in ECM composition affect functions of the cells forming the BBB via a multitude of ECM receptors that transduce signals to coordinate cellular responses with changes in their environment. Moreover, expression of ECM receptors and secretion of ECM proteins by cells at the BBB is tightly regulated at different developmental stages or during disease. Therefore, the presence of multiple ECM components at the brain microvasculature in close contact with the cells forming the BBB represent an ideal system to regulate the dynamic changes of BBB functions. Indeed, changes in BBB functions correlate with changes in ECM composition. This is evident by the reduction of ECM ligands, such as laminin, fibronectin, and collagen IV, and their receptors that coincides with increased BBB permeability in several stroke models (Figure 1) (Hamann et al., 1995; Tagaya et al., 2001; Fukuda et al., 2004). Moreover, the functional importance of integrins and ECM proteins in BBB functions was demonstrated in genetic models (Arikawa-Hirasawa et al., 1999; Smyth et al., 1999; Dong et al., 2002; McCarty et al., 2002; Gould et al., 2006). Notably, ECM proteins can activate integrin receptors to regulate cell survival, motility and responses to growth factors (Milner et al., 1999; Milner and Campbell, 2002; ffrench-Constant and Colognato, 2004; Adams et al., 2007b). Thus, ECM proteins and their receptors are emerging multifaceted regulators of BBB functions.
Increased BBB permeability during disease alters the ECM composition of the CNS due to leakage of plasma proteins and deposition of fibrin, which mediates proinflammatory and neurodegenerative effects by direct interactions with integrin receptors in neurons and glia (Figure 2). Studies using fibrinogen KO mice, fibrinogen mutants that do not bind integrin receptors, or fibrin-depleting anti-coagulants demonstrated a causative role for fibrin in animal models of MS (Adams et al., 2007a), AD (Cortes-Canteli et al., 2010), inhibition of remyelination (Akassoglou et al., 2002), astrocyte scar formation (Schachtrup et al., 2010), and ischemia (Adhami et al., 2006). Further studies with genetically modified animals for other ECM proteins will elucidate their role in BBB functions and their contribution during disease. Integrins are excellent therapeutic targets since their activity can be modulated by monoclonal antibodies, many of which are already used in clinical applications. Since increased BBB permeability is a hallmark of several diseases including stroke, MS, and AD, targeting the interactions of ECM proteins with their integrin receptors could potentially lead to novel therapeutic agents for neurologic, neuroinflammatory, and neurodegenerative diseases.
Acknowledgments
We thank John Carroll for graphics and Gary Howard for editorial assistance. K.A. is supported by National Institutes of Health/National Institute of Neurological Disorders and Stroke R01 Grants NS051470, NS052189 and NS066361.
Abbreviations
- ABC
ATP-binding cassette
- AD
Alzheimer’s Disease
- AJ
adherens junctions
- BBB
blood-brain barrier
- BCEC
brain capillary endothelial cells
- BM
basement membranes
- CNS
central nervous system
- CSF
cerebrospinal fluid
- EC
endothelial cell
- ECM
extracellular matrix
- EGF
Epidermal Growth Factor
- GLUT-1
glucose transporter 1
- HUVEC
human umbilical vein endothelial cell
- JAM
junctional adhesion molecule
- KO
knock-out
- LAM
leukocyte adhesion molecule
- MS
Multiple Sclerosis
- MCAO
middle cerebral artery occlusion
- OPN
osteopontin
- PA
plasminogen activator
- PAI-1
plasminogen activator inhibitor-1
- PDGF
Platelet-Derived Growth Factor
- PDGFRβ
Platelet-Derived Growth Factor Receptor beta
- SPARC
Secreted Protein Acidic and Rich in Cysteine
- TEER
transendothelial electrical resistance
- TEM
transmission electron microscopy
- TGF-β
Transforming Growth Factor-beta
- TJ
tight junction
- VEGF
Vascular Endothelial Growth Factor
- ZO
zona occludens
REFERENCES
- Abbott NJ, Ronnback L, Hansson E. Astrocyte-endothelial interactions at the blood-brain barrier. Nat Rev Neurosci. 2006;7:41–53. doi: 10.1038/nrn1824. [DOI] [PubMed] [Google Scholar]
- Adams RA, Passino M, Sachs BD, Nuriel T, Akassoglou K. Fibrin mechanisms and functions in nervous system pathology. Mol Interv. 2004;4:163–176. doi: 10.1124/mi.4.3.6. [DOI] [PubMed] [Google Scholar]
- Adams RA, Bauer J, Flick MJ, Sikorski SL, Nuriel T, Lassmann H, Degen JL, Akassoglou K. The fibrin-derived gamma377-395 peptide inhibits microglia activation and suppresses relapsing paralysis in central nervous system autoimmune disease. J Exp Med. 2007a;204:571–582. doi: 10.1084/jem.20061931. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Adams RA, Schachtrup C, Davalos D, Tsigelny I, Akassoglou K. Fibrinogen signal transduction as a mediator and therapeutic target in inflammation: Lessons from Multiple Sclerosis. Curr Med Chem. 2007b;14:2925–2936. doi: 10.2174/092986707782360015. [DOI] [PubMed] [Google Scholar]
- Adhami F, Liao G, Morozov YM, Schloemer A, Schmithorst VJ, Lorenz JN, Dunn RS, Vorhees CV, Wills-Karp M, Degen JL, Davis RJ, Mizushima N, Rakic P, Dardzinski BJ, Holland SK, Sharp FR, Kuan C-Y. Cerebral Ischemia-Hypoxia Induces Intravascular Coagulation and Autophagy. Am J Pathol. 2006;169:566–583. doi: 10.2353/ajpath.2006.051066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Adibhatla RM, Hatcher JF. Tissue plasminogen activator (tPA) and matrix metalloproteinases in the pathogenesis of stroke: therapeutic strategies. CNS Neurol Disord Drug Targets. 2008;7:243–253. doi: 10.2174/187152708784936608. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Agrawal S, Anderson P, Durbeej M, van Rooijen N, Ivars F, Opdenakker G, Sorokin LM. Dystroglycan is selectively cleaved at the parenchymal basement membrane at sites of leukocyte extravasation in experimental autoimmune encephalomyelitis. J Exp Med. 2006;203:1007–1019. doi: 10.1084/jem.20051342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Akassoglou K, Yu WM, Akpinar P, Strickland S. Fibrin inhibits peripheral nerve remyelination by regulating Schwann cell differentiation. Neuron. 2002;33:861–875. doi: 10.1016/s0896-6273(02)00617-7. [DOI] [PubMed] [Google Scholar]
- Al Ahmad A, Taboada CB, Gassmann M, Ogunshola OO. Astrocytes and pericytes differentially modulate blood-brain barrier characteristics during development and hypoxic insult. J Cereb Blood Flow Metab. 2010 doi: 10.1038/jcbfm.2010.148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alamowitch S, Plaisier E, Favrole P, Prost C, Chen Z, Van Agtmael T, Marro B, Ronco P. Cerebrovascular disease related to COL4A1 mutations in HANAC syndrome. Neurology. 2009;73:1873–1882. doi: 10.1212/WNL.0b013e3181c3fd12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alghisi GC, Ponsonnet L, Ruegg C. The integrin antagonist cilengitide activates alphaVbeta3, disrupts VE-cadherin localization at cell junctions and enhances permeability in endothelial cells. PLoS One. 2009;4:e4449. doi: 10.1371/journal.pone.0004449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Allaman I, Belanger M, Magistretti PJ. Astrocyte-neuron metabolic relationships: for better and for worse. Trends Neurosci. 2011;34:76–87. doi: 10.1016/j.tins.2010.12.001. [DOI] [PubMed] [Google Scholar]
- Allt G, Lawrenson JG. Pericytes: cell biology and pathology. Cells Tissues Organs. 2001;169:1–11. doi: 10.1159/000047855. [DOI] [PubMed] [Google Scholar]
