Abstract
The objective of this study is to investigate the relationships among frontotemporal fiber tract compromise and task-switching performance in healthy controls and patients with temporal lobe epilepsy (TLE). We performed diffusion tensor imaging (DTI) on 30 controls and 32 patients with TLE (15 left TLE). Fractional anisotropy (FA) was calculated for four fiber tracts [uncinate fasciculus (UncF), arcuate fasciculus (ArcF), dorsal cingulum (CING), and inferior fronto-occipital fasciculus (IFOF)]. Participants completed the Trail Making Test-B (TMT-B) and Verbal Fluency Category Switching (VFCS) test. Multivariate analyses of variances (MANOVAs) were performed to investigate group differences in fiber FA and set-shifting performances. Canonical correlations were used to examine the overall patterns of structural-cognitive relationships and were followed by within-group bivariate correlations. We found a significant canonical correlation between fiber FA and task-switching performance. In controls, TMT-B correlated with left IFOF, whereas VFCS correlated with FA of left ArcF and left UncF. These correlations were not significant in patients with TLE. We report significant correlations between frontotemporal fiber tract integrity and set-shifting performance in healthy controls that appear to be absent or attenuated in patients with TLE. These findings suggest a breakdown of typical structure-function relationships in TLE that may reflect aberrant developmental or degenerative processes.
Keywords: Diffusion tensor, Uncinate, Cingulate, Frontal lobe, Arcuate, IFOF
INTRODUCTION
Patients with refractory temporal lobe epilepsy (TLE) often exhibit significant executive dysfunction above and beyond the impairments typically associated with medial temporal lobe compromise. Past research has provided substantial evidence showing significant executive functioning deficits in TLE patients, including problems with fluency, set-shifting, selective attention, and impulsive thinking (e.g., Cahn-Weiner, Wittenberg, & McDonald, 2009; Drake, Allegri, & Thomson, 2000; Garcia Espinosa et al., 2010; Hermann, Seidenberg, Lee, Chan, & Rutecki, 2007; Kim, Lee, Yoo, Kang, & Lee, 2007; Lopes, Simoes, Robalo, Fineza, & Goncalves, 2010; Martin et al., 2000). In fact, recent findings by Cahn-Weiner et al. (2009) have challenged the existing viewthat problems in executive functioning can be used to reliably discriminate patients with frontal lobe epilepsy (FLE) from those with TLE (but see McDonald, Delis, Norman, Tecoma, & Iragui-Madoz, 2005).
In recent years, advanced quantitative neuroimaging has provided new insights into the possible mechanisms leading to executive dysfunction in TLE, demonstrating significant gray and white matter frontotemporal atrophy in TLE patients (e.g., Guimaraes et al., 2007; Riederer et al., 2008; Seidenberg et al., 2005). For example, studies examining regional cortical thinning in patients with TLE have shown significant thinning in frontal lobe regions including the precentral and paracentral gyrus, inferior frontal gyrus (pars opercularis), orbitofrontal, and inferior medial prefrontal cortex (Bernhardt et al., 2009; Bernhardt, Bernasconi, Concha, & Bernasconi, 2010; Lin et al., 2007; McDonald, Hagler, et al., 2008; Mueller et al., 2009). In addition, studies examining gray and white matter atrophy in TLE have shown volume reductions in the ipsilateral middle frontal lobe and the cingulum bundle (Guimaraes et al., 2007; Riederer et al., 2008) and there is some evidence that these reductions are greater in patients with left TLE (Coan, Appenzeller, Bonilha, Li, & Cendes, 2009; Riederer et al., 2008).
Diffusion tensor imaging (DTI) and MRI-based tractography have provided novel tools for probing white matter microstructure and evaluating the integrity of white matter pathways in the brain. Tractography, which uses voxel-level directional diffusion tensor information to generate virtual three dimensional white matter maps (Yogarajah and Duncan, 2008), has been established as a reliable method for quantifying disease-related variations in white-matter tracts in TLE patients (Diehl et al., 2008; Focke et al., 2008; Hagler et al., 2009; McDonald, Ahmadi, et al., 2008) and may be more sensitive to regional changes than reductions in white matter volume (Hutchinson et al., 2010). In particular, Lin, Riley, Juranek and Cramer (2008) applied DTI to patients with TLE and showed reduced white matter integrity (i.e., fractional anisotropy; FA) in the uncinate (UncF) and arcuate fasciculus (ArcF), with reduction in the former correlating with earlier age of onset. Using a similar method, Ahmadi et al. (2009) found widespread reductions in fiber tract FA ipsilateral to the seizure focus and reported that these FA reductions were greatest in patients with left TLE, particularly in the UncF and the parahippocampal fibers. Aging and schizophrenia research have also shown that reductions in FA in several fiber tracts, including the cingulum bundle and UncF, are likely to be associated with a decline in executive functioning (Burns et al., 2003; Charlton, Barrick, Lawes, Markus, & Morris, 2010; Voineskos et al., 2010; Zahr, Rohlfing, Pfefferbaum, & Sullivan, 2009). Thus, it appears likely that compromise to tracts that project to the frontal lobe can help explain executive dysfunction in TLE and can be captured by DTI.
DTI with tractography has recently been used to explore how white matter integrity and the integrity of distal functional networks relate to verbal memory, fluency and language impairments in TLE (Bonelli et al., 2010; Diehl et al., 2010; McDonald, Ahmadi, et al., 2008; Protzner & McAndrews, 2011; Trebuchon-Da Fonseca et al., 2009; Yogarajah et al., 2010). However, except for a few studies (Dulay et al., 2009; Riley et al., 2010; Wang et al., 2007), there is limited research in patients with TLE on the relationship between white-matter integrity and executive dysfunction. In addition, most studies have combined patients with left and right TLE in their analyses rather than investigating possible differences between subgroups in their DTI-cognitive associations. In this study, we assess how compromise to frontal lobe white matter tracts relates to executive functioning in TLE. In particular, we focus on two set-shifting tasks—a visuomotor set-shifting (Trail Making Test, Part B; TMT-B) and a verbal set-shifting task (Verbal Fluency Category Switching; VFCS). We seek to test if decreases in the fractional anisotropy of tracts that project to the frontal lobes are related to task switching performance overall, and then differentially for controls versus right TLE versus left TLE patients.
We hypothesize that integrity of the frontotemporal fiber tracts will explain a significant amount of variation in task switching performance. Given literature that shows greater cognitive impairment (e.g., Hamberger & Seidel, 2003; McDonald, Delis, Kramer, Tecoma, & Iragui, 2008; Pereira et al., 2010) and white matter atrophy (Ahmadi et al., 2009; Coan et al., 2009) in patients with left relative to right TLE, we expect the relationship between fiber track FA and task switching to be the more pronounced in patients with left TLE due to a greater range in their cognitive and fiber tract FA values.
