Abstract
Background
Hospitalized heart failure patients have a high readmission rate. We sought to determine the independent risk due to central sleep apnea (CSA) of readmission in patients with systolic heart failure (SHF)
Methods and Results
Prospective observational cohort study of hospitalized patients with SHF. Patients underwent sleep studies during the hospitalization and were followed for 6 months to determine their rate of cardiac readmissions. 784 consecutive patients were included. 165 patients had CSA and 139 had no sleep disordered breathing (SDB). The remainder had obstructive sleep apnea (OSA). The rate ratio for 6 months cardiac readmissions was 1.53 (95% CI (1.1, 2.2), p=.03) in CSA patients compared to no SDB. This rate ratio is adjusted for systolic function, type of cardiomyopathy, age, weight, sex, diabetes, coronary disease, length of stay, admission sodium, creatinine, hemoglobin, blood pressure and discharge medications. Severe OSA was also an independent predictor of readmissions with an adjusted rate ratio of 1.49 (p=.04).
Conclusion
In this first evaluation of the impact of SDB on cardiac readmissions in heart failure, CSA was an independent risk factor for 6 month cardiac readmissions. The effect size of CSA exceeded that of all known predictors of heart failure readmissions.
Keywords: Sleep Disordered breathing, Central sleep apnea, Obstructive sleep apnea, heart failure, readmissions
INTRODUCTION
An increase in the incidence of heart failure in recent decades is attributed to improved survival of cardiovascular disease, increased prevalence of diabetes(1), and aging of the population(2). The clinical course of heart failure syndromes is characterized by recurrent hospitalizations(3) accounting for a significant portion of the human and most of the economic burden of heart failure(3). In addition, admissions for heart failure are the most common hospitalizations in Medicare patients(4). Attention has focused in recent years on evaluating predictors of heart failure readmissions as an indicator of quality of care and of patient morbidity(5, 6). Several predictors of readmission have been identified; many of which are non-modifiable demographic, physiological, or functional factors(7, 8). Most of the interventions that are likely to improve outcomes such as b-blockers, angiotensin converting enzyme inhibitors, close follow up, and multi-disciplinary teams are already part of the standard of care(9, 10). Identification of independent risk factors that are modifiable may provide clinicians with an effective intervention to decrease readmissions(11).
Sleep Disordered breathing (SDB) is highly prevalent in patients with heart failure(12, 13). In particular, central sleep apnea (CSA) is common (20-40%)(14) in patients with advanced systolic heart failure and has been associated with negative prognosis in these patients(15). New therapeutic modalities were introduced for CSA that are starting to demonstrate some benefit(16). The negative impact of CSA may be most pronounced during hospitalizations and in the period following discharge in heart failure patients.
From this background, we sought to evaluate the effect of CSA on cardiac readmission risk in hospitalized patients with heart failure. We reasoned that if CSA was independently associated with an increase in heart failure related readmissions, then in-hospital diagnosis of CSA could identify higher risk patients, and treatment of CSA might decrease cardiac readmissions.
METHODS
Participants
All patients who were hospitalized at the Ohio State University Medical Center (OSUMC) Heart Hospital with a diagnosis of decompensated heart failure between January 2007 and March 2010 were targeted for this study. Only patients with left ventricular ejection fraction (LVEF) less than or equal to 45% were included in this study. The sleep study orders are part of the admission order set for heart failure at the OSUMC Heart Hospital. Subsequently, they are activated in all patients who have heart failure as an admission diagnosis(12) on the first or second night of hospitalization. Sleep studies may not have been done if the patient declined or a shortage of devices precluded completion of the study during the hospitalization.
Sleep studies and group definitions
The sleep studies were cardiorespiratory devices (Stardust II, Respironics, Inc., Murrysville, PA) attended by trained night shift nurses who note the patient's sleep time and any interruptions to sleep. We considered the recordings interpretable if they included at least two hours of uninterrupted observed sleep. Segments of the study that included simultaneously more than one missing signal were subtracted from the recording time. An intact effort signal was required for any segment to be interpretable. SDB was defined as an Apnea Hypopnea Index (AHI) ≥15 events/hour. Respiratory event scoring and classification of OSA and CSA was according to standard clinical guidelines(17). An AHI cutoff of 15 events/hour was selected for the in-hospital study to mitigate against an expected increase in respiratory control instability and oxygen desaturation during the heart failure episode. Heart failure patients with AHI ≤ 15 events/hour served as our comparison group for the CSA group.
This inpatient testing method has been previously validated against polysomnography (PSG) (18) and is accepted for the diagnosis of obstructive sleep apnea (OSA)(19). We have validated the sensitivity for OSA of this method in hospitalized heart failure patients as well(12). We also evaluated the predictive value of this inpatient approach for CSA. From our cohort of patients who underwent inpatient sleep testing and lived within 100 miles from the hospital draw area (determined by phone area code), we invited patients who were classified as CSA on the inpatient testing in the first two years of testing (2007-2009) to return for outpatient polysomnography within 6-12 months of discharge. Of the 78 invited, 43 patients returned for their validation PSG. Only 2/43 had AHI<5 on the PSG. Both of these patients’ normalized AHI were explained by near complete normalization of LVEF at the time of the PSG. The remainder of the patients continued to be classified as CSA on the PSG.
