Abstract
This article reviews the latest developments in computational cardiology. It focuses on the contribution of cardiac modelling to the development of new therapies as well as the advancement of existing ones for cardiac arrhythmias and pump dysfunction. Reviewed are cardiac modelling efforts aimed at advancing and optimizing existent therapies for cardiac disease (defibrillation, ablation of ventricular tachycardia, and cardiac resynchronization therapy) and at suggesting novel treatments, including novel molecular targets, as well as efforts to use cardiac models in stratification of patients likely to benefit from a given therapy, and the use of models in diagnostic procedures.
Introduction
The iterative interaction between experimentation and simulation has long played a central role in the advancement of biological sciences. Among computational models of the various physiological systems, the heart is the most highly advanced example of a virtual organ, capable of integrating data at multiple scales, from genes to the whole organ.1 State-of-the-art whole-heart models of electrophysiology and electromechanics are currently being used to study a wide range of mechanisms in the workings of the normal and the diseased heart.2 The focus of this review is on the contribution of heart computational models to the treatment of the diseased heart, i.e. on the computational medicine aspect of cardiac modelling applications. Reviewed below are cardiac modelling efforts aimed at advancing and optimizing existing therapies for cardiac disease and at suggesting novel treatments, including novel molecular targets, as well as efforts to use cardiac models in stratification of patients likely to benefit from a given therapy, and the use of models in diagnostic procedures.
Subject-specific biophysically-detailed modelling of the heart for optimization and advancement of therapies for arrhythmias and pump dysfunction
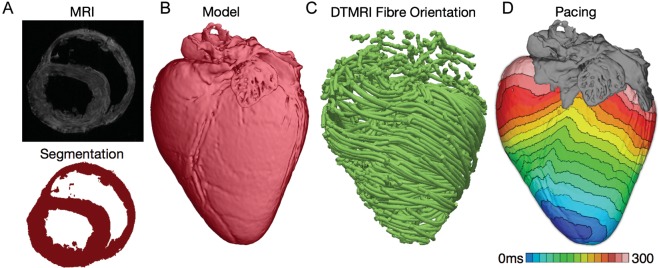
Recent years have witnessed revolutionary advances in imaging, including ex vivo structural and diffusion tensor (DT) magnetic resonance imaging (MRI) that facilitate acquisition of the intact structure of explanted hearts with high resolution. Leveraging these advances, a new generation of whole-heart image-based models with unprecedented detail have emerged.3,4 Figure 1A presents the development of one such model, of the normal canine heart, together with the activation map resulting from paced propagation in this heart. Such ex vivo heart models are currently being used, in combination with experimental electrophysiological data, to provide a better understanding of the role of the individual morphology of the infarct region in the generation and maintenance of infarct-related ventricular tachycardia (VT), the most frequent clinical ventricular arrhythmia, present in 64% of patients with ventricular rhythm disorder and in 89% of patients with sudden cardiac death.5 Using a multi-scale model of the infarcted pig ventricles reconstructed from ex vivo MRI and DTMRI data, the study by Pop et al.6 demonstrated a good correspondence between in-silico and experimental electroanatomical voltage maps, and was able to successfully predict infarct-related VT inducibility after programmed electrical stimulation. Arevalo et al.7 examined the role of the extent of the infarct border zone in arrhythmogenesis, establishing that a minimum volume of remodelled tissue is needed for VT maintenance. Such simulation methodology could have a major clinical impact in predicting the optimal targets of catheter ablation of infarct-related VT in individual hearts, should the methodology be able to reconstruct patient hearts from clinical imaging data and evaluate the three-dimensional (3D) patterns of infarct-related VT8 in the patient. The first attempts in this direction have already been made. Relan et al.9 used a hybrid X-ray and MR environment to image a patient heart, which was further personalized with voltage measurements. The results demonstrated that the heart model could successfully be used to assess infarct-related VT inducibility from sites not accessible in the clinic.
Figure 1.
Magnetic resonance imaging-based high-resolution model of electrical activation in the normal canine heart. (A) Segmentation of the myocardium. (B) Reconstructed canine heart geometry. (C) Fibre orientation in the canine heart as determined from the diffusion-tensor magnetic resonance imaging data. (D) Paced activation in the heart.
Computational modelling of the electrophysiology of the human atria is also becoming an important component in the evaluation and advancement of therapeutic strategies, as recent state-of-the-art models can accurately simulate the complex spatio-temporal dynamics of atrial arrhythmias. Similar to ventricular modelling methodology, biophysically detailed atrial models are reconstructed from MRI10 and computerized tomography (CT)11 scans. Contemporary atrial models allow for realistic simulation of electro cardiograph (ECG) signals,12 monophasic action potentials,13 and electrograms,14 serving as an important tool for analysing the aetiologies that underlie electrical signals obtained clinically. For example, simulation studies of bipolar electrograms in atrial tissue have found that fibroblast proliferation and microscale obstacles (such as collagenous septa) may be responsible for the fractionation of electrograms15 during atrial fibrillation (AF), signals which guide catheter ablation of AF. Studies involving simulation of ECG signals in atrial models have helped optimize procedures such as haemodialysis therapy (which can cause AF)16 and predicting the optimal position and direction of one-channel bipolar ECGs.17 Full utilization of multi-scale atrial models in the clinic for preventative, diagnostic, or therapeutic purposes will require the generation of patient-specific atrial models; early efforts in this direction are already underway.18 An example of a patient-specific model with the fibrosis regions delineated is shown in Figure 2.
Figure 2.
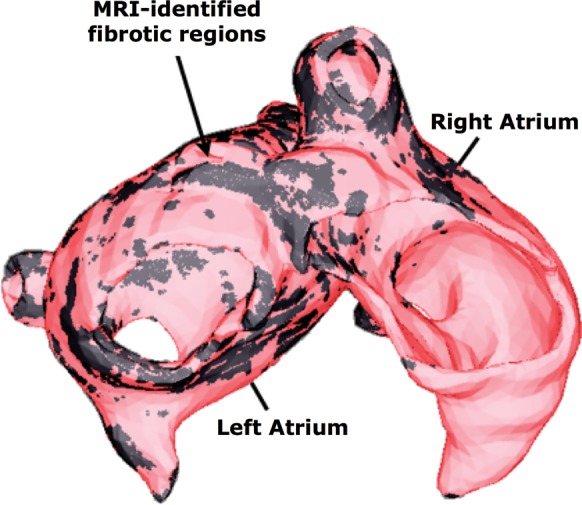
A reconstruction of patient atria with fibrotic remodelling from magnetic resonance imaging scans. MRI data courtesy of Dr R. Macleod, University of Utah.
