Abstract
Aims
To demonstrate the utility of genetically engineered excitable cells for studies of basic electrophysiology and cardiac cell therapy.
Methods and results
‘Zig-zag’ networks of neonatal rat ventricular myocytes (NRVMs) were micropatterned onto thin elastomeric films to mimic the slow action potential (AP) conduction found in fibrotic myocardium. Addition of genetically engineered excitable human embryonic kidney cells (HEK-293 cells) (‘Ex-293’ cells stably expressing Kir2.1, Nav1.5, and Cx43 channels) increased both cardiac conduction velocity by 370% and twitch force amplitude by 64%. Furthermore, we stably expressed mutant Nav1.5 [A1924T (fast sodium channel mutant (substitution of alanine by threonine at amino acid 1924)] channels with hyperpolarized steady-state activation and showed that, despite a 71.6% reduction in peak INa, these cells propagated APs at the same velocity as the wild-type Nav1.5-expressing Ex-293 cells. Stable expression of Cav3.3 (T-type voltage-gated calcium) channels in Ex-293 cells (to generate an ‘ExCa-293’ line) significantly increased their AP duration and reduced repolarization gradients in cocultures of these cells and NRVMs. Additional expression of an optogenetic construct [ChIEF (light-gated Channelrhodopsin mutant)]enabled light-based control of AP firing in ExCa-293 cells.
Conclusion
We show that, despite being non-contractile, genetically engineered excitable cells can significantly improve both electrical and mechanical function of engineered cardiac tissues in vitro. We further demonstrate the utility of engineered cells for tissue-level studies of basic electrophysiology and cardiac channelopathies. In the future, this novel platform could be utilized in the high-throughput design of new genetically encoded indicators of cell electrical function, validation, and improvement of computer models of AP conduction, and development of novel engineered somatic cell therapies for the treatment of cardiac infarction and arrhythmias.
Keywords: Cardiac cell therapy, Ion channels, Optogenetics, Genetic engineering, Biosensors
Introduction
A heart attack usually results in a large loss of cardiac muscle tissue that cannot be endogenously repaired by limited cardiomyocyte proliferation1 or stem cell recruitment.2 As a result, the workload of the surviving myocardium becomes increased, which ultimately leads to congestive heart failure (CHF), a condition often associated with life-threatening arrhythmias. The transplantation of exogenous stem cells in the heart has been recently pursued in clinical trials as a method to recover impaired heart function.3 So far, the use of different stem cells (from muscle,4–6 bone marrow,7,8 peripheral blood,9,10 or heart)11,12 has shown encouraging but moderate results, prompting the quest for more effective therapies.13 One proposed approach is the genetic reprogramming of adult fibroblasts to induced pluripotent stem cells14,15 followed by cardiogenic differentiation and implantation.16 In addition, Srivastava's group has recently demonstrated direct reprogramming of cardiac fibroblasts into cardiomyocyte-like cells in vivo by retroviral delivery of three transcription factors.17 While such reprogrammed cells were remarkably similar to adult cardiomyocytes, the efficiency of this process was relatively low and questioned by others18 and potential arrrhythmogenic events were not studied in detail.
Despite significant promise, stem cell therapies for heart disease involve potential risks of: (i) tumorigenicity (if a fraction of implanted cells retain pluripotency),19 (ii) arrhythmogenicity (if the implanted cells create localized structural and functional heterogeneities within the host myocardium),20 and (iii) low efficacy (if the implanted cells are functionally immature).21,22 In particular, arrhythmias could result from host–donor mismatch in: (i) cell size, shape, and alignment, (ii) gap junction number, type, and distribution, (iii) membrane electrical properties including both excitability and refractoriness, (iv) intracellular Ca2+ handling, (v) automaticity, and (vi) paracrine actions.23 For example, several studies have shown that human pluripotent stem cell-derived cardiomyocytes are functionally heterogeneous, spontaneously active, have rudimentary Ca2+ handling,24,25 and propagate action potentials (APs) at velocities of only a few cm/s.26,27 Even if genetically improved before implantation,28 these immature cells would likely continue to heterogeneously differentiate and proliferate in vivo. Thus, the described arrhythmogenic and tumorigenic risks could be both unpredictable and sustained.
An alternative approach to stem cell-based cardiac therapies is the implantation of somatic cells genetically engineered to exert a specific electrical influence on host cardiomyocytes. This approach may allow the development of safe and efficient cell therapies that would target a particular cardiac electrophysiological phenotype (atria, pulmonary veins, ventricular epi- or endocardium, Purkinje system, and sinoatrial or atrioventricular node, etc.) or a particular cardiac disease. Several proof-of-concept studies have demonstrated the capability of engineered cells to electrotonically bridge small conduction gaps29–31 and locally modify cardiac automaticity32–34 and repolarization.35,36 For example, unexcitable human embryonic kidney cells (HEK-293), human cervical cells (HeLa), and mesenchymal stem cells (MSCs) were genetically engineered to express hyperpolarization-activated cyclic nucleotide-gated (HCN) ion channels, known to generate the cardiac pacemaker current (If), and used to induce cardiac automaticity in vitro.33,34 When implanted into canine left ventricle, HCN-expressing MSCs supported ectopic cardiac pacemaking for several weeks.34 Furthermore, Yankelson et al.35 showed that mouse fibroblast cells (NIH-3T3), genetically engineered to express inward (Kir2.1) or delayed (Kv1.3) rectifier potassium channels, prolonged the cardiac effective refractory period and reduced ventricular tachycardia upon implantation into pig myocardium. Although these cells had a stable and relatively uniform phenotype, they remained unexcitable and thus would be likely to impede cardiac conduction and contraction for graft sizes larger than a few hundred microns.30,31 Nevertheless, these studies suggested that the implantation of somatic cells with genetically engineered electrical properties is a promising strategy for improving compromised electrical function in the heart.37
We recently showed for the first time that human unexcitable somatic cells can be genetically engineered into an autonomous source of electrically excitable and conducting cells.38 This was achieved by stably coexpressing the proteins for an inward rectifying potassium channel (Kir2.1), a voltage-gated sodium channel (Nav1.5), and a gap junction channel (connexin-43, Cx43) in HEK-293 cells. Moreover, starting from a single genetically engineered cell, we derived an excitable cell line named ‘Ex-293’ and showed that it can successfully bridge large, cm-sized conduction gaps within engineered cardiac tissues in vitro.38 In the current study, we further demonstrate the utility of these cells in basic electrophysiological studies and cardiac cell therapies. Specifically, we explored the hypothesis that despite their non-contractile nature, Ex-293 cells would improve both electrical and mechanical function of cardiac tissue by electrically resynchronizing activation of previously disconnected cardiomyocytes. Through the combined use of patch-clamp recordings in single cells and AP mapping in engineered tissues, we further demonstrated that engineered excitable cells can be used to directly study roles of Nav1.5 channel mutations in AP conduction. Finally, we examined the potential to modify and control the duration and initiation of APs in engineered excitable cells via the expression of T-type calcium and light-activated ion channels.
Methods
Generation and maintenance of engineered excitable cells
Creation and characterization of the monoclonal (i.e. deriving from a single cell) ‘Ex-293’ cell line (a HEK-293 cell line stably expressing Kir2.1, Nav1.5, and Cx43 channels) was previously described.38 For the current study, a plasmid encoding a mutant Nav1.5 channel (‘A1924T’ provided by Drs Walter Chazin and Svetlana Stepanovic, Vanderbilt University) was expressed in a stable HEK-293 cell line already expressing Kir2.1 and Cx43 channels. A monoclonal A1924T-expressing excitable cell line (‘A1924T/Ex-293’) was then compared with the wild-type Nav1.5-expressing Ex-293 cells. In addition, the Ex-293 cells were also genetically engineered to overexpress a human T-type voltage-gated calcium channel (Cav3.3, encoded by the CACNA1I gene and provided by Dr Edward Perez-Reyes, University of Virginia), and a monoclonal cell line was derived that stably expressed Kir2.1, Nav1.5, Cx43, and Cav3.3 channels. This line was named ‘ExCa-293’ to indicate the addition of Ca2+ current. The ExCa-293 line was then transiently transfected with an engineered light-gated Channelrhodopsin gene construct (ChIEF-tdTomato, provided by Dr Roger Tsien, University of California, San Diego) to evaluate the potential for light-controlled AP activation. Engineered HEK-293 cells were plated individually for patch-clamp analysis or used to create confluent isotropic cell monolayers as previously described.38
Isolation and culture of neonatal rat ventricular myocytes
All animals were treated in accordance with protocols approved by the Duke University Institutional Animal Care and Use Committee (IACUC). Cardiac cells were isolated from 2-day-old neonatal rat ventricles by enzymatic digestion and enriched using differential preplating, as previously described.39 Neonatal rat ventricular myocytes (NRVMs) were initially seeded and cultured in cardiac growth media supplemented with 100 µM bromodeoxyuridine (BrdU, Sigma) to inhibit fibroblast proliferation. Bromodeoxyuridine was removed 24 h later and media exchanged with HEK/NRVM culture media (low-glucose Dulbecco's modified eagle medium, containing 5% fetal bovine serum (FBS), 25 U/mL penicillin and 25 µg/mL streptomycin).