Data collection and outcome measurements
Once our study patients were identified from our screening procedure, their cardiac readmissions over a 6-month period after discharge were tracked. The dates of cardiac readmissions were generated electronically for every patient using ICD codes with prefix 410-414 or 425-428. Two research coordinators, who were not directly aware of the patient's SDB status, confirmed the cardiac nature of every admission using the following criteria. All admissions had to be to a primary cardiology service in the OSU Heart Hospital. Elective admissions and admissions for procedures were excluded. Mortality data were obtained from the electronic medical records and from the state of Ohio's Vital Statistics website. If a cardiac admission to an outside facility was noted in our medical records, we recorded it if it complied with the criteria mentioned above. It was possible that some patients were admitted to other institutions within the 6 months follow up period without any notation in our records. However, most patients have established care in our Heart Hospital facilities and so they continued seeking care here after the initial hospitalization episode. Also, the 6 month follow up period was recent enough after the initial hospitalization that patients were being transferred back from outside facilities to our institution for continuity of care. To address the possibility of missing readmissions, we contacted a subgroup of 194 of the study cohort who were hospitalized and underwent sleep studies between March 2010 and September 2010 and found that less than 4% of their 6 month cardiac readmissions were not recorded in our system.
The study protocol was approved by the OSU Institutional Review Board [2007H0043] and is listed under clinical trials number [NCT00701038]. This study complies with the Declaration of Helsinki.
Statistical design and analysis
Our primary purpose was to compare post-discharge cardiac readmission numbers (rates) of screen identified CSA with patients who screened negative for SDB (no SDB). In multivariable modeling of the rates, we adjusted for a set of covariates that were found previously to distinguish severity or to predict negative outcomes in heart failure patients (7, 20, 21). We also added covariates that were significantly different at baseline between CSA and no SDB patients (Table 1). The covariates used in the model were LVEF, age, body mass index, sex, creatinine, diabetes, type of cardiomyopathy, coronary artery disease, discharge systolic blood pressure (<110 vs. ≥ 110), discharge angiotensin-converting enzyme inhibitors or angiotensin receptor blockers, discharge beta blocker, initial length of stay, admission sodium, and admission hemoglobin.
Table-1.
Comparison of baseline characteristics by Sleep Disordered Breathing group
| Mean (SD) or n (%) | |||
|---|---|---|---|
| Patient Characteristic | CSA (n=165) | OSA (n=480) | No SDB (n=139) |
| Age | 60.3 (15.0)* | 59.5 (13.4)† | 54.2 (15.9) |
| Sex (male) | 137 (83%)*† | 347 (72%) † | 70 (50%) |
| Cardiomyopathy | |||
| Ischemic | 106 (64%) | 312 (65%) | 75 (54%) |
| Dilated | 35 (21%)* | 121 (25%) | 47 (34%) |
| Others | 24 (15%) | 47 (10%) | 17 (12%) |
| LVEF | 22.2 (9.4) *‡ | 25.4 (10.7)† | 29.2 (10.3) |
| BMI kg/cm2 | 28.4 (6.2) ‡ | 31.2 (7.8) | 29.8 (10.4) |
| Length of Stay | 9.6 (10.7) | 8.8 (10.9) | 8.6 (9.7) |
| Creatinine (mg/dL) | 1.39 (0.75) | 1.38 (0.82) | 1.32 (0.74) |
| Coronary artery disease | 107 (65%) | 341 (71%) † | 79 (57%) |
| Diabetes | 57 (35%) ‡ | 227 (47%) † | 41 (30%) |
| Discharged on ACEI or ARB | 133 (81%) | 407 (85%) † | 106 (76%) |
| Discharged on beta-blockers | 150 (91%) | 436 (91%) † | 118 (85%) |
| Discharge SBP < 110 mmHg | 51 (31%) | 135 (28%) | 46 (33%) |
| Admission Na | 136.8 (4.2) | 136.5 (3.7) | 136.6 (3.5) |
| Admission Hemoglobin | 12.2 (1.8) | 12.1 (2.0) | 12.1 (2.0) |
P<0.05 for the comparison between CSA and the NO SDB group
P<0.05 for the comparison between OSA and the NO SDB group
P<0.05 for the comparison between CSA and OSA groups
BMI; body mass index; SDB: sleep disordered breathing; CSA: central sleep apnea; OSA: obstructive sleep apnea. LVEF: Left ventricular ejection fraction; ARB: angiotensin receptor blocker; ACEI: angiotensin-converting enzyme inhibitor, SBP: systolic blood pressure.
The rationale in adjusting for these was to estimate CSA's independent contribution to the prediction of cardiac readmissions. The number of cardiac readmissions in a six-month period for each patient was the dependent variable. The numbers of cardiac readmissions were modeled using the Negative Binomial distribution, and so groups were compared on their rates of readmission (22, 23) (Proc Genmod, SAS 9.2, SAS Inc, Cary NC, 2009). The Negative Binomial distribution for the readmission counts can be justified based on the assumption that given a patient's particular rate of readmission, a Poisson distribution could adequately represent that person's number of readmissions, and if the distribution of rates across patients followed a Gamma distribution, the observed number of readmissions across all patients should follow a Negative Binomial(22, 23). For those who died before 6 months or were lost to follow up before 6 months post discharge, rates were based on their specific follow-up time. We also compared cardiac readmission rates of OSA patients to no SDB patients to identify commonalities and differences from the CSA
Since BNP is used as an indicator of decompensation, it was important to address its possible role as a covariate. There was substantial missing data for BNP (admission BNP was not available for 58 CSA patients and 80 patients without SDB). The missing admission BNP was attributed to practice variation and was not correlated to underlying severity of heart failure. There was no difference in LVEF, age, type of cardiomyopathy or length of stay among patients with recorded BNP and those without. Therefore, our first multivariable model excluded this covariate. A second model included BNP by using a multiple imputation technique to adjust for missing data bias(24) (Proc MI/MIANALYZE, SAS 9.2, SAS Inc, Cary NC,2009). We applied the same approach to the 91 patients without 6 months follow up. The missing follow up was equally distributed among the three groups (negative, CSA, and OSA). There were no significant differences in baseline characteristics between those with complete follow up and those without.