- Annes JP, Munger JS, Rifkin DB. Making sense of latent TGFbeta activation. J Cell Sci. 2003;116:217–224. doi: 10.1242/jcs.00229. [DOI] [PubMed] [Google Scholar]
- Arikawa-Hirasawa E, Watanabe H, Takami H, Hassell JR, Yamada Y. Perlecan is essential for cartilage and cephalic development. Nat Genet. 1999;23:354–358. doi: 10.1038/15537. [DOI] [PubMed] [Google Scholar]
- Armulik A, Genove G, Mae M, Nisancioglu MH, Wallgard E, Niaudet C, He L, Norlin J, Lindblom P, Strittmatter K, Johansson BR, Betsholtz C. Pericytes regulate the blood-brain barrier. Nature. 2010;468:557–561. doi: 10.1038/nature09522. [DOI] [PubMed] [Google Scholar]
- Arnold SA, Rivera LB, Miller AF, Carbon JG, Dineen SP, Xie Y, Castrillon DH, Sage EH, Puolakkainen P, Bradshaw AD, Brekken RA. Lack of host SPARC enhances vascular function and tumor spread in an orthotopic murine model of pancreatic carcinoma. Dis Model Mech. 2010;3:57–72. doi: 10.1242/dmm.003228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Asher RA, Morgenstern DA, Fidler PS, Adcock KH, Oohira A, Braistead JE, Levine JM, Margolis RU, Rogers JH, Fawcett JW. Neurocan is upregulated in injured brain and in cytokine-treated astrocytes. J Neurosci. 2000;20:2427–2438. doi: 10.1523/JNEUROSCI.20-07-02427.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bagley RG, Weber W, Rouleau C, Teicher BA. Pericytes and endothelial precursor cells: cellular interactions and contributions to malignancy. Cancer Res. 2005;65:9741–9750. doi: 10.1158/0008-5472.CAN-04-4337. [DOI] [PubMed] [Google Scholar]
- Bandopadhyay R, Orte C, Lawrenson JG, Reid AR, De Silva S, Allt G. Contractile proteins in pericytes at the blood-brain and blood-retinal barriers. J Neurocytol. 2001;30:35–44. doi: 10.1023/a:1011965307612. [DOI] [PubMed] [Google Scholar]
- Banerjee S, Bhat MA. Neuron-glial interactions in blood-brain barrier formation. Annu Rev Neurosci. 2007;30:235–258. doi: 10.1146/annurev.neuro.30.051606.094345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barber AJ, Lieth E. Agrin accumulates in the brain microvascular basal lamina during development of the blood-brain barrier. Dev Dyn. 1997;208:62–74. doi: 10.1002/(SICI)1097-0177(199701)208:1<62::AID-AJA6>3.0.CO;2-#. [DOI] [PubMed] [Google Scholar]
- Baumann E, Preston E, Slinn J, Stanimirovic D. Post-ischemic hypothermia attenuates loss of the vascular basement membrane proteins, agrin and SPARC, and the blood-brain barrier disruption after global cerebral ischemia. Brain Res. 2009;1269:185–197. doi: 10.1016/j.brainres.2009.02.062. [DOI] [PubMed] [Google Scholar]
- Bell RD, Winkler EA, Sagare AP, Singh I, LaRue B, Deane R, Zlokovic BV. Pericytes control key neurovascular functions and neuronal phenotype in the adult brain and during brain aging. Neuron. 2010;68:409–427. doi: 10.1016/j.neuron.2010.09.043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Booth NA, Anderson JA, Bennett B. Platelet release protein which inhibits plasminogen activators. J Clin Pathol. 1985;38:825–830. doi: 10.1136/jcp.38.7.825. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Booth NA. Fibrinolysis and thrombosis. Baillieres Best Pract Res Clin Haematol. 1999;12:423–433. doi: 10.1053/beha.1999.0034. [DOI] [PubMed] [Google Scholar]
- Brachvogel B, Pausch F, Farlie P, Gaipl U, Etich J, Zhou Z, Cameron T, von der Mark K, Bateman JF, Poschl E. Isolated Anxa5+/Sca-1+ perivascular cells from mouse meningeal vasculature retain their perivascular phenotype in vitro and in vivo. Exp Cell Res. 2007;313:2730–2743. doi: 10.1016/j.yexcr.2007.04.031. [DOI] [PubMed] [Google Scholar]
- Brekken RA, Sage EH. SPARC, a matricellular protein: at the crossroads of cell-matrix. Matrix Biol. 2000;19:569–580. doi: 10.1016/s0945-053x(00)00105-0. [DOI] [PubMed] [Google Scholar]
- Burgess RW, Skarnes WC, Sanes JR. Agrin isoforms with distinct amino termini: differential expression, localization, and function. J Cell Biol. 2000;151:41–52. doi: 10.1083/jcb.151.1.41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cambier S, Gline S, Mu D, Collins R, Araya J, Dolganov G, Einheber S, Boudreau N, Nishimura SL. Integrin alpha(v)beta8-mediated activation of transforming growth factor-beta by perivascular astrocytes: an angiogenic control switch. Am J Pathol. 2005;166:1883–1894. doi: 10.1016/s0002-9440(10)62497-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Candelario-Jalil E, Yang Y, Rosenberg GA. Diverse roles of matrix metalloproteinases and tissue inhibitors of metalloproteinases in neuroinflammation and cerebral ischemia. Neuroscience. 2009;158:983–994. doi: 10.1016/j.neuroscience.2008.06.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cardoso FL, Brites D, Brito MA. Looking at the blood-brain barrier: molecular anatomy and possible investigation approaches. Brain Res Rev. 2010;64:328–363. doi: 10.1016/j.brainresrev.2010.05.003. [DOI] [PubMed] [Google Scholar]
- Caruso RA, Fedele F, Finocchiaro G, Pizzi G, Nunnari M, Gitto G, Fabiano V, Parisi A, Venuti A. Ultrastructural descriptions of pericyte/endothelium peg-socket interdigitations in the microvasculature of human gastric carcinomas. Anticancer Res. 2009;29:449–453. [PubMed] [Google Scholar]
- Carvey PM, Hendey B, Monahan AJ. The blood-brain barrier in neurodegenerative disease: a rhetorical perspective. J Neurochem. 2009;111:291–314. doi: 10.1111/j.1471-4159.2009.06319.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chang DI, Hosomi N, Lucero J, Heo JH, Abumiya T, Mazar AP, del Zoppo GJ. Activation systems for latent matrix metalloproteinase-2 are upregulated immediately after focal cerebral ischemia. J Cereb Blood Flow Metab. 2003;23:1408–1419. doi: 10.1097/01.WCB.0000091765.61714.30. [DOI] [PubMed] [Google Scholar]
- Chen W, Ma Q, Suzuki H, Hartman R, Tang J, Zhang JH. Osteopontin reduced hypoxia-ischemia neonatal brain injury by suppression of apoptosis in a rat pup model. Stroke. 2011;42:764–769. doi: 10.1161/STROKEAHA.110.599118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clark AW, Krekoski CA, Bou SS, Chapman KR, Edwards DR. Increased gelatinase A (MMP-2) and gelatinase B (MMP-9) activities in human brain after focal ischemia. Neurosci Lett. 1997;238:53–56. doi: 10.1016/s0304-3940(97)00859-8. [DOI] [PubMed] [Google Scholar]
- Colgan OC, Collins NT, Ferguson G, Murphy RP, Birney YA, Cahill PA, Cummins PM. Influence of basolateral condition on the regulation of brain microvascular endothelial tight junction properties and barrier function. Brain Res. 2008;1193:84–92. doi: 10.1016/j.brainres.2007.11.072. [DOI] [PubMed] [Google Scholar]
- Cortes-Canteli M, Paul J, Norris EH, Bronstein R, Ahn HJ, Zamolodchikov D, Bhuvanendran S, Fenz KM, Strickland S. Fibrinogen and beta-amyloid association alters thrombosis and fibrinolysis: a possible contributing factor to Alzheimer's disease. Neuron. 2010;66:695–709. doi: 10.1016/j.neuron.2010.05.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Costell M, Gustafsson E, Aszodi A, Morgelin M, Bloch W, Hunziker E, Addicks K, Timpl R, Fassler R. Perlecan maintains the integrity of cartilage and some basement membranes. J Cell Biol. 1999;147:1109–1122. doi: 10.1083/jcb.147.5.1109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Daneman R, Zhou L, Kebede AA, Barres BA. Pericytes are required for blood-brain barrier integrity during embryogenesis. Nature. 2010;468:562–566. doi: 10.1038/nature09513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dejana E, Tournier-Lasserve E, Weinstein BM. The control of vascular integrity by endothelial cell junctions: molecular basis and pathological implications. Dev Cell. 2009;16:209–221. doi: 10.1016/j.devcel.2009.01.004. [DOI] [PubMed] [Google Scholar]