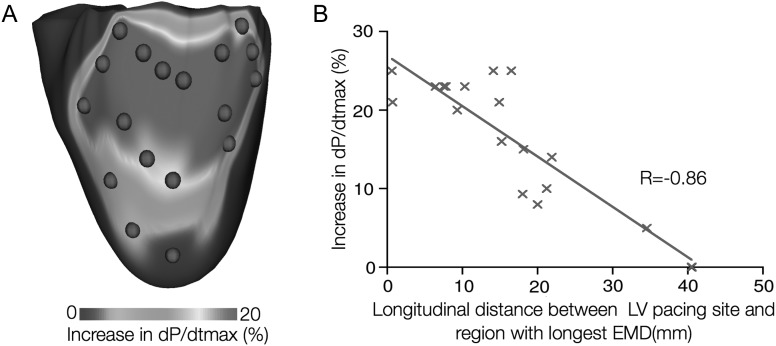
Finally, biophysically detailed electromechanical models of the heart, which represent the most sophisticated models of the heart developed so far,19 have also been harnessed in the development of therapies for pump dysfunction. Cardiac resynchronization therapy (CRT) employs bi-ventricular pacing to re-coordinate the contraction of the failing heart. Electromechanical modelling studies have provided insight into the mechanisms that govern CRT efficacy. Kerckhoffs et al.20,21 have demonstrated that improvement of ventricular function following CRT in the failing heart with left bundle branch block (LBBB) is diminished with increasing infarct size and that infarct location also affects the response to CRT. Niederer et al.22 revealed that the haemodynamic benefit from CRT is improved when length-dependent tension regulation is attenuated; the study suggested that a compromised Frank–Starling mechanism (the organ-level equivalent of length-dependent tension regulation) could be a clinical metric in identifying heart failure patients as potential responders to CRT. Electromechanical models of the heart have also been used as test beds to understand how different pacing parameters affect CRT efficacy.23,24 Constantino et al.25 recently proposed a strategy to optimize the response to CRT that involves placing the Left Ventricle (LV) pacing electrode at a location that targets the regions with the longest electromechanical delay; Figure 3 presents simulation results demonstrating that maximal haemodynamic benefit occurred when the LV pacing site was located near the base and mid-ventricle, which was within the region of longest electromechanical delay. Finally, patient-specific models of hearts with contractile dyssnchrony have been recently developed,26,27 holding high promise to become an important clinical tool in the treatment of dyssycnhronous heart failure.
Figure 3.
Optimizing the placement of the Left Ventricle (LV) pacing electrode for cardiac resynchronization therapy in the failing canine heart. (A) Map of the percentage increase in dP/dtmax as a function of the LV pacing site (P is pressure). Red dots denote LV pacing sites. (B) Correlation of longitudinal distance between LV pacing site and region with longest EMD, and percentage increase in dP/dtmax.
Biophysically-detailed computational modelling of the heart as a testbed for new molecular therapies
A major avenue of scientific inquiry in computational cardiology relates the binding/unbinding of drugs to molecular target(s) to the instigation, termination or prevention of cardiac arrhythmias. In this section we focus on examples where emergent behaviour, resulting from integrations across the scales of biological hierarchy, has shed new light on existing or novel drug actions for treatment of arrhythmia.
At the level of the ion channel, Markov models with state specific drug binding/unbinding have been used to test hypotheses regarding the mechanisms of drug effects on macroscopic currents. Since the arrhythmogenic long QT syndrome (LQTS) type 2 is characterized by loss of repolarizing rapid delayed rectifier K+ current, IKr,28 a straightforward approach for the prevention of LQT-2 arrhythmia, therefore, would be pharmacological enhancement of IKr. Perry et al.29 explored the novel compound RPR260243, shown to enhance IKr, represented by the KCNH2 isoform 1a current expressed in xenopus oocytes. Rate constants in a proposed modal gating scheme were determined to best fit the experimental data. The model revealed that IKr enhancement could be explained by dose-dependent loss of deactivation; point mutation analysis provided the structural mechanism underlying this model prediction. Going a step further, Sale et al.30 simulated the effects of the drug E4031, known to block IKr in its open state in a use-dependent manner31 on the human ventricular action potential. Drug E4031 is known to block IKr in its open state, in a use-dependent manner.31 Previously it was unclear as to why isoform 1a current, which is smaller than 1a/1b combination, was more sensitive to E4031. The Markov model for 1a-alone vs. 1a/1b mechanistically explained the discrepancy, and related action potential prolongation with E4031 as seen experimentally.
Type 3 LQTS is brought on by enhancement of the non-inactivating, or late Na+ current (INa).28 Using Markov models, Clancy et al.32 compared the effect of two INa blocking drugs, lidocaine and mexiletine, revealing that mexiletine preferentially binds to the population of channels undergoing late burst opening; the latter events are dangerously common in LQT3, leading to arrhythmogenic early afterdepolarizations (EADs). In contrast, lidocaine preferentially binds to channels during the rapid activation/inactivation phase of INa. The model showed that there are mexiletine doses that selectively remove late current and EADs without detrimental effect to excitability.
Nesterenko et al.33 also drew connections between drug binding kinetics and emergent effects on the action potential (AP) in the investigation of the novel anti-arrhythmic drug ranolazine. Among its many targets,34 ranolazine reduces late INa in atrial-selective fashion.35 Nesterenko et al. explained the mechanism behind this selectivity by introducing the concept of a ‘pre-open’ state to the INa Markov model. The effect of late INa block by ranolazine on tissue electrophysiology was examined by Morita et al.,36 who demonstrated that late INa block suppresses EADs that lead to focal reentry after hydrogen peroxide application, as observed in experiments. Importantly, ranolazine is a mild IKr blocker at clinical doses, an effect of major significance for drug safety. Rodriguez et al.37 proposed that computer modelling of multichannel affecting drugs, such as ranolazine, could be a testbed for determining the utility of new or previously rejected compounds or drug combination approaches, with modelling as a force against rigid standards, and toward rational, more holistic drug candidate selection. Fundamentally, this is the modelling approach of Sarkar and Sobie38 whose recent article explores basic mechanisms by which interrelated model parameters contribute to the consequence of IKr block, a phenomenon known as the ‘repolarization reserve’.39 The article makes the important discovery that subtle changes in ion channel substrate can have profound and indirect effects on the response to drugs. Simulating the subtle differences between species40 and the effects of sex hormones41,42 also demonstrated changes in drug block effects. Personalized medicine requires clear delineation of the subtle interspecies and inter-individual differences, which determine outcomes; this delineation is made possible in part by mechanistic simulation.
