Abstract
Aims
The aims of the study are to develop a cost-minimization analysis from the hospital perspective and a cost-effectiveness analysis from the third payer standpoint, based on direct estimates of costs and QOL associated with remote follow-ups, using Merlin@home and Merlin.net, compared with standard ambulatory follow-ups, in the management of ICD and CRT-D recipients.
Methods and results
Remote monitoring systems can replace ambulatory follow-ups, sparing human and economic resources, and increasing patient safety. TARIFF is a prospective, controlled, observational study aimed at measuring the direct and indirect costs and quality of life (QOL) of all participants by a 1-year economic evaluation. A detailed set of hospitalized and ambulatory healthcare costs and losses of productivity that could be directly influenced by the different means of follow-ups will be collected. The study consists of two phases, each including 100 patients, to measure the economic resources consumed during the first phase, associated with standard ambulatory follow-ups, vs. the second phase, associated with remote follow-ups.
Conclusion
Remote monitoring systems enable caregivers to better ensure patient safety and the healthcare to limit costs. TARIFF will allow defining the economic value of remote ICD follow-ups for Italian hospitals, third payers, and patients. The TARIFF study, based on a cost-minimization analysis, directly comparing remote follow-up with standard ambulatory visits, will validate the cost effectiveness of the Merlin.net technology, and define a proper reimbursement schedule applicable for the Italian healthcare system.
Trial registration: NCT01075516.
Keywords: Telemonitoring, Telemedicine, Remote monitoring, Implantable cardioverter defibrillator, Cardiac resynchronization therapy
Background
Telemonitoring to verify the proper functions of implantable cardioverter defibrillators (ICD) with or without cardiac resynchronization therapy (CRT-D) is becoming a standard means of ambulatory follow-up. It allows the remote home monitoring of patients and spares caregivers' time. The continuous, wireless monitoring of important clinical variables and device functions, and immediate therapeutic responses, might improve the patient's prognosis and quality of life (QOL) and, by lowering the rates of hospitalizations, might represent a significant costs saving for the healthcare system. It might also decrease the utilization of emergency services, or number of non-scheduled ambulatory visits1 and increase the productivity and efficiency of medical facilities.
As several clinical trials have shown that primary prevention with ICD and CRT-D implants increases the survival of patients suffering from heart failure,2–5 it is strongly recommended by the recently updated practice guidelines issued by the European Society of Cardiology.6 Consequently, the rate of device implantation and follow-ups in Italy have grown significantly in recent years.7 In the current clinical practice for CRT-D and ICD recipients, regular follow-ups are recommended every 3 or 6 months,8 although a large proportion of patients, particularly when suffering from heart failure, need additional visits for worsening of their health status. Therefore, ambulatory clinics are at high risk of becoming overloaded and unable to properly follow all patients, thus lowering the quality of healthcare and increasing the waiting time for services.
A solution to this problem is being proposed with the implementation of remote monitoring systems,9 which, besides limiting the number of scheduled follow-ups, might lower the number of emergency visits and hospitalizations by the early detection of arrhythmias and progression of heart failure, thus reducing healthcare costs for patients, caregivers, and hospitals, and alleviating the economic burden imposed on national healthcare systems.10–12 In addition, an early reaction to clinical events might improve clinical outcomes and patient QOL.13–15
The Merlin.net System (St Jude Medical, Sylmar, CA) has been adopted by several medical institutions to enable the remote follow-up of devices and collect diagnostic and device status information via programmed remote transmissions.16–19
As the cost effectiveness of remote monitoring systems has not been widely studied, new evaluations of their economic value are needed. TARIFF is designed to compare direct and indirect costs and benefits of on-site vs. remote follow-ups, including QOL, hospitalizations, and ambulatory visits. Furthermore, the study intends to study the cost and the value of remote monitoring of healthcare services, to help the Italian medical system make decisions regarding their reimbursement and supply to the hospitals.
Design and methods
Remote monitoring system
The Merlin.Net™ PCN is a dedicated, virtual data collection centre, accessible via an Internet site protected by a username and password. The Merlin.Net transfers data collected either on-site or remotely, using the Merlin@home transmitter, which communicates, on a daily basis and without patient interaction, with the implanted devices, notifying every pre-defined clinical and technical issue. The data are automatically transmitted via wireless telemetry between Merlin@home and the implanted devices, and sent to Merlin.net via a standard landline or via the Global System for Mobile Communication network.