METHODS
Participants
This study was approved by the ethical standards committee on human experimentation at University of California, San Diego (UCSD) and completed according to the standards established in the Helsinki Declaration. Written informed consent was obtained from all participants. Thirty-two patients with refractory TLE (ages, 19–64 years) and 30 right-handed healthy controls (ages, 18–54 years) were included in the study. Control subjects were recruited through open advertisement and screened for past or present neurological or psychiatric conditions; those with a history of neurological or psychiatric illness, or who were currently experiencing clinical levels of depression (Beck Depression Inventory-2 score >13), were excluded from the study. All TLE patients were either undergoing or had previously completed presurgical evaluation at the UCSD Epilepsy Center and diagnosed by a board-certified neurologists (E.S.T. and V.J.I.) with expertise in epileptology. Fifteen left TLE and 17 right TLE patients were identified by unequivocal left or right seizure onsets during ictal recordings in a video-EEG monitoring unit (with scalp and foramen ovale electrodes), seizure semiology, and neuroimaging results. None of the patients exhibited dual pathology on MRI. Due to the limitations imposed by the clinical assessment schedules, both tests could not be performed on every patient, creating size and membership differences between patient groups: 24 patients were included in the TMT-B analysis; 26 patients were included in the VFCS analysis; 11 left TLE and 9 right TLE patients had information for both neuropsychological measures. Demographic information for controls and patients with TLE are presented in Table 1a. There were no significant differences among the groups in terms of age, education, or gender distribution (see Table 1a). In addition, t tests revealed no difference between patients with left and right TLE in terms of age of seizure onset, seizure frequency, disease duration, and number of current medications (all p-values >.05). Chi square tests revealed no difference in the proportion of patients with a history of febrile seizures, presence of generalized tonic-clonic seizures, history of status epilepticus, or MTS (all p-values > .05; see Table 1b). Thirteen patients had undergone the intracarotid amobarbital procedure (IAP) and all were identified as left language dominant.
Table 1a.
Sample demographics
| N | Female | Age |
Education |
|||
|---|---|---|---|---|---|---|
| % | M | SD | M | SD | ||
| Controls | 30 | 53.3 | 35.6 | 11.6 | 14.6 | 1.9 |
| Left TLE | 15 | 53.3 | 36.0 | 11.8 | 13.7 | 1.7 |
| Right TLE | 17 | 52.9 | 36.6 | 12.0 | 14.1 | 1.8 |
| Group ANOVAs/chi square tests | χ2=.001 | F=.044 | F=1.148 | |||
| P=.99 | p=.96 | p=.32 | ||||
Table 1b.
Sample clinical characteristics
| Age of onset |
Duration |
# meds |
SzFreq |
MTS |
Febrile1 |
HxSE2 |
|||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| M | SD | M | SD | M | SD | M | SD | % | % | % | |
| Left TLE | 15.53 | 12.9 | 20.1 | 12.8 | 2.2 | 0.9 | 9.1 | 9.3 | 80.0 | 21.5 | 20.0 |
| Right TLE | 15.29 | 11.5 | 20.9 | 14.4 | 2.4 | 0.6 | 5.2 | 3.9 | 58.8 | 31.3 | 16.7 |
| Group ANOVAs/chi square tests | t=.056 | t=−.181 | t=−.586 | t=1.693 | χ2=1.663 | χ2=.368 | χ2=.041 | ||||
| p=.96 | p=.86 | p=.56 | p=.10 | p=.20 | p=.54 | p=.84 | |||||
Only available for 14/15 L-TLE and 16/17 R-TLE patients.
Only available for 10/15 L-TLE and 12/17 R-TLE patients.
Procedure
Image acquisition
Magnetic resonance imaging was performed on a General Electric 1.5 T EXCITE HD scanner with an 8-channel phased-array head coil. Image acquisitions included a conventional 3-plane localizer, GE calibration scan, two T1-weighted 3D structural scans (TE = 4.9 ms, TR = 10.7 ms, TI = 1 s, flip angle = 8°, bandwidth = 31.25 Hz/pixel, FOV = 25.6 cm, matrix = 256 × 192, slice thickness = 1.0 mm), and three diffusion-weighted (DW) sequences. Diffusion data were acquired using single-shot echo-planar imaging with isotropic 2.5 mm voxels (matrix size = 96 × 96, FOV = 24 cm, 47 axial slices, slice thickness = 2.5 mm, partial k-space acquisition, TE = 75.6 ms, TR = 12.3 s), covering the entire cerebrum and brainstem without gaps. One volume series was acquired with 51 diffusion gradient directions using a b-value of 1000 mm2/s with an additional b = 0 volume. For use in nonlinear B0 distortion correction, two additional b = 0 volumes were acquired, with either forward or reverse phase-encode polarity. The imaging protocol was identical for all participants, and all patients were seizure-free for a minimum of 24 hr before the MRI scan to avoid the possible effects of acute postictal changes on diffusion parameters (Yogarajah and Duncan, 2008).
Image processing
Image files in DICOM format were transferred to a Linux workstation for processing with a customized, automated, processing stream written in MATLAB and C++. The two T1-weighted images were rigid body registered to each other and reoriented into a common space, roughly similar to alignment based on the AC-PC line. Images were corrected for non-linear warping caused by non-uniform fields created by the gradient coils (Jovicich et al., 2006). Image intensities were corrected for spatial sensitivity inhomogeneities in the 8-channel head coil by normalizing with the ratio of a body coil scan to a head coil scan. Five pre-processing steps were performed on the diffusion-weighted images. (1) Image distortion in the diffusion-weighed (DW) volumes caused by eddy currents was minimized by nonlinear optimization. (2) Within-scan head motion was removed by calculating diffusion tensors, synthesizing of DW volumes from those tensors, and rigid body registering each data volume to its corresponding synthesized volume. (3) Head motion between scans was removed by rigid body registration between the b = 0 images of each DW scan. (4) Image distortion caused by magnetic susceptibility artifacts was minimized with a nonlinear B0-unwarping method using paired images with opposite phase-encode polarities (Holland, Kuperman, & Dale, 2010). (5) Images were resampled using linear interpolation to 1.875 mm3 isotropic voxels.
Fiber tracking and calculations
Fiber tract FA values are scalars ranging from 0 to 1 that represent the propensity of molecules to diffuse in an anisotropic manner where the zero value indicates similar diffusion in all directions while a value of one represents diffusion along a single, preferred axis (Basser, Mattiello, & LeBihan, 1994). Fiber tracts were derived using a probabilistic diffusion tensor atlas that was based on manual tracings (Hagler et al., 2009). To obtain the atlas, all fiber tracts were first traced in DTI Studio (John Hopkins University, Baltimore, MD) by the same image analyst. Manual tracings were performed according to the multiple region of interest (ROI) procedure described by Wakana, Jiang, Nagae-Poetscher, van Zijl, & Mori (2004), using an FA threshold of 0.15. This method has been shown to have very high intra- and inter-rater reproducibility for all fiber tracts included (k = .69–.99). Data from this manual training set were used to create a probabilistic fiber atlas that consisted of averaged information about the locations and local orientations of 13 chosen fiber tracts. Fiber ROIs were derived from the atlas for single subjects using both T1-weighted images and orientation estimates from diffusion tensor calculations. The T1-weighted images were used to map the brain into a common space and diffusion tensor orientation estimates were compared to the atlas to obtain a relative probability that a voxel belongs to a particular fiber given the similarity of diffusion orientations. Results obtained from the atlas-generated fibers were highly correlated with those from manually tracings. However, the automated results used to calculate FA have the advantage of eliminating the time demands of manual tracings.
In the current study, the following four fiber tracts were selected because of their frontal lobe connections and possible contribution to executive functioning (see Figure 1):
Uncinate fasciculus (UncF): originates by two divisions from the orbital and polar frontal cortex. It first runs backward, enters the external capsule, and then arches around the limen insulae as a compact tract, entering the temporal pole to project to the uncus, amygdala, and hippocampus (Klingler & Gloor, 1960).