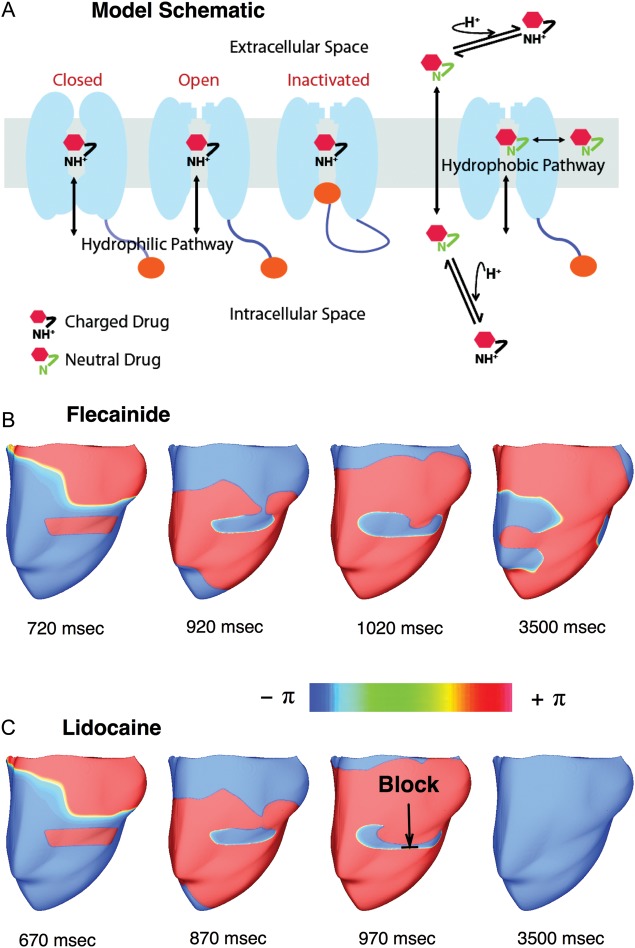
Relating effects of drugs on ion channels beyond the AP requires virtual tissue or whole heart organ simulation, to examine arrhythmia onset, termination and prevention. Recently, Benson et al.43 related the effects of d-sotalol, an IKr blocker, and amiodarone, a complex multichannel effector, to arrhythmia formation in the heterogenous canine ventricular wedge. An emergent finding was the understanding of how the vulnerable window is enhanced by d-sotalol, but reduced by amiodarone due to different effects of the drugs on different cell types. Whereas the drug models used by Benson et al. were implemented by simple conduction scaling, a new study by Moreno et al.44 has incorporated both state-dependent Markov modelling of drug effects and full integration to the human AP, human tissue, and finally realistic MRI-based human heart. This is the first instance of such massive integration across the space and time scales at play. Moreno et al. showed that the effects of flecainide and lidocaine on INa block are globally similar in response to dynamic protocols. However, clinical trials have shown previously that flecainide tended to be pro-arrhythmic at therapeutic doses, while lidocaine was not. Moreno et al. results make clear that neither simple reduction in sodium conductance, nor single cell simulation can resolve this paradox. At the macroscopic scale, the vulnerable window was greater for flecainide than for lidocaine (especially in heart failure simulations due to shortened diastole) and reentrant arrhythmia in the ventricle persisted (Figure 4). At the microscopic scale, Markov models explained that this was due to the relatively slow accumulation of and recovery from use-dependent block with flecainide.
Figure 4.
Drug-related arrhythmias. (A) Schematic for drug binding to sodium channels. Maps of the phase variable in (B) Sustained figure-of-eight reentry with 2 μM flecainide, and (C) Non-sustained reentry with 20 μM lidocaine, following premature stimuli (S2). Sustained reentry occurred when applying S2 within the vulnerable window (VW) of the model with 2 μM flecainide (VW = 660–735 ms), but not for the model with 20 μM lidocaine (VW = 630 –685 ms). Modified with permission from ref.44
Biophysically detailed computational modelling of novel defibrillation therapies
Controlling the complex spatio-temporal dynamics underlying life-threatening cardiac arrhythmias such as fibrillation is extremely difficult because of the non-linear interaction of excitation waves in a heterogeneous anatomical substrate. In the absence of a better strategy, strong electrical shocks have remained the only reliable treatment for ventricular fibrillation. Over the years, biophysically detailed multi-scale models of defibrillation have made major contributions to understanding how defibrillation shocks used in clinical practice interact with cardiac tissue.45,46 Recently, however, defibrillation modelling has focused on the development of new methodologies for low-voltage termination of lethal arrhythmias or for applying defibrillation in novel, less damaging ways.
Based on simulations of the response of the myocardium to shocks,47 the study by Luther et al.48 offered a method for low-voltage control of atrial fibrillation using a sequence of low-energy electric field pulses. Mechanistically, following a series of low-voltage shocks, heterogeneities in the myocardium become sources of activation; the many pulses of the field recruit many such activation sites. The study demonstrated that the geometric structure of the coronary vasculature (one type of heterogeneity in the heart) allowed for the recruitment of a correspondingly dense and widespread series of wave sources inside the myocardium. These distributed wave sources acted as non-invasive, intramural multi-site pacing, which effectively terminated the wavefronts of fibrillation.
A recent novel approach to defibrillation also relied on biophysically detailed modelling of heart electrophysiology. The study by Tandri et al.,49 was based on the premise that sustained kilohertz-range alternating current (AC) fields have been known to instantaneous and reversibly block electrical conduction in nerve tissue. The article provided proof of the concept that electric fields, such as those used for neural block, when applied to cardiac tissue, similarly produce reversible block of cardiac impulse propagation and lead to successful defibrillation, and that this methodology could potentially be a safer means for terminating life-threatening reentrant arrhythmias. The data revealed a previously unrecognized capacity for myocardial cells to be placed in an extended but immediately reversible state of refractoriness by an applied electric field. The imposed refractory state blocked all wave propagation and resulted in termination of reentrant arrhythmias, without impairment of subsequent cellular electrical function or initiation of post-shock fibrillatory activity. Since the same AC fields block neural and cardiac activity equally well, the proposed defibrillation methodology could possibly be utilized to achieve high-voltage yet painless defibrillation.