RESULTS
Participants’ Characteristics
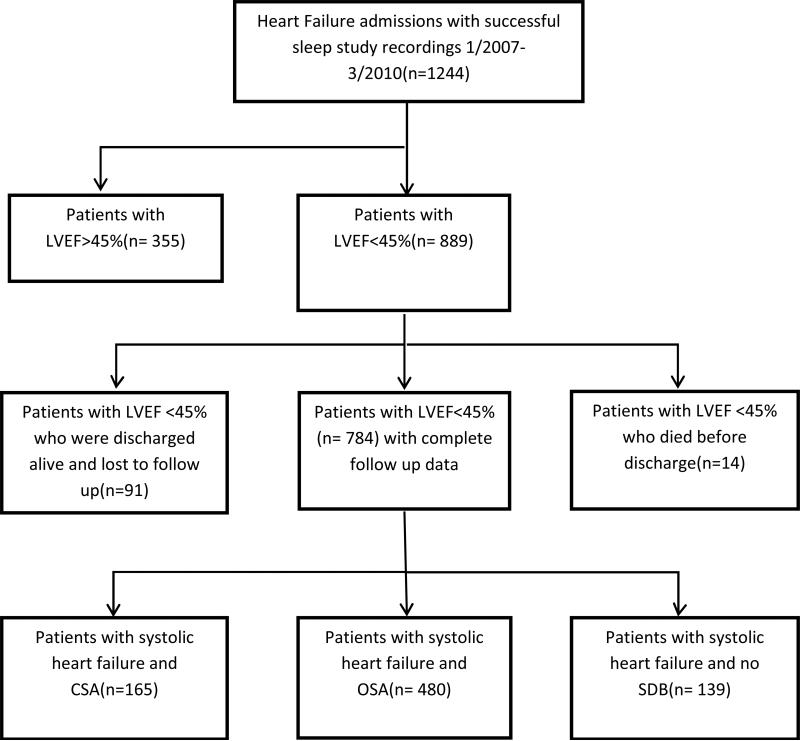
Successful sleep studies were performed on 1244 consecutive patients with systolic heart failure between January 2007 and March 2010. Of those, 889 patients had an LVEF≤45%. Fourteen patients died before discharge and were excluded from analysis (11 with OSA, 2 without SDB, and 1 with CSA). Another 91 patients had no recorded follow up or mortality data and could not be reached by phone, and in a sensitivity analysis we imputed the readmissions for them as addressed above. These patients were 18 CSA patients (11%), 21 patients with no sleep disordered breathing (13%); and 52 OSA patients (10%). Figure 1 describes the disposition of all patients in the study. The remaining 784 patients were included in the following primary analysis. Table 1 provides a comparison of the characteristics of the three groups. Note that we adjust for differences among the groups in our multivariable models. The prevalence and type of SDB in this population was similar to our previous findings(12) and other reports in similar populations(13).
Figure-1. Selection and disposition of all patients.
LVEF: Left ventricular ejection fraction; SDB: Sleep Disordered breathing; CSA: central sleep apnea; OSA: obstructive sleep apnea
Fourteen patients died before discharge and were excluded from analysis (11 with OSA, 1 without SDB, and 1 with CSA). Another 91 patients had no recorded follow up or mortality data and could not be contacted, so missing data strategy was applied as described in the methods section (18 CSA patients, 21 patients with no SDB, and 57 OSA patients).
Effect of CSA on cardiac readmissions
CSA was a predictor of 6 month cardiac readmission (univariable rate ratio: 1.63, p=0.01), and proved to be an independent predictor after adjustment for measured covariates as detailed in Table 2 (adjusted rate ratio 1.53, p=.03). In other words, adjusting for all these covariates resulted in a small decrease in the rate ratio, despite the possibility that we overcorrected for covariates that might be intervening variables. We also addressed the rate ratio for more immediate cardiac readmissions (within 1 month), and found that it was similar to the 6-month (univariable rate ratio 2.0 (95%CI 1.1, 3.5), and after covariate adjustment was 1.5 (95%CI 0.9, 2.8). This size of the CSA rate ratio was comparable to an LVEF rate ratio. (When we dichotomized LVEF at its median value (LVEF=25%), the lower LVEF group rate ratio was 1.4). Using the model with multiple imputations for those without BNP on admission, the rate ratio for CSA was 1.49. With multiple imputations for those with missing follow up data (91 patients) the rate ratio for CSA was 1.43. When we used multiple imputations for both BNP and missing follow up the rate ratio remained at 1.43.
Table-2.
Effect of CSA on 6 month Cardiac readmissions
| Model | Rate Ratio (confidence interval), p value |
|---|---|
| Univariable model | 1.63 (1.1, 2.4), p=. 01 |
| Multivariable model (adjusted for listed covariates*) | 1.53 (1.1, 2.2), p=. 03 |
In addition to SDB group, the model included the following variables:
Left ventricular ejection fraction, age, body mass index, sex, creatinine, diabetes, type of cardiomyopathy, coronary artery disease, discharge SBP (<110 vs. ≥ 110), discharge angiotensin-converting enzyme inhibitors or angiotensin receptor blockers, discharge beta blocker, initial length of stay, admission sodium, and admission hemoglobin.
To determine the sensitivity of our results to the use of the 50% cutoff for central apneas in the classification of CSA, we lowered the ratio to 45% (central apneas ≥45% of total apneas). With the lower cutoff, we included 20 more patients for a total of 185 patients. The adjusted rate ratio for this group was still at RR = 1.48.