- Del Zoppo GJ, Milner R, Mabuchi T, Hung S, Wang X, Koziol JA. Vascular matrix adhesion and the blood-brain barrier. Biochem Soc Trans. 2006;34:1261–1266. doi: 10.1042/BST0341261. [DOI] [PubMed] [Google Scholar]
- Ding Y, Clark JC. Cerebrovascular injury in stroke. Neurol Res. 2006;28:3–10. doi: 10.1179/016164106X91799. [DOI] [PubMed] [Google Scholar]
- Dohgu S, Takata F, Yamauchi A, Nakagawa S, Egawa T, Naito M, Tsuruo T, Sawada Y, Niwa M, Kataoka Y. Brain pericytes contribute to the induction and up-regulation of blood-brain barrier functions through transforming growth factor-beta production. Brain Res. 2005;1038:208–215. doi: 10.1016/j.brainres.2005.01.027. [DOI] [PubMed] [Google Scholar]
- Dong L, Chen Y, Lewis M, Hsieh JC, Reing J, Chaillet JR, Howell CY, Melhem M, Inoue S, Kuszak JR, DeGeest K, Chung AE. Neurologic defects and selective disruption of basement membranes in mice lacking entactin-1/nidogen-1. Lab Invest. 2002;82:1617–1630. doi: 10.1097/01.lab.0000042240.52093.0f. [DOI] [PubMed] [Google Scholar]
- Dore-Duffy P, Owen C, Balabanov R, Murphy S, Beaumont T, Rafols JA. Pericyte migration from the vascular wall in response to traumatic brain injury. Microvasc Res. 2000;60:55–69. doi: 10.1006/mvre.2000.2244. [DOI] [PubMed] [Google Scholar]
- Dore-Duffy P, Katychev A, Wang X, Van Buren E. CNS microvascular pericytes exhibit multipotential stem cell activity. J Cereb Blood Flow Metab. 2006;26:613–624. doi: 10.1038/sj.jcbfm.9600272. [DOI] [PubMed] [Google Scholar]
- Ek CJ, Dziegielewska KM, Stolp H, Saunders NR. Functional effectiveness of the blood-brain barrier to small water-soluble molecules in developing and adult opossum (Monodelphis domestica) J Comp Neurol. 2006;496:13–26. doi: 10.1002/cne.20885. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ellis V, Behrendt N, Dano K. Plasminogen activation by receptor-bound urokinase. A kinetic study with both cell-associated and isolated receptor. J Biol Chem. 1991;266:12752–12758. [PubMed] [Google Scholar]
- Ellison JA, Velier JJ, Spera P, Jonak ZL, Wang X, Barone FC, Feuerstein GZ. Osteopontin and its integrin receptor alpha(v)beta3 are upregulated during formation of the glial scar after focal stroke. Stroke. 1998;29:1698–1706. doi: 10.1161/01.str.29.8.1698. discussion 170. [DOI] [PubMed] [Google Scholar]
- Ellison JA, Barone FC, Feuerstein GZ. Matrix remodeling after stroke. De novo expression of matrix proteins and integrin receptors. Ann N Y Acad Sci. 1999;890:204–222. doi: 10.1111/j.1749-6632.1999.tb07996.x. [DOI] [PubMed] [Google Scholar]
- Engelhardt B. Development of the blood-brain barrier. Cell Tissue Res. 2003;314:119–129. doi: 10.1007/s00441-003-0751-z. [DOI] [PubMed] [Google Scholar]
- Engelhardt B, Sorokin L. The blood-brain and the blood-cerebrospinal fluid barriers: function and dysfunction. Semin Immunopathol. 2009;31:497–511. doi: 10.1007/s00281-009-0177-0. [DOI] [PubMed] [Google Scholar]
- ffrench-Constant C, Colognato H. Integrins: versatile integrators of extracellular signals. Trends Cell Biol. 2004;14:678–686. doi: 10.1016/j.tcb.2004.10.005. [DOI] [PubMed] [Google Scholar]
- Fisher M. Pericyte signaling in the neurovascular unit. Stroke. 2009;40:S13–S15. doi: 10.1161/STROKEAHA.108.533117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Flick MJ, Du X, Witte DP, Jirouskova M, Soloviev DA, Busuttil SJ, Plow EF, Degen JL. Leukocyte engagement of fibrin(ogen) via the integrin receptor alphaMbeta2/Mac-1 is critical for host inflammatory response in vivo. J Clin Invest. 2004;113:1596–1606. doi: 10.1172/JCI20741. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Friedlander DR, Milev P, Karthikeyan L, Margolis RK, Margolis RU, Grumet M. The neuronal chondroitin sulfate proteoglycan neurocan binds to the neural cell adhesion molecules Ng-CAM/L1/NILE and N-CAM, and inhibits neuronal adhesion and neurite outgrowth. J Cell Biol. 1994;125:669–680. doi: 10.1083/jcb.125.3.669. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fukuda S, Fini CA, Mabuchi T, Koziol JA, Eggleston LL, Jr, del Zoppo GJ. Focal cerebral ischemia induces active proteases that degrade microvascular matrix. Stroke. 2004;35:998–1004. doi: 10.1161/01.STR.0000119383.76447.05. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gautam M, Noakes PG, Moscoso L, Rupp F, Scheller RH, Merlie JP, Sanes JR. Defective neuromuscular synaptogenesis in agrin-deficient mutant mice. Cell. 1996;85:525–535. doi: 10.1016/s0092-8674(00)81253-2. [DOI] [PubMed] [Google Scholar]
- George EL, Georges-Labouesse EN, Patel-King RS, Rayburn H, Hynes RO. Defects in mesoderm, neural tube and vascular development in mouse embryos lacking fibronectin. Development. 1993;119:1079–1091. doi: 10.1242/dev.119.4.1079. [DOI] [PubMed] [Google Scholar]
- Gesemann M, Brancaccio A, Schumacher B, Ruegg MA. Agrin is a high-affinity binding protein of dystroglycan in non-muscle tissue. J Biol Chem. 1998;273:600–605. doi: 10.1074/jbc.273.1.600. [DOI] [PubMed] [Google Scholar]
- Giancotti FG, Ruoslahti E. Integrin signaling. Science. 1999;285:1028–1032. doi: 10.1126/science.285.5430.1028. [DOI] [PubMed] [Google Scholar]
- Goldblum SE, Ding X, Funk SE, Sage EH. SPARC (secreted protein acidic and rich in cysteine) regulates endothelial cell shape and barrier function. Proc Natl Acad Sci U S A. 1994;91:3448–3452. doi: 10.1073/pnas.91.8.3448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gong Y, Kim SO, Felez J, Grella DK, Castellino FJ, Miles LA. Conversion of Glu-plasminogen to Lys-plasminogen is necessary for optimal stimulation of plasminogen activation on the endothelial cell surface. J Biol Chem. 2001;276:19078–19083. doi: 10.1074/jbc.M101387200. [DOI] [PubMed] [Google Scholar]
- Gorlatov S, Medved L. Interaction of fibrin(ogen) with the endothelial cell receptor VE-cadherin: mapping of the receptor-binding site in the NH2-terminal portions of the fibrin beta chains. Biochemistry. 2002;41:4107–4116. doi: 10.1021/bi0160314. [DOI] [PubMed] [Google Scholar]
- Gould DB, Phalan FC, van Mil SE, Sundberg JP, Vahedi K, Massin P, Bousser MG, Heutink P, Miner JH, Tournier-Lasserve E, John SW. Role of COL4A1 in small-vessel disease and hemorrhagic stroke. N Engl J Med. 2006;354:1489–1496. doi: 10.1056/NEJMoa053727. [DOI] [PubMed] [Google Scholar]
- Gravanis I, Tsirka SE. Tissue-type plasminogen activator as a therapeutic target in stroke. Expert Opin Ther Targets. 2008;12:159–170. doi: 10.1517/14728222.12.2.159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grimpe B, Probst JC, Hager G. Suppression of nidogen-1 translation by antisense targeting affects the adhesive properties of cultured astrocytes. Glia. 1999;28:138–149. doi: 10.1002/(sici)1098-1136(199911)28:2<138::aid-glia5>3.0.co;2-8. [DOI] [PubMed] [Google Scholar]
- Halfter W, Dong S, Yip YP, Willem M, Mayer U. A critical function of the pial basement membrane in cortical histogenesis. J Neurosci. 2002;22:6029–6040. doi: 10.1523/JNEUROSCI.22-14-06029.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hallmann R, Horn N, Selg M, Wendler O, Pausch F, Sorokin LM. Expression and function of laminins in the embryonic and mature vasculature. Physiol Rev. 2005;85:979–1000. doi: 10.1152/physrev.00014.2004. [DOI] [PubMed] [Google Scholar]