Biophysically detailed computational modelling of the heart in risk stratification for arrhythmias
Robust methods for stratifying the risk of lethal cardiac arrhythmias decrease morbidity and mortality in patients with cardiovascular disease and reduce health care costs.50 The most widely used approaches currently used for stratifying risk of cardiac arrhythmias involve testing for abnormalities in the ECG, then using the results to identify patients who would benefit from Implantable Cardioverter-Defibrillator (ICD) therapy. Electrocardiogram-based risk stratification methods scan for abnormalities in ventricular depolarization (late potentials51 and a fractionated QRS complex52) and repolarization (T-wave alternans,53 QT variability or dispersion54,55). However, the mechanisms underlying these ECG indices, and their relationship to lethal cardiac arrhythmias, are not fully understood. This lack of knowledge likely explains why results of clinical trials to correlate surface EGG indices to lethal cardiac arrhythmias are often contradictory.50 Computational models of the heart have made inroads in this clinical cardiology arena.43,56–62
Research has reported a strong correlation between increased arrhythmia risk and the presence of T-wave alternans.63,64 In the clinical setting, testing for microvolt T-wave alternans (MTWA) has particularly shown promise for dichotomizing patients that would and would not benefit from ICD therapy.65,66 However, the mechanistic basis of MTWA preceding lethal ventricular arrhythmias has been under debate. Until recently, it was believed that a steep action potential duration (APD) restitution (>1) at rapid heart rates67 produces alternans in APD that underlie T-wave alternans and the genesis of fibrillation.68 However, MTWA is most successful in stratifying risk in patients at heart rates <110 b.p.m., where APD restitution is flat.69 Computational models of the LV wall in combination with clinical data revealed that abnormal handing of intracellular calcium underlies alternans in action potential voltage, which result in MTWA at heart rates <110 b.p.m;56,57 abnormalities in intracellular calcium have long been linked to ventricular fibrillation.70,71 Computational modelling studies have also shown that under the conditions of abnormal calcium dynamics, T-wave alternans magnitude is enhanced by structural heterogeneities in the myocardium.58
A recent study used an MRI-based computational model of the human ventricles to demonstrate that detecting instabilities in the QT interval in clinical ECGs can predict VT onset, particularly in patients with acute myocardial infarction.62 Thanks to the ease with which the frequency and degree of premature activations could be controlled in the model, the study found that increased frequency of premature activation can precede the onset of VT, with premature activations placing the system in a state where the QT interval is unstable. Therefore, screening the QT interval of the ECG for instabilities using the novel algorithm developed by Chen and Trayanova72 could potentially be a robust risk stratification method for patients with acute myocardial infarction. These studies pave the way for executing computer simulations to determine patient-specific thresholds for arrhythmia stratification ECG indices, rather than relying on clinical guidelines based on large and diverse patient cohorts. Another approach for stratifying the risk of lethal cardiac arrhythmias that has recently gained traction is the use of computer models to predict arrhythmia outcome in patients that exhibit potentially lethal mutations in genes encoding cardiac proteins associated with long-QT syndrome.43,59–61 These studies chart new directions for future genotype-based risk stratification and personalized gene therapy.
Inverse problem in electrocardiography: computational modelling of the heart as a diagnostic tool
Since the development of the ECG over a century ago, clinicians have sought to gain insight into the spatiotemporal patterns of electrical activation in the heart by interpreting non-invasively recorded signals. Inverse electrocardiography extends this inquiry, combining body surface electrograms and patient-specific anatomical data with state-of-the-art computational techniques. From a mathematical standpoint, the reconstruction of signals in the domain of the heart is ill-posed, in that it can easily be corrupted by low-amplitude electric noise or minute positional errors.
The application of inverse electrocardiography in humans has been led by the Rudy lab, whose electrocardiographic imaging (ECGI) method computes epicardial extracellular potential distributions. ECGI has been performed on Wolff–Parkinson–White (WPW) patients before and after accessory pathway ablation,73 to characterize the size and extent of scar in post-myocardial infarction patients,74 to identify responders to CRT therapy,75 and to non-invasively map infarct-related ventricular arrhythmias.76 Finally, the technique was used in a wide variety of AF patients to demonstrate that multiple AF mechanisms (isthmus propagation, macroscopic reentry, multiple coexisting wavelets, and wave break) often occur simultaneously in the same patient.77
Studies by the teams of He, Oosterom, Berger, and Kalinin78–82 have advanced non-invasive electrocardiographic imaging beyond mapping of epicardial electrograms to intramural or endocardial electrical activity. The inverse methodology by the He team reconstructs an equivalent current density field throughout the ventricles, from which 3D maps of activation sequence are generated; while it has not been yet utilized in patient studies, the technique was able to accurately pinpoint sources of endocardial ectopy in pigs83 and to image activation sequences during pacing and VT in both rabbits84 and dogs.85 Kalinin's approach82 to map epicardial and endocardial electrograms has been applied in 200 patients in Russian hospitals.86 Similarly, the methodology developed by Berger et al.81 has been used to identify pre-excitation sites in WPW patients and to provide detail on simultaneous endocardial and epicardial activation sequences during CRT.79
Concluding remarks
Modern cardiac research has increasingly recognized that appropriate models and simulation can help interpret an array of experimental data and dissect important mechanisms and relationships. Advances in computer modelling have made major contributions to the understanding of cardiac function in health and disease. This review focuses on another aspect of computational modelling of the heart: its contribution towards improving the clinical practice of cardiology. The augmented role of cardiac modelling in the development of new therapies for cardiac dysfunction and diagnostic modalities arises from its function as the framework that unifies diverse cardiac electrophysiology and electromechanics insight. Multiscale, multiphysics models that incorporate electromechanical and structural remodelling in cardiac disease are well poised to become a first line of screening for new therapies and approaches, including pharmacological intervention. Furthermore, riding on the heels of diagnostic developments that stem from mathematical modelling and simulation will be new approaches to patient screening and diagnosis. The journey has begun.
Conflict of interest: N.A.T. is cofounder of Cardiosolv LLC. Cardiosolv was not involved in this research. Other authors: none declared.
Funding
This research was supported by NIH R01 award HL103428 and NSF awards CBET-0933029 and CDI-1124804 to NAT; Ruth L. Kirschstein National Research Service Predoctoral Awards HL099275 (to HJA) and HL103090 (to JC); AHA Predoctoral Fellowship 10PRE3650037 (to JDB); and a postdoctoral fellowship from the Natural Sciences and Engineering Research Council of Canada (to PMB).