Fabrication of Ex-293 or ExCa-293 island/neonatal rat ventricular myocyte cocultures
A circular polydimethylsiloxane (PDMS) ring (∼1 cm-diameter and 1 mm-wide) was adhered onto the middle of a 22 mm-diameter fibronectin-coated (15 μg/mL) coverslip. Ex-293 or ExCa-293 cells were seeded inside the ring while freshly isolated NRVMs were seeded around the ring. The ring was removed the following day to allow engineered HEK-293 cells to outgrow and form a confluent interface with NRVMs. The coculture was maintained in HEK/NRVM media and used for optical mapping of AP propagation.
Fabrication of zig-zag neonatal rat ventricular myocyte networks on thin elastomeric films
Aclar® coverslips (25 mm diameter) were coated with a ∼35 μm thick layer of PDMS using a photoresist spinner (Headway Research). After curing for 2 h at 80°C, the spun PDMS film was scored into a rectangular strip (20 × 12.5 mm) and two narrow (2 mm wide, 1 mm tall) PDMS blocks (anchors) were glued (using uncured PDMS) to the opposite ends of each film to provide holders for force testing and prevent the films from rolling when lifted from the substrate (Figure 1A and C). Prior to cell seeding, the PDMS films were exposed to UV/ozone for 8 min and then coated for 30 min with 5 μg/mL collagen I (rat tail, BD Biosciences, suspended in 0.02 N acetic acid) at room temperature followed by multiple rinses in phosphate-buffered saline (PBS). Micropatterened zig-zag network of fibronectin (30 μg/mL) lines (100 μm wide parallel lines spaced 100 μm apart and connected by staggered 100 μm wide bridges spaced 6 mm apart, Figure 1B) was stamped onto the collagen-coated PDMS films.39 The films were treated with 0.2% w/v Pluronic F-127 (Molecular Probes) for 30 min to inhibit non-specific cell attachment and washed with PBS. Neonatal rat ventricular myocytes were then seeded at a density of 1.9 × 103 cells per mm2 in cardiac growth media followed by a switch to 2% FBS media on Culture day 2.39 On Culture day 4, Ex-293 cells were dissociated using CellStripper™ (Mediatech, Inc.), plated onto the NRVM zig-zag patterns at a density of 0.7 × 103 cells per mm2 and cultured in HEK/NRVM culture media. The next day, the rectangular thin film was carefully lifted from the Aclar® substrate using forceps and the PDMS anchors for support. The following day (Culture day 6), the control zig-zag NRVM monocultures and NRVM+Ex-293 cocultures were assessed for AP propagation and active force generation using our previously published methods.39–41
Figure 1.
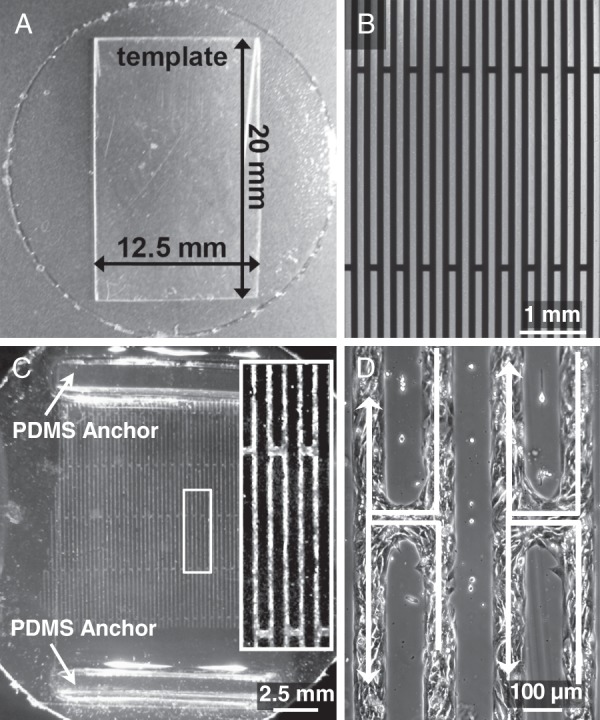
Fabrication of thin films with zig-zag cardiac networks. (A) A 25 mm Aclar® coverslip coated with a ∼35 µm thick layer of polydimethylsiloxane was scored along an overlaid template to yield a thin elastic film. (B) Photomask with zig-zag pattern used in soft lithography procedures. Dark area corresponds to fibronectin pattern printed on the polydimethylsiloxane film. (C) A polydimethylsiloxane film with 4-day-old zig-zag culture. Polydimethylsiloxane anchors provided structural support during transport and force testing. Inset shows a close-up of the culture. (D) A higher magnification image of neonatal rat ventricular myocytes in zig-zag pattern. White arrows denote tortuous path during transverse impulse propagation from right to left.
Optical mapping of action potential propagation
Cell cultures were stained with a voltage-sensitive dye, Di-4 ANEPPS, and transmembrane voltage was optically mapped using a 504-channel photodiode array (Redshirt Imaging) as previously described.38,39 Fluorescence signals were acquired at a 2.4 kHz sampling rate with a spatial resolution of 750 µm. Electrical conduction was initiated using a 1.2× threshold stimulus delivered by a bipolar point electrode in the monolayers and island cocultures or line electrode in the zig-zag cultures. Data was processed, displayed, and analysed using custom-made MATLAB software. Longitudinal and transverse conduction velocities (CV) and average AP duration at 80% repolarization (APD80) were derived as previously described.38,39
Assessment of force generation in zig-zag cultures
Isometric measurement of contractile force generation in the zig-zag cultures was performed as previously described.41,42 Briefly, one end of the film was fixed within the measurement bath, while the other end was pinned onto a floating PDMS platform connected to a sensitive force transducer. The films were stretched to 106% of their initial length using a computer-controlled linear actuator (Thorlabs, Inc.). Cultures were paced at 1 Hz rate by a bipolar line electrode positioned at longitudinal or transverse edge of the film. The voltage output from the force transducer was recorded using a DAM50 amplifier (World Precision Instruments) and a custom Labview (National Instruments) acquisition program. MATLAB was used to analyse the amplitude and duration of generated twitch force traces.41,42
Sharp electrode and patch clamp recordings of membrane voltage and currents
Membrane currents, AP generation, and resting membrane potential (RMP) were assessed in engineered HEK-293 cells as previously described.38 Briefly, sharp microelectrode recording of propagated APs was performed at 35°C in monolayers of ExCa-293 cells stimulated (1 Hz) by a point electrode. Nickel chloride (NiCl2, 10 mM) was used to inhibit calcium current during recording. Whole-cell patch clamp recordings were performed at room temperature using a Multiclamp 700B amplifier (Axon Instruments) and analysed with the WinWCP software package (provided by Dr John Dempster, University of Strathclyde). The internal patch and external bath solutions, voltage protocols, and analytical methods for recording whole-cell sodium current (INa), inward-rectifier current (IK1), or RMP were similar to those previously described.38 Light-activated inward currents and resulting AP activation in ExCa-293 cells expressing the ChIEF construct were recorded using voltage-clamp and current-clamp patch-clamp modes, respectively. Cells were illuminated with blue light (450 ± 40 nm) pulses with duration controlled by a fast electronic shutter (Optiquip) operated using pulse protocols designed in WinWCP software.