- Hamann GF, Okada Y, Fitridge R, del Zoppo GJ. Microvascular basal lamina antigens disappear during cerebral ischemia and reperfusion. Stroke. 1995;26:2120–2126. doi: 10.1161/01.str.26.11.2120. [DOI] [PubMed] [Google Scholar]
- Hamann GF, Liebetrau M, Martens H, Burggraf D, Kloss CU, Bultemeier G, Wunderlich N, Jager G, Pfefferkorn T. Microvascular basal lamina injury after experimental focal cerebral ischemia and reperfusion in the rat. J Cereb Blood Flow Metab. 2002;22:526–533. doi: 10.1097/00004647-200205000-00004. [DOI] [PubMed] [Google Scholar]
- Harhaj NS, Antonetti DA. Regulation of tight junctions and loss of barrier function in pathophysiology. Int J Biochem Cell Biol. 2004;36:1206–1237. doi: 10.1016/j.biocel.2003.08.007. [DOI] [PubMed] [Google Scholar]
- Haseloff RF, Blasig IE, Bauer HC, Bauer H. In search of the astrocytic factoRs) modulating blood-brain barrier functions in brain capillary endothelial cells in vitro. Cell Mol Neurobiol. 2005;25:25–39. doi: 10.1007/s10571-004-1375-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heye N, Cervos-Navarro J. Microthromboemboli in acute infarcts: analysis of 40 autopsy cases. Stroke. 1996;27:431–434. doi: 10.1161/01.str.27.3.431. [DOI] [PubMed] [Google Scholar]
- Hoylaerts M, Rijken DC, Lijnen HR, Collen D. Kinetics of the activation of plasminogen by human tissue plasminogen activator. Role of fibrin. J Biol Chem. 1982;257:2912–2919. [PubMed] [Google Scholar]
- Huber JD, Egleton RD, Davis TP. Molecular physiology and pathophysiology of tight junctions in the blood-brain barrier. Trends Neurosci. 2001;24:719–725. doi: 10.1016/s0166-2236(00)02004-x. [DOI] [PubMed] [Google Scholar]
- Hynes RO. Integrins: versatility, modulation, and signaling in cell adhesion. Cell. 1992;69:11–25. doi: 10.1016/0092-8674(92)90115-s. [DOI] [PubMed] [Google Scholar]
- Hynes RO. Integrins: bidirectional, allosteric signaling machines. Cell. 2002;110:673–687. doi: 10.1016/s0092-8674(02)00971-6. [DOI] [PubMed] [Google Scholar]
- Iozzo RV, Cohen IR, Grassel S, Murdoch AD. The biology of perlecan: the multifaceted heparan sulphate proteoglycan of basement membranes and pericellular matrices. Biochem J. 1994;302(Pt 3):625–639. doi: 10.1042/bj3020625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Israeli D, Tanne D, Daniels D, Last D, Shneor R, Guez D, Landau E, Roth Y, Ocherashvilli A, Bakon M, Hoffman C, Weinberg A, Volk T, Mardor Y. The application of MRI for depiction of subtle blood brain barrier disruption in stroke. Int J Biol Sci. 2010;7:1–8. doi: 10.7150/ijbs.7.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Janzer RC, Raff MC. Astrocytes induce blood-brain barrier properties in endothelial cells. Nature. 1987;325:253–257. doi: 10.1038/325253a0. [DOI] [PubMed] [Google Scholar]
- Johansson PA, Dziegielewska KM, Liddelow SA, Saunders NR. The blood-CSF barrier explained: when development is not immaturity. Bioessays. 2008;30:237–248. doi: 10.1002/bies.20718. [DOI] [PubMed] [Google Scholar]
- Kang SH, Kramer JM. Nidogen is nonessential and not required for normal type IV collagen localization in Caenorhabditis elegans. Mol Biol Cell. 2000;11:3911–3923. doi: 10.1091/mbc.11.11.3911. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kastrup A, Groschel K, Ringer TM, Redecker C, Cordesmeyer R, Witte OW, Terborg C. Early disruption of the blood-brain barrier after thrombolytic therapy predicts hemorrhage in patients with acute stroke. Stroke. 2008;39:2385–2387. doi: 10.1161/STROKEAHA.107.505420. [DOI] [PubMed] [Google Scholar]
- Kermode AG, Thompson AJ, Tofts P, MacManus DG, Kendall BE, Kingsley DP, Moseley IF, Rudge P, McDonald WI. Breakdown of the blood-brain barrier precedes symptoms and other MRI signs of new lesions in multiple sclerosis. Pathogenetic and clinical implications. Brain. 1990;113:1477–1489. doi: 10.1093/brain/113.5.1477. [DOI] [PubMed] [Google Scholar]
- Kniesel U, Wolburg H. Tight junctions of the blood-brain barrier. Cell Mol Neurobiol. 2000;20:57–76. doi: 10.1023/A:1006995910836. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Knoll R, Postel R, Wang J, Kratzner R, Hennecke G, Vacaru AM, Vakeel P, Schubert C, Murthy K, Rana BK, Kube D, Knoll G, Schafer K, Hayashi T, Holm T, Kimura A, Schork N, Toliat MR, Nurnberg P, Schultheiss HP, Schaper W, Schaper J, Bos E, Den Hertog J, van Eeden FJ, Peters PJ, Hasenfuss G, Chien KR, Bakkers J. Laminin-alpha4 and integrin-linked kinase mutations cause human cardiomyopathy via simultaneous defects in cardiomyocytes and endothelial cells. Circulation. 2007;116:515–525. doi: 10.1161/CIRCULATIONAHA.107.689984. [DOI] [PubMed] [Google Scholar]
- Knox SM, Whitelock JM. Perlecan: how does one molecule do so many things? Cell Mol Life Sci. 2006;63:2435–2445. doi: 10.1007/s00018-006-6162-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kondo T, Kinouchi H, Kawase M, Yoshimoto T. Astroglial cells inhibit the increasing permeability of brain endothelial cell monolayer following hypoxia/reoxygenation. Neurosci Lett. 1996;208:101–104. doi: 10.1016/0304-3940(96)12555-6. [DOI] [PubMed] [Google Scholar]
- Kose N, Asashima T, Muta M, Iizasa H, Sai Y, Terasaki T, Nakashima E. Altered expression of basement membrane-related molecules in rat brain pericyte, endothelial, and astrocyte cell lines after transforming growth factor-beta1 treatment. Drug Metab Pharmacokinet. 2007;22:255–266. doi: 10.2133/dmpk.22.255. [DOI] [PubMed] [Google Scholar]
- Koto T, Takubo K, Ishida S, Shinoda H, Inoue M, Tsubota K, Okada Y, Ikeda E. Hypoxia disrupts the barrier function of neural blood vessels through changes in the expression of claudin-5 in endothelial cells. Am J Pathol. 2007;170:1389–1397. doi: 10.2353/ajpath.2007.060693. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kwok J, Fawcett J. Dev Neurobiol. 2011 doi: 10.1002/dneu.20974. XXXSpecial-Issue: regeneration. in press. [DOI] [PubMed] [Google Scholar]
- Kwon I, Kim EH, del Zoppo GJ, Heo JH. Ultrastructural and temporal changes of the microvascular basement membrane and astrocyte interface following focal cerebral ischemia. J Neurosci Res. 2009;87:668–676. doi: 10.1002/jnr.21877. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lai CH, Kuo KH. The critical component to establish in vitro BBB model: Pericyte. Brain Res Brain Res Rev. 2005;50:258–265. doi: 10.1016/j.brainresrev.2005.07.004. [DOI] [PubMed] [Google Scholar]
- Lam CK, Yoo T, Hiner B, Liu Z, Grutzendler J. Embolus extravasation is an alternative mechanism for cerebral microvascular recanalization. Nature. 2010;465:478–482. doi: 10.1038/nature09001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Languino LR, Plescia J, Duperray A, Brian AA, Plow EF, Geltosky JE, Altieri DC. Fibrinogen mediates leukocyte adhesion to vascular endothelium through an ICAM-1-dependent pathway. Cell. 1993;73:1423–1434. doi: 10.1016/0092-8674(93)90367-y. [DOI] [PubMed] [Google Scholar]
- Languino LR, Duperray A, Joganic KJ, Fornaro M, Thornton GB, Altieri DC. Regulation of leukocyte-endothelium interaction and leukocyte transendothelial migration by intercellular adhesion molecule 1- fibrinogen recognition. Proc Natl Acad Sci U S A. 1995;92:1505–1509. doi: 10.1073/pnas.92.5.1505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lassmann H, Zimprich F, Vass K, Hickey WF. Microglial cells are a component of the perivascular glia limitans. J Neurosci Res. 1991;28:236–243. doi: 10.1002/jnr.490280211. [DOI] [PubMed] [Google Scholar]