We did not hypothesize an effect of CSA on mortality during this short 6- month follow up. In our exploratory analysis, we found no significant difference in 6-month mortality between CSA and no SDB groups. The mortality odds ratio (comparing CSA to no SDB) was 1.1 (95%CI 0.6, 2.0), (p=0.7). Six-month mortality was 16% in CSA, and 15% in those with no SDB.
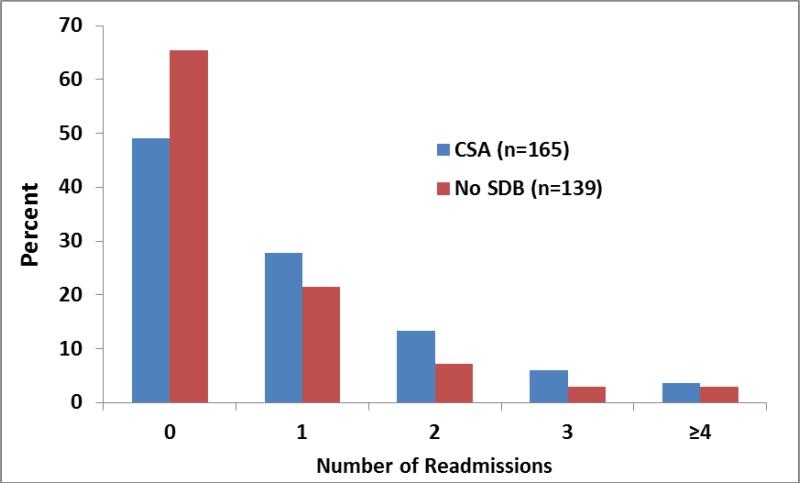
Among the covariates used in the multivariable modeling, the predictors, other than CSA, that achieved p<0.05 significance for 6 month cardiac readmissions after adjustment for all other covariates in the model (Figure 3), were lower LVEF (p=.01), younger age (p=.003), longer initial length of stay (p=.02), and lower admission hemoglobin (p<.0001). Figure 2 shows the distribution of cardiac readmission counts for the CSA and no SDB groups. Note that the Negative Binomial distribution fits the counts almost perfectly. For the CSA patients each observed count was within 1% of the counts fitted by the Negative Binomial, and similarly for the no SDB group, they were within 2.5%.
Figure-2. Comparison of Distribution of Cardiac readmissions in 6 months between patients with CSA and patients with no SDB.
Distribution of cardiac readmission counts within 6 months; SDB: Sleep Disordered breathing; CSA: central sleep apnea; Note the higher percent of patients readmitted for each count in the CSA group.
Exploration of the effect of OSA on cardiac readmissions
In the exploratory analysis for OSA, the 6 month cardiac readmission rate (compared to no SDB) was higher, but not significantly (cardiac readmissions rate ratio: 1.32 p=.10). However, there was a stronger effect on cardiac readmission in patients with more severe OSA. For those with AHI≥30 events/ hour the rate ratio was 1.49 (95% CI, 1.0, 2.2), p=.04, but for those with AHI<30 the rate ratio was 1.22 (95% CI, 0.9, 1.8), p=.28. Half of the OSA patients had an AHI< 30. In contrast, only 17% of CSA patients had AHI< 30.
Because the effect of OSA on readmissions depends on its severity, we sought to further explore the effect of OSA in patients with more severe systolic dysfunction. We found that in OSA patients with AHI≥30 and EF < 25 % the cardiac readmission rate ratio was 1.8 (95%CI 1.0, 3.2), while for those with AHI≥30 but EF≥25, the rate ratio was 1.1 (95% CI 0.7, 1.7).
DISCUSSION
This is the first evaluation of the impact of CSA on post discharge cardiac readmission risk in patients with heart failure. In this prospective study of previously unscreened hospitalized systolic heart failure patients, CSA identified during the hospitalization was an important independent predictor of 6-month cardiac readmission with a rate 43-50% higher than the no SDB comparison group. We also found that when AHI was >30 events/hour, OSA was an independent predictor of 6-month cardiac readmissions. Adjustment to all known predictors of cardiac readmissions made only a minor (less than 10%) decrease in these effect sizes.
The occurrence of CSA during hospitalizations with heart failure episodes may be promoted by the increased cardiac filling pressure(25), fluid shift(26) and impaired cerebral vasomotion(27). These abnormalities may be ameliorated after resolution of the decompensation episode, decreasing the severity or the persistence of CSA itself. However, CSA remains a prevalent respiratory disorder in stable and optimally treated outpatients with heart failure(14, 28). It is likely that a significant degree of CSA would persist in our patients into the stable outpatient setting and can be treated with newer pressure delivery devices that target the respiratory control instability (16, 29). Our validation procedure confirmed that CSA was indeed still present in all the patients whose cardiac dysfunction persisted through their outpatient polysomnography. The prevalence and distribution of SDB in hospitalized heart failure have been reported previously in studies that were designed specifically for that (13, 30). It must be noted, however, that discrepancies in prevalence and distribution of SDB in this population have already been reported. Recent reports suggest that increased filling pressures as is the case during decompensation may actually increase upper airway collapsibility and give rise to OSA (26). Simultaneously, the interstitial edema (25) can give rise to central apnea in these patients. The increased weight of the population may simply account for a significant increase in reported OSA cases in patients with heart failure. Finally, we caution that scoring criteria for OSA and CSA vary among centers particularly regarding use of hypopneas in the classification(17).
CSA is likely to exert has multiple negative neurohumoral and functional effects in heart failure patients. CSA is associated with episodic hypoxia which increases the sympathetic tone(31, 32) and may worsen the vascular endothelial function and increase the cardiac afterload(33). Patients with systolic heart failure and CSA have increased sympathetic activity compared to those with heart failure and no CSA (34, 35). Increased sympathetic tone in CSA patients results in increased ventricular irritability and arrhythmia (36), which also responds to treatment of CSA (37). Another important effect of CSA on heart failure patients appears to be decreased functional capacity. Patients with heart failure and CSA have worse exercise capacity than those without CSA(38), which improves with treatment of CSA(39, 40).