Immunostaining and image acquisition
Ex-293 and NRVM cocultures were immunostained as previously described.38 Primary anti-sarcomeric α-actinin (Sigma, EA-53 mouse monoclonal) antibody was applied overnight at 4°C. Secondary antibodies, including Alexa Fluor 594 (chicken anti-mouse) and fluorescein-isothiocyanate-conjugated anti-green fluorescent protein (α-GFP) were applied for 1 h at room temperature. Images were acquired and processed with IPLab software (BioVision Technologies) using a charge-coupled device camera (Cooke SensiCam QE) connected to an inverted fluorescence microscope (Nikon TE2000).
Statistical analysis
Data are presented as mean ± s.e.m. and were evaluated for statistical significance using an unpaired two-tailed t-test or analysis of variance followed by Tukey's post hoc test for multiple comparisons. Statistical significance was defined as ^P < 0.05, #P < 0.01, or *P < 0.001.
Results
Ex-293 cells improve transverse electrical conduction and force of contraction in ‘zig-zag’ cardiomyocyte cultures
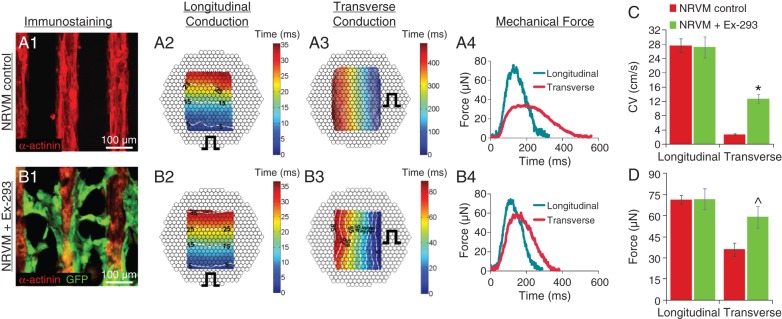
We have previously shown that Ex-293 cells are capable of actively bridging large, centimeter-sized conduction gaps between isolated regions of engineered cardiac tissue.38 In this study, we hypothesized that restored continuous cardiac conduction and concomitant resynchronization of cardiomyocyte activity would also yield enhanced cardiac contractile function, despite the fact that Ex-293 cells are non-contractile. To test this hypothesis, we designed an in vitro model of tortuous impulse conduction by creating a ‘zig-zag’ network of NRVMs (Figure 1). Longitudinal line stimulation (perpendicular to continuous NRVM strands, Figure 2A2) in these cultures yielded rapid AP conduction (velocity of 27.7 ± 2.0 cm/s, n = 4) while transverse line stimulation (parallel to NRVM strands, Figure 2A3) resulted in very slow conduction (2.7 ± 0.3 cm/s) as activation occurred tortuously through the zig-zag-patterned cells. The effective ratio of longitudinal to transverse conduction (anisotropy ratio) was 11.9 ± 1.66. When the electrically excitable Ex-293 cells (identified by GFP labelling) were added onto the zig-zag NRVM culture, they adhered to the NRVMs and bridged the acellular spaces between the NRVM strands (Figure 2B1). Longitudinal CV in these ‘NRVM+Ex-293’ cocultures remained similar to that in the control NRVM monocultures (Figure 2B2), while transverse CV significantly increased (to 12.7 ± 1.2 cm/s, Figure 2B3), showing that Ex-293 cells electrically coupled to NRVMs and increased the transverse connectivity between the adjacent NRVM strands. This increase in the transverse CV (Figure 2C) also decreased the effective anisotropy ratio to 2.2 ± 0.36 (n = 3 for all).
Figure 2.
Addition of Ex-293 cells improves transverse conduction and contractile force output of zig-zag neonatal rat ventricular myocyte cultures. (A1–A3) Control neonatal rat ventricular myocyte zig-zag cultures (A1) exhibit rapid longitudinal (A2) and slow transverse (A3) conduction during optical mapping (note prolonged time scale in A3). Pulse sign indicates position of line electrode. (A4) Neonatal rat ventricular myocyte control cultures generated lower force amplitudes with longer duration when stimulated transversely vs. longitudinally. (B1–B3) Neonatal rat ventricular myocyte zig-zag networks seeded with Ex-293 cells (GFP+, B1) yielded similar longitudinal (B2) yet much faster transverse (B3) conduction compared with neonatal rat ventricular myocyte control. (B4) Force amplitudes and durations in the NRVM+Ex-293 networks were higher and shorter in the transverse direction compared with neonatal rat ventricular myocyte control. (C, D) Average conduction velocity (C) and maximum force amplitude (D) resulting from longitudinal or transverse stimulation in neonatal rat ventricular myocyte control and NRVM+Ex-293 networks. Unpaired t-tests, n = 3–8, *P < 0.001 and ^P < 0.05 compared with neonatal rat ventricular myocyte control cultures during transverse stimulation.
Using this culture system, we further tested whether the addition of Ex-293 cells to zig-zag NRVM cultures also increased cardiac contractile force generation. In control NRVM cultures, longitudinal stimulation produced twitch forces with approximately twice higher amplitude (71.3 ± 3.0 vs. 36.1 ± 4.7 µN, n = 8) and twice shorter duration (216.9 ± 8.1 vs. 420.6 ± 21.1 ms, n = 8) than transverse stimulation (Figure 2A4 and D). When Ex-293 cells were added to the zig-zag NRVM cultures, longitudinal stimulation was found to yield contractile forces similar to those of control cultures (Figure 2B4 and D) while transverse stimulation yielded a 63.7% increase in the twitch amplitude compared with the NRVM controls (59.1 ± 7.6 µN, n = 4 vs. 36.1 ± 4.7 µN, n = 8) and 23.8% decrease in the twitch duration (Figure 2B4 and D). Taken together, these proof-of-principle studies showed that despite being non-contractile, Ex-293 cells successfully improved not only the electrical but also the mechanical function of the cardiomyocyte networks.
Comparison of mutant A1924T vs. wild-type Nav1.5-expressing engineered excitable cell lines
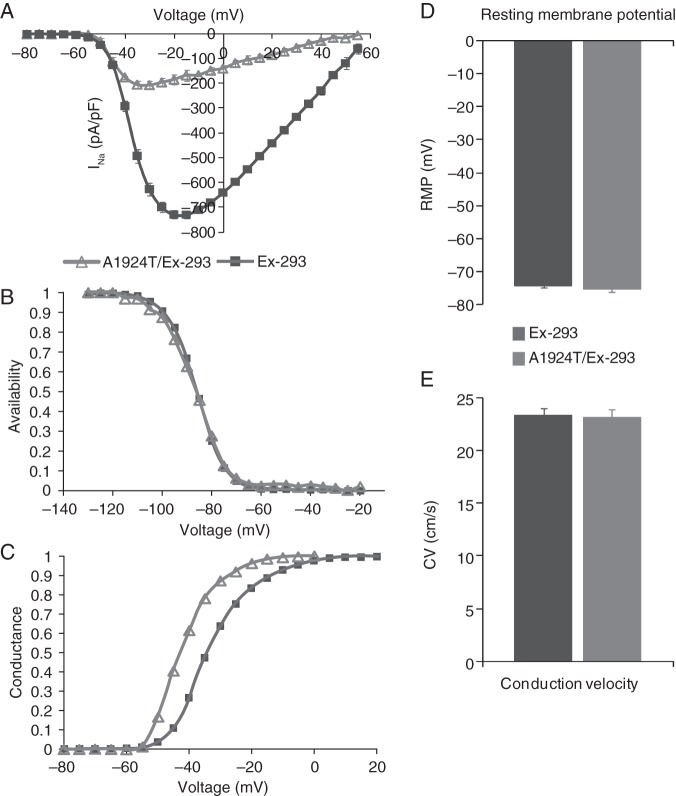
We also utilized engineered HEK-293 cells to examine the specific influence that mutated sodium channels would have upon multicellular AP propagation. Whole-cell patch clamp recordings revealed that the peak current density (pA/pF) of INa in the monoclonal A1924T/Ex-293 cell line (−206.95 ± 17.67 pA/pF, n = 4) was 3.52 times (71.6%) smaller than that of the Ex-293 cell line (−728.92 ± 17.28 pA/pF, n = 6; Figure 3A). Furthermore, peak INa in A1924T/Ex-293 cells was shifted to more hyperpolarized test potentials (∼−30 vs. −20 mV in Ex-293 cells). While there was no significant difference in channel availability (Figure 3B), analysis of steady-state sodium channel kinetics revealed a −9.49 ± 1.56 mV shift in the V1/2 of activation in the A1924T/Ex-293 cells as compared with Ex-293 cells (Figure 3C). These results were consistent with previous characterization of the A1924T mutation heterologously expressed in Xenopus oocytes.43
Figure 3.