- Ley K, Laudanna C, Cybulsky MI, Nourshargh S. Getting to the site of inflammation: the leukocyte adhesion cascade updated. Nat Rev Immunol. 2007;7:678–689. doi: 10.1038/nri2156. [DOI] [PubMed] [Google Scholar]
- Lijnen HR. Elements of the fibrinolytic system. Ann N Y Acad Sci. 2001;936:226–236. doi: 10.1111/j.1749-6632.2001.tb03511.x. [DOI] [PubMed] [Google Scholar]
- Liotta LA, Goldfarb RH, Brundage R, Siegal GP, Terranova V, Garbisa S. Effect of plasminogen activator (urokinase), plasmin, and thrombin on glycoprotein and collagenous components of basement membrane. Cancer Res. 1981;41:4629–4636. [PubMed] [Google Scholar]
- Lukes A, Mun-Bryce S, Lukes M, Rosenberg GA. Extracellular matrix degradation by metalloproteinases and central nervous system diseases. Mol Neurobiol. 1999;19:267–284. doi: 10.1007/BF02821717. [DOI] [PubMed] [Google Scholar]
- Mark KS, Davis TP. Cerebral microvascular changes in permeability and tight junctions induced by hypoxia-reoxygenation. Am J Physiol Heart Circ Physiol. 2002;282:H1485–H1494. doi: 10.1152/ajpheart.00645.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mathiisen TM, Lehre KP, Danbolt NC, Ottersen OP. The perivascular astroglial sheath provides a complete covering of the brain microvessels: an electron microscopic 3D reconstruction. Glia. 2010;58:1094–1103. doi: 10.1002/glia.20990. [DOI] [PubMed] [Google Scholar]
- McCarty JH, Monahan-Earley RA, Brown LF, Keller M, Gerhardt H, Rubin K, Shani M, Dvorak HF, Wolburg H, Bader BL, Dvorak AM, Hynes RO. Defective associations between blood vessels and brain parenchyma lead to cerebral hemorrhage in mice lacking alphav integrins. Mol Cell Biol. 2002;22:7667–7677. doi: 10.1128/MCB.22.21.7667-7677.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McCarty JH, Lacy-Hulbert A, Charest A, Bronson RT, Crowley D, Housman D, Savill J, Roes J, Hynes RO. Selective ablation of alphav integrins in the central nervous system leads to cerebral hemorrhage, seizures, axonal degeneration and premature death. Development. 2005;132:165–176. doi: 10.1242/dev.01551. [DOI] [PubMed] [Google Scholar]
- McCarty JH. Integrin-mediated regulation of neurovascular development, physiology and disease. Cell Adh Migr. 2009;3:211–215. doi: 10.4161/cam.3.2.7767. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McGeer PL, Zhu SG, Dedhar S. Immunostaining of human brain capillaries by antibodies to very late antigens. J Neuroimmunol. 1990;26:213–218. doi: 10.1016/0165-5728(90)90003-6. [DOI] [PubMed] [Google Scholar]
- Melgar MA, Rafols J, Gloss D, Diaz FG. Postischemic reperfusion: ultrastructural blood-brain barrier and hemodynamic correlative changes in an awake model of transient forebrain ischemia. Neurosurgery. 2005;56:571–581. doi: 10.1227/01.neu.0000154702.23664.3d. [DOI] [PubMed] [Google Scholar]
- Meller R, Stevens SL, Minami M, Cameron JA, King S, Rosenzweig H, Doyle K, Lessov NS, Simon RP, Stenzel-Poore MP. Neuroprotection by osteopontin in stroke. J Cereb Blood Flow Metab. 2005;25:217–225. doi: 10.1038/sj.jcbfm.9600022. [DOI] [PubMed] [Google Scholar]
- Mettouchi A, Klein S, Guo W, Lopez-Lago M, Lemichez E, Westwick JK, Giancotti FG. Integrin-specific activation of Rac controls progression through the G(1) phase of the cell cycle. Mol Cell. 2001;8:115–127. doi: 10.1016/s1097-2765(01)00285-4. [DOI] [PubMed] [Google Scholar]
- Mi H, Haeberle H, Barres BA. Induction of astrocyte differentiation by endothelial cells. J Neurosci. 2001;21:1538–1547. doi: 10.1523/JNEUROSCI.21-05-01538.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miles LA, Plow EF. Binding and activation of plasminogen on the platelet surface. J Biol Chem. 1985;260:4303–4311. [PubMed] [Google Scholar]
- Milner R, Huang X, Wu J, Nishimura S, Pytela R, Sheppard D, ffrench-Constant C. Distinct roles for astrocyte alphavbeta5 and alphavbeta8 integrins in adhesion and migration. J Cell Sci. 1999;12(Pt 23):4271–4279. doi: 10.1242/jcs.112.23.4271. [DOI] [PubMed] [Google Scholar]
- Milner R, Relvas JB, Fawcett J, ffrench-Constant C. Developmental Regulation of alphav Integrins Produces Functional Changes in Astrocyte Behavior. Mol Cell Neurosci. 2001;18:108–118. doi: 10.1006/mcne.2001.1003. [DOI] [PubMed] [Google Scholar]
- Milner R, Campbell IL. Developmental regulation of beta1 integrins during angiogenesis in the central nervous system. Mol Cell Neurosci. 2002;20:616–626. doi: 10.1006/mcne.2002.1151. [DOI] [PubMed] [Google Scholar]
- Milner R, Hung S, Wang X, Berg GI, Spatz M, del Zoppo GJ. Responses of endothelial cell and astrocyte matrix-integrin receptors to ischemia mimic those observed in the neurovascular unit. Stroke. 2008a;39:191–197. doi: 10.1161/STROKEAHA.107.486134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Milner R, Hung S, Wang X, Spatz M, del Zoppo GJ. The rapid decrease in astrocyte-associated dystroglycan expression by focal cerebral ischemia is protease-dependent. J Cereb Blood Flow Metab. 2008b;28:812–823. doi: 10.1038/sj.jcbfm.9600585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miner JH, Cunningham J, Sanes JR. Roles for laminin in embryogenesis: exencephaly, syndactyly, and placentopathy in mice lacking the laminin alpha5 chain. J Cell Biol. 1998;143:1713–1723. doi: 10.1083/jcb.143.6.1713. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miner JH, Li C, Mudd JL, Go G, Sutherland AE. Compositional and structural requirements for laminin and basement membranes during mouse embryo implantation and gastrulation. Development. 2004;131:2247–2256. doi: 10.1242/dev.01112. [DOI] [PubMed] [Google Scholar]
- Miranti CK, Brugge JS. Sensing the environment: a historical perspective on integrin signal transduction. Nat Cell Biol. 2002;4:E83–E90. doi: 10.1038/ncb0402-e83. [DOI] [PubMed] [Google Scholar]
- Miyagoe Y, Hanaoka K, Nonaka I, Hayasaka M, Nabeshima Y, Arahata K, Takeda S. Laminin alpha2 chain-null mutant mice by targeted disruption of the Lama2 gene: a new model of merosin (laminin 2)-deficient congenital muscular dystrophy. FEBS Lett. 1997;415:33–39. doi: 10.1016/s0014-5793(97)01007-7. [DOI] [PubMed] [Google Scholar]
- Miyamoto S, Teramoto H, Gutkind JS, Yamada KM. Integrins can collaborate with growth factors for phosphorylation of receptor tyrosine kinases and MAP kinase activation: roles of integrin aggregation and occupancy of receptors. J Cell Biol. 1996;135:1633–1642. doi: 10.1083/jcb.135.6.1633. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moore CJ, Winder SJ. Dystroglycan versatility in cell adhesion: a tale of multiple motifs. Cell Commun Signal. 2010;8:3. doi: 10.1186/1478-811X-8-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morancho A, Rosell A, Garcia-Bonilla L, Montaner J. Metalloproteinase and stroke infarct size: role for anti-inflammatory treatment? Ann N Y Acad Sci. 2010;1207:123–133. doi: 10.1111/j.1749-6632.2010.05734.x. [DOI] [PubMed] [Google Scholar]
- Moro L, Venturino M, Bozzo C, Silengo L, Altruda F, Beguinot L, Tarone G, Defilippi P. Integrins induce activation of EGF receptor: role in MAP kinase induction and adhesion-dependent cell survival. Embo J. 1998;17:6622–6632. doi: 10.1093/emboj/17.22.6622. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mosesson MW. Fibrinogen and fibrin structure and functions. J Thromb Haemost. 2005;3:1894–1904. doi: 10.1111/j.1538-7836.2005.01365.x. [DOI] [PubMed] [Google Scholar]