The Merlin.Net website is similar to a mailbox interface, where the user can examine recent transmissions or direct alerts. The data collected on Merlin.Net can also be exported to an Electronic Health Record (EHR), for a complete patient data management.
Study design
TARIFF is an observational, prospective, multicenter study designed to compare the costs and benefits of on-site vs. remote follow-ups. This study is conducted at six Italian medical centres and has been approved by the institutional ethics committees of each participating centre. The enrolment of patients began in December 2009 and both enrolment phases will end before the end of 2011.
The purposes of the study are to (i) measure the incremental economic value of remote monitoring of ICD and CRT-D for Italian hospitals, third payers, and patients, and (ii) develop, from the hospital perspective, a cost-minimization and use analysis based on the QOL associated with remote follow-up of ICD and CRT-D, using Merlin@home and Merlin.net, compared with standard, on-site patient care. The primary endpoint of the study is to measure, under real-life circumstances, the incremental costs and benefits incurred by the Italian healthcare system for the implementation of each method of follow-up. The secondary study endpoint is to compare, from the hospital point of view, the incremental costs represented by each method of follow-up, including assessments of QOL, using the EQ-5D questionnaire.20 The patients are enrolled within 2–8 weeks after device implant and followed for 12 months.
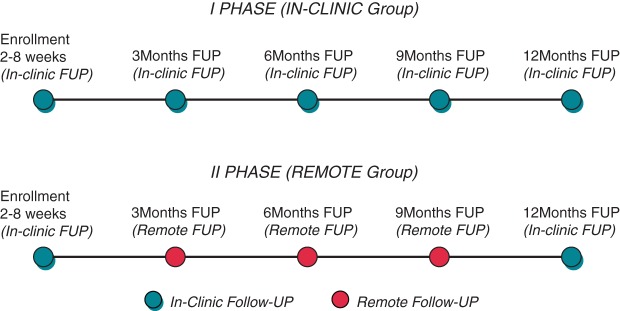
The study is divided between two consecutive phases. In Phase I, ∼100 consecutive patients are followed according to a standard on-site ambulatory follow-up at 3, 6, 9, and 12 months after implantation of a device. In Phase II, another group of 100 consecutive patients are followed remotely. At the enrollment visit, the patients receive the transmitter programmed for remote transmissions at 3, 6, and 9 months after device implant. At 12 months of follow-up, the patients are scheduled to be seen in the ambulatory department. The patients enrolled in Phase II are monitored daily remotely by programmable alerts throughout the 1-year study; each time that the device's parameters are out of the programmed range, an alert message is automatically sent to the clinic or to the physician in charge through e-mail or fax. Each patient is instructed to keep a diary during each 3 months period of follow-up to record information regarding interim hospitalizations, emergency department visits, adverse events, changes in medication regimens, EQ-5D questionnaire, and visits with other healthcare professionals. In Figure 1 the flow chart of the study is summarized.
Figure 1.
Study flow chart.
Patient selection
Patients who meet the following criteria are eligible to participate in the study:
(i) men and non-pregnant women > 18 years of age; (ii) implantation of an ICD or CRT-D for standard indications within 2–8 weeks; (iii) willing and able to be followed by the same medical centre throughout the study; and (iv) capable of understanding and completing the EQ-5D questionnaire.
Follow-up data collection
Eligible patients who meet all the inclusion criteria are enrolled after they have signed a written, informed consent approved by the local ethics committee (LEC). During the enrollment visit, baseline data are collected, including demographics, medical history, estimation of New York Heart Association functional class, drug therapy, QOL assessment, using the EQ-5D questionnaire, economic costs incurred, and productivity loss for patients and caregivers. At each on-site ambulatory visit in Phase I and remote follow-up visit in Phase II, the following data are collected: (i) changes in drug therapy; (ii) interim medical history, medical tests, and examinations performed and any other clinical activity occurring in the last 3 months; and (iii) QOL assessment. At the final 1 year visit, precisely the same follow-up data are collected in both study groups.
All hospitalizations, unscheduled visits, and transmissions triggered by the patient or by the physician, and all adverse events are recorded on dedicated case report forms and referred to the LEC as required.
Data analysis
All costs, clinical outcome data, and QOL assessments recorded in both study phases are measured and compared. The monetary values of variables are assessed, when appropriate. If, globally, telemonitoring is found advantageous and less expensive for the patient than standard on-site, ambulatory follow-up, it is defined as the dominant method.
Therefore, the incremental cost-effectiveness or cost–utility ratio is calculated in order to estimate the incremental cost per effectiveness additional unit.