Electrophysiological comparison of mutant A1924T Nav1.5-expressing vs. wild-type Nav1.5-expressing excitable cells. (A–C) INa current–voltage relationship (A), steady-state inactivation (B), and steady-state activation (C) in monoclonal A1924T/Ex-293 (n = 4) vs. wild-type Ex-293 (n = 6) cells. (D) Resting membrane potential in A1924T/Ex-293 (n = 5) and Ex-293 (n = 27) cells. (E) Conduction velocity recorded during optical mapping of action potential propagation in A1924T/Ex-293 (n = 4) and Ex-293 (n = 39) cell monolayers. Results compared in (D) and (E) were not statistically different.
To examine whether the alternate expression of the A1924T mutant affected the expression or function of Kir2.1 channels, patch clamp recordings of IK1 were performed and compared with those in Ex-293 cells. No significant changes in the magnitude or current–voltage profile of IK1 were observed (not shown) and consequently, the resting membrane potentials in the A1924T/Ex-293 cells (75.3 ± 0.8 mV, n = 5) was similar to that of Ex-293 cells (−74.2 ± 0.7 mV, n = 27, Figure 3D). Optical mapping of transmembrane potentials in confluent monolayers of A1924T/Ex-293 cells showed rapid and uniform AP propagation upon stimulation similar to that seen in Ex-293 monolayers (not shown). Interestingly, despite a 71.6% reduction in peak INa, A1924T/Ex-293 monolayers had CVs (23.1 ± 0.4 cm/s, n = 4) comparable with those of Ex-293 monolayers (23.3 ± 0.6 cm/s, n = 39, Figure 3E), which likely resulted from the increased excitability of A1924T/Ex-293 cells.
Prolongation of Ex-293 action potential duration by expression of Cav3.3 channels
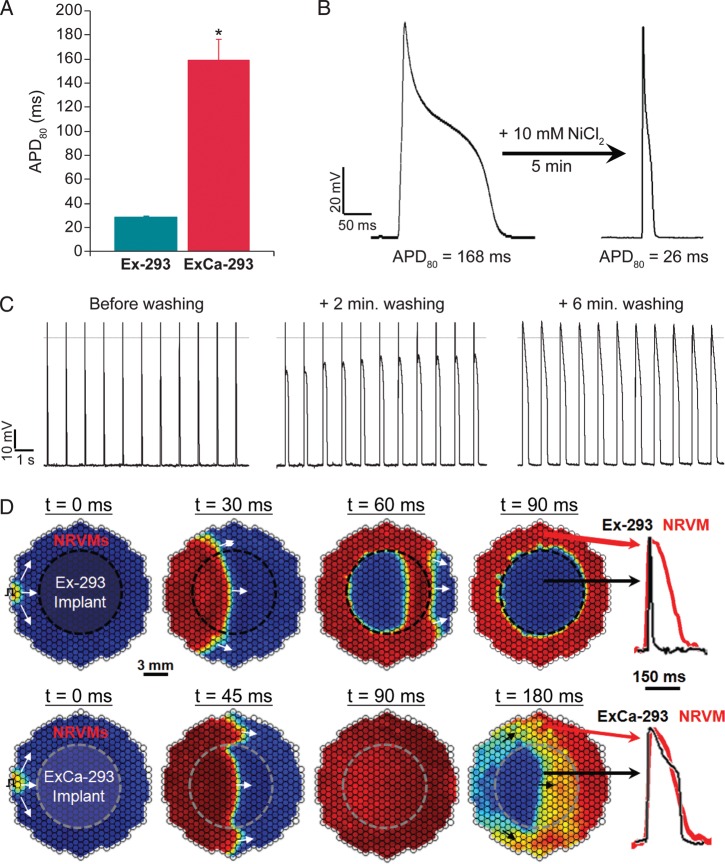
To demonstrate that the relatively short APD of engineered cells could be prolonged to levels measured in native cardiomyocytes, we chose to additionally overexpress a voltage-gated calcium channel in Ex-293 cells. Although L-type Ca current plays a pivotal role in the plateau phase of the cardiac AP,44 the alpha subunit of L-type calcium channels requires coexpression of beta subunits to be functional in heterologous expression systems.45 We therefore chose to stably express the alpha subunit of T-type calcium channels (Cav3.3) because it can autonomously form functional channels in HEK-293 cells.46 The derived T-type calcium current (ICa,T)-expressing Ex-293 cell lines showed significant prolongation of the APD compared to Ex-293 cells. One monoclonal Ex-293+Cav3.3 line (named ‘ExCa-293’) was selected for further studies based on its stability of APD during steady pacing and similar APDs (159.1 ± 19.2 ms, n = 5; Figure 4A) to those usually measured in our NRVM cultures (140–170 ms). Inhibition of ICa,T by 10 mM NiCl247 decreased the APD of ExCa-293 cells to the levels measured in Ex-293 cells (Figure 4B). Washing off the NiCl2-containing Tyrode's solution during steady 1 Hz pacing yielded recovery of the ExCa-293 APD to original levels (Figure 4C).
Figure 4.
Expression of T-type calcium current (ICa,T) in Ex-293 cells generates the ‘ExCa-293’ cell line with longer action potential duration. (A) The action potential duration in ExCa-293 monolayers (n = 5) is significantly longer than in Ex-293 monolayers (n = 39, *P < 0.001). (B) Inhibition of ICa,T by application of NiCl2 shortens the action potential duration to levels measured in Ex-293 cells. (C) Wash-out of NiCl2 reverses the action potential duration to original levels. (D) Time sequence of isovoltage frames during impulse conduction in neonatal rat ventricular myocyte cultures with a central region made of either Ex-293 (top panels) or ExCa-293 (bottom panels) cells. Red denotes peak and blue rest of the action potential. Action potential traces shown on right are from sites indicated by arrows.
We also created cell cocultures in which a central and circular (1 cm in diameter) Ex-293 or ExCa-293 cell monolayer was surrounded by a confluent monolayer of NRVMs. In this setting, APs induced in the NRVM area rapidly propagated through the Ex-293 region demonstrating a strong electrical integration between the two cell types (Figure 4D, top panels). While activation proceeded smoothly, the disparity between the Ex-293 and NRVM APD (Figure 4D, top rightmost panel) resulted in the generation of sharp repolarization gradients (with the rapidly repolarized central Ex-293 region next to still active NRVMs, Figure 4D, top panels). In certain conditions (e.g. premature stimulation) this electrical heterogeneity supported the formation of reentrant arrhythmias (not shown). However, when the central region consisted of ExCa-293 cells, dispersion of repolarization across the coculture was significantly reduced (Figure 4D, bottom panels) due to similar APDs between engineered cells and NRVMs (Figure 4D, bottom rightmost panel).
Expression of ChIEF in ExCa-293 cells enables light-based control of action potential initiation and duration
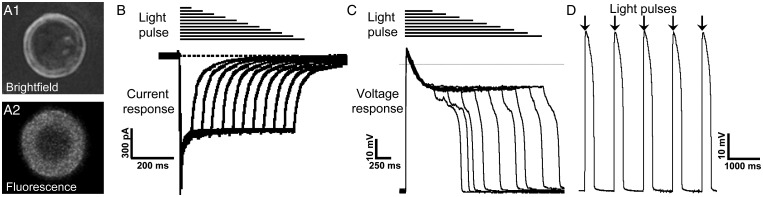
To explore whether APs in excitable engineered cells could be triggered via light-activated channels, we transiently expressed the Channelrhodopsin mutant, ChIEF,48 into ExCa-293 cells (Figure 5A1 and 2) and applied brief pulses of blue light during voltage- and current-clamp recordings. Our results show that ChIEF activation by pulsed light generates inward currents in ExCa-293 cells (Figure 5B) that are sufficient to both drive repetitive AP firing and control APD (Figure 5C and D).