References
- 1.Noble D. Modeling the heart – from genes to cells to the whole organ. Science. 2002;295:1678–82. doi: 10.1126/science.1069881. [DOI] [PubMed] [Google Scholar]
- 2.Trayanova NA. Whole-heart modeling: applications to cardiac electrophysiology and electromechanics. Circ Res. 2011;108:113–28. doi: 10.1161/CIRCRESAHA.110.223610. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Vadakkumpadan F, Arevalo H, Prassl AJ, Chen J, Kickinger F, Kohl P, et al. Image-based models of cardiac structure in health and disease. Wiley Interdiscip Rev Syst Biol Med. 2010;2:489–506. doi: 10.1002/wsbm.76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bishop MJ, Plank G, Burton RA, Schneider JE, Gavaghan DJ, Grau V, et al. Development of an anatomically detailed MRI-derived rabbit ventricular model and assessment of its impact on simulations of electrophysiological function. Am J Physiol Heart Circ Physiol. 2010;298:H699–718. doi: 10.1152/ajpheart.00606.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Stevenson WG, Brugada P, Waldecker B, Zehender M, Wellens HJ. Clinical, angiographic, and electrophysiologic findings in patients with aborted sudden death as compared with patients with sustained ventricular tachycardia after myocardial infarction. Circulation. 1985;71:1146–52. doi: 10.1161/01.cir.71.6.1146. [DOI] [PubMed] [Google Scholar]
- 6.Pop M, Sermesant M, Mansi T, Crystal E, Ghate S, Peyrat J, et al. Correspondence between simple 3-D MRI-based computer models and in-vivo EP measurements in swine with chronic infarctions. IEEE Trans Biomed Eng., 2011;58:3483–6. doi: 10.1109/TBME.2011.2168395. [DOI] [PubMed] [Google Scholar]
- 7.Arevalo H, Plank G, Helm P, Halperin H, Trayanova N. Volume of peri-infarct zone determines arrhythmogenesis in infarcted heart. Heart Rhythm. 2009;6:S232–3. [Google Scholar]
- 8.Ashikaga H, Arevalo H, Vadakkumpadan F, Blake R, Berger R, Calkins H, et al. MRI-based patient-specific virtual electrophysiology laboratory for scar-related ventricular tachycardia. Circulation. 2011;124:A541. [Google Scholar]
- 9.Relan J, Chinchapatnam P, Sermesant M, Rhode K, Ginks M, Delingette H, et al. Coupled personalization of cardiac electrophysiology models for prediction of ischaemic ventricular tachycardia. J. R. Soc. Interface Focus. 2011;1:396–407. doi: 10.1098/rsfs.2010.0041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Virag N, Jacquemet V, Henriquez CS, Zozor S, Blanc O, Vesin JM, et al. Study of atrial arrhythmias in a computer model based on magnetic resonance images of human atria. Chaos. 2002;12:754–63. doi: 10.1063/1.1483935. [DOI] [PubMed] [Google Scholar]
- 11.Di Martino ES, Bellini C, Schwartzman DS. In vivo porcine left atrial wall stress: computational model. J Biomech. 2011;44:2589–94. doi: 10.1016/j.jbiomech.2011.08.023. [DOI] [PubMed] [Google Scholar]
- 12.Jacquemet V, van Oosterom A, Vesin JM, Kappenberger L. Analysis of electrocardiograms during atrial fibrillation. A biophysical model approach. IEEE Eng Med Biol Mag. 2006;25:79–88. doi: 10.1109/emb-m.2006.250511. [DOI] [PubMed] [Google Scholar]
- 13.Vigmond EJ, Leon LJ. Electrophysiological basis of mono-phasic action potential recordings. Med Biol Eng Comput. 1999;37:359–65. doi: 10.1007/BF02513313. [DOI] [PubMed] [Google Scholar]
- 14.Vigmond EJ, Tsoi V, Yin Y, Page P, Vinet A. Estimating atrial action potential duration from electrograms. IEEE Trans Biomed Eng. 2009;56:1546–55. doi: 10.1109/TBME.2009.2014740. [DOI] [PubMed] [Google Scholar]
- 15.Jacquemet V, Henriquez CS. Genesis of complex fractionated atrial electrograms in zones of slow conduction: a computer model of microfibrosis. Heart Rhythm. 2009;6:803–10. doi: 10.1016/j.hrthm.2009.02.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Krueger MW, Severi S, Rhode K, Genovesi S, Weber FM, Vincenti A, et al. Alterations of atrial electrophysiology related to hemodialysis session: insights from a multiscale computer model. J Electrocardiol. 2011;44:176–83. doi: 10.1016/j.jelectrocard.2010.11.016. [DOI] [PubMed] [Google Scholar]
- 17.Lim KM, Hong SB, Jeon JW, Gyung MS, Ko BH, Bae SK, et al. Predicting the optimal position and direction of a ubiquitous ECG using a multi-scale model of cardiac electrophysiology. Conf Proc IEEE Eng Med Biol Soc. 2011;2011:993–6. doi: 10.1109/IEMBS.2011.6090230. [DOI] [PubMed] [Google Scholar]
- 18.MacLeod RS, Blauer J, Kholmovski E, Ranjan R, Marrouche N, Trayanova NA, et al. Subject specific, image based analysis and modeling in patients with atrial fibrillation from MRI. 2012 9th IEEE International Symposium on Biomedical Imaging (ISBI), p. 1364. http://ieeexplore.ieee.org/xpls/abs_all.jsp?arnumber=6235820 . [Google Scholar]
- 19.Gurev V, Lee T, Constantino J, Arevalo H, Trayanova NA. Models of cardiac electromechanics based on individual hearts imaging data: image-based electromechanical models of the heart. Biomech Model Mechanobiol. 2011;10:295–306. doi: 10.1007/s10237-010-0235-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Kerckhoffs R, McCulloch A, Omens J, Mulligan L. Effect of pacing site and infarct location on regional mechanics and global hemodynamics in a model based study of heart failure. Lect Notes Comput Sci. 2007;4466:350–60. [Google Scholar]
- 21.Kerckhoffs RC, McCulloch AD, Omens JH, Mulligan LJ. Effects of biventricular pacing and scar size in a computational model of the failing heart with left bundle branch block. Med Image Anal. 2009;13:362–9. doi: 10.1016/j.media.2008.06.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Niederer SA, Plank G, Chinchapatnam P, Ginks M, Lamata P, Rhode KS, et al. Length-dependent tension in the failing heart and the efficacy of cardiac resynchronization therapy. Cardiovasc Res. 2011;89:336–43. doi: 10.1093/cvr/cvq318. [DOI] [PubMed] [Google Scholar]