Arcuate fasciculus (ArcF): operationalized as the temporal section of the superior longitudinal fasciculus, it originates in the ventrolateral frontal cortex (i.e., pars opercularis) and runs backward toward the parietal lobe to arch around the sylvian fissure, then changes its course to run forward and finally reach the posterior part of the superior temporal gyrus and middle temporal gyrus (Catani, Jones, & Ffytche, 2005).
Inferior fronto-occipital fasciculus (IFOF): originates from the ventrolateral and dorsolateral frontal lobe, runs posteriorly just superior and medial to the UncF. Its fibers pack and enter the anterior floor of the external capsule just before it arches around the limen insulae. Fibers of the IFOF then re-expand to end in the basal temporo-occipital regions, including the fusiform and lingual gyri (Martino, Brogna, Robles, Vergani, & Duffau, 2010).
Cingulum, the cingulate gyrus part (CING): the C-shaped bundle of long association fibers that lie alongside the cingulate gyrus and arch inferiorly around the splenium of the corpus callosum, excluding the part that extends toward the parahippocampal gyrus and the entorhinal cortex (Wakana et al., 2004).
Fig. 1.
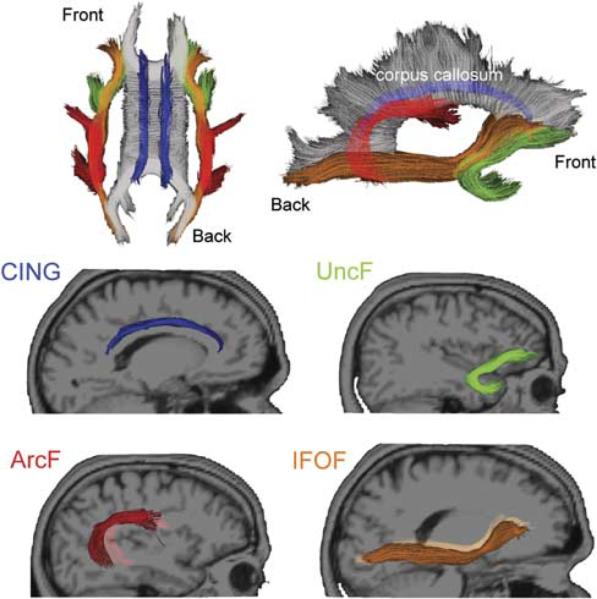
Fibers that project to the frontal lobe selected for the analysis. Top row shows the selected fibers from superior horizontal and mid-sagittal views. Middle and bottom rows present the individual fibers from sagittal view. CING appears in blue; UncF in green; ArcF in red and IFOF in orange.
In addition, we included the corticospinal tract (CST), defined as the projection fibers that descend inferiorly to the spinal cord from the motor cortex through the cerebral peduncle of the brainstem, as a “control tract” in our analysis to test the specificity of relationships between frontal fiber tract integrity and set-shifting performance (Wakana et al., 2004). For all tracts, data points more than 3 standard deviations from the mean were identified as outliers and removed from the analysis. This approach resulted in the removal of only two data points.
Neuropsychological testing
We selected two set-shifting tests from the Delis-Kaplan Executive Function System (D-KEFS) family of tests for their relevance to frontal lobe dysfunction: TMT-B and the VFCS (Delis, Kaplan, & Kramer, 2001). TMT-B is a timed, visuomotor number-letter sequencing procedure that measures visuomotor set-shifting. VFCS evaluates an examinee's ability to alternate between saying words from two different, overlearned semantic categories as quickly as possible in 60 s. Both tests were performed on healthy controls and patients at the time of MRI scanning as a part of a larger battery of tests that assess language, memory, and motor functioning. Tests were scored using standard procedures and age-scaled scores were used as the primary dependent variables in the statistical analysis.
Statistical Analysis
We performed a three-group (groups: control, left TLE, right TLE) between-subjects multivariate analysis of variance (MANOVA) for each hemisphere on four dependent variables, consisting of UncF, ArcF, IFOF, and CING FA. A separate MANOVA was performed to examine group differences on our two task-switching measures. We used Wilk's criterion (Λ) as the omnibus test statistic for all MANOVAs. For significant MANOVAs, we performed individual univariate ANOVAs for each fiber tract using Bonferroni corrected α = .0125 (4 fibers per hemisphere) and α = .025 (2 task-switching measures). Pearson correlations were performed within each patient group to investigate the relationship between clinical variables (i.e., age of seizure onset, disease duration, seizure frequency) and fiber tract integrity.
To determine whether an omnibus relationship existed between frontotemporal fiber tract FA and set-shifting performance, a canonical correlation analysis was conducted for each hemisphere across groups using the four fiber tract FA values (set 1) and the 2 task-switching variables (set 2). When the multivariate analysis was significant, bivariate within-group correlations were performed, correcting for false discovery rate (FDR). An additional canonical correlation was calculated using the corticospinal tract FA (set 3) and the 2 task-switching variables (set 2) to assess the specificity of our findings. To control for multiple comparisons, canonical R significance was assessed using α = .025, Bonferroni corrected for the two hemispheres.
RESULTS
Group Differences in FA and Task-Switching Performance
The results of the Box's M test of homogeneity of covariance (all p-values >.19) and Levene's homogeneity test (all p-values >.02) were not statistically significant for either hemisphere, indicating that our data met the homogeneity assumptions for MANOVA. The omnibus MANOVA on fiber tract FA was significant, revealing group differences in overall fiber tract FA for both hemispheres [left hemisphere F(8,110) = 3.43; p = .001; partial η2 = .200; right hemisphere F(8,110) = 3.09; p = .004; partial η2 = .183]. The effect sizes were moderate in magnitude. Univariate follow-up ANOVAs revealed significant bilateral differences for ArcF (left: p < .001; partial η2 = .249; right: p = .006; partial η2 = .160), IFOF (left: p < .001; partial η2 = .309; right: p = .001; partial η2 = .220), left Unc (p < .001; partial η2 = .287), and right CING (p = .001; partial η2 = .229) and right CING (p = .001; partial η2 = .229). The effect sizes were all in the moderate range and, with the exception of CING, were larger on the left hemisphere. Post hoc contrasts showed significantly (all p-values <.006) lower FA values for patients with left TLE as compared to controls bilaterally in all four tracts while control versus right TLE differences were not significant for any of the tracts in either hemisphere after Bonferroni correction. Left TLE patients also had significantly lower FA values in comparison to patients with right TLE in all left hemispheric tracts except for the CING (UncF p = .018; ArcF p = .003; IFOF p = .002; CING p = .052) and only in ArcF and IFOF in the right hemisphere (UncF p = .126; ArcF p = .008; IFOF p = .013; CING p = .030). These results are summarized in Table 2a and 2b.
Table 2a.