Figure 5.
Expression of the ChIEF Channelrhodopsin mutant enables light-activated action potential firing in ExCa-293 cells. (A1, A2) An ExCa-293 cell expressing ChIEF-tdTomato (A1) identified by the expression of red fluorescence (A2). (B and C) Blue light (450 ± 40 nm) pulses of increasing duration (horizontal black lines) generate longer-lasting inward currents (B) and action potentials (C) in ExCa-293 cells. (D) Train of action potentials triggered by 20 ms blue light pulses delivered at 1 Hz rate (black arrows).
Discussion
Stem cell therapies hold significant potential to restore normal structure and function to the diseased heart.13 However, these therapies may be proarrhythmic or inefficient because implanted cells, while often capable of electromechanical coupling with host cardiomyocytes, may have heterogeneous and immature functional phenotypes.20,23,49 Recent proof-of-concept studies using genetically engineered unexcitable cells to locally modify cardiomyocyte electrical properties34,35 and passively bridge short cardiac conduction defects30,31 have suggested a new mode of therapy where somatic cells could be engineered with electrical properties tailored to treat specific cardiac arrhythmias or enhance conduction following myocardial infarction. We recently showed that unexcitable human somatic cells can be genetically engineered into an autonomous source of electrically excitable and conducting cells and then characterized their electrical properties.38 Here we further demonstrate the utility of these cells for studies of basic electrophysiology and cardiac cell therapies.
Resynchronization of cardiac conduction by engineered excitable cells improves cardiac contractile function
Although the engineered excitable Ex-293 cells38 are non-contractile, we hypothesized that their ability to electrically couple isolated populations of cardiomyocytes would enhance cardiac mechanical function by synchronizing cardiomyocyte contractions. This hypothesis was tested in an in vitro model of the tortuous ‘zig-zag’ pattern of electrical conduction characteristic of highly fibrotic heart tissue.50,51 We utilized elastic thin PDMS films as a culture substrate based on the methods of Feinberg et al.52 which enabled us to simultaneously measure AP propagation and generation of contractile force. Interestingly, the average force amplitudes we measured during longitudinal stimulation in the zig-zag NRVM patterns (71.3 µN) were comparable with those that Feinberg et al.52 measured in confluent anisotropic monolayers (74.2 µN). Furthermore, adding the Ex-293 cells in this assay did not impair longitudinal propagation velocity or resulting force of contraction but significantly improved the transverse conduction (2.7 to 12.7 cm/s) and resulting contractile output (64% increase in twitch amplitude). This effect could be attributed to resynchronized activation of adjacent NRVM strands that was also evident from the decreased twitch duration during transverse propagation in the presence of Ex-293 cells. These experiments suggested that implantation of non-contractile excitable cells or the genetic modification of endogenous fibroblasts to establish direct paths of fast AP conduction may both improve slow cardiac conduction and enhance the mechanical function of diseased myocardium.
Use of the engineered excitable cell platform for channelopathy analysis
We further envisioned that engineered excitable cells could be used as a platform for correlating ion channel biophysics at the cellular scale to AP conduction at the tissue scale. To test this concept, we generated a stable monoclonal HEK-293 cell line expressing both Kir2.1 channels and Cx43 gap junctions that could be further modified by the additional expression of depolarizing currents (using wild-type or mutated sodium and/or calcium channels) to enable studies of the role these currents have in AP conduction. For example, in this study, we expressed a previously studied43,53 and clinically-relevant Nav1.5 channel mutation (‘A1924T’) known to cause a hyperpolarizing shift in steady-state INa activation, and explored if changes in the kinetics of an expressed mutant channel are predictive of changes in AP conduction. The results of this study showed that even with a 71.6% reduction in peak INa compared with Ex-293 cells, the monoclonal A1924T/Ex-293 cells propagated APs at the same velocity. By showing that these two lines had the same resting potential, we eliminated the possibility that sodium channel availability differed between the two lines. Thus, the obtained results could be directly attributed to the hyperpolarizing (−9.5 mV) shift in steady-state sodium channel activation of the A1924T Nav1.5 channels which effectively reduced the excitation threshold in these cells by enabling a higher fraction of mutant channels to activate upon depolarization compared with what normally occurred in wild-type cells.
Based on the lower peak INa of the A1924T/Ex-293 vs. Ex-293 cells, we assume that the mutant cell line had a substantially lower number of sodium channels expressed on the membrane due to weaker expression of the A1924T sodium channel gene. However, this would need to be confirmed by quantitative reverse transcriptase polymerase chain reaction analysis. Assuming that the mutant channel gene was expressed at a lower level suggests that stronger channel expression, to better match the 15.5 nA peak INa of the Ex-293 cells (by for example using stronger gene promoters),54 would generate increased AP upstroke velocity and yield faster AP propagation in A1924T cells. Thus, the expression of mutated sodium channels in engineered cells may be a promising approach for deriving engineered somatic cells that support physiologically relevant CVs (e.g. closer to the average of ∼50 cm/s measured in human ventricular tissue).55
Introduction of T-type Ca2+ current into the Ex-293 cell platform
In this study, we have also shown that the APD of Ex-293 cells can be prolonged through the additional expression of voltage-gated T-type calcium channels (Cav3.3). While the derived monoclonal Ex-293+Cav3.3 cell line (‘ExCa-293’) exhibited significantly longer APD as compared with Ex-293 cells, the ExCa-293 cells also exhibited APD variability (159.1 ± 19.2 ms) even within the same cell during constant rate stimulation indicating potentially irregular endogenous calcium-handling processes and susceptibility to calcium overload. HEK-293 cells have been shown to lack major isoforms of the sodium-calcium exchanger (NCX)56 which has a significant role in regulating intracellular calcium levels. Furthermore, the high input resistance at positive membrane potentials in Ex-293 cells is expected to make the APD of these cells very sensitive to the addition of any inward currents during early repolarization. This is in contrast to cardiac myocytes where additional repolarizing potassium currents (e.g. Ito, IKr, IKs, and IKur) ensure stable repolarization despite the presence of inward calcium currents.
In Figure 4D we also presented a proof-of-concept in vitro cardiac cell therapy study using a ‘graft’ of either Ex-293 or ExCa-293 cells within an NRVM monolayer. The disparity between the short repolarization time of the Ex-293 cells compared with that of the surrounding NRVMs (Figure 4D, top panels) increased the potential for post-refractory reexcitation and generation of arrhythmias. Recently, similar concerns were raised about the potential dispersion of repolarization that may result upon implantation of Cx43-expressing skeletal myoblasts (having a relatively short APD) into human myocardial tissue.57 As we have demonstrated through the use of the Cav3.3-expressing ExCa-293 cells, the use of ion channels that modify APD and/or refractoriness in genetically engineered somatic cells provides a strategy to tune the electrical properties of engineered donor cells to better match those of host myocardial tissue and reduce the potential for arrhythmia induction upon implantation. Complications regarding variability in APD due to inadequate calcium handling may be overcome, for example, by the additional expression of the NCX exchanger or use of somatic cells that inherently have more efficient mechanisms for regulating intracellular calcium.
Genetically engineered excitable cells for use in optogenetics
Recently, optogenetic approaches have been proposed for the potential development of light-driven cardiac pacing.58,59 While the use of light would not directly enable responsiveness of the pacing rate to endogenous autonomic regulation, it is still an exciting approach to generate, in a spatially and temporally controlled manner, APs through the direct activation of membrane channels rather than the traditional use of extracellular electrodes. We have shown here that it is possible to control the activation and duration of APs within the engineered ExCa-293 cell line by the additional expression of the Channelrhodopsin mutant, ChIEF, and use of blue light pulses. The future use of these cells and cell micropatterning techniques may allow systematic studies of how the specific ion currents and geometry of the pacemaking region influence the initiation and propagation of the pacemaking beat.