If the efficacy and clinical outcomes are similar with both methods, the analysis will be limited to a comparison of costs, in order to identify the least expensive treatments.
Economic evaluation: resource utilization and costs
Besides clinical outcomes, resource utilization and QOL data are collected on each patient. The collection of resource utilization includes (i) hospitalizations; (ii) emergency department, family physician, specialist, ambulatory department, and other healthcare professional visits; (iii) medical tests and procedures, and surgical interventions; and (iv) names and dosages of prescriptions and over-the-counter medications, as recorded in the patient expense log. Information regarding loss of productivity is estimated separately as days lost from (i) paid employment of the patient or the caregiver, (ii) homemaking activities; and (iii) volunteer activities. The base case analysis includes all costs. Costs incurred for emergency department visits and hospitalizations are calculated by reviews of medical records. Productivity losses are estimated separately as days lost from paid employment for the patient or caregiver, days lost from homemaking activities, and days lost from volunteer activities.
Sources of unit prices for this analysis: The professional fees are obtained from the National Health Service records or from a survey of local commercial providers. Costs of ambulatory and emergency department visits, laboratory tests and procedures, and hospitalization are obtained from the hospitals participating in the study. Costs of over-the-counter pharmaceuticals are obtained from the hospital databases and prescription costs from the patients during the follow-ups. Costs for analysing online data when remote monitoring system was used for follow-up are evaluated according to time spent by physicians and allied professionals involved in data analysis. Losses of productivity are calculated from patient interviews during the scheduled follow-up. Among the large number of (i) drug and dosages combinations, (ii) tests and procedures, and (iii) hospitalizations recorded in the case report forms, the most frequent unit costs are presented. All costs are expressed in 2011 euros.
Sample size calculation
A sample size of 200 patients (∼100 patients for each phase of the study), including an up to 15% dropout rate at 12 months of follow-up, was calculated from a review of several publications on the economics of telemonitoring in countries other than Italy.11,21 These assumptions were particularly considered in the REFORM study,21 which followed for 12 months, at 3 month intervals (i) a group with conventional ambulatory visits vs. (ii) a group followed by remote monitoring. This German study found costs differences between the two follow-up methods, which were useful to calculate the sample size. With 94 patients included in the analysis, a single group t-test at a 5% two-sided significance level has an 80% power to detect an 10% difference between the two groups, with a common standard deviation (SD) assumed to be €400. Further assuming a 10% dropout rate, ∼100 patients for each phase in the study population are needed to reach an 80% power to detect a 10% difference between the two groups. A 12-month follow-up was calculated to detect all the variables defined in the study objectives.
Statistical analysis, stochastic uncertainty, and sensitivity analysis
Resource utilization data are presented as counts and percentages, and the continuous cost and utility variables are presented as mean ± SD. The differences in counts for resource utilization are compared, using the χ2 test. As the costs and QOL estimates tend to be skewed, these variables are fitted, using gamma distributions and arithmetic means for across-groups comparisons. Cost-effectiveness acceptability curves are typically interpreted as the probability that one treatment is cost effective at a given willingness-to-pay threshold, based on stochastic uncertainty. As the curves are based on expectations, these results are not entered in a statistical analysis.
The estimated cost and outcome data for each patient are used to assess the stochastic uncertainty by estimating 1000 bootstrapped samples (i.e. sampling with replacement). For a visual representation of this patient-level uncertainty, the results are plotted on the cost-effectiveness plane. The cost-effectiveness acceptability curves are calculated, to show the probability of remote follow-ups to be more cost effective than conventional follow-ups, as a function of society's willingness-to-pay for a QALY gained. Deterministic sensitivity analyses will be performed to ascertain the methodological uncertainty.
Discussion
Remote monitoring is becoming the standard means of following recipients of cardiac implantable electronic devices, and has been included in the international professional practice guidelines.8 Large randomized clinical trials have shown that the substitution of remote for on-site ambulatory follow-ups effectively lowers the healthcare source consumption without compromising patient safety.14 Furthermore, the reaction time to detected events can be significantly shortened by remote monitoring.13 An early reaction to device malfunction or to adverse clinical events may have an effect on patient outcome.15–18
From this perspective, the Merlin.net system is specifically designed to allow early interventions following daily alerts, previously programmed by physicians, according to the patient and device characteristics. The Merlin.net site also represents a virtual data collection centre, easily and ubiquitously accessible via a simple Internet connection, because this allows physicians to know important device diagnostic data and to react in case of alerts due to patient's or device's issues. The TARIFF study will be essential to ascertain the speed of physician's reaction to direct incoming alerts, at a minimum cost and for a better patient's outcome.