- 23.Kerckhoffs RC, Lumens J, Vernooy K, Omens JH, Mulligan LJ, Delhaas T, et al. Cardiac resynchronization: insight from experimental and computational models. Prog Biophys Mol Biol. 2008;97:543–61. doi: 10.1016/j.pbiomolbio.2008.02.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Niederer SA, Shetty AK, Plank G, Bostock J, Razavi R, Smith NP, et al. Biophysical modeling to simulate the response to multisite left ventricular stimulation using a quadripolar pacing lead. Pacing Clin Electrophysiol. 2012;35:204–214. doi: 10.1111/j.1540-8159.2011.03243.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Constantino J, Hu Y, Trayanova NA. A computational approach to understanding the cardiac electromechanical activation sequence in the normal and failing heart, with translation to the clinical practice of CRT. Prog Biophys Mol Biol. 2012 doi: 10.1016/j.pbiomolbio.2012.07.009. http://www.sciencedirect.com/science/article/pii/S0079610712000545 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Aguado-Sierra J, Krishnamurthy A, Villongco C, Chuang J, Howard E, Gonzales MJ, et al. Patient-specific modeling of dyssynchronous heart failure: a case study. Prog Biophys Mol Biol. 2011;107:147–55. doi: 10.1016/j.pbiomolbio.2011.06.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Sermesant M, Chabiniok R, Chinchapatnam P, Mansi T, Billet F, Moireau P, et al. Patient-specific electromechanical models of the heart for the prediction of pacing acute effects in CRT: a preliminary clinical validation. Med Image Anal. 2012;16:201–15. doi: 10.1016/j.media.2011.07.003. [DOI] [PubMed] [Google Scholar]
- 28.Schwartz PJ, Priori SG, Spazzolini C, Moss AJ, Vincent GM, Napolitano C, et al. Genotype-phenotype correlation in the long-QT syndrome: gene-specific triggers for life-threatening arrhythmias. Circulation. 2001;103:89–95. doi: 10.1161/01.cir.103.1.89. [DOI] [PubMed] [Google Scholar]
- 29.Perry M, Sachse FB, Sanguinetti MC. Structural basis of action for a human ether-a-go-go-related gene 1 potassium channel activator. Proc Natl Acad Sci USA. 2007;104:13827–32. doi: 10.1073/pnas.0703934104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Sale H, Wang J, O'Hara TJ, Tester DJ, Phartiyal P, He JQ, et al. Physiological properties of hERG 1a/1b heteromeric currents and a hERG 1b-specific mutation associated with Long-QT syndrome. Circ Res. 2008;103:e81–95. doi: 10.1161/CIRCRESAHA.108.185249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Spector PS, Curran ME, Keating MT, Sanguinetti MC. Class III antiarrhythmic drugs block HERG, a human cardiac delayed rectifier K+ channel. Open-channel block by methanesulfonanilides. Circ Res. 1996;78:499–503. doi: 10.1161/01.res.78.3.499. [DOI] [PubMed] [Google Scholar]
- 32.Clancy CE, Zhu ZI, Rudy Y. Pharmacogenetics and anti-arrhythmic drug therapy: a theoretical investigation. Am J Physiol Heart Circ Physiol. 2007;292:H66–75. doi: 10.1152/ajpheart.00312.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Nesterenko VV, Zygmunt AC, Rajamani S, Belardinelli L, Antzelevitch C. Mechanisms of atrial-selective block of Na channels by ranolazine: II. Insights from a mathematical model. Am J Physiol Heart Circ Physiol. 2011;301:H1615–24. doi: 10.1152/ajpheart.00243.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Antzelevitch C, Belardinelli L, Zygmunt AC, Burashnikov A, Di Diego JM, Fish JM, et al. Electrophysiological effects of ranolazine, a novel antianginal agent with antiarrhythmic properties. Circulation. 2004;110:904–10. doi: 10.1161/01.CIR.0000139333.83620.5D. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Burashnikov A, Di Diego JM, Zygmunt AC, Belardinelli L, Antzelevitch C. Atrium-selective sodium channel block as a strategy for suppression of atrial fibrillation: differences in sodium channel inactivation between atria and ventricles and the role of ranolazine. Circulation. 2007;116:1449–57. doi: 10.1161/CIRCULATIONAHA.107.704890. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Morita N, Lee JH, Xie Y, Sovari A, Qu Z, Weiss JN, et al. Suppression of re-entrant and multifocal ventricular fibrillation by the late sodium current blocker ranolazine. J Am Coll Cardiol. 2011;57:366–75. doi: 10.1016/j.jacc.2010.07.045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Rodriguez B, Burrage K, Gavaghan D, Grau V, Kohl P, Noble D. The systems biology approach to drug development: application to toxicity assessment of cardiac drugs. Clin Pharmacol Ther. 2010;88:130–4. doi: 10.1038/clpt.2010.95. [DOI] [PubMed] [Google Scholar]
- 38.Sarkar AX, Sobie EA. Regression analysis for constraining free parameters in electrophysiological models of cardiac cells. PLoS Comput Biol. 2010;6:e1000914. doi: 10.1371/journal.pcbi.1000914. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Roden DM, Yang T. Protecting the heart against arrhythmias: potassium current physiology and repolarization reserve. Circulation. 2005;112:1376–8. doi: 10.1161/CIRCULATIONAHA.105.562777. [DOI] [PubMed] [Google Scholar]
- 40.O'Hara T, Rudy Y. Quantitative comparison of cardiac ventricular myocyte electrophysiology and response to drugs in human and non-human species. Am J Physiol Heart Circ Physiol. 2011;302:H1023–H1030. doi: 10.1152/ajpheart.00785.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Nakamura H, Kurokawa J, Bai CX, Asada K, Xu J, Oren RV, et al. Progesterone regulates cardiac repolarization through a nongenomic pathway: an in vitro patch-clamp and computational modeling study. Circulation. 2007;116:2913–22. doi: 10.1161/CIRCULATIONAHA.107.702407. [DOI] [PubMed] [Google Scholar]
- 42.Yang PC, Kurokawa J, Furukawa T, Clancy CE. Acute effects of sex steroid hormones on susceptibility to cardiac arrhythmias: a simulation study. PLoS Comput Biol. 2010;6:e1000658. doi: 10.1371/journal.pcbi.1000658. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Benson AP, Al-Owais M, Holden AV. Quantitative prediction of the arrhythmogenic effects of de novo hERG mutations in computational models of human ventricular tissues. Eur Biophys J. 2011;40:627–39. doi: 10.1007/s00249-010-0663-2. [DOI] [PubMed] [Google Scholar]