Future applications of genetically engineered excitable cells
We have shown that, along with being excitable, our reproducible and immortalized cell platform allows robust expression of additional proteins which could be useful in the rapid development of new genetically encoded indicators of membrane potential or intracellular ion concentrations.60,61 Similarly, additional expression of wild-type or mutated ion channels, their auxiliary subunits, different gap junctions, or scaffolding proteins62,63 may help reveal the mechanistic roles of these proteins in cardiac conduction by combined use of patch clamp and optical mapping techniques in preparations made of the same engineered cells. These and other electrophysiological studies in this simplified and highly homogenous excitable cell system may also help validate and improve computational models of AP conduction.
Importantly, unlike the use of HEK-293 cells in this study, the genetic engineering of somatic cells for cardiac therapy would require the use of a readily accessible cell source that is contact-inhibited and terminally differentiated. Nevertheless, the proof-of-concept experiments presented here support the development of autologous somatic tissue grafts that can be rendered electrically excitable and conducting via a minimum set of genetic modifications. These biosynthetic cells and tissues may offer a safe and efficient alternative (or complement) to stem cell-based cardiac therapies. For example, engineered somatic cells may have a low metabolic demand due to their non-contractile nature and this may enhance their survival when transplanted into the injured heart. In addition, utilization of the same genetic engineering principles as explored here may enable the development of potential gene therapies targeted to endogenous cardiac fibroblasts for antiarrhythmic resynchronization of cardiomyocyte activity. Taken together, we believe that the versatile engineered cell platform presented in this study will foster the future design of novel electrophysiological experiments, measurement and modeling tools, and cardiac therapeutic approaches.
Funding
This work was supported by predoctoral fellowships from the National Science Foundation and American Heart Association to R.D.K. and research grants from National Institutes of Health (HL106203, HL104326 and HL083342) to N.B.
Acknowledgements
We thank Ava Krol for NRVM isolation, Denny Himel for help with cell micropatterning, Mark Juhas and Weining Bian for assistance with the force measurements, Woohyun Yoon for help with optogenetic constructs, and Nima Badie for assistance with optical mapping analysis.
Conflict of interest: none declared.
References
- 1.Anversa P, Kajstura J. Ventricular myocytes are not terminally differentiated in the adult mammalian heart. Circ Res. 1998;83:1–14. doi: 10.1161/01.res.83.1.1. doi:10.1161/01.RES.83.1.1. [DOI] [PubMed] [Google Scholar]
- 2.Laflamme MA, Myerson D, Saffitz JE, Murry CE. Evidence for cardiomyocyte repopulation by extracardiac progenitors in transplanted human hearts. Circ Res. 2002;90:634–40. doi: 10.1161/01.res.0000014822.62629.eb. doi:10.1161/01.RES.0000014822.62629.EB. [DOI] [PubMed] [Google Scholar]
- 3.Menasche P, Hagege AA, Scorsin M, Pouzet B, Desnos M, Duboc D, et al. Myoblast transplantation for heart failure. Lancet. 2001;357:279–80. doi: 10.1016/S0140-6736(00)03617-5. doi:10.1016/S0140-6736(00)03617-5. [DOI] [PubMed] [Google Scholar]
- 4.Menasche P, Alfieri O, Janssens S, McKenna W, Reichenspurner H, Trinquart L, et al. The Myoblast Autologous Grafting in Ischemic Cardiomyopathy (MAGIC) Trial. First randomized placebo-controlled study of myoblast transplantation. Circulation. 2008 doi: 10.1161/CIRCULATIONAHA.107.734103. [DOI] [PubMed] [Google Scholar]
- 5.Duckers HJ, Houtgraaf J, Hehrlein C, Schofer J, Waltenberger J, Gershlick A, et al. Final results of a phase IIa, randomised, open-label trial to evaluate the percutaneous intramyocardial transplantation of autologous skeletal myoblasts in congestive heart failure patients: the SEISMIC trial. EuroIntervention. 2011;6:805–12. doi: 10.4244/EIJV6I7A139. doi:10.4244/EIJV6I7A139. [DOI] [PubMed] [Google Scholar]
- 6.Durrani S, Konoplyannikov M, Ashraf M, Haider KH. Skeletal myoblasts for cardiac repair. Regen Med. 2010;5:919–32. doi: 10.2217/rme.10.65. doi:10.2217/rme.10.65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Perin EC, Willerson JT, Pepine CJ, Henry TD, Ellis SG, Zhao DX, et al. Effect of transendocardial delivery of autologous bone marrow mononuclear cells on functional capacity, left ventricular function, and perfusion in chronic heart failure: the FOCUS-CCTRN trial. JAMA. 2012;307:1717–26. doi: 10.1001/jama.2012.418. doi:10.1001/jama.2012.418. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Dill T, Schachinger V, Rolf A, Mollmann S, Thiele H, Tillmanns H, et al. Intracoronary administration of bone marrow-derived progenitor cells improves left ventricular function in patients at risk for adverse remodeling after acute ST-segment elevation myocardial infarction: results of the Reinfusion of Enriched Progenitor cells And Infarct Remodeling in Acute Myocardial Infarction study (REPAIR-AMI) cardiac magnetic resonance imaging substudy. Am Heart J. 2009;157:541–7. doi: 10.1016/j.ahj.2008.11.011. doi:10.1016/j.ahj.2008.11.011. [DOI] [PubMed] [Google Scholar]
- 9.Losordo DW, Schatz RA, White CJ, Udelson JE, Veereshwarayya V, Durgin M, et al. Intramyocardial transplantation of autologous CD34+ stem cells for intractable angina: a phase I/IIa double-blind, randomized controlled trial. Circulation. 2007;115:3165–72. doi: 10.1161/CIRCULATIONAHA.106.687376. doi:10.1161/CIRCULATIONAHA.106.687376. [DOI] [PubMed] [Google Scholar]
- 10.Tatsumi T, Ashihara E, Yasui T, Matsunaga S, Kido A, Sasada Y, et al. Intracoronary transplantation of non-expanded peripheral blood-derived mononuclear cells promotes improvement of cardiac function in patients with acute myocardial infarction. Circ J. 2007;71:1199–207. doi: 10.1253/circj.71.1199. doi:10.1253/circj.71.1199. [DOI] [PubMed] [Google Scholar]
- 11.Bolli R, Chugh AR, D'Amario D, Loughran JH, Stoddard MF, Ikram S, et al. Cardiac stem cells in patients with ischaemic cardiomyopathy (SCIPIO): initial results of a randomised phase 1 trial. Lancet. 2011;378:1847–57. doi: 10.1016/S0140-6736(11)61590-0. doi:10.1016/S0140-6736(11)61590-0. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 12.Makkar RR, Smith RR, Cheng K, Malliaras K, Thomson LE, Berman D, et al. Intracoronary cardiosphere-derived cells for heart regeneration after myocardial infarction (CADUCEUS): a prospective, randomised phase 1 trial. Lancet. 2012;379:895–904. doi: 10.1016/S0140-6736(12)60195-0. doi:10.1016/S0140-6736(12)60195-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Laflamme MA, Murry CE. Heart regeneration. Nature. 2011;473:326–35. doi: 10.1038/nature10147. doi:10.1038/nature10147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Yu J, Vodyanik MA, Smuga-Otto K, Antosiewicz-Bourget J, Frane JL, Tian S, et al. Induced pluripotent stem cell lines derived from human somatic cells. Science. 2007;318:1917–20. doi: 10.1126/science.1151526. doi:10.1126/science.1151526. [DOI] [PubMed] [Google Scholar]