- Motamed K, Sage EH. SPARC inhibits endothelial cell adhesion but not proliferation through a tyrosine phosphorylation-dependent pathway. J Cell Biochem. 1998;70:543–552. doi: 10.1002/(sici)1097-4644(19980915)70:4<543::aid-jcb10>3.0.co;2-i. [DOI] [PubMed] [Google Scholar]
- Mu D, Cambier S, Fjellbirkeland L, Baron JL, Munger JS, Kawakatsu H, Sheppard D, Broaddus VC, Nishimura SL. The integrin alpha(v)beta8 mediates epithelial homeostasis through MT1-MMP-dependent activation of TGF-beta1. J Cell Biol. 2002;157:493–507. doi: 10.1083/jcb.200109100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mu Z, Yang Z, Yu D, Zhao Z, Munger JS. TGFbeta1 and TGFbeta3 are partially redundant effectors in brain vascular morphogenesis. Mech Dev. 2008;125:508–516. doi: 10.1016/j.mod.2008.01.003. [DOI] [PubMed] [Google Scholar]
- Munger JS, Huang X, Kawakatsu H, Griffiths MJ, Dalton SL, Wu J, Pittet JF, Kaminski N, Garat C, Matthay MA, Rifkin DB, Sheppard D. The integrin alpha v beta 6 binds and activates latent TGF beta 1: a mechanism for regulating pulmonary inflammation and fibrosis. Cell. 1999;96:319–328. doi: 10.1016/s0092-8674(00)80545-0. [DOI] [PubMed] [Google Scholar]
- Murray V, Norrving B, Sandercock PA, Terent A, Wardlaw JM, Wester P. The molecular basis of thrombolysis and its clinical application in stroke. J Intern Med. 2010;267:191–208. doi: 10.1111/j.1365-2796.2009.02205.x. [DOI] [PubMed] [Google Scholar]
- Murshed M, Smyth N, Miosge N, Karolat J, Krieg T, Paulsson M, Nischt R. The absence of nidogen 1 does not affect murine basement membrane formation. Mol Cell Biol. 2000;20:7007–7012. doi: 10.1128/mcb.20.18.7007-7012.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakagawa S, Deli MA, Nakao S, Honda M, Hayashi K, Nakaoke R, Kataoka Y, Niwa M. Pericytes from brain microvessels strengthen the barrier integrity in primary cultures of rat brain endothelial cells. Cell Mol Neurobiol. 2007;27:687–694. doi: 10.1007/s10571-007-9195-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Neuwelt EA, Bauer B, Fahlke C, Fricker G, Iadecola C, Janigro D, Leybaert L, Molnar Z, O'Donnell ME, Povlishock JT, Saunders NR, Sharp F, Stanimirovic D, Watts RJ, Drewes LR. Engaging neuroscience to advance translational research in brain barrier biology. Nat Rev Neurosci. 2011;12:169–182. doi: 10.1038/nrn2995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nguyen JV, Soto I, Kim KY, Bushong EA, Oglesby E, Valiente-Soriano FJ, Yang Z, Davis CH, Bedont JL, Son JL, Wei JO, Buchman VL, Zack DJ, Vidal-Sanz M, Ellisman MH, Marsh-Armstrong N. Myelination transition zone astrocytes are constitutively phagocytic and have synuclein dependent reactivity in glaucoma. Proc Natl Acad Sci U S A. 2011;108:1176–1181. doi: 10.1073/pnas.1013965108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nimmerjahn A, Kirchhoff F, Helmchen F. Resting microglial cells are highly dynamic surveillants of brain parenchyma in vivo. Science. 2005;308:1314–1318. doi: 10.1126/science.1110647. [DOI] [PubMed] [Google Scholar]
- Ninomia T, Wang L, Kumar SR, Kim A, Zlokovic BV. Brain injury and cerebrovascular fibrin deposition correlate with reduced antithrombotic brain capillary functions in a hypertensive stroke model. J Cereb Blood Flow Metab. 2000;20:998–1009. doi: 10.1097/00004647-200006000-00012. [DOI] [PubMed] [Google Scholar]
- Nishimura SL. Integrin-mediated transforming growth factor-beta activation, a potential therapeutic target in fibrogenic disorders. Am J Pathol. 2009;175:1362–1370. doi: 10.2353/ajpath.2009.090393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nishioku T, Dohgu S, Takata F, Eto T, Ishikawa N, Kodama KB, Nakagawa S, Yamauchi A, Kataoka Y. Detachment of brain pericytes from the basal lamina is involved in disruption of the blood-brain barrier caused by lipopolysaccharide-induced sepsis in mice. Cell Mol Neurobiol. 2009;29:309–316. doi: 10.1007/s10571-008-9322-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Noell S, Fallier-Becker P, Deutsch U, Mack AF, Wolburg H. Agrin defines polarized distribution of orthogonal arrays of particles in astrocytes. Cell Tissue Res. 2009;337:185–195. doi: 10.1007/s00441-009-0812-z. [DOI] [PubMed] [Google Scholar]
- Okada Y, Copeland BR, Fitridge R, Koziol JA, del Zoppo GJ. Fibrin contributes to microvascular obstructions and parenchymal changes during early focal cerebral ischemia and reperfusion. Stroke. 1994;25:1847–1853. doi: 10.1161/01.str.25.9.1847. discussion 1853-1844. [DOI] [PubMed] [Google Scholar]
- Okada Y, Copeland BR, Hamann GF, Koziol JA, Cheresh DA, del Zoppo GJ. Integrin alphavbeta3 is expressed in selected microvessels after focal cerebral ischemia. Am J Pathol. 1996;149:37–44. [PMC free article] [PubMed] [Google Scholar]
- Owens T, Bechmann I, Engelhardt B. Perivascular spaces and the two steps to neuroinflammation. J Neuropathol Exp Neurol. 2008;67:1113–1121. doi: 10.1097/NEN.0b013e31818f9ca8. [DOI] [PubMed] [Google Scholar]
- Paul J, Strickland S, Melchor JP. Fibrin deposition accelerates neurovascular damage and neuroinflammation in mouse models of Alzheimer's disease. J Exp Med. 2007;204:1999–2008. doi: 10.1084/jem.20070304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paulus W, Baur I, Schuppan D, Roggendorf W. Characterization of integrin receptors in normal and neoplastic human brain. Am J Pathol. 1993;143:154–163. [PMC free article] [PubMed] [Google Scholar]
- Peppiatt CM, Howarth C, Mobbs P, Attwell D. Bidirectional control of CNS capillary diameter by pericytes. Nature. 2006;443:700–704. doi: 10.1038/nature05193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Petzelbauer P, Zacharowski PA, Miyazaki Y, Friedl P, Wickenhauser G, Castellino FJ, Groger M, Wolff K, Zacharowski K. The fibrin-derived peptide Bbeta15-42 protects the myocardium against ischemia-reperfusion injury. Nat Med. 2005;11:298–304. doi: 10.1038/nm1198. [DOI] [PubMed] [Google Scholar]
- Pinkstaff JK, Detterich J, Lynch G, Gall C. Integrin subunit gene expression is regionally differentiated in adult brain. J Neurosci. 1999;19:1541–1556. doi: 10.1523/JNEUROSCI.19-05-01541.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Poschl E, Schlotzer-Schrehardt U, Brachvogel B, Saito K, Ninomiya Y, Mayer U. Collagen IV is essential for basement membrane stability but dispensable for initiation of its assembly during early development. Development. 2004;131:1619–1628. doi: 10.1242/dev.01037. [DOI] [PubMed] [Google Scholar]
- Proctor JM, Zang K, Wang D, Wang R, Reichardt LF. Vascular development of the brain requires beta8 integrin expression in the neuroepithelium. J Neurosci. 2005;25:9940–9948. doi: 10.1523/JNEUROSCI.3467-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Quaegebeur A, Segura I, Carmeliet P. Pericytes: blood-brain barrier safeguards against neurodegeneration? Neuron. 2010;68:321–323. doi: 10.1016/j.neuron.2010.10.024. [DOI] [PubMed] [Google Scholar]
- Ranby M. Studies on the kinetics of plasminogen activation by tissue plasminogen activator. Biochim Biophys Acta. 1982;704:461–469. doi: 10.1016/0167-4838(82)90068-1. [DOI] [PubMed] [Google Scholar]
- Rascher G, Fischmann A, Kroger S, Duffner F, Grote EH, Wolburg H. Extracellular matrix and the blood-brain barrier in glioblastoma multiforme: spatial segregation of tenascin and agrin. Acta Neuropathol. 2002;104:85–91. doi: 10.1007/s00401-002-0524-x. [DOI] [PubMed] [Google Scholar]