There are two implications for our findings; the first is that CSA is an independent predictor of cardiac readmission that can be used to identify patients at risk for negative outcomes following the heart failure episode. The second implication is that CSA, a potentially treatable disorder, may be targeted to positively affect the post-discharge outcome in heart failure episodes. These two implications are applicable to OSA as well. OSA is more likely to be an independent disorder and has an effective treatment. We have previously found that in-hospital treatment of OSA patients can improve discharge LVEF in a similar group of patients with systolic heart failure(41). It must be noted that to date there are no adequately powered randomized controlled trials demonstrating positive effect for the treatment of CSA in heart failure patients. CPAP is not beneficial in these patients(35), and the recently introduced adaptive servo ventilators are just starting to show promise in small studies(40, 42). The recent findings of vast under-diagnosis of SDB in heart failure patients(43), along with our findings may support a systematic approach to the diagnosis of SDB during hospitalizations with decompensated heart failure. Javaheri et al demonstrated an association between CSA and increased mortality in patients with systolic heart failure(15). The 6 month mortality rate in the overall group approached that in a recent report on a similar population of hospitalized patients(44). We did not expect an effect for CSA on mortality to be apparent in the 6 month follow up period in these patients with severe chronic disease and other significant comorbidities.
Only 4% of the CSA patients were put on treatment for a few weeks at the end of the 6 months follow up period, and we included them in the analysis. We reasoned that we would only introduce a slight conservative bias toward the null hypothesis by including them. The same applied to the OSA patients, in whom a larger percentage (13.6%) were treated for OSA and less were verifiably adherent to their device therapy. We did not use the polysomnography due to cost and inconvenience in the hospitalized setting. We had the intention to use a practical and broadly generalizable method for the diagnosis in this high risk population. The finding of an important clinical outcome such as increased readmissions in patients identified as having CSA on this inpatient test provides additional clinical validation for this diagnostic method.
It is critical to view CSA in this study as a manifestation of respiratory control instability during heart failure hospitalization that predicts a significant increase in morbidity, specifically, increased 6 month cardiac readmission rate. The effect of treatment of CSA after discharge on cardiac readmissions should be addressed in subsequent work. The main contribution of this study is in identifying a novel and potentially modifiable predictor of heart failure readmissions. To date, there are no studies evaluating the effect of CSA on cardiac readmissions. The design included consecutive hospitalized patients without prior screening, providing clarity in the definition of the target population. The prospective design of this study is also a strength that contributes to the validity of its findings.
Table-3.
Rate ratios for readmission for all other covariates in the multivariable model
| Readmission risk factor | Rate Ratio1 (95% confidence interval) | p-value |
|---|---|---|
| LVEF | 0.73 (0.6, 0.9) | 0.01 |
| Age | 0.71 (0.6, 0.9) | 0.01 |
| Initial length of stay | 1.28 (1.1, 1.6) | 0.01 |
| Admission hemoglobin | 0.58 (0.5, 0.7) | <0.001 |
| SBP | 1.0 (0.9, 1.2) | 0.59 |
| Creatinine | 1.2 (0.8, 1.9) | 0.29 |
| BMI | 0.8 (0.6, 1.1) | 0.12 |
| Gender (male vs female) | 1.16 (0.9, 1.5) | 0.26 |
| CAD | 0.98 (0.7, 1.4) | 0.90 |
| Cardiomyopathy (ischemic vs. nonischemic) | 1.14 (0.8, 1.6) | 0.48 |
| Cardiomyopathy (other vs. nonischemic) | 1.18 (0.8, 1.7) | 0.42 |
| Diabetes | 1.1 (0.8, 1.3) | 0.68 |
| Discharge beta-blocker | 1.3 (0.9, 2.0) | 0.23 |
| Discharge ARB or ACEI | 0.8 (0.6, 1.1) | 0.19 |
| Sodium | 1.1 (0.9, 1.4) | 0.39 |
Rate ratios are for a 2 standard deviation increase for these continuously measured risk factors (2 SD= LVEF 21%, age 29 years, Initial LOS 22 days, Admission Hemoglobin 3.8 g/dL, High SBP 21.9 mm Hg, Creatinine 2.7 mg/dL, BMI 16 kg/cm2, Sodium 7.8 mg/dl), the remaining covariates were dichotomized or trichotomized (cardiomyopathy).
LVEF: Left ventricular ejection fraction; SBP: systolic blood pressure; BMI: Body mass index; CAD: Coronary Artery Disease; ARB: Angiotensin receptor blocker; ACEI: Angiotensin converting enzyme inhibitor.
Acknowledgments
FUNDING SOURCES
This research was supported by a grant from National Institute of Health [R21 HL092480] RNK, DJ, WTA, KP, and VB. The research was also supported by the National Center for Research Resources [ULRR025755] RNK DJ KP. The content is the sole responsibility of the authors and does not represent the official views of NCRR or NIH.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
DISCLOSURES
CONFLICT OF INTEREST
None of the authors have any relevant conflicts of interest.
AUTHOR CONTRIBUTION
All authors contributed to the manuscript in accordance with the ICMJE authorship guidelines. RK, DJ, WA designed the research and analysis; RK drafted the manuscript and DJ reviewed, revised critically, and approved. WA reviewed, revised and approved. BP/JW collected the data and scored the sleep studies (data interpretation) and contributed to writing the methods section and reviewing and revising the manuscript. VB contributed to the design of the research, the interpretation of data, and to reviewing and approving the manuscript. RK had full access to the data at all stages and guarantees its integrity.