Few data have been published regarding the cost-effectiveness of remote monitoring in standard clinical practice, and reimbursement continues to be an issue in several European countries, including Italy. Furthermore, there is a need to accurately define the reimbursement value of remote follow-ups, as the periodic interrogations require time and dedicated personnel. In this respect, the failure to communicate the value of remote monitoring by industry, because of market competition and early marketing of the technology, is eroding the perceived value of innovation, thus limiting the availability of funding for innovative and valuable improvements.
The TARIFF observational study, based on the prospective comparison of two groups, will enable an assessment of the real-life added value of remote monitoring, considering also its value from the perspective of the different Italian stakeholders for a broader adoption and for a better organization of the healthcare assistance.
Future advances in remote care technologies allowing the monitoring of not only device function, but also the periodic evaluation of vital signs or specific disease indicators, such as fluid accumulation or left atrial pressure monitoring, will add further value to remote monitoring, more and more influencing the patient's prognosis besides improving QOL.
Conclusions
Although the benefits conferred by remote monitoring have already been assessed on the basis of patient outcomes, its economic added value remains to be shown. The TARIFF study, based on a cost-minimization analysis, directly comparing remote follow-up with standard ambulatory visits, will hopefully validate the cost effectiveness of the Merlin.net technology, and define a proper reimbursement schedule applicable for the Italian healthcare system.
Funding
Funding to pay the open access publication charge was provided by St.Jude Medical Italia, sponsor of the TARIFF Registry.
Author's contributions
R.P.R., P.S., T.G., and A.P. wrote the manuscript, which was reviewed and approved by all other authors. All authors have contributed to the design of the study. Rodolphe Ruffy reviewed this manuscript for style and language.
Acknowledgement
The authors thank Cristina Giretti for the contribution to the setting up the economic aspects of this study.
Conflict of interests: P.S., T.G., and A.P. are employees of St Jude Medical.
References
- 1.Cleland JGF, Lewinter C, Goode KM. Telemonitoring for heart failure: the only feasible option for good universal care? Eur J Heart Fail. 2009;11:227–8. doi: 10.1093/eurjhf/hfp027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Moss AJ, Hall WJ, Cannom DS, Daubert JP, Higgins SL, Klein H, et al. Improved survival with an implanted defibrillator in patients with coronary disease at high risk for ventricular arrhythmia. Multicenter Automatic Defibrillator Implantation Trial Investigators. N Engl J Med. 1996;335:1933–40. doi: 10.1056/NEJM199612263352601. [DOI] [PubMed] [Google Scholar]
- 3.Buxton AE, Lee KL, Fisher JD, Josephson ME, Prystowsky EN, Hafley G. A randomized study of the prevention of sudden death in patients with coronary artery disease. Multicenter Unsustained Tachycardia Trial Investigators. N Engl J Med. 1999;341:1882–90. doi: 10.1056/NEJM199912163412503. [DOI] [PubMed] [Google Scholar]
- 4.Moss AJ, Hall WJ, Cannom DS, Daubert JP, Higgins SL, Klein H, et al. Multicenter automatic defibrillator implantation trial II investigators. Prophylactic implantation of a defibrillator in patients with myocardial infarction and reduced ejection fraction. N Engl J Med. 2002;346:877–83. doi: 10.1056/NEJMoa013474. [DOI] [PubMed] [Google Scholar]
- 5.Bardy GH, Lee KL, Mark DB, Poole JE, Packer DL, Boineau R, et al. Sudden Cardiac Death in Heart Failure Trial (SCD-HeFT) Investigators. Amiodarone or an implantable cardioverter-defibrillator for congestive heart failure. N Engl J Med. 2005;352:225–37. doi: 10.1056/NEJMoa043399. [DOI] [PubMed] [Google Scholar]
- 6.Dickstein K, Vardas PE, Auricchio A, Daubert JC, Linde C, McMurray J, et al. 2010 Focused Update of ESC Guidelines on device therapy in heart failure: an update of the 2008 ESC Guidelines for the diagnosis and treatment of acute and chronic heart failure and the 2007 ESC Guidelines for cardiac and resynchronization therapy. Europace. 2010;12:1526–36. doi: 10.1093/europace/euq392. [DOI] [PubMed] [Google Scholar]