- 15.Takahashi K, Tanabe K, Ohnuki M, Narita M, Ichisaka T, Tomoda K, et al. Induction of pluripotent stem cells from adult human fibroblasts by defined factors. Cell. 2007;131:861–72. doi: 10.1016/j.cell.2007.11.019. doi:10.1016/j.cell.2007.11.019. [DOI] [PubMed] [Google Scholar]
- 16.Park IH, Zhao R, West JA, Yabuuchi A, Huo H, Ince TA, et al. Reprogramming of human somatic cells to pluripotency with defined factors. Nature. 2008;451:141–6. doi: 10.1038/nature06534. doi:10.1038/nature06534. [DOI] [PubMed] [Google Scholar]
- 17.Qian L, Huang Y, Spencer CI, Foley A, Vedantham V, Liu L, et al. In vivo reprogramming of murine cardiac fibroblasts into induced cardiomyocytes. Nature. 2012 doi: 10.1038/nature11044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Chen JX, Krane M, Deutsch MA, Wang L, Rav-Acha M, Gregoire S, et al. Inefficient Reprogramming of Fibroblasts into Cardiomyocytes Using Gata4, Mef2c, and Tbx5. Circ Res. 2012 doi: 10.1161/CIRCRESAHA.112.270264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Nussbaum J, Minami E, Laflamme MA, Virag JA, Ware CB, Masino A, et al. Transplantation of undifferentiated murine embryonic stem cells in the heart: teratoma formation and immune response. FASEB J. 2007;21:1345–57. doi: 10.1096/fj.06-6769com. doi:10.1096/fj.06-6769com. [DOI] [PubMed] [Google Scholar]
- 20.Liao SY, Liu Y, Siu CW, Zhang Y, Lai WH, Au KW, et al. Proarrhythmic risk of embryonic stem cell-derived cardiomyocyte transplantation in infarcted myocardium. Heart Rhythm. 2010;7:1852–9. doi: 10.1016/j.hrthm.2010.09.006. doi:10.1016/j.hrthm.2010.09.006. [DOI] [PubMed] [Google Scholar]
- 21.Xi J, Khalil M, Shishechian N, Hannes T, Pfannkuche K, Liang H, et al. Comparison of contractile behavior of native murine ventricular tissue and cardiomyocytes derived from embryonic or induced pluripotent stem cells. FASEB J. 2010;24:2739–51. doi: 10.1096/fj.09-145177. doi:10.1096/fj.09-145177. [DOI] [PubMed] [Google Scholar]
- 22.Binah O, Dolnikov K, Sadan O, Shilkrut M, Zeevi-Levin N, Amit M, et al. Functional and developmental properties of human embryonic stem cells-derived cardiomyocytes. J Electrocardiol. 2007;40:S192–6. doi: 10.1016/j.jelectrocard.2007.05.035. doi:10.1016/j.jelectrocard.2007.05.035. [DOI] [PubMed] [Google Scholar]
- 23.Macia E, Boyden PA. Stem cell therapy is proarrhythmic. Circulation. 2009;119:1814–23. doi: 10.1161/CIRCULATIONAHA.108.779900. doi:10.1161/CIRCULATIONAHA.108.779900. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Lieu DK, Liu J, Siu CW, McNerney GP, Tse HF, Abu-Khalil A, et al. Absence of transverse tubules contributes to non-uniform Ca(2+) wavefronts in mouse and human embryonic stem cell-derived cardiomyocytes. Stem cells Dev. 2009;18:1493–500. doi: 10.1089/scd.2009.0052. doi:10.1089/scd.2009.0052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Lee YK, Ng KM, Lai WH, Chan YC, Lau YM, Lian Q, et al. Calcium homeostasis in human induced pluripotent stem cell-derived cardiomyocytes. Stem Cell Rev. 2011;7:976–86. doi: 10.1007/s12015-011-9273-3. doi:10.1007/s12015-011-9273-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Satin J, Kehat I, Caspi O, Huber I, Arbel G, Itzhaki I, et al. Mechanism of spontaneous excitability in human embryonic stem cell derived cardiomyocytes. J Physiol. 2004;559:479–96. doi: 10.1113/jphysiol.2004.068213. doi:10.1113/jphysiol.2004.068213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Kehat I, Khimovich L, Caspi O, Gepstein A, Shofti R, Arbel G, et al. Electromechanical integration of cardiomyocytes derived from human embryonic stem cells. Nat Biotechnol. 2004;22:1282–9. doi: 10.1038/nbt1014. doi:10.1038/nbt1014. [DOI] [PubMed] [Google Scholar]
- 28.Liu J, Lieu DK, Siu CW, Fu JD, Tse HF, Li RA. Facilitated maturation of Ca2+ handling properties of human embryonic stem cell-derived cardiomyocytes by calsequestrin expression. Am J Physiol Cell Physiol. 2009;297:C152–9. doi: 10.1152/ajpcell.00060.2009. doi:10.1152/ajpcell.00060.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Hofshi A, Itzhaki I, Gepstein A, Arbel G, Gross GJ, Gepstein L. A combined gene and cell therapy approach for restoration of conduction. Heart Rhythm. 2011;8:121–30. doi: 10.1016/j.hrthm.2010.10.011. doi:10.1016/j.hrthm.2010.10.011. [DOI] [PubMed] [Google Scholar]
- 30.Gaudesius G, Miragoli M, Thomas SP, Rohr S. Coupling of cardiac electrical activity over extended distances by fibroblasts of cardiac origin. Circ Res. 2003;93:421–8. doi: 10.1161/01.RES.0000089258.40661.0C. doi:10.1161/01.RES.0000089258.40661.0C. [DOI] [PubMed] [Google Scholar]
- 31.Klinger R, Bursac N. Cardiac cell therapy in vitro: reproducible assays for comparing the efficacy of different donor cells. IEEE Eng Med Biol Mag. 2008;27:72–80. doi: 10.1109/MEMB.2007.913849. doi:10.1109/MEMB.2007.913849. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.de Boer TP, van Veen TA, Houtman MJ, Jansen JA, van Amersfoorth SC, Doevendans PA, et al. Inhibition of cardiomyocyte automaticity by electrotonic application of inward rectifier current from Kir2.1 expressing cells. Med Biol Eng Comput. 2006;44:537–42. doi: 10.1007/s11517-006-0059-8. doi:10.1007/s11517-006-0059-8. [DOI] [PubMed] [Google Scholar]
- 33.Valiunas V, Kanaporis G, Valiuniene L, Gordon C, Wang HZ, Li L, et al. Coupling an HCN2-expressing cell to a myocyte creates a two-cell pacing unit. J Physiol. 2009;587:5211–26. doi: 10.1113/jphysiol.2009.180505. doi:10.1113/jphysiol.2009.180505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Potapova I, Plotnikov A, Lu Z, Danilo P, Jr, Valiunas V, Qu J, et al. Human mesenchymal stem cells as a gene delivery system to create cardiac pacemakers. Circ Res. 2004;94:952–9. doi: 10.1161/01.RES.0000123827.60210.72. doi:10.1161/01.RES.0000123827.60210.72. [DOI] [PubMed] [Google Scholar]
- 35.Yankelson L, Feld Y, Bressler-Stramer T, Itzhaki I, Huber I, Gepstein A, et al. Cell therapy for modification of the myocardial electrophysiological substrate. Circulation. 2008;117:720–31. doi: 10.1161/CIRCULATIONAHA.106.671776. doi:10.1161/CIRCULATIONAHA.106.671776. [DOI] [PubMed] [Google Scholar]
- 36.Feld Y, Melamed-Frank M, Kehat I, Tal D, Marom S, Gepstein L. Electrophysiological modulation of cardiomyocytic tissue by transfected fibroblasts expressing potassium channels: a novel strategy to manipulate excitability. Circulation. 2002;105:522–9. doi: 10.1161/hc0402.102661. doi:10.1161/hc0402.102661. [DOI] [PubMed] [Google Scholar]
- 37.Gepstein L, Feld Y, Yankelson L. Somatic gene and cell therapy strategies for the treatment of cardiac arrhythmias. Am J Physiol Heart Circ Physiol. 2004;286:H815–22. doi: 10.1152/ajpheart.00962.2003. doi:10.1152/ajpheart.00962.2003. [DOI] [PubMed] [Google Scholar]
- 38.Kirkton RD, Bursac N. Engineering biosynthetic excitable tissues from unexcitable cells for electrophysiological and cell therapy studies. Nat Commun. 2011;2:300. doi: 10.1038/ncomms1302. doi:10.1038/ncomms1302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Badie N, Scull JA, Klinger RY, Krol A, Bursac N. Conduction block in micropatterned cardiomyocyte cultures replicating the structure of ventricular cross-sections. Cardiovasc Res. 2012;93:263–71. doi: 10.1093/cvr/cvr304. doi:10.1093/cvr/cvr304. [DOI] [PubMed] [Google Scholar]