REFERENCES
- 1.From AM, Leibson CL, Bursi F, Redfield MM, Weston SA, Jacobsen SJ, Rodeheffer RJ, Roger VL. Diabetes in heart failure: prevalence and impact on outcome in the population. Am J Med. 2006 Jul;119(7):591–9. doi: 10.1016/j.amjmed.2006.05.024. [DOI] [PubMed] [Google Scholar]
- 2.Barker WH, Mullooly JP, Getchell W. Changing incidence and survival for heart failure in a well-defined older population, 1970-1974 and 1990-1994. Circulation. 2006 Feb 14;113(6):799–805. doi: 10.1161/CIRCULATIONAHA.104.492033. [DOI] [PubMed] [Google Scholar]
- 3.Lloyd-Jones D, Adams RJ, Brown TM, Carnethon M, Dai S, De Simone G, Ferguson TB, Ford E, Furie K, Gillespie C, Go A, Greenlund K, Haase N, Hailpern S, Ho PM, Howard V, Kissela B, Kittner S, Lackland D, Lisabeth L, Marelli A, McDermott MM, Meigs J, Mozaffarian D, Mussolino M, Nichol G, Roger VL, Rosamond W, Sacco R, Sorlie P, Thom T, Wasserthiel-Smoller S, Wong ND, Wylie-Rosett J. Heart disease and stroke statistics--2010 update: a report from the American Heart Association. Circulation. 2010 Feb 23;121(7):e46–e215. doi: 10.1161/CIRCULATIONAHA.109.192667. [DOI] [PubMed] [Google Scholar]
- 4.Jencks SF, Williams MV, Coleman EA. Rehospitalizations among patients in the Medicare fee-for-service program. N Engl J Med. 2009 Apr 2;360(14):1418–28. doi: 10.1056/NEJMsa0803563. [DOI] [PubMed] [Google Scholar]
- 5.Muzzarelli S, Leibundgut G, Maeder MT, Rickli H, Handschin R, Gutmann M, Jeker U, Buser P, Pfisterer M, Brunner-La Rocca HP. Predictors of early readmission or death in elderly patients with heart failure. Am Heart J. 2010 Aug;160(2):308–14. doi: 10.1016/j.ahj.2010.05.007. [DOI] [PubMed] [Google Scholar]
- 6.Hernandez AF, Greiner MA, Fonarow GC, Hammill BG, Heidenreich PA, Yancy CW, Peterson ED, Curtis LH. Relationship between early physician follow-up and 30-day readmission among Medicare beneficiaries hospitalized for heart failure. Jama. 2010 May 5;303(17):1716–22. doi: 10.1001/jama.2010.533. [DOI] [PubMed] [Google Scholar]
- 7.Fonarow GC, Abraham WT, Albert NM, Stough WG, Gheorghiade M, Greenberg BH, O'Connor CM, Pieper K, Sun JL, Yancy CW, Young JB. Factors identified as precipitating hospital admissions for heart failure and clinical outcomes: findings from OPTIMIZE-HF. Arch Intern Med. 2008 Apr 28;168(8):847–54. doi: 10.1001/archinte.168.8.847. [DOI] [PubMed] [Google Scholar]
- 8.Fonarow GC. The relationship between body mass index and mortality in patients hospitalized with acute decompensated heart failure. Am Heart J. 2007 Nov;154(5):e21. doi: 10.1016/j.ahj.2007.07.043. [DOI] [PubMed] [Google Scholar]
- 9.Fonarow GC, Abraham WT, Albert NM, Gattis Stough W, Gheorghiade M, Greenberg BH, O'Connor CM, Pieper K, Sun JL, Yancy CW, Young JB. Influence of a performance-improvement initiative on quality of care for patients hospitalized with heart failure: results of the Organized Program to Initiate Lifesaving Treatment in Hospitalized Patients With Heart Failure (OPTIMIZE-HF). Arch Intern Med. 2007 Jul 23;167(14):1493–502. doi: 10.1001/archinte.167.14.1493. [DOI] [PubMed] [Google Scholar]
- 10.Fonarow GC, Abraham WT, Albert NM, Stough WG, Gheorghiade M, Greenberg BH, O'Connor CM, Sun JL, Yancy C, Young JB. Carvedilol use at discharge in patients hospitalized for heart failure is associated with improved survival: an analysis from Organized Program to Initiate Lifesaving Treatment in Hospitalized Patients with Heart Failure (OPTIMIZE-HF). Am Heart J. 2007 Jan;153(1):82, e1–11. doi: 10.1016/j.ahj.2006.10.008. [DOI] [PubMed] [Google Scholar]
- 11.Fonarow GC, Abraham WT, Albert NM, Stough WG, Gheorghiade M, Greenberg BH, O'Connor CM, Pieper K, Sun JL, Yancy C, Young JB. Association between performance measures and clinical outcomes for patients hospitalized with heart failure. Jama. 2007 Jan 3;297(1):61–70. doi: 10.1001/jama.297.1.61. [DOI] [PubMed] [Google Scholar]
- 12.Khayat R, Jarjoura D, Patt B, Yamokoski T, Abraham WT. In-hospital Testing for Sleep-disordered Breathing in Hospitalized Patients With Decompensated Heart Failure: Report of Prevalence and Patient Characteristics. Journal of Cardiac Failure. 2009 doi: 10.1016/j.cardfail.2009.05.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Paulino A, Damy T, Margarit L, Stoica M, Deswarte G, Khouri L, Vermes E, Meizels A, Hittinger L, d'Ortho MP. Prevalence of sleep-disordered breathing in a 316-patient French cohort of stable congestive heart failure. Arch Cardiovasc Dis. 2009 Mar;102(3):169–75. doi: 10.1016/j.acvd.2008.12.006. [DOI] [PubMed] [Google Scholar]
- 14.Sin DD, Fitzgerald F, Parker JD, Newton G, Floras JS, Bradley TD. Risk factors for central and obstructive sleep apnea in 450 men and women with congestive heart failure. Am J Respir Crit Care Med. 1999 Oct;160(4):1101–6. doi: 10.1164/ajrccm.160.4.9903020. [DOI] [PubMed] [Google Scholar]
- 15.Javaheri S, Shukla R, Zeigler H, Wexler L. Central sleep apnea, right ventricular dysfunction, and low diastolic blood pressure are predictors of mortality in systolic heart failure. J Am Coll Cardiol. 2007 May 22;49(20):2028–34. doi: 10.1016/j.jacc.2007.01.084. [DOI] [PubMed] [Google Scholar]
- 16.Teschler H, Dohring J, Wang YM, Berthon-Jones M. Adaptive pressure support servo-ventilation: a novel treatment for Cheyne-Stokes respiration in heart failure. Am J Respir Crit Care Med. 2001 Aug 15;164(4):614–9. doi: 10.1164/ajrccm.164.4.9908114. [DOI] [PubMed] [Google Scholar]
- 17.Medicine AAoS. AASM The AASM Manual for the Scoring of Sleep and Associated Events: Rules, Terminology and Technical Specifications. 2007.