Summary statistics for FA and neuropsychological tests
| Controls |
L-TLE |
R-TLE |
ANOVA |
|||||||
|---|---|---|---|---|---|---|---|---|---|---|
| M | SD | M | SD | M | SD | F | P | partial η2 | ||
| Uncinate FA | RH | 0.37451 | 0.0207 | 0.3520 | 0.0184 | 0.3659 | 0.0352 | 4.12 | .021 | 0.124 |
| LH | 0.39061 | 0.0246 | 0.3482r | 0.0294 | 0.3721 | 0.0303 | 11.66 | .000 | 0.287 | |
| Arcuate FA | RH | 0.41761 | 0.0219 | 0.3913r | 0.0324 | 0.4179 | 0.0290 | 5.52 | .006 | 0.160 |
| LH | 0.43151 | 0.0189 | 0.3942r | 0.0373 | 0.4241 | 0.0278 | 9.60 | .000 | 0.249 | |
| IFOFFA | RH | 0.41511 | 0.0199 | 0.3832r | 0.0253 | 0.4062 | 0.0327 | 8.18 | .001 | 0.220 |
| LH | 0.42511 | 0.0197 | 0.3802r | 0.0360 | 0.4125 | 0.0307 | 12.98 | .000 | 0.309 | |
| Cingulum FA | RH | 0.39661 | 0.0360 | 0.3483 | 0.0411 | 0.3779 | 0.0342 | 8.61 | .001 | 0.229 |
| LH | 0.41661 | 0.0487 | 0.3743 | 0.0420 | 0.4091 | 0.0508 | 3.72 | .030 | 0.114 | |
| TMT-B | 11.5331 | 2.240 | 8.818 | 2.136 | 9.857 | 1.952 | ||||
| % impaired* | 31% | 20% | χ2=0.34; p=.56 | |||||||
| VF Switching | 12.0661,r | 2.033 | 7.545 | 3.615 | 9.143 | 3.024 | ||||
| % impaired* | 42% | 30% | χ2=0.32; p=.57 | |||||||
Pairwise comparison to left TLE significant (p<.025).
Tairwise comparison to right TLE significant (p<.025).
Impairment defined clinically as age-scaled score <7.
Table 2b.
MANOVA results
| MANOVA |
||||||||
|---|---|---|---|---|---|---|---|---|
| DVs | IVs | df | Wilk's λ | F | P | η 2 | Box's M | Sig. |
| Right hemisphere FA: UncF, ArcF, IFOF, CING | Group | 8, 110 | 0.667 | 3.086 | .004 | 0.183 | 26.705 | .258 |
| Left hemisphere FA: UncF, ArcF, IFOF, CING | Group | 8, 110 | 0.641 | 3.428 | .001 | 0.200 | 28.783 | .185 |
| Task-switching performance: TMT-B and VFCS | Group | 4, 88 | 0.584 | 6.787 | .000 | 0.236 | 7.532 | .344 |
| Corticospinal tract FA: left and right hemisphere | Group | 4, 116 | 0.894 | 1.679 | .160 | 0.055 | 33.972 | .000 |
MANOVA on task-switching performance (TMT-B and VFCS scaled scores) showed significant group differences with a moderate effect size, F(4,88)=6.79; p<.001; partial η2=.236. Post hoc contrasts showed that on TMT-B, left TLE patients performed significantly worse than healthy controls (p=.001) while there was no significant difference between left versus right TLE patients or right TLE patients versus controls. For VFCS performance, both left TLE (p<.001) and right TLE (p<.01) patients had significantly lower scores than healthy controls, with no significant difference observed between the two patient groups.
Canonical Correlations Between FA and Task-Switching Performance
A canonical correlation analysis was conducted using four white-matter integrity (FA) variables (set 1) and two variables representing performance on task-switching tasks (set 2) for each hemisphere. The analysis yielded one statistically and practically significant canonical variate pair for each hemisphere. The standardized canonical correlations and loadings for both set 1 and 2 variables, together with the canonical adequacy coefficients of the canonical variates, are provided in Table 3a.
Table 3a.
Canonical Correlations
| Set 1 |
Set 2 |
Canonical correlation |
||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Var | β | r | Var | β | r | Rc | df | CAC | Wilk's λ | F | P | η 2 |
| Left UncF | .354 | .850 | 0.653 | 8 | 68.05% | .496 | 4.505 | .000 | .504 | |||
| Left ArcF | .833 | .976 | ||||||||||
| Left IFOF | −.263 | .783 | TMT-B | −.023 | .478 | 61.41% | ||||||
| Left CING | .139 | .659 | VFCS | 1.011 | .999 | |||||||
| Right UncF | −.261 | .567 | 0.646 | 8 | 54.79% | .523 | 3.923 | .001 | .477 | |||
| Right ArcF | .807 | .919 | ||||||||||
| Right IFOF | .011 | .595 | TMT-B | .111 | .550 | 63.64% | ||||||
| Right CING | .488 | .819 | VFCS | .943 | .995 | |||||||
| Left CsT | n/a | n/a | TMT-B | .217 | .650 | 0.386 | 2 | 69.37% | .851 | 3.94 | .027 | .149 |
| VFCS | .875 | .982 | ||||||||||
| Right CsT | n/a | n/a | TMT-B | .445 | .793 | 0.387 | 2 | 73.95% | .850 | 3.97 | .026 | .150 |
| VFCS | .702 | .922 | ||||||||||
Using a cutoff value of |.30| for practical significance, the UncF and ArcF were the only two fibers significantly associated with this canonical variate in the left hemisphere based on the standardized canonical coefficients; the ArcF and CING were the only two significantly associated in the right hemisphere. The strong canonical loadings for both TMT-B and VFCS in both hemispheres suggest that the canonical variate represented overall task-switching performance.
We found a strong correlation between the set of fiber tract FA measures in each hemisphere [left hemisphere Rc=.653; F(8) 5 4.36; p=.001; η2=.504; right hemisphere Rc=.646; F(8)=3.92; p=.001; η2=.477] and our pair of task-switching measures. For the set of left hemisphere fibers, 43% of the variance was associated with our pair of task-switching measures. For the set of right hemisphere fibers, 42% of the variance was associated with the pair of task-switching measures.
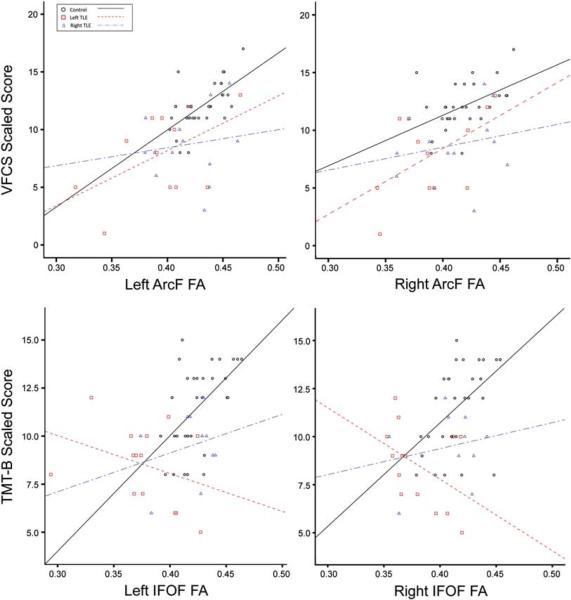
FDR-corrected bivariate correlations showed a significant TMT-B-left IFOF relationship and a significant VFCS-left ArcF and VFCS-left UncF relationship only for the control group. There were no significant correlations for left or right TLE patients in either hemisphere using an FDR-correction. Bivariate correlations are presented in Table 3b.
Table 3b.