- 44.Moreno JD, Zhu ZI, Yang PC, Bankston JR, Jeng MT, Kang C, et al. A computational model to predict the effects of class I anti-arrhythmic drugs on ventricular rhythms. Sci Transl Med. 2011;3:98ra83. doi: 10.1126/scitranslmed.3002588. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Ashihara T, Constantino J, Trayanova NA. Tunnel propagation of postshock activations as a hypothesis for fibrillation induction and isoelectric window. Circ Res. 2008;102:737–45. doi: 10.1161/CIRCRESAHA.107.168112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Constantino J, Long Y, Ashihara T, Trayanova NA. Tunnel propagation following defibrillation with ICD shocks: hidden postshock activations in the left ventricular wall underlie isoelectric window. Heart Rhythm. 2010;7:953–61. doi: 10.1016/j.hrthm.2010.03.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Pumir A, Nikolski V, Horning M, Isomura A, Agladze K, Yoshikawa K, et al. Wave emission from heterogeneities opens a way to controlling chaos in the heart. Phys Rev Lett. 2007;99:208101. doi: 10.1103/PhysRevLett.99.208101. [DOI] [PubMed] [Google Scholar]
- 48.Luther S, Fenton FH, Kornreich BG, Squires A, Bittihn P, Hornung D, et al. Low-energy control of electrical turbulence in the heart. Nature. 2011;475:235–9. doi: 10.1038/nature10216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Tandri H, Weinberg SH, Chang KC, Zhu R, Trayanova NA, Tung L, et al. Reversible cardiac conduction block and defibrillation with high-frequency electric field. Sci Transl Med. 2011;3:102ra96. doi: 10.1126/scitranslmed.3002445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Goldberger JJ, Buxton AE, Cain M, Costantini O, Exner DV, Knight BP, et al. Risk stratification for arrhythmic sudden cardiac death: identifying the roadblocks. Circulation. 2011;123:2423–30. doi: 10.1161/CIRCULATIONAHA.110.959734. [DOI] [PubMed] [Google Scholar]
- 51.Kuchar DL, Thorburn CW, Sammel NL. Prediction of serious arrhythmic events after myocardial infarction: signal-averaged electrocardiogram, Holter monitoring and radionuclide ventriculography. J Am Coll Cardiol. 1987;9:531–8. doi: 10.1016/s0735-1097(87)80045-1. [DOI] [PubMed] [Google Scholar]
- 52.Das MK, Khan B, Jacob S, Kumar A, Mahenthiran J. Significance of a fragmented QRS complex versus a Q wave in patients with coronary artery disease. Circulation. 2006;113:2495–501. doi: 10.1161/CIRCULATIONAHA.105.595892. [DOI] [PubMed] [Google Scholar]
- 53.Rosenbaum DS, Jackson LE, Smith JM, Garan H, Ruskin JN, Cohen RJ. Electrical alternans and vulnerability to ventricular arrhythmias. N Engl J Med. 1994;330:235–41. doi: 10.1056/NEJM199401273300402. [DOI] [PubMed] [Google Scholar]
- 54.Berger RD, Kasper EK, Baughman KL, Marban E, Calkins H, Tomaselli GF. Beat-to-beat QT interval variability: novel evidence for repolarization lability in ischemic and nonischemic dilated cardiomyopathy. Circulation. 1997;96:1557–65. doi: 10.1161/01.cir.96.5.1557. [DOI] [PubMed] [Google Scholar]
- 55.Couderc JP, Zareba W, McNitt S, Maison-Blanche P, Moss AJ. Repolarization variability in the risk stratification of MADIT II patients. Europace. 2007;9:717–23. doi: 10.1093/europace/eum131. [DOI] [PubMed] [Google Scholar]
- 56.Narayan SM, Bayer JD, Lalani G, Trayanova NA. Action potential dynamics explain arrhythmic vulnerability in human heart failure: a clinical and modeling study implicating abnormal calcium handling. J Am Coll Cardiol. 2008;52:1782–92. doi: 10.1016/j.jacc.2008.08.037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Bayer JD, Narayan SM, Lalani GG, Trayanova NA. Rate-dependent action potential alternans in human heart failure implicates abnormal intracellular calcium handling. Heart Rhythm. 2010;7:1093–101. doi: 10.1016/j.hrthm.2010.04.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Doshi AN, Idriss SF. Effect of resistive barrier location on the relationship between T-wave alternans and cellular repolarization alternans: a 1-D modeling study. J Electrocardiol. 2010;43:566–71. doi: 10.1016/j.jelectrocard.2010.07.019. [DOI] [PubMed] [Google Scholar]
- 59.Zhao JT, Hill AP, Varghese A, Cooper AA, Swan H, Laitinen-Forsblom PJ, et al. Not all hERG pore domain mutations have a severe phenotype: G584S has an inactivation gating defect with mild phenotype compared to G572S, which has a dominant negative trafficking defect and a severe phenotype. J Cardiovasc Electrophysiol. 2009;20:923–30. doi: 10.1111/j.1540-8167.2009.01468.x. [DOI] [PubMed] [Google Scholar]
- 60.Jons C, O-Uchi J, Moss AJ, Reumann M, Rice JJ, Goldenberg I, et al. Use of mutant-specific ion channel characteristics for risk stratification of long QT syndrome patients. Sci Transl Med. 2011;3:76ra28. doi: 10.1126/scitranslmed.3001551. [DOI] [PubMed] [Google Scholar]
- 61.O'Hara T, Rudy Y. Arrhythmia formation in subclinical (‘silent’) long QT syndrome requires multiple insults: quantitative mechanistic study using the KCNQ1 mutation Q357R as example. Heart Rhythm. 2012;9:275–82. doi: 10.1016/j.hrthm.2011.09.066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Chen X, Hu Y, Fetics BJ, Berger RD, Trayanova NA. Unstable QT interval dynamics precedes ventricular tachycardia onset in patients with acute myocardial infarction: a novel approach to detect instability in QT interval dynamics from clinical ECG. Circ Arrhythm Electrophysiol. 2011;4:858–66. doi: 10.1161/CIRCEP.110.961763. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Narayan SM. T-wave alternans and the susceptibility to ventricular arrhythmias. J Am Coll Cardiol. 2006;47:269–81. doi: 10.1016/j.jacc.2005.08.066. [DOI] [PubMed] [Google Scholar]