- 18.Santos-Silva R, Sartori DE, Truksinas V, Truksinas E, Alonso FF, Tufik S, Bittencourt LR. Validation of a portable monitoring system for the diagnosis of obstructive sleep apnea syndrome. Sleep. 2009 May 1;32(5):629–36. doi: 10.1093/sleep/32.5.629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Collop NA, Anderson WM, Boehlecke B, Claman D, Goldberg R, Gottlieb DJ, Hudgel D, Sateia M, Schwab R. Clinical guidelines for the use of unattended portable monitors in the diagnosis of obstructive sleep apnea in adult patients. Portable Monitoring Task Force of the American Academy of Sleep Medicine. J Clin Sleep Med. 2007 Dec 15;3(7):737–47. [PMC free article] [PubMed] [Google Scholar]
- 20.O'Connor CM, Abraham WT, Albert NM, Clare R, Gattis Stough W, Gheorghiade M, Greenberg BH, Yancy CW, Young JB, Fonarow GC. Predictors of mortality after discharge in patients hospitalized with heart failure: an analysis from the Organized Program to Initiate Lifesaving Treatment in Hospitalized Patients with Heart Failure (OPTIMIZE-HF). Am Heart J. 2008 Oct;156(4):662–73. doi: 10.1016/j.ahj.2008.04.030. [DOI] [PubMed] [Google Scholar]
- 21.Abraham WT, Fonarow GC, Albert NM, Stough WG, Gheorghiade M, Greenberg BH, O'Connor CM, Sun JL, Yancy CW, Young JB. Predictors of in-hospital mortality in patients hospitalized for heart failure: insights from the Organized Program to Initiate Lifesaving Treatment in Hospitalized Patients with Heart Failure (OPTIMIZE-HF). J Am Coll Cardiol. 2008 Jul 29;52(5):347–56. doi: 10.1016/j.jacc.2008.04.028. [DOI] [PubMed] [Google Scholar]
- 22.Lawless JE. Negative Binomial and Mixed Poisson Regression. The Canadian Journal of Statistics. 1987;15(3):209–25. [Google Scholar]
- 23.Glynn RJ, Buring JE. Ways of measuring rates of recurrent events. BMJ. 1996 Feb 10;312(7027):364–7. doi: 10.1136/bmj.312.7027.364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Little RJAaR DB. Statistical Analysis with Missing Data. second ed. John Wiley & Sons; New York: 2002. [Google Scholar]
- 25.Solin P, Bergin P, Richardson M, Kaye DM, Walters EH, Naughton MT. Influence of pulmonary capillary wedge pressure on central apnea in heart failure. Circulation. 1999 Mar 30;99(12):1574–9. doi: 10.1161/01.cir.99.12.1574. [DOI] [PubMed] [Google Scholar]
- 26.Yumino D, Redolfi S, Ruttanaumpawan P, Su MC, Smith S, Newton GE, Mak S, Bradley TD. Nocturnal rostral fluid shift: a unifying concept for the pathogenesis of obstructive and central sleep apnea in men with heart failure. Circulation. 2010 Apr 13;121(14):1598–605. doi: 10.1161/CIRCULATIONAHA.109.902452. [DOI] [PubMed] [Google Scholar]
- 27.Xie A, Skatrud JB, Khayat R, Dempsey JA, Morgan B, Russell D. Cerebrovascular response to carbon dioxide in patients with congestive heart failure. Am J Respir Crit Care Med. 2005 Aug 1;172(3):371–8. doi: 10.1164/rccm.200406-807OC. [DOI] [PubMed] [Google Scholar]
- 28.Javaheri S, Parker TJ, Liming JD, Corbett WS, Nishiyama H, Wexler L, Roselle GA. Sleep apnea in 81 ambulatory male patients with stable heart failure. Types and their prevalences, consequences, and presentations. Circulation. 1998 Jun 2;97(21):2154–9. doi: 10.1161/01.cir.97.21.2154. [DOI] [PubMed] [Google Scholar]
- 29.Pepperell JC, Maskell NA, Jones DR, Langford-Wiley BA, Crosthwaite N, Stradling JR, Davies RJ. A randomized controlled trial of adaptive ventilation for Cheyne-Stokes breathing in heart failure. Am J Respir Crit Care Med. 2003 Nov 1;168(9):1109–14. doi: 10.1164/rccm.200212-1476OC. [DOI] [PubMed] [Google Scholar]
- 30.Khayat RN, Jarjoura D, Patt B, Yamokoski T, Abraham WT. In-hospital testing for sleep-disordered breathing in hospitalized patients with decompensated heart failure: report of prevalence and patient characteristics. J Card Fail. 2009 Nov;15(9):739–46. doi: 10.1016/j.cardfail.2009.05.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Katragadda S, Xie A, Puleo D, Skatrud JB, Morgan BJ. Neural mechanism of the pressor response to obstructive and nonobstructive apnea. J Appl Physiol. 1997 Dec;83(6):2048–54. doi: 10.1152/jappl.1997.83.6.2048. [DOI] [PubMed] [Google Scholar]