FA-task correlations
| Uncinate |
Arcuate |
IFOF |
Cingulum |
|||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| RH |
LH |
RH |
LH |
RH |
LH |
RH |
LH |
|||||||||
| r | p | r | p | r | p | r | p | r | p | r | p | r | p | r | p | |
| TMT-B (ss) | ||||||||||||||||
| Controls | .431 | .016 | .263 | .161 | .331 | .074 | .285 | .128 | .477 | .008 | .530 | .003 | .102 | .592 | −.061 | .750 |
| L-TLE | −.586 | .028 | −.401 | .156 | −.188 | .521 | −.211 | .468 | −.438 | .117 | −.338 | .238 | −.244 | .401 | −.440 | .132 |
| R-TLE | .485 | .131 | .275 | .442 | .375 | .285 | .204 | .573 | .210 | .561 | .247 | .491 | .374 | .321 | .658 | .039 |
| VFCS (ss) | ||||||||||||||||
| Controls | .284 | .128 | .526 | .003 | .472 | .009 | .620 | .000 | .269 | .151 | .460 | .011 | .259 | .166 | .275 | .142 |
| L-TLE | −.147 | .647 | .159 | .622 | .535 | .073 | .516 | .086 | −.128 | .692 | .143 | .657 | .524 | .080 | .314 | .348 |
| R-TLE | .045 | .879 | −.276 | .340 | .215 | .460 | .142 | .627 | −.081 | .784 | −.171 | .559 | −.421 | .152 | .044 | .881 |
Follow-up canonical analyses exploring the specificity of our results to the frontotemporal regions by testing the corticospinal tract–task switching relationship yielded non-significant results in both hemispheres (both p-values >.025).
Bivariate correlations with clinical seizure variables revealed that in left TLE, an earlier age of seizure onset was related to lower FA of the left (r=.58; p<.05) and right (r=.55; p<.05) UncF and longer disease duration was related to lower FA of the left cingulum (r=−.57; p<.05), left UncF (r=−.62; p<.05), right UncF (r=−.56; p<.05), and left IFOF (r=−.57; p<.05). In patients with right TLE, longer disease duration was related to lower FA of the right IFOF (r=.53; p<.05). No other clinical variables correlated with fiber tract FA and none of the correlations survived correction for multiple comparisons.
DISCUSSION
In this study, we used DTI with probabilistic atlas-based fiber tract segmentation to analyze the correlation between FA of four cortical association tracts that connect frontal regions to more posterior ones (i.e., UncF, CING, IFOF, ArcF) and task-switching performance in healthy controls and patients with TLE. In line with previous studies (Ahmadi et al., 2009; Lin et al., 2008; Riley et al., 2010), our data confirmed that patients with TLE exhibit white-matter abnormalities (i.e., reduced FA) in association with fiber tracts that project to the frontal lobes. However, our post hoc comparisons established that the FA values for the left TLE patients significantly differ from those of controls both ipsilaterally and contralaterally, whereas the right TLE's FA values did not significantly differ from healthy controls' after correcting for multiple comparisons, despite being numerically lower. These findings are consistent with some MRI and DTI literature that has identified significantly greater gray and white matter atrophy in patients with left TLE, both ipsilateral and contralateral to the seizure focus (Ahmadi et al., 2009; Bonilha et al., 2007; Keller & Roberts, 2008). Moreover, our post hoc analyses indicates that the structural differences between patients with left versus right TLE are not well-explained by differences in disease severity, given the lack of group differences in key clinical variables. Although the proportion of patients with MTS did not differ between groups, inspection of Table 1b reveals a tendency for a greater proportion of patients with left TLE to have MTS, suggesting some additional disease burden that could be contributing to their total structural and cognitive compromise. However, post hoc analyses including only those patients with MTS (12 left TLE; 10 right TLE) did not change the pattern of results. These data are consistent with other studies demonstrating greater structural compromise in patients with left TLE in the absence of differences in clinical indicators of disease severity (Riederer et al., 2008).
Results from the canonical analysis indicated significant omnibus relationships (Rc>.64 for both hemispheres) between FA in our cluster of frontotemporal fiber tracts and task-switching performance. This strong relationship was largely accounted for by the positive relationship between VFCS performance and FA of the left UncF and bilateral ArcF FA. Furthermore, the canonical relationship between our control fiber tract (i.e., corticospinal tract) and set-shifting was not significant. These data indicate that variability in frontotemporal fiber tract integrity can influence task-shifting performance and that this relationship is at least somewhat specific. However, when analyses were considered separately for each group and task, only the correlations within the healthy controls remained significant after correcting for multiple comparisons. In particular, higher FA values of the left ArcF and left UncF were associated with stronger performances on VFCS, whereas higher FA values of the left IFOF were associated with stronger performances on TMT-B. The VFCS-left ArcF association is not surprising since the ArcF connects left frontal areas (i.e., Broca's) to left temporal (i.e., Wernicke's) and temporo-parietal regions involved in lexico-semantic processing (e.g., comprehension, production; Catani, Howard, Pajevic, & Jones, 2002). Similarly, the UncF connects the orbitofrontal areas to anterior and medial temporal areas important for verbal episodic and working memory (Charlton et al., 2010). The involvement of both intrahemipsheric, associative white matter tracts connecting language- and memory-dominant left hemispheric areas underlies a task like VFCS, which requires overt word generation subject to certain categorical rules that must be held in working memory by the participant. While previous studies of UncF integrity have reported strong correlations with the fiber's FA and verbal memory performance (Charlton et al., 2010; Diehl et al., 2008; McDonald, Ahmadi, et al., 2008), our findings are novel for supporting the possible role of the left UncF in task-switching, and suggest some material specificity of this fiber with respect to verbal set-shifting performance.
For TMT-B, FA of the left IFOF was the only fiber tract that survived FDR-correction in controls, supporting previous aging research that this fiber tract plays an important role in visuomotor set-shifting (Perry et al., 2009). Furthermore, there was a strong trend for the right IFOF to correlate with TMT-B, albeit to a lesser degree (p=.008). The IFOF is the main white matter pathway connecting the dorsolateral and ventrolateral frontal cortex to posterior temporal (i.e., middle and inferior temporal and fusiform gyri) and inferior occipital areas (i.e., lingual gyrus) with some parietal fibers that project toward the temporo-parietal junction (Catani et al., 2002; Martino et al., 2010). It has been implicated in various processes that include semantic processing (Martino et al., 2010) and top-down modulation of attention in visual and visuomotor tasks (Urbanski et al., 2008). Within this context, these correlations may reflect the importance of this fiber tract to top-down attentional processes that underlie visuomotor set-shifting: IFOF's possible bilateral role in TMT-B performance may reflect the joint verbal (i.e., letter sequencing) and visuospatial attentional demands of the task, contrasting the involvement of left fiber tracts only for in VFCS task, which has primarily verbal demands.
Despite the significant and tract-specific relationships that emerged in our healthy controls, none of the correlations between frontotemporal fiber tract FA and set-shifting survived FDR-correction in either patient group. Although patients with left and right TLE groups showed significantly poorer VFCS performance, and patients with left TLE showed poorer TMT-B performance and greater structural compromise relative to controls, a reliable relationship between these structure-cognitive variables did not emerge. Inspection of Figure 2 suggests that these relationships varied substantially for our patients, ranging from attenuated (i.e., VFCS-ArcF FA) to approaching the opposite direction in left TLEs (i.e., TMT-B-IFOF FA). While the reason for these altered patterns in patients is unclear, one possibility is that frontotemporal fiber tract damage in TLE disrupts the normal substrates involved in set-shifting, forcing patients to rely on alternate, albeit less efficient, strategies and/or brain networks for task completion. This could involve the recruitment of more widespread cortical or subcortical networks or the engagement of posterior brain regions than were captured by our DTI analysis. This is supported by some recent data showing small to modest correlations between executive dysfunction in TLE and reduced FA in the cerebellum (Riley et al., 2010), increased mean diffusivity in the posterior limbs of the internal capsule (Wang et al., 2010) and decreased connectivity between the caudate nucleus and broad regions of the dorsolateral prefrontal cortex (Riley, Moore, Cramer, & Lin, 2011). It is possible that the developmentally disruptive nature of TLE results in greater variability among patients in frontal lobe white matter development and the strategies that are learned for successful task completion. Although contrary to our initial hypothesis, a similar breakdown of structure-function relationship has been shown by Hermann et al. (2006) who reported a pattern of normal white matter volume-cognitive function associations in healthy children that were absent in children with new-onset epilepsy. Our data extend the literature by revealing a breakdown in the typical prefrontal-set-shifting relationships in patients with TLE at the specific fiber tract level.