- Redzic Z. Molecular biology of the blood-brain and the blood-cerebrospinal fluid barriers: similarities and differences. Fluids Barriers CNS. 2011;8:3. doi: 10.1186/2045-8118-8-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Risau W, Lemmon V. Changes in the vascular extracellular matrix during embryonic vasculogenesis and angiogenesis. Dev Biol. 1988;125:441–450. doi: 10.1016/0012-1606(88)90225-4. [DOI] [PubMed] [Google Scholar]
- Rouach N, Giaume C. Connexins and gap junctional communication in astrocytes are targets for neuroglial interaction. Prog Brain Res. 2001;132:203–214. doi: 10.1016/S0079-6123(01)32077-0. [DOI] [PubMed] [Google Scholar]
- Ryu JK, Davalos D, Akassoglou K. Fibrinogen signal transduction in the nervous system. J Thromb Haemost 7 Suppl. 2009;1:151–154. doi: 10.1111/j.1538-7836.2009.03438.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sachs BD, Baillie GS, McCall JR, Passino MA, Schachtrup C, Wallace DA, Dunlop AJ, MacKenzie KF, Klussmann E, Lynch MJ, Sikorski SL, Nuriel T, Tsigelny I, Zhang J, Houslay MD, Chao MV, Akassoglou K. p75 neurotrophin receptor regulates tissue fibrosis through inhibition of plasminogen activation via a PDE4/cAMP/PKA pathway. J Cell Biol. 2007;177:1119–1132. doi: 10.1083/jcb.200701040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sahni A, Arevalo MT, Sahni SK, Simpson-Haidaris PJ. The VE-cadherin binding domain of fibrinogen induces endothelial barrier permeability and enhances transendothelial migration of malignant breast epithelial cells. Int J Cancer. 2009;125:577–584. doi: 10.1002/ijc.24340. [DOI] [PubMed] [Google Scholar]
- Sandoval KE, Witt KA. Blood-brain barrier tight junction permeability and ischemic stroke. Neurobiol Dis. 2008;32:200–219. doi: 10.1016/j.nbd.2008.08.005. [DOI] [PubMed] [Google Scholar]
- Saunders NR, Ek CJ, Habgood MD, Dziegielewska KM. Barriers in the brain: a renaissance? Trends Neurosci. 2008;31:279–286. doi: 10.1016/j.tins.2008.03.003. [DOI] [PubMed] [Google Scholar]
- Schachtrup C, Lu P, Jones LL, Lee JK, Lu J, Sachs BD, Zheng B, Akassoglou K. Fibrinogen inhibits neurite outgrowth via beta3 integrin-mediated phosphorylation of the EGF receptor. Proc Natl Acad Sci U S A. 2007;104:11814–11819. doi: 10.1073/pnas.0704045104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schachtrup C, Ryu JK, Helmrick MJ, Vagena E, Galanakis DK, Degen JL, Margolis RU, Akassoglou K. Fibrinogen triggers astrocyte scar formation by promoting the availability of active TGF-beta after vascular damage. J Neurosci. 2010;30:5843–5854. doi: 10.1523/JNEUROSCI.0137-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schneller M, Vuori K, Ruoslahti E. Alphavbeta3 integrin associates with activated insulin and PDGFbeta receptors and potentiates the biological activity of PDGF. Embo J. 1997;16:5600–5607. doi: 10.1093/emboj/16.18.5600. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scholler K, Trinkl A, Klopotowski M, Thal SC, Plesnila N, Trabold R, Hamann GF, Schmid-Elsaesser R, Zausinger S. Characterization of microvascular basal lamina damage and blood-brain barrier dysfunction following subarachnoid hemorrhage in rats. Brain Res. 2007;1142:237–246. doi: 10.1016/j.brainres.2007.01.034. [DOI] [PubMed] [Google Scholar]
- Schultz DR, Arnold PI. Properties of four acute phase proteins: C-reactive protein, serum amyloid A protein, alpha 1-acid glycoprotein, and fibrinogen. Semin Arthritis Rheum. 1990;20:129–147. doi: 10.1016/0049-0172(90)90055-k. [DOI] [PubMed] [Google Scholar]
- Schwartz MA. Integrins, oncogenes, and anchorage independence. J Cell Biol. 1997;139:575–578. doi: 10.1083/jcb.139.3.575. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schwartz MA, Ginsberg MH. Networks and crosstalk: integrin signalling spreads. Nat Cell Biol. 2002;4:E65–E68. doi: 10.1038/ncb0402-e65. [DOI] [PubMed] [Google Scholar]
- Shattil SJ, Hoxie JA, Cunningham M, Brass LF. Changes in the platelet membrane glycoprotein IIb.IIIa complex during platelet activation. J Biol Chem. 1985;260:11107–11114. [PubMed] [Google Scholar]
- Sheehan JJ, Tsirka SE. Fibrin-modifying serine proteases thrombin, tPA, and plasmin in ischemic stroke: a review. Glia. 2005;50:340–350. doi: 10.1002/glia.20150. [DOI] [PubMed] [Google Scholar]
- Shen W, Li S, Chung SH, Zhu L, Stayt J, Su T, Couraud PO, Romero IA, Weksler B, Gillies MC. Tyrosine phosphorylation of VE-cadherin and claudin-5 is associated with TGF-beta1-induced permeability of centrally derived vascular endothelium. Eur J Cell Biol. 2011;90:323–332. doi: 10.1016/j.ejcb.2010.10.013. [DOI] [PubMed] [Google Scholar]
- Siddharthan V, Kim YV, Liu S, Kim KS. Human astrocytes/astrocyte-conditioned medium and shear stress enhance the barrier properties of human brain microvascular endothelial cells. Brain Res. 2007;1147:39–50. doi: 10.1016/j.brainres.2007.02.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sidelmann JJ, Gram J, Jespersen J, Kluft C. Fibrin clot formation and lysis: basic mechanisms. Semin Thromb Hemost. 2000;26:605–618. doi: 10.1055/s-2000-13216. [DOI] [PubMed] [Google Scholar]
- Sixt M, Engelhardt B, Pausch F, Hallmann R, Wendler O, Sorokin LM. Endothelial cell laminin isoforms, laminins 8 and 10, play decisive roles in T cell recruitment across the blood-brain barrier in experimental autoimmune encephalomyelitis. J Cell Biol. 2001;153:933–946. doi: 10.1083/jcb.153.5.933. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith MA, Hilgenberg LG. Agrin in the CNS: a protein in search of a function? Neuroreport. 2002;13:1485–1495. doi: 10.1097/00001756-200208270-00001. [DOI] [PubMed] [Google Scholar]
- Smyth N, Vatansever HS, Murray P, Meyer M, Frie C, Paulsson M, Edgar D. Absence of basement membranes after targeting the LAMC1 gene results in embryonic lethality due to failure of endoderm differentiation. J Cell Biol. 1999;144:151–160. doi: 10.1083/jcb.144.1.151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sofroniew MV, Vinters HV. Astrocytes: biology and pathology. Acta Neuropathol. 2010;119:7–35. doi: 10.1007/s00401-009-0619-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sorokin L, Girg W, Gopfert T, Hallmann R, Deutzmann R. Expression of novel 400-kDa laminin chains by mouse and bovine endothelial cells. Eur J Biochem. 1994;223:603–610. doi: 10.1111/j.1432-1033.1994.tb19031.x. [DOI] [PubMed] [Google Scholar]
- Stewart PA, Wiley MJ. Developing nervous tissue induces formation of blood-brain barrier characteristics in invading endothelial cells: a study using quail--chick transplantation chimeras. Dev Biol. 1981;84:183–192. doi: 10.1016/0012-1606(81)90382-1. [DOI] [PubMed] [Google Scholar]
- Sundberg C, Rubin K. Stimulation of beta1 integrins on fibroblasts induces PDGF independent tyrosine phosphorylation of PDGF beta-receptors. J Cell Biol. 1996;132:741–752. doi: 10.1083/jcb.132.4.741. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tabrizi P, Wang L, Seeds N, McComb JG, Yamada S, Griffin JH, Carmeliet P, Weiss MH, Zlokovic BV. Tissue plasminogen activator (tPA) deficiency exacerbates cerebrovascular fibrin deposition and brain injury in a murine stroke model: studies in tPA-deficient mice and wild-type mice on a matched genetic background. Arterioscler Thromb Vasc Biol. 1999;19:2801–2806. doi: 10.1161/01.atv.19.11.2801. [DOI] [PubMed] [Google Scholar]