- 64.Qu Z, Xie Y, Garfinkel A, Weiss JN. T-wave alternans and arrhythmogenesis in cardiac diseases. Front Physiol. 2010;1:154. doi: 10.3389/fphys.2010.00154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Bloomfield DM, Bigger JT, Steinman RC, Namerow PB, Parides MK, Curtis AB, et al. Microvolt T-wave alternans and the risk of death or sustained ventricular arrhythmias in patients with left ventricular dysfunction. J Am Coll Cardiol. 2006;47:456–63. doi: 10.1016/j.jacc.2005.11.026. [DOI] [PubMed] [Google Scholar]
- 66.Hohnloser SH, Ikeda T, Cohen RJ. Evidence regarding clinical use of microvolt T-wave alternans. Heart Rhythm. 2009;6:S36–44. doi: 10.1016/j.hrthm.2008.10.011. [DOI] [PubMed] [Google Scholar]
- 67.Weiss JN, Karma A, Shiferaw Y, Chen PS, Garfinkel A, Qu Z. From pulsus to pulseless: the saga of cardiac alternans. Circ Res. 2006;98:1244–53. doi: 10.1161/01.RES.0000224540.97431.f0. [DOI] [PubMed] [Google Scholar]
- 68.Pastore JM, Girouard SD, Laurita KR, Akar FG, Rosenbaum DS. Mechanism linking T-wave alternans to the genesis of cardiac fibrillation. Circulation. 1999;99:1385–94. doi: 10.1161/01.cir.99.10.1385. [DOI] [PubMed] [Google Scholar]
- 69.Narayan SM, Franz MR, Lalani G, Kim J, Sastry A. T-wave alternans, restitution of human action potential duration, and outcome. J Am Coll Cardiol. 2007;50:2385–92. doi: 10.1016/j.jacc.2007.10.011. [DOI] [PubMed] [Google Scholar]
- 70.Weiss JN, Nivala M, Garfinkel A, Qu Z. Alternans and arrhythmias: from cell to heart. Circ Res. 2011;108:98–112. doi: 10.1161/CIRCRESAHA.110.223586. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Merchant FM, Armoundas AA. Role of substrate and triggers in the genesis of cardiac alternans, from the myocyte to the whole heart: implications for therapy. Circulation. 2012;125:539–49. doi: 10.1161/CIRCULATIONAHA.111.033563. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Chen X, Trayanova NA. A novel methodology for assessing the bounded-input bounded-output instability in QT interval dynamics: application to clinical ECG with ventricular tachycardia. IEEE Trans Biomed Eng. 2011;59:2111–2117. doi: 10.1109/TBME.2011.2170837. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Ghosh S, Rhee EK, Avari JN, Woodard PK, Rudy Y. Cardiac memory in patients with Wolff-Parkinson-White syndrome: noninvasive imaging of activation and repolarization before and after catheter ablation. Circulation. 2008;118:907–15. doi: 10.1161/CIRCULATIONAHA.108.781658. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Cuculich PS, Zhang J, Wang Y, Desouza KA, Vijayakumar R, Woodard PK, et al. The electrophysiological cardiac ventricular substrate in patients after myocardial infarction: noninvasive characterization with electrocardiographic imaging. J Am Coll Cardiol. 2011;58:1893–902. doi: 10.1016/j.jacc.2011.07.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Ghosh S, Silva JN, Canham RM, Bowman TM, Zhang J, Rhee EK, et al. Electrophysiologic substrate and intraventricular left ventricular dyssynchrony in nonischemic heart failure patients undergoing cardiac resynchronization therapy. Heart Rhythm. 2011;8:692–9. doi: 10.1016/j.hrthm.2011.01.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Wang Y, Cuculich PS, Zhang J, Desouza KA, Vijayakumar R, Chen J, et al. Noninvasive electroanatomic mapping of human ventricular arrhythmias with electrocardiographic imaging. Sci Transl Med. 2011;3:98ra84. doi: 10.1126/scitranslmed.3002152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Cuculich PS, Wang Y, Lindsay BD, Faddis MN, Schuessler RB, Damiano RJ, Jr, et al. Noninvasive characterization of epicardial activation in humans with diverse atrial fibrillation patterns. Circulation. 2010;122:1364–72. doi: 10.1161/CIRCULATIONAHA.110.945709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.van Dam PM, Oostendorp TF, Linnenbank AC, van Oosterom A. Non-invasive imaging of cardiac activation and recovery. Ann Biomed Eng. 2009;37:1739–56. doi: 10.1007/s10439-009-9747-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Berger T, Pfeifer B, Hanser FF, Hintringer F, Fischer G, Netzer M, et al. Single-veat noninvasive imaging of ventricular endocardial and epicardial activation in patients undergoing CRT. PLoS One. 2011;6:e16255. doi: 10.1371/journal.pone.0016255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Han C, Liu C, Pogwizd S, He B. Noninvasive three-dimensional cardiac activation imaging on a rabbit model. Conf Proc IEEE Eng Med Biol Soc. 2009;2009:3271–3. doi: 10.1109/IEMBS.2009.5333511. [DOI] [PubMed] [Google Scholar]
- 81.Berger T, Fischer G, Pfeifer B, Modre R, Hanser F, Trieb T, et al. Single-beat noninvasive imaging of cardiac electrophysiology of ventricular pre-excitation. J Am Coll Cardiol. 2006;48:2045–52. doi: 10.1016/j.jacc.2006.08.019. [DOI] [PubMed] [Google Scholar]
- 82.Kalinin A. Iterative algorithm for the inverse problem of electrocardiography in a medium with piecewise-constant electrical conductivity. Comput Math Model. 2011;22:30–4. [Google Scholar]
- 83.Lai D, Liu C, Eggen MD, Iaizzo PA, He B. Localization of endocardial ectopic activity by means of noninvasive endocardial surface current density reconstruction. Phys Med Biol. 2011;56:4161–76. doi: 10.1088/0031-9155/56/13/027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Han C, Pogwizd SM, Killingsworth CR, He B. Noninvasive imaging of three-dimensional cardiac activation sequence during pacing and ventricular tachycardia. Heart Rhythm. 2011;8:1266–72. doi: 10.1016/j.hrthm.2011.03.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Han C, Pogwizd SM, Killingsworth CR, He B. Noninvasive reconstruction of the three-dimensional ventricular activation sequence during pacing and ventricular tachycardia in the canine heart. Am J Physiol Heart Circ Physiol. 2012;302:H244–52. doi: 10.1152/ajpheart.00618.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Bokeriia LA, Revishvili A, Kalinin AV, Kalinin VV, Liadzhina OA, Fetisova EA. Hardware-software system for noninvasive electrocardiographic examination of heart based on inverse problem of electrocardiography. Med Tekh. 2008;6:1–7. [PubMed] [Google Scholar]