Fig. 2.
Scatter plots showing significant fiber FA and task-switching performance association. Top row shows the relationship between TMT-B performance and IFOF FA. Bottom row shows the relationship between VFCS and ArcF FA. Circles show values for health controls; squares for left TLE patients; triangles for right TLE patients. Best fit lines (solid: controls; dashed: left TLE patients; dotted-dashed: right TLE patients) approximate the correlational relationships per group. In both cases, a significant FA-task-switching performance is observed only for healthy controls.
In summary, we report the presence of specific fiber tract-cognitive relationships in healthy controls that appear to be absent or significantly attenuated in patients with left and right TLE. This disruption of the typical structure function-relationships is interesting given previous assumptions that frontotemporal fiber tract compromise is responsible for executive dysfunction in TLE. However, several limitations to our study should be noted. First, our results are limited by a relatively small sample size, which limits our ability to test more complex models of the link between fiber tract integrity and neuropsychological measures. In addition, executive functions are multi-faceted in nature and it is likely that set-shifting tasks recruit a wide network of regions in patient populations (e.g., Martin et al., 2000; Wang et al., 2007), making these associations complex and perhaps more subtle than that can be captured by exploring damage to specific tracts. The limited sample size also makes it difficult to balance type I and type II errors with the use of a Bonferroni corrected, conservative alpha. To that extent, we apply a false discovery rate (FDR) correction to our correlation results, which still yields very conservative results. Nonetheless, our sample size is comparable to those of other neuroimaging studies with strict patient selection criteria (i.e., video-EEG diagnosed unilateral TLE with no evidence of dual pathology). Further research with larger samples is required to test the validity of our results, using multiple measures of executive functioning, and examining how the relative contributions of regional gray/white matter damage contributes to different components of executive functioning. In addition, it is possible that subregions of various fiber tracts, rather than the entire fiber tract ROI, would show stronger associations with specific measures of set-shifting or other measures of executive functioning. Additional research to explore these associations and relate them to functional integrity of brain networks using multimodal structural and functioning neuroimaging is on-going in our lab.
ACKNOWLEDGMENTS
This work from supported by the National Institute of Health (C.R.M., grant number K23NS056091 and C.R.M., grant number R01NS065838-01A1). We also greatly acknowledge support from GE Healthcare.
Footnotes
Authors report no conflicts of interest.
REFERENCES
- Ahmadi ME, Hagler DJ, Jr., McDonald CR, Tecoma ES, Iragui VJ, Dale AM, Halgren E. Side matters: Diffusion tensor imaging tractography in left and right temporal lobe epilepsy. AJNR American Journal of Neuroradiology. 2009;30(9):1740–1747. doi: 10.3174/ajnr.A1650. doi:10.3174/ajnr.A1650. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Basser PJ, Mattiello J, LeBihan D. MR diffusion tensor spectroscopy and imaging. Journal of Biophysics. 1994;66(1):259–267. doi: 10.1016/S0006-3495(94)80775-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bernhardt BC, Bernasconi N, Concha L, Bernasconi A. Cortical thickness analysis in temporal lobe epilepsy: Reproducibility and relation to outcome. Neurology. 2010;74(22):1776–1784. doi: 10.1212/WNL.0b013e3181e0f80a. doi:10.1212/WNL.0b013e3181e0f80a. [DOI] [PubMed] [Google Scholar]
- Bernhardt BC, Worsley KJ, Kim H, Evans AC, Bernasconi A, Bernasconi N. Longitudinal and cross-sectional analysis of atrophy in pharmacoresistant temporal lobe epilepsy. Neurology. 2009;72(20):1747–1754. doi: 10.1212/01.wnl.0000345969.57574.f5. doi:10.1212/01.wnl.0000345969.57574.f5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bonelli SB, Powell RH, Yogarajah M, Samson RS, Symms MR, Thompson PJ, Duncan JS. Imaging memory in temporal lobe epilepsy: Predicting the effects of temporal lobe resection. Brain. 2010;133(Pt 4):1186–1199. doi: 10.1093/brain/awq006. doi:10.1093/brain/awq006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bonilha L, Alessio A, Rorden C, Baylis G, Damasceno BP, Min LL, Cendes F. Extrahippocampal gray matter atrophy and memory impairment in patients with medial temporal lobe epilepsy. Human Brain Mapping. 2007;28(12):1376–1390. doi: 10.1002/hbm.20373. doi:10.1002/hbm.20373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burns J, Job D, Bastin ME, Whalley H, Macgillivray T, Johnstone EC, Lawrie SM. Structural disconnectivity in schizophrenia: A diffusion tensor magnetic resonance imaging study. The British Journal of Psychiatry. 2003;182:439–443. [PubMed] [Google Scholar]
- Cahn-Weiner DA, Wittenberg D, McDonald C. Everyday cognition in temporal lobe and frontal lobe epilepsy. Epileptic Disorders. 2009;11(3):222–227. doi: 10.1684/epd.2009.0265. doi:10.1684/epd.2009.0265. [DOI] [PubMed] [Google Scholar]
- Catani M, Howard RJ, Pajevic S, Jones DK. Virtual in vivo interactive dissection of white matter fasciculi in the human brain. Neuroimage. 2002;17(1):77–94. doi: 10.1006/nimg.2002.1136. [DOI] [PubMed] [Google Scholar]
- Catani M, Jones DK, Ffytche DH. Perisylvian language networks of the human brain. Annals of Neurology. 2005;57:8–16. doi: 10.1002/ana.20319. [DOI] [PubMed] [Google Scholar]
- Charlton RA, Barrick TR, Lawes IN, Markus HS, Morris RG. White matter pathways associated with working memory in normal aging. Cortex. 2010;46(4):474–489. doi: 10.1016/j.cortex.2009.07.005. doi:10.1016/j.cortex.2009.07.005. [DOI] [PubMed] [Google Scholar]
- Coan AC, Appenzeller S, Bonilha L, Li LM, Cendes F. Seizure frequency and lateralization affect progression of atrophy in temporal lobe epilepsy. Neurology. 2009;73(11):834–842. doi: 10.1212/WNL.0b013e3181b783dd. doi:10.1212/WNL.0b013e3181b783dd. [DOI] [PubMed] [Google Scholar]
- Delis DC, Kaplan E, Kramer JH. The Delis-Kaplan Executive Function System. The Psychological Corporation; San Antonio, TX: 2001. [Google Scholar]