- Tagaya M, Haring HP, Stuiver I, Wagner S, Abumiya T, Lucero J, Lee P, Copeland BR, Seiffert D, del Zoppo GJ. Rapid loss of microvascular integrin expression during focal brain ischemia reflects neuron injury. J Cereb Blood Flow Metab. 2001;21:835–846. doi: 10.1097/00004647-200107000-00009. [DOI] [PubMed] [Google Scholar]
- Tao-Cheng JH, Nagy Z, Brightman MW. Tight junctions of brain endothelium in vitro are enhanced by astroglia. J Neurosci. 1987;7:3293–3299. doi: 10.1523/JNEUROSCI.07-10-03293.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tilling T, Korte D, Hoheisel D, Galla HJ. Basement membrane proteins influence brain capillary endothelial barrier function in vitro. J Neurochem. 1998;71:1151–1157. doi: 10.1046/j.1471-4159.1998.71031151.x. [DOI] [PubMed] [Google Scholar]
- Tilling T, Engelbertz C, Decker S, Korte D, Huwel S, Galla HJ. Expression and adhesive properties of basement membrane proteins in cerebral capillary endothelial cell cultures. Cell Tissue Res. 2002;310:19–29. doi: 10.1007/s00441-002-0604-1. [DOI] [PubMed] [Google Scholar]
- Ugarova TP, Lishko VK, Podolnikova NP, Okumura N, Merkulov SM, Yakubenko VP, Yee VC, Lord ST, Haas TA. Sequence gamma 377–395(P2), but not gamma 190–202(P1), is the binding site for the alpha MI-domain of integrin alpha M beta 2 in the gamma C-domain of fibrinogen. Biochemistry. 2003;42:9365–9373. doi: 10.1021/bi034057k. [DOI] [PubMed] [Google Scholar]
- van Horssen J, Bo L, Vos CM, Virtanen I, de Vries HE. Basement membrane proteins in multiple sclerosis-associated inflammatory cuffs: potential role in influx and transport of leukocytes. J Neuropathol Exp Neurol. 2005;64:722–729. doi: 10.1097/01.jnen.0000173894.09553.13. [DOI] [PubMed] [Google Scholar]
- Vincent AJ, Lau PW, Roskams AJ. SPARC is expressed by macroglia and microglia in the developing and mature nervous system. Dev Dyn. 2008;237:1449–1462. doi: 10.1002/dvdy.21495. [DOI] [PubMed] [Google Scholar]
- Wagner S, Tagaya M, Koziol JA, Quaranta V, del Zoppo GJ. Rapid disruption of an astrocyte interaction with the extracellular matrix mediated by integrin alpha 6 beta 4 during focal cerebral ischemia/reperfusion. Stroke. 1997;28:858–865. doi: 10.1161/01.str.28.4.858. [DOI] [PubMed] [Google Scholar]
- Wang J, Milner R. Fibronectin promotes brain capillary endothelial cell survival and proliferation through alpha5beta1 and alphavbeta3 integrins via MAP kinase signalling. J Neurochem. 2006;96:148–159. doi: 10.1111/j.1471-4159.2005.03521.x. [DOI] [PubMed] [Google Scholar]
- Wang JF, Zhang XF, Groopman JE. Stimulation of beta 1 integrin induces tyrosine phosphorylation of vascular endothelial growth factor receptor-3 and modulates cell migration. J Biol Chem. 2001;276:41950–41957. doi: 10.1074/jbc.M101370200. [DOI] [PubMed] [Google Scholar]
- Wang Q, Tang XN, Yenari MA. The inflammatory response in stroke. J Neuroimmunol. 2007;184:53–68. doi: 10.1016/j.jneuroim.2006.11.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang S, Voisin MB, Larbi KY, Dangerfield J, Scheiermann C, Tran M, Maxwell PH, Sorokin L, Nourshargh S. Venular basement membranes contain specific matrix protein low expression regions that act as exit points for emigrating neutrophils. J Exp Med. 2006a;203:1519–1532. doi: 10.1084/jem.20051210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang X, Louden C, Yue TL, Ellison JA, Barone FC, Solleveld HA, Feuerstein GZ. Delayed expression of osteopontin after focal stroke in the rat. J Neurosci. 1998a;18:2075–2083. doi: 10.1523/JNEUROSCI.18-06-02075.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang X, Lo EH. Triggers and mediators of hemorrhagic transformation in cerebral ischemia. Mol Neurobiol. 2003;28:229–244. doi: 10.1385/MN:28:3:229. [DOI] [PubMed] [Google Scholar]
- Wang Y, Jin G, Miao H, Li JY, Usami S, Chien S. Integrins regulate VE-cadherin and catenins: dependence of this regulation on Src, but not on Ras. Proc Natl Acad Sci U S A. 2006b;103:1774–1779. doi: 10.1073/pnas.0510774103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang YF, Tsirka SE, Strickland S, Stieg PE, Soriano SG, Lipton SA. Tissue plasminogen activator (tPA) increases neuronal damage after focal cerebral ischemia in wild-type and tPA-deficient mice. Nat Med. 1998b;4:228–231. doi: 10.1038/nm0298-228. [DOI] [PubMed] [Google Scholar]
- Wolburg H, Lippoldt A. Tight junctions of the blood-brain barrier: development, composition and regulation. Vascul Pharmacol. 2002;38:323–337. doi: 10.1016/s1537-1891(02)00200-8. [DOI] [PubMed] [Google Scholar]
- Wolburg H, Noell S, Wolburg-Buchholz K, Mack A, Fallier-Becker P. Agrin, aquaporin-4, and astrocyte polarity as an important feature of the blood-brain barrier. Neuroscientist. 2009;15:180–193. doi: 10.1177/1073858408329509. [DOI] [PubMed] [Google Scholar]
- Yamada KM, Even-Ram S. Integrin regulation of growth factor receptors. Nat Cell Biol. 2002;4:E75–E76. doi: 10.1038/ncb0402-e75. [DOI] [PubMed] [Google Scholar]
- Yamagata K, Tagami M, Nara Y, Mitani M, Kubota A, Fujino H, Numano F, Kato T, Yamori Y. Astrocyte-conditioned medium induces blood-brain barrier properties in endothelial cells. Clin Exp Pharmacol Physiol. 1997;24:710–713. doi: 10.1111/j.1440-1681.1997.tb02117.x. [DOI] [PubMed] [Google Scholar]
- Yamagata K, Tagami M, Takenaga F, Yamori Y, Itoh S. Hypoxia-induced changes in tight junction permeability of brain capillary endothelial cells are associated with IL-1beta and nitric oxide. Neurobiol Dis. 2004;17:491–499. doi: 10.1016/j.nbd.2004.08.001. [DOI] [PubMed] [Google Scholar]
- Yang Z, Mochalkin I, Doolittle RF. A model of fibrin formation based on crystal structures of fibrinogen and fibrin fragments complexed with synthetic peptides. Proc Natl Acad Sci U S A. 2000;97:14156–14161. doi: 10.1073/pnas.97.26.14156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yurchenco PD, Amenta PS, Patton BL. Basement membrane assembly, stability and activities observed through a developmental lens. Matrix Biol. 2004;22:521–538. doi: 10.1016/j.matbio.2003.10.006. [DOI] [PubMed] [Google Scholar]
- Yurchenco PD, Patton BL. Developmental and pathogenic mechanisms of basement membrane assembly. Curr Pharm Des. 2009;15:1277–1294. doi: 10.2174/138161209787846766. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zaccaria ML, Di Tommaso F, Brancaccio A, Paggi P, Petrucci TC. Dystroglycan distribution in adult mouse brain: a light and electron microscopy study. Neuroscience. 2001;104:311–324. doi: 10.1016/s0306-4522(01)00092-6. [DOI] [PubMed] [Google Scholar]
- Zhang Y, Barres BA. Astrocyte heterogeneity: an underappreciated topic in neurobiology. Curr Opin Neurobiol. 2010;20:588–594. doi: 10.1016/j.conb.2010.06.005. [DOI] [PubMed] [Google Scholar]
- Zhang ZG, Chopp M, Goussev A, Lu D, Morris D, Tsang W, Powers C, Ho KL. Cerebral microvascular obstruction by fibrin is associated with upregulation of PAI-1 acutely after onset of focal embolic ischemia in rats. J Neurosci. 1999;19:10898–10907. doi: 10.1523/JNEUROSCI.19-24-10898.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhu J, Motejlek K, Wang D, Zang K, Schmidt A, Reichardt LF. beta8 integrins are required for vascular morphogenesis in mouse embryos. Development. 2002;129:2891–2903. doi: 10.1242/dev.129.12.2891. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zlokovic BV. The blood-brain barrier in health and chronic neurodegenerative disorders. Neuron. 2008;57:178–201. doi: 10.1016/j.neuron.2008.01.003. [DOI] [PubMed] [Google Scholar]