- 7.Boriani G, Auricchio A, Klersy C, Kirchhof P, Brugada J, Morgan J, et al. Healthcare personnel resource burden related to in-clinic follow-up of cardiovascular implantable electronic devices: a European Heart Rhythm Association and Eucomed joint survey. Europace. 2011;13:1166–73. doi: 10.1093/europace/eur026. [DOI] [PubMed] [Google Scholar]
- 8.Wilkoff BL, Auricchio A, Brugada J, Cowie M, Ellenbogen KA, Gillis MA, et al. HRS/EHRA expert consensus on the monitoring of cardiovascular implantable electronic devices (CIEDs): description of techniques, indications, personnel, frequency and ethical considerations. Heart Rhythm. 2008;5:907–25. doi: 10.1016/j.hrthm.2008.04.013. [DOI] [PubMed] [Google Scholar]
- 9.Lazarus A. Remote, wireless, ambulatory monitoring of implantable pacemakers, cardioverter defibrillators, and cardic resynchronization therapy systems: analysis of a worldwide database. Pacing Clin Electrophysiol. 2007;30:1424. doi: 10.1111/j.1540-8159.2007.00595.x. [DOI] [PubMed] [Google Scholar]
- 10.Sharpe N. Heart failure management, a broader view required. Eur Heart J. 1998;19:975. doi: 10.1053/euhj.1998.1053. [DOI] [PubMed] [Google Scholar]
- 11.Clark RA, Inglis SC, McAlister FA, Cleland J, Stewart S. Telemonitoring or structured telephone support programmes for patients with chronic heart failure: systematic review and meta-analysis. BMJ. 2007;334:942. doi: 10.1136/bmj.39156.536968.55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Halimi F, Cantu F. Remote monitoring for active cardiovascular implantable electonic devices: a European Survey. Europace. 2010;12:1778–80. doi: 10.1093/europace/euq399. [DOI] [PubMed] [Google Scholar]
- 13.Crossley GH, Boyle A, Vitense A, Chang Y. Mead RH for the CONNECT Investigators, The CONNECT (Clinical Evaluation of Remote Notification to Reduce Time to Clinical Decision) Trial: The value of wireless remote monitoring with automatic clinician alerts. J Am Coll Cardiol. 2011;57:1182–9. doi: 10.1016/j.jacc.2010.12.012. [DOI] [PubMed] [Google Scholar]
- 14.Varma N, Michalski J, Epstein AE, Schweikert R. Automatic remote monitoring of implantable cardioverter-defibrillator lead and generator performance the Lumos-T safely RedUceS RouTine. Circulation. 2010;122:428–36. doi: 10.1161/CIRCEP.110.951962. [DOI] [PubMed] [Google Scholar]
- 15.Spencker S, Coban N, Koch L, Schirdewan A, Müller D. Potential role of home monitoring to reduce inappropriate shocks in implantable cardioverterdefibrillator patients due to lead failure. Europace. 2009;11:483–8. doi: 10.1093/europace/eun350. [DOI] [PubMed] [Google Scholar]
- 16.Reynolds DW, Jayaprasad N, Francis J. Remote monitoring of implantable cardioverter defibrillator. Indian Pacing Electrophysiol J. 2006;6:186–8. [PMC free article] [PubMed] [Google Scholar]
- 17.Ricci RP, Morichelli L, Santini M. Remote control of implanted devices through home monitoring technology improves detection and clinical management of atrial fibrillation. Europace. 2009;11:54–61. doi: 10.1093/europace/eun303. [DOI] [PubMed] [Google Scholar]
- 18.Ricci RP, Morichelli L, Gargaro A, Laudadio MT, Santini M. Home monitoring in patients with implantable cardiac devices: is there a potential reduction of stroke risk? Results from a computer model tested through Monte Carlo simulations. J Cardiovas Electrophysiol. 2009;20:1244–51. doi: 10.1111/j.1540-8167.2009.01543.x. [DOI] [PubMed] [Google Scholar]
- 19.Burri H, Senouf D. Remote monitoring and follow-up of pacemakers and implantable cardioverter defibrillators. Europace. 2009;11:701–9. doi: 10.1093/europace/eup110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. www.euroqol.org. ‘© 1990 EuroQol Group. EQ-5D™ is a trade mark of the EuroQol Group’.
- 21.Elsner CH, Sommer P, Piorkowski C, Taborsky M, Neuser H, Bytesnik J, et al. A Prospective Multicenter Comparison Trial of home monitoring against regular follow-up in MADIT II patients: additional visits and cost impact. Comput Cardiol. 2006;33:241–4. [Google Scholar]