- Diehl B, Busch RM, Duncan JS, Piao Z, Tkach J, Luders HO. Abnormalities in diffusion tensor imaging of the uncinate fasciculus relate to reduced memory in temporal lobe epilepsy. Epilepsia. 2008;49(8):1409–1418. doi: 10.1111/j.1528-1167.2008.01596.x. doi:10.1111/j.1528-1167.2008.01596.x. [DOI] [PubMed] [Google Scholar]
- Diehl B, Piao Z, Tkach J, Busch RM, LaPresto E, Najm I, Luders H. Cortical stimulation for language mapping in focal epilepsy: Correlations with tractography of the arcuate fasciculus. Epilepsia. 2010;51(4):639–646. doi: 10.1111/j.1528-1167.2009.02421.x. doi:10.1111/j.1528-1167. 2009.02421.x. [DOI] [PubMed] [Google Scholar]
- Drake M, Allegri RF, Thomson A. Executive cognitive alteration of prefrontal type in patients with mesial temporal lobe epilepsy. [Alteracion cognitiva ejecutiva de tipo prefrontal en pacientes con epilepsia del lobulo temporal mesial] Medicina. 2000;60(4):453–456. [PubMed] [Google Scholar]
- Dulay MF, Verma A, Karmonik C, Kawai M, Xue Z, Strutt AM, Grossman RG. Frontal lobe functional correlates of diffusion tensor imaging in temporal lobe epilepsy (abstract) Epilepsia. 2009;50(Suppl. 11):79. [Google Scholar]
- Focke NK, Yogarajah M, Bonelli SB, Bartlett PA, Symms MR, Duncan JS. Voxel-based diffusion tensor imaging in patients with mesial temporal lobe epilepsy and hippocampal sclerosis. Neuroimage. 2008;40(2):728–737. doi: 10.1016/j.neuroimage.2007.12.031. doi:10.1016/j.neuroimage. 2007.12.031. [DOI] [PubMed] [Google Scholar]
- Garcia Espinosa A, Andrade Machado R, Borges Gonzalez S, Garcia Gonzalez ME, Perez Montoto A, Toledo Sotomayor G. Wisconsin card sorting test performance and impulsivity in patients with temporal lobe epilepsy: Suicidal risk and suicide attempts. Epilepsy & Behavior. 2010;17(1):39–45. doi: 10.1016/j.yebeh.2009.09.010. doi:10.1016/j.yebeh.2009.09.010. [DOI] [PubMed] [Google Scholar]
- Guimaraes CA, Bonilha L, Franzon RC, Li LM, Cendes F, Guerreiro MM. Distribution of regional gray matter abnormalities in a pediatric population with temporal lobe epilepsy and correlation with neuropsychological performance. Epilepsy & Behavior. 2007;11(4):558–566. doi: 10.1016/j.yebeh.2007.07.005. doi:10.1016/j.yebeh.2007.07.005. [DOI] [PubMed] [Google Scholar]
- Hagler DJ, Jr., Ahmadi ME, Kuperman J, Holland D, McDonald CR, Halgren E, Dale AM. Automated white-matter tractography using a probabilistic diffusion tensor atlas: Application to temporal lobe epilepsy. Human Brain Mapping. 2009;30(5):1535–1547. doi: 10.1002/hbm.20619. doi:10.1002/hbm.20619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hamberger MJ, Seidel WT. Auditory and visual naming tests: Normative and patient data for accuracy, response time, and tip-of-the-tongue. Journal of the International Neuropsychological Society. 2003;9(3):479–489. doi: 10.1017/s135561770393013x. [DOI] [PubMed] [Google Scholar]
- Hermann B, Jones J, Sheth R, Dow C, Koehn M, Seidenberg M. Children with new-onset epilepsy: Neuropsychological status and brain structure. Brain. 2006;129:2609–2619. doi: 10.1093/brain/awl196. doi:10.1093/brain/awl196. [DOI] [PubMed] [Google Scholar]
- Hermann B, Seidenberg M, Lee EJ, Chan F, Rutecki P. Cognitive phenotypes in temporal lobe epilepsy. Journal of the International Neuropsychological Society. 2007;13(1):12–20. doi: 10.1017/S135561770707004X. doi:10.1017/S135561770707004X. [DOI] [PubMed] [Google Scholar]
- Holland D, Kuperman JM, Dale AM. Efficient correction of inhomogeneous static magnetic field-induced distortion in echo planar imaging. Neuroimage. 2010;50(1):175–183. doi: 10.1016/j.neuroimage.2009.11.044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hutchinson E, Pulsipher D, Dabbs K, Myers y Gutierrez A, Sheth R, Jones J, Hermann B. Children with new-onset epilepsy exhibit diffusion abnormalities in cerebral white matter in the absence of volumetric differences. Epilepsy Research. 2010;88:208–214. doi: 10.1016/j.eplepsyres.2009.11.011. doi:10.1016.j.eplepsyres.2009.11.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jovicich J, Czanner S, Greve D, Haley E, Kouwe A, Gollub R, Dale A. Reliability in multi-site structural MRI studies: Effects of gradient non-linearity correction on phantom and human data. Neuroimage. 2006;30(2):436–443. doi: 10.1016/j.neuroimage.2005.09.046. [DOI] [PubMed] [Google Scholar]
- Keller SS, Roberts N. Voxel-based morphometry of temporal lobe epilepsy: An introduction and review of the literature. Epilepsia. 2008;49(5):741–757. doi: 10.1111/j.1528-1167.2007.01485.x. doi:10.1111/j.1528-1167. 2007.01485.x. [DOI] [PubMed] [Google Scholar]
- Kim CH, Lee SA, Yoo HJ, Kang JK, Lee JK. Executive performance on the Wisconsin card sorting test in mesial temporal lobe epilepsy. European Neurology. 2007;57:39–46. doi: 10.1159/000097009. doi:10.1159/000097009. [DOI] [PubMed] [Google Scholar]
- Klingler J, Gloor P. The connections of the amygdala and of the anterior temporal cortex in the human brain. Journal of Comparative Neurology. 1960;115:333–369. doi: 10.1002/cne.901150305. [DOI] [PubMed] [Google Scholar]
- Lin JJ, Riley JD, Juranek J, Cramer SC. Vulnerability of the frontal-temporal connections in temporal lobe epilepsy. Epilepsy Research. 2008;82(2–3):162–170. doi: 10.1016/j.eplepsyres.2008.07.020. doi:10.1016/j.eplepsyres.2008.07.020. [DOI] [PubMed] [Google Scholar]
- Lin JJ, Salamon N, Lee AD, Dutton RA, Geaga JA, Hayashi KM, Thompson PM. Reduced neocortical thickness and complexity mapped in mesial temporal lobe epilepsy with hippocampal sclerosis. Cerebral Cortex. 2007;17:2007–2018. doi: 10.1093/cercor/bhl109. doi:10.1093/cercor/bhl109. [DOI] [PubMed] [Google Scholar]
- Lopes AF, Simoes MM, Robalo CN, Fineza I, Goncalves OB. Neuropsychological evaluation in children with epilepsy: Attention and executive functions in temporal lobe epilepsy. [Evaluacion neuropsicologica en ninos con epilepsia: atencion y funciones ejecutivas en epilepsia del lobulo temporal] Revista De Neurologia. 2010;50(5):265–272. [PubMed] [Google Scholar]
- Martin RC, Sawrie SM, Edwards R, Roth DL, Faught E, Kuzniecky RI, Gilliam FG. Investigation of executive function change following anterior temporal lobectomy: Selective normalization of verbal fluency. Neuropsychology. 2000;14(4):501–508. doi: 10.1037//0894-4105.14.4.501. [DOI] [PubMed] [Google Scholar]
- Martino J, Brogna C, Robles SG, Vergani F, Duffau H. Anatomic dissection of the inferior fronto-occipital fasciculus revisited in the lights of brain stimulation data. Cortex. 2010;46(5):691–699. doi: 10.1016/j.cortex.2009.07.015. doi:10.1016/j.cortex.2009.07.015. [DOI] [PubMed] [Google Scholar]