- 40.Hinds S, Bian W, Dennis RG, Bursac N. The role of extracellular matrix composition in structure and function of bioengineered skeletal muscle. Biomaterials. 2011;32:3575–83. doi: 10.1016/j.biomaterials.2011.01.062. doi:10.1016/j.biomaterials.2011.01.062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Liau B, Christoforou N, Leong KW, Bursac N. Pluripotent stem cell-derived cardiac tissue patch with advanced structure and function. Biomaterials. 2011;32:9180–7. doi: 10.1016/j.biomaterials.2011.08.050. doi:10.1016/j.biomaterials.2011.08.050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Bian W. Durham, NC: Biomedical Engineering. Duke University; 2011. Tissue Engineering of a Differentiated Skeletal Muscle Construct with Controllable Structure and Function; p. 221. PhD Thesis. [Google Scholar]
- 43.Rook MB, Bezzina Alshinawi C, Groenewegen WA, van Gelder IC, van Ginneken AC, Jongsma HJ, et al. Human SCN5A gene mutations alter cardiac sodium channel kinetics and are associated with the Brugada syndrome. Cardiovasc Res. 1999;44:507–17. doi: 10.1016/s0008-6363(99)00350-8. doi:10.1016/S0008-6363(99)00350-8. [DOI] [PubMed] [Google Scholar]
- 44.Grant AO. Cardiac ion channels. Circ Arrhythm Electrophysiol. 2009;2:185–94. doi: 10.1161/CIRCEP.108.789081. doi:10.1161/CIRCEP.108.789081. [DOI] [PubMed] [Google Scholar]
- 45.Babai N, Kanevsky N, Dascal N, Rozanski GJ, Singh DP, Fatma N, et al. Anion-sensitive regions of L-type CaV1.2 calcium channels expressed in HEK293 cells. PLoS One. 2010;5:e8602. doi: 10.1371/journal.pone.0008602. doi:10.1371/journal.pone.0008602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Cribbs LL, Lee JH, Yang J, Satin J, Zhang Y, Daud A, et al. Cloning and characterization of alpha1H from human heart, a member of the T-type Ca2+ channel gene family. Circ Res. 1998;83:103–9. doi: 10.1161/01.res.83.1.103. doi:10.1161/01.RES.83.1.103. [DOI] [PubMed] [Google Scholar]
- 47.Lee JH, Gomora JC, Cribbs LL, Perez-Reyes E. Nickel block of three cloned T-type calcium channels: low concentrations selectively block alpha1H. Biophys J. 1999;77:3034–42. doi: 10.1016/S0006-3495(99)77134-1. doi:10.1016/S0006-3495(99)77134-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Lin JY, Lin MZ, Steinbach P, Tsien RY. Characterization of engineered channelrhodopsin variants with improved properties and kinetics. Biophys J. 2009;96:1803–14. doi: 10.1016/j.bpj.2008.11.034. doi:10.1016/j.bpj.2008.11.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Halbach M, Pfannkuche K, Pillekamp F, Ziomka A, Hannes T, Reppel M, et al. Electrophysiological maturation and integration of murine fetal cardiomyocytes after transplantation. Circ Res. 2007;101:484–92. doi: 10.1161/CIRCRESAHA.107.153643. doi:10.1161/CIRCRESAHA.107.153643. [DOI] [PubMed] [Google Scholar]
- 50.Koura T, Hara M, Takeuchi S, Ota K, Okada Y, Miyoshi S, et al. Anisotropic conduction properties in canine atria analyzed by high- resolution optical mapping: preferential direction of conduction block changes from longitudinal to transverse with increasing age. Circulation. 2002;105:2092–8. doi: 10.1161/01.cir.0000015506.36371.0d. doi:10.1161/01.CIR.0000015506.36371.0D. [DOI] [PubMed] [Google Scholar]
- 51.de Bakker JM, van Capelle FJ, Janse MJ, Tasseron S, Vermeulen JT, de Jonge N, et al. Slow conduction in the infarcted human heart. ‘Zigzag’ course of activation. Circulation. 1993;88:915–26. doi: 10.1161/01.cir.88.3.915. doi:10.1161/01.CIR.88.3.915. [DOI] [PubMed] [Google Scholar]
- 52.Feinberg AW, Feigel A, Shevkoplyas SS, Sheehy S, Whitesides GM, Parker KK. Muscular thin films for building actuators and powering devices. Science. 2007;317:1366–70. doi: 10.1126/science.1146885. doi:10.1126/science.1146885. [DOI] [PubMed] [Google Scholar]
- 53.Potet F, Chagot B, Anghelescu M, Viswanathan PC, Stepanovic SZ, Kupershmidt S, et al. Functional Interactions between Distinct Sodium Channel Cytoplasmic Domains through the Action of Calmodulin. J Biol Chem. 2009;284:8846–54. doi: 10.1074/jbc.M806871200. doi:10.1074/jbc.M806871200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Schlabach MR, Hu JK, Li M, Elledge SJ. Synthetic design of strong promoters. Proc Natl Acad Sci USA. 2010;107:2538–43. doi: 10.1073/pnas.0914803107. doi:10.1073/pnas.0914803107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Taggart P, Sutton PM, Opthof T, Coronel R, Trimlett R, Pugsley W, et al. Inhomogeneous transmural conduction during early ischaemia in patients with coronary artery disease. J Mol Cell Cardiol. 2000;32:621–30. doi: 10.1006/jmcc.2000.1105. doi:10.1006/jmcc.2000.1105. [DOI] [PubMed] [Google Scholar]
- 56.Ander BP, Hurtado C, Raposo CS, Maddaford TG, Deniset JF, Hryshko LV, et al. Differential sensitivities of the NCX1.1 and NCX1.3 isoforms of the Na+-Ca2+ exchanger to alpha-linolenic acid. Cardiovasc Res. 2007;73:395–403. doi: 10.1016/j.cardiores.2006.09.013. doi:10.1016/j.cardiores.2006.09.013. [DOI] [PubMed] [Google Scholar]
- 57.Cohen IS, Rosen AB, Gaudette GR. A Caveat Emptor for myocardial regeneration: mechanical without electrical recovery will not suffice. J Mol Cell Cardiol. 2007;42:285–8. doi: 10.1016/j.yjmcc.2006.11.001. doi:10.1016/j.yjmcc.2006.11.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Jia Z, Valiunas V, Lu Z, Bien H, Liu H, Wang HZ, et al. Stimulating Cardiac Muscle by Light: Cardiac Optogenetics by Cell Delivery. Circ Arrhythm Electrophysiol. 2011 doi: 10.1161/CIRCEP.111.964247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Bruegmann T, Malan D, Hesse M, Beiert T, Fuegemann CJ, Fleischmann BK, et al. Optogenetic control of heart muscle in vitro and in vivo. Nat Methods. 2010;7:897–900. doi: 10.1038/nmeth.1512. doi:10.1038/nmeth.1512. [DOI] [PubMed] [Google Scholar]
- 60.Palmer AE, Qin Y, Park JG, McCombs JE. Design and application of genetically encoded biosensors. Trends Biotechnol. 2011;29:144–52. doi: 10.1016/j.tibtech.2010.12.004. doi:10.1016/j.tibtech.2010.12.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Mutoh H, Perron A, Akemann W, Iwamoto Y, Knopfel T. Optogenetic monitoring of membrane potentials. Exp Physiol. 2011;96:13–8. doi: 10.1113/expphysiol.2010.053942. doi:10.1113/expphysiol.2010.053942. [DOI] [PubMed] [Google Scholar]
- 62.Milstein ML, Musa H, Balbuena DP, Anumonwo JM, Auerbach DS, Furspan PB, et al. Dynamic reciprocity of sodium and potassium channel expression in a macromolecular complex controls cardiac excitability and arrhythmia. Proc Natl Acad Sci USA. 2012 doi: 10.1073/pnas.1109370109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Mohler PJ, Rivolta I, Napolitano C, LeMaillet G, Lambert S, Priori SG, et al. Nav1.5 E1053K mutation causing Brugada syndrome blocks binding to ankyrin-G and expression of Nav1.5 on the surface of cardiomyocytes. Proc Natl Acad Sci USA. 2004;101:17533–8. doi: 10.1073/pnas.0403711101. doi:10.1073/pnas.0403711101. [DOI] [PMC free article] [PubMed] [Google Scholar]