- 32.Fletcher EC. Effect of episodic hypoxia on sympathetic activity and blood pressure. Respir Physiol. 2000 Feb;119(2-3):189–97. doi: 10.1016/s0034-5687(99)00114-0. [DOI] [PubMed] [Google Scholar]
- 33.MacCarthy PA, Shah AM. Impaired endothelium-dependent regulation of ventricular relaxation in pressure-overload cardiac hypertrophy. Circulation. 2000 Apr 18;101(15):1854–60. doi: 10.1161/01.cir.101.15.1854. [DOI] [PubMed] [Google Scholar]
- 34.Naughton MT, Benard DC, Liu PP, Rutherford R, Rankin F, Bradley TD. Effects of nasal CPAP on sympathetic activity in patients with heart failure and central sleep apnea. Am J Respir Crit Care Med. 1995 Aug;152(2):473–9. doi: 10.1164/ajrccm.152.2.7633695. [DOI] [PubMed] [Google Scholar]
- 35.Bradley TD, Logan AG, Kimoff RJ, Series F, Morrison D, Ferguson K, Belenkie I, Pfeifer M, Fleetham J, Hanly P, Smilovitch M, Tomlinson G, Floras JS. Continuous positive airway pressure for central sleep apnea and heart failure. N Engl J Med. 2005 Nov 10;353(19):2025–33. doi: 10.1056/NEJMoa051001. [DOI] [PubMed] [Google Scholar]
- 36.Leung RS, Diep TM, Bowman ME, Lorenzi-Filho G, Bradley TD. Provocation of ventricular ectopy by cheyne-stokes respiration in patients with heart failure. Sleep. 2004 Nov 1;27(7):1337–43. doi: 10.1093/sleep/27.7.1337. [DOI] [PubMed] [Google Scholar]
- 37.Javaheri S. Effects of continuous positive airway pressure on sleep apnea and ventricular irritability in patients with heart failure. Circulation. 2000 Feb 1;101(4):392–7. doi: 10.1161/01.cir.101.4.392. [DOI] [PubMed] [Google Scholar]
- 38.Arzt M, Harth M, Luchner A, Muders F, Holmer SR, Blumberg FC, Riegger GA, Pfeifer M. Enhanced ventilatory response to exercise in patients with chronic heart failure and central sleep apnea. Circulation. 2003 Apr 22;107(15):1998–2003. doi: 10.1161/01.CIR.0000065227.04025.C2. [DOI] [PubMed] [Google Scholar]
- 39.Arzt M, Schulz M, Wensel R, Montalvan S, Blumberg FC, Riegger GA, Pfeifer M. Nocturnal continuous positive airway pressure improves ventilatory efficiency during exercise in patients with chronic heart failure. Chest. 2005 Mar;127(3):794–802. doi: 10.1378/chest.127.3.794. [DOI] [PubMed] [Google Scholar]
- 40.Oldenburg O, Schmidt A, Lamp B, Bitter T, Muntean BG, Langer C, Horstkotte D. Adaptive servoventilation improves cardiac function in patients with chronic heart failure and Cheyne-Stokes respiration. Eur J Heart Fail. 2008 Jun;10(6):581–6. doi: 10.1016/j.ejheart.2008.04.007. [DOI] [PubMed] [Google Scholar]
- 41.Khayat RN, Abraham WT, Patt B, Pu M, Jarjoura D. In-hospital treatment of obstructive sleep apnea during decompensation of heart failure. Chest. 2009 Oct;136(4):991–7. doi: 10.1378/chest.09-0597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Yoshihisa A, Shimizu T, Owada T, Nakamura Y, Iwaya S, Yamauchi H, Miyata M, Hoshino Y, Sato T, Suzuki S, Sugimoto K, Yamaki T, Kunii H, Nakazato K, Suzuki H, Saitoh S, Takeishi Y. Adaptive servo ventilation improves cardiac dysfunction and prognosis in chronic heart failure patients with Cheyne-Stokes respiration. Int Heart J. 2011;52(4):218–23. doi: 10.1536/ihj.52.218. [DOI] [PubMed] [Google Scholar]
- 43.Javaheri S, Caref EB, Chen E, Tong KB, Abraham WT. Sleep apnea testing and outcomes in a large cohort of medicare beneficiaries with newly diagnosed heart failure. Am J Respir Crit Care Med. 2011 Feb 15;183(4):539–46. doi: 10.1164/rccm.201003-0406OC. [DOI] [PubMed] [Google Scholar]
- 44.O'Connor CM, Hasselblad V, Mehta RH, Tasissa G, Califf RM, Fiuzat M, Rogers JG, Leier CV, Stevenson LW. Triage after hospitalization with advanced heart failure: the ESCAPE (Evaluation Study of Congestive Heart Failure and Pulmonary Artery Catheterization Effectiveness) risk model and discharge score. J Am Coll Cardiol. 2010 Mar 2;55(9):872–8. doi: 10.1016/j.jacc.2009.08.083. [DOI] [PMC free article] [PubMed] [Google Scholar]