- McDonald CR, Ahmadi ME, Hagler DJ, Tecoma ES, Iragui VJ, Gharapetian L, Halgren E. Diffusion tensor imaging correlates of memory and language impairments in temporal lobe epilepsy. Neurology. 2008;71(23):1869–1876. doi: 10.1212/01.wnl.0000327824.05348.3b. doi:10.1212/01.wnl.0000327824.05348.3b. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McDonald CR, Delis DC, Kramer JH, Tecoma ES, Iragui VJ. A componential analysis of proverb interpretation in patients with frontal lobe epilepsy and temporal lobe epilepsy: Relationships with disease-related factors. The Clinical Neuropsychologist. 2008;22(3):480–496. doi: 10.1080/13854040701363828. doi:10.1080/13854040701363828. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McDonald CR, Delis DC, Norman MA, Tecoma ES, Iragui-Madoz VI. Is impairment in set-shifting specific to frontal-lobe dysfunction? evidence from patients with frontal-lobe or temporal-lobe epilepsy. Journal of the International Neuropsychological Society. 2005;11(4):477–481. [PubMed] [Google Scholar]
- McDonald CR, Hagler DJ, Jr., Ahmadi ME, Tecoma E, Iragui V, Gharapetian L, Halgren E. Regional neocortical thinning in mesial temporal lobe epilepsy. Epilepsia. 2008;49(5):794–803. doi: 10.1111/j.1528-1167.2008.01539.x. doi:10.1111/j.1528-1167.2008.01539.x. [DOI] [PubMed] [Google Scholar]
- Mueller SG, Laxer KD, Barakos J, Cheong I, Garcia P, Weiner MW. Widespread neocortical abnormalities in temporal lobe epilepsy with and without mesial sclerosis. Neuroimage. 2009;46(2):353–359. doi: 10.1016/j.neuroimage.2009.02.020. doi:10.1016/j.neuroimage.2009.02.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pereira FR, Alessio A, Sercheli MS, Pedro T, Bilevicius E, Rondina JM, Cendes F. Asymmetrical hippocampal connectivity in mesial temporal lobe epilepsy: Evidence from resting state fMRI. BMC Neuroscience. 2010;11:66. doi: 10.1186/1471-2202-11-66. doi:10.1186/1471-2202-11-66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perry ME, McDonald CR, Hagler DJ, Jr., Gharapetian L, Kuperman JM, McEvoy LK. White matter tracts associated with set-shifting in healthy aging. Neuropsychologia. 2009;47(13):2835–2842. doi: 10.1016/j.neuropsychologia.2009.06.008. doi:10.1016/j.neuropsychologia.2009.06.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Protzner AB, McAndrews MP. Network alterations supporting word retrieval in patients with medial temporal lobe epilepsy. Journal of Cognitive Neuroscience. 2011;23:2605–2619. doi: 10.1162/jocn.2010.21599. doi:10.1162/jocn.2010.21599. [DOI] [PubMed] [Google Scholar]
- Riederer F, Lanzenberger R, Kaya M, Prayer D, Serles W, Baumgartner C. Network atrophy in temporal lobe epilepsy: A voxel-based morphometry study. Neurology. 2008;71(6):419–425. doi: 10.1212/01.wnl.0000324264.96100.e0. doi:10.1212/01.wnl.0000324264.96100.e0. [DOI] [PubMed] [Google Scholar]
- Riley JD, Franklin DL, Choi V, Kim RC, Binder DK, Cramer S, Lin JJ. Altered white matter integrity in temporal lobe epilepsy: Association with cognitive and clinical profiles. Epilepsia. 2010;51(4):536–545. doi: 10.1111/j.1528-1167.2009.02508.x. doi:10.1111/j.1528-1167. 2009.02508.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Riley JD, Moore S, Cramer S, Lin JJ. Caudate atrophy and impaired frontostriatal connections are linked to executive dysfunction in temporal lobe epilepsy. Epilepsy & Behavior. 2011;21(1):80–87. doi: 10.1016/j.yebeh.2011.03.013. doi:10.1016/j.yebeh.2011.03.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seidenberg M, Kelly KG, Parrish J, Geary E, Dow C, Rutecki P, Hermann B. Ipsilateral and contralateral MRI volumetric abnormalities in chronic unilateral temporal lobe epilepsy and their clinical correlates. Epilepsia. 2005;46(3):420–430. doi: 10.1111/j.0013-9580.2005.27004.x. doi:10.1111/j.0013-9580.2005.27004.x. [DOI] [PubMed] [Google Scholar]
- Trebuchon-Da Fonseca A, Guedj E, Alario FX, Laguitton V, Mundler O, Chauvel P, Liegeois-Chauvel C. Brain regions underlying word finding difficulties in temporal lobe epilepsy. Brain. 2009;132(Pt 10):2772–2784. doi: 10.1093/brain/awp083. doi:10.1093/brain/awp083. [DOI] [PubMed] [Google Scholar]
- Urbanski M, Thiebaut de Schotten M, Rodrigo S, Catani M, Oppenheim C, Touze E, Bartolomeo P. Brain networks of spatial awareness: Evidence from diffusion tensor imaging tractography. Journal of Neurology, Neurosurgery, and Psychiatry. 2008;79(5):598–601. doi: 10.1136/jnnp.2007.126276. doi:10.1136/jnnp.2007.126276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Voineskos AN, Lobaugh NJ, Bouix S, Rajji TK, Miranda D, Kennedy JL, Shenton ME. Diffusion tensor tractography findings in schizophrenia across the adult lifespan. Brain. 2010;133(Pt 5):1494–1504. doi: 10.1093/brain/awq040. doi:10.1093/brain/awq040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wakana S, Jiang H, Nagae-Poetscher LM, van Zijl PC, Mori S. Fiber tract-based atlas of human white matter anatomy. Radiology. 2004;230:77–87. doi: 10.1148/radiol.2301021640. doi:10.1148/radiol.2301021640. [DOI] [PubMed] [Google Scholar]
- Wang XQ, Lang SY, Lu H, Ma L, Mao YL, Yang F. Executive function impairment in patients with temporal lobe epilepsy: Neuropsychological and diffusion-tensor imaging study. Zhonghua Yi Xue Za Zhi. 2007;87(45):3183–3187. [PubMed] [Google Scholar]
- Wang XQ, Lang SY, Lu H, Ma L, Mao YL, Yang F. Changes in extratemporal integrity and cognition in temporal lobe epilepsy: a diffusion tensor imaging study. Neurology India. 2010;58(6):891–899. doi: 10.4103/0028-3886.73739. doi:10.4103/0028-3886.73739. [DOI] [PubMed] [Google Scholar]
- Yogarajah M, Duncan JS. Diffusion-based magnetic resonance imaging and tractography in epilepsy. Epilepsia. 2008;49:189–200. doi: 10.1111/j.1528-1167.2007.01378.x. [DOI] [PubMed] [Google Scholar]
- Yogarajah M, Focke NK, Bonelli SB, Thompson P, Vollmar C, McEvoy AW, Duncan JS. The structural plasticity of white matter networks following anterior temporal lobe resection. Brain. 2010;133(Pt 8):2348–2364. doi: 10.1093/brain/awq175. doi:10.1093/brain/awq175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zahr NM, Rohlfing T, Pfefferbaum A, Sullivan EV. Problem solving, working memory, and motor correlates of association and commissural fiber bundles in normal aging: A quantitative fiber tracking study. NeuroImage. 2009;44(3):1050–1062. doi: 10.1016/j.neuroimage.2008.09.046. doi:10.1016/j.neuroimage.2008.09.046. [DOI] [PMC free article] [PubMed] [Google